Abstract
Background
Outbreaks of enteroviral meningitis occur periodically and may lead to hospitalization and severe disease.
Objective
To analyze and describe the meningitis outbreak in patients hospitalized in Israel in 2021–2022, during the COVID-19 pandemic.
Results
In December 2021, before the emergence of the SARS-CoV-2 omicron variant, an off-season increase in enterovirus (EV) infections was observed among patients hospitalized with meningitis. In January 2022, enterovirus cases decreased by 66% in parallel with the peak of the Omicron wave, and then increased rapidly by 78% in March (compared with February) after a decline in Omicron cases. Sequencing of the enterovirus-positive samples showed a dominance of echovirus 6 (E-6) (29%) before and after the Omicron wave. Phylogenetic analysis found that all 29 samples were very similar and all clustered in the E-6 C1 subtype. The main E-6 symptoms observed were fever and headache, along with vomiting and neck stiffness. The median patient age was 25 years, with a broad range (0–60 years).
Conclusion
An upsurge in enterovirus cases was observed after the decline of the SARS-CoV-2 omicron wave. The dominant subtype was E-6, which was present prior to the emergence of the omicron variant, but increased rapidly only after the omicron wave decline. We hypothesize that the omicron wave delayed the rise in E-6-associated meningitis.
Keywords: Enterovirus, Meningitis, Echovirus-6, COVID-19, Omicron variant
1. Introduction
Enteroviruses circulate year-round, with peaks usually occurring in the summer [1,2]. The enterovirus B species includes all the echovirus types (echovirus 1–7, E9, E11 – E21, E24 – E27, E29 – E33) that are associated with aseptic meningitis and encephalitis [1]. Echovirus 6 (E-6) was found responsible for several meningitis-enteroviral outbreaks and it is one of the most common causes of meningitis [3], [4], [5], [6]. Enteroviruses are transmitted mainly via the fecal-oral route. However, transmission via the respiratory route have also been documented [4]. Enteroviruses can infiltrate the central nervous system (CNS) through infection of cerebral vascular endothelial cells which cross the blood brain barrier into the CNS. Enteroviral meningitis infections are usually detected by reverse transcriptase-polymerase chain reaction (RT-PCR) of cerebrospinal fluid (CSF) samples and are based on distinctive clinical symptoms, including fever, headache, neck stiffness, arthralgia/myalgia, vomiting/nausea and photophobia.
The COVID-19 pandemic led to changes in the incidence of infectious diseases, and many respiratory diseases were less prevalent or nearly absent during the pandemic [7]. Social distancing, hand washing and mask usage gradually curbed the spread of viral agents, and marked reductions in the incidence of common respiratory viral pathogens during most of 2020 and early 2021 have been described [7]. However, the effect of the pandemic on enteroviruses has not been described, despite its spread through respiratory pathways.
The present study describes an Echovirus 6 (E-6)-associated meningitis outbreak in hospitalized patients in Israel. We suggest that, in addition to respiratory viruses, the COVID-19 pandemic also impacted enterovirus infection patterns.
2. Methods
2.1. Patients and samples
Sheba Medical Center (SMC) is the largest tertiary medical center in Israel, providing medical aid to all residents of Israel and the Palestinian Authority. SMC treats over 1 million patients annually and is therefore representative of the overall picture of enteroviral meningitis in the country.
A retrospective analysis of CSF samples collected from March 2021 to April 2022 included 1846 patients that presented with clinical symptoms characteristic of aseptic meningitis such as fever, headache, vomiting and neck stiffness (Supplementary 2, Table S5). All samples were sent immediately for routine evaluation for the presence of enteroviruses. From 1846 CSF samples, 98 (5% of all samples) were positive for enterovirus. Only 63 samples were sequenced due to low viral and technical difficulties (Supplementary data 3)
The male to female ratio and age distribution of patients positive for enterovirus are presented in Table 1 .
Table 1.
Positive enterovirus samples detected in Sheba Medical Center.
| Number of patients | % | |
|---|---|---|
| Total positive patients | 98/1846 | 5 |
| Sex ratio (male:female) | 63:35 | |
| Age (Years) | ||
| <2 | 32/98 | 32.7 |
| 2–4 | 9/98 | 9.2 |
| 5–10 | 7/98 | 7.1 |
| 11–20 | 4/98 | 4.1 |
| 21–40 | 37/98 | 37.8 |
| 40–60 | 6/98 | 6.1 |
| >65 | 3/98 | 3.1 |
2.2. SARS-CoV-2 data analysis
The data regarding SARS-CoV-2 cases in Israel was retrieved from the Israeli Ministry of Health (IMH). Positive SARS-CoV-2 infections are updated daily. (https://datadashboard.health.gov.il/COVID-19/general) (Supplementary data 2, Table S5).
