Abstract
Perivascular adipose tissue (PVAT) refers to the aggregate of adipose tissue surrounding the vasculature, exhibiting the phenotypes of white, beige and brown adipocytes. PVAT has emerged as an active modulator of vascular homeostasis and pathogenesis of cardiovascular diseases in addition to its structural role to provide mechanical support to blood vessels. More specifically, PVAT is closely involved in the regulation of reactive oxygen species (ROS) homeostasis and inflammation along the vascular tree, through the tight interaction between PVAT and cellular components of the vascular wall. Furthermore, the phenotype-genotype of PVAT at different regions of vasculature varies corresponding to different cardiovascular risks. During ageing and obesity, the cellular proportions and signaling pathways of PVAT vary in favor of cardiovascular pathogenesis by promoting ROS generation and inflammation. Physiological means and drugs that alter PVAT mass, components and signaling may provide new therapeutic insights in the treatment of cardiovascular diseases. In this review, we aim to provide an updated understanding towards PVAT in the context of redox regulation, and to highlight the therapeutic potential of targeting PVAT against cardiovascular complications.
Keywords: Perivascular adipose tissue, Oxidative stress, Inflammation, Cardiovascular disease, Ageing, Obesity
Graphical abstract
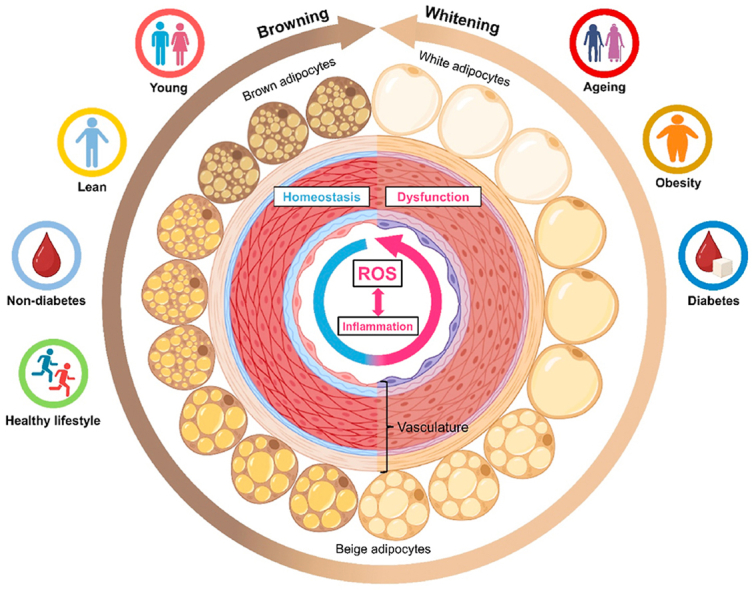
PVAT homeostasis and dysfunction associated with ROS and inflammation.Different body status and lifestyles contribute to either the homeostasis or dysfunction of PVAT, by modulating oxidative stress, inflammation and browning/whitening of PVAT.
Highlights
-
•
PVAT regulates ROS homeostasis and inflammation via interaction with vascular cells.
-
•
During ageing and obesity, PVAT favors cardiovascular disease pathogenesis.
-
•
PVAT phenotype-genotype differs in different vascular regions.
-
•
Certain physiological means can alter PVAT phenotype-genotype.
Abbreviations
- AGE
advanced glycation end product
- Ang II
angiotensin II
- AT1R
Ang II type 1 receptor
- ATF3
activating transcription factor 3
- BAT
brown adipose tissue
- BeAT
beige adipose tissue
- BMAL1
brain and muscle Arnt-like protein-1
- BMP4
bone morphogenetic protein 4
- COX-1
cyclooxygenase 1
- CT
computed tomography
- CXCL
C-X-C motif chemokine ligand
- EC
endothelial cell
- eNOS
endothelial nitric oxide synthase
- ER
endoplasmic reticulum
- ETC
electron transport chain
- FAI
fat attenuation index
- HFD
high fat diet
- IL
interleukin
- MCP-1
monocyte chemoattractant protein-1
- MnSOD
manganese superoxide dismutase
- NO
nitric oxide
- NOX
NADPH oxidase
- PAI-1
plasminogen activator inhibitor
- PGC-1α
peroxisome proliferator activated receptor gamma coactivator 1α
- PPARγ
peroxisome proliferator activated receptor gamma
- PVAT
perivascular adipose tissue
- RAAS
renin-angiotensin-aldosterone system
- ROS
reactive oxygen species
- SIRT1
sirtuin 1
- TNFα
tumor necrosis factor α
- VEGF
vascular endothelial growth factor
- VSMC
vascular smooth muscle cell
- WAT
white adipose tissue
1. Introduction
In vascular system, most blood vessels are surrounded to varied degrees by a functionally specialized aggregate of adipose tissue, termed as perivascular adipose tissue (PVAT) [1]. Distinct from general adipose depots, PVAT stays in close proximity to tunica adventitia of small-, medium-, and large-diameter arteries, serving as a pivotal endocrine/paracrine organ for the regulation of cardiometabolic homeostasis [2]. In addition to its structural role on vascular support, PVAT is tightly involved in the modulation of vascular homeostasis, and vascular dysfunction in associated with cardiometabolic diseases [3]. Upon the stress of cardiovascular risk factors (e.g. obesity and smoking), PVAT can act as a negative modulator to favor the progression of type 2 diabetes mellitus and cardiovascular diseases, like atherosclerosis, hypertension and coronary artery disease [4].
PVAT shows phenotypic, genotypic and functional heterogeneity, depending on the sites of vasculature where PVAT exists [5]. Majority of PVATs, particularly those around thoracic aorta, display certain defining hallmarks of brown adipose tissue (BAT), including expression of thermogenic genes (e.g. Ucp-1), presence of numerous lipid droplets, and high mitochondrial content [6]. Besides, certain PVAT, predominantly mesenteric PVAT, is more-white adipose tissue (WAT)-like, due to lower expression of thermogenic genes like UCP-1, presence of larger lipid droplets, and low mitochondrial number [7]. Meanwhile, certain PVAT, like coronary PVAT, is more beige adipose tissue (BeAT)-like because some brown adipocyte-associated genes, such as UCP-1 and carnitine palmitoyltransferase 1B, are differentially expressed when compared to those of BAT [8]. Since PVAT is developmentally distinct from predefined adipose tissues, PVAT can be considered as the fourth type of adipose tissue [9].
PVAT can contribute to the accumulation of reactive oxygen species (ROS) and inflammation in the vasculature. Depending on health conditions, PVAT may elicit a net beneficial or harmful effect on the vasculature [10]. Under physiological conditions, PVAT can elicit anticontractile effects on arteries by serving as a source of vasodilatory factors, like nitric oxide (NO), leptin and angiotensin-1 to 7, to mitigate vascular injury [11]. Under disease states, PVAT can generate ROS, such as superoxide anion and hydrogen peroxide, to injure vascular cells. Major sources of ROS in PVAT originate from NADPH oxidase, mitochondria and endothelial nitric oxide synthase (eNOS) uncoupling [12]. During the pathogenesis of cardiovascular diseases, dysfunctional PVAT might also secrete pro-inflammatory factors, like leptin [13], tumor necrosis factor α (TNFα), and monocyte chemoattractant protein-1 (MCP-1) to promote vascular inflammation [14]. Through different signaling pathways, elevated ROS production in vasculature drives vascular inflammation, and vice versa [15]. PVAT-triggered oxidative stress and inflammation in vascular beds promote dysfunction of vascular cells so as to accelerate the progression of cardiovascular diseases.
2. PVAT and vascular cells
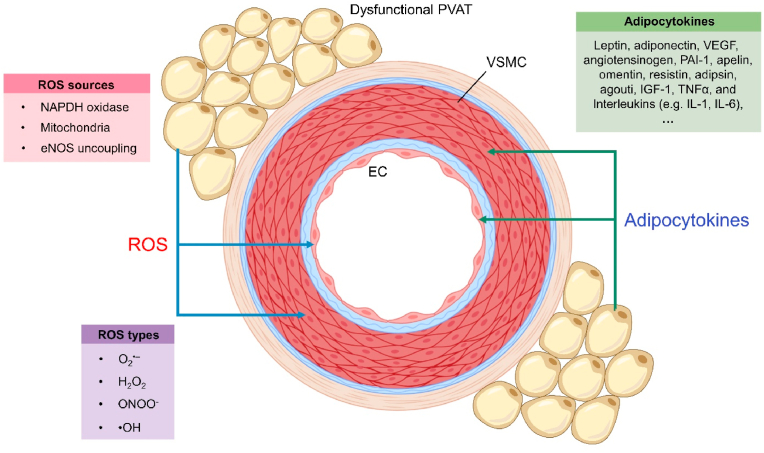
PVAT is tightly involved in the regulation of ROS homeostasis and inflammation in vascular beds, through close interaction with cellular components of the vascular wall, including vascular smooth muscle cells (VSMCs), endothelial cells (ECs) and immune cells. PVAT-generated ROS, especially hydrogen peroxide, can diffuse towards vascular cells to cause oxidative damage and impair their function (Fig. 1). Besides, PVAT-derived substances and factors can also trigger inflammation in the vascular cells (Fig. 1), where the interaction between oxidative stress and inflammation further injures vascular cells to increase disease risks [16].
Fig. 1.
ROS and adipocytokines generated by dysfunctional PVAT. Upon PVAT dysfunction, PVAT generates ROS through multiple sources and derives various adipocytokines to influence the homeostasis of vascular cells, particularly VSMCs and ECs.
