Abstract
Background:
Pesticide exposure has been associated with adverse health effects. We evaluated relationships between proximity to agricultural insecticide applications and insecticides in household dust, accounting for land use and wind direction.
Methods:
We measured concentrations (ng/g) of nine insecticides in carpet-dust samples collected from 598 California homes. Using a geographic information system (GIS), we integrated the California Pesticide Use Reporting (CPUR) database to estimate agricultural use within residential buffers with radii of 0.5 to 4km. We calculated the density of use (kg/km2) during 30-, 60-, 180-, and 365-day periods prior to dust collection and evaluated relationships between three density metrics (CPUR unit-based, agricultural land area adjusted, and average daily wind direction adjusted) and dust concentrations. We modeled natural-log transformed concentrations using Tobit regression for carbaryl, chlorpyrifos, cypermethrin, diazinon, and permethrin. Odds of detection were modeled with logistic regression for azinphos-methyl, cyfluthrin, malathion, and phosmet. We adjusted for season, year, occupation, and home/garden uses.
Results:
Chlorpyrifos use within 1–4km was associated with 1 to 2-times higher dust concentrations in both the 60- and 365-day periods. Carbaryl applications within 2–4km of homes 60-days prior to dust collection were associated with 3 to 7-times higher concentrations and the 4km trend was strongest using the wind-adjusted metric (p-trend=0.04). For diazinon, there were 2-times higher concentrations for the 60-day metrics in the 2km buffer and for the CPUR and wind-adjusted metrics within 4km. Cyfluthrin, phosmet, and azinphos-methyl applications within 4km in the prior 365-days were associated with 2-, 6-, and 3-fold higher odds of detection, respectively.
Conclusions:
Agricultural use of six of the nine insecticides within 4km is an important determinant of indoor contamination. Our findings demonstrated that GIS-based metrics for quantifying potential exposure to fugitive emissions from agriculture should incorporate tailored distances and time periods and support wind-adjustment for some, but not all insecticides.
Keywords: Pesticides, Insecticides, Exposure assessment, Environmental epidemiology, Dust, Agriculture
1. Introduction
In the United States (U.S.) pesticides are frequently applied to crops to control weeds and insects to produce greater yields. Annually, 899 million pounds of active ingredient are typically applied to the approximately 20% of U.S. land used for agriculture (Atwood and Paisley-Jones 2017; Bigelow and Borchers 2017). Pesticides have the potential to travel beyond the treated area through wind drift and volatilization that causes deposition in adjacent agricultural and non-agricultural areas (Farha et al. 2016; Unsworth et al. 1999). As a result, individuals living in proximity to agricultural areas with pesticide applications can be exposed through inhalation, dermal, and ingestion pathways. Epidemiologic studies have indicated that exposures to agricultural pesticides through residential proximity to pesticide applications may be associated with childhood cancer (Booth et al. 2015; Park et al. 2020; Patel et al. 2020; Reynolds et al. 2005), adult cancer (Cockburn et al. 2011; Swartz et al. 2022; Tayour et al. 2019; VoPham et al. 2015), reproductive outcomes (Eskenazi et al. 2004; Gemmill et al. 2013; Rull et al. 2006), neurobehavioral outcomes in children (Coker et al. 2017; Gunier et al. 2017a; Gunier et al. 2017b; Hyland et al. 2021; Ongono et al. 2021; Rowe et al. 2016; Shelton et al. 2014; von Ehrenstein et al. 2019), depression in adults (Furlong et al. 2020), and Parkinson’s disease (Brouwer et al. 2017; Costello et al. 2009; van der Mark et al. 2012; Wang et al. 2014).
Investigating the contribution of agricultural pesticide applications to residential exposures is challenging in large part due to the lack of detailed pesticide use reporting. In California, the largest agricultural state in the U.S. (United States Department of Agriculture 2019), the California Department of Pesticide Regulation has required reporting of all agricultural pesticide applications since 1990 for public land survey sections (PLSS; approximately 1 square mile). The California Pesticide Use Reporting (CPUR) database (California Department of Pesticide Regulation 2020b) records identify the active ingredient, date of application, crop treated, and the PLSS section. In 2017, nearly one-quarter of the pesticides applied in the U.S. were used in California (California Department of Pesticide Regulation 2020a).
Using a geographic information system (GIS), CPUR and land use data can be used to estimate pesticide use around residences (Gunier et al. 2011; Nuckols et al. 2007; Rull and Ritz 2003). A study of 89 homes in agricultural areas of central and northern California found that pesticide use within 1,250 meters of the residence was a significant determinant of concentrations of pesticide active ingredients for two herbicides, one fungicide, and two of the four insecticides assessed (Gunier et al. 2011). Here, we build on this prior work by investigating a larger number of insecticides, using expanded buffer sizes, and incorporating agricultural land use and wind direction into the exposure metrics. Our aim was to evaluate agricultural insecticide use near residences and the importance of timing of insecticide applications, location of agricultural land, and wind direction as determinants of concentrations of insecticides in homes.
2. Methods
2.1. Study Population
2.1.1. California Childhood Leukemia Study (CCLS)
Our analysis includes 577 residences from the California Childhood Leukemia Study (CCLS). The CCLS is a population-based case-control study of childhood leukemia in 35 counties in the Central Valley San Francisco Bay area (Chang et al. 2006; Ma et al. 2004; Ma et al. 2005). In 2001 to 2007, CCLS participants who were < 8 years old at diagnosis and residentially stable were invited to participate in a second interview (i.e., living in the same home at the initial and second interviews) (Ward et al. 2009) in which a carpet dust sample was collected (Colt et al. 2008). The CCLS study protocol was approved by the institutional review boards at the University of California, Berkeley, the California Committee for Protection of Human Subjects, and the National Cancer Institute.
2.1.2. Fresno Agricultural Pesticide Study (FAPS)
The Fresno Agricultural Pesticide Study (FAPS) in Fresno County, California, was a complementary exposure study conducted within the same period using the same sampling and laboratory analysis protocol and similar questionnaires (Deziel et al. 2013; Gunier et al. 2011). Interviews in 21 homes took place from 2003 to 2005. The FAPS study protocol was approved by the institutional review boards at Colorado State and Fresno State Universities and the National Cancer Institute.
2.2. Interviews and Dust Sampling
In both studies, interviewers took a Global Positioning System (GPS) reading just outside of the home. Experienced interviewers asked participants about their pesticide use using standardized questions and visual aids that identified different types of pests. Home and garden use was ascertained by asking about specific pest treatments in the previous twelve months including treatments for ants/cockroaches, bees/wasps/hornets, flea/ticks in the home and on pets, flies/mosquitos, indoor insects, lawn and garden weeds, and professional indoor and outdoor treatments (Deziel et al. 2015). Participants were also asked if they removed their shoes before entering the home, and if in the previous twelve months anyone in the home had worked as a 1) farmer/farm or ranch worker; 2) gardener, landscaper, nursery worker, or groundskeeper; 3) packer or agricultural worker; or 4) pesticide handler or mixer.
The methods for dust sampling have been previously described (Colt et al. 2008). In short, a carpet dust sample was collected using a high-volume surface sampler vacuum (HVS3; Cascade Stack Sampling System, Venice, FL) for all the FAPS and most of the CCLS participants; if the amount of HVS3 dust was insufficient, household vacuum dust was collected by removing the used bag or emptying the loose dust from the canister into a sealable polyethylene bag. When scheduling the interview, participants were instructed not to change their vacuum bag for at least a week before the scheduled interview date. In the FAPS, the sample room was selected from the rooms that were located on the side of the home facing crops. In the CCLS, parents were asked to identify the room in which their child spent most of their waking time in the year prior to the diagnosis or reference date. If the room had a carpet or area rug measuring at least 0.84 square meters (nine square feet) and it was present before the reference date, a dust sample was collected. In the final analytic sample 168 samples (28%) were from the household vacuum and 430 (72%) were from the HVS3.
2.3. Laboratory Analysis
We measured 13 insecticides that had agricultural use in the study area counties during the study period. Allethrin, azinphos-methyl, carbaryl, chlorpyrifos, cyfluthrin, cypermethrin, deltamethrin, diazinon, malathion, permethrin, phosmet, propoxur, and tetramethrin were detected in >5% of dust samples and were included in our analyses. Isomers were measured for allethrin (1 and 2), cyfluthrin (1–4), cypermethrin (1–4), tetramethrin (1 and 2), and permethrin (cis and trans). Dust samples were shipped to the Battelle Memorial Institute (Columbus, OH) where they were stored in −20° Celsius freezers until analysis. Methods describing the laboratory quantification methods (Colt et al. 2008), quality control procedures, and detection limits have been previously described (Madrigal et al. 2021). Briefly, the 13 insecticides were extracted using a hexane:acetone solution. Extracts were quantified using gas chromatography/mass spectrometry. Detection limits ranged from 2 ng/g dust for permethrin to 100 ng/g dust for azinphos-methyl (Table 1) and mean sample recoveries ranged from 73% for malathion, phosmet, diazinon and chlorpyrifos to 122% for azinphos-methyl. The mean percent differences for within batch duplicates ranged from 0.5% for tetramethrin 1 to 38% for carbaryl.
Table 1.
Detection limits, percent detections, and concentrations for 13 agricultural insecticides measured in 598 dust samples from California homes
| Insecticide | Detection limit (ng/g) | Detected (%) | Median (interquartile range) concentration among homes with detection (ng/g)a |
|---|---|---|---|
| Allethrinb | 20 | 7.4 | 251.8 (86.1–615.8) |
| Azinphos-methyl | 100 | 7.5 | 126.2 (80.3–219.7) |
| Malathion | 10 | 8.7 | 93.7 (58.0–192.1) |
| Deltamethrin | 50 | 11.9 | 452.1 (182.8–1046.3) |
| Tetramethrinb | 2 | 12.2 | 116.9 (39.8–350.3) |
| Cyfluthrinb | 20 | 26.1 | 468.8 (221.4–946.8) |
| Phosmet | 25 | 28.1 | 22.4 (11.1–72.9) |
| Cypermethrinb | 20 | 49.5 | 569.6 (251.4–1366.1) |
| Propoxur | 5 | 66.7 | 22.3 (9.2–53.8) |
| Carbaryl | 2 | 68.9 | 30.2 (13.3–91.9) |
| Diazinon | 2 | 80.4 | 15.9 (6.2–45.1) |
| Chlorpyrifos | 5 | 89.6 | 32.9 (15.8–87.7) |
| Permethrinb | 2 | 99.8 | 1069.5 (387.5–4296.0) |
Distribution among residences with insecticide concentrations above the detection limit
Isomers have been summed
2.4. Pesticide Use Metrics
We obtained CPUR data for insecticide applications for years 1999–2007 to correspond with the 365-day period prior to the dust collection. Data included the insecticide applied, application date, pounds applied, and PLSS area. As described previously (Gunier et al. 2011; Nuckols et al. 2007), we used a geographic information system (GIS) to create a CPUR density metric for each insecticide. This metric is defined as the sum of area-weighted kilograms of insecticide active ingredient applied in a circular buffer around a participant’s residence within a time period that intersected the PLSS, divided by the area of the buffer. We created metrics for buffers with radii of 0.5-, 1-, 2-, and 4-km (buffer areas: 0.79 km2, 3.14 km2, 12.57 km2, and 50.27 km2, respectively) for each of four time periods (30, 60, 180, 365 days before dust collection).
