Abstract
Purpose:
To investigate the relationship of anti-VEGF treatment discontinuation with baseline factors and outcomes in eyes treated initially with aflibercept or bevacizumab for macular edema from central or hemiretinal vein occlusion.
Design:
Long-term follow-up after a randomized clinical trial from 64 US centers.
Methods:
Analysis included 150 SCORE2 Month 60 completers classified into three groups: discontinued treatment early, treated intermittently, and treated continuously. Outcomes included visual acuity (VA) and central subfield thickness (CST).
Results:
Those who discontinued treatment early were younger (60.9 years, versus 66.7 and 70.5 for the treated intermittently and treated continuously groups; P=0.001), and 17.4% were black, compared to 19.5% and 4.7% for the treated intermittently and treated continuously groups (P=0.006). At Month 60, the discontinued treatment early group had a higher proportion with complete resolution of macular edema (69.6%) than those treated intermittently (15.0%) and treated continuously (15.7%) (P<0.001). Least squares means analyses over follow-up demonstrated that the discontinued treatment early group had lower mean CST (257μm) than the treated intermittently (CST=303μm, P=0.02) and treated continuously (CST=300μm, P=0.01) groups.
Conclusions:
Compared to those treated continuously, those who discontinued treatment early were younger and more likely black. The discontinued treatment early group had a higher proportion with complete resolution of macular edema at Month 60, and lower mean CST over follow-up, but not better VA, than the treated continuously and treated intermittently groups. Results support the need for continued monitoring and individualized treatment for patients treated with anti-VEGF for macular edema from central or hemiretinal vein occlusion.
Trial Registration:
Clinical trial identifier at clinicaltrials.gov: NCT01969708
Graphical Abstract
This analysis investigates the relationship of anti-VEGF treatment discontinuation with baseline factors and outcomes in eyes with macular edema from central retinal or hemiretinal vein occlusion. Those who were younger or black were more likely to discontinue treatment early. The discontinued treatment early group had a lower mean central retinal thickness and a significantly higher proportion of participants with complete resolution of macular edema at Month 60 compared to those treated intermittently or continuously.
Introduction
The primary outcome results of the Study of COmparative Treatments for REtinal Vein Occlusion 2 (SCORE2) demonstrated that bevacizumab, a frequently used off-label anti-vascular endothelial growth factor (anti-VEGF) treatment, is noninferior to aflibercept, an anti-VEGF treatment approved by the United States Food and Drug Administration, with respect to visual acuity letter score (VALS) at 6 months in eyes with macular edema associated with central retinal vein occlusion (CRVO) or hemiretinal vein occlusion (HRVO).1 Further, at 24 months and 60 months post-randomization (12 months and 48 months, respectively, after cessation of the SCORE2 protocol-defined treatment schedule), participants initially randomized to aflibercept and those initially randomized to bevacizumab had similar VALS and central retinal thickness outcomes.2,3 SCORE2 demonstrated that VALS improved substantially when patients were treated per protocol through Month 12, with lessened improvement after Month 12, when treatment was switched to investigator discretion and fewer treatments were received, although VALS remained markedly improved over baseline through Month 60.2,3 Although most participants received treatment during each of the 5 study years, there was a wide range in the number of treatments participants received per year after Month 12, with 34% of participants receiving no treatment in year 5.3 The current study investigates, among participants in SCORE2, the relationship of treatment discontinuation with (a) baseline factors and (b) longitudinal VALS and central subfield thickness (CST) on optical coherence tomography.
Methods
SCORE2 included a prospective long-term observational cohort study that followed a randomized clinical trial. The study adhered to the tenets of the Declaration of Helsinki4 and is registered on http://www.clinicaltrials.gov (identifier: NCT01969708). The study was approved by either a site-specific or a centralized institutional review board (Advarra, Columbia, Maryland), and written informed consent was obtained from all participants.