2.1. PVAT-generated ROS
Multiple types of ROS are generated by dysfunctional PVAT. In addition to superoxide anion (O2•–), hydrogen peroxide (H2O2) can be produced within PVAT [17]. Notably, superoxide can be converted to the highly reactive nitrogen species peroxynitrite (ONOO−) in the presence of NO, whereas H2O2 is further converted to hydroxyl radical (•OH) which oxidizes DNA and lipids to aggravate cellular injury [18]. In the presence of cardiovascular risk factors (e.g. obesity and ageing), redox balance in PVAT is disturbed leading to overproduction of ROS. Major ROS sources in PVAT include NADPH oxidase, mitochondria and eNOS uncoupling [12].
The NADPH oxidase (NOX) family is one of the major vascular ROS-generating sources, where NOX uses NADPH as an electron donor to catalyze superoxide production [19]. NOX1, NOX2, NOX4 and NOX5 are expressed in vascular cells [19], while NOX1, NOX2 and NOX4, but not NOX5, are detected in PVAT [20]. Additionally, NOX4 can also release H2O2, when compared to NOX1-3, and NOX5 that generate superoxide [21]. Similar to brown and beige adipocytes, adipocytes in PVAT also generate mitochondrial ROS to increase oxidative stress. During ATP production by oxidative phosphorylation in mitochondria, ROS like superoxide and H2O2 are formed from electron transport chain (ETC) [22]. Costa et al. provided experimental clues to indicate mitochondria as a ROS source in PVAT and they showed that mitochondrial ETC in thoracic PVAT can generate H2O2 for the modulation of vascular smooth muscle contractility, by using uncouplers of oxidative phosphorylation [23]. More importantly, deficiency of the thermogenic gene UCP-1 results in an overproduction of mitochondrial ROS [24].
eNOS is an oxidoreductase homodimer which catalyzes the conversion of O2 and l-arginine to l-citrulline and NO [25]. eNOS uncoupling refers to the switch of enzymatic activity from a NO- to a predominantly superoxide-generating enzyme, especially when the substrate l-arginine and the eNOS cofactor tetrahydrobiopterin (BH4) are at suboptimal levels [26]. eNOS expression has been identified in thoracic PVAT. Of note, any cardiovascular risk factors that lower availability of l-arginine and BH4 promote eNOS uncoupling and therefore superoxide production [7]. Consequently, the overproduced ROS by PVAT during pathological conditions cause oxidative damage to adjacent vascular cells.
2.2. PVAT-derived factors
PVAT acting as an autocrine/paracrine organ modulates vascular ROS production and inflammation through secreting adipocytokines, the constellation of adipokines and inflammatory cytokines [27]. PVAT can release many adipocytokines, including leptin, adiponectin, vascular endothelial growth factor (VEGF), angiotensinogen, resistin, omentin, plasminogen activator inhibitor (PAI-1), insulin-like growth factor-1 (IGF-1), TNFα and interleukins, such as interleukin 1 (IL-1) and IL-6 [8]. Particularly, certain adipocytokines (e.g. adiponectin and angiotensin-1 to 7) are involved in the mediation of anti-contractile properties of PVAT by opening voltage-gated KV channels of adjacent vascular smooth muscle cells [28], and contributing to the regulation of arterial blood pressure [29]. Certain adipocytokines elicit an autocrine effect to modulate the metabolism in PVAT adipocyte. For instance, PVAT-derived leptin can stimulate NO production in perivascular adipocytes [30], where NO is a critical vasodilator and serves as an important antioxidant by scavenging ROS in the vascular system [31].
In a paracrine manner, PVAT-derived adipocytokines reach vascular cells, and regulate the migration and infiltration of immune cells to modulate vascular oxidative stress and inflammation. Leptin plays a pivotal role in triggering the production of pro-inflammatory cytokines (e.g. TNFα and IL-6) from immune cells including monocytes, macrophages and leukocytes [32], where these cytokines directly activate inflammatory signaling in VSMCs and ECs. Clinically, a leptin-inflammation-fibrosis-hypoxia axis was observed in coronary PVAT from patients undergoing coronary artery bypass surgery [33], implying a pro-inflammatory microenvironment around the vasculature.
Adiponectin is known to be a humoral vasodilator which relaxes arteries through opening KV channels [34]. During obesity, the expression of the anti-inflammatory adiponectin in PVAT is reduced [35], accounting for elevated inflammation in ECs and macrophages [36]. Meanwhile, resistin can facilitate macrophage infiltration and increase the expression of inflammatory markers in ECs [37]. Besides, omentin is reported to alleviate endothelial dysfunction by inhibiting endoplasmic reticulum (ER) stress and oxidative stress, possibly through the AMPK/PPARδ signaling pathway. Omentin was also shown to decrease the expression of inflammatory markers in both macrophages and ECs [38]. In VSMCs, omentin suppresses TNFα-upregulated NOX activity, ROS production and inflammation [39]. These previous findings highlight the contribution of PVAT-derived factors in mediating vascular oxidative stress and inflammation.
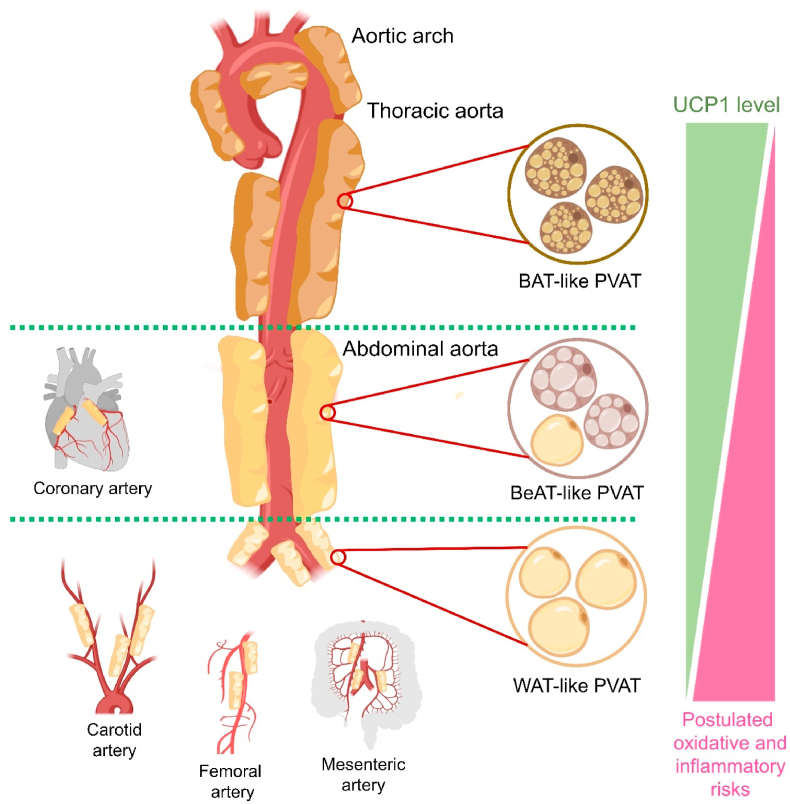
3. PVAT at different regions of vasculature
In different anatomic locations, PVATs are constituted by different cell types [40]. Around different regions of the vascular tree, PVAT can be classified as ‘WAT-like’, ‘BAT-like’ and ‘BeAT-like’, exhibiting different phenotype-genotype (Fig. 2) [41]. Heterogenous PVATs are believed to be associated with distinct paracrine functions [42]. It is reasonable to postulate that different types of PVAT shall correspond to different cardiovascular risks, since their capability to generate ROS and inflammatory secretome are different. Around the thoracic aortas of rodents, the thoracic PVAT is more BAT-like in terms of morphology and function [43]. However, whether thoracic PVAT in humans is also BAT-like remains debatable [44]. Meanwhile, PVAT surrounding small arteries like carotid, femoral and mesenteric arteries are mostly WAT-like [45]. Notably, PVAT around abdominal aorta displays the features of both WAT and BAT, hinting that abdominal PVAT is potentially more BeAT-like [41]. Interestingly, adipose tissue is absent around the coronary artery from murine [41], but present around those from humans and larger experimental animals, such as pigs [46], and rabbits [47]. In humans, coronary PVAT is more phenotypically close to BeAT [48]. However, the morphology of coronary PVAT among larger experimental animals is less well defined.
Fig. 2.
Regional difference of PVAT and corresponding risks. Different regions of the vasculature are surrounded by distinct PVATs associated with different phenotype-genotypes. The color differences of the adipose tissues reflect different proportions of brown adipocytes. WAT-like PVAT shall confer relatively higher oxidative and inflammatory risks.
From the extensive research on the browning and whitening of adipocytes, the capabilities of adipose tissues to produce ROS and induce inflammation in adjacent tissues can be approximately deduced. Different types of adipocytes are associated with differential levels of UCP1 (BAT > BeAT > WAT), where UCP1 upregulation often accounts for a decreased ROS production in adipocytes [49], implying that the hierarchies of ROS production in adipose tissues are as follows: WAT > BeAT > BAT. Compared to BeAT and BAT, WAT is associated with a high pro-inflammatory profile, characterized by elevated production of adipocytokines related to inflammatory modulation and enhanced infiltration of immune cells [50]. Moreover, deficiency of UCP1 has been shown to exacerbate ER stress and inflammation in BAT [51], where the crosstalk between ER stress and oxidative stress often triggers inflammatory response in different disease states [52]. More importantly, UCP1 deficiency in PVAT was found to cause NLRP3-inflammasome hyperactivation and IL-1β release, aggravating endothelial dysfunction [24]. These findings suggest that UCP1 deficiency potentially promotes inflammation in adipose and adjacent tissues, and the hierarchies of inflammatory profiles in adipose are as follows: WAT > BeAT > BAT. Therefore, it is also reasonable to postulate that WAT-like PVAT shall confer higher oxidative and inflammatory risks.