2.4.1. CPUR Unit-based Density Metric
The CPUR density metric was computed for each residence, insecticide active ingredient, and buffer as follows (“CPUR method”; Equation 1, Figure 1): , where k is the insecticide active ingredient used in n sections intersected by the buffer around the residence, Aj is the acreage of section j within the buffer, Tj is the total acreage of section j, and Xj is the kilograms of insecticide applied in the section, j, during specific time periods.
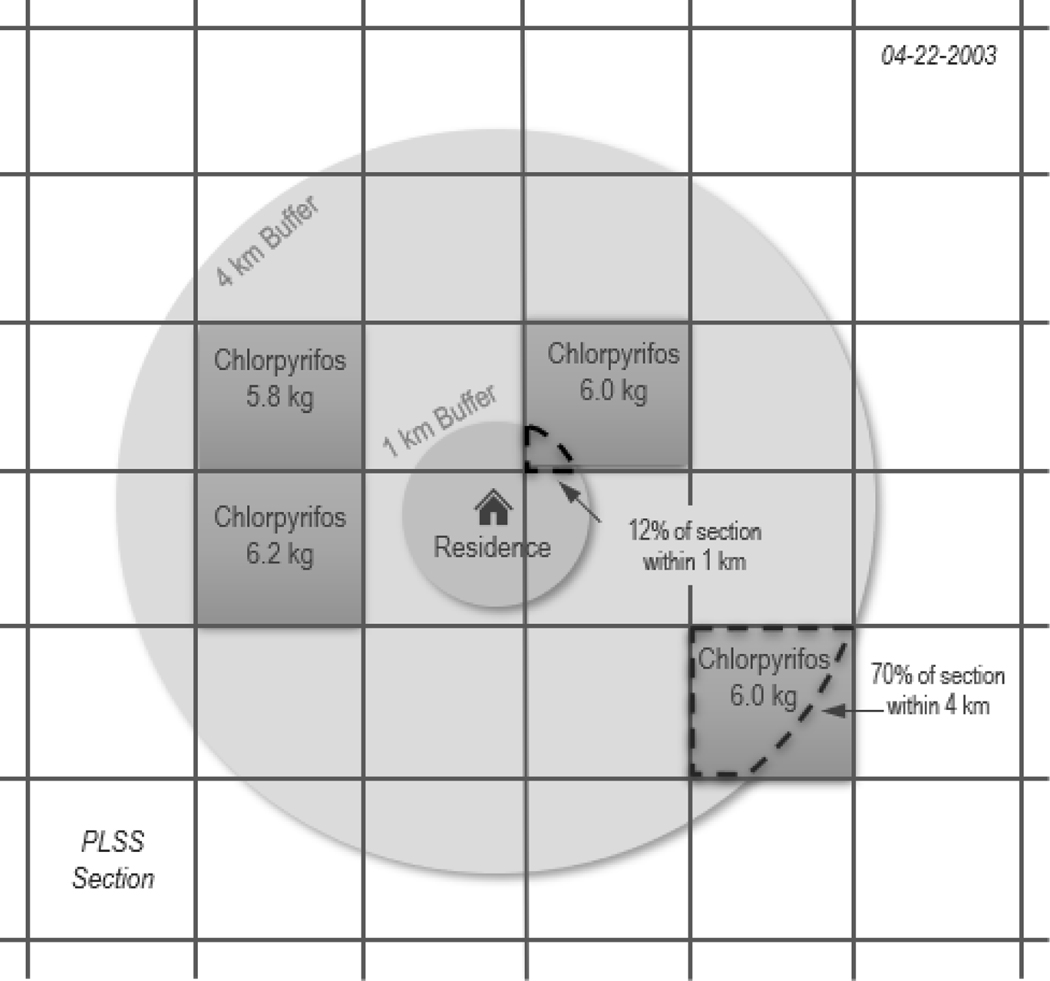
Figure 1.
Illustration of the CPUR density metric for the 1km and 4km buffer around the residence on one day for four application sites. In the 1km buffer, one application of chlorpyrifos is included after weighting the 6.0 kg applied by 12% due to most of the public land survey section (PLSS) being outside of the buffer. In the 4km buffer, three applications of chlorpyrifos are included in entirety and one application is weighted by 70% due to 30% of the PLSS being outside of the buffer.
2.4.2. Crop Area-adjusted CPUR Metric
This metric refines estimates of pesticide use density by restricting the effective area of pesticide application to areas of agricultural land with reported use of our study insecticides by section and time period in the CPUR database. To identify areas of agricultural land, we used information on pasture/hay and row crops (e.g., vegetables, grains, orchards, and vineyards), termed agricultural land hereafter, from the National Land Cover Database (NLCD) (30 m resolution) versions 2001, 2004, and 2006 (Fry et al. 2011; Homer et al. 2007; Xian et al. 2009). We linked residence locations to the version of the database that most closely corresponded to the period prior to the dust collection (e.g., visits that took place in 2003 were linked to the 2001 NLCD). Areas of agricultural land that intersected the buffers within each PLSS were summed. We excluded CPUR applications for which no agricultural land was identified by NLCD within the buffer (31% and 7% of all CPUR reported applications of our study insecticides in the 0.5km and 4km buffers, respectively). We computed a crop-area density metric (CROP-A; Figure 2) by multiplying the proportion of agricultural land within the buffer by the amount of insecticide applied to the section, summing across all the sections within the buffer, and dividing by the agricultural land area (Equation 2): , where k is the insecticide active ingredient used on crop type i in the n sections intersected by the buffer around the residence; m is the total number of crop types on which insecticide k was applied in section j, Aij is the acreage of crop type i within section j within the buffer, Tij is the total acreage of crop types i within section j, and Xij is the kilograms of insecticide applied to crop type i in section j.
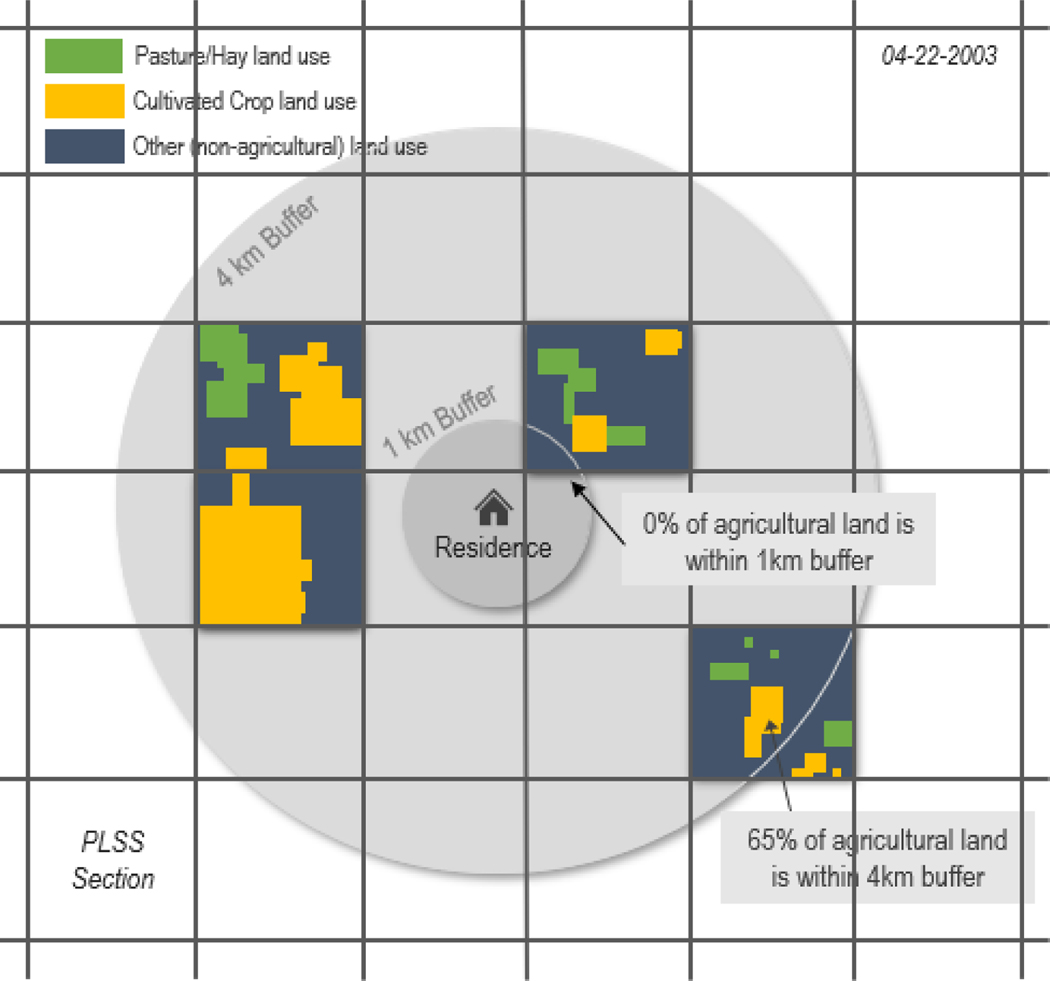
Figure 2.
Illustration of the CROP-A metric for the 1km and 4km buffer around the residence on one day for four application sites. The applications of chlorpyrifos are matched to the acres of pasture/hay and cultivated crops identified in each public land survey section (PLSS) within the buffer. The metric is refined by weighting the amount of chlorpyrifos applied by the proportion of pasture/hay and cultivated crops (i.e., agricultural land) identified in each PLSS within the buffer.
2.4.3. Wind-adjusted CPUR Metric
To account for potential insecticide drift due to wind conditions on the date of application and subsequent days until the date of residential dust collection, we created a metric that adjusted for the proportion of days the home was downwind of insecticide applications (Figure 3). Information on daily wind direction was derived from the North American Regional Reanalysis (NARR) database which provides meteorological data at a spatial resolution of 32km × 32km and temporal resolution of 1 day (Mesinger et al. 2006). Daily NARR wind raster data at the 32km scale were used to determine the daily wind direction at the PLSS centroids that were intersected by the buffers. We determined the direction between the centroid of each portion of the section within the buffer relative to the home and weighted insecticide use in each section according to the percentage of time the wind blew from those locations during the period between application and dust collection. We considered the contributing area for pesticide drift to be within a 90° downwind ‘capture zone’ wedge centered on the wind direction and anchored at the section centroid. The wind-adjusted CPUR density metric (W-CPUR) was computed for each insecticide active ingredient as follows (Equation 3): , where k is the insecticide active ingredient used in n sections intersected by the buffer around the residence, Aj is the acreage of section j within the buffer, Tj is the total acreage of section j, Xj is the kilograms of insecticide applied in the section j, and Wj is the proportion of days the home was within 90° downwind of the centroid of section j during the specific time period. Daily average wind direction estimated at the centroid of each PLSS was assumed constant over the entire PLSS.
