Abstract
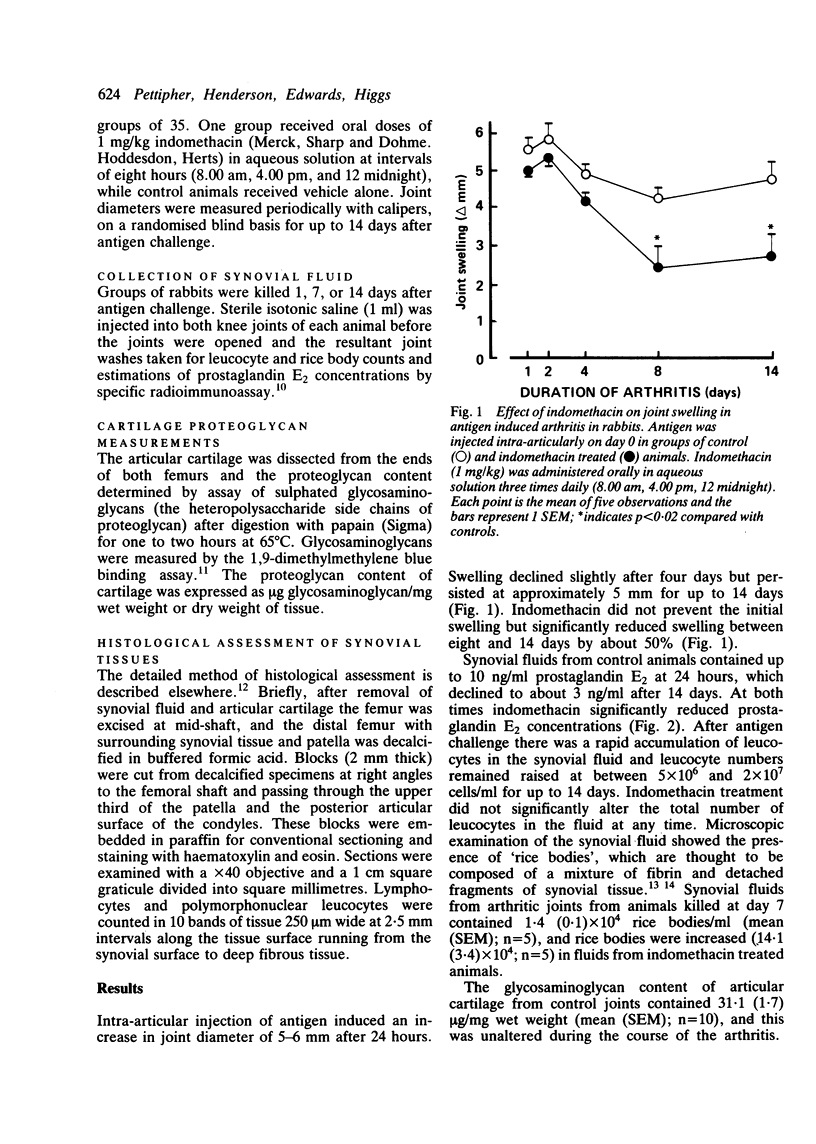
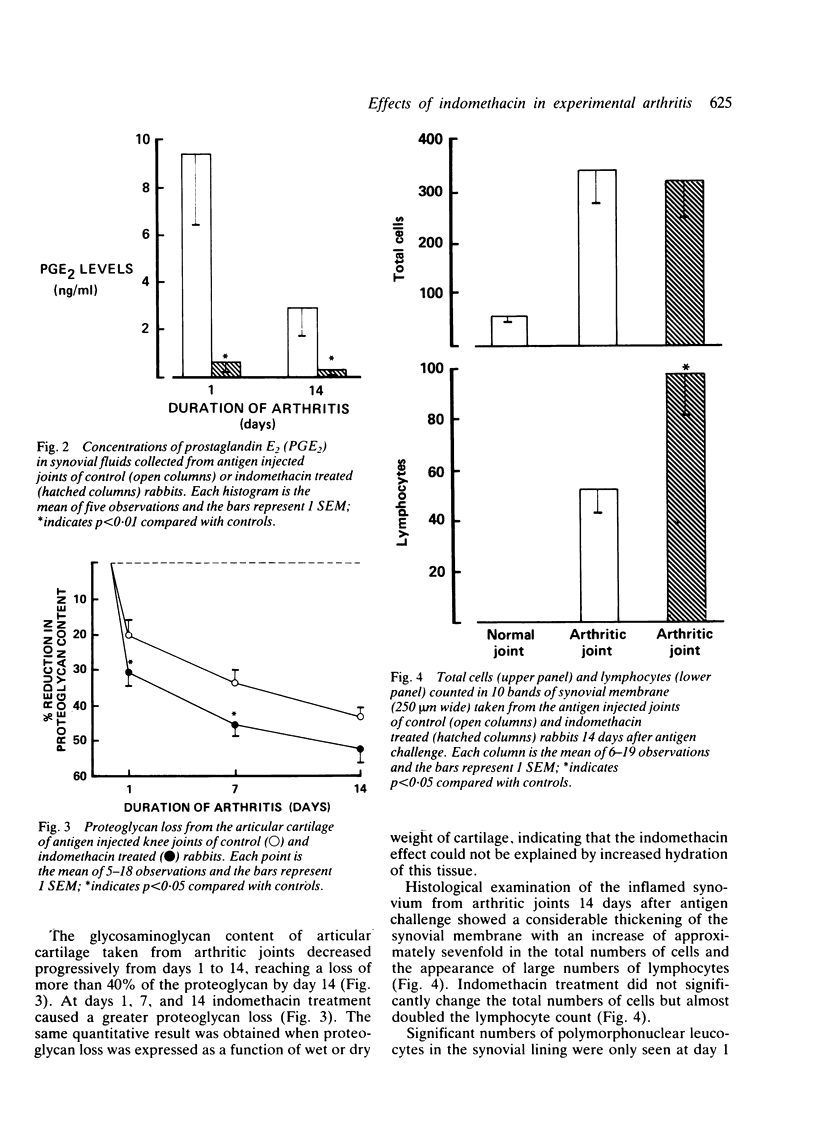
The effects of indomethacin on antigen induced arthritis in rabbits have been investigated. Arthritis was induced in the knee joints of sensitised rabbits by intra-articular injection of antigen. Swelling of the joints was measured for 14 days after antigen challenge, and groups of animals were killed on days 1, 7, or 14 for collection of synovial fluids and tissues. Indomethacin (1 mg/kg, three times daily) reduced joint swelling and the prostaglandin E2 concentrations in synovial fluid. In addition, indomethacin increased the loss of proteoglycan from articular cartilage and the numbers of lymphocytes in the inflamed synovial lining. These findings suggest that the symptomatic benefits of indomethacin and related drugs in inflammatory arthritis may be achieved at the expense of significant adverse effects on joint tissues.
Full text
PDF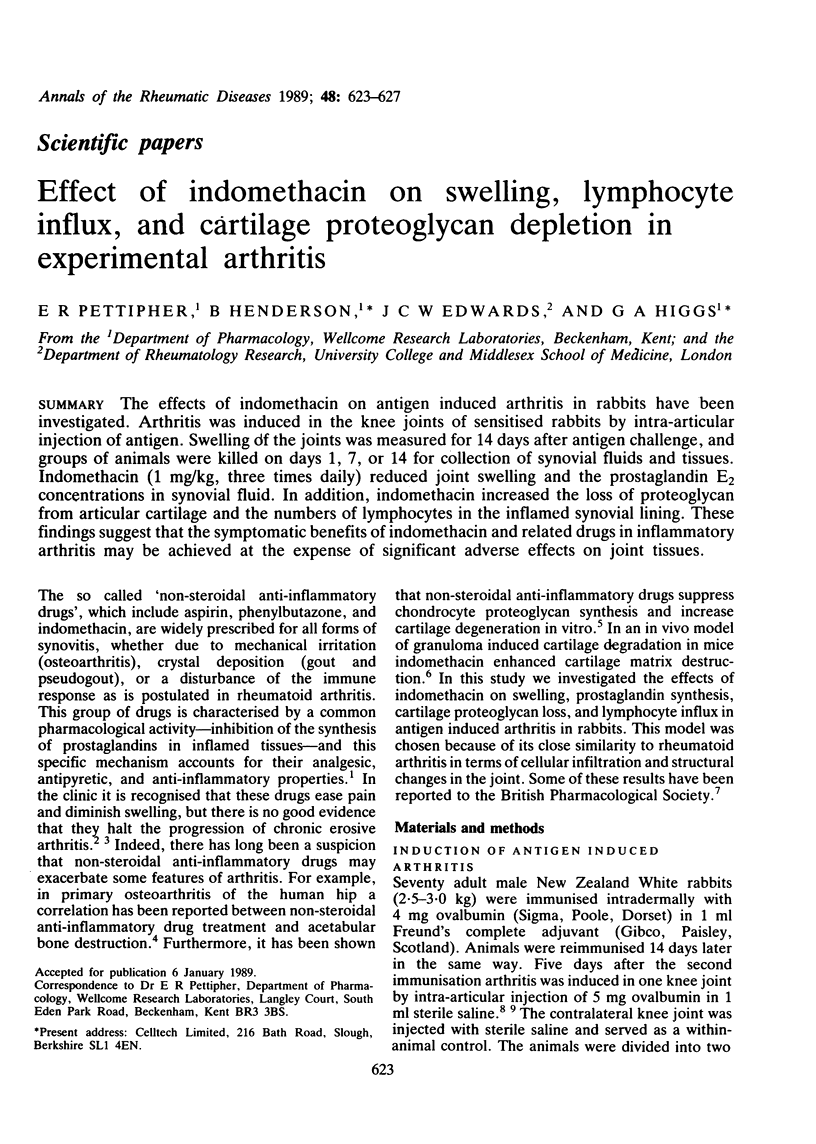


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blackham A., Farmer J. B., Radziwonik H., Westwick J. The role of prostaglandins in rabbit monoarticular arthritis. Br J Pharmacol. 1974 May;51(1):35–44. doi: 10.1111/j.1476-5381.1974.tb09629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottomley K. M., Griffiths R. J., Rising T. J., Steward A. A modified mouse air pouch model for evaluating the effects of compounds on granuloma induced cartilage degradation. Br J Pharmacol. 1988 Mar;93(3):627–635. doi: 10.1111/j.1476-5381.1988.tb10320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt K. D. Effects of nonsteroidal anti-inflammatory drugs on chondrocyte metabolism in vitro and in vivo. Am J Med. 1987 Nov 20;83(5A):29–34. doi: 10.1016/0002-9343(87)90848-5. [DOI] [PubMed] [Google Scholar]
