HIGHLIGHTS
-
•
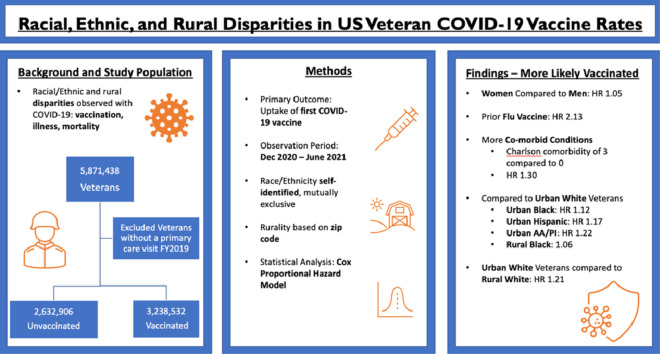
A total of 55.2% of 5.8 million veterans received ≥1 COVID-19 vaccine in the first 6 months.
-
•
Vaccinated veterans were older, were more likely men, and had more comorbidities.
-
•
Urban Black, Hispanic, and Asian American Pacific Islander veterans were more likely to receive a vaccine.
-
•
Veterans in rural areas were less likely than urban veterans to receive a vaccine.
-
•
Rural Black veterans remained more likely to be vaccinated than White urban veterans.
Keywords: COVID-19, vaccine, health disparities, veterans, rurality
Abstract
Introduction
Race-, ethnicity-, and rurality-related disparities in COVID-19 vaccine uptake have been documented in the U.S. We determined whether these disparities existed among patients at the Department of Veterans Affairs, the largest healthcare system in the U.S.
Methods
Using Department of Veterans Affairs Corporate Data Warehouse data, we included 5,871,438 patients (9.4% women) with at least 1 primary care visit in 2019 in a retrospective cohort study. Each patient was assigned a single race and ethnicity, which were mutually exclusive, self-reported categories. Rurality was based on the 2019 home address at the ZIP code level. Our primary outcome was time to first COVID-19 vaccination between December 15, 2020 and June 15, 2021. Additional covariates included age (in years), sex, geographic region (North Atlantic, Midwest, Southeast, Pacific, continental), smoking status (current, former, never), Charlson Comorbidity Index (based on ≥1 inpatient or 1 outpatient International Classification of Diseases codes), service connection (any/none, using standardized Department of Veterans Affairs cut offs for disability compensation), and influenza vaccination in 2019–2020 (yes/no).
Results
Compared with unvaccinated patients, those vaccinated (n=3,238,532; 55.2%) were older (mean age in years vaccinated=66.3 [SD=14.4] vs unvaccinated=57.7 [18.0], p<0.0001). They were more likely to identify as Black (18.2% vs 16.1%, p<0.0001), Hispanic (7.0% vs 6.6%, p<0.0001), or Asian American Pacific Islander (2.0% vs 1.7%, p<0.0001). In addition, they were more likely to reside in urban settings (68.0% vs 62.8, p<0.0001). Relative to non-Hispanic White urban veterans, the reference group for whom race/ethnicity–urban/rural hazard ratios were reported, all urban race/ethnicity groups were associated with increased likelihood for vaccination except American Indian/Alaskan Native groups. Urban Black groups and rural Black groups were 12% (hazard ratio=1.12; 95% CI=1.12, 1.13) and 6% (hazard ratio=1.06; 95% CI=1.05, 1.06) more likely to receive a first vaccination than White urban groups. Urban Hispanic, Asian American Pacific Islander, and mixed groups were more likely to receive vaccination, whereas rural members of these groups were less likely (Hispanic: urban hazard ratio=1.17; 95% CI=1.16, 1.18, rural hazard ratio=0.98; 95% CI=0.97, 0.99; Asian American Pacific Islander: urban hazard ratio=1.22; 95% CI=1.21, 1.23, rural hazard ratio=0.86; 95% CI=0.84, 0.88). Rural White veterans were 21% less likely to receive an initial vaccine than urban White veterans (hazard ratio=0.79; 95% CI=0.78, 0.79). American Indian/Alaskan Native groups were less likely to receive vaccination regardless of rurality: urban hazard ratio=0.93 (95% CI=0.91, 0.95) and rural hazard ratio=0.76 (95% CI=0.74, 0.78).
Conclusions
Urban Black, Hispanic, and Asian American Pacific Islander veterans were more likely than their urban White counterparts to receive a first vaccination; all rural race/ethnicity groups except Black patients had a lower likelihood for vaccination than urban White patients. A better understanding of disparities and rural outreach will inform equitable vaccine distribution.