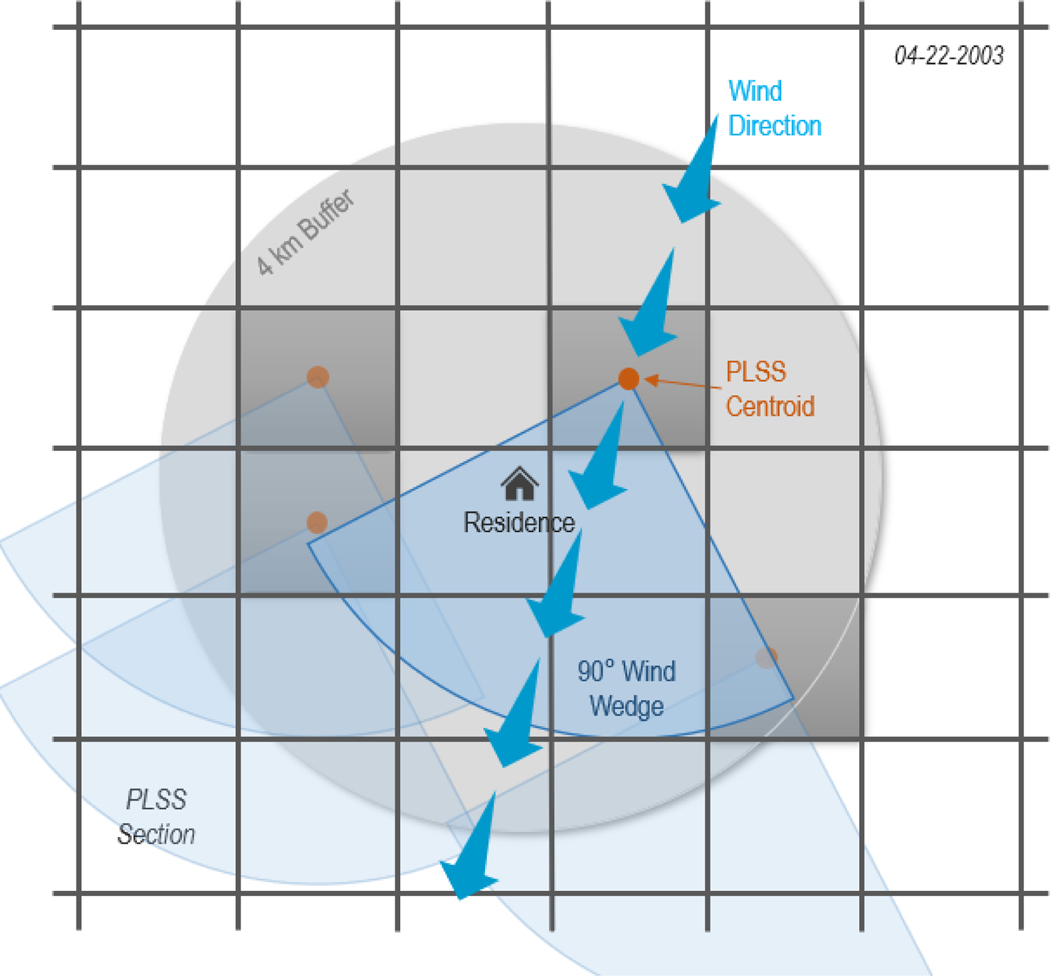
Figure 3.
Illustration of the W-CPUR metric within a 4km buffer around the residence on one day for four application sites. The wind direction is determined for each day between application and dust collection for each public land survey section (PLSS) centroid with a chlorpyrifos application within the buffer to calculate the proportion of days the home was downwind of the application site.
2.5. Statistical Analyses
We computed detection frequencies and determined that the insecticide dust concentrations (ng/g) were not normally distributed; therefore, non-parametric tests were used to compare distributions. For insecticides that had at least a 40% detection rate in the dust (cypermethrin, propoxur, carbaryl, diazinon, chlorpyrifos, permethrin), we used a single imputation method (Lubin et al. 2004) to assign values for each sample where the insecticide was below the detection limit. We included isomers of each insecticide as covariates in the imputation model; all insecticide isomers were summed (cyfluthrin, cypermethrin, tetramethrin, permethrin) for analysis.
We examined the number of residences with agricultural insecticide applications within each of the 0.5km to 4km buffers. Four insecticides were excluded from further analysis due to their limited applications in the buffers surrounding homes in our study population (allethrin (zero homes), deltamethrin (1–6 homes within 0.5–4km), propoxur (zero homes), and tetramethrin (1 home within 0.5–4km). For the other nine insecticides, we calculated the median density (kg/km2) within each buffer for each metric (CPUR, CROP-A, and W-CPUR). We computed Spearman rank correlations to evaluate the associations between the three metrics for each insecticide, buffer, and time period. Then we used the 365-day CPUR metric to compare dust detection frequencies among participants with and without insecticide applications, and for the five insecticides detected in ≥40% of dust samples we compared median dust concentrations.
For active ingredients detected in <40% of dust samples (azinphos-methyl, malathion, cyfluthrin, phosmet), we used logistic regression to individually model the odds of each insecticide being detected in the dust (detect vs. non-detect; dependent variable). For active ingredients detected in >40% of dust samples (cypermethrin, carbaryl, diazinon, chlorpyrifos, permethrin), we used Tobit regression to individually model the natural log transformed dust concentrations (dependent variable) and exponentiated the β-coefficients to show the associated unit increase in the predicted dust value. In both the logistic and Tobit regression models we assessed the CPUR, CROP-A, and W-CPUR metrics (independent variables) separately for each active ingredient as an indicator variable (any applications vs. none) for each buffer and time period. In addition, we categorized each metric as no use within the buffer, ≤ median, and > median for insecticides with 10–25% of homes with potential exposure, and as tertiles with a separate non-exposed category for insecticides with more than 25% of homes exposed. Median and tertile cutpoints were based on the distribution of the CPUR metric. We examined the following covariates using a forward selection approach: year (continuous variable from 2001 to 2006, centered by subtracting 2000) and season (winter, spring, summer, and fall) of dust collection, and binary variables for self-reported usual removal of shoes before entering the home, home and garden use of products for the control of specific pests (ants, bees, fleas/ticks, and flies) and weeds, and agricultural occupations. Any covariate with a p-value ≤0.1 was retained in the final multivariable model. For each insecticide, we examined associations for consistency between time periods, buffer sizes, and metrics, with presentation of the strongest statistically significant associations for each insecticide in the main results table. All analyses were conducted in SAS version 9.4 (Cary, NC).
3. Results
The insecticides with the lowest detection frequencies were allethrin (7.4%), azinphos-methyl (7.5%), and malathion (8.7%); whereas, detections were highest for diazinon (80.4%), chlorpyrifos (89.6%), and permethrin (99.8%) (Table 1). Among homes with these insecticides detected in the dust, median concentrations ranged from 15.9 ng/g for diazinon to 1069.5 ng/g for permethrin.
Agricultural insecticide applications in the 365 days before dust collection (Table 2) were most frequent for chlorpyrifos in each buffer (CPUR metric: 0.5km: 20%, 2km: 36%, and 4km: 48%); whereas azinphos-methyl was the least applied. For the 0.5 km buffer, the median density (CPUR metric) in exposed homes ranged from 0.1 kg/km2 for cyfluthrin to 6.5 kg/km2 for phosmet. For the 4km buffer, the densities ranged from 0.03 kg/km2 for cyfluthrin to 3.4 kg/km2 for chlorpyrifos. For the CROP-A metric, the proportion of exposed homes was smaller but most median densities were similar to those for the CPUR metric. The W-CPUR metric generally had the lowest density compared to the CPUR and CROP-A metrics, and the prevalence of exposure was generally lower than that of the CPUR metric, but higher than the CROP-A metric. As expected, the number of applications and densities decreased for 30, 60, and 180 days before dust collection (data not shown).
Table 2.
Percent of homes with insecticide applications and density of insecticidea use (kg/km2) within 0.5km, 2km, and 4km among homes with CPUR applications within 365 days before dust collection for three exposure metrics (CPURb, CROP-Ac, and W-CPURd)
| Insecticide | Metric | Residences within 0.5km, n (%) | Median (IQR) density of use 0.5km, kg/km2 | Residences within 2km, n (%) | Median (IQR) density of use 2km, kg/km2 | Residences within 4km, n (%) | Median (IQR) density of use 4km, kg/km2 |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Azinphos-methyl | CPUR | 13 (2.2) | 1.8 (1.1–5.6) | 49 (8.2) | 0.7 (0.2–2.4) | 101 (16.9) | 0.6 (0.1–1.1) |
| CROP-A | 13 (2.2) | 2.2 (0.2–8.0) | 43 (7.2) | 0.7 (0.3–2.0) | 97 (16.2) | 0.6 (0.1–1.1) | |
| W-CPUR | 12 (2.0) | 0.5 (0.2–0.9) | 41 (6.9) | 0.1 (0.01–0.5) | 91 (15.2) | 0.06 (0.01–0.3) | |
|
| |||||||
| Carbaryl | CPUR | 30 (5.0) | 2.5 (0.9–11.4) | 102 (17.1) | 0.4 (0.1–2.2) | 184 (30.8) | 0.4 (0.05–1.5) |
| CROP-A | 23 (3.8) | 2.0 (0.7–12.6) | 91 (15.2) | 0.4 (0.1–3.1) | 173 (28.9) | 0.4 (0.1–1.5) | |
| W-CPUR | 26 (4.3) | 0.7 (0.05–2.0) | 95 (15.9) | 0.1 (0.01–0.3) | 171 (28.6) | 0.1 (0.01–0.3) | |
|
| |||||||
| Chlorpyrifos | CPUR | 121 (20.2) | 5.5 (1.2–14.0) | 216 (36.1) | 3.4 (0.7–12.4) | 287 (48.0) | 3.4 (0.3–10.4) |
| CROP-A | 95 (15.9) | 5.6 (1.3–16.8) | 193 (32.3) | 4.6 (1.2–12.8) | 265 (44.3) | 3.9 (0.5–12.1) | |
| W-CPUR | 114 (19.1) | 1.0 (0.13–3.3) | 212 (35.4) | 0.7 (0.1–3.1) | 280 (46.8) | 0.7 (0.04–3.3) | |
|
| |||||||
| Cyfluthrin | CPUR | 36 (6.0) | 0.1 (0.02–0.3) | 100 (16.7) | 0.03 (0.004–0.1) | 168 (28.1) | 0.03 (0.003–0.1) |