SCORE2 methods have been described in detail.5 Between September 17, 2014 and November 18, 2015, a total of 362 patients (305 with CRVO and 57 with HRVO) were randomly assigned to receive intravitreal injection of bevacizumab (1.25 mg) or aflibercept (2.0 mg) at randomization and every 4 weeks through Month 5. The primary outcome was change from baseline in best-corrected electronic Early Treatment Diabetic Retinopathy Study (e-ETDRS) VALS at Month 6, with a non-inferiority margin of 5.1,5 Following assessment of the primary outcome at Month 6, participants originally assigned to aflibercept who met the protocol-defined criteria for a good response were re-randomized to either continuing aflibercept every 4 weeks (n=79) versus changing to a treat and extend (TAE) regimen (n=80); participants originally assigned to aflibercept who met the protocol-defined criteria for a poor or marginal response at 6 months (n=15) were to receive a dexamethasone implant. Participants originally assigned to bevacizumab who met the protocol-defined criteria for a good response were re-randomized to either continuing bevacizumab every 4 weeks (n=67) versus changing to a TAE regimen (n=67); participants originally assigned to bevacizumab who met the protocol-defined criteria for a poor or marginal response at 6 months (n=39) were to receive aflibercept. SCORE2 participants’ last visit as part of the SCORE2 protocol-defined treatment schedule was at Month 12.
After Month 12, there was no protocol-defined treatment schedule. Rather, physicians could treat as they deemed necessary, using any commercially available drug (including non-study drug or no drug) based on their typical practice and on any schedule, and patients were followed at visits through Month 60 as part of the SCORE2 Long-term Follow-up (SCORE2 LTF). Study data included all interventions administered to the study eye for treatment of macular edema secondary to CRVO or HRVO (including injections given at non-study offices, provided they were documented in the medical record). At Months 0, 6, 12, 24, 36, 48, and 60, data were collected on best-corrected electronic Early Treatment Diabetic Retinopathy Study (E-ETDRS) VALS, CST assessed by spectral domain optical coherence tomography (SD-OCT), and eye examinations. SD-OCT images were sent to the Reading Center at the University of Wisconsin-Madison for grading. At each annual in-person visit with the participant, and via telephone call with the site clinical coordinator at Months 18, 30, 42, and 54, there was a medical record review of new ocular conditions, procedures, and other new health conditions occurring since the preceding annual visit. Data for these analyses were frozen on May 6, 2021.
Statistical Analyses
Statistical analyses were limited to the 150 SCORE2 participants who completed the Month 60 visits, referred to as Month 60 completers. To facilitate statistical analyses, operational definitions were created using patterns of treatment for macular edema over 6 periods of SCORE2 follow-up based on calendar dates: Months 0 to 6, Months 7 to 12, Months 13 to 24, Months 25 to 36, Months 37 to 48, and Months 48 to 60. Three mutually exclusive treatment pattern groups were created based on treatment discontinuation status:
Discontinued treatment early: 23 SCORE2 completers who were treated at least once within Months 0 to 6 and at least once within Months 7 to 12, but who were never treated again between Months 12 and 60.
Treated continuously: 86 SCORE2 completers who were treated at least once in each of the 6 periods.
Treated intermittently: 41 SCORE2 completers not in the other 2 groups. Because all SCORE2 completers were treated at least once within Months 0 to 6 and at least once within Months 7 to 12, this final group consists of those treated in at least one, but not all, of the four annual periods after Month 12.
Analysis of variance and chi-square tests were used to assess whether the three treatment pattern groups differed in baseline characteristics, characteristics measured at Month 1 after response to first treatment, and characteristics at the Month 60 visit. Univariate logistic regression analyses were also performed to compare the discontinued treatment early and treated continuously groups, with respect to their association with baseline and Month 1 characteristics. In these logistic regression analyses, discontinued treatment early was coded as 1 and treated continuously was coded as 0. Multiple logistic regression analyses were also performed to identify the strongest predictors. Least square means were estimated from longitudinal mixed model regressions for the VALS and CST post-baseline outcomes on the independent values of baseline value (VALS or CST), visit month (categorical), the 3 groups defined by treatment patterns, and the visit month-by-group interaction. The longitudinal mixed models assumed the covariance between time points t and s of a given participant was σ2ρ|t−s|. The least square means with accompanying pairwise-difference p-values help assess whether these outcomes differ among the 3 treatment pattern groups. All results should be considered post-hoc, and no controlling for multiple testing was performed due to the exploratory nature of the analysis. All analyses were performed in SAS 9.4.
