Abstract
Purpose:
American Indians/Alaska Native (AI/AN) persons are disproportionately affected by hepatitis C virus (HCV). The Northwest Portland Area Indian Health Board Indian Country Extension for Community Healthcare Outcomes (ECHO) telehealth clinic supports primary care providers (PCPs) in treating HCV. We evaluated the extent to which Indian Country ECHO increases access to HCV treatment and holistically serves AI/AN patients.
Methods:
We conducted a retrospective descriptive analysis of Indian Country ECHO treatment recommendations from 2017 to 2021. Recommendations were classified into the following categories: HCV treatment with direct-acting antiviral medication, prevention, substance use disorder treatment, lab or imaging orders, pharmacological considerations, behavior changes, other, and referral. Subanalysis of treatment recommendations was completed for patients with cirrhosis.
Findings:
Of the 776 patients from 77 Indian Health System facilities who presented at Indian Country ECHO, 718 (93%) received treatment recommendations. Most patients (93%) received recommendations for HCV treatment by their PCP; only 3% received a recommendation for referral to a hepatologist or liver transplant center for additional care. Most patients received at least 1 recommendation beyond the scope of HCV treatment provision. Cirrhosis criteria were met by 8% of patients, of which 80% received recommendations for HCV treatment by their PCP and 25% received recommendations for referral to a specialist for additional care.
Conclusions:
Most patients presented at the Indian Country ECHO received recommendations for HCV treatment by their PCP, along with recommendations beyond the scope of HCV. Indian Country ECHO telehealth clinic provides comprehensive recommendations to effectively integrate evidence-based HCV treatment with holistic care at the primary care level.
Keywords: access to care, American Indian/Alaska Native, Extension for Community Healthcare Outcomes (ECHO), hepatitis C, telehealth
INTRODUCTION
In the United States, an estimated 2.4 million individuals are impacted by chronic infection with hepatitis C virus (HCV),1 with American Indian and Alaska Native (AI/AN) persons disproportionately affected.2 HCV is the leading cause of liver cancer and liver transplants.3 Untreated HCV can progress to cirrhosis, resulting in an increased risk of developing complications of liver disease, including hepatic decompensation or hepatocellular carcinoma. However, HCV is curable. Second-generation direct-acting antiviral (DAA) medications are increasingly available and highly effective, with a cure rate of 90%-95%.4–6 Successful HCV treatment greatly reduces liver-related and all-cause mortality for HCV-infected individuals.2 For individuals with cirrhosis, HCV treatment dramatically reduces rates of hepatic decompensation, hepatocellular carcinoma, and liver-related mortality.7–11
AI/AN persons have the highest HCV-related mortality rate (8.63 per 100,000) of any racial and ethnic group in the United States and more than double the national rate (3.33 per 100,000).2 Despite knowledge of HCV-related morbidity and mortality in AI/AN persons, treatment rates remain low. A retrospective cohort study of HCV treatment initiation in AI/ANs in North Dakota showed that only 22 of 124 (18%) AI/AN persons with HCV received treatment.12 Reasons for not receiving HCV treatment included lack of access to specialists, signs of advanced liver fibrosis, active substance use, and cost.12 However, one limitation of this study is that it was published before the adoption of the second-generation DAAs for HCV treatment.
The primary health system for AI/AN persons is the Indian Health System, a network of facilities comprised of the Indian Health Service, Tribal, and Urban Indian organizations. Due to the inordinate rates of HCV infection and mortality for AI/AN persons, HCV treatment is an Indian Health System priority.13 Beyond the disproportionate mortality rates, developing a response for the Indian Health System is important because of the multiple levels of influencing factors, including individual characteristics and resources, service environment attributes, and the macro-level context that impact all aspects of the HCV care continuum.14
One important facet of the Indian Health System response to addressing HCV is telementoring support through the Extension for Community Healthcare Outcomes (ECHO) model. The ECHO model was developed in 2003 at the University of New Mexico to increase rural clinicians’ ability to treat HCV and has since expanded nationally and internationally.15 In 2017, the Northwest Portland Area Indian Health Board established its first Indian Country ECHO program. The Indian Country ECHO model connects primary care providers (PCPs) at Indian Health System facilities across the country to multidisciplinary specialists via virtual teleECHO clinics. The ECHO model brings specialty care directly to patients in their own communities. It has already been shown to eliminate the treatment barriers of inaccessibility and lack of communication between care teams associated with referral to specialty care.16 Previous research has also demonstrated that patients without cirrhosis treated for HCV with DAAs had similar treatment outcomes when treated by their PCP compared to specialty clinicians.17,18
While the ECHO model has been successful at increasing rates of HCV treatment and cure in patients without cirrhosis,17,18 there is limited research demonstrating ECHO’s success in treating HCV in patients with cirrhosis. Patients with cirrhosis have previously been excluded from studies comparing treatment outcomes between ECHO and specialty care due to cirrhosis being a risk factor for poor treatment outcomes.17,19 AI/AN persons have markedly higher age-adjusted death rates due to chronic liver disease and cirrhosis (45.2 age-adjusted deaths per 100,000 population) compared to the population overall (11.1 age-adjusted deaths per 100,000 population).20 Given the higher rates of cirrhosis and chronic liver disease in AI/AN persons, the Indian Country ECHO can be an important tool for PCPs within the Indian Health System, especially in rural settings with limited access to specialty care.