Graphical abstract
INTRODUCTION
Disparities related to coronavirus disease 2019 (COVID-19) are present in the U.S., with race/ethnicity (RE) and rurality influencing the likelihood of vaccination, illness, and mortality. In the first months of the pandemic, data from the Centers for Disease Control and Prevention (CDC) showed that Black and Hispanic adults were more likely to test positive, be hospitalized, and die from the virus.1, 2, 3 However, early evidence from the Department of Veterans Affairs (VA), the largest integrated healthcare system in the U.S., showed that such disparities were attenuated among veterans in VA care, substantiating previous observations that health disparities tend to be less in the VA than in the private sector.4,5
COVID-19 vaccination offers significant protection against the virus.6 Unfortunately, vaccine allocation during the first 6 months of distribution showed similar disparities to both disease incidence and outcomes among the general U.S. adult population. CDC data show that Black adults received proportionally fewer first doses of COVID-19 vaccine than their non-Hispanic White counterparts during the initial phase of the vaccine roll out.7 As of June 15, 2021, initial vaccinations were received by 8.8% of U.S. adults who identified as Black despite their making up 12.4% of the total U.S. adult population. Disparities were less evident in Hispanic populations, with 17.2% of vaccinated adults identified as Hispanic while accounting for 17.6% of the total population. In comparison, 61.2% of vaccinated adults were White despite making up 59.4% of the total adult population. This inequity is hypothesized to arise from ongoing structural racism fomenting barriers to health care and potentially greater vaccine hesitancy among people of color8 but is not insurmountable. In a national study of older adults (aged ≥65 years) receiving vaccinations through the VA during the first 2 months of the COVID-19 vaccine campaign (December 15, 2020–February 23, 2020), vaccination was more likely among individuals who identified as Black, Hispanic, or Asian American Pacific Islander (AA/PI) than among those who identified as White.9
Rural communities represent another vulnerable group experiencing higher rates of COVID-19 infection, hospitalization, and death throughout the pandemic. For example, among veterans living with chronic viruses such as HIV in rural areas, entry into care is often delayed.10 Greater rurality was associated with a higher likelihood of poor health outcomes among patients infected with COVID-19 and a lower likelihood of receiving the vaccine on the basis of CDC data at the county level.11 In addition, rural residents were more likely to have to travel to nonadjacent counties to receive a vaccine. Therefore, rurality may be a potent barrier to initial vaccine uptake, and it requires greater efforts for rural residents to obtain vaccination.11
Distribution of the COVID-19 vaccine in the U.S. was a coordinated but complex effort. Although the federal government was tasked with approving the 3 widely circulated vaccines, they were allocated weekly to each state and territory with distribution then determined by individual jurisdictions. Because of vaccine scarcity, state and local vaccine availability was inconsistent during the first months of vaccine allocation.12
In contrast, the VA provides care to over 9 million veterans across nearly 1,300 sites in every state in the U.S.; it is the largest single healthcare system in the U.S. with relatively broad outreach and includes the Office of Rural Health. These and other programs in the VA may mitigate disparities by improving access to care. We hypothesized that RE disparities in initial COVID-19 vaccination would be mitigated in a national cohort of U.S. veterans and that rurality would modify the associations between RE and vaccination.
METHODS
Study Sample
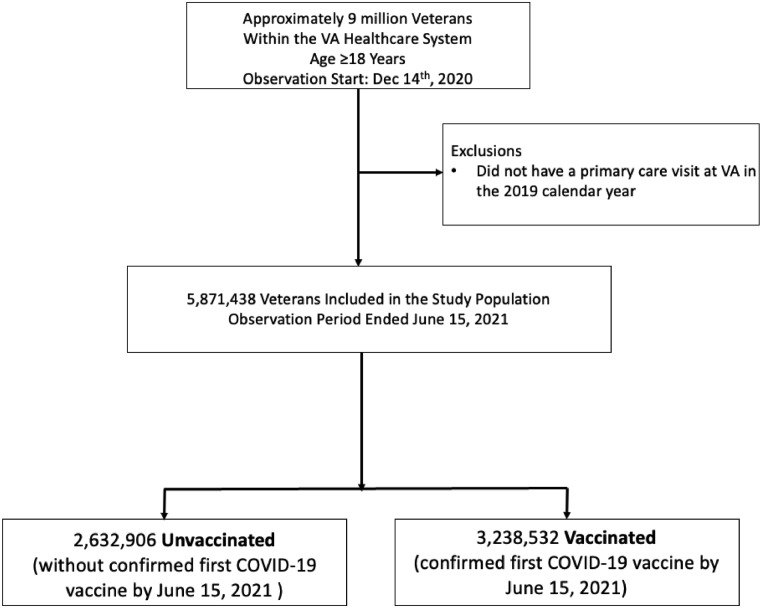
In this retrospective cohort study, we included demographic and clinical data for 5.8 million veterans with a VA primary care visit during the 2019 calendar year (all aged ≥18 years). To receive care at the VA, it is necessary to be aged ≥18 years to serve as an active-duty member of the Armed Forces (Figure 1). All data were obtained from the Corporate Data Warehouse (CDW). We identified individuals who received a first COVID-19 vaccine dose during the observation period (December 15, 2020–June 15, 2021). The CDW includes data for vaccine receipt both within the VA and non-VA facilities, although data are not available for veterans who receive care through the Indian Health Service (IHS). Vaccination distribution at VA primarily followed CDC phased allocation process, beginning on December 15, 2020,13 although age-based eligibility was broadened earlier, and a COVID-19 vaccine was available to all veterans by March 24, 2021 (all U.S. adults on April 19, 2021).14
Figure 1.
Patient selection.
Measures
Initial COVID-19 vaccine receipt was the primary outcome and was ascertained, including non-VA vaccination as detailed earlier; VA uses a validated algorithm to identify COVID-19 vaccination. Vaccination data are updated weekly and cleaned to eliminate duplicate entries.15 The VA immunization database includes data on COVID-19 vaccines administered at the VA as well as vaccines administered outside the VA using linked U.S. national pharmacy databases and patient self-report recorded by VA providers. Using time to first-vaccination as the outcome, patients were censored with the first COVID vaccination, death, or the end of the observation period, whichever came first as of June 15, 2021.
Our predictors of interest were RE and rurality. RE was self-reported in CDW and categorized into a single mutually exclusive group of non-Hispanic Black (Black), Hispanic, non-Hispanic White, AA/PI, American Indian/Alaskan Native (AI/AN), mixed (for individuals who self-identified as multiple races/ethnicities), and other (included declined to answer and unknown by patient and missing).16 Owing to the unknown nature and potential heterogeneity of the Other RE group, we excluded the Other group for modeling purposes.17
Rurality was based on the 2019 home address at the ZIP code level and was defined as urban, rural, and highly rural on the basis of rural-urban commuting area codes, where an area is defined as rural if it contains fewer than 35 people per square mile.18 We folded highly rural into the rural category because it contained extremely few residents.