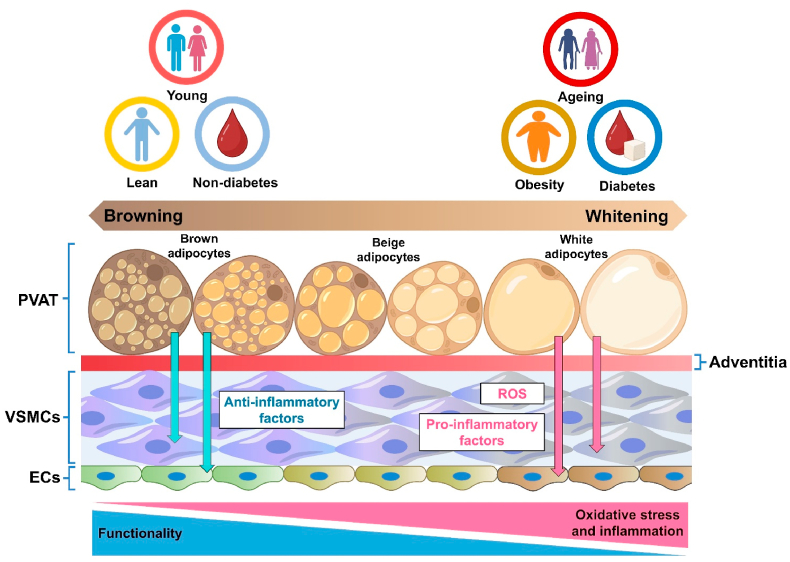
4. Ageing and PVAT
As a critical regulator on vascular homeostasis, PVAT actively contributes to the vascular dysfunction during ageing (Fig. 3). During ageing, vascular dysfunction can be attributed to decreased endothelial function, ROS accumulation, chronic inflammation and structural remodeling in vasculature [53]. Dysfunctional PVAT during ageing overproduces ROS and secretes adipocytokines to mediate the signaling and inflammation of adjacent vascular layers, leading to the dysfunctions of both VSMCs and endothelial cells [54]. Numerous studies indicate that aged PVAT directly causes vascular dysfunction. For instance, the anticontractile effect of mesenteric PVAT was remarkably reduced in senescence-accelerated prone mice, an ageing-related model of vascular dysfunction [55]. More recently, a study reveals that ageing induces PVAT dysfunction and impairs vascular function via KV7.3-5 channels through modulating inflammatory and metabolic processes in PVAT [56], where KV channels are commonly expressed in VSMCs of different arteries [57]. Moreover, transplantation studies on adipose tissue also suggest the harmful effects of aged PVAT on vascular function. Transplantation of PVAT from aged donor mice increased the aortic pulse wave velocity, a non-invasive clinical measure on arterial stiffness, in young recipient mice. Transplantation of aged PVAT also increased thickness of aortic wall and lumen diameter, and caused the accumulation of collagens in arterial adventitial layer of young recipient mice. In addition, prior treatment of the superoxide scavenger TEMPOL alleviated the stiffness-promoting effect of aged PVAT in young recipient mice [58].
Fig. 3.
The influence of body status on PVAT function. Ageing, obesity and diabetes enhance the whitening of PVAT, where WAT-like PVAT promotes oxidative stress and inflammation in the vasculature, triggering the dysfunction of vascular cells, including VSMCs and ECs.
Several mechanisms contribute to vascular dysfunction induced by aged PVAT. During ageing, higher ROS production by PVAT causes direct damage on adjacent vascular cells. In PVAT of obese aged mice (2-year-old), NOX upregulation, eNOS downregulation and increased inflammation were noted [59]. Additionally, increased amount of adipocytokines secreted by PVAT also exacerbate vascular dysfunction. Higher levels of pro-inflammatory cytokines and chemokines were found in the conditioned media of PVAT isolated from obese aged mice [59], hinting the secretome tole of PVAT during ageing. Compared to young mice, increased amount of pro-inflammatory cytokines secreted by PVAT was reported in aged mice based on cytokine array data. Notably, higher levels of IL-6, granulocyte macrophage colony stimulating factor, MCP-1, C-X-C motif chemokine ligand (CXCL) 1 and CXCL2 in aged mice were observed [58].
During ageing, diminished BAT activity [60], and augmented BAT whitening [61] shall contribute to the impairment of vascular function and serve as driving factors of cardiovascular pathogenesis. In the BAT-like periaortic adipose tissue of aged human individuals, reduced BAT activity was observed [62]. Importantly, both unilocular white adipocytes and multilocular brown adipocytes were found in the periaortic adipose tissue isolated from aged mice [63], where whitening of adipose tissue correlates to increased production of ROS and pro-inflammatory cytokines [64]. Consistently, elevated lipid deposition and upregulated expression of CD11c, a macrophage marker, were found in the PVAT surrounding thoracic aortas of aged rats [63]. Therefore, therapeutic strategies that can re-establish browning and promote BAT activity in PVAT might alleviate ageing-associated vascular dysfunction and lower the risk of cardiovascular diseases. Further extensive studies are still needed to develop feasible drug delivery systems for PVAT-targeted therapy.
5. Obesity and PVAT
Similar to other adipose tissues, high energy intake (e.g. high fat diet feeding) can also increase PVAT mass and cause whitening of PVAT [65]. During obesity, PVAT remarkably loses its anti-contractile property [66] (Fig. 3), contributing to the pathogenesis of hypertensive cardiovascular diseases. The critical role of dysfunctional PVAT in accelerating vascular dysfunction has been clearly elucidated in preclinical studies, where removal of PVAT from the vasculature causes no difference in vasodilatation of vascular rings from lean and obese mice [67]. Besides, co-incubation of aortic PVAT from high fat diet (HFD)-fed rats and mesenteric arteries significantly impairs vascular relaxation [65].
Importantly, obesity-induced PVAT dysfunction impairs vascular function through multiple mechanisms by causing ROS accumulation and inflammation. Whitening of PVAT during obesity results in eNOS uncoupling and diminished NO bioavailability because of l-arginine deficiency [68], where these outcomes are directly related to the buildup of oxidative stress. Additionally, obesity-associated PVAT dysfunction alters energy signaling and metabolism of adjacent vascular cells. Co-incubation of PVAT from HFD-fed rats and VSMCs significantly inhibited AMPK activation and promoted mTOR phosphorylation [65], where the AMPK and mTOR signaling pathways are often closely related to ROS modulation [69]. The renin-angiotensin-aldosterone system (RAAS) is tightly related to the regulation of vascular homeostasis and hence blood pressure [70]. Different RAAS components can be produced by adipose tissues, including PVAT, and these components contribute to the development of arterial stiffness and hypertension [71]. In obese patients, adipocyte hypertrophy significantly elevated the generation of angiotensin II (Ang II), angiotensinogen and aldosterone in PVAT [72]. Through Ang II type 1 receptor (AT1R), Ang II increases oxidative stress and lowers NO bioavailability by suppressing eNOS activity in vascular endothelial cells [73]. Besides, Ang II also promotes the generation of vasoconstrictors, including endothelin-1 and cyclooxygenase 1 (COX-1)-derived prostanoids to induce oxidative stress and impair vascular function [74]. Deficiency of bone morphogenetic protein 4 (BMP4) in PVAT was shown to increase the levels of Ang II and angiotensinogen, and vascular oxidative stress [75].
Obesity-induced PVAT dysfunction causes inflammation in surrounding microenvironment by promoting the infiltration of immune cells, like monocytes, macrophages and dendritic cells. During obesity, the abundance and activation status of resident immune cells in PVAT greatly vary. For instance, hyperactivation of regulatory T cells and M2 macrophages were observed in thoracic and mesenteric PVATs of HFD-fed Dahl salt-sensitive hypertensive rats [76]. These immune cells and the inflamed PVAT account for high levels of pro-inflammatory cytokines and adipokines, like MCP-1, TNFα, IL-6, leptin, visfatin and resistin, in the microenvironment. By contrast, anti-inflammatory adipocytokines, particularly adiponectin, are reduced in dysfunctional PVAT [77]. Consequently, inflammation in obese PVAT exacerbates generation of ROS, particularly O2•– and H2O2 [78]. Pharmacologically, co-incubation of obese PVAT-containing aortic segments with free radical scavengers and TNFα inhibitor could remarkably restore vascular function [79]. Coherently, deficiency of TNFα receptor in PVAT from obese mice also reduced ROS production and vasocontraction [79]. Meanwhile, deficiency of IL-18 in PVAT was associated with impaired anticontractile activity, decreased manganese superoxide dismutase (MnSOD) expression, mitochondrial deformation and enhanced whitening in PVAT [80]. These studies highlight the connection between oxidative stress and inflammation in PVAT and adjacent vasculature particularly during the development of vascular dysfunction. Therapeutic approaches that promote browning in PVAT might attenuate oxidative stress and inflammation to lower cardiovascular risks.
Furthermore, epigenetic changes in PVAT adipocytes might also underlie obesity-related vascular abnormalities. In a previous study, epigenetic repression of peroxisome proliferator activated receptor gamma coactivator 1α (PGC-1α) in PVAT was shown to impair the expression of both UCP1 and peroxisome proliferator activated receptor gamma (PPARγ) in epicardial adipose tissues from HFD-fed rats [81]. Another recent study suggests that activating transcription factor 3 (ATF3) might play a role in epigenetic control in PVAT, eliciting an anti-inflammatory effect against obesity-related vascular impairment, although the detailed mechanism remains largely unknown [82]. Further extensive studies are required to extend the role and mechanism of epigenetic regulation in obese PVAT in related to ROS buildup and inflammation modulation.