2.3. Nucleic acid extraction and real-time PCR (qRT-PCR)
CSF samples were extracted using NucliSENSE easyMAG (BioMerieux, France) according to the manufacturer's instructions. Samples were then screened for the presence of enterovirus using quantitative PCR (qPCR). A duplex reaction was used that contains the primers and probes for general enterovirus [8] and phage MS-2 detection. The MS-2 RNA was spiked into the samples prior to the extraction process and was used as a control for the integrity of the extraction [9]. The primers sequences were as follows: Enterovirus (EV) forward primer: 5′p-CCCTGAATGCGGCTAATCC-3′p, EV reverse primer: 5′p-ATTGTCA CCATAAGCAGCCA-3′p, EV probe: FAM-5′-AACCGACTACTTTGGGTGTCCGTGTTTC-‘3–BHQ-1, MS-2 forward: 5p’-TGCTCGCGGATACCCG-3′p, MS-2 reverse: 5′p-AACTTGC GTTCTCGAGCGAT-3′p, MS-2 probe: VIC-5′-ACCTCGGGTTTCCGTCTTGCTCGT-‘3-NFQ. The reaction components were assembled in the following volumes and concentrations: Reliance 4X master mix (Bio-Rad, www.bio-rad.com): 5 µl, EV forward primer: 375 nM final concentration, EV reverse primer: 1.125 µM final concentration, EV probe: 250 nM final concentration, MS-2 primers: 150 nM each final concentration, MS-2 probe: 100 nM final concentration, PCR-grade H2O to a final volume of 20 µl.
Reaction conditions were as follows: 50 °C for 15 min., 95 °C for 10 min., 45 cycles X [95 °C for 10 s, 60 °C for 30 s]. Fluorescence was read in each cycle after the 60 °C step. The reaction was run in a Bio-Rad CFX-96 thermal cycler. Results were analyzed using the Bio Rad CFX Maestro software.
2.4. Enterovirus sequencing
Genomic viral RNA was reverse-transcribed to cDNA as described by Nix et al. [10], after which, first and second PCR amplifications were performed using FastStart Taq DNA polymerase (Roche Applied Science, Germany) [10]. The PCR products were then separated on a 2% agarose gel and visualized using agarose gel electrophoresis. PCR products were then purified with Ilustra ExoProStar enzyme (Merck, Germany). The DNA templates were sequenced using the BigDye Terminator v1.1 kit on an ABI Prism 3100 automated sequencer (Applied Biosystems, USA). All procedures were conducted in accordance with the manufacturer's instructions with minor modifications, as described by Nix et al. [10].
2.5. Phylogenetic analysis
Positive Echovirus-6 samples were sequenced and analyzed using Sequencher (Gene Codes Corporation, USA) and a phylogenetic tree was constructed using MEGA (Molecular Evolutionary Genetic Analysis) software, version 7. The Echovirus-6 samples that were analyzed belong to two different timelines, 29 samples were sequenced in 2020–2021 (ON960565–93) and eight samples were sequenced in 2017 (OP391717–24). Additionally, 20 representative sequences were retrieved from GenBank for the phylogenetic analysis.