Results
Baseline characteristics were compared across the three subsets of Month 60 completers defined by their treatment patterns (Table 1). Those who discontinued treatment early were younger at baseline (mean age of 60.9 years, versus 66.7 and 70.5 years for the treated intermittently and treated continuously groups, respectively; P=0.001) for the difference across all 3 groups). Race also differed among the 3 groups of Month 60 completers: 17% of the 23 who discontinued treatment early and 20% of the 41 treated intermittently were black, while only 5% of the 86 who were treated continuously were black (P=0.006). When examining pairwise differences between the treatment continuously and discontinued early groups, those who discontinued treatment early were more likely to have diabetes (48%) compared to those treated continuously (24%; P<0.001). Further, those who discontinued treatment early had a shorter mean duration of macular edema at baseline (mean of 1.1 months) compared to those treated continuously (9.1 months;P=0.03). Variables related to treatment response at Month 1 (VALS, CST, complete resolution of macular edema) did not differ among the 3 groups. Lastly, when examining treatment response at Month 60, the last follow-up visit, VALS was highest and the proportion of participants with complete resolution of macular edema was highest among those who discontinued treatment early (mean VALS of 73.1 letters, 69.6% with complete resolution of macular edema), and lowest among those treated intermittently (mean VALS of 57.4 letters, 15% with complete resolution of macular edema); those treated continuously had values in between the other two groups (mean VALS of 65.7 letters, 15.7% with complete resolution of macular edema) (P=0.03 and P<0.001 for VALS and complete resolution of macular edema, respectively). Lastly, at Month 60, the mean CST was 209μm among those who discontinued treatment early compared to 275μm among those treated continuously (P<0.001 for the pairwise comparison).
Table 1.
Comparison Between Treatment Pattern Groups with Respect To Baseline and Post-baseline Variables of Interest
| Treated Continuously N= 86 | Discontinued Treatment Early N=23 | Treated Intermittently N=41 | P-Value - Difference among 3 groups* | P-Value — Treated Continuously vs Discontinued treatment early* | |
|---|---|---|---|---|---|
| Baseline Characteristics | |||||
| Age, Years - Mean (SD) | 70.5 (10.0) | 60.9 (13.3) | 66.7 (12.1) | 0.001 | <0.001 |
| Female, N (%) | 39 (45.3%) | 8 (34.8%) | 19 (46.3%) | 0.62 | 0.36 |
| Other | 5 (5.8%) | 5 (21.7%) | 4 (9.8%) | ||
| Hispanic ethnicity | 5 (5.8%) | 3 (13.0%) | 7 (17.1%) | 0.12 | 0.24 |
| CRVO disease type | 76 (88.4%) | 19 (82.6%) | 32 (78.0%) | 0.31 | 0.46 |
| Mean disease duration (months), Mean (SD) | 9.1 (17.7) | 1.1 (2.2) | 6.4 (11.9) | 0.07 | <0.001 |
| w/ Diabetes | 21 (24.4%) | 11 (47.8%) | 13 (31.7%) | 0.09 | 0.03 |
| w/ Hypertensive | 62 (72.1%) | 17 (73.9%) | 31 (75.6%) | 0.91 | 0.86 |
| Aflibercept assignment | 40 (46.5%) | 11 (47.8%) | 22 (53.7%) | 0.75 | 0.91 |
| Prior anti-VEGF treatment | 32 (37.2%) | 4 (17.4%) | 13 (31.7%) | 0.20 | 0.073 |
| Visual Acuity Letter Score, Mean (SD) | 52.6 (14.7) | 51.0 (15.4) | 48.2 (14.7) | 0.31 | 0.66 |