- DUMONDE D. C., GLYNN L. E. The production of arthritis in rabbits by an immunological reaction to fibrin. Br J Exp Pathol. 1962 Aug;43:373–383. [PMC free article] [PubMed] [Google Scholar]
- Edwards J. C., Read N., Trefty B., Coulstock J., Henderson B. Quantitative histological analysis of antigen-induced arthritis in the rabbit. Br J Exp Pathol. 1988 Oct;69(5):739–748. [PMC free article] [PubMed] [Google Scholar]
- Farndale R. W., Buttle D. J., Barrett A. J. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986 Sep 4;883(2):173–177. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- Goodwin J. S., Bankhurst A. D., Messner R. P. Suppression of human T-cell mitogenesis by prostaglandin. Existence of a prostaglandin-producing suppressor cell. J Exp Med. 1977 Dec 1;146(6):1719–1734. doi: 10.1084/jem.146.6.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart F. D., Huskisson E. C. Non-steroidal anti-inflammatory drugs. Current status and rational therapeutic use. Drugs. 1984 Mar;27(3):232–255. doi: 10.2165/00003495-198427030-00004. [DOI] [PubMed] [Google Scholar]
- Higgs G. A., Moncada S., Vane J. R. Eicosanoids in inflammation. Ann Clin Res. 1984;16(5-6):287–299. [PubMed] [Google Scholar]
- Kunkel S. L., Chensue S. W., Phan S. H. Prostaglandins as endogenous mediators of interleukin 1 production. J Immunol. 1986 Jan;136(1):186–192. [PubMed] [Google Scholar]