3. Results
3.1. Circulation of enterovirus during the Omicron wave of the COVID-19 pandemic
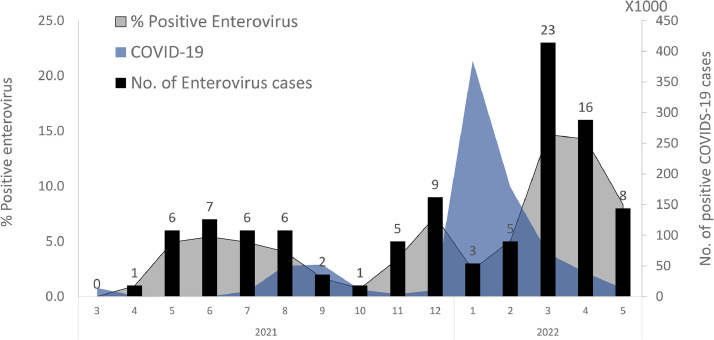
The COVID-19 pandemic began at the end of 2019 in the city of Wuhan, China. The rate of detected cases of COVID-19 fluctuated between 2019 and 2022 as a result of lockdowns, vaccine operations and the penetration of new variants [11]. In December 2021, before the emergence of the SARS-CoV-2 omicron variant, an off-season increase in enterovirus cases was recorded (Fig. 1 ). However, as Omicron cases surged, the number of enterovirus infections dramatically decreased by more than 60%. In March 2022, after the decline in incidence of omicron cases, the number of positive enterovirus cases increased, and was higher compared to the low circulation seen in previous years during the same period (Supplementary data 2, Tables S1 and S2). The largest increase in positive enteroviral cases occurred after the omicron wave waned.
Fig. 1.
Enterovirus incidence in 2021–2022 during the delta and omicron SARS-CoV-2 waves. The number of positive enterovirus cases is presented by black columns, the percentage of enterovirus-positive cases is shown in blue and the number of new COVID-19 cases per week is shown in gray.
3.2. Enterovirus subtyping
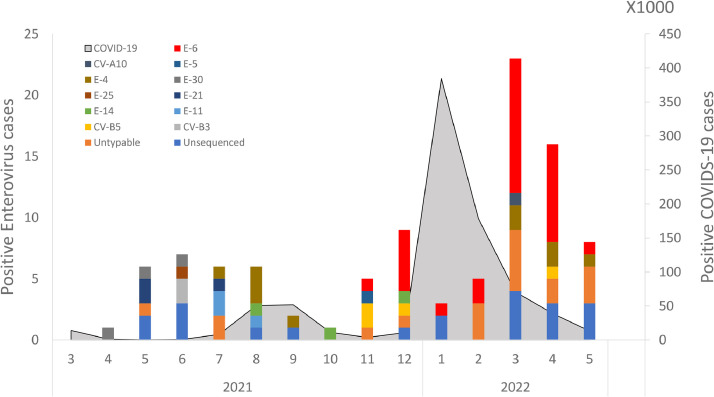
The genus Enterovirus belongs to the Picornaviridae family, which includes more than 100 subtypes [1]. Fig. 2 shows the distribution of different enterovirus strains identified in positive CSF samples. While no Echovirus 6 (E-6) strains were detected until November 2021, by December, E-6, was present in 55% of hospitalized meningitis patients (n = 5). In January-February 2022, only 8 cases of meningitis were detected in parallel to the rapid increase in the Omicron variant. As the Omicron wave waned in March 2022, the prevalence of enteroviral meningitis increased immediately, with E-6 accounting for 52% of these infections (n = 12). As of May 2022, E-6 derived enteroviral meningitis had declined (data not shown).
Fig. 2.
The monthly detection rates of various enterovirus subtypes and the number of new COVID-19 cases (gray). E-6 infection rates are colored in red.
3.3. Clinical and genomic characterization of E-6 cases
From the CSF samples that were found positive for enterovirus, 29 of them belonged to E-6. These cases series were clinically analyzed (Table 2 A-B). The median patient age was 25 years, with a broad range (0–60 years). The main symptoms were fever (89.7%), headache (86.2), vomiting (41.4%) and neck stiffness (41.4%), with no respiratory symptoms recorded. The median duration of fever was at least one day and median hospitalization duration was 3 days. All patients had a benign clinical course, and were discharged without sequelae. Laboratory data (Table 2B) showed that the median white blood cell count in CSF was 91.5 (cells/mm3) (median range 1–1436) and glucose and protein levels were 61.5 (mg/dl) (median range 28–89) and 64.5 (mg/dl) (median range 23–133), respectively. Peripheral white blood cell and neutrophil counts were 9 (K/µl) (median range 5–15) and 7 (K/µl) (median range 2–11), respectively. Additionally, none of the patients were immunocompromised.
Table 2.