| Central Subfield thickness, μm – Mean (SD) | 658 (232) | 705 (268) | 682 (214) | 0.67 | 0.42 |
| Month 01 Values | |||||
| 10 or more | 50 (58.1%) | 17 (73.9%) | 28 (70.0%) | ||
| CST < 300 μm at M01 | 62 (73.8%)** | 17 (73.9%) | 24 (61.5%)*** | 0.35 | 0.99 |
| Complete macular resolution at M01 | 24 (27.9%) | 7 (30.4%) | 7 (17.9%)*** | 0.42 | 0.81 |
| Month 60 Values | |||||
| Visual Acuity Letter Score, Mean (SD) | 65.7 (21.3) | 73.1 (24.4) | 57.4 (26.7) | 0.03 | 0.16 |
| Central Subfield thickness, μm – Mean (SD) | 275 (141) | 209 (39) | 262 (101) | 0.07 | <0.001 |
| Complete macular resolution at M60 | 13(15.7%)! | 16(69.6%) | 6(15.0%)!! | <0.001 | <.0001 |
P-values derived from ANOVA/t-test for continuous measures and Chi-Square tests for categorical.
Denominator is 84.
Denominator is 39.
denominator is 83.
denominator is 40.
The univariate logistic regression analyses identifying characteristics among Month 60 completers that are associated with discontinued treatment early compared to those treated continuously (Table 2) agreed roughly with the group-specific pairwise tests of Table 1 in that younger age (OR=0.93; P=0.0008), race (black versus white – OR=5.50, P=0.03; other versus white – OR=5.50, P=0.01); presence of diabetes (OR=2.84; P=0.03), and lower Month 60 CST (OR=0.28 per 100μm; P=0.02) were significantly associated with discontinued treatment early at the nominal level of 0.05 in both tables. In contrast, disease duration did not reach statistical significance in the logistic regression analyses. Because Month 60 CST is not useful for early prediction of future treatment discontinuation status, we included only age, race, disease duration, and diabetes as covariates in the multiple logistic regression analysis (Table 3). Of these predictors, only younger age (OR=0.94; P=0.01) and race for the black versus white comparison (OR=6.51; P=0.04) remained significantly associated with discontinued treatment early versus treated continuously in the multiple logistic regression analysis. To further illustrate the dependence of the relationship of treatment discontinuation status upon age, 23% of completers (N=17/23) who were younger than the median age of 68 years discontinued treatment early, while only 8% (N=6/23) of those older than 68 years discontinued treatment early.
Table 2:
Univariate Logistic Regression Analyses Identifying Characteristics Associated with Discontinued Treatment Early Compared to Those Treated Continuously
| Odds Ratio* | Lower 95% Confidence Limit | Upper 95% Confidence Limit | P-Value | |
|---|---|---|---|---|
| Baseline Variable | ||||
| Age, Years | 0.93 | 0.89 | 0.97 | 0.0008 |
| Female vs Male | 0.64 | 0.25 | 1.67 | 0.37 |
| Other vs White | 5.50 | 1.41 | 21.52 | 0.01 |
| Hispanic ethnicity vs non-hispanic | 2.43 | 0.54 | 11.03 | 0.25 |
| CRVO disease type | 1.60 | 0.45 | 5.66 | 0.47 |
| Disease duration (months) | 0.88 | 0.76 | 1.02 | 0.09 |
| w/ Diabetes vs not | 2.84 | 1.09 | 7.37 | 0.03 |
| w/ Hypertensive vs not | 1.10 | 0.39 | 3.11 | 0.86 |
| Aflibercept assignment vs bevacizumab | 1.05 | 0.42 | 2.65 | 0.91 |
| Prior anti-VEGF treatment vs not | 0.36 | 0.11 | 1.14 | 0.08 |
| Visual Acuity Letter Score | 0.99 | 0.96 | 1.02 | 0.65 |