| CROP-A | 29 (4.8) | 0.08 (0.03–0.3) | 91 (15.2) | 0.04 (0.004–0.1) | 154 (25.7) | 0.03 (0.004–0.1) | |
| W-CPUR | 31 (5.2) | 0.01 (0.001–0.05) | 95 (15.9) | 0.003 (0.001–0.01) | 162 (27.1) | 0.003 (0.0003–0.02) | |
|
| |||||||
| Cypermethrin | CPUR | 28 (4.7) | 0.2 (0.07–1.0) | 68 (11.4) | 0.2 (0.05–0.7) | 106 (17.7) | 0.1 (0.02–0.4) |
| CROP-A | 19 (3.2) | 0.2 (0.05–0.6) | 59 (9.9) | 0.2 (0.1–0.6) | 99 (16.6) | 0.1 (0.02–0.4) | |
| W-CPUR | 26 (4.3) | 0.04 (0.01–0.11) | 62 (10.4) | 0.02 (0.01–0.1) | 96 (16.0) | 0.02 (0.003–0.1) | |
|
| |||||||
| Diazinon | CPUR | 61 (10.2) | 3.0 (0.4–7.3) | 162 (27.1) | 1.0 (0.1–4.2) | 238 (40.0) | 0.8 (0.05–3.5) |
| CROP-A | 42 (7.0) | 3.5 (0.8–7.7) | 139 (23.2) | 1.2 (0.2–4.8) | 211 (35.3) | 1.0 (0.1–4.2) | |
| W-CPUR | 57 (9.5) | 0.4 (0.06–2.1) | 158 (26.4) | 0.1 (0.01–0.8) | 232 (38.8) | 0.1 (0.005–0.7) | |
|
| |||||||
| Malathion | CPUR | 30 (5.0) | 1.9 (0.2–14.6) | 98 (16.4) | 0.7 (0.09–5.2) | 189 (31.6) | 0.3 (0.04–1.7) |
| CROP-A | 24 (4.0) | 1.6 (0.2–13.4) | 87 (14.5) | 0.7 (0.1–5.1) | 170 (28.4) | 0.4 (0.1–2.0) | |
| W-CPUR | 29 (4.8) | 0.2 (0.03–3.3) | 95 (15.9) | 0.1 (0.01–0.9) | 175 (29.3) | 0.05 (0.004–0.4) | |
|
| |||||||
| Permethrin | CPUR | 48 (8.0) | 0.6 (0.1–2.3) | 137 (22.9) | 0.1 (0.02–0.7) | 209 (35.0) | 0.1 (0.02–0.7) |
| CROP-A | 37 (6.2) | 0.5 (0.1–2.7) | 127 (21.2) | 0.1 (0.02–0.8) | 200 (33.4) | 0.1 (0.02–0.7) | |
| W-CPUR | 45 (7.5) | 0.07 (0.02–0.57) | 128 (21.4) | 0.04 (0.005–0.2) | 201 (33.6) | 0.03 (0.003–0.2) | |
|
| |||||||
| Phosmet | CPUR | 48 (8.0) | 6.5 (0.8–17.7) | 112 (18.7) | 2.5 (0.5–11.7) | 178 (30.0) | 1.4 (0.2–7.5) |
| CROP-A | 38 (6.3) | 7.1 (3.0–19.1) | 103 (17.2) | 3.6 (0.5–12.9) | 165 (27.6) | 1.8 (0.3–7.7) | |
| W-CPUR | 46 (7.7) | 1.2 (0.1–3.6) | 106 (17.7) | 0.3 (0.03–2.1) | 166 (27.8) | 0.4 (0.03–2.0) | |
Four insecticides were excluded from further analysis due to their limited applications in the buffers surrounding homes in our study population (allethrin (zero homes), deltamethrin (1–6 homes within 0.5–4km), propoxur (zero homes), and tetramethrin (1 home within 0.5–4km)
CPUR= CPUR Metric (kg/km2): insecticide use density proportional to the area of the buffer
CROP-A= Crop area adjusted CPUR Metric (kg/km2): insecticide use density proportional to the area of cultivated crops and pasture/hay in the buffer
W-CPUR= Wind-adjusted CPUR Metric (kg/km2): insecticide use density proportional to the area of the buffer, weighted to account for the proportion of days the residence was downwind of the section in which the insecticide was applied during the period between application and dust collection
We evaluated the Spearman rank correlations between the pesticide use density metrics for each insecticide (Supplemental Table 1). Correlations for the 30-day period were similar to those for 60-days, and correlations for the 180-day period were similar to those for 365-days. Therefore, we present results only for 60-day and 365-day metrics. Correlations between CPUR and CROP-A were high (ρs≥0.67) for all the insecticides except for 60-day carbaryl and 60- and 365-day diazinon, malathion, and permethrin in the 0.5km buffer. Likewise, correlations between CPUR and W-CPUR were high (ρs≥0.67) except for 60-day and 365-day cypermethrin and phosmet metrics in the 0.5km buffers and 60-day azinphos-methyl, carbaryl, cypermethrin, and malathion metrics in the 2km buffers. Correlations between CROP-A and CPUR-W were ρs<0.67 for carbaryl, chlorpyrifos, cypermethrin, diazinon, malathion, and phosmet (0.5km/60day metrics) and for azinphos-methyl, carbaryl, cypermethrin, and malathion (2km/60-day metrics). All correlations were high between all the metrics in the 4km buffer.
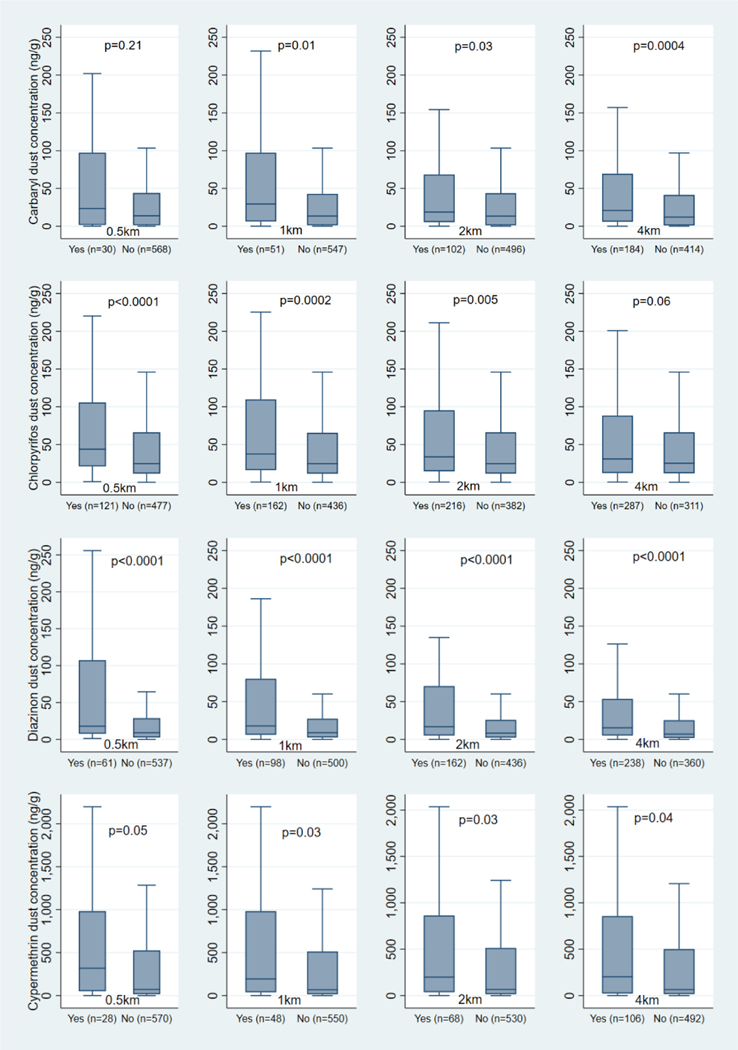
For the five insecticides with >40% detections, we compared concentrations in homes with and without nearby use based on the CPUR metric in the prior 365 days. Generally, median concentrations in exposed homes decreased slightly (carbaryl, chlorpyrifos, cypermethrin) or stayed the same (diazinon, permethrin) with increasing buffer size. In the 0.5km buffer, chlorpyrifos, cypermethrin, and diazinon concentrations were 2–4 times higher in homes with agricultural applications compared to homes without (Figure 4). Median carbaryl, chlorpyrifos, and diazinon concentrations were 1.5–2 times greater in homes with applications, whereas cypermethrin concentrations were three times greater in homes with agricultural applications within the 1–4km buffers. Permethrin concentrations did not differ among homes with and without nearby insecticide use (not shown).
Figure 4.
Distributions of carbaryl, chlorpyrifos, diazinon, and cypermethrin concentrations in house dust of homes with (Yes) and without (No) nearby agricultural use in the prior 365 days
Yes=Homes with CPUR applications (exposed); No=Homes without CPUR applications (unexposed); p-values from Wilcoxon rank sum test used to compare the distribution of the insecticide concentration in the dust among homes classified as exposed and those classified as unexposed; dust concentrations with values 1.5 times the IQR above the upper quartile or below the lower quartile were excluded from the graphs.
When we evaluated 30-, 60- and 180-day time periods between application and dust for these insecticides, only carbaryl showed substantial differences from the 365 results. In the 30 and 60 days before dust collection, carbaryl concentrations were 2.5–11 times greater in homes with applications (Supplemental Table 2). The only other notable differences from the 365-day results were for cypermethrin and permethrin. Homes with cypermethrin applications within 180 days in the 4km buffer had 3-times higher median dust concentrations compared to homes without applications (p=0.04), but there were no differences for the other buffers and time periods (not shown). Permethrin concentrations in the 60-day period within 2 km and in the 30-day period within 2–4 km, were 2 times higher among unexposed homes (p<0.05; data not shown).
Results of multivariable models for carbaryl, chlorpyrifos, cypermethrin, and diazinon for 60-day CPUR, CROP-A, and W-CPUR metrics are shown in Table 3. All three metrics were associated with concentrations of carbaryl, diazinon, and chlorpyrifos dust concentrations. For carbaryl, the metrics were associated with 3–5 times significantly greater concentrations in the 4km buffers and the linear trend across density categories was only significant for the W-CPUR metric (p-trend=0.04; Table 3). In the 2km buffer, the 60-day metrics were associated with elevated concentrations. Year, season, and shoe removal were inversely associated and use of flea or tick products and treatments for lawn/garden weeds were positively associated with carbaryl concentrations. Associations for the 365-day metrics were positive but weaker than the 60-day metrics and none were significant (Supplemental Table 3).
Table 3.
