Abstract
Objective:
To test the hypothesis that genital skin and male urethra affected by lichen sclerosus (LS) has increased collagen content and altered collagen structure.
Methods:
We used picrosirius red to stain and image collagen in human urethral, vulvar, and foreskin specimens with and without LS. Using Image J software, we quantified and compared 1) collagen content (using two metrics: collagen proportionate area [CPA] and collagen fiber count), 2) collagen fiber length and width, and 3) collagen structure using the texture analysis technique gray level co-localization matrix (GLCM) with respect to LS status and tissue type.
Results:
We analyzed 23 LS specimens (vulva n=9, urethra n=7, foreskin n=7) and 29 non-LS specimens (vulva n=9, urethra n=7, foreskin n=13). Fiber count and CPA were significantly higher in all LS specimens compared to non-LS specimens (CPA: mean±SD 0.971±0.03 vs 0.948±0.02, p < 0.007; fiber count: mean±SD = 2906±127 vs 2509±78 fibers; p = 0.003). Collagen fiber width and length were similar with respect to LS status. GLCM analysis showed decreased inverse difference moment and increased entropy in LS tissues indicative of less homogeneous and more disorganized tissue structure (p<0.001).
Conclusions:
LS tissues have greater collagen content compared to non-LS tissues. Quantitative assessment of collagen organization, using GLCM, revealed less homogeneity and more disorganization of collagen in LS compared to non-LS tissues. Taken together, our findings suggest that alterations in physical tissue properties seen in LS may be due to both increased collagen abundance and altered structure.
Keywords: Lichen sclerosus, urethral stricture, fibrosis, extracellular matrix
Introduction
Lichen sclerosis (LS) is a debilitating chronic inflammatory and fibrotic disease of the anogenital skin which causes significant urinary and sexual dysfunction. LS is more common in women than men (6:1), and the prevalence is estimated to be between 1:300 and 1:1000 (1). In affected men, LS is associated with severe urethral fibrosis and associated stricture disease in up to 30% of cases (2). LS associated urethral strictures are refractory to surgical reconstruction (urethroplasty) failing up to 70% of the time (2, 3). The pathogenesis of LS and the mechanisms by which it causes urethral fibrosis is unknown and represents a critical knowledge gap that impedes development of alternative treatment strategies for this debilitating disease.
Fibrotic diseases are defined by excessive deposition of fibrillar collagen. Collagen is the primary component of the extra cellular matrix (ECM) which serves as a scaffold to support tissue architecture. Dysregulation of collagen production and metabolism result in collagen accumulation (fibrosis) that disrupt organ structure and function. For example, excessive collagen type I synthesis drives myocardial fibrosis that results in heart failure (4) and renal collagen deposition sustains pathological responses of the glomerulus and thereby implicated in progression of chronic kidney disease (5). More recently, altered collagen and ECM structure in fibrotic diseases has been recognized as a critical determinant of tissue mechanical properties and thereby a central driver of disease pathogenesis (6, 7). Changes in collagen structure usually result from post-translational crosslinking of collagen fibrils by an enzyme family called LOX/LOXL which results in both increased collagen fiber width and increased tissue stiffness (8, 9). Alterations in collagen organization, and increased crosslinking in particular, are often more powerful determinants of tissue stiffness than total collagen content as has been demonstrated in both Duchenne Muscular Dystrophy and Idiopathic Pulmonary Fibrosis among other diseases (10). Further, targeting the mechanisms driving altered collagen structure represents an untapped therapeutic target in fibrotic diseases.
Alterations in collagen content and structure have not been well studied in LS and associated urethral stricture disease and those that have been done have yielded mixed results (3, 11). Therefore, we compared collagen content and structure in three human tissue types (vulva, foreskin, and male urethra) affected and unaffected by LS. We hypothesize that genital skin and male urethra affected by LS has increased total collagen content, wider collagen fibers (reflective of increased collagen crosslinking), and altered collagen structure compared to non-LS tissues, recapitulating the pattern seen in other fibrotic diseases.
Materials and Methods
Patient identification and sample collection
Following Institutional Review Board (IRB) approval (IRB registration number IRB00003739) we used procedural billing data to identify and include adult patients (age >18yrs) who underwent vulvar biopsy, circumcision, urethroplasty, or urethral biopsy at our institution from 2015–2020 with available pathologic tissue. Due to the rarity of LS we cross-referenced these results with pathology records to identify specimens with mention of either “lichen sclerosus” or “balanitis xerotica obliterans” (a historic synonym for LS) in the pathology report. Pathology reports were reviewed and specimens with a diagnosis of malignancy or carcinoma in situ were excluded. Ten specimens per tissue type (urethra, vulva, foreskin) without mention of LS or BXO were randomly selected as non-LS samples. All pathologic tissues were then re-reviewed with a Genitourinary Pathologist (WH) who was blinded to the original pathology report to determine the diagnosis of LS based on the presence of epidermal atrophy, loss of dermal rete pegs, hyperkeratosis, dermal hyalinization, and presence of inflammatory infiltrate (12). Based on this review, patients were classified as either “LS” or “non-LS.” Eight samples, one LS and 7 non-LS were excluded due to inadequate tissue for analysis.