6. Diabetes and PVAT
Similar to obesity, PVAT dysfunction is also observed during the progression of diabetes mellitus (Fig. 3). However, since obesity is considered as a key modifiable risk factor for the onset and development of diabetes mellitus [83], it is reasonable to postulate that dysfunctional PVAT during diabetes might partially confer harmful effects on the vasculature through similar mechanisms. Indeed, diabetic patients and animal models are often associated with obesity, making it difficult to solely investigate the role of diabetic PVAT. Of note, certain animal and human studies have suggested that PVAT dysfunction during diabetes impairs vascular homeostasis by increasing oxidative stress and inflammation. Whitening of PVAT has also been observed in diabetic mouse model (e.g. diabetic db/db mice) [84], implying increased cardiovascular risks.
In 2020, Azul et al. used a nonobese rodent model of type 2 diabetes mellitus (i.e. Goto-Kakizaki diabetic rats) to study the role of diabetic PVAT in vascular dysfunction. This study showed that the presence of periaortic PVAT in nonobese diabetic rats significantly impaired vascular function, increased aortic PVAT level of nitrotyrosine, suppressed antioxidant enzymes (e.g. MnSOD and catalase) in thoracic PVAT, and upregulated the expression of inflammatory markers (e.g. MCP-1 and CD36) in diabetic PVAT [85]. These results imply the harmful effects of diabetic PVAT on the vasculature. Another human study showed a positive correlation between adiponectin level in PVAT and O2•– derived by NADPH oxidase. This study also provided experimental clues to indicate that reduced levels of adiponectin stimulated NADPH oxidase in patients with type 2 diabetes, where the diabetic PVAT could sense the elevated activity of NADPH oxidase to induce adiponectin upregulation [86]. These results highlight the participation of PVAT-derived adipocytokine in the modulation of vascular function during diabetes mellitus. In another study using db/db mice, diabetic PVAT was found to be associated with whitening (i.e. decreased UCP1 expression), macrophage polarization to the pro-inflammatory M1 phenotype, and upregulation of pro-inflammatory cytokines (e.g. IL-6, TNFα and Interferon-γ) [84], although the contribution of obesity could not be fully ruled out in db/db mice. Future studies shall compare the harmful roles of obese and diabetic PVAT.
7. Clinical implications of PVAT dysfunction
Clinically, PVAT dysfunction participates in different cardiovascular complications, such as atherosclerotic and hypertensive cardiovascular diseases [87]. The contribution of ROS overproduction and inflammation induced by PVAT dysfunction during cardiovascular pathogenesis cannot be overlooked. Mechanistically, dysfunctional PVAT is associated with augmented secretion of both pro-atherogenic and pro-angiogenic adipocytokines (e.g. MCP-1, VEGF and TNFα), which exacerbate atherogenesis by inducing vascular inflammation, unfavorable VSMC proliferation and vasa varosum neovascularization [88]. Coherently, in patients with large artery atherosclerotic stroke, PVAT density around carotid artery was found higher than those with other stroke etiologies [89]. Besides, increased epicardial adipose tissue mass was shown positively associated with the development of high-risk plaque in a meta-analysis covering 3772 patients [90].
Aortic aneurysm refers to the weakening and the consequential outward bulge of arterial wall. Due to similar risk factors (e.g. hypercholesterolemia and hypertension), patients with atherosclerosis are more vulnerable to aortic aneurysm [91]. In patients with abdominal aortic aneurysm, increased density of PVAT was found in aneurysm sites [92]. Since chronic inflammation is a hallmark of aortic aneurysm [92], it is reasonable to postulate that deposition of dysfunctional PVAT is contributory to aortic aneurysm pathophysiology. Another clinical study has proposed that histopathological evaluation of PVAT deposits might potentially be a non-invasive strategy to estimate inflammation in the vasculature [93]. Particularly, PVAT mapping by coronary computed tomography (CT) angiography was suggested to be one of the feasible means to evaluate coronary inflammation and atherosclerotic development. Briefly, coronary inflammation and atherogenesis-associated phenotypic changes in adjacent PVAT can be detected as perivascular attenuation gradients by CT angiography, where such gradients can be captured and quantified by fat attenuation index (FAI) [94]. Inflammatory signals from the vascular wall induce structural modifications in surrounding PVAT, resulting in a gradient of adipocyte sizes around inflamed arteries, where adipocyte sizes correlate to different ratios of liquid and aqueous phases. Meanwhile, FAI refers to the visualization tool which measures weighted three-dimensional perivascular attenuation gradients around the arteries. An inflamed artery is associated with distinct FAI mapping pattern, and disrupted balance of liquid and aqueous phases in surrounding PVAT, when compared to a healthy artery [95]. In a previous large cohort study involving 3912 patients, higher values of CT angiography-based perivascular fat attenuation index were found associated with increased risk of cardiac mortality [96]. These clinical studies highlight the diagnostic potential of dysfunctional PVAT in inflammation-related cardiovascular diseases.
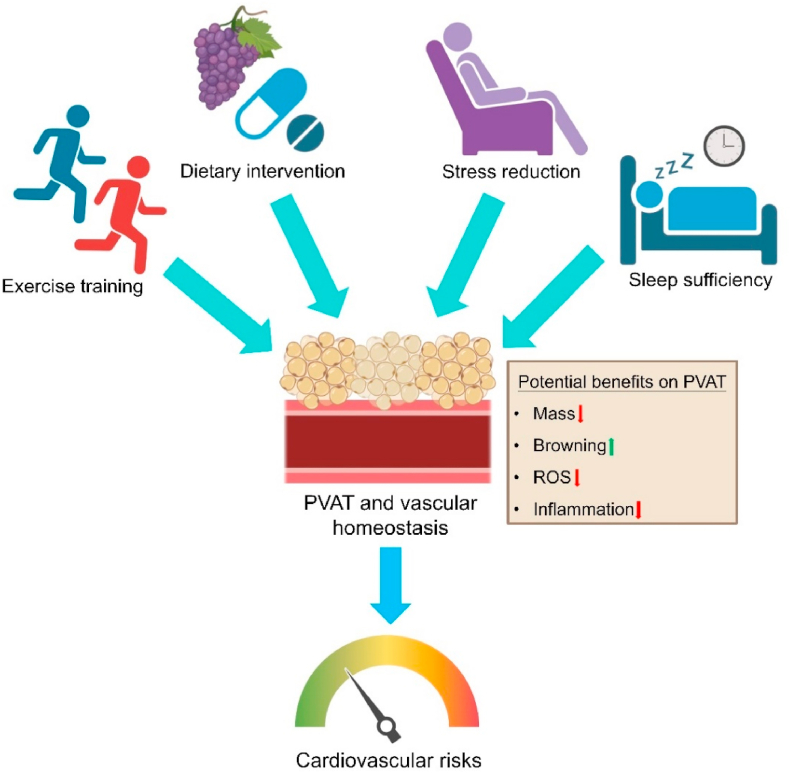
8. Physiological means to alter PVAT function
PVAT dysfunction, characterized by disturbed secretion of adipocytokines along with endothelial dysfunction, is believed to increase cardiovascular risks [97]. Due to lack of effective pharmacological and genetic interventions to specifically target PVAT, other physiological means shall be considered to ameliorate PVAT dysfunction for better cardiovascular health. In particular, exercise training, dietary interventions, stress reduction and sleep management can both directly and indirectly, through PVAT, improve cardiovascular health (Fig. 4). These healthier lifestyles can attenuate ROS buildup and inflammation in PVAT, and can promote the ‘re-browning’ of PVAT to indirectly improve cardiovascular homeostasis.
Fig. 4.
Physiological means to improve PVAT homeostasis. Exercise training, dietary intervention, stress reduction and sleep sufficiency can potentially improve homeostasis of PVAT and vascular system through different mechanisms, thus beneficial to cardiovascular health.
Physical activity reduces the mass and promote browning of adipose tissues [98]. Therefore, it is reasonable to postulate that exercise can fundamentally retard the negative effects of WAT-like PVAT on the vasculature. Indeed, many studies have elucidated the benefits of exercise on PVAT in different animal models. Chronic running exercise has been shown to facilitate re-browning of periaortic PVAT by upregulating UCP1 expression in diabetic mice [84]. In addition, chronic aerobic exercise can suppress ROS level in periaortic PVAT of obese mice, as shown by dihydroethidium staining [99]. An 8-week aerobic training also upregulated eNOS expression and NO level, while downregulated thrombospondin 1 and TNFα expressions in thoracic PVAT of obese rats [100]. Recently, chronic aerobic exercise was shown to raise PVAT adiponectin level in an induced mouse model of heart failure [101], suggesting that exercise can improve the paracrine function of PVAT. Moreover, continuous and interval running exercise could limit PVAT-secreted advanced glycation end products (AGEs) in swine [46], where accumulated AGEs in the vasculature perturb cell surface of ECs to cause oxidative and inflammatory insults [15]. Exercise training was also associated with improved proteosome function [100], where proteosome is tightly involved in the regulation of inflammatory response [102]. These animal studies highlight the antioxidant and anti-inflammatory effects of exercise on PVAT.
Dietary interventions, that utilize nutraceuticals or active ingredients extracted from foods, might improve PVAT function and alter PVAT phenotype. Hesperidin is a flavonoid commonly found in vegetables and fruits. Notably, hesperidin has been shown to partially ameliorate aortic stiffness by reducing PVAT-derived AGEs [103]. Resveratrol is one of the natural phytoalexins eliciting numerous beneficial effects on cardiovascular system [104]. A previous study suggested that resveratrol can improve PVAT function by retarding inflammation, potentially via a AMPK/sirtuin 1 (SIRT1)-dependent manner [105]. Despite the presence of eNOS in PVAT, whether resveratrol can enhance eNOS activity and prevent eNOS uncoupling in PVAT is still debatable [106].
Mental stress has been indicated to exacerbate obesity through promoting adipocyte hypertrophy, whitening and inflammation of adipose tissues [107]. A previous study showed that chronic mental stress impaired PVAT function and induced the production of inflammatory cytokines from PVAT in a rat model of induced depression [108], implying a possible linkage of mental stress and inflammatory state in PVAT and the vasculature. In the same study, aerobic exercise training partially rescued PVAT function and attenuated aortic stiffness [108], hinting that stress relief shall be beneficial on PVAT function and hence cardiovascular health.