Multivariable associationsa of 60-day insecticide density metrics (kg/km2) with concentrations of four insecticides detected in ≥40% of house-dust samples
| Insecticide | Buffer | 60-day CPURb | 60-day CROP-Ac | 60-day W-CPURd | |||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Exposure density (kg/km2) vs. 0 | Exp β (95% CI) | Exposure density (kg/km2) vs. 0 | Exp β (95% CI) | Exposure density (kg/km2) vs. 0 | Exp β (95% CI) | ||
|
|
|||||||
| Cypermethrin e | 0.5km | >0 | 0.8 (0.1–9.6) | >0 | 0.3 (0.0–8.3) | >0 | 4.9 (0.2–100.4) |
|
| |||||||
| 1km | >0 | 0.8 (0.1–5.5) | >0 | 1.6 (0.2–15.4) | >0 | 1.3 (0.1–14.4) | |
|
| |||||||
| 2km | >0 | 1.4 (0.3–6.5) | >0 | 1.5 (0.3–7.6) | >0 | 1.9 (0.3–10.9) | |
|
| |||||||
| 4km | >0 | 1.7 (0.5–5.8) | >0 | 2.3 (0.7–7.7) | >0 | 1.3 (0.3–5.5) | |
|
| |||||||
| Carbaryl f | 0.5km | >0 | 10.3 (0.7–143.0) | >0 | 10.3 (0.7–143.0) | >0 | 27.8 (0.8–913.1) |
|
| |||||||
| 1km | >0 | 6.4 (0.8–53.9) | >0 | 6.4 (0.8–53.9) | >0 | 8.5 (0.7–103.2) | |
|
| |||||||
| 2km | >0 | 6.3 (1.7–24.0) | >0 | 5.1 (1.2–21.1) | >0 | 6.9 (1.3–36.0) | |
|
| |||||||
| 4km | ≤0.11 > 0.11 | 4.2 (1.3–14.2) 3.4 (1.0–12.2) p-trend=0.05 |
≤0.13 > 0.13 |
5.0 (1.4–17.4) 3.1 (0.9–11.3) p-trend=0.08 |
≤0.04 > 0.04 | 1.8 (0.4–8.1) 4.7 (1.1–20.5) p-trend=0.04 |
|
|
| |||||||
| Diazinon g | 0.5km | >0 | 3.1 (1.3–7.6) | >0 | 2.5 (0.9–7.3) | >0 | 2.8 (1.1–7.5) |
|
| |||||||
| 1km | >0 | 1.7 (0.9–3.3) | >0 | 2.5 (1.2–5.2) | >0 | 1.3 (0.6–2.6) | |
|
| |||||||
| 2km | ≤ 0.4 > 0.4 |
0.9 (0.4–1.9) 2.5 (1.2–4.9) p-trend=0.01 |
≤ 0.5 > 0.5 |
1.2 (0.6–2.4) 2.5 (1.2–5.1) p-trend=0.01 |
≤ 0.11 > 0.11 |
0.8 (0.4–1.9) 2.4 (1.1–5.3) p-trend=0.03 |
|
|
| |||||||
| 4km | ≤ 0.3 > 0.3 |
1.4 (0.8–2.5) 2.0 (1.1–3.5) p-trend=0.03 |
≤ 0.4 > 0.4 |
1.8 (1.0–3.4) 1.8 (1.0–3.3) p-trend=0.07 |
≤ 0.05 > 0.05 |
1.4 (0.7–2.7) 2.2 (1.1–4.1) p-trend=0.02 |
|
|
| |||||||
| Chlorpyrifos h | 0.5km | >0 | 1.4 (0.9–2.2) | >0 | 1.2 (0.7–2.1) | >0 | 1.1 (0.6–1.9) |
|
| |||||||
| 1km | ≤ 1.3 > 1.3 |
1.1 (0.7–1.9) 1.8 (1.0–3.0) p-trend=0.04 |
≤ 0.9 > 0.9 |
1.3 (0.7–2.4) 1.5 (0.9–2.7) p-trend=0.14 |
≤ 0.3 > 0.3 |
0.9 (0.5–1.6) 1.8 (1.0–3.4) p-trend=0.06 |
|
|
| |||||||
| 2km | ≤ 0.93 > 0.93 |
1.0 (0.7–1.5) 1.4 (0.9–2.2) p-trend=0.09 |
≤ 1.1 > 1.1 |
1.2 (0.8–1.9) 1.5 (1.0–2.3) p-trend=0.08 |
≤ 0.3 > 0.3 |
1.0 (0.6–1.6) 1.3 (0.8–2.1) p-trend=0.25 |
|
|
| |||||||
| 4km | ≤ 0.23 0.24–1.7 > 1.7 |
0.8 (0.5–1.2) 1.1 (0.7–1.7) 1.7 (1.1–2.6) p-trend=0.01 |
≤ 0.30 0.31–1.9 > 1.9 |
0.9 (0.6–1.4) 1.1 (0.7–1.8) 1.8 (1.2–2.9) p-trend=0.006 |
≤ 0.05 0.06–0.6 > 0.6 |
0.8 (0.5–1.3) 1.4 (0.9–2.3) 1.9 (1.2–2.9) p-trend=0.006 |
|
For each insecticide, three separate Tobit regression models were used to model the natural log transformed dust concentrations (dependent variable) with each density metric (CPUR, CROP-A, and W-CPUR) comparing >0 or density categories to homes with no use. A forward approach was used to evaluate covariates. Any covariate with a p-value ≤0.1 was retained in the final multivariable model.
CPUR= CPUR Metric (kg/km2): insecticide use density proportional to the area of the buffer
CROP-A= Crop area adjusted CPUR Metric (kg/km2): insecticide use density proportional to the area of cultivated crops and pasture/hay with insecticide use in the buffer
W-CPUR= Wind-adjusted CPUR Metric (kg/km2): insecticide use density proportional to the area of the buffer, weighted to account for the proportion of days the residence was downwind of the section in which the insecticide was applied during the period between application and dust collection
Adjusted for the use of flea or tick products, and treatments for ants and flies that were positively associated with cypermethrin concentrations.
Adjusted for year, season, and shoe removal that were inversely associated with carbaryl concentrations and use of flea or tick products, and treatments for lawn/garden weeds that were positively associated with carbaryl concentrations.
Adjusted for year and season that were inversely associated with concentrations and use of flea or tick products and farm working occupation that were positively associated with concentrations.
Adjusted for year that was inversely associated with concentrations and farm working occupation that was positively associated.
For diazinon, we observed a 2- to 2.5-fold increase with significant linear trends for the CPUR and W-CPUR metrics in the 4km buffer and for all metrics in the 2 km buffer (Table 3). In the 1km buffer, the CROP-A metric was a predictor of dust concentrations, whereas the CPUR and the W-CPUR metrics were positive but not statistically significant. The 365-day metrics in the 1km buffer were predictors of dust concentrations but only the W-CPUR metric had a significant linear trend (Supplemental Table 3). In the other buffers, we observed elevated concentrations for all metrics, but none of the trends were significant.
For chlorpyrifos, all three 4km metrics showed associations with concentrations that were of similar magnitude with significant trends across density tertiles (Table 3). In the 1km buffer, the CPUR and W-CPUR metrics were predictors compared to the CROP-A metric. The 365-day chlorpyrifos metrics showed similar patterns to the 60-day metrics and similar magnitudes of association with >median and the highest tertile categories (Supplemental Table 3).
For cypermethrin, none of the metrics predicted dust concentrations but household use of flea/tick products, and treatments for ants and flies were positively associated with concentrations (Table 3). Results for the 365-day metric were similar (Supplemental Table 3).
None of the CPUR metrics during any period or buffer size were predictors of permethrin concentrations (Supplemental Table 3). Year of dust collection, use of flea or tick products, and treatments for ants, bees, and flies were positively associated with permethrin dust concentrations.
For the four insecticides detected in <40% of homes, the percent detections in homes with and without CPUR applications in the prior 365 days are shown in Supplemental Table 4. Azinphos-methyl was detected more frequently in exposed homes compared to unexposed homes for the 2–4km buffers. Cyfluthrin detections were more frequent in exposed homes in the 0.5 and 1km buffers, but not the larger buffers. There was no difference in the proportion detected in exposed and unexposed homes for malathion for any buffer. For phosmet, exposed homes had higher detection frequencies than unexposed homes for all buffers. The patterns were similar for earlier time periods (not shown).
With these four insecticides, we did not have sufficient exposure data to model more than a yes/no agricultural exposure variable for the 60-day metrics, therefore we present the results of the 365-day metrics. For cyfluthrin, the 365-day 0.5km CPUR, CROP-A and W-CPUR metrics showed the strongest associations with the odds of detection of cyfluthrin, and the 4km metrics showed significant trends with increasing density except for the cyfluthrin W-CPUR metric (Table 4). For the 60-day metrics (Supplemental Table 5) odds of detection were about six-fold higher for the 0.5km CPUR and CROP-A metrics, but there was no association with the W-CPUR metric. Point estimates were non-significant for the other buffers for all of the metrics. Year of dust collection, use of flea or tick products, and treatments for lawn/garden weeds were positively associated with cyfluthrin dust detections.
Table 4.
Multivariable associationsa of 365-day insecticide metrics with detections of three insecticides detected in <40% of house-dust samples
| Insecticide | Buff er | 365-day CPURb | 365-day CROP-Ac | 365-day W-CPURd | |||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Exposure density (kg/km2) vs. 0 | OR (95% CI) detect vs. non-detect | Exposure density (kg/km2) vs. 0 | OR (95% CI) detect vs. non-detect | Exposure density (kg/km2) vs. 0 | OR (95% CI) detect vs. non-detect | ||
|
| |||||||
| Cyfluthrin e | 0.5km | >0 | 3.0 (1.5–6.0) | >0 | 2.5 (1.1–5.4) | >0 | 2.4 (1.1–5.0) |
|
| |||||||
| 1km | >0 | 1.7 (1.0–3.1) | >0 | 1.6 (0.8–2.9) | >0 | 1.6 (0.8–2.9) | |
|
| |||||||
| 2km | ≤ 0.03 | 1.1 (0.6–2.2) | ≤ 0.04 | 1.3 (0.6–2.5) | ≤0.003 | 1.3 (0.6–2.4) | |
| > 0.03 | 1.9 (1.0–3.6) | > 0.04 | 1.8(0.9–3.4) | > 0.003 | 1.5(0.8–2.9) | ||
| p-trend=0.04 | p-trend=0.10 | p-trend=0.22 | |||||
|
| |||||||
| 4km | ≤0.007 0.008–0.05 > 0.05 |
1.0 (0.5–1.9) 1.0 (0.5–1.9) 2.2 (1.2–4.0) |
≤0.011 0.12–0.06 > 0.06 |
1.1 (0.6–2.2) 0.9 (0.4–1.8) 2.4 (1.3–4.4) |
<0.001 0.001–0.01 > 0.01 |
1.0 (0.5–2.0) 1.4 (0.7–2.6) 1.5 (0.8–2.8) |
|
| p-trend=0.01 | p-trend=0.01 | p-trend=0.19 | |||||
|
|
|||||||
| Phosmet f | 0.5km | >0 | 4.9 (2.6–9.4) | >0 | 5.5 (2.7–11.3) | >0 | 4.5 (2.3–8.7) |
|
| |||||||
| 1km | ≤ 3.4 | 1.9 (0.9–4.2) | ≤ 4.0 | 3.1 (1.4–7.0) | ≤ 0.7 | 2.0 (0.9–4.5) | |
| > 3.4 | 7.7 (3.6–16.4) | > 4.0 | 7.5 (3.4–16.9) | > 0.7 | 6.4 (3.0–13.8) | ||
| p-trend=<0.0001 | p-trend=<0.0001 | p-trend=<0.0001 | |||||
|
| |||||||
| 2km | ≤ 2.4 | 3.2 (1.7–6.0) | ≤ 3.6 | 3.6 (1.9–6.9) | ≤ 0.3 | 3.0 (1.6–5.7) | |
| > 2.4 | 7.0 (3.7–13.3) p-trend=<0.0001 |
> 3.6 | 7.3 (3.7–14.2) p-trend=<0.0001 |
> 0.3 | 7.6 (3.9–14.7) p-trend=<0.0001 |
||
|
| |||||||
| 4km | ≤ 0.4 0.5–4.9 > 4.9 |
1.2 (0.6–2.4) 2.4 (1.3–4.6) 6.4 (3.4–11.9) |
≤ 0.4 0.5–5.4 > 5.4 |
1.2 (0.6–2.5) 3.2 (1.7–6.2) 6.6 (3.5–12.7) |
< 0.07 0.07–1.1 > 1.1 |
0.6 (0.3–1.5) 3.6 (1.9–6.9) 6.3 (3.3–12.1) |
|
| p-trend=<0.0001 | p-trend=<0.0001 | p-trend=<0.0001 | |||||
|
|
|||||||
| Azinphos-methyl g | 0.5k | >0 | 1.0 (0.1–8.1) | >0 | 1.0 (0.1–8.1) | >0 | 1.1 (0.1–8.9) |
|
| |||||||
| 1km | >0 | 2.0 (0.6–7.1) | >0 | 1.5 (0.3–6.6) | >0 | 2.3 (0.6–8.0) | |
|
| |||||||
| 2km | >0 | 2.7 (1.2–6.2) | >0 | 3.2 (1.4–7.4) | >0 | 2.8 (1.2–6.8) | |
|
| |||||||
| 4km | ≤ 0.6 > 0.6 |
2.5 (1.0–6.0) 3.0 (1.3–6.9) |
≤ 0.6 > 0.6 |
2.6 (1.1–6.3) 3.1 (1.4–7.3) |
≤ 0.06 > 0.06 |
2.2 (0.9–5.6) 2.7 (1.1–6.6) |
|
| p-trend=0.01 | p-trend=0.01 | p-trend=0.03 | |||||
For each insecticide, three separate logistic regression models were used to model the odds of detection (dependent variable) with each density metrics (CPUR, CROP-A, and W-CPUR) comparing >0 or density categories to homes with no use. A forward approach was used to evaluate covariates. Any covariate with a p-value ≤0.1 was retained in the final multivariable model.