Collagen staining
We stained collagen with picrosirius red (PSR) which has a high affinity and specificity for fibrillar collagen and is commonly used to quantify collagen content in multiple tissue types (13). Five-micron formalin-fixed, paraffin embedded tissue sections were deparaffinized at room temperature in two washes of xylene (3 min) and then rehydrated through a graded series of ethanol (100%, 75%, 50%; all 5 min) to distilled water. Sections were incubated at room temperature for 1 hour in a solution of 0.5g of Direct Red 80 (Sigma-Aldrich, St. Louis, MO) in 500mL of saturated picric acid. The slides were washed twice with acidified water (0.5% acetic acid) for 5 minutes each and then dehydrated in ascending concentrations of ethanol (50%, 75%, 100%; 4 quick dips) and cleared in xylene. Sections were covered with Richard-Allan toluene-based mounting medium (4112APG; Thermo Fisher Scientific) before coverslipping.
Image acquisition, processing, and analysis
Picrosirius red (PSR) stained tissues were imaged using fluorescent microscopy as previously described (14). Briefly, PSR stained tissues were imaged using an Eclipse E600 microscope (Nikon) with a 20x dry objective lens (Plan Fluor NA = 0.50; Nikon) for collagen content analysis and a 40x dry objective lens (Plan Fluor NA = 0.75; Nikon) for collagen structure analysis. PSR fluorescence was detected using a Y-2E/C TX Red filter cube and tissue autofluorescence was detected using a B-2 E/C FITC filter cube (Chroma Technology Corp; Bellows Fall, VT). The PSR fluorescence and tissue autofluorescence images were captured using a CoolSnap DYNO camera (Photometrics) and NIS-Elements D v4.6 imaging software (Nikon) at 1920 × 1460 pixel resolution. Five randomly selected stromal regions of interest (ROIs) immediately adjacent to the epithelial surface were captured using a 20x objective for content analysis and three ROIs were captured using a 40x objective for fiber characteristics. Exposure time was kept constant for all images, and brightness and contrast adjustments that optimize signal-to noise-ratio were kept constant using Adobe Photoshop software (Adobe Systems; San Jose, CA).
The PSR fluorescence and tissue autofluorescence images were superimposed using an Image J-based analysis package (15). Images were processed as red and green images with PSR fluorescence occupying the red channel and tissue autofluorescence occupying the green channel. PSR fluorescence was isolated using Image J and the PSR Isolation plugin. For collagen content analysis, the resulting monochrome image was processed with Image J and the Black Background Quantification plugin was used to quantify the total number of black and color pixels. Total collagen content was defined as the proportion of color pixels compared to the total number of pixels for each image. For collagen structure analysis, the PSR isolated monochrome images were analyzed using CT-FIRE software (LOCI; Madison, WI). This software detects and quantifies collagen fiber-like metrics in images, including fiber length, diameter, and density (15). Values for collagen content, fiber length, fiber count, and fiber thickness obtained for each ROI were used to calculate a mean value for each subject. Mean values for each parameter were compared between LS and no-LS tissues using the Student’s T-test and the Mann-Whitney U test where appropriate.
Texture analysis
Gray level co-occurrence matrix (GLCM) is a second order statistical method that measures and reports on the spatial relationship between image pixels. The matrix elements consist of the number of occurrences of different pairs of pixel values across the image. GLCM textures were calculated in Image J (open-source image analysis platform) using a custom macro. This was built with the Texture Analyzer plugin (Julio E. Cabrera, version v0.4 2006/07/07) to output five standard parameters: Angular Second Moment (ASM), Inverse Difference Moment (IDM), Contrast, Entropy, and Correlation. Previously, GLCM has been shown to discriminate differences in collagen structure across regions of normal and high-grade serous ovarian cancer tissue (16).
Results
Collagen content
We analyzed 52 total specimens which included 23 non-LS specimens (9 vulva, 7 urethra, 7 foreskin) and 29 LS specimens (9 vulva, 7 urethra, 13 foreskin). Average patient age was 57.5 (range 19–90) years. We hypothesized that total collagen content is greater in LS compared to non-LS tissues. To test this, we used two quantitative metrics to compare collagen content. The first was collagen proportionate area (CPA) which is the proportion of each region of interest stained with PSR. This method has been validated for use in multiple other fibrotic tissue types (lung, liver, kidney) (17, 18). The second metric was collagen fiber count defined as the number of individual collagen fibers in a given slide area.