- Lewis G. P., Barrett M. L. Immunosuppressive actions of prostaglandins and the possible increase in chronic inflammation after cyclo-oxygenase inhibitors. Agents Actions. 1986 Oct;19(1-2):59–65. doi: 10.1007/BF01977259. [DOI] [PubMed] [Google Scholar]
- Muirden K. D., Mills K. W. Do lymphocytes protect the rheumatoid joint? Br Med J. 1971 Oct 23;4(5781):219–221. doi: 10.1136/bmj.4.5781.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman N. M., Ling R. S. Acetabular bone destruction related to non-steroidal anti-inflammatory drugs. Lancet. 1985 Jul 6;2(8445):11–14. doi: 10.1016/s0140-6736(85)90058-3. [DOI] [PubMed] [Google Scholar]
- Pettipher E. R., Higgs G. A., Henderson B. Interleukin 1 induces leukocyte infiltration and cartilage proteoglycan degradation in the synovial joint. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8749–8753. doi: 10.1073/pnas.83.22.8749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popert A. J., Scott D. L., Wainwright A. C., Walton K. W., Williamson N., Chapman J. H. Frequency of occurrence, mode of development, and significance or rice bodies in rheumatoid joints. Ann Rheum Dis. 1982 Apr;41(2):109–117. doi: 10.1136/ard.41.2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon J. A. A radioimmunoassay for 6-keto-prostaglandin F1alpha. Prostaglandins. 1978 Mar;15(3):383–397. doi: 10.1016/0090-6980(78)90122-3. [DOI] [PubMed] [Google Scholar]
- Wright V., Amos R. Do drugs change the course of rheumatoid arthritis? Br Med J. 1980 Apr 5;280(6219):964–966. doi: 10.1136/bmj.280.6219.964-a. [DOI] [PMC free article] [PubMed] [Google Scholar]