CPUR= CPUR Metric (kg/km2): insecticide use density proportional to the area of the buffer
CROP-A= Crop area adjusted CPUR Metric (kg/km2): insecticide use density proportional to the area of cultivated crops and pasture/hay with insecticide use in the buffer
W-CPUR= Wind-adjusted CPUR Metric (kg/km2): insecticide use density proportional to the area of the buffer, weighted to account for the proportion of days the residence was downwind of the section in which the insecticide was applied during the period between application and dust collection
Detected in 26% of samples. Adjusted for year of dust collection, use of flea or tick products, and treatments for lawn/garden weeds that were positively associated with detection.
Detected in 28% of samples. Adjusted for year and season of dust collection, and farm working occupation that were positively associated with detection.
Detected in 7% of samples. None of the covariates were predictors of detections.
For phosmet, the CPUR, CROP-A and W-CPUR 1, 2 and 4km metrics were each associated with 6–8 times higher odds of dust detection (all p-values for trend <0.0001; Table 4). Each of the 0.5km buffer metrics was associated with 4–5 times higher odds of detection. The 60-day metrics (Supplemental Table 5) showed similar associations with higher odds of detection compared to unexposed homes for all metrics, although ORs were somewhat stronger than the 365-day estimates. Year, season of dust collection, and occupation as a farmworker were positively associated with dust detection.
For azinphos-methyl, the 4km 365-day metrics were predictors of dust detections with significant trends across the median exposure categories (Table 4). There was no association with the 0.5km-1km metrics, but all metrics were predictors of dust detections in 2km buffer. We were limited in our ability to examine the metrics using the 60-day period due to the small number of homes with azinphos-methyl applications (0.5km: 2 exposed homes; 4km: 31 exposed homes; Supplemental Table 5). No covariates were predictors of azinphos-methyl concentrations.
There were no associations with any metric for malathion in the 365- (not shown) or 60-day time periods (Supplemental Table 5). Home use of products for the control of bees and lawn/garden weeds, as well as having someone in the home who identified as a farmworker were predictors of malathion detections.
4. Discussion
We estimated agricultural insecticide use around participant’s homes and evaluated whether nearby agricultural applications were predictors of concentrations of insecticide active ingredients in house dust. Our analyses expand upon prior work by Gunier et al. (2011) in this study population by including additional insecticide active ingredients and using data from a greater number of homes (598 vs. 89). In addition to a CPUR metric, we created two additional metrics using information on agricultural land use and wind direction contemporaneous with the time between insecticide application and dust collection. We showed that agricultural applications of carbaryl, diazinon, chlorpyrifos, cyfluthrin, phosmet, and azinphos-methyl were associated with concentrations or detections in the home. Comparing the three different metrics across four time periods and four buffer sizes using multivariable models, we found that varying the time between agricultural use and dust collection and adjusting for wind direction was particularly important for carbaryl applications. Generally, wind-adjusted carbaryl applications within 2–4km of homes within 30- and 60-days of dust collection showed the strongest associations.
We evaluated insecticide applications during the 30-, 60-, 180-, and 365-day periods prior to dust collection. Varying the time period did not impact the associations with cypermethrin, permethrin, and malathion applications that were not associated with dust levels during any time period, while chlorpyrifos applications were associated during all time periods. Chlorpyrifos has a field dissipation half-life of around 40 days, which may be why we observed an association during any time period (USDA Agricultural Research Service 1995b). For carbaryl and diazinon, associations differed by the time period. Consistent with our prior work (Gunier et al. 2011), we observed no associations between carbaryl applications within 180 and 365-days of dust collection. When we examined shorter periods of 30- and 60-days between application and dust collection, the density of carbaryl use at all buffer sizes was a predictor of the dust concentrations. For diazinon, use in the prior 30- and 60-days was a more consistent predictor of dust concentrations. Both carbaryl and diazinon have short field dissipation halflives of 4–13 and 7 days (USDA Agricultural Research Service 1995a; c), respectively, which may be why we only observed associations for these active ingredients in the shorter time periods.
Numerous prior studies have shown higher levels of pesticides in dust samples taken from homes close to agricultural activities compared to non-proximal homes (Dereumeaux et al. 2020). In a meta-regression of published data, Deziel et al. showed that house dust pesticide concentrations decreased sharply and non-linearly with increasing distance from treated fields that was linear on a log-log scale (Deziel et al. 2017). Using data from 89 (15%) of the homes in our study, Gunier et al. examined a 365- and 180-day CPUR metric and a metric that accounted for the location of crops at the time of the dust sampling and found that chlorpyrifos and phosmet but not carbaryl or diazinon concentrations were higher in homes with agricultural insecticide use within a 1.25km buffer (Gunier et al. 2011). The null findings for carbaryl and diazinon may have been due to the longer time periods between application and dust collection used in the study; in our study we also did not observe associations with carbaryl in the 180- or 365-day time periods. For diazinon, the 60-day metrics were significant predictors of dust concentrations. Several exposure studies demonstrate findings generally consistent with ours, including a California study of 504 house dust samples found that agricultural applications of chlorpyrifos but not diazinon or permethrin within 3 miles (4.8km) of the home were associated with dust concentrations (Harnly et al. 2009). Also consistent with the results of our study is a study of 61 homes that showed those within 200 feet of a pesticide-treated fruit orchard had higher median chlorpyrifos, azinphos-methyl, and phosmet concentrations levels in their house dust compared to homes more than 200 feet from an orchard (Fenske et al. 2002; Lu et al. 2000; Simcox et al. 1995).
We explored associations using buffer sizes ranging from 0.5 to 4km. Numerous studies have shown that exposure to pesticides is greater at closer residential distances to agricultural fields (Dereumeaux et al. 2020; Nuckols et al. 2007; Obendorf et al. 2006; Ward et al. 2006), but little is known about the optimal distance to use for each insecticide. The distance from the source of the agricultural insecticide application may be an important determinant of the presence and concentration of active ingredients in the home environment, and may vary by insecticide, yet many studies that use spatial exposure metrics consider only one buffer size in their analyses. A recent review of residential exposure to agricultural pesticides found that only 25% of studies that used GIS-based exposure metrics explored more than one buffer size (Teysseire et al. 2020). Factors that influence each ingredient’s ability to persist and be transported by wind or water flow from agricultural land include temperature, metabolism in plants and soil microorganisms, and the chemical properties of the active ingredient such as half-life in soil, vapor pressure, octanol/water partition coefficient, and water solubility (Bennett et al. 1998; Farha et al. 2016; Gavrilescu 2005; Juraske et al. 2008; Van Eerd et al. 2003). This indicates that an individualized modeling approach may need to be tailored to each pesticide to better understand the association between location of applications and residential exposure. This consideration is supported by our study demonstrating that agricultural applications predicted dust concentrations in the home at different distances and time periods for different insecticides. Our findings highlight the need to explore different exposure time periods and buffer sizes for each active ingredient to accurately characterize the potential for non-occupational human exposure.
In this study we attempted to enhance the exposure assessment based on CPUR use by incorporating data on agricultural land use and wind conditions. Although studies have shown that land use information such as crop field area and distance from the home to a crop field (Nuckols et al. 2007; Rull and Ritz 2003; Vannier et al. 2020) and wind direction (Gunier et al. 2018; Rowe et al. 2016; Sagiv et al. 2019) can be incorporated into exposure metrics for use in epidemiologic studies, few have compared different metrics to demonstrate the utility of incorporating additional information to refine the metric. Off-target pesticide exposure has been linked to wind conditions (Pfleeger et al. 2006) and modelling studies suggests that pesticide dispersion is strongly affected by meteorological conditions (e.g., wind direction) during application (Costanzini et al. 2018), highlighting the need to evaluate the impact of incorporating wind direction into exposure metrics. In this study, incorporating agricultural land use categories led to similar estimates of insecticide density between the crop-area adjusted and the CPUR metric. Generally, the density estimates for the wind metric were smaller than the crop-adjusted and CPUR metrics, since the weighting we applied reduced the prevalence of exposure. When we assessed correlations between the three metrics, most of the insecticides showed moderate to high correlation between the three metrics across the different buffer sizes and time periods. The weakest correlations were observed with the CROP-A metric, which may be due to limitations in the land use data that was available for linkage. Despite differences in the density estimates among the three metrics, the resulting associations between each metric and dust concentrations were similar to each other with few exceptions. For example, for the 60-day 4km carbaryl estimates only the wind-adjusted metric shows a positive trend. The 60-day diazinon CPUR and wind-adjusted metrics were associated with dust concentrations in the 4km buffer, but the crop-area adjusted metric was not. We did not observe many other differences in the associations between insecticide applications and dust concentrations using the different metrics. This suggests that the simpler CPUR metric could provide a reasonable estimate of exposure for use in epidemiologic studies investigating links between agricultural insecticide use and health outcomes. However, it should be noted that the CPUR metric is based on a robust and mandatory pesticide use reporting program administered only in the State of California, USA. The results of our study underscore the need for similar data resources in all regions where cultivated and residential land uses are highly integrated.
Strengths of this study include the availability of a comprehensive database of agricultural insecticide use for the study area that included detailed information on the types and quantities of insecticides applied to crops including both spot spraying and broadcast spraying for insect control. We used data from a large number of homes, allowing us to investigate relationships with a diverse set of insecticides, including those that were less frequently applied. Further, our study included many homes from both urban, suburban, and rural areas and we were able to vary the time period between insecticide application and dust collection. We had self-reported information on home and garden use of insecticides and whether household members had occupational exposures, allowing us to evaluate additional factors as determinants of the insecticide concentrations in the house dust and control for these factors in multivariate models.