Overall, CPA was significantly greater in LS compared to non-LS tissues (mean±SD 0.971±0.03 vs 0.948±0.02, p < 0.007). When each tissue type was compared individually, collagen content in urethra and foreskin LS tissues was significantly greater than in non-LS tissues (urethra – 0.979±0.01 vs 0.953±0.02, p=0.02; foreskin – 0.974±0.03 vs 0.951±0.02, p = 0.027). Vulvar LS tissues also had increased CPA compared to non-LS tissues (0.961±0.06 vs 0.941±0.02) but this difference was not statistically significant (p = 0.385) (Figure 1). Similarly, there were more collagen fibers in LS tissues than in non-LS tissues (mean±SE = 2906±127 vs 2509±78 fibers; p = 0.003). When collagen fiber counts were compared across individual tissue types with respect to LS status we found that LS tissues consistently had more collagen fibers but this difference was statistically significant only for foreskin tissues (2722±154.8 vs 2161±42.24 fibers; p=0.003) (Figure 1).
Figure 1.
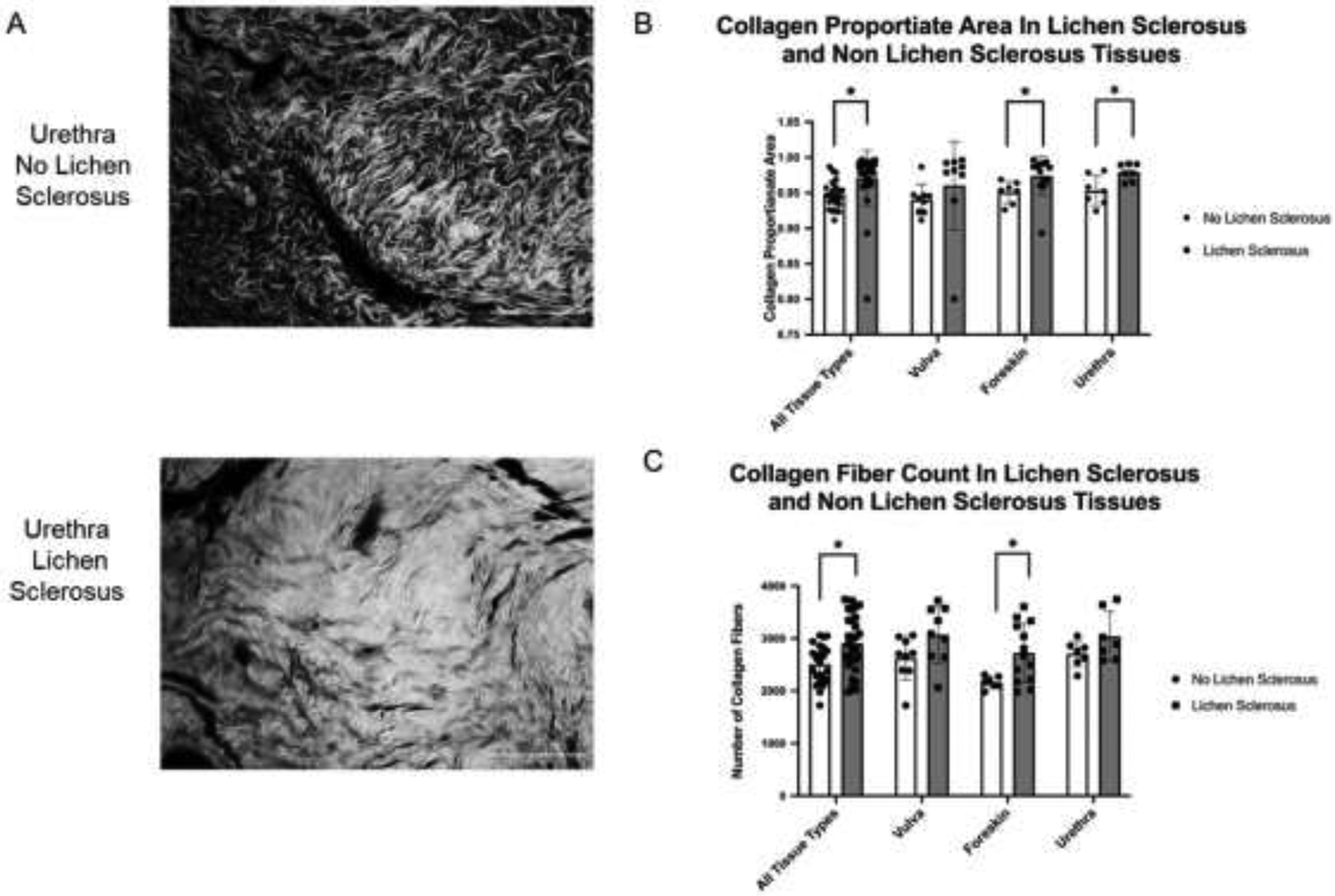
Comparison of collagen content in human vulva, urethra, and foreskin with and without LS. (A) Representative fluorescent microscopy images (20x) of PSR stained urethral collagen demonstrating increased collagen content in LS compared to non-LS tissue. (B) Comparison of mean collagen proportionate area in tissues with and without LS. Taken together, LS tissues had higher mean collagen proportionate area compared to non-LS tissues (0.971 vs 0.948, respectively; p=0.007). When segregated by tissue type urethra and foreskin LS tissues had significantly higher collagen proportionate areas (urethra – 0.979 vs 0.953, p=0.02; foreskin – 0.974 vs 0.951, p = 0.027) while vulvar tissues were no different with respect to LS status (0.961 vs 0.941, p = 0.385). (C) Comparison of collagen fiber count in tissues with and without LS. Taken together, LS tissues had higher mean collagen fiber counts compared to non LS tissues (2906 vs 2509 fibers, p = 0.003). When segregated by tissue type foreskin LS tissues had higher mean number of collagen fibers (2722 vs 2161 fibers, p = 0.004) while vulva and urethra were similar with respect to LS status (vulva – 3071 vs 2625 fibers, p = 0.07; urethra – 3036 vs 2707, p = 0.139). Brackets indicate pairwise comparisons with “*” indicating p < 0.05. LS = lichen sclerosus, PSR = picrosirius red. Scale bar = 100 μm.