This retrospective descriptive analysis of Indian Country ECHO clinic treatment recommendations has 3 objectives. First, to determine what percentage of patients presented during an ECHO session received DAA recommendations for HCV treatment by their PCP. Second, to quantify and describe recommendations that are beyond the scope of DAA prescription for HCV treatment to analyze how Indian Country ECHO provides holistic care beyond the scope of HCV treatment. Third, to analyze treatment recommendations for patients with cirrhosis to determine how ECHO served this subset of patients with increased risk for complications. This analysis will provide an indication of program effectiveness in treating HCV in the AI/AN patient population and the extent to which ECHO provides care beyond the scope of HCV treatment.
METHODS
ECHO clinic format
Indian Country ECHO consisted of 1-5 (average of 3) monthly hour-long virtual telehealth clinics. Each clinic is comprised of didactic presentations, deidentified patient case presentations, and an overview of specialist recommendations. ECHO virtual telehealth clinics used Zoom Video Communications© (Zoom, San Jose, California) to connect PCPs serving AI/AN patients to a multidisciplinary team of specialists, including physicians, pharmacists, and nurse practitioners that provided comprehensive treatment recommendations.
ECHO case forms and treatment recommendation collection
We conducted a retrospective analysis of patients and treatment recommendations presented at the Indian Country ECHO from February 2017 to March 2021. All ECHO patients and treatment recommendations were inputted into a collective dataset for categorization and analysis. This study was approved by the Portland Area Indian Health Service Institutional Review Board #1632040-2.
Treatment recommendation categorization
Indian Country ECHO treatment recommendations were tailored to meet each patient’s specific needs for HCV treatment and general non-HCV-related care. Holistic, non-HCV-related care included recommendations for disease prevention, patient behavior changes, and treatment of coexisting chronic conditions. Three individuals involved in the Indian Country ECHO program were involved in the creation of the treatment recommendation categories, with all coauthors reviewing the treatment recommendation categories. Two of these individuals then independently reviewed all sessions and coded treatment recommendations. If there were disagreements or uncertainty regarding categorization, group discussions among coders were conducted to resolve issues. To assess interrater agreement, both reviewers independently reviewed and coded 10% of the data. Interrater agreement regarding treatment recommendation coding occurred for 89% of treatment recommendations assessed. Discrepancies were resolved either through discussion among coders or, if not discussed, coding priority went to the original coder.
There were two levels of treatment recommendation categorization: primary-level codes and secondary-level codes. Primary codes comprised of broad categories into the following: HCV treatment, prevention, substance use treatment, workup, pharmacological changes, behavior changes, referral, and other (Table 1). The primary codes for HCV treatment, referral, and other had no associated secondary codes. Prevention, substance use treatment, workup, pharmacological changes, and behavior changes primary codes were comprised of combining multiple secondary codes. Secondary codes were more specific and detailed than primary codes, allowing for additional and more nuanced analyses. The prevention primary code combined hepatitis A vaccination, hepatitis B vaccination, and hepatocellular carcinoma screening secondary codes. The primary code for substance use disorder treatment comprised of combining the following secondary codes: evaluation and treatment of substance use disorder treatment, harm reduction services, and behavioral health referral. The workup primary code included both lab and imaging order secondary codes. Medication changes and drug-drug interaction secondary codes combined to create the pharmacological changes primary code. Lastly, the behavior change primary code combined patient education and lifestyle modification secondary codes. Additional subanalysis was conducted for recommendations for medication changes specific to recommendations for naloxone and pre-exposure prophylaxis (PrEP) for human immunodeficiency virus. Treatment recommendation data assessment included the overall number of recommendations, number of recommendations per case, and percentage of patients receiving treatment recommendations for each primary coding category.
TABLE 1.
ECHO treatment recommendation category descriptions
| Treatment recommendation primary codes | Primary code description | Treatment recommendation secondary codes | Secondary code descriptions |
|---|---|---|---|
| Hepatitis C treatment | Recommendations for HCV treatment via direct-acting antiviral agents, ribavirin, and/or interferon | No secondary codes | |
|
| |||
| Lab or imaging orders | Any lab or imaging orders | Lab order | Any lab orders including FibroScan® and FibroTest® |
|
| |||
| Imaging order | Any imaging orders including endoscopy or ultrasound | ||
|
| |||
| Pharmacological considerations | Starting, stopping, changing dose of medication, or information about drug-drug interactions | Medication changes | Starting, stopping, or changing dose of a medication |
|
| |||
| Drug-drug interactions | Information about drug-drug interactions | ||
|
| |||
| Prevention | Recommendations for hepatitis A or B vaccinations or hepatocellular carcinoma screening | Hepatitis A vaccination | Recommendations for hepatitis A screening and vaccination |
| Hepatitis B vaccination | Recommendations for hepatitis B screening and vaccination | ||
| Hepatocellular carcinoma screening | Recommendations for hepatocellular carcinoma screening or surveillance | ||
|
| |||
| Behavior changes | Recommendations for educating or encouraging patients on lifestyle changes | Patient education | Educating patient about information related to hepatitis treatment, HCV transmission risk, and behaviors that increase liver damage |