We included covariates that were likely to contribute to vaccine uptake. Smoking status was ascertained using Health Factors Data19; cigarette smoking Health Factors Data are used nationally by the VA Healthcare System, and responses are categorized as current, former, or never smoker on the basis of self-report. We used the most frequent response to define the variable at the patient level.19 To adjust for comorbidities, we used baseline Charlson Comorbidity Index (CCI) at the time of enrollment in the study during their primary care visit in calendar year 201920 (comorbidities determined by at least 1 inpatient or 2 outpatient International Classification of Diseases codes for the conditions before baseline and CCI categorized as 0, 1, 2, and ≥3).21 As a proxy for overall receptivity to vaccinations, influenza vaccination uptake during the 2019–2020 influenza season was determined using individual patient vaccination data in the electronic health record.22 We also included level of service connectedness as a means of determining disability compensation from active duty in the Armed Forces (none, <50%, or ≥50%). Other covariates included age (in years; as a continuous variable), sex, and geographic region (North Atlantic, Southeast, Midwest, Pacific, continental).
Statistical Analysis
We used descriptive statistics to measure the central tendency for continuous variables and chi-square for categorical variables. For continuous variables, we used t test, and for non-normal variables, we used the Wilcoxon rank sums test. We used a Cox proportional hazards model to evaluate the associations between RE and rurality groups and the time to receipt of the initial COVID-19 vaccine dose over 6 months. The start of follow-up (or time zero) was defined as the first date of COVID-19 vaccine becoming available in the U.S., namely December 15, 2020.
Veterans were followed until receiving the first COVID-19 vaccination dose, death, or reaching the end of 6 months without vaccination, whichever came first. Adjusted hazard ratios (HRs) were estimated after accounting for age, sex, smoking status, CCI, previous influenza vaccination and service connectedness, and geographic region, with the use of robust variance estimators to account for potential within-facility clustering. Because of a significant interaction between RE and rurality (p<0.05), a composite variable accounting for RE by rurality interactions was included in the final multivariable models to allow for direct comparisons across RE and rurality combinations, with non-Hispanic White Urban patients as the reference group.
Sensitivity Analyses
Because we suspected that previous vaccine behavior was important, we conducted Cox models by previous influenza vaccination status. In addition, separate models by sex were performed to determine whether the direction and strength of associations with RE and rurality persisted in women only. Finally, we conducted Cox models, including the Other RE category in the models.
All analyses were performed using SAS, Version 9.4 (SAS Institute, Cary, NC). Proportional hazard assumptions were checked using cumulative martingale residuals and Kolmogorov-type supremum tests. Statistical significance for hypothesis testing was based on a 2-sided p<0.05.
RESULTS
Our study population included 5,871,438 veterans (9.4% women), of whom 55.2% received at least 1 dose of a COVID-19 vaccine. We excluded a group that we categorized as Other and that composed 5.7% of the study population for whom RE was missing.17 Among this group were patients who declined to answer the question about RE identity (n=109,966, 1.9%), who did not know their RE identity (n=38,804; 0.7%), or whom their RE was truly missing (n=182,191; 3.2%).
Vaccinated veterans were older (mean age in years=66.3 [SD=14.4] vs unvaccinated=57.7 [SD=18.0], p<0.001). Most veterans were White (66.7%), and vaccinated veterans were more likely to self-identify as Black (vaccinated=18.2% vs unvaccinated=16.1%, p<0.001). Vaccinated veterans were less likely to reside in rural settings (vaccinated=30.9% vs unvaccinated=35.7%, p<0.001). Vaccinated veterans had a greater comorbidity burden, represented by higher CCI, than unvaccinated veterans (Table 1).
Table 1.
Characteristics of the Study Population
| Characteristics | Vaccinated n=2,632,906 |
Vaccinated n=3,238,532 |
Total N=5,871,438 |
p-Value |
|---|---|---|---|---|
| Age, years, mean (SD) | 57.7 (18.0) | 66.3 (14.4) | 62.4 (16.7) | <0.0001 |
| Age group (%) | <0.0001 | |||
| <45 | 29.7 | 10.6 | 19.1 | |
| 45–54 | 14.6 | 10.3 | 12.2 | |
| 55–64 | 16.8 | 18.5 | 17.8 | |
| 65–74 | 22.7 | 36.7 | 30.4 | |
| ≥75 | 16.2 | 23.9 | 20.5 | |
| Sex, n (%) | <0.0001 | |||
| Female | 279,943 (10.6) | 269,247 (8.3) | 549,190 (9.4) | |
| Male | 2,352,958 (89.4) | 2,969,285 (91.7) | 5,322,243 (90.6) | |
| Race and ethnicity (%) | ||||
| White | 66.9 | 66.7 | 66.8 | |
| Black | 16.1 | 18.2 | 17.2 | |
| Hispanic | 6.6 | 7.0 | 6.9 | |
| Asian/Pacific Islander | 1.7 | 2.0 | 1.9 | |
| American Indian/Alaskan Native | 0.8 | 0.6 | 0.7 | |
| Mixed | 0.9 | 0.8 | 0.8 | |
| Other (missing, declined answer, unknown to patient) | 7.0 | 4.7 | 5.7 | |
| Urban versus rural (%) | <0.0001 | |||
| Urban | 62.8 | 68.0 | 65.7 | |
| Rural | 35.7 | 30.9 | 33.0 | |
| Highly rural | 1.5 | 1.1 | 1.3 | |
| Previous influenza vaccination (2019–2020 season), n (%) | 30.0 | 62.0 | 47.9 | <0.0001 |
| Charlson Comorbidity Index, median (IQR) | 0 [0–1] | 1 [0–2] | 0 [0–1] | <0.0001 |
| Charlson Comorbidity Index (%) | <0.0001 | |||
| 0 | 67.2 | 46.8 | 56.0 | |
| 1 | 16.1 | 22.0 | 19.4 | |
| 2 | 6.8 | 11.2 | 9.2 | |
| ≥3 | 9.9 | 20.0 | 15.4 | |
| Geographic region | <0.0001 | |||
| North Atlantic | 21.0 | 22.5 | 21.8 | |
| Midwest | 20.8 | 20.9 | 20.9 | |
| Southwest | 20.6 | 21.2 | 20.9 | |
| Pacific | 17.6 | 18.4 | 18.1 | |
| Continental | 20.0 | 17.0 | 18.3 | |
| Service connectedness | ||||
| None | 36.1 | 36.2 | 36.2 | |
| <50% | 23.2 | 22.6 | 22.9 | |
| ≥50% | 40.7 | 41.2 | 40.9 |
Adjusted models determined that Black, Hispanic, and AA/PI individuals were more likely to receive a vaccination than White individuals, whereas AI/AN veterans had decreased likelihood of vaccination regardless of rurality. Rural residence was associated with a lower likelihood of receiving at least 1 dose of vaccine (HR=0.80; CI=0.80, 0.81).