A and B. Clinical and laboratory data of the patients infected with E-6.
| A. | Number of patients | % |
|---|---|---|
| Number of patients | 29/98 | |
| Age (Median) | 25 | (Range 0–60) |
| Signs and symptoms | ||
| Fever | 26/29 | 89.7 |
| Headache | 25/29 | 86.2 |
| Vomiting | 12/29 | 41.4 |
| Neck stiffness | 12/29 | 41.4 |
| Kernig sign | 3/29 | 10.3 |
| Photo/phonophobia | 10/29 | 34.5 |
| Brudzinski's sign | 3/29 | 10.3 |
| Duration of fever (median range in days) | and 1 | (1–14) |
| Duration of hospitalization (median and range in days) | 3 | (1–6) |
| B. | Laboratory data of E-6 cases | |
|---|---|---|
| Median | Range | |
| CSF white cell count (cells/mm3) | 91.5 | (1–1436) |
| CSF glucose (mg/dl) | 61.5 | (28–89) |
| CSF protein (mg/dl) | 64.5 | (23–133) |
| CRP (mg/l) | 19 | (1–142) |
| Peripheral white blood cell count (K/µl) | 9 | (5–15) |
| Peripheral neutrophil count (K/µl) | 7 | (2–11) |
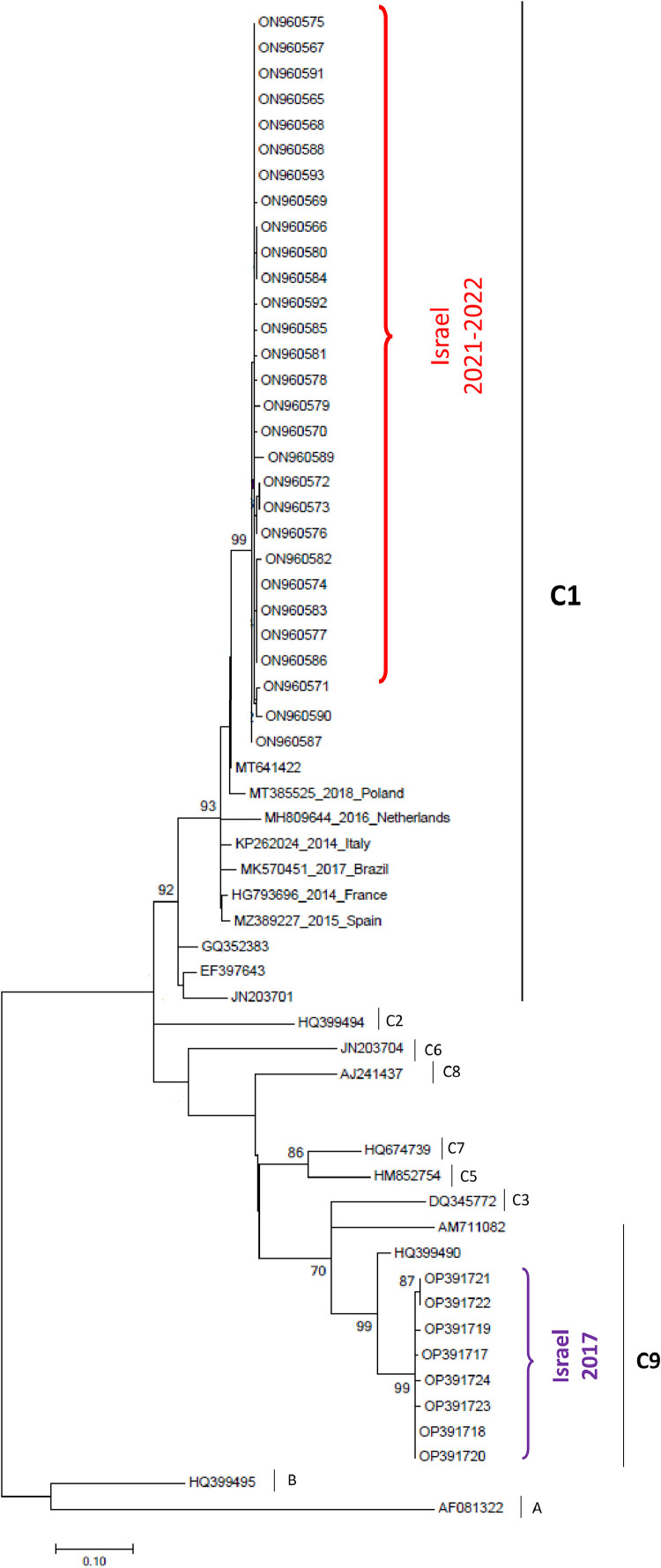
Construction of a phylogenetic tree (Fig. 3 ) showed that all 2022 E-6 samples to cluster in the C1 lineage, consistent with clinical sample E-6 viruses observed in England, Poland, Brazil, Spain and Japan [[4], [5], [6],12]. However, the eight samples collected during 2017 in Israel belonged to the C9 lineage.