| Lifestyle modifications | Encouragement of lifestyle changes including tobacco, marijuana, or alcohol cessation | ||
|
| |||
| Other | Treatment recommendations that did not meet criteria for any of the other categories | No secondary codes | |
|
| |||
| Substance use treatment | Recommendations for referral to safe syringe program, referral to a behavioral health specialist, or substance use treatment | Evaluation and treatment of substance use | Engagement with patient regarding substance use, evaluating/diagnosing patient for substance use disorder, and encouragement of patient engagement with substance use treatment programs |
| Harm reduction | Information regarding syringe service programs, needle exchange, safe injection sites, and harm reduction kits | ||
| Behavioral health referral | Referral or encouraged engagement with behavioral health | ||
|
| |||
| Refer to specialist | Referral to a liver transplant center or hepatologist | No secondary codes | |
Subanalysis of patients with indeterminant fibrosis and evidence of cirrhosis
The American Association for the Study of Liver Diseases defines uncomplicated, noncirrhotic patients as persons 18 years of age who have not been previously treated for HCV and have no evidence of cirrhosis.21 Evidence of cirrhosis was defined as having any of the following: a Fibrosis-4 (Fib-4) score greater than 3.25,22 transient elastography indicating cirrhosis, noninvasive serologic testing (FibroSure® or FibroTest) indicating cirrhosis, or clinical evidence of cirrhosis (liver nodularity and/or splenomegaly on imaging, or platelet counts less than 150,000/mm3).21 To increase specificity for this study, patients were considered to have cirrhosis if they had a Fib-4 score above 3.25 and at least one of the following: platelet counts less than 150,000/mm3, FibroSure®/FibroTest result indicating cirrhosis, or imaging results demonstrating cirrhosis. Patients with cirrhosis were compared to patients without fibrosis and patients with indeterminant fibrosis. Patients without fibrosis were defined as having a Fib-4 score below 1.45, platelet counts above 150,000/mm3, and no imaging or serologic testing showing evidence of cirrhosis. Patients with discordant Fib-4 scores and platelet counts were considered to have indeterminant fibrosis status.
The P values were 2-sided, and P<.05 was considered statistically significant when comparing male and female demographic data from patient case forms. Chi-squares tests were used to test for differences between no fibrosis, indeterminant, and cirrhosis comparison groups regarding categorical treatment recommendation data. To adjust for the threat of multiple comparison, we conducted a strict Bonferroni correction to change the statistical threshold to 0.006 (0.05/8 comparisons) for significant differences between comparison groups. Stata, version 17 (StataCorp LLC©), was used for the statistical analyses.
RESULTS
Most patients presented at ECHO received the recommendations for PCPs to treat HCV
A total of 776 patients from 77 Indian Health System facilities were scheduled for presentation at Indian Country ECHO during the study period. Two-thirds (51, 66%) of facilities were rural as defined by the US Health Resources & Services Administration “Rural Urban Commuting Area” criteria.23 A slight majority of patients were males (55%) compared to females (41%). The average male age (46.6±12.6 years) was significantly older than females (42.7±13.1 years) (P<.05). Additionally, two trans males were presented and 27 patients did not have sex disclosed on case forms.
Signed treatment recommendations for 718 patients (93%) were collected and analyzed. The remaining 58 patients (7%) did not include treatment recommendations, primarily due to transfer to different ECHO programs (54%) or for clinician schedule conflicts (46%). Of the 718 patients with completed treatment recommendations, 93% were recommended to proceed with HCV treatment, while 7% required further workup before an HCV treatment recommendation could be provided. Approximately half of the patients (27, 52%) requiring additional workup were subsequently represented at the Indian Country ECHO and received recommendations for HCV treatment by their PCP.
Of the 666 patients who received HCV treatment recommendations (93%), all but two patients received recommendations that PCPs complete HCV treatment. A small proportion (19, 3%) of patients received recommendations to refer to a hepatologist or liver transplant center. These patients either met the criteria for cirrhosis (68%) or indeterminant fibrosis (32%). Most of these patients (84%) still received HCV treatment recommendations in addition to a referral to a specialist. Those who did not receive HCV treatment recommendations required additional workup and received recommendations to present the case at a following Indian Country ECHO. The reasons for referral were liver transplant consultations (79%) or general hepatology consultations (21%) to further evaluate ascites or decompensation to determine appropriate HCV care.
Treatment recommendations not related to HCV treatment
The total number of treatment recommendations given was 4,176 with a median of 5 recommendations per patient case (interquartile range [IQR] 4-8, range 1-18) (Figure 1). Treatment recommendations were comprehensive and often extended beyond the scope of HCV treatment to include prevention-based, behavior change, other, and substance use treatment recommendation. Overall, only 701 of 4,176 (17%) treatment recommendations were specific to DAAs prescribed for HCV treatment (Table 2). Most patients (682, 95%) received at least 1 treatment recommendation that did not directly pertain to HCV treatment. Over half of the patients (56%) received at least 1 prevention-based treatment recommendation. This included 38% getting a hepatitis A vaccination recommendation, 20% getting a hepatitis B vaccination recommendation, and 18% of patients getting a cancer screening or surveillance recommendation.
FIGURE 1.