Collagen structure
We hypothesized collagen fibers are wider and longer in LS compared to non-LS tissues as this is reflective of increased fiber cross linking (9). We found that collagen fiber width and length are similar between LS and non-LS tissues causing us to reject our hypothesis (Figure 2). However, visual inspection of the PSR stained images showed very high density and overlap of collagen fibers and subjective differences in collagen organization between LS and non-LS tissues. Because of this we were concerned that curvelet analysis may lack sensitivity to differences in individual fiber characteristics due to poor curvelet fitting to individual collagen fibers. Therefore, we used gray level co-occurrence matrix analysis (GLCM) to assess differences in the structural organization of collagen fibers.
Figure 2.
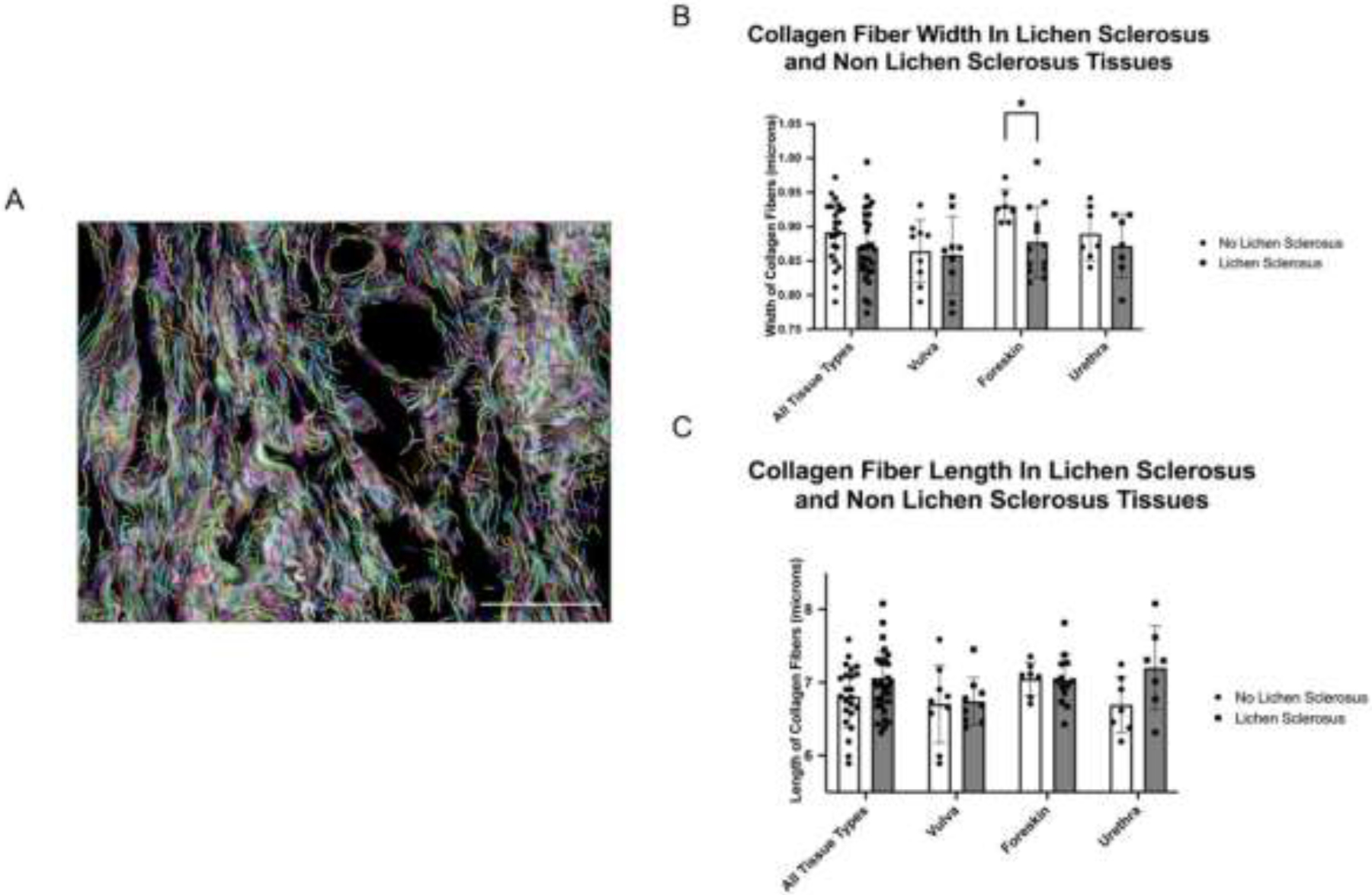
Comparison of collagen fiber width and length in human vulva, urethra, and foreskin tissue with and without LS. (A) Representative 40x image demonstrating an area of subepithelial collagen deposition with superimposed curvelets from the CT-FIRE software package. Each colored curvelet is fit to an individual collagen fiber and analysis of these curvelets yield the data presented in the remainder of the figure. (B) Mean collagen fiber width was similar across all tissue types with respect to LS status (p=0.111). However, when individual tissue types were examined foreskin tissues with LS were found to have significantly