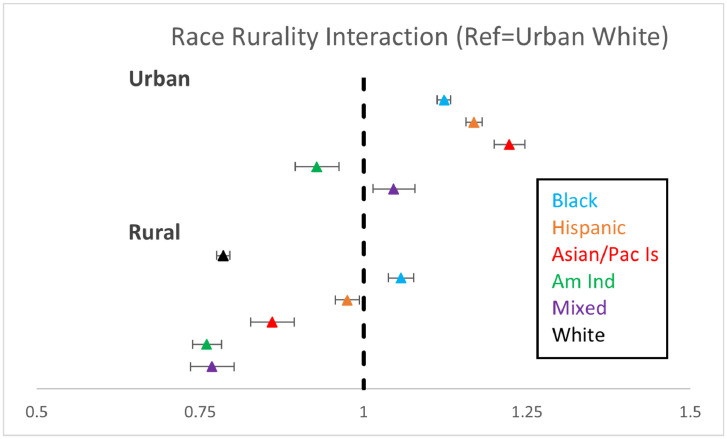
In the final models including interactions between RE Х rurality, urban Black individuals were 12% more likely to get vaccinated than urban White individuals; in addition, the only rural RE group more likely to be vaccinated than urban White individuals were rural Black veterans (urban Black HR=1.12; 95% CI=1.12, 1.13, rural Black HR=1.06; 95% CI=1.05, 1.06) (Table 2 and Figure 2). Other groups varied by RE and rurality compared with urban White (rural White HR=0.78, 95% CI=0.78, 0.79; rural Hispanic HR=0.98, 95% CI=0.97, 0.99; and urban Hispanic HR=1.17, 95% CI=1.16, 1.18); among AA/PI and mixed individuals, urban veterans were more likely to receive first vaccination, but rural veterans were less likely to receive first COVID vaccination (rural AA/PI HR=0.86, 95% CI=0.84, 0.88 and urban AA/PI HR=1.22, 95% CI=1.21, 1.23; rural mixed HR=0.77, 95% CI=0.75, 0.79 and urban mixed HR=1.05, 95% CI=1.03, 1.06). AI/AN individuals were less likely to receive the first dose of vaccine than their White counterparts, regardless of rurality (rural AI/AN HR=0.76, 95% CI=0.74, 0.78 and urban HR=0.93, 95% CI=0.91, 0.95).
Table 2.
Factors Associated With Uptake of COVID-19 Vaccine During the First 6 Months of Vaccine Availability in VA (Dec 14, 2020–Jun 15, 2021), Cox Model (N=5,478,830)
| Factors | Hazard ratio | 95% CI |
|---|---|---|
| Female versus male | 1.05 | 1.05, 1.05 |
| Previous influenza vaccination (2019–2020 season) | 2.13 | 2.12, 2.13 |
| Charlson Comorbidity Index (ref=0) | ||
| 1 | 1.18 | 1.17, 1.18 |
| 2 | 1.22 | 1.21, 1.22 |
| ≥3 | 1.30 | 1.29, 1.30 |
| Urban (ref=urban White) | ||
| Urban Black | 1.12 | 1.12, 1.13 |
| Urban Hispanic | 1.17 | 1.16, 1.18 |
| Urban Asian/Pacific Islander | 1.22 | 1.21, 1.23 |
| Urban American Indian/Alaskan Native | 0.93 | 0.91, 0.95 |
| Urban mixed | 1.05 | 1.03, 1.06 |
| Rural White | 0.79 | 0.78, 0.79 |
| Rural Black | 1.06 | 1.05, 1.06 |
| Rural Hispanic | 0.98 | 0.97, 0.99 |
| Rural Asian/Pacific Islander | 0.86 | 0.84, 0.88 |
| Rural American Indian/Alaskan Native | 0.76 | 0.74, 0.78 |
| Rural mixed | 0.77 | 0.75, 0.79 |
| Age, years | 1.03 | 1.03, 1.03 |
| Geographic region (ref=North Atlantic) | ||
| Midwest | 1.02 | 1.01, 1.03 |
| Southeast | 1.00 | 0.99, 1.00 |
| Pacific | 1.04 | 1.04, 1.04 |
| Continental | 0.92 | 0.91, 0.92 |
| Service connected (ref=none) | ||
| <50% | 1.13 | 1.13, 1.14 |
| ≥50% | 1.23 | 1.22, 1.23 |
Dec, December; Jun, June; VA, Department of Veterans Affairs.
Figure 2.
Forest plot of race and ethnicity interactions with rurality and associations with first COVID-19 vaccination among veterans
Note: Analysis was adjusted for age, sex, Charlson, geographic region, service connection, time-to-vaccination (in days), and previous influenza vaccination during the 2020 influenza season.
Am Ind, American Indian/Alaskan Native; Asian/Pac, Asian American Pacific Islander; Dec, December; VA, Department of Veterans Affairs.
Other covariates associated with vaccination included increasing age (HR=1.03, 95% CI=1.03, 1.03), female sex (HR=1.05, 95% CI=1.05, 1.06), greater disease burden compared with CCI=0 (CCI=1 HR=1.18, 95% CI=1.17, 1.18; CCI=2 HR=1.22, 95% CI=1.21, 1.22; CCI ≥3 HR=1.30, 95% CI=1.29, 1.30), previous influenza vaccination (HR=2.13, 95% CI=2.12, 2.13), and geographic region compared with North Atlantic (Midwest HR=1.02, 95% CI=1.01, 1.03; Southeast HR=1.00, 95% CI=0.99, 1.00; Pacific HR=1.04, 95% CI=1.04, 1.04; continental HR=0.92, 95% CI=0.91, 0.92) (Table 2).