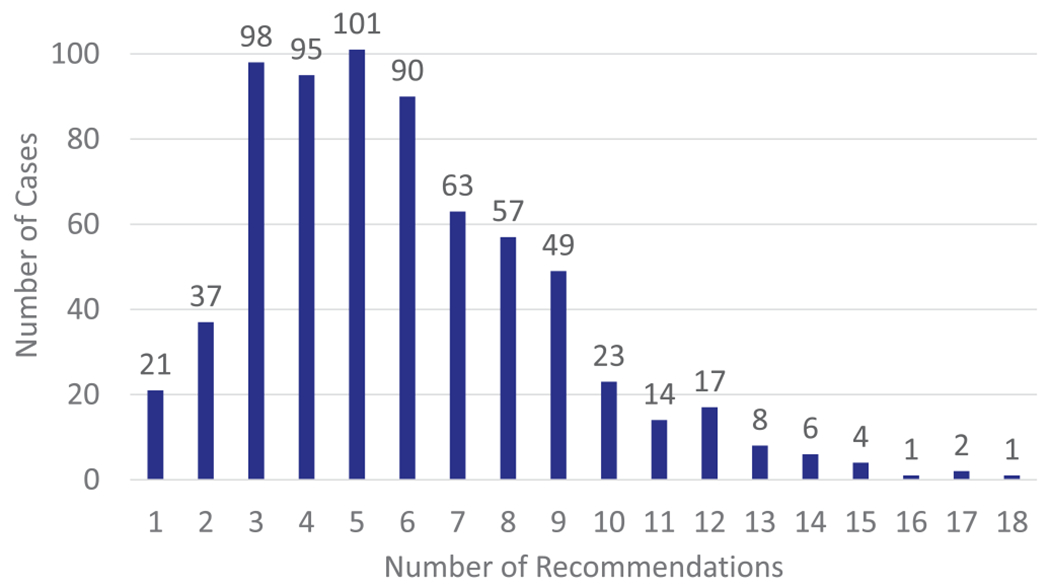
Histogram of the number of treatment recommendation per case. Most patients (95%) received at least 1 treatment recommendation that did not directly pertain to HCV treatment. Over half (56%) of the patients received between 3 and 6 treatment recommendations.
TABLE 2.
Treatment recommendation characteristics for all patients, patients with no evidence of fibrosis, and patients with evidence of cirrhosis
| Treatment recommendation primary codes | Percent of patients getting recommendation (%) | P-value | |||
|---|---|---|---|---|---|
| All patients (n = 718) | Patients with no evidence of fibrosis (n = 357) | Patients with indeterminant fibrosis(n = 272) | Patients with evidence of cirrhosis (n = 61) | ||
| Hepatitis C treatment | 95 | 97 | 88 | 80 | *P = .002 |
| Lab or imaging orders | 64 | 55 | 77 | 82 | *P<.001 |
| Pharmacological considerations | 62 | 45 | 62 | 57 | P = .94 |
| Prevention | 56 | 51 | 66 | 70 | *P<.001 |
| Behavior changes | 50 | 53 | 49 | 36 | P = .165 |
| Other | 42 | 37 | 42 | 56 | P = .017 |
| Substance use treatment | 34 | 35 | 28 | 33 | P = .097 |
| Refer to specialist | 3 | 0.1 | 3 | 25 | *P<.001 |
Indicates statistical significance P<.006.
About 17% of patients received more than one prevention-based recommendation. Half of the patients received a behavior change recommendation, with 41% receiving a lifestyle modification recommendation and 10% receiving a patient education recommendation. One-third of patients (34%) received a substance use disorder treatment recommendation, primarily for behavioral health referral (21%), evaluation or treatment for substance use (20%), and harm reduction (15%). About 15% of patients received more than one substance use disorder treatment recommendation.
We noted that 42% of participants had a recommendation in the “other” category. The 3 most recurrent “other” recommendations were depression or anxiety evaluation and treatment (15%), ECHO staff contact information for re-presenting the patient at ECHO (15%), and HCV screening for family members (12%). Additional, non-HCV-related care included prescription recommendations for naloxone or buprenorphine for 14% of patients and prescription recommendations for PrEP for 9% of patients.
Differences in treatment recommendations based on the extent of fibrosis
Approximately half of the patients who received treatment recommendations met the criteria for no fibrosis (357, 52%). About 40% of patients (272) were considered to have indeterminant fibrosis. A small but meaningful number of patients met the criteria for cirrhosis (61, 8%) and were provided treatment recommendations (Figure 2).
FIGURE 2.