Fig. 3.
Phylogenetic tree of the E-6 samples detected in this study (Samples from 2021 to 2022 assigned in red and samples from 2017 assigned in purple), in comparison with other E-6 subtypes.
4. Discussion
Enterovirus is one of the most common causes of aseptic meningitis/encephalitis, along with herpesvirus and arboviruses [13]. The frequency of viral infection varies by season, geographic location and immunocompetency status. According to epidemiological studies in Korea, Russia, The Netherlands and Spain, enterovirus infections primarily occur during the summer [4,[14], [15], [16]]. However, the present study shows an increase in enterovirus cases during the spring (March-April) of 2022, whereas the same period in 2018–2021 had significantly lower infection rates (Supplementary 1, Table S2), which indicates an off-season infection wave.
Subtyping of enterovirus-positive samples found an increase from November (1 case) to December 2021 (5 cases) in E-6 cases. The number of infections decreased in January-February 2022 and increased again in March and April. In parallel, the Omicron variant of SARS-CoV-2 spread worldwide, leading to the highest infection rates experienced worldwide to date [11]. In Israel, as in other countries, the Omicron variant superseded the preceding variant (Delta), with infection rates peaking in January 2022 [17]. Public health measures including masks and social distancing for unvaccinated individuals were mitigated in February 2022 as SARS-CoV-2 cases decreased [18].
The decline in the number of enterovirus cases may be related to the December 2021-January 2022 omicron wave. Our hypothesis, however, has several limitations, including the small sample size in this study, the unique circumstances of a pandemic outbreak (COVID-19) alongside a meningitis outbreak in hospitalized patients, which is difficult to compare with other meningitis outbreaks.
To understand how SARS-CoV-2 may influence the infection rate of E-6, it is important to note that enterovirus infections are usually transmitted by direct contact with respiratory secretions or stool. Hence, the public health measures introduced to reduce SARS-CoV-2 infection would likely reduce enterovirus infections as well.
Another possible alternative is viral interference between enterovirus and SARS-CoV-2. As enteroviruses are shed in respiratory secretions and stool, person-to-person infection is the main mode of transportation for the virus [19]. Person-to-person infection results from the introduction of the infected stool/mucus into an uninfected person through the oral cavity. This assumption coincides with reports of other respiratory viruses [20,21] that were attenuated during the pandemic.
Although several studies showed that viral meningitis triggered by enterovirus occurs mainly in younger children (<6 years) [19], in this study, we observed a similar number of enterovirus infections in the 21–40 years (37.8%) and ≤4 years (41.9%) age groups. This difference may be explained by the fact that 29% of the patients were infected with E-6, which are consistent with other studies that showed a wide age range of E-6-infected patients [22]. The sex ratio also suggested that males (63%) are more susceptible to enteroviral meningitis, as is seen for other enteroviral infections [23,24].
Genetically, all 29 samples collected between 2021 and 2022 belonged to the E-6 subtype C1, while all 2017 samples belonged to the C9 subtype. In other words, the current outbreak, which started in November 2021 and continued through 2022 during the Omicron wave, was unique and was not affected by previously circulating echoviruses. The C1 subtype was also found to circulate in other outbreaks such as the Netherlands (2014–2016), where it was associated with neurological symptoms [23]. In Israel, E-6 was detected in November 2021, peaked in March 2022, and decreased through June and July 2022 (data not shown). It is still too early to conclude whether E-6 will continue its circulation or disappear. E-6 circulation was also found in Cyprus between 2015 and 2017. The enterovirus most frequently detected in Cyprus was echovirus 30 (E-30), which is dominant in Europe, while the second most common enterovirus detected was E-6 (14.2%) [22]. Interestingly, the phylogenetic analysis of the Cyprus samples classified them as the C1 subtype, as seen in the current work (C9). Another episode of circulation of E-6 associated with viral meningitis was observed during the early summer (May to July) of 2017 in China [23].