Our study has several limitations. Although we were able to link the pesticide application data with available land use and wind information to further refine our exposure metrics, the accuracy and resolution of these data impacts the quality of the subsequent exposure metrics. Our dust collections spanned 2001–2007, but land use information was only available for 2001, 2004, and 2006. California agriculture is characterized by multiple crops per field within an annual cycle as well as relatively frequent replacement/interchange of orchards, berries, and grapes within a short time based on market demand. The land use data may not have accurately captured crop locations for crops that were rotated during months/years without available land use data. Prior studies have accounted for potential downwind pesticide drift from application sites to homes by incorporating historical wind direction data from the nearest meteorological station to each residence (Gunier et al. 2018; Rowe et al. 2016; Sagiv et al. 2019). These studies used a 45° wedge, which is more conservative than our 90° wedge. In our study we modeled the wind from the source to the residence and ascertained wind direction from a low-resolution atmospheric and land surface hydrology dataset available at a 32km scale. Given the scale of our data, it is possible that data from the nearest meteorological station may have resulted in different estimates of the number of days downwind, but station data is also limited by the variation in distance between the station and the residence. We did not account for wind speed in our wind-adjusted metric, a factor that could also impact pesticide drift. Our study relates agricultural insecticide use to concentrations measured in house dust, and it is not clear how levels in house dust relate to levels measured in biological samples taken from residents of the home. Research is needed to relate dust levels in the home to exposures measured in biological samples. Our findings for some insecticides (e.g., cypermethrin, phosmet) were limited by sparse data due to infrequent applications during the shorter time periods and/or in smaller buffer sizes. For example, although cypermethrin was detected in 49% of dust samples, this level of detection is likely due to its use in products around the home as only 28 homes (5%) had applications in the 0.5km buffer within the 365 days before dust collection.
5. Conclusions
For six of the nine insecticides, agricultural use within 4km of a home was a significant determinant of indoor contamination. Dust concentrations and detections were higher in homes with nearby agricultural insecticide use compared to homes without use for carbaryl, diazinon, chlorpyrifos, cyfluthrin, phosmet, and azinphos-methyl. Our findings demonstrated the utility of GIS-based metrics for quantifying potential exposure to fugitive insecticide emissions from cultivated agriculture but indicated that associations with measured levels of insecticides in homes varies depending on buffer size (i.e., defined proximity) and the time elapsed between application and house dust collection. Our findings suggest inclusion of wind enhanced prediction for some, but not all insecticides studied. Taken together, our results imply that GIS-based exposure metrics used in epidemiologic studies should be tailored to the fate and transport characteristics of each insecticide.
Supplementary Material
Acknowledgements
This research was partially supported by the Intramural Research Program of the National Cancer Institute (NCI) (Project Z01 CP01012522), National Institutes of Health and through NCI subcontracts 7590-S-04 (University of California, Berkeley) and 7590-S-01 (Battelle Memorial Institute) under NCI contract N02-CP-11015 (Westat). This research was also financially supported by National Institute of Environmental Health Sciences grants R01ES009137 and P-42-ES-04705-18 (University of California, Berkeley) and NCI grant 5R01CA092683-03 (Colorado State University). We thank Joanne S. Colt formerly of the Intramural Research Program of NCI for her contributions to the design and conduct of the CCLS study, Erin M. Bell formerly of the Intramural Research Program of NCI for her contributions to the design and conduct of the FAPS study, Matthew Airola of Westat for his GIS support, and Shannon Merkle at Information Management Services for programming and data management support. This research could not have been conducted without the important support from our clinical collaborators and participating hospitals which include: University of California Davis Medical Center (Dr. Jonathan Ducore), University of California San Francisco (Dr. Mignon Loh and Dr Katherine Matthay), Children’s Hospital of Central California (Dr. Vonda Crouse), Lucile Packard Children’s Hospital (Dr. Gary Dahl), Children’s Hospital Oakland (Dr. James Feusner), Kaiser Permanente Sacramento (Dr. Vincent Kiley), Kaiser Permanente Santa Clara (Dr. Carolyn Russo and Dr. Alan Wong), Kaiser Permanente San Francisco (Dr. Kenneth Leung), and Kaiser Permanente Oakland (Dr. Stacy Month), and the families of the study participants. We also wish to acknowledge the effort and dedication our all our collaborators at the Northern California Childhood Leukemia Study who helped make this study possible, and the staff at the Battelle Memorial Institute, Columbus, Ohio who performed laboratory analyses. The authors declare that they have no actual or potential competing financial interests.
Footnotes
Credit authorship contribution statement:
Jessica M. Madrigal: Software, formal analysis, writing -original draft; Robert B. Gunier: Conceptualization, exposure assessment, writing – review & editing; Rena R. Jones: Writing – review & editing; Abigail Flory: Software, exposure assessment, writing – review & editing; Catherine Metayer: writing – review & editing, funding acquisition; J.R. Nuckols: Conceptualization, exposure assessment, writing – review & editing, funding acquisition; Mary H. Ward: Conceptualization, supervision, validation, writing – review & editing, funding acquisition
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Atwood D; Paisley-Jones C.Pesticides Industry Sales and Usage: 2008 and 2012 Market Estimates.: United States Environmental Protection Agency; 2017 [Google Scholar]
- Bennett DH; McKone TE; Matthies M; Kastenberg WE General formulation of characteristic travel distance for semivolatile organic chemicals in a multimedia environment. Environmental Science and Technology 1998;32:4023–4030 [Google Scholar]
- Bigelow DP; Borchers A.Major Uses of Land in the United States, 2012,. EIB-178: U.S. Department of Agriculture, Economic Research Service, ; 2017 [Google Scholar]
- Booth BJ; Ward MH; Turyk ME; Stayner LT Agricultural crop density and risk of childhood cancer in the midwestern United States: an ecologic study. Environmental health : a global access science source 2015;14:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwer M; Huss A; van der Mark M; Nijssen PCG; Mulleners WM; Sas AMG; van Laar T; de Snoo GR; Kromhout H; Vermeulen RCH Environmental exposure to pesticides and the risk of Parkinson’s disease in the Netherlands. Environment international 2017;107:100–110 [DOI] [PubMed] [Google Scholar]
- California Department of Pesticide Regulation. Pesticide Use Reporting - 2017 Summary Data. 2020a [Google Scholar]
- California Department of Pesticide Regulation. Pesticide Use Reporting (PUR). 2020b [Google Scholar]
- Chang JS; Selvin S; Metayer C; Crouse V; Golembesky A; Buffler PA Parental smoking and the risk of childhood leukemia. American journal of epidemiology 2006;163:1091–1100 [DOI] [PubMed] [Google Scholar]
- Cockburn M; Mills P; Zhang X; Zadnick J; Goldberg D; Ritz B.Prostate cancer and ambient pesticide exposure in agriculturally intensive areas in California. American journal of epidemiology 2011;173:1280–1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coker E; Gunier R; Bradman A; Harley K; Kogut K; Molitor J; Eskenazi B.Association between Pesticide Profiles Used on Agricultural Fields near Maternal Residences during Pregnancy and IQ at Age 7 Years. International journal of environmental research and public health 2017;14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colt JS; Gunier RB; Metayer C; Nishioka MG; Bell EM; Reynolds P; Buffler PA; Ward MH Household vacuum cleaners vs. the high-volume surface sampler for collection of carpet dust samples in epidemiologic studies of children. Environmental health : a global access science source 2008;7:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costanzini S; Teggi S; Bigi A; Ghermandi G; Filippini T; Malagoli C; Nannini R; Vinceti M. Atmospheric Dispersion Modelling and Spatial Analysis to Evaluate Population Exposure to Pesticides from Farming Processes. 2018;9:38 [Google Scholar]
- Costello S; Cockburn M; Bronstein J; Zhang X; Ritz B.Parkinson’s disease and residential exposure to maneb and paraquat from agricultural applications in the central valley of California. American journal of epidemiology 2009;169:919–926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dereumeaux C; Fillol C; Quenel P; Denys S.Pesticide exposures for residents living close to agricultural lands: A review. Environment international 2020;134:105210 [DOI] [PubMed] [Google Scholar]
- Deziel NC; Colt JS; Kent EE; Gunier RB; Reynolds P; Booth B; Metayer C; Ward MH Associations between self-reported pest treatments and pesticide concentrations in carpet dust. Environmental health : a global access science source 2015;14:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deziel NC; Freeman LEB; Graubard BI; Jones RR; Hoppin JA; Thomas K; Hines CJ; Blair A; Sandler DP; Chen H; Lubin JH; Andreotti G; Alavanja MCR; Friesen MC Relative Contributions of Agricultural Drift, Para-Occupational, and Residential Use Exposure Pathways to House Dust Pesticide Concentrations: Meta-Regression of Published Data. Environmental health perspectives 2017;125:296–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deziel NC; Ward MH; Bell EM; Whitehead TP; Gunier RB; Friesen MC; Nuckols JR Temporal variability of pesticide concentrations in homes and implications for attenuation bias in epidemiologic studies. Environmental health perspectives 2013;121:565–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B; Harley K; Bradman A; Weltzien E; Jewell NP; Barr DB; Furlong CE; Holland NT Association of in utero organophosphate pesticide exposure and fetal growth and length of gestation in an agricultural population. Environmental health perspectives 2004;112:1116–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farha W; Abd El-Aty AM; Rahman MM; Shin H-C; Shim J-H An overview on common aspects influencing the dissipation pattern of pesticides: a review. Environmental Monitoring and Assessment 2016;188:693. [DOI] [PubMed] [Google Scholar]
- Fenske RA; Lu C; Barr D; Needham L.Children’s exposure to chlorpyrifos and parathion in an agricultural community in central Washington State. Environmental health perspectives 2002;110:549–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry JA; Xian G; Jin S; Dewitz JA; Homer CG; Yang L; Barnes CA; Herold ND; Wickham JD Completion of the 2006 National Land Cover Database for the conterminous United States. Photogrammetric Engineering and Remote Sensing 2011;77:858–864 [Google Scholar]
- Furlong MA; Paul KC; Cockburn M; Bronstein J; Keener A; Rosario ID; Folle AD; Ritz B.Ambient Pyrethroid Pesticide Exposures in Adult Life and Depression in Older Residents of California’s Central Valley. Environmental epidemiology (Philadelphia, Pa) 2020;4:e123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrilescu M.Fate of pesticides in the environment and its bioremediation. Engineering in Life Sciences 2005;5:497–526 [Google Scholar]