increased collagen fiber width compared to non-LS tissues (0.877 vs 0.93 microns, p = 0.006). (C) Comparison of collagen fiber lengths across vulvar, foreskin, and urethral tissues did not display significant differences with regard to LS status. Brackets indicate pairwise comparisons with “*” indicating p <0.05. LS = lichen sclerosus. Scale bar = 50 μm.
We used GLCM to assess two metrics. The first is entropy which measures image orderliness by examining the probability of pixel pair occurrences. The value of entropy increases when pair occurrences are less common. The second is inverse difference moment (IDM) which quantifies the homogeneity of an image by weighing the diagonal of the matrix the highest, thus more pixel similarity results in higher IDM. Using GLCM analysis, we found that LS tissue has more entropy and less IDM than non LS tissue. These results suggest decreased homogeneity of collagen structure and more disorganized structure in LS tissues compared to non-LS tissues (Figure 3).
Figure 3.
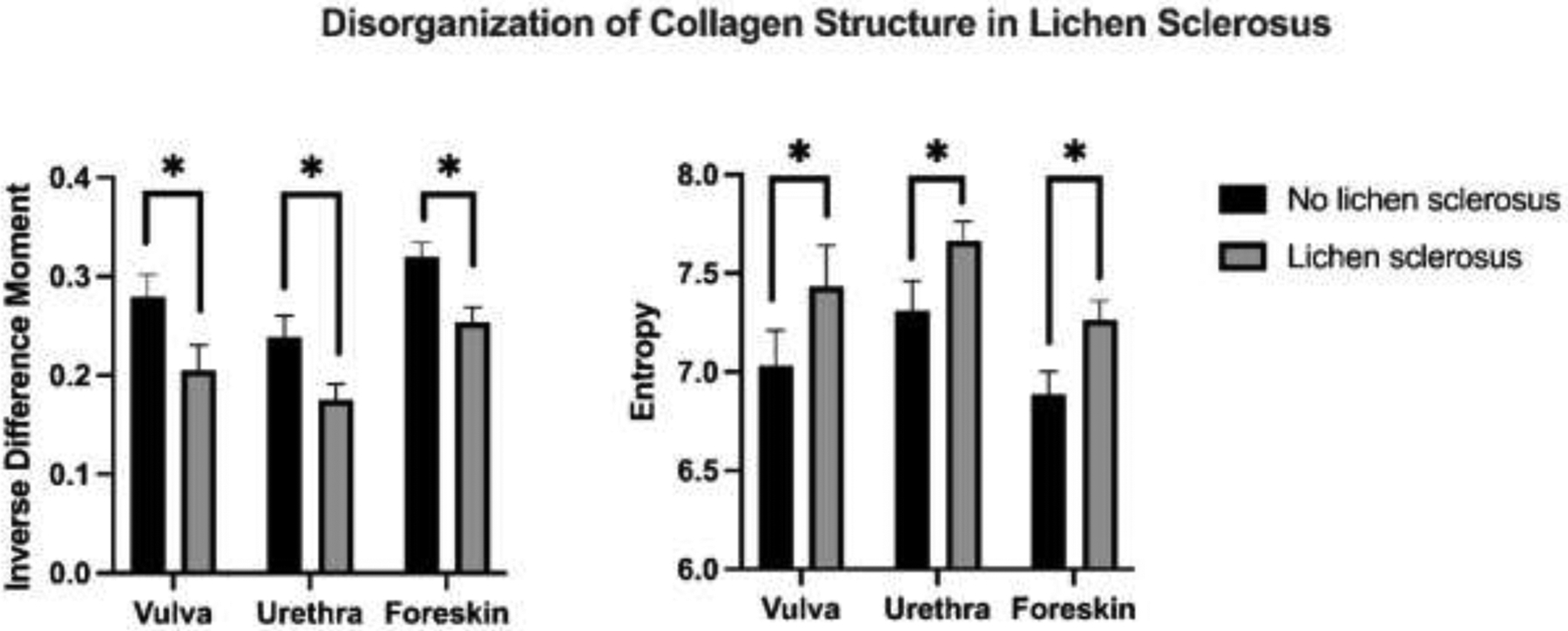
Comparison of inverse difference moment (IDM) and entropy in human vulva, urethra, and foreskin tissues with and without LS. There is a consistent decrease in IDM and increase in entropy in LS compared to non-LS tissues. This reflects decreased homogeneity and disorganization of collagen structure and may imply alternative pathways for collagen production and remodeling in LS. * indicates p<0.001 in pairwise comparisons.