In sensitivity analyses stratified by influenza vaccination, similar associations were observed for RE and COVID-19 vaccination regardless of influenza vaccination during the preceding influenza season except for rural Black previously influenza-vaccinated individuals who had HR similar to that of urban White previously influenza-vaccinated individuals (1.01, 95% CI=1.00, 1.02) (Table 3). Separate models stratified by sex similarly found consistent associations, although the effects of RE and rurality were attenuated in women, with rural Black women having HR similar to that of urban White women (0.99, 95% CI=0.97, 1.01) (Table 3).
Table 3.
Cox Models Stratified by Relevant Previous Influenza Vaccination and Sex
| Influenza vaccination (2019–2020)a |
Sexb |
|||
|---|---|---|---|---|
| Race and ethnicity and rurality | Yes | No | Women | Men |
| Rural | ||||
| Black | 1.01 (1.00, 1.02) | 1.12 (1.11, 1.13) | 0.99 (0.97, 1.01) | 1.06 (1.05, 1.07) |
| Hispanic | 0.97 (0.96, 0.99) | 0.98 (0.96, 0.99) | 0.91 (0.88, 0.94) | 0.98 (0.97, 0.99) |
| AA/PI | 0.85 (0.82, 0.87) | 0.88 (0.85, 0.91) | 0.85 (0.79, 0.91) | 0.86 (0.84, 0.88) |
| AI/AN | 0.80 (0.78, 0.82) | 0.71 (0.68, 0.73) | 0.72 (0.67, 0.77) | 0.76 (0.74, 0.78) |
| Mixed | 0.79 (0.78, 0.81) | 0.73 (0.70, 0.76) | 0.76 (0.70, 0.82) | 0.77 (0.75, 0.79) |
| White | 0.80 (0.80, 0.81) | 0.75 (0.74, 0.75) | 0.76 (0.75, 0.76) | 0.79 (0.78, 0.79) |
| Urban | ||||
| Black | 1.09 (1.08, 1.09) | 1.17 (1.16, 1.17) | 1.01 (1.00, 1.02) | 1.14 (1.13, 1.14) |
| Hispanic | 1.12 (1.11, 1.12) | 1.25 (1.24, 1.26) | 1.09 (1.07, 1.11) | 1.18 (1.17, 1.18) |
| AA/PI | 1.15 (1.13, 1.16) | 1.36 (1.34, 1.38) | 1.13 (1.10, 1.16) | 1.23 (1.22, 1.24) |
| AI/AN | 0.94 (0.91, 0.96) | 0.92 (0.89, 0.95) | 0.93 (0.88, 0.98) | 0.92 (0.90, 0.94) |
| Mixed | 1.02 (1.00, 1.04) | 1.08 (1.05, 1.10) | 1.00 (0.97, 1.04) | 1.05 (1.03, 1.07) |
Models also adjusted for age, sex, Charlson, geographic region, and percentage service connected.
Models also adjusted for age, previous influenza vaccination, Charlson, geographic region, and service connected.
AA/PI, Asian American Pacific Islander; AI/AN, American Indian/Alaskan Native.
Finally, in sensitivity analyses including the Other RE category, the primary associations did not change; veterans included in the Other category were less likely to be vaccinated (urban-Other OR=0.67, 95% CI=0.66, 0.67 and rural-Other OR=0.83, 95% CI=0.83, 0.84) (Appendix Table 1, available online).
DISCUSSION
Whereas vaccination disparities have been prevalent in non-White populations in the general U.S. population, Black, Hispanic, and AA/PI veterans receiving care within VA were more likely to receive a COVID-19 vaccine during the first 6 months of the vaccination campaign. Of note, our observation period extends to the time when vaccinations were available to all U.S. adults.
Our findings build on a recent study among those receiving care in VA examining disparities in vaccination uptake through February 23, 2021, before the vaccine was available to all U.S. adults and all veterans. Similar to our results, their investigation found that Black, Hispanic, and AA/PI veterans were more likely to receive a COVID-19 vaccine, whereas AI/AN patients were less likely. The authors concluded that the VA's proactive vaccine distribution strategy may have mitigated disparities.9 For context, as vaccine supply increased nationally, RE disparities in vaccination status in the U.S. decreased after the first 6 months, although they were not eliminated.1 Our work adds to these initial observations by examining RE differences by urban compared with those by rural settings and finding that vaccination disparities are most prominent in rural settings in all RE except for Black-rural veterans.
There are likely several reasons why the VA successfully mitigated disparities during the initial phase of vaccine allocation. Proposed strategies for health equity have focused on a combination of factors: building trust, engaging local leaders, and eliminating barriers to care.23,24 We speculate that the VA addressed these key components with a centralized, coordinated, national campaign deployed by locally engaged facilities with trusted primary care providers. This coordinated effort included active outreach to veterans once they were eligible for the COVID-19 vaccine and expanding services to include weekend vaccination clinics.25 The VA's ability to track vaccine uptake for millions of patients and contact those who had not received a vaccine with information both about the vaccine and about the logistics for getting one may have led to more equitable and rapid outreach to patients of color. This is in contrast to the non-veteran population, who relied on state and local governments to distribute vaccines to hospitals and healthcare facilities, which have historically been less accessible to communities of color owing to decades of underinvestment in their healthcare.26
It is possible that veterans, regardless of RE, are less likely to experience vaccine hesitancy. Early data, as noted by the Secretary of Veterans Affairs, Denis McDonough, showed less vaccine hesitancy among veterans of color than among Black U.S. adults in the general population.27 Owing to an established relationship, veterans of all RE may be more likely to trust both their specific VA physician and the VA as a whole.27,28 The observation that veterans are less hesitant about receiving vaccines may also be due to vaccination requirements for service in the Armed Forces. Although improved access to care and vaccine information were likely contributors to improved vaccine uptake among veterans of color, vaccine hesitancy among communities of color may contribute to national disparities in vaccine receipt. Multiple studies have shown that even when there is ample availability of vaccines, Black and Hispanic U.S. adults are less likely to obtain a vaccine owing to greater hesitancy.29, 30, 31 This hesitancy may stem from decades of systemic racism within the healthcare system32,33 and may be mitigated with effective community engagement and trusted allies in health care.