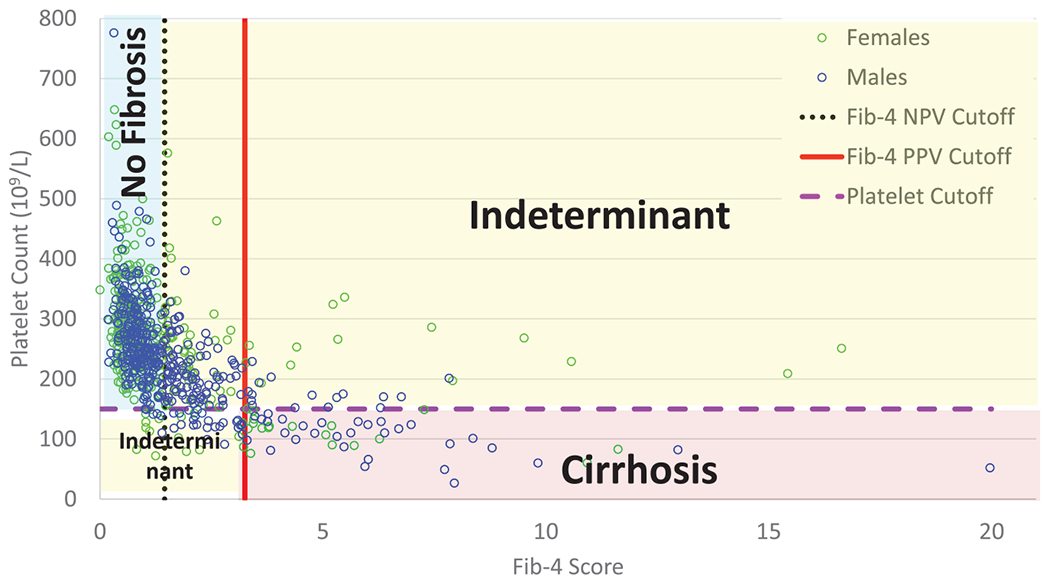
Distribution of patients meeting criteria for cirrhosis versus no cirrhosis based on Fib-4 score and platelet count. A small portion of patients (8%) met the study criteria for cirrhosis, while 40% had indeterminant fibrosis, and 52% had no evidence of fibrosis. Fibrosis-4 cutoffs were based on the previous literature showing that fibrosis-4 scores less than 1.45 had a negative predictive value of 90% to exclude advanced fibrosis and fibrosis-4 scores greater than 3.25 had a 97% specificity for advanced fibrosis22. Platelet counts below 150 (x109/L) are a known complication of cirrhosis and sign of decreased liver function21. Patients were also considered to have cirrhosis if imaging, FibroSure®, or FibroTest® results indicated cirrhosis.
The median number of treatment recommendations per case for patients with cirrhosis (7, IQR 5-9) was greater than patients without fibrosis (5, IQR 3-7) and patients with indeterminant fibrosis (6, IQR 4-8), but did not meet our threshold for statistical significance (P<.006). Of the patients with cirrhosis who received treatment recommendations through ECHO, 79% received recommendations to treat HCV by their PCP (Table 2). The other 21% of patients with cirrhosis received recommendations for further workup before recommendations for HCV treatment could be provided. Comparatively, 88% of patients with indeterminant fibrosis received recommendations for HCV treatment by their PCP, and 12% required further workup before HCV recommendation provision. Many patients with indeterminant fibrosis (58%) received recommendations for an additional workup to assess the extent of fibrosis, including FibroSure®/FibroTest or additional imaging. A greater percentage of patients with cirrhosis (25%) received a referral to a specialist or liver transplant center compared to patients with no fibrosis (0.1%) and patients with indeterminant fibrosis (3%). Of the 25% of patients with cirrhosis who received a referral to a hepatologist or liver transplant center for additional care, most of these patients (84%) also received HCV treatment recommendations independent of that referral. Additionally, 26% of patients with cirrhosis were presented at an ECHO clinic on multiple occasions for follow-up recommendations.
The frequency of the type of treatment recommendation was compared between patients without fibrosis, patients with indeterminant fibrosis, and patients with cirrhosis (Table 2). There was a significant difference between comparison groups for hepatitis C treatment, lab or imaging orders, prevention, and referral recommendations (P <.006). There was no significant difference between comparison groups for substance use treatment, pharmacologic consideration, other, and behavior change recommendations (P >.006).
DISCUSSION
This is the first analysis of HCV and non-HCV-related treatment recommendations given in an ECHO clinic specifically established for AI/AN persons who are disproportionately affected by HCV-related morbidity and mortality. This analysis suggests that ECHO increases access to HCV treatment in the unique setting of the Indian Health System. The vast majority of patients (93%) presented at the Indian Country ECHO received treatment recommendation for HCV treatment through their PCP. These findings support other studies that have demonstrated that ECHO increases PCP capacity to treat HCV,24 expands the number of Indian Health System, tribal, and urban facilities that provide HCV treatment,25 and obtains HCV cure rates comparable to specialists.18
The ECHO model increases access to HCV care by addressing important previously identified barriers to HCV care, including the need for referral, transportation, travel costs, and lack of access to specialists.12,26 Prior to the initiation of the Indian Country ECHO, a facility-based assessment of the Indian Health Service found only 8% of survey respondents provided HCV treatment at their facility and that most (69%) referred to a specialist for HCV treatment.26 Barriers to getting HCV care from referrals were patient ineligibility for treatment due to active substance use, transportation, and cost.26 This study noted that most referral facilities for HCV care were far from Indian Health System facilities with only 19% being less than 50 miles away, 27% being between 50 and 100 miles away, 27% being more than 100 miles away, and the remaining 27% being unsure of the distance to the nearest referral facility.27 Another study of focus groups exploring barriers to accessing health care for AI/AN patients noted the barrier of finding transportation to health care facilities, especially if individuals do not have access to cars.28 Transportation difficulties were found to influence decision-making on where to receive health care, with participants choosing to receive health care at facilities closer in distance with perceived inferior care over facilities further away with perceived superior care.28 These barriers to HCV care through referrals are compounded by the time between referral for specialty care and the receipt of evaluation by a specialist having the highest attrition rate in the HCV care continuum.29 ECHO eliminates these barriers to HCV treatment by connecting PCPs at Indian Health Service, tribal, and urban facilities, particularly those in rural areas, directly to specialists. Indian Country ECHO is successful at achieving this goal. A survey of 44 clinicians who participate in Indian Country ECHO found that most respondents reported beginning treating HCV at their facility subsequent to attending ECHO clinics.25