- Gemmill A; Gunier RB; Bradman A; Eskenazi B; Harley KG Residential proximity to methyl bromide use and birth outcomes in an agricultural population in California. Environmental health perspectives 2013;121:737–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunier RB; Bradman A; Castorina R; Holland NT; Avery D; Harley KG; Eskenazi B.Residential proximity to agricultural fumigant use and IQ, attention and hyperactivity in 7-year old children. Environmental research 2017a;158:358–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunier RB; Bradman A; Harley KG; Kogut K; Eskenazi B.Prenatal Residential Proximity to Agricultural Pesticide Use and IQ in 7-Year-Old Children. Environmental health perspectives 2017b;125:057002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunier RB; Raanan R; Castorina R; Holland NT; Harley KG; Balmes JR; Fouquette L; Eskenazi B; Bradman A.Residential proximity to agricultural fumigant use and respiratory health in 7-year old children. Environmental research 2018;164:93–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunier RB; Ward MH; Airola M; Bell EM; Colt J; Nishioka M; Buffler PA; Reynolds P; Rull RP; Hertz A; Metayer C; Nuckols JR Determinants of agricultural pesticide concentrations in carpet dust. Environmental health perspectives 2011;119:970–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harnly ME; Bradman A; Nishioka M; McKone TE; Smith D; McLaughlin R; Kavanagh-Baird G; Castorina R; Eskenazi B.Pesticides in dust from homes in an agricultural area. Environmental science & technology 2009;43:8767–8774 [DOI] [PubMed] [Google Scholar]
- Homer CG; Dewitz J; Fry J; Coan M; Hossain N; Larson C; Herold N; McKerrow AJ; VanDriel JN; Wickham J.Completion of the 2001 National Land Cover Database for the conterminous United States. Photogrammetric Engineering and Remote Sensing 2007;73:337–341 [Google Scholar]
- Hyland C; Bradshaw PT; Gunier RB; Mora AM; Kogut K; Deardorff J; Sagiv SK; Bradman A; Eskenazi B.Associations between pesticide mixtures applied near home during pregnancy and early childhood with adolescent behavioral and emotional problems in the CHAMACOS study. Environmental epidemiology (Philadelphia, Pa) 2021;5:e150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juraske R; Antón A; Castells F.Estimating half-lives of pesticides in/on vegetation for use in multimedia fate and exposure models. Chemosphere 2008;70:1748–1755 [DOI] [PubMed] [Google Scholar]
- Lu C; Fenske RA; Simcox NJ; Kalman D.Pesticide exposure of children in an agricultural community: evidence of household proximity to farmland and take home exposure pathways. Environmental research 2000;84:290–302 [DOI] [PubMed] [Google Scholar]
- Lubin JH, Colt JS, Camann D, Davis S, Cerhan JR, Severson RK, Bernstein L, Hartge P, 2004. Epidemiologic evaluation of measurement data in the presence of detection limits. Environmental health perspectives 112, 1691–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X; Buffler PA; Layefsky M; Does MB; Reynolds P.Control selection strategies in case-control studies of childhood diseases. American journal of epidemiology 2004;159:915–921 [DOI] [PubMed] [Google Scholar]
- Ma X; Buffler PA; Wiemels JL; Selvin S; Metayer C; Loh M; Does MB; Wiencke JK Ethnic difference in daycare attendance, early infections, and risk of childhood acute lymphoblastic leukemia. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 2005;14:1928–1934 [DOI] [PubMed] [Google Scholar]
- Madrigal JM; Jones RR; Gunier RB; Whitehead TP; Reynolds P; Metayer C; Ward MH Residential exposure to carbamate, organophosphate, and pyrethroid insecticides in house dust and risk of childhood acute lymphoblastic leukemia. Environmental research 2021;201:111501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesinger F; DiMego G; Kalnay E; Mitchell K; Shafran PC; Ebisuzaki W; Jovi D.a.; Woollen J; Rogers E; Berbery EH; Ek MB; Fan Y; Grumbine R; Higgins W; Li H; Lin Y; Manikin G; Parrish D; Shi W.North American Regional Reanalysis %J Bulletin of the American Meteorological Society. 2006;87:343–360 [Google Scholar]
- Nuckols JR; Gunier RB; Riggs P; Miller R; Reynolds P; Ward MH Linkage of the California Pesticide Use Reporting Database with spatial land use data for exposure assessment. Environmental health perspectives 2007;115:684–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obendorf SK; Lemley AT; Hedge A; Kline AA; Tan K; Dokuchayeva T.Distribution of pesticide residues within homes in central New York State. Archives of environmental contamination and toxicology 2006;50:31–44 [DOI] [PubMed] [Google Scholar]
- Ongono JS; Michelon C; Béranger R; Cadot E; Simoncic V; Loubersac J; Mortamais M; Baghdadli A.Association between residential proximity to agricultural crops and adaptive behaviors in children with autism spectrum disorder from the French ELENA cohort. Journal of psychiatric research 2021;145:197–204 [DOI] [PubMed] [Google Scholar]
- Park AS; Ritz B; Yu F; Cockburn M; Heck JE Prenatal pesticide exposure and childhood leukemia - A California statewide case-control study. International journal of hygiene and environmental health 2020;226:113486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel DM; Gyldenkærne S; Jones RR; Olsen SF; Tikellis G; Granström C; Dwyer T; Stayner LT; Ward MH Residential proximity to agriculture and risk of childhood leukemia and central nervous system tumors in the Danish national birth cohort. Environment international 2020;143:105955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfleeger TG; Olszyk D; Burdick CA; King G; Kern J; Fletcher J.Using a Geographic Information System to identify areas with potential for off-target pesticide exposure. Environmental toxicology and chemistry 2006;25:2250–2259 [DOI] [PubMed] [Google Scholar]
- Reynolds P; Von Behren J; Gunier RB; Goldberg DE; Harnly M; Hertz A.Agricultural Pesticide Use and Childhood Cancer in California. 2005;16:93–100 [DOI] [PubMed] [Google Scholar]
- Rowe C; Gunier R; Bradman A; Harley KG; Kogut K; Parra K; Eskenazi B.Residential proximity to organophosphate and carbamate pesticide use during pregnancy, poverty during childhood, and cognitive functioning in 10-year-old children. Environmental research 2016;150:128–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rull RP; Ritz B.Historical pesticide exposure in California using pesticide use reports and land-use surveys: an assessment of misclassification error and bias. Environmental health perspectives 2003;111:1582–1589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rull RP; Ritz B; Shaw GM Neural tube defects and maternal residential proximity to agricultural pesticide applications. American journal of epidemiology 2006;163:743–753 [DOI] [PubMed] [Google Scholar]
- Sagiv SK; Bruno JL; Baker JM; Palzes V; Kogut K; Rauch S; Gunier R; Mora AM; Reiss AL; Eskenazi B.Prenatal exposure to organophosphate pesticides and functional neuroimaging in adolescents living in proximity to pesticide application. Proceedings of the National Academy of Sciences of the United States of America 2019;116:18347–18356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton JF; Geraghty EM; Tancredi DJ; Delwiche LD; Schmidt RJ; Ritz B; Hansen RL; Hertz-Picciotto I.Neurodevelopmental disorders and prenatal residential proximity to agricultural pesticides: the CHARGE study. Environmental health perspectives 2014;122:1103–1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simcox NJ; Fenske RA; Wolz SA; Lee IC; Kalman DA Pesticides in household dust and soil: exposure pathways for children of agricultural families. Environmental health perspectives 1995;103:1126–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz SJ; Morimoto LM; Whitehead TP; DeRouen MC; Ma X; Wang R; Wiemels JL; McGlynn KA; Gunier R; Metayer C.Proximity to endocrine-disrupting pesticides and risk of testicular germ cell tumors (TGCT) among adolescents: A population-based case-control study in California. International journal of hygiene and environmental health 2022;239:113881 [DOI] [PubMed] [Google Scholar]
- Tayour C; Ritz B; Langholz B; Mills PK; Wu A; Wilson JP; Shahabi K; Cockburn M.A case-control study of breast cancer risk and ambient exposure to pesticides. Environmental epidemiology (Philadelphia, Pa) 2019;3:e070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teysseire R; Manangama G; Baldi I; Carles C; Brochard P; Bedos C; Delva F.Assessment of residential exposures to agricultural pesticides: A scoping review. PloS one 2020;15:e0232258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States Department of Agriculture. United States Department of Agriculture/Economic Research Service, Farm Income and Wealth Statistics. 2019 [Google Scholar]
- Unsworth JB; Wauchope RD; Klein A-W; Dorn E; Zeeh B; Yeh SM; Akerblom M; Racke KD; Rubin B.Significance of the Long Range Transport of Pesticides in the Atmosphere %J Pure and Applied Chemistry. 1999;71:1359 [Google Scholar]
- U.S. Department of Agriculture. 1995a. Pesticide Properties Database: Carbaryl. Available: https://www.ars.usda.gov/northeast-area/beltsville-md-barc/beltsville-agricultural-research-center/adaptive-cropping-systems-laboratory/docs/ppd/pesticide-list/ [accessed 9 June 2022].
- U.S. Department of Agriculture. 1995b. Pesticide Properties Database: Chlorpyrifos. Available: https://www.ars.usda.gov/northeast-area/beltsville-md-barc/beltsville-agricultural-research-center/adaptive-cropping-systems-laboratory/docs/ppd/pesticide-list/ [accessed 9 June 2022].
- U.S. Department of Agriculture. 1995c. Pesticide Properties Database: Diazinon. Available: https://www.ars.usda.gov/northeast-area/beltsville-md-barc/beltsville-agricultural-research-center/adaptive-cropping-systems-laboratory/docs/ppd/pesticide-list/ [accessed 9 June 2022].
- van der Mark M; Brouwer M; Kromhout H; Nijssen P; Huss A; Vermeulen R.Is pesticide use related to Parkinson disease? Some clues to heterogeneity in study results. Environmental health perspectives 2012;120:340–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Eerd LL; Hoagland RE; Zablotowicz RM; Hall JC Pesticide metabolism in plants and microorganisms. Weed Science 2003;51 [Google Scholar]
- Vannier C; Chevrier C; Hubert-Moy L.Role of land use and land cover in residential exposures to agricultural pesticide models. International journal of environmental health research 2020:1–22 [DOI] [PubMed] [Google Scholar]
- von Ehrenstein OS; Ling C; Cui X; Cockburn M; Park AS; Yu F; Wu J; Ritz B.Prenatal and infant exposure to ambient pesticides and autism spectrum disorder in children: population based case-control study. BMJ (Clinical research ed) 2019;364:l962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VoPham T; Brooks MM; Yuan JM; Talbott EO; Ruddell D; Hart JE; Chang CC; Weissfeld JL Pesticide exposure and hepatocellular carcinoma risk: A case-control study using a geographic information system (GIS) to link SEER-Medicare and California pesticide data. Environmental research 2015;143:68–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A; Cockburn M; Ly TT; Bronstein JM; Ritz B.The association between ambient exposure to organophosphates and Parkinson’s disease risk. Occupational and environmental medicine 2014;71:275–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward MH; Colt JS; Metayer C; Gunier RB; Lubin J; Crouse V; Nishioka MG; Reynolds P; Buffler PA Residential exposure to polychlorinated biphenyls and organochlorine pesticides and risk of childhood leukemia. Environmental health perspectives 2009;117:1007–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward MH; Lubin J; Giglierano J; Colt JS; Wolter C; Bekiroglu N; Camann D; Hartge P; Nuckols JR Proximity to crops and residential exposure to agricultural herbicides in iowa. Environmental health perspectives 2006;114:893–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xian G; Homer C; Fry J.Updating the 2001 National Land Cover Database land cover classification to 2006 by using Landsat imagery change detection methods. Remote Sensing of Environment 2009;113:1133–1147 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.