There are several factors that are hypothesized to contribute to the higher rate of vaccination among AA/PI veterans and in the U.S. adult population as a whole. Multiple surveys have estimated vaccine hesitancy within AA/PI to be approximately 25%, which is lower than those of other RE groups.34 The observed higher relative rate of vaccination may be partially explained by a relatively large proportion of the AA/PI community working in health care with exposure to patients who were ill with the COVID-19 virus. In addition, language-concordant information on vaccines was widely available for AA/PI individuals owing to efforts by community organizers.35
The lower relative rates of vaccination among AI/AN veterans in our study are discordant with national data. Contrary to initial concerns about vaccine hesitancy among AI/AN adults, as a group, they have been vaccinated at higher rates than their White counterparts owing to the proactive distribution of vaccines by the Indian Health Services (IHS).36,37 Overall, CDC data show that AI/AN U.S. adults have received COVID-19 vaccines at a higher rate than the general U.S. population.7 Many members of the AI/AN community who live in rural settings receive their care through the IHS. If AI/AN veterans received their vaccine through the IHS, those data may not be captured in our outcome ascertainment, which may explain the differences in our findings.
Rurality is an often recognized barrier to equitable health care. In our study, overall vaccinations in rural communities were lower than in urban areas. This observation is likely due to challenges in accessing care in the setting of greater distances traveled for vaccine administration. Similar disparities have been reported for preventive care such as lung cancer screening.38 CDC data showed that rural residents more often had to travel beyond adjacent counties to receive a vaccine and that rural residents who infrequently traveled outside of their home county were less likely to be vaccinated. Barriers to care among persons residing in rural areas are especially difficult to overcome for older U.S. adults as well as for persons without health insurance or healthcare. Furthermore, numerous polls have shown greater vaccine hesitancy in rural communities. A recent poll by the Kaiser Family Foundation showed that 21% of respondents from rural areas would definitely not get a vaccine, compared with 10% among urban respondents.39 Although the VA has an institutional goal to provide accessible health care to veterans in rural settings, additional strategies and outreach may be necessary to overcome barriers to accessing health care within rural communities.
Unsurprisingly, previous influenza vaccination was most strongly associated with COVID-19 vaccination. In sensitivity analyses stratified by previous influenza vaccination, we found a persistently higher likelihood of COVID-19 vaccination in non-White veterans, regardless of previous influenza vaccine receipt.
Limitations
There are several limitations to this study. First, our primary outcome was the uptake of a single dose of vaccine over a relatively short 6-month period rather than full vaccination series adherence and boosting; longer observation time may be especially relevant to examine vaccination disparities in younger age groups. Furthermore, we did not determine severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection rates before initial vaccination and how this might also influence the likelihood of vaccination subsequently. However, we chose to examine the first phase of vaccination allocation because it was more useful in determining disparities owing to the greatest vaccine scarcity during the initial vaccine distribution phase; future work should examine how vaccination was also associated with previous SARS-CoV-2 infection as well as rates of breakthrough infections among those vaccinated and boosted. In addition, our data may not include all veterans who received vaccines outside of the VA system. It is possible that veterans received a vaccine outside of the VA because they were increasingly available in their community. However, this is unlikely to have occurred differentially by race and ethnicity. Throughout the observation period, the necessary criteria to qualify for receipt of a vaccine changed, and the vaccine was not strictly available to all patients at the start of the observation period. However, vaccine eligibility was fluid from the first day of availability, where comorbid burden and employment status were frequent justifications for earlier vaccination in younger age groups. In addition, owing to the initial scarce vaccine supply, staff avoided vaccine waste earlier on and offered vaccinations more liberally than an age-alone cut off. In the first 30 days of vaccination, 17% of vaccinations went to veterans aged <55 years. These factors made it difficult to model for changing vaccine eligibility, which could have created an immortal time bias in our analysis. Finally, although there were fewer women than men in this investigation, we considered vaccination patterns stratified by sex and found overall similar although attenuated associations among women.
CONCLUSIONS
Efficient, equitable vaccine distribution is crucial to combating the COVID-19 pandemic and promoting public health. Our investigation may offer insights into how to improve vaccine uptake for our nation as a whole. As millions more vaccines are administered across the country, including boosters, more data are needed to determine whether this pattern persists among veterans and whether there are ways to utilize these data to circumvent inequities in the general U.S. adult population. Our data show that a proactive approach in a well-developed, primary care–focused healthcare system; reducing barriers; and addressing vaccine hesitancy may help improve vaccination rates among people of color. In addition, our data show that inequities between rural and urban residents persist despite eliminating some barriers to accessing care and vast outreach efforts. For the health and well-being of our country, it is essential to better understand how to effectively increase vaccination rates in Black and Hispanic communities, which have been disproportionately affected by the pandemic.
Acknowledgments
ACKNOWLEDGMENTS
The authors thank Kwan Hur, Francesca Cunningham, and the Center for Medication Center/Pharmacy Benefits Management Services of the Department of Veterans Affairs (VA) Central Office for their review and insights into COVID-19 vaccine distribution reporting for this manuscript as well as their tireless work for VA.
The views expressed in this manuscript represent those of the authors and do not necessarily represent those of the VA.