DISCUSSION
Collagen content and structure are poorly studied in LS and often with conflicting results. We quantitatively compared collagen content and structure in vulvar, foreskin, and urethral tissues affected and unaffected by LS. We found that LS tissues had greater collagen content compared to non-LS tissues which is consistent with other fibrotic diseases. Quantitative assessment of collagen organization, using GLCM, revealed less homogeneity and more disorganization of collagen in LS compared to non-LS tissues. Taken together, our findings suggest that alterations in physical tissue properties seen in LS may be due to both increased collagen abundance and altered structure.
There are few prior studies characterizing LS collagen content and structure. Electron microscopy studies reveal a greater density of dermal collagen in vulvar LS compared to non-diseased control tissues (19). An immunohistochemical study revealed an abnormal distribution of collagen I and III in vulvar LS, although individual collagen fibers were not distinguished in this study, perhaps due to the markedly increased fiber density (20). Godoy and colleagues showed in a case-control format similar to the present study that LS tissues had decreased elastic fibers, a “non-homogeneous” distribution of collagen, and increased expression of collagens I, III, and V with increased density of collagen V on 3D reconstruction (21). An additional immunohistochemical study examining expression of basement membrane proteins noted more abundant collagen IV and VII (both of which constitute the structural scaffold of the ECM) in LS tissues (22). In summary, prior studies of LS describe qualitative increases in collagen content and alterations in ultrastructure and our results are concordant with this literature. However, our study is the first to quantify increases in collagen content and alterations in collagen organization. Further, prior studies have been limited to vulvar and foreskin tissues, and we include urethra as LS related urethral stricture is particularly challenging to manage clinically. We found that while LS tissues overall have increased collagen content compared to non-LS tissue, there is variation across tissues types with significant quantitative collagen increases only in foreskin and urethra but not vulva. This suggests that the pathologic effects of LS vary across tissue types and possibly biologic sex. The mechanisms driving these differences are worthy of further study to inform the best application of existing treatments and development of new targeted therapies for LS.
It is controversial if the increased collagen content seen in LS results from increased collagen production, impaired degradation, or both. A study by Oikarinen and colleagues demonstrated increased collagen production in LS and colocalization of fibroblasts and type I procollagen cDNA on in situ hybridization (23). However, the authors did not identify active fibroblasts characterized by TGF-β expression in regions of histological inflammation. The authors suggest that alternative inflammatory pathways may account for fibrosis in LS. Other studies have concluded that collagen biosynthesis is not increased in LS. One study of foreskin specimens affected by LS actually reported that new collagen synthesis was decreased in LS compared to unaffected controls on hydoxyproline assay (24). These prior studies have examined a small number of patients with different tissue types. Therefore, it is possible that variation across multiple LS tissue types accounts for differences and/or that selection bias in how patients were recruited for study confounded these results. While our study does not provide data regarding the mechanisms underlying increased collagen abundance, we plan to pursue this as a future direction.
We demonstrate altered collagen structure in LS across multiple tissue types. In other fibrotic disorders, such as idiopathic pulmonary fibrosis, altered collagen structure most often results from post-translational modification. The most common of these is cross linking of collagen fibrils which is positively associated with increased tissue stiffness (25). Further, there is mounting evidence that increased matrix stiffness can promote self-sustaining progressive fibrosis such as that seen in LS tissues (26, 27). Based on our data demonstrating a consistent increase in collagen content and structural alterations we postulate that LS related fibrosis results from both increased collagen production and altered post-translational modification of collagen fibrils. To further investigate this, we plan to assess LOX/LOXL, the enzyme responsible for collagen cross linking, in LS tissues and perform ex-vivo mechanical studies with atomic force microscopy to quantify differences in tissue stiffness and correlate these with changes in collagen cross linking.