Sleep deprivation induces obesity though multiple mechanisms [109]. Sleep deprivation causes psychological stress [110], where such stress further exacerbates obesity. Although there is no direct clue to show that sleep deprivation increases PVAT mass, certain studies implied that sleep-related disorders and signaling pathways are involved in the regulation of PVAT function. In pregnant murine, pre-existing obstructive sleep apnoea was shown to impair PVAT function and reduce adiponectin secretion from PVAT in male offspring [111]. In another study, disturbance of circadian clock in PVAT due to deficiency of brain and muscle Arnt-like protein-1 (BMAL1) dysregulated vasoactivity and blood pressure [112]. This study implied that sleep disturbance due to shift work or jet lag shall pose threat on cardiovascular health. Hence, it is rational to postulate that proper sleep management and prevention of sleep disorders shall confer beneficial effects on PVAT function.
9. Conclusion
In addition to a structural unit surrounding the vascular tree, PVAT serves as an endocrine/paracrine component in regulating vascular homeostasis. PVAT plays a modulatory role in the redox homeostasis and inflammation of vasculature, where the vicious cycle between oxidative stress and inflammation further aggravates the progression of cardiovascular diseases. Notably, PVAT interacts with cellular components of vasculature, including VSMCs, ECs and immune cells, during the regulation of vascular homeostasis. PVAT at different regions of vasculature is associated with different phenotypes and genotypes. During different physical conditions, such as ageing and obesity, the cellular proportions and phenotype-genotype of PVAT vary to increase the risks of cardiovascular diseases. Clinically, PVAT dysfunction is observed in multiple cardiovascular diseases. Physiological activities, like physical activity, and certain medications could alter the mass and phenotype-genotype of PVAT. As a master endocrine organ, PVAT-related study shall open up new opportunities for identifying therapeutic strategies against cardiovascular complications.
Author contributions
Conceptualization, C.K.C. and Y.H.; writing-original draft preparation, C.K.C., H.D. and M.J.; writing-review and editing, C.K.C., H.Y., M.G. and Y.H.; supervision, Y.H.; funding acquisition, Y.H. All authors have read and agreed to the published version of manuscript.
Funding
This work was supported by Research Grants Council of Hong Kong (PDFS 2022/23, SRFS2021-4S04, 14109720, 14100121, 11103222, T24-508/22-N), and CityU Start-up Fund. Our work was also funded by the Deutsche Forschungsgemeinschaft (DFG) – GO766/22-3, 15-2, 26-1.
Declaration of competing interest
The authors declare no competing interests.
Acknowledgements
We thank the members of the Y.H. group for the helpful discussions and critical comments. Figures were created with BioRender.com.
Contributor Information
Chak Kwong Cheng, Email: andy.ckcheng@cityu.edu.hk.
Yu Huang, Email: yu.huang@cityu.edu.hk.
Data availability
No data was used for the research described in the article.
References
- 1.Gollasch M. Adipose-vascular coupling and potential therapeutics. Annu. Rev. Pharmacol. Toxicol. 2017;57:417–436. doi: 10.1146/ANNUREV-PHARMTOX-010716-104542. [DOI] [PubMed] [Google Scholar]
- 2.Scheja L., Heeren J. The endocrine function of adipose tissues in health and cardiometabolic disease. Nat. Rev. Endocrinol. 2019;15:507–524. doi: 10.1038/s41574-019-0230-6. [DOI] [PubMed] [Google Scholar]
- 3.Fleenor B.S., Carlini N.A., Ouyang A., Harber M.P. Perivascular adipose tissue-mediated arterial stiffening in aging and disease: an emerging translational therapeutic target? Pharmacol. Res. 2022;178 doi: 10.1016/J.PHRS.2022.106150. [DOI] [PubMed] [Google Scholar]
- 4.Liu Y., Sun Y., Hu C., Liu J., Gao A., Han H., Chai M., Zhang J., Zhou Y., Zhao Y. Perivascular adipose tissue as an indication, contributor to, and therapeutic target for atherosclerosis. Front. Physiol. 2020;11 doi: 10.3389/FPHYS.2020.615503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li X., Ma Z., Zhu Y.Z. Regional heterogeneity of perivascular adipose tissue: morphology, origin, and secretome. Front. Pharmacol. 2021;12 doi: 10.3389/FPHAR.2021.697720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adachi Y., Ueda K., Nomura S., Ito K., Katoh M., Katagiri M., Yamada S., Hashimoto M., Zhai B., Numata G., Otani A., Hinata M., Hiraike Y., Waki H., Takeda N., Morita H., Ushiku T., Yamauchi T., Takimoto E., Komuro I. Beiging of perivascular adipose tissue regulates its inflammation and vascular remodeling. Nat. Commun. 2022;13:5117. doi: 10.1038/s41467-022-32658-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Man A.W.C., Zhou Y., Xia N., Li H. Endothelial nitric oxide synthase in the perivascular adipose tissue. Biomedicines. 2022;10:1754. doi: 10.3390/BIOMEDICINES10071754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng C.K., Bakar H.A., Gollasch M., Huang Y. Perivascular adipose tissue: the sixth man of the cardiovascular system. Cardiovasc. Drugs Ther. 2018;32:481–502. doi: 10.1007/S10557-018-6820-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hildebrand S., Stümer J., Pfeifer A. PVAT and its relation to Brown, beige, and white adipose tissue in development and function. Front. Physiol. 2018;9:70. doi: 10.3389/FPHYS.2018.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.dosReis Costa D.E.F., Silveira A.L.M., Campos G.P., Nóbrega N.R.C., deAraújo N.F., deFigueiredo Borges L., dosSantos Aggum Capettini L., Ferreira A.V.M., Bonaventura D. High-carbohydrate diet enhanced the anticontractile effect of perivascular adipose tissue through activation of renin-angiotensin system. Front. Physiol. 2021;11 doi: 10.3389/FPHYS.2020.628101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gálvez-Prieto B., Somoza B., Gil-Ortega M., García-Prieto C.F., delas Heras A.I., González M.C., Arribas S., Aranguez I., Bolbrinker J., Kreutz R., Ruiz-Gayo M., Fernández-Alfonso M.S. Anticontractile effect of perivascular adipose tissue and leptin are reduced in hypertension. Front. Pharmacol. 2012;3:103. doi: 10.3389/FPHAR.2012.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Victorio J.A., Davel A.P. Perivascular adipose tissue oxidative stress on the pathophysiology of cardiometabolic diseases. Curr. Hypertens. Rev. 2020;16:192–200. doi: 10.2174/1573402115666190410153634. [DOI] [PubMed] [Google Scholar]
- 13.Luo J.D., Zhang G.S., Chen M.S. Leptin and cardiovascular disease: response to therapeutic interventions. Circulation. 2008;117:3238–3249. doi: 10.1161/CIRCULATIONAHA.107.741645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim H.W., Shi H., Winkler M.A., Lee R., Weintraub N.L. Perivascular adipose tissue and vascular perturbation/atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2020;40:2569–2576. doi: 10.1161/ATVBAHA.120.312470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng C.K., Shang W., Liu J., Cheang W.S., Wang Y., Xiang L., Lau C.W., Luo J.Y., Ng C.F., Huang Y., Wang L. Activation of AMPK/miR-181b Axis alleviates endothelial dysfunction and vascular inflammation in diabetic mice. Antioxidants. 2022;11:1137. doi: 10.3390/ANTIOX11061137/S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steven S., Frenis K., Oelze M., Kalinovic S., Kuntic M., Jimenez M.T.B., Vujacic-Mirski K., Helmstädter J., Kröller-Schön S., Münzel T., Daiber A. Vascular inflammation and oxidative stress: major triggers for cardiovascular disease. Oxid. Med. Cell. Longev. 2019;2019 doi: 10.1155/2019/7092151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao Y.J., Lu C., Su L.Y., Sharma A.M., Lee R.M.K.W. Modulation of vascular function by perivascular adipose tissue: the role of endothelium and hydrogen peroxide. Br. J. Pharmacol. 2007;151:323–331. doi: 10.1038/SJ.BJP.0707228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ayala-Lopez N., Thompson J.M., Watts S.W. Perivascular adipose tissue’s impact on norepinephrine-induced contraction of mesenteric resistance arteries. Front. Physiol. 2017;8:37. doi: 10.3389/FPHYS.2017.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Touyz R.M., Anagnostopoulou A., Camargo L.L., Rios F.J., Montezano A.C. Vascular biology of superoxide-generating NADPH oxidase 5-implications in hypertension and cardiovascular disease. Antioxidants Redox Signal. 2019;30:1027–1040. doi: 10.1089/ARS.2018.7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quesada I., Cejas J., García R., Cannizzo B., Redondo A., Castro C. Vascular dysfunction elicited by a cross talk between periaortic adipose tissue and the vascular wall is reversed by pioglitazone. Cardiovasc. Ther. 2018;36 doi: 10.1111/1755-5922.12322. [DOI] [PubMed] [Google Scholar]