This work was supported by VA Office of Women's Health Services operational funds through the Office of Patient Care Services (MOU XVA 65–107 to MMF and BBM); the Pain Research, Informatics, Multi-morbidities, and Education Center to ECD, LAB, BB, CB, KMA; VA/RR&D RX003666-01 to KC; the Claude D. Pepper Older Americans Independence Center (Number P30AG21342 NIH/National Institute on Aging to LH); and the National Institute on Alcohol Abuse and Alcoholism (U24-AA020794, U01-AA020790, U10 AA013566 to ACJ).
Declaration of interest: none.
CRediT AUTHOR STATEMENT
Ethan Bernstein: Conceptualization, Writing – original draft. Eric C. DeRycke: Data curation, Formal analysis, Validation. Ling Han: Data curation, Formal analysis, Validation. Melissa M. Farmer: Writing – review & editing. Lori A. Bastian: Supervision, Writing – review & editing. Bevanne Bean-Mayberry: Writing – review & editing. Brett Bade: Writing – review & editing, Supervision. Cynthia Brandt: Writing – review & editing. Kristina Crothers: Writing – review & editing. Melissa Skanderson: Writing – review & editing. Christopher Ruser: Writing – review & editing. Juliette Spelman: Writing – review & editing. Isabel S. Bazan: Writing – review & editing. Amy C. Justice: Writing – review & editing, Supervision. Christopher T. Rentsch: Supervision, Writing – review & editing. Kathleen M. Akgün: Supervision, Writing – review & editing.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.focus.2023.100094.
Appendix. Supplementary materials
REFERENCES
- 1.COVID-19. COVID Data Tracker: Health Equity Data. Centers for Disease Control and Prevention. https://covid.cdc.gov/covid-data-tracker/#health-equity-data. Updated April 15, 2023. Accessed December 21, 2021.
- 2.Mackey K, Ayers CK, Kondo KK, et al. Racial and ethnic disparities in COVID-19-related infections, hospitalizations, and deaths : a systematic review. Ann Intern Med. 2021;174(3):362–373. doi: 10.7326/M20-6306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mude W, Oguoma VM, Nyanhanda T, Mwanri L, Njue C. Racial disparities in COVID-19 pandemic cases, hospitalisations, and deaths: a systematic review and meta-analysis. J Glob Health. 2021;11:05015. doi: 10.7189/jogh.11.05015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rentsch CT, Kidwai-Khan F, Tate JP, et al. Patterns of COVID-19 testing and mortality by race and ethnicity among United States veterans: a nationwide cohort study. PLoS Med. 2020;17(9) doi: 10.1371/journal.pmed.1003379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peterson K, Anderson J, Boundy E, Ferguson L, McCleery E, Waldrip K. Mortality disparities in racial/ethnic minority groups in the Veterans Health Administration: an evidence review and map. Am J Public Health. 2018;108(3):e1–e11. doi: 10.2105/AJPH.2017.304246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.COVID-19 vaccines effectiveness. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/effectiveness/work.html. Updated April 15, 2023. Accessed December 21, 2021.
- 7.Demographic characteristics of people receiving COVID-19 vaccinations in the United States. Centers for Disease Control and Prevention.https://covid.cdc.gov/covid-data-tracker/#vaccination-demographics-trends. Updated April 15, 2023. Accessed November 28, 2021.
- 8.Jimenez ME, Rivera-Núñez Z, Crabtree BF, et al. Black and Latinx community perspectives on COVID-19 mitigation behaviors, testing, and vaccines. JAMA Netw Open. 2021;4(7) doi: 10.1001/jamanetworkopen.2021.17074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haderlein TP, Wong MS, Jones KT, Moy EM, Yuan AH, Washington DL. Racial/Ethnic Variation in Veterans Health Administration COVID-19 vaccine uptake. Am J Prev Med. 2022;62(4):596–601. doi: 10.1016/j.amepre.2021.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ohl M, Lund B, Belperio PS, et al. Rural residence and adoption of a novel HIV therapy in a national, equal-access healthcare system. AIDS Behav. 2013;17(1):250–259. doi: 10.1007/s10461-011-0107-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel B. Disparities in COVID-19 vaccination coverage between urban and rural Coutines- United States. Centers for Disease Control and Prevention. https://www.cdc.gov/mmwr/volumes/70/wr/mm7020e33.html. Updated April 15, 2023. Accessed November 28, 2021.
- 12.Michaud J, Kates J. Kaiser Family Foundation; San Francisco, CA: 2020. Distributing a COVID-19 vaccine across the U.S.-A Look at key issues.https://www.kff.org/report-section/distributing-a-covid-19-vaccine-across-the-u-s-a-look-at-key-issues-issue-brief/ Published October 20, 2020. Accessed September 5, 2021. [Google Scholar]
- 13.Dooling K. Centers for Disease Control and Prevention; Atlanta, GA: 2020. Phased allocation of COVID-19 vaccines.https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2020-11/COVID-04-Dooling.pdf Published November 23, 2020. Accessed December 3, 2021. [Google Scholar]
- 14.The White House . The White House; Washington, DC: 2021. Remarks by President Biden Marking the 150 millionth COVID-19 vaccine shot [press release]https://www.whitehouse.gov/briefing-room/speeches-remarks/2021/04/06/remarks-by-president-biden-marking-the-150-millionth-covid-19-vaccine-shot/ Published April 6, 2021. Accessed December 3, 2021. [Google Scholar]
- 15.Corporate data warehouse. U.S. Department of Veterans Affairs, Health Services Research & Development. https://www.hsrd.research.va.gov/for_researchers/cdw.cfm. Updated April 7, 2023. Accessed February 24, 2022.
- 16.Ferguson JM, Justice AC, Osborne ThasMagid HSA, Purnell AL, Rentsch CT. Geographic and temporal variation in racial and ethnic disparities in SARS-CoV-2 positivity between February 2020 and August 2021 in the United States. Sci Rep. 2022;12(1):273. doi: 10.1038/s41598-021-03967-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hernandez SE, Sylling PW, Mor MK, et al. Developing an algorithm for combining race and ethnicity data sources in the Veterans Health Administration. Mil Med. 2020;185(3–4):e495–e500. doi: 10.1093/milmed/usz322. [DOI] [PubMed] [Google Scholar]
- 18.Economic Research Service U.S. Department of Agriculture. https://www.ers.usda.gov/. Updated March 22, 2023. Accessed March 22, 2023.