Our study has several limitations which deserve mention. First as a retrospective study of archival pathology specimens it is subject to inherent selection bias. However, due to the relative rarity of LS in male patients obtaining sufficient tissue for analysis in a prospective manner represents a significant pragmatic barrier. Second, our study used non-LS tissues as a comparison group rather than true normal tissue - the collection of which is limited by clinical equipoise. While this may obscure differences accounted for by variation across tissue types we predict that comparing diseased tissues affected and unaffected by LS is clinically relevant and that use of true normal tissue as a comparison group would likely amplify the magnitude of observed differences. Third, our microscopy based approach does not allow for chemical quantification of collagen content nor does it permit analysis of differences in collagen transcription or post translational modification. Prior studies have validated that CPA and curvelet analysis reflect chemically measured collagen content (28). As a future direction we aim to characterize differences in collagen gene expression as well as LOX/LOXL expression which is responsible for collagen fibril cross linking in order to further parse the relative contribution of increased collagen production and cross linking in LS pathogenesis.
Conclusion
Our study demonstrates, for the first time, a quantitative increase in collagen content and altered collagen structure in LS across multiple human tissue types including urethra. These data are novel in LS, but consistent with findings in other fibrotic disorders. As a future direction we plan to further probe the molecular mechanisms underlying both increased collagen abundance and post-translational modifications of collagen fibrils leading to altered structure. By coupling these mechanistic studies with ex-vivo study of the mechanical properties of tissue, specifically urethra, we hope to identify pathways which are fruitful therapeutic targets in treatment of LS related fibrosis.
Funding Source Declaration:
Matthew Grimes, MD reports a relationship with National Institute of Diabetes and Digestive and Kidney Diseases that includes funding grants.
Official Title: Institutional Career Development Award
Sponsor Award: 5K12DK100022
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Granieri MA, Peterson AC, Madden-Fuentes RJ. Effect of Lichen Sclerosis on Success of Urethroplasty. Urol Clin North Am. 2017;44(1):77–86. [DOI] [PubMed] [Google Scholar]
- 2.Fergus KB, Lee AW, Baradaran N, Cohen AJ, Stohr BA, Erickson BA, et al. Pathophysiology, Clinical Manifestations, and Treatment of Lichen Sclerosus: A Systematic Review. Urology. 2020;135:11–9. [DOI] [PubMed] [Google Scholar]
- 3.Levy A, Browne B, Fredrick A, Stensland K, Bennett J, Sullivan T, et al. Insights into the Pathophysiology of Urethral Stricture Disease due to Lichen Sclerosus: Comparison of Pathological Markers in Lichen Sclerosus Induced Strictures vs Nonlichen Sclerosus Induced Strictures. J Urol. 2019;201(6):1158–63. [DOI] [PubMed] [Google Scholar]
- 4.Querejeta R, Lopez B, Gonzalez A, Sanchez E, Larman M, Martinez Ubago JL, et al. Increased collagen type I synthesis in patients with heart failure of hypertensive origin: relation to myocardial fibrosis. Circulation. 2004;110(10):1263–8. [DOI] [PubMed] [Google Scholar]
- 5.Duffield JS. Cellular and molecular mechanisms in kidney fibrosis. J Clin Invest. 2014;124(6):2299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lacomb R, Nadiarnykh O, Campagnola PJ. Quantitative second harmonic generation imaging of the diseased state osteogenesis imperfecta: experiment and simulation. Biophys J. 2008;94(11):4504–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nadiarnykh O, Plotnikov S, Mohler WA, Kalajzic I, Redford-Badwal D, Campagnola PJ. Second harmonic generation imaging microscopy studies of osteogenesis imperfecta. J Biomed Opt. 2007;12(5):051805. [DOI] [PubMed] [Google Scholar]
- 8.Liu SB, Ikenaga N, Peng ZW, Sverdlov DY, Greenstein A, Smith V, et al. Lysyl oxidase activity contributes to collagen stabilization during liver fibrosis progression and limits spontaneous fibrosis reversal in mice. FASEB J. 2016;30(4):1599–609. [DOI] [PubMed] [Google Scholar]
- 9.Wollensak G, Wilsch M, Spoerl E, Seiler T. Collagen fiber diameter in the rabbit cornea after collagen crosslinking by riboflavin/UVA. Cornea. 2004;23(5):503–7. [DOI] [PubMed] [Google Scholar]