- 21.Nisimoto Y., Diebold B.A., Constentino-Gomes D., Lambeth J.D. Nox4: a hydrogen peroxide-generating oxygen sensor. Biochemistry. 2014;53:5111–5120. doi: 10.1021/BI500331Y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thomas C., Mackey M.M., Diaz A.A., Cox D.P. Hydroxyl radical is produced via the Fenton reaction in submitochondrial particles under oxidative stress: implications for diseases associated with iron accumulation. Redox Rep. 2009;14:102–108. doi: 10.1179/135100009X392566. [DOI] [PubMed] [Google Scholar]
- 23.Costa R.M., Filgueira F.P., Tostes R.C., Carvalho M.H.C., Akamine E.H., Lobato N.S. H2O2 generated from mitochondrial electron transport chain in thoracic perivascular adipose tissue is crucial for modulation of vascular smooth muscle contraction. Vasc. Pharmacol. 2016;84:28–37. doi: 10.1016/J.VPH.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 24.Gu P., Hui X., Zheng Q., Gao Y., Jin L., Jiang W., Zhou C., Liu T., Huang Y., Liu Q., Nie T., Wang Y., Wang Y., Zhao J., Xu A. Mitochondrial uncoupling protein 1 antagonizes atherosclerosis by blocking NLRP3 inflammasome-dependent interleukin-1β production. Sci. Adv. 2021;7 doi: 10.1126/SCIADV.ABL4024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vanhoutte P.M., Zhao Y., Xu A., Leung S.W.S. Thirty years of saying NO: sources, fate, actions, and misfortunes of the endothelium-derived vasodilator mediator. Circ. Res. 2016;119:375–396. doi: 10.1161/CIRCRESAHA.116.306531. [DOI] [PubMed] [Google Scholar]
- 26.Yang Y.M., Huang A., Kaley G., Sun D. eNOS uncoupling and endothelial dysfunction in aged vessels. Am. J. Physiol. Heart Circ. Physiol. 2009;297:H1829–H1836. doi: 10.1152/AJPHEART.00230.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qi X.Y., Qu S.L., Xiong W.H., Rom O., Chang L., Jiang Z.S. Perivascular adipose tissue (PVAT) in atherosclerosis: a double-edged sword, Cardiovasc. Diabetologe. 2018;17:134. doi: 10.1186/S12933-018-0777-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsvetkov D., Tano J.Y., Kassmann M., Wang N., Schubert R., Gollasch M. The role of DPO-1 and xe991-sensitive potassium channels in perivascular adipose tissue-mediated regulation of vascular tone. Front. Physiol. 2016;7:335. doi: 10.3389/FPHYS.2016.00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zavaritskaya O., Zhuravleva N., Schleifenbaum J., Gloe T., Devermann L., Kluge R., Mladenov M., Frey M., Gagov H., Fésüs G., Gollasch M., Schubert R. Role of KCNQ channels in skeletal muscle arteries and periadventitial vascular dysfunction. Hypertension. 2013;61:151–159. doi: 10.1161/HYPERTENSIONAHA.112.197566. [DOI] [PubMed] [Google Scholar]
- 30.Fernández-Alfonso M.S., Gil-Ortega M., García-Prieto C.F., Aranguez I., Ruiz-Gayo M., Somoza B. Mechanisms of perivascular adipose tissue dysfunction in obesity. Internet J. Endocrinol. 2013;2013 doi: 10.1155/2013/402053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arora D., Jain P., Singh N., Kaur H., Bhatla S.C. Mechanisms of nitric oxide crosstalk with reactive oxygen species scavenging enzymes during abiotic stress tolerance in plants. Free Radic. Res. 2016;50:291–303. doi: 10.3109/10715762.2015.1118473. [DOI] [PubMed] [Google Scholar]
- 32.Chen Y., Qin Z., Wang Y., Li X., Zheng Y., Liu Y. Role of inflammation in vascular disease-related perivascular adipose tissue dysfunction. Front. Endocrinol. 2021;12 doi: 10.3389/FENDO.2021.710842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Drosos I., Chalikias G., Pavlaki M., Kareli D., Epitropou G., Bougioukas G., Mikroulis D., Konstantinou F., Giatromanolaki A., Ritis K., Münzel T., Tziakas D., Konstantinides S., Schäfer K. Differences between perivascular adipose tissue surrounding the heart and the internal mammary artery: possible role for the leptin-inflammation-fibrosis-hypoxia axis. Clin. Res. Cardiol. 2016;105:887–900. doi: 10.1007/S00392-016-0996-7. [DOI] [PubMed] [Google Scholar]
- 34.Fésüs G., Dubrovska G., Gorzelniak K., Kluge R., Huang Y., Luft F.C., Gollasch M. Adiponectin is a novel humoral vasodilator. Cardiovasc. Res. 2007;75:719–727. doi: 10.1016/J.CARDIORES.2007.05.025. [DOI] [PubMed] [Google Scholar]
- 35.Xia N., Li H. The role of perivascular adipose tissue in obesity‐induced vascular dysfunction. Br. J. Pharmacol. 2017;174:3425–3442. doi: 10.1111/BPH.13650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ouchi N., Walsh K. A novel role for adiponectin in the regulation of inflammation, Arterioscler. Thromb. Vasc. Biol. 2008;28:1219–1221. doi: 10.1161/ATVBAHA.108.165068. [DOI] [PubMed] [Google Scholar]
- 37.Cho Y., Lee S.E., Lee H.C., Hur J., Lee S., Youn S.W., Lee J., Lee H.J., Lee T.K., Park J., Hwang S.J., Kwon Y.W., Cho H.J., Oh B.H., Park Y.B., Kim H.S. Adipokine resistin is a key player to modulate monocytes, endothelial cells, and smooth muscle cells, leading to progression of atherosclerosis in rabbit carotid artery. J. Am. Coll. Cardiol. 2011;57:99–109. doi: 10.1016/J.JACC.2010.07.035. [DOI] [PubMed] [Google Scholar]
- 38.Rami A.Z.A., Hamid A.A., Anuar N.N.M., Aminuddin A., Ugusman A. Exploring the relationship of perivascular adipose tissue inflammation and the development of vascular pathologies. Mediat. Inflamm. 2022;2022 doi: 10.1155/2022/2734321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kazama K., Usui T., Okada M., Hara Y., Yamawaki H. Omentin plays an anti-inflammatory role through inhibition of TNF-α-induced superoxide production in vascular smooth muscle cells. Eur. J. Pharmacol. 2012;686:116–123. doi: 10.1016/J.EJPHAR.2012.04.033. [DOI] [PubMed] [Google Scholar]
- 40.Watts S.W., Gollasch M. Editorial: perivascular adipose tissue (PVAT) in health and disease. Front. Physiol. 2018;9:1004. doi: 10.3389/FPHYS.2018.01004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brown N.K., Zhou Z., Zhang J., Zeng R., Wu J., Eitzman D.T., Chen Y.E., Chang L. Perivascular adipose tissue in vascular function and disease: a review of current research and animal models. Arterioscler. Thromb. Vasc. Biol. 2014;34:1621–1630. doi: 10.1161/ATVBAHA.114.303029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gil-Ortega M., Somoza B., Huang Y., Gollasch M., Fernández-Alfonso M.S. Regional differences in perivascular adipose tissue impacting vascular homeostasis. Trends Endocrinol. Metabol. 2015;26:367–375. doi: 10.1016/J.TEM.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 43.Fitzgibbons T.P., Kogan S., Aouadi M., Hendricks G.M., Straubhaar J., Czech M.P. Similarity of mouse perivascular and brown adipose tissues and their resistance to diet-induced inflammation. Am. J. Physiol. Heart Circ. Physiol. 2011;301:H1425–H1437. doi: 10.1152/AJPHEART.00376.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fitzgibbons T.P., Czech M.P. Epicardial and perivascular adipose tissues and their influence on cardiovascular disease: basic mechanisms and clinical associations. J. Am. Heart Assoc. 2014;3 doi: 10.1161/JAHA.113.000582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Horimatsu T., Kim H.W., Weintraub N.L. The role of perivascular adipose tissue in non-atherosclerotic vascular disease. Front. Physiol. 2017;8:969. doi: 10.3389/FPHYS.2017.00969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ouyang A., Dylan Olver T., Emter C.A., Fleenor B.S. Chronic exercise training prevents coronary artery stiffening in aortic-banded miniswine: role of perivascular adipose-derived advanced glycation end products. J. Appl. Physiol. 2019;127:816–827. doi: 10.1152/JAPPLPHYSIOL.00146.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Payne G.A., Borbouse L., Kumar S., Neeb Z., Alloosh M., Sturek M., Tune J.D. Epicardial perivascular adipose-derived leptin exacerbates coronary endothelial dysfunction in metabolic syndrome via a protein kinase C-beta pathway. Arterioscler. Thromb. Vasc. Biol. 2010;30:1711–1717. doi: 10.1161/ATVBAHA.110.210070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Owen M.K., Noblet J.N., Sassoon D.J., Conteh A.M., Goodwill A.G., Tune J.D. Perivascular adipose tissue and coronary vascular disease. Arterioscler. Thromb. Vasc. Biol. 2014;34:1643–1649. doi: 10.1161/ATVBAHA.114.303033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Demine S., Renard P., Arnould T. Mitochondrial uncoupling: a key controller of biological processes in physiology and diseases. Cells. 2019;8:795. doi: 10.3390/CELLS8080795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Corrêa L.H., Heyn G.S., Magalhaes K.G. The impact of the adipose organ plasticity on inflammation and cancer progression. Cells. 2019;8:662. doi: 10.3390/CELLS8070662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bond L.M., Burhans M.S., Ntambi J.M. Uncoupling protein-1 deficiency promotes brown adipose tissue inflammation and ER stress. PLoS One. 2018;13 doi: 10.1371/JOURNAL.PONE.0205726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dandekar A., Mendez R., Zhang K. Cross talk between ER stress, oxidative stress, and inflammation in health and disease. Methods Mol. Biol. 2015;1292:205–214. doi: 10.1007/978-1-4939-2522-3_15. [DOI] [PubMed] [Google Scholar]