Although many outbreaks of enteroviral meningitis in hospitalized patients are caused by echovirus, the potential of severe disease is rather low. However, severe outcomes have been reported in susceptible neonate populations [25] . Interestingly, during the COVID-19 pandemic, the number of infant hospitalizations with enteroviral meningitis decreased and in SMC, only a few cases of enteroviral-associated meningitis were detected (Supplementary 1, Table S1).
5. Conclusions
Detection of echovirus-6-associated with meningitis increased in Israel toward the end of 2021, but then decreased in parallel with the rise of the Omicron wave, which peaked in January 2022. After the Omicron wave waned, E-6 C1 began circulating again and was identified in almost 30% of the patients hospitalized with meningitis. Herein, we suggest that the SARS-CoV-2 omicron variant had an influence on this outbreak. The effects of social distancing versus viral interference requires further investigation.
Funding source
None
Ethical approval
The institutional review board (IRB) of SMC approved this study (Helsinki Number SMC-8311-21). CSF samples were collected as part of the routine sampling performed in the clinical virology laboratory. The samples were tested and analyzed for the presence of enterovirus as part of routine testing. The work described herein is an anonymous retrospective study, hence, informed consent (either written or verbal) was not required.
CRediT authorship contribution statement
Ilana S. Fratty: Data curation, Formal analysis, Methodology, Project administration, Writing – review & editing. Or Kriger: Formal analysis, Methodology. Leah Weiss: Project administration. Rinat Vasserman: Project administration. Oran Erster: Writing – original draft. Ella Mendelson: Validation. Danit Sofer: Validation. Merav Weil: Data curation, Supervision.
Declaration of Competing Interest
None to declare.
Acknowledgements
Not applicable.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jcv.2023.105425.
Appendix. Supplementary materials
References
- 1.Nikonov O.S., Chernykh E.S., Garber M.B., Nikonova E.Y. Enteroviruses: classification, diseases they cause, and approaches to development of antiviral drugs. Biochemistry. 2017;82(13):1615–1631. doi: 10.1134/S0006297917130041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wright W.F., Pinto C.N., Palisoc K., Baghli S. Viral (aseptic) meningitis: a review. J. Neurol. Sci. 2019;398:176–183. doi: 10.1016/j.jns.2019.01.050. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization (WHO). Enterovirus surveillance guidelines. 2015.
- 4.Monge S., Benschop K., Soetens L., Pijnacker R., Hahne S., Wallinga J., et al. Echovirus type 6 transmission clusters and the role of environmental surveillance in early warning, the Netherlands, 2007 to 2016. Euro Surveill. 2018;23(45) doi: 10.2807/1560-7917.ES.2018.23.45.1800288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gambaro F., Perez A.B., Aguera E., Prot M., Martinez-Martinez L., Cabrerizo M., et al. Genomic surveillance of enterovirus associated with aseptic meningitis cases in southern Spain, 2015-2018. Sci. Rep. 2021;11(1):21523. doi: 10.1038/s41598-021-01053-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Toczylowski K., Wieczorek M., Bojkiewicz E., Wietlicka-Piszcz M., Gad B., Sulik A. Pediatric enteroviral central nervous system infections in bialystok, poland: epidemiology, viral types, and drivers of seasonal variation. Viruses. 2020;12(8) doi: 10.3390/v12080893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olsen S.J., Winn A.K., Budd A.P., Prill M.M., Steel J., Midgley C.M., et al. Changes in influenza and other respiratory virus activity during the COVID-19 pandemic-United States, 2020-2021. Am. J. Transplant. 2021;21(10):3481–3486. doi: 10.1111/ajt.16049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verstrepen W.A., Bruynseels P., Mertens A.H. Evaluation of a rapid real-time RT-PCR assay for detection of enterovirus RNA in cerebrospinal fluid specimens. J. Clin. Virol. 2002;25(1):S39–S43. doi: 10.1016/s1386-6532(02)00032-x. Suppl. [DOI] [PubMed] [Google Scholar]