- 10.Sahani R, Wallace CH, Jones BK, Blemker SS. Diaphragm muscle fibrosis involves changes in collagen organization with mechanical implications in Duchenne muscular dystrophy. J Appl Physiol (1985). 2022;132(3):653–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Da-Silva EA, Sampaio FJ, Dornas MC, Damiao R, Cardoso LE. Extracellular matrix changes in urethral stricture disease. J Urol. 2002;168(2):805–7. [PubMed] [Google Scholar]
- 12.Erickson BA, Tesdahl BA, Voznesensky MA, Breyer BN, Voelzke BB, Alsikafi NF, et al. Urethral lichen sclerosus under the microscope: a survey of academic pathologists. Can J Urol. 2018;25(3):9328–33. [PubMed] [Google Scholar]
- 13.Huang Y, de Boer WB, Adams LA, MacQuillan G, Rossi E, Rigby P, et al. Image analysis of liver collagen using sirius red is more accurate and correlates better with serum fibrosis markers than trichrome. Liver Int. 2013;33(8):1249–56. [DOI] [PubMed] [Google Scholar]
- 14.Wegner KA, Keikhosravi A, Eliceiri KW, Vezina CM. Fluorescence of Picrosirius Red Multiplexed With Immunohistochemistry for the Quantitative Assessment of Collagen in Tissue Sections. J Histochem Cytochem. 2017;65(8):479–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bauman TM, Nicholson TM, Abler LL, Eliceiri KW, Huang W, Vezina CM, et al. Characterization of fibrillar collagens and extracellular matrix of glandular benign prostatic hyperplasia nodules. PLoS One. 2014;9(10):e109102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rentchler EC, Gant KL, Drapkin R, Patankar M, P JC. Imaging Collagen Alterations in STICs and High Grade Ovarian Cancers in the Fallopian Tubes by Second Harmonic Generation Microscopy. Cancers (Basel). 2019;11(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calvaruso V, Burroughs AK, Standish R, Manousou P, Grillo F, Leandro G, et al. Computer-Assisted Image Analysis of Liver Collagen: Relationship to Ishak Scoring and Hepatic Venous Pressure Gradient. Hepatology. 2009;49(4):1236–44. [DOI] [PubMed] [Google Scholar]
- 18.Courtoy GE, Leclercq I, Froidure A, Schiano G, Morelle J, Devuyst O, et al. Digital Image Analysis of Picrosirius Red Staining: A Robust Method for Multi-Organ Fibrosis Quantification and Characterization. Biomolecules. 2020;10(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Almeida HL Jr., Bicca Ede B, Breunig Jde A, Rocha NM, Silva RM. Scanning electron microscopy of lichen sclerosus. An Bras Dermatol. 2013;88(2):247–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farrell AM, Dean D, Millard PR, Charnock FM, Wojnarowska F. Alterations in fibrillin as well as collagens I and III and elastin occur in vulval lichen sclerosus. J Eur Acad Dermatol. 2001;15(3):212–7. [DOI] [PubMed] [Google Scholar]
- 21.Godoy CA, Teodoro WR, Velosa AP, Garippo AL, Eher EM, Parra ER, et al. Unusual remodeling of the hyalinization band in vulval lichen sclerosus by type V collagen and ECM 1 protein. Clinics (Sao Paulo). 2015;70(5):356–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marren P, Dean D, Charnock M, Wojnarowska F. The basement membrane zone in lichen sclerosus: an immunohistochemical study. Br J Dermatol. 1997;136(4):508–14. [PubMed] [Google Scholar]
- 23.Oikarinen A, Sandberg M, Hurskainen T, Kinnunen T, Kallioinen M. Collagen biosynthesis in lichen sclerosus et atrophicus studied by biochemical and in situ hybridization techniques. Acta Derm Venereol Suppl (Stockh). 1991;162:3–12. [PubMed] [Google Scholar]
- 24.Panizzon R, Vuorio T, Bruckner-Tuderman L. Collagen biosynthesis and type I and type III procollagen mRNA in lichen sclerosus et atrophicus. Arch Dermatol Res. 1990;282(7):480–3. [DOI] [PubMed] [Google Scholar]
- 25.Jones MG, Andriotis OG, Roberts JJ, Lunn K, Tear VJ, Cao L, et al. Nanoscale dysregulation of collagen structure-function disrupts mechano-homeostasis and mediates pulmonary fibrosis. Elife. 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu F, Lagares D, Choi KM, Stopfer L, Marinkovic A, Vrbanac V, et al. Mechanosignaling through YAP and TAZ drives fibroblast activation and fibrosis. Am J Physiol-Lung C. 2015;308(4):L344–L57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parker MW, Rossi D, Peterson M, Smith K, Sikstrom K, White ES, et al. Fibrotic extracellular matrix activates a profibrotic positive feedback loop. J Clin Invest. 2014;124(4):1622–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsochatzis E, Bruno S, Isgro G, Hall A, Theocharidou E, Manousou P, et al. Collagen proportionate area is superior to other histological methods for sub-classifying cirrhosis and determining prognosis. J Hepatol. 2014;60(5):948–54. [DOI] [PubMed] [Google Scholar]