- 53.Ungvari Z., Tarantini S., Donato A.J., Galvan V., Csiszar A. Mechanisms of vascular aging. Circ. Res. 2018;123:849–867. doi: 10.1161/CIRCRESAHA.118.311378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Queiroz M., Sena C.M. Perivascular adipose tissue in age-related vascular disease. Ageing Res. Rev. 2020;59 doi: 10.1016/J.ARR.2020.101040. [DOI] [PubMed] [Google Scholar]
- 55.Agabiti-Rosei C., Favero G., DeCiuceis C., Rossini C., Porteri E., Rodella L.F., Franceschetti L., Maria Sarkar A., Agabiti-Rosei E., Rizzoni D., Rezzani R. Effect of long-term treatment with melatonin on vascular markers of oxidative stress/inflammation and on the anticontractile activity of perivascular fat in aging mice. Hypertens. Res. 2017;40:41–50. doi: 10.1038/HR.2016.103. [DOI] [PubMed] [Google Scholar]
- 56.Wang Y., Yildiz F., Struve A., Kassmann M., Markó L., Köhler M.B., Luft F.C., Gollasch M., Tsvetkov D. Aging affects KV7 channels and perivascular adipose tissue-mediated vascular tone. Front. Physiol. 2021;12 doi: 10.3389/FPHYS.2021.749709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tsvetkov D., Kaßmann M., Tano J.Y., Chen L., Schleifenbaum J., Voelkl J., Lang F., Huang Y., Gollasch M. Do KV 7.1 channels contribute to control of arterial vascular tone? Br. J. Pharmacol. 2017;174:150–162. doi: 10.1111/BPH.13665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fleenor B.S., Eng J.S., Sindler A.L., Pham B.T., Kloor J.D., Seals D.R. Superoxide signaling in perivascular adipose tissue promotes age-related artery stiffness. Aging Cell. 2014;13:576–578. doi: 10.1111/ACEL.12196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bailey-Downs L.C., Tucsek Z., Toth P., Sosnowska D., Gautam T., Sonntag W.E., Csiszar A., Ungvari Z. Aging exacerbates obesity-induced oxidative stress and inflammation in perivascular adipose tissue in mice: a paracrine mechanism contributing to vascular redox dysregulation and inflammation. J. Gerontol. A. Biol. Sci. Med. Sci. 2013;68:780–792. doi: 10.1093/GERONA/GLS238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yoneshiro T., Aita S., Matsushita M., Okamatsu-Ogura Y., Kameya T., Kawai Y., Miyagawa M., Tsujisaki M., Saito M. Age-related decrease in cold-activated brown adipose tissue and accumulation of body fat in healthy humans. Obesity. 2011;19:1755–1760. doi: 10.1038/OBY.2011.125. [DOI] [PubMed] [Google Scholar]
- 61.Pan X.X., Yao K.L., Yang Y.F., Ge Q., Zhang R., Gao P.J., Ruan C.C., Wu F. Senescent T cell induces Brown adipose tissue “whitening” via secreting IFN-γ. Front. Cell Dev. Biol. 2021;9 doi: 10.3389/FCELL.2021.637424/FULL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Police S.B., Thatcher S.E., Charnigo R., Daugherty A., Cassis L.A. Obesity promotes inflammation in periaortic adipose tissue and angiotensin II-induced abdominal aortic aneurysm formation. Arterioscler. Thromb. Vasc. Biol. 2009;29:1458–1464. doi: 10.1161/ATVBAHA.109.192658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Padilla J., Jenkins N.T., Vieira-Potter V.J., Harold Laughlin M. Divergent phenotype of rat thoracic and abdominal perivascular adipose tissues. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013;304:R543–R552. doi: 10.1152/AJPREGU.00567.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kotzbeck P., Giordano A., Mondini E., Murano I., Severi I., Venema W., Cecchini M.P., Kershaw E.E., Barbatelli G., Haemmerle G., Zechner R., Cinti S. Brown adipose tissue whitening leads to brown adipocyte death and adipose tissue inflammation. J. Lipid Res. 2018;59:784–794. doi: 10.1194/JLR.M079665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ma L., Ma S., He H., Yang D., Chen X., Luo Z., Liu D., Zhu Z. Perivascular fat-mediated vascular dysfunction and remodeling through the AMPK/mTOR pathway in high-fat diet-induced obese rats. Hypertens. Res. 2010;33:446–453. doi: 10.1038/HR.2010.11. [DOI] [PubMed] [Google Scholar]
- 66.Ramirez J.G., O’Malley E.J., Ho W.S.V. Pro‐contractile effects of perivascular fat in health and disease. Br. J. Pharmacol. 2017;174:3482–3495. doi: 10.1111/BPH.13767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gil-Ortega M., Stucchi P., Guzmán-Ruiz R., Cano V., Arribas S., González M.C., Ruiz-Gayo M., Fernández-Alfonso M.S., Somoza B. Adaptative nitric oxide overproduction in perivascular adipose tissue during early diet-induced obesity. Endocrinology. 2010;151:3299–3306. doi: 10.1210/EN.2009-1464. [DOI] [PubMed] [Google Scholar]
- 68.Zaborska K.E., Wareing M., Austin C. Comparisons between perivascular adipose tissue and the endothelium in their modulation of vascular tone. Br. J. Pharmacol. 2017;174:3388–3397. doi: 10.1111/BPH.13648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pasha M., Eid A.H., Eid A.A., Gorin Y., Munusamy S. Sestrin2 as a novel biomarker and therapeutic target for various diseases. Oxid. Med. Cell. Longev. 2017;2017 doi: 10.1155/2017/3296294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Poznyak A.V., Bharadwaj D., Prasad G., Grechko A.V., Sazonova M.A., Orekhov A.N. Renin-angiotensin system in pathogenesis of atherosclerosis and treatment of CVD. Int. J. Mol. Sci. 2021;22:6702. doi: 10.3390/IJMS22136702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stanek A., Brożyna-Tkaczyk K., Myśliński W. The role of obesity-induced perivascular adipose tissue (PVAT) dysfunction in vascular homeostasis. Nutrients. 2021;13:3843. doi: 10.3390/NU13113843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Briones A.M., Cat A.N.D., Callera G.E., Yogi A., Burger D., He Y., Corrêa J.W., Gagnon A.M., Gomez-Sanchez C.E., Gomez-Sanchez E.P., Sorisky A., Ooi T.C., Ruzicka M., Burns K.D., Touyz R.M. Adipocytes produce aldosterone through calcineurin-dependent signaling pathways: implications in diabetes mellitus-associated obesity and vascular dysfunction. Hypertension. 2012;59:1069–1078. doi: 10.1161/HYPERTENSIONAHA.111.190223. [DOI] [PubMed] [Google Scholar]
- 73.Ding J., Yu M., Jiang J., Luo Y., Zhang Q., Wang S., Yang F., Wang A., Wang L., Zhuang M., Wu S., Zhang Q., Xia Y., Lu D. Angiotensin II decreases endothelial nitric oxide synthase phosphorylation via AT1R nox/ROS/PP2A pathway. Front. Physiol. 2020;11 doi: 10.3389/FPHYS.2020.566410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wilcox C.S., Wang C., Wang D. Endothelin-1-Induced microvascular ROS and contractility in angiotensin-II-infused mice depend on COX and TP receptors. Antioxidants. 2019;8:193. doi: 10.3390/ANTIOX8060193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mu W.-J., Song Y.-J., Yang L.-J., Qian S.-W., Yang Q.-Q., Liu Y., Tang Q.-Q., Tang Y. Bone morphogenetic protein 4 in perivascular adipose tissue ameliorates hypertension through regulation of angiotensinogen. Front. Cardiovasc. Med. 2022;9 doi: 10.3389/FCVM.2022.1038176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kumar R.K., Yang Y., Contreras A.G., Garver H., Bhattacharya S., Fink G.D., Rockwell C.E., Watts S.W. Phenotypic changes in T cell and macrophage subtypes in perivascular adipose tissues precede high-fat diet-induced hypertension. Front. Physiol. 2021;12 doi: 10.3389/FPHYS.2021.616055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chang L., Garcia-Barrio M.T., Chen Y.E. Perivascular adipose tissue regulates vascular function by targeting vascular smooth muscle cells, arterioscler. Thromb. Vasc. Biol. 2020;40:1094–1109. doi: 10.1161/ATVBAHA.120.312464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Barp C.G., Bonaventura D., Assreuy J., No, Ros R.A.S., Pvat More than a soup of letters. Front. Physiol. 2021;12 doi: 10.3389/FPHYS.2021.640021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.daCosta R.M., Fais R.S., Dechandt C.R.P., Louzada-Junior P., Alberici L.C., Lobato N.S., Tostes R.C. Increased mitochondrial ROS generation mediates the loss of the anti-contractile effects of perivascular adipose tissue in high-fat diet obese mice. Br. J. Pharmacol. 2017;174:3527–3541. doi: 10.1111/BPH.13687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li W., Jin D., Takai S., Hayakawa T., Ogata J., Yamanishi K., Yamanishi H., Okamura H. Impaired function of aorta and perivascular adipose tissue in IL-18-deficient mice. Am. J. Physiol. Heart Circ. Physiol. 2019;317:H1142–H1156. doi: 10.1152/AJPHEART.00813.2018. [DOI] [PubMed] [Google Scholar]