- 9.Dreier J., Stormer M., Kleesiek K. Use of bacteriophage MS2 as an internal control in viral reverse transcription-PCR assays. J. Clin. Microbiol. 2005;43(9):4551–4557. doi: 10.1128/JCM.43.9.4551-4557.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nix W.A., Oberste M.S., Pallansch M.A. Sensitive, seminested PCR amplification of VP1 sequences for direct identification of all enterovirus serotypes from original clinical specimens. J. Clin. Microbiol. 2006;44(8):2698–2704. doi: 10.1128/JCM.00542-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koelle K., Martin M.A., Antia R., Lopman B., Dean N.E. The changing epidemiology of SARS-CoV-2. Science. 2022;375(6585):1116–1121. doi: 10.1126/science.abm4915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wieczorek M., Figas A., Krzysztoszek A. Enteroviruses associated with aseptic meningitis in Poland, 2011-2014. Pol. J. Microbiol. 2016;65(2):231–235. doi: 10.5604/17331331.1204485. [DOI] [PubMed] [Google Scholar]
- 13.Costa B.K.D., Sato D.K. Viral encephalitis: a practical review on diagnostic approach and treatment. J. Pediatr. (Rio J) 2020;96(1):12–19. doi: 10.1016/j.jped.2019.07.006. Suppl. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kang H.J., Yoon Y., Lee Y.P., Kim H.J., Lee D.Y., Lee J.W., et al. A different epidemiology of Enterovirus A and Enterovirus B co-circulating in Korea, 2012-2019. J Pediatr. Infect. Dis. Soc. 2021;10(4):398–407. doi: 10.1093/jpids/piaa111. [DOI] [PubMed] [Google Scholar]
- 15.Akhmadishina L.V., Govorukhina M.V., Kovalev E.V., Nenadskaya S.A., Ivanova O.E., Lukashev A.N. Enterovirus A71 Meningoencephalitis Outbreak, Rostov-on-Don, Russia, 2013. Emerg. Infect. Dis. 2015;21(8):1440–1443. doi: 10.3201/eid2108.141084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Casas-Alba D., de Sevilla M.F., Valero-Rello A., Fortuny C., Garcia-Garcia J.J., Ortez C., et al. Outbreak of brainstem encephalitis associated with enterovirus-A71 in Catalonia, Spain (2016): a clinical observational study in a children's reference centre in Catalonia. Clin. Microbiol. Infect. 2017;23(11):874–881. doi: 10.1016/j.cmi.2017.03.016. [DOI] [PubMed] [Google Scholar]
- 17.Israel Ministry of Health Control (IMoHC), https://datadashboard.health.gov.il/COVID-19/; 2021.
- 18.Israel Minstry of Health. 01.02.2022. https://www.gov.il/en/departments/news,.
- 19.BKrong N., Minh N.N.Q., Qui P.T., Chau T.T.H., Nghia H.D.T., Do L.A.H., et al. Enterovirus serotypes in patients with central nervous system and respiratory infections in Viet Nam 1997-2010. Virol. J. 2018;15(1):69. doi: 10.1186/s12985-018-0980-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fratty I.S., Reznik-Balter S., Nemet I., Atari N., Kliker L., Sherbany H., et al. Outbreak of Influenza and other respiratory viruses in hospitalized patients alongside the SARS-CoV-2 pandemic. Front. Microbiol. 2022;13 doi: 10.3389/fmicb.2022.902476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drori Y., Jacob-Hirsch J., Pando R., Glatman-Freedman A., Friedman N., Mendelson E., et al. Influenza A virus inhibits RSV infection via a two-wave expression of IFIT proteins. Viruses. 2020;12(10) doi: 10.3390/v12101171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Richter J., Tryfonos C., Christodoulou C. Molecular epidemiology of enteroviruses in Cyprus 2008-2017. PLoS ONE. 2019;14(8) doi: 10.1371/journal.pone.0220938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun Y., Miao Z., Yan J., Gong L., Chen Y., Mao H., et al. Sero-molecular epidemiology of enterovirus-associated encephalitis in Zhejiang Province, China, from 2014 to 2017. Int. J. Infect. Dis. 2019;79:58–64. doi: 10.1016/j.ijid.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 24.Benschop K.S., Geeraedts F., Beuvink B., Spit S.A., Fanoy E.B., Claas E.C., et al. Increase in ECHOvirus 6 infections associated with neurological symptoms in the Netherlands, June to August 2016. Euro Surveill. 2016;21(39) doi: 10.2807/1560-7917.ES.2016.21.39.30351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siafakas N., Goudesidou M., Gaitana K., Gounaris A., Velegraki A., Pantelidi K., et al. Successful control of an echovirus 6 meningitis outbreak in a neonatal intensive care unit in central Greece. Am. J. Infect. Control. 2013;41(11):1125–1128. doi: 10.1016/j.ajic.2013.02.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.