- 19.McGinnis KA, Brandt CA, Skanderson M, et al. Validating smoking data from the Veteran's Affairs Health Factors dataset, an electronic data source. Nicotine Tob Res. 2011;13(12):1233–1239. doi: 10.1093/ntr/ntr206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47(11):1245–1251. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 21.Justice AC, McGinnis KA, Skanderson M, et al. Towards a combined prognostic index for survival in HIV infection: the role of ‘non-HIV’ biomarkers. HIV Med. 2010;11(2):143–151. doi: 10.1111/j.1468-1293.2009.00757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leuchter RK, Jackson NJ, Mafi JN, Sarkisian CA. Association between Covid-19 vaccination and influenza vaccination rates. N Engl J Med. 2022;386(26):2531–2532. doi: 10.1056/NEJMc2204560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Balasuriya L, Santilli A, Morone J, et al. COVID-19 vaccine acceptance and access among Black and Latinx communities. JAMA Netw Open. 2021;4(10) doi: 10.1001/jamanetworkopen.2021.28575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bibbins-Domingo K, Petersen M, Havlir D. Taking vaccine to where the –virus is - equity and effectiveness in coronavirus vaccinations. JAMA Health Forum. 2021;2(2) doi: 10.1001/jamahealthforum.2021.0213. [DOI] [PubMed] [Google Scholar]
- 25.VA Weekend Vaccine Clinic. U.S. Department of Veterans Affairs. https://www.va.gov/connecticut-health-care/events/covid-19-vaccine-booster-clinic/. Updated April 15, 2023. Accessed November 28, 2021
- 26.Gillispie-Bell V. The contrast of color: why the Black community continues to suffer health disparities. Obstet Gynecol. 2021;137(2):220–224. doi: 10.1097/AOG.0000000000004226. [DOI] [PubMed] [Google Scholar]
- 27.Wentling N. VA Seeing no difference in coronavirus vaccine reluctance based on race. Stars And Stripes Magazine. March 1, 2021 https://www.stripes.com/va-seeing-no-difference-in-coronavirus-vaccine-reluctance-based-on-race-1.663982?utm_medium=email&utm_source=Stars+and+Stripes+Emails&utm_campaign=Daily+Headlines Accessed December 3, 2021. [Google Scholar]
- 28.Jasuja GK, Meterko M, Bradshaw LD, et al. Attitudes and intentions of U.S. veterans regarding COVID-19 vaccination [published correction appears in JAMA Netw Open. 2021;4(12):e2141483] JAMA Netw Open. 2021;4(11) doi: 10.1001/jamanetworkopen.2021.32548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khubchandani J, Macias Y. COVID-19 vaccination hesitancy in Hispanics and African-Americans: a review and recommendations for practice. Brain Behav Immun Health. 2021;15 doi: 10.1016/j.bbih.2021.100277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baack BN, Abad N, Yankey D, et al. COVID-19 vaccination coverage and intent among adults aged 18–39 years - United States, March–May 2021. MMWR Morb Mortal Wkly Rep. 2021;70(25):928–933. doi: 10.15585/mmwr.mm7025e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kricorian K, Turner K. COVID-19 vaccine acceptance and beliefs among Black and Hispanic Americans. PLoS One. 2021;16(8) doi: 10.1371/journal.pone.0256122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Katz RV, Green BL, Kressin NR, et al. The legacy of the Tuskegee Syphilis Study: assessing its impact on willingness to participate in biomedical studies. J Health Care Poor Underserved. 2008;19(4):1168–1180. doi: 10.1353/hpu.0.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rusoja EA, Thomas BA. The COVID-19 pandemic, Black mistrust, and a path forward. EClinicalMedicine. 2021;35 doi: 10.1016/j.eclinm.2021.100868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ta Park VM, Dougan M, Meyer OL, et al. Vaccine willingness: findings from the COVID-19 effects on the mental and physical health of Asian Americans & Pacific Islanders survey study (COMPASS) Prev Med Rep. 2021;23 doi: 10.1016/j.pmedr.2021.101480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Quach T, Ðoàn LN, Liou J, Ponce NA. A rapid assessment of the impact of COVID-19 on Asian Americans: cross-sectional survey study. JMIR Public Health Surveill. 2021;7(6):e23976. doi: 10.2196/23976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hilla L, Artiga S. Kaiser Family Foundation; San Francisco, CA: 2021. COVID-19 vaccination among American Indian and Alaska Native People.https://www.kff.org/racial-equity-and-health-policy/issue-brief/covid-19-vaccination-american-indian-alaska-native-people/#:∼:text=Federal%20data%20show%20that%2032,people%20compared%20to%20other%20groups Published April 9, 2021. Accessed December 3, 2021. [Google Scholar]
- 37.Foxworth R, Redvers N, Moreno MA, Lopez-Carmen VA, Sanchez GR, Shultz JM. Covid-19 vaccination in American Indians and Alaska natives - lessons from effective community responses. N Engl J Med. 2021;385(26):2403–2406. doi: 10.1056/NEJMp2113296. [DOI] [PubMed] [Google Scholar]
- 38.Kunitomo Y, Bade B, Gunderson CG, et al. Racial differences in adherence to lung cancer screening follow-up: a systematic review and meta-analysis. Chest. 2022;161(1):266–275. doi: 10.1016/j.chest.2021.07.2172. [DOI] [PubMed] [Google Scholar]
- 39.Kirzinger A, Muñana C, Brodie M. Kaiser Family Foundation; San Francisco, CA: 2021. Vaccine hesitancy in rural American.https://www.kff.org/coronavirus-covid-19/poll-finding/vaccine-hesitancy-in-rural-america/ Published January 7, 2021. Accessed December 3, 2021. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.