These results also documented that Indian Country ECHO provides holistic care beyond the scope of HCV treatment. Half of the patients receiving treatment recommendations received prevention-based or lifestyle change treatment recommendations. Prevention-based recommendations for hepatitis A and B vaccination can further reduce the rate of progression of chronic liver disease and viral hepatitis superinfections that can cause acute liver failure.21,30,31 Hepatocellular carcinoma screening and surveillance for patients with cirrhosis has been shown to improve overall survival.32 Behavior change recommendations, such as weight loss, alcohol cessation, and tobacco cessation, have been shown to be helpful in slowing the rate of progression of liver disease.21,33,34 While active substance use has been cited as a barrier to HCV treatment for some providers,12,26,35 it was not a barrier to treatment for patients presented at Indian Country ECHO. One-third of patients received recommendations for substance use treatment inclusive of substance use evaluation and treatment, harm reduction information, and behavioral health referral. This is critical given that injection drug use is the most common risk factor for HCV infection,36 and ongoing nonsterile injection practices increase the risk of HCV reinfection.37 Comprehensive integrated care for patients with coexisting HCV and substance use has been shown to increase the rates of HCV treatment initiation and sustained viral response.38 Substance use treatment and harm reduction education can help reduce the spread of HCV and reduce individual risk of HCV reinfection. Behavioral health integration to HCV care has been shown to help address patient psychiatric and substance use comorbidities and increase HCV treatment readiness and outcomes.39,40 Thus, clinicians presenting patients at ECHO for HCV treatment not only received guidance for HCV treatment but also received additional holistic, evidence-based, patient-centered recommendations inclusive of preventative health and substance use services.
This study also found that ECHO can serve patients with indeterminant fibrosis and evidence of cirrhosis. Nearly, 40% of patients were considered to have indeterminant fibrosis due to discordant Fib-4 scores, platelet counts, and no additional testing or imaging for fibrosis staging. Most patients with indeterminant fibrosis (88%) received recommendations for HCV treatment by their PCP, while 12% required additional workup, and 3% required referral to a specialist. Of the small but important proportion of patients with evidence of cirrhosis, most (80%) received recommendations for HCV treatment and a quarter (25%) received a recommendation for a referral, usually to establish care at a liver transplant center. These results correspond to another study that compared HCV treatment and cure rates between ECHO and a liver transplant center in difficult-to-access patient populations in Australia.41 Both study arms had similar rates of cirrhosis. The study demonstrated comparable rates of HCV treatment initiation, completion, and loss to follow-up, but no subanalysis for HCV treatment results for patients with cirrhosis was reported.41 Given that incidence rates of HCV and mortality rates for cirrhosis and liver cancer are increasing in the United States,36,42,43 ECHO may help expand access to care for patients with progressing fibrosis and cirrhosis. One study exploring increasing access to specialty care for advanced liver disease in the veteran population through ECHO found that almost 30% of requested cases could be completed through ECHO, resulting in quicker consultation time and decreased travel time for patients.44
Study limitations include the retrospective nature of the study, the inability to assess treatment recommendation implementation, and HCV cure rates. A small number of patients 28 (4%) had completed treatment recommendation but missing data to determine the extent of fibrosis. Thus, our results may slightly overestimate or underestimate the actual number of patients with indeterminant fibrosis and cirrhosis. To account for the threat of multiple comparisons, the statistical threshold for significance was adjusted from P = .05 to P = .006. This study may have missed truly significant differences in treatment recommendations between comparison groups because we increased our threshold for significance. Follow-up studies surveying PCPs who attended Indian Country ECHO sessions and patients who were presented as cases on how treatment recommendations were implemented in real-life clinical practice are needed. There may be additional barriers to HCV treatment that occur after the ECHO clinic that may reduce rates of HCV treatment in this patient population. A survey of 44 clinicians who participated in Indian Country ECHO cited that the continued barriers to HCV treatment were lack of access to HCV medications, need for more frequent/regular access to specialists, difficulty getting patients into care/follow-up, and limited clinical time for HCV.25 Access to HCV treatment medication was noted as a particularly important barrier due to third-party payers requiring late-stage liver disease and/or extensive periods of documented sobriety to cover HCV treatment.
Future studies should investigate treatment outcomes stratified by liver fibrosis staging, patient characteristic variables that impact treatment outcomes, and a comparison of treatment outcomes of patients with cirrhosis initially treated in the ECHO clinic versus those referred to a specialist. Indian Country ECHO plans to partner with other ECHO programs to collect additional patient characteristic and treatment recommendation data to further investigate how ECHO use differs based on the Center for Disease Control & Prevention region. Furthermore, an economic evaluation of the impacts of improved access and outcomes would be extremely beneficial to all health systems, particularly for those in rural settings.
CONCLUSIONS
Our study further supports research demonstrating how ECHO virtual telehealth clinics increase PCP capacity to treat HCV, even in patients with coexisting complex chronic conditions including cirrhosis. By relying on PCPs rather than referrals to specialty care, access to HCV treatment is considerably increased and is one means of addressing the significant HCV-related health disparities among AI/AN persons.
ACKNOWLEDGMENTS
The authors express their very great appreciation to the Northwest Portland Area Indian Health Board Tribal Delegates for their continued support to eliminate health disparities and improve the quality of life of American Indians and Alaska Natives.