- 81.Shore A., Karamitri A., Kemp P., Speakman J.R., Lomax M.A. Role of Ucp1 enhancer methylation and chromatin remodelling in the control of Ucp1 expression in murine adipose tissue. Diabetologia. 2010;53:1164–1173. doi: 10.1007/S00125-010-1701-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li H.F., Liu H.T., Chen P.Y., Lin H., Tseng T.L. Role of PVAT in obesity-related cardiovascular disease through the buffering activity of ATF3. iScience. 2022;25 doi: 10.1016/J.ISCI.2022.105631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Grant B., Sandelson M., Agyemang-Prempeh B., Zalin A. Managing obesity in people with type 2 diabetes. Clin. Med. 2021;21 doi: 10.7861/CLINMED.2021-0370. e327–e231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang J., Polaki V., Chen S., Bihl J.C. Exercise improves endothelial function associated with alleviated inflammation and oxidative stress of perivascular adipose tissue in type 2 diabetic mice. Oxid. Med. Cell. Longev. 2020;2020 doi: 10.1155/2020/8830537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Azul L., Leandro A., Boroumand P., Klip A., Seiça R., Sena C.M. Increased inflammation, oxidative stress and a reduction in antioxidant defense enzymes in perivascular adipose tissue contribute to vascular dysfunction in type 2 diabetes. Free Radic. Biol. Med. 2020;146:264–274. doi: 10.1016/J.FREERADBIOMED.2019.11.002. [DOI] [PubMed] [Google Scholar]
- 86.Antonopoulos A.S., Margaritis M., Coutinho P., Shirodaria C., Psarros C., Herdman L., Sanna F., DeSilva R., Petrou M., Sayeed R., Krasopoulos G., Lee R., Digby J., Reilly S., Bakogiannis C., Tousoulis D., Kessler B., Casadei B., Channon K.M., Antoniades C. Adiponectin as a link between type 2 diabetes and vascular NADPH oxidase activity in the human arterial wall: the regulatory role of perivascular adipose tissue. Diabetes. 2015;64:2207–2219. doi: 10.2337/DB14-1011. [DOI] [PubMed] [Google Scholar]
- 87.Hu H., Garcia-Barrio M., Jiang Z.S., Chen Y.E., Chang L. Roles of perivascular adipose tissue in hypertension and atherosclerosis, antioxid. Redox Signal. 2021;34:736–749. doi: 10.1089/ARS.2020.8103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ahmadieh S., Kim H.W., Weintraub N.L. Potential role of perivascular adipose tissue in modulating atherosclerosis. Clin. Sci. (Lond). 2020;134:3–13. doi: 10.1042/CS20190577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jin J., Huang R., Chen Q., Ke B., Tao T., Zhao R., He X. Carotid artery perivascular adipose tissue density relates to recanalization and clinical outcome after mechanical thrombectomy. Front. Aging Neurosci. 2021;13 doi: 10.3389/FNAGI.2021.761248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nerlekar N., Brown A.J., Muthalaly R.G., Talman A., Hettige T., Cameron J.D., Wong D.T.L. Association of epicardial adipose tissue and high‐risk plaque characteristics: a systematic review and meta‐analysis. J. Am. Hear. Assoc. Cardiovasc. Cerebrovasc. Dis. 2017;6 doi: 10.1161/JAHA.117.006379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cheng C.K., Lin X., Pu Y., Tse J.K.Y., Wang Y., Zhang C.L., Cao X., Lau C.W., Huang J., He L., Luo J.Y., Shih Y.T., Wan S., Ng C.F., Wang L., Ma R.C.W., Chiu J.J., Chan T.F., Yu Tian X., Huang Y. SOX4 is a novel phenotypic regulator of endothelial cells in atherosclerosis revealed by single-cell analysis. J. Adv. Res. 2023;43:187–203. doi: 10.1016/J.JARE.2022.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dias-Neto M., Meekel J.P., vanSchaik T.G., Hoozemans J., Sousa-Nunes F., Henriques-Coelho T., Lely R.J., Wisselink W., Blankensteijn J.D., Yeung K.K. High density of periaortic adipose tissue in abdominal aortic aneurysm. Eur. J. Vasc. Endovasc. Surg. 2018;56:663–671. doi: 10.1016/J.EJVS.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 93.Gaibazzi N., Tuttolomondo D., Nicolini F., Tafuni A., Sartorio D., Martini C., Maestri F., Gallingani A., DeFilippo M., Corradi D. The histopathological correlate of peri-vascular adipose tissue attenuation on computed tomography in surgical ascending aorta aneurysms: is this a measure of tissue inflammation? Diagnostics. 2021;11:1799. doi: 10.3390/DIAGNOSTICS11101799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Klüner L.V., Oikonomou E.K., Antoniades C. Assessing cardiovascular risk by using the fat attenuation index in coronary CT angiography. Radiol. Cardiothorac. Imaging. 2021;3 doi: 10.1148/RYCT.2021200563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kotanidis C.P., Antoniades C. Perivascular fat imaging by computed tomography (CT): a virtual guide. Br. J. Pharmacol. 2021;178:4270–4290. doi: 10.1111/BPH.15634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Oikonomou E.K., Marwan M., Desai M.Y., Mancio J., Alashi A., Hutt Centeno E., Thomas S., Herdman L., Kotanidis C.P., Thomas K.E., Griffin B.P., Flamm S.D., Antonopoulos A.S., Shirodaria C., Sabharwal N., Deanfield J., Neubauer S., Hopewell J.C., Channon K.M., Achenbach S., Antoniades C. Non-invasive detection of coronary inflammation using computed tomography and prediction of residual cardiovascular risk (the CRISP CT study): a post-hoc analysis of prospective outcome data. Lancet. 2018;392:929–939. doi: 10.1016/S0140-6736(18)31114-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lian X., Gollasch M. A clinical perspective: contribution of dysfunctional perivascular adipose tissue (PVAT) to cardiovascular risk. Curr. Hypertens. Rep. 2016;18:82. doi: 10.1007/S11906-016-0692-Z. [DOI] [PubMed] [Google Scholar]
- 98.Boa B.C.S., Yudkin J.S., vanHinsbergh V.W.M., Bouskela E., Eringa E.C. Exercise effects on perivascular adipose tissue: endocrine and paracrine determinants of vascular function. Br. J. Pharmacol. 2017;174:3466–3481. doi: 10.1111/BPH.13732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sousa A.S., Sponton A.C.S., Trifone C.B., Delbin M.A. Aerobic exercise training prevents perivascular adipose tissue-induced endothelial dysfunction in thoracic aorta of obese mice. Front. Physiol. 2019;10:1009. doi: 10.3389/FPHYS.2019.01009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.DeVallance E., Branyan K.W., Lemaster K.C., Anderson R., Marshall K.L., Olfert I.M., Smith D.M., Kelley E.E., Bryner R.W., Frisbee J.C., Chantler P.D. Exercise training prevents the perivascular adipose tissue-induced aortic dysfunction with metabolic syndrome. Redox Biol. 2019;26 doi: 10.1016/J.REDOX.2019.101285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shi N., Xia J., Wang C., Zhou J., Huang J., Hu M., Liao J. Aerobic exercise prevents arterial stiffness and attenuates hyperexcitation of sympathetic nerves in perivascular adipose tissue of mice after transverse aortic constriction. Int. J. Mol. Sci. 2022;23 doi: 10.3390/IJMS231911189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Goetzke C.C., Ebstein F., Kallinich T. Role of proteasomes in inflammation. J. Clin. Med. 2021;10:1783. doi: 10.3390/JCM10081783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ouyang A., Garner T.B., Fleenor B.S. Hesperidin reverses perivascular adipose-mediated aortic stiffness with aging. Exp. Gerontol. 2017;97:68–72. doi: 10.1016/J.EXGER.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cheng C.K., Luo J.Y., Lau C.W., Chen Z.Y., Tian X.Y., Huang Y. Pharmacological basis and new insights of resveratrol action in the cardiovascular system. Br. J. Pharmacol. 2020;177:1258–1277. doi: 10.1111/BPH.14801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sun Y., Li J., Xiao N., Wang M., Kou J., Qi L., Huang F., Liu B., Liu K. Pharmacological activation of AMPK ameliorates perivascular adipose/endothelial dysfunction in a manner interdependent on AMPK and SIRT1. Pharmacol. Res. 2014;89:19–28. doi: 10.1016/J.PHRS.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 106.Xia N., Förstermann U., Li H. Effects of resveratrol on eNOS in the endothelium and the perivascular adipose tissue. Ann. N. Y. Acad. Sci. 2017;1403:132–141. doi: 10.1111/NYAS.13397. [DOI] [PubMed] [Google Scholar]
- 107.Gianotti L., Belcastro S., D’Agnano S., Tassone F. The stress Axis in obesity and diabetes mellitus: an update. Endocrine. 2021;2:334–347. doi: 10.3390/ENDOCRINES2030031. [DOI] [Google Scholar]
- 108.DeVallance E.R., Branyan K.W., Olfert I.M., Pistilli E.E., Bryner R.W., Kelley E.E., Frisbee J.C., Chantler P.D. Chronic stress induced perivascular adipose tissue impairment of aortic function and the therapeutic effect of exercise. Exp. Physiol. 2021;106:1343–1358. doi: 10.1113/EP089449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cooper C.B., Neufeld E.V., Dolezal B.A., Martin J.L. Sleep deprivation and obesity in adults: a brief narrative review. BMJ Open Sport Exerc. Med. 2018;4 doi: 10.1136/BMJSEM-2018-000392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nollet M., Wisden W., Franks N.P. Sleep deprivation and stress: a reciprocal relationship. Interface Focus. 2020;10 doi: 10.1098/RSFS.2019.0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Badran M., Yassin B.A., Lin D.T.S., Kobor M.S., Ayas N., Laher I. Gestational intermittent hypoxia induces endothelial dysfunction, reduces perivascular adiponectin and causes epigenetic changes in adult male offspring. J. Physiol. 2019;597:5349–5364. doi: 10.1113/JP277936. [DOI] [PubMed] [Google Scholar]
- 112.Chang L., Xiong W., Zhao X., Fan Y., Guo Y., Garcia-Barrio M., Zhang J., Jiang Z., Lin J.D., Chen Y.E. Bmal1 in perivascular adipose tissue regulates resting-phase blood pressure through transcriptional regulation of angiotensinogen. Circulation. 2018;138:67–79. doi: 10.1161/CIRCULATIONAHA.117.029972. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.