| Central Subfield thickness, per 100 μm | 1.08 | 0.90 | 1.31 | 0.42 |
| M01 Variables | ||||
| Delta VALS at M01: 5 – 9 vs < 5 letters | 1.00 | 0.18 | 5.6 | 1.00 |
| Delta VALS at M01: > = 10 vs < 5 letters | 2.04 | 0.53 | 7.79 | 0.29 |
| CST<300 at M01 vs not | 1.01 | 0.35 | 2.87 | 0.99 |
| Complete macular resolution at M01 vs N | 1.13 | 0.41 | 3.09 | 0.81 |
| M60 Variables | ||||
| Visual Acuity Letter Score | 1.02 | 0.99 | 1.05 | 0.16 |
| Central Subfield thickness, per 100 μm | 0.28 | 0.09 | 0.82 | 0.02 |
| Complete macular resolution at M60 vs N | 12.31 | 4.23 | 35.79 | <.0001 |
Odds Ratio defined as the ratio of the odds of discontinuing treatment early given the presence of the characteristic and the odds of discontinuing treatment early, given the absence of the characteristic. For continuous characteristics, the odds ratio is the odds of treatment discontinuation early for every unit increase with the continuous variable.
Table 3:
Multivariate Logistic Regression Analyses Identifying Baseline and M01 Characteristics Associated with Discontinued Treatment Early Compared to Those Treated Continuously
| Variable | Odds Ratio | Lower 95% Confidence Limit | Lower 95% Confidence Limit | P-Value |
|---|---|---|---|---|
| Age (years) | 0.94 | 0.90 | 0.987 | 0.01 |
| Disease duration (months) | 0.91 | 0.80 | 1.04 | 0.18 |
| w/ Diabetes vs not | 1.56 | 0.52 | 4.71 | 0.43 |
| Race Black vs White | 6.15 | 1.12 | 33.74 | 0.04 |
| Race Other vs White | 2.62 | 0.58 | 11.93 | 0.21 |
The longitudinal mixed model analyses and accompanying least square means (Table 4) suggest that for VALS and change of VALS from baseline over time, there is little difference among the three groups based on treatment discontinuation status in VALS or in change of VALS from baseline. This is supported by Figure 1, with its extensive overlap in contemporaneous pointwise 95% confidence intervals. The longitudinal analysis also suggests that the discontinued treatment early group has a lower mean CST over follow-up (mean of 257μm) and a greater mean decrease from baseline CST (mean change of 443μm) compared with the treated continuously (P=0.01 for CST and P=0.02 for change from baseline in CST) and treated intermittently groups (P=0.02 for CST and P=0.02 for change from baseline in CST), which did not differ significantly from each other in CST outcomes over follow-up (treated continuously: mean=300μm and mean change=−397μm; treated intermittently: mean=303μm and mean change=−394μm). Figure 2 illustrates these findings for CST, which graphically shows lower mean and greater mean change from baseline in CST for the discontinued early group compared with the other two groups.
Table 4:
Longitudinal Analyses and Least Square Means and Pairwise Comparisons of 3 Groups Defined by Treatment Patterns for Visual Acuity and Central Subfield Thickness Post-baseline Outcomes
| Outcome | 1. Treated Continuously | 2. Discontinued Treatment Early | 3. Treated Intermittently | P-value 1 vs 2 | P-value 1 vs 3 | P-value 2 vs 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LS Mean | Lower 95% CI | Upper 95% CI | LS Mean | Lower 95% CI | Upper 95% CI | LS Mean | Lower 95% CL | Upper 95% CL | ||||
| VALS | 68.6 | 65.8 | 71.4 | 73.2 | 67.8 | 78.6 | 67.4 | 63.4 | 71.5 | 0.14 | 0.65 