FUNDING
Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number S06GM127164. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The work reported here was supported by the Indian Health Service and Minority HIV/AIDS Fund. XAL was supported by AHRQ K12 HS026370.
Funding information
National Institute of General Medical Sciences of the National Institutes of Health, Grant/Award Number: S06GM127164; NW Center of Excellence & K12 in Patient-Centered Learning Health Systems Science, Grant/Award Number: AHRQK12HS026370; Indian Health Service and Minority HIV/AIDS Fund
Footnotes
CONFLICT OF INTEREST
No authors have conflicts of interest relevant to this work.
REFERENCES
- 1.Schillie SWC, Osborne M, Wesolowski L, Ryerson AB. CDC Recommendations for Hepatitis C Screening Among Adults – United States, 2020. [DOI] [PMC free article] [PubMed]
- 2.Centers for Disease Control and Prevention. 2019 Viral Hepatitis Surveillance Report. 2021. Accessed April 2022, https://www.cdc.gov/hepatitis/statistics/SurveillanceRpts.html
- 3.Ly KN, Hughes EM, Jiles RB, Holmberg SD. Rising mortality associated with hepatitis C virus in the United States, 2003–2013. Clin Infect Dis. 2016;62(10):1287–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.AASLD-IDSA. HCV Testing and Linkage to Care. Recommendations for Testing, Managing, and Treating Hepatitis C. Accessed April 15, http://www.hcvguidelines.org/full-report/hcv-testing-and-linkage-care. 2022.
- 5.Afdhal N, Zeuzem S, Kwo P, et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370(20):1889–1898. [DOI] [PubMed] [Google Scholar]
- 6.Yin S, Barker L, White JZ, Jiles RB. Sofosbuvir-based regimens for chronic hepatitis C in a well-insured U.S. population: patient characteristics, treatment adherence, effectiveness, and health care costs, 2013–2015. J Manag Care Spec Pharm. 2019;25(2):195–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Backus LI, Boothroyd DB, Phillips BR, Belperio P, Halloran J, Mole LA. A sustained virologic response reduces risk of all-cause mortality in patients with hepatitis C. Clin Gastroenterol Hepatol. 2011;9(6):509–516. [DOI] [PubMed] [Google Scholar]
- 8.Dienstag JL, Ghany MG, Morgan TR, et al. A prospective study of the rate of progression in compensated, histologically advanced chronic hepatitis C. Hepatology. 2011;54(2):396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mira JA, Rivero-Juarez A, Lopez-Cortes LF, et al. Benefits from sustained virologic response to pegylated interferon plus ribavirin in HIV/hepatitis C virus-coinfected patients with compensated cirrhosis. Clin Infect Dis. 2013;56(11):1646–1653. [DOI] [PubMed] [Google Scholar]
- 10.Morgan RL, Baack B, Smith BD, Yartel A, Pitasi M, Falck-Ytter Y. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med. 2013;158(5 Pt 1):329–337. [DOI] [PubMed] [Google Scholar]
- 11.van der Meer AJ, Veldt BJ, Feld JJ, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA. 2012;308(24):2584–2593. [DOI] [PubMed] [Google Scholar]
- 12.Hossain S, Jalil S, Guerrero DM, Sahmoun AE. Challenges of hepatitis C treatment in Native Americans in two North Dakota medical facilities. Rural Remote Health. 2014;14(3):2982. [PubMed] [Google Scholar]
- 13.Weahkee PDDMD. Hepatitis C: Universal Screening and Treatment. 2019.
- 14.Hoj SB, Jacka B, Minoyan N, Artenie AA, Bruneau J. Conceptualising access in the direct-acting antiviral era: an integrated framework to inform research and practice in HCV care for people who inject drugs. Int J Drug Policy. 2019;72:11–23. [DOI] [PubMed] [Google Scholar]
- 15.Arora S, Kalishman S, Thornton K, et al. Expanding access to hepatitis C virus treatment–Extension for Community Healthcare Outcomes (ECHO) project: disruptive innovation in specialty care. Hepatology. 2010;52(3):1124–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beste LA, Glorioso TJ, Ho PM, et al. Telemedicine specialty support promotes hepatitis C treatment by primary care providers in the Department of Veterans Affairs. Am J Med. 2017;130(4):432–438. [DOI] [PubMed] [Google Scholar]
- 17.Syed TA, Bashir MH, Farooqui SM, et al. Treatment outcomes of hepatitis C-infected patients in specialty clinic vs. primary care physician clinic: a comparative analysis. Gastroenterol Res Pract. 2019;2019:8434602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arora S, Thornton K, Murata G, et al. Outcomes of treatment for hepatitis C virus infection by primary care providers. N Engl J Med. 2011;364(23):2199–2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sangiovanni A, Prati GM, Fasani P, et al. The natural history of compensated cirrhosis due to hepatitis C virus: a 17-year cohort study of 214 patients. Hepatology. 2006;43(6):1303–1310. [DOI] [PubMed] [Google Scholar]
- 20.NCFH Statistics. Age-Adjusted Death Rates for Selected Causes of Death, by Sex, Race, and Hispanic Origin: United States, Selected Years 1950–2018. Hyattsville, MD; 2019. [Google Scholar]
- 21.Ghany MG, Morgan TR, Panel A-IHCG. Hepatitis C Guidance 2019 Update: American Association for the Study of Liver Diseases-Infectious Diseases Society of America Recommendations for Testing, Managing, and Treating Hepatitis C Virus Infection. Hepatology. 2020;71(2):686–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sterling RK, Lissen E, Clumeck N, et al. Development of a simple non-invasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43(6):1317–1325. [DOI] [PubMed] [Google Scholar]
- 23.Services USDoHaH. Defining Rural Population. Accessed May 13, 2022. https://www.hrsa.gov/rural-health/about-us/what-is-rural
- 24.Mitruka K, Thornton K, Cusick S, et al. Expanding primary care capacity to treat hepatitis C virus infection through an evidence-based care model-Arizona and Utah, 2012–2014. MMWR Morb Mortal Wkly Rep. 2014;63(18):393–398. [PMC free article] [PubMed] [Google Scholar]
- 25.Stephens D, Leston J, Terrault NA, et al. An evaluation of hepatitis C virus telehealth services serving tribal communities: patterns of usage, evolving needs, and barriers. J Public Health Manag Pract. 2019;25(Suppl 5):S97–S100. [DOI] [PubMed] [Google Scholar]
- 26.Reilley B, Leston J, Redd JT, Geiger R. Lack of access to treatment as a barrier to HCV screening: a facility-based assessment in the Indian health service. J Public Health Manag Pract. 2014;20(4):420–423. [DOI] [PubMed] [Google Scholar]
- 27.Amoako A, Ortiz-Paredes D, Engler K, Lebouche B, Klein MB. Patient and provider perceived barriers and facilitators to direct acting antiviral hepatitis C treatment among priority populations in high income countries: a knowledge synthesis. Int J Drug Policy. 2021;96:103247. [DOI] [PubMed] [Google Scholar]
- 28.Shah VO, Ghahate DM, Bobelu J, et al. Identifying barriers to healthcare to reduce health disparity in Zuni Indians using focus group conducted by community health workers. Clin Transl Sci. 2014;7(1):6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reader SW, Kim HS, El-Serag HB, Thrift AP. Persistent challenges in the hepatitis C virus care continuum for patients in a Central Texas Public Health System. Open Forum Infect Dis. 2020;7(8):ofaa322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang JY, Lee SD, Tsai YT, Lo KJ, Chiang BN. Fulminant hepatitis A in chronic HBV carrier. Dig Dis Sci. 1986;31(1):109–111. [DOI] [PubMed] [Google Scholar]
- 31.Riley TR 3rd, Bhatti AM. Preventive strategies in chronic liver disease: part I. Alcohol, vaccines, toxic medications and supplements, diet and exercise. Am Fam Physician. 2001;64(9):1555–1560. [PubMed] [Google Scholar]
- 32.Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68(2):723–750. [DOI] [PubMed] [Google Scholar]
- 33.Hezode C, Lonjon I, Roudot-Thoraval F,et al. Impact of smoking on histological liver lesions in chronic hepatitis C. Gut. 2003;52(1):126–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hickman IJ, Clouston AD, Macdonald GA, et al. Effect of weight reduction on liver histology and biochemistry in patients with chronic hepatitis C. Gut. 2002;51(1):89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stephenson J Former addicts face barriers to treatment for HCV. JAMA. 2001;285(8):1003–1005. [DOI] [PubMed] [Google Scholar]
- 36.Centers for Disease Control and Prevention. 2019 Viral Hepatitis Surveillance Report. 2021. Accessed May 20, 2022. https://www.cdc.gov/hepatitis/statistics/SurveillanceRpts.htm
- 37.Martinello M, Grebely J, Petoumenos K, et al. HCV reinfection incidence among individuals treated for recent infection. J Viral Hepat. 2017;24(5):359–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ho SB, Brau N, Cheung R, et al. Integrated care increases treatment and improves outcomes of patients with chronic hepatitis C virus infection and psychiatric illness or substance abuse. Clin Gastroenterol Hepatol. 2015;13(11):2005–2014. [DOI] [PubMed] [Google Scholar]
- 39.Cos TA, Bartholomew TS, Huynh KJ. Role of behavioral health providers in treating hepatitis C. Prof Psychol: Res Pract. 2019;50(4):246. [Google Scholar]
- 40.Grosgebauer K, Bartholomew TS, Huynh K, Cos T. Impact of behavioral health consultation on hepatitis C treatment outcomes at a Federally Qualified Health Center;Philadelphia, PA. J Prim Prev. 2021;42(2):203–215. [DOI] [PubMed] [Google Scholar]
- 41.Mohsen W, Chan P, Whelan M, et al. Hepatitis C treatment for difficult to access populations: can telementoring (as distinct from telemedicine) help? Intern Med J. 2019;49(3):351–357. [DOI] [PubMed] [Google Scholar]
- 42.Tapper EB, Parikh ND. Mortality due to cirrhosis and liver cancer in the United States, 1999–2016: observational study. BMJ. 2018;362:k2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paik JM, Golabi P, Younossi Y, Saleh N, Nhyira A, Younossi ZM. The growing burden of disability related to chronic liver disease in the United States: data from the Global Burden of Disease Study 2007–2017. Hepatol Commun. 2021;5(5):749–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Glass LM, Waljee AK, McCurdy H, Su GL, Sales A. Specialty care access network-extension of community healthcare outcomes model program for liver disease improves specialty care access. Dig Dis Sci. 2017;62(12):3344–3349. [DOI] [PubMed] [Google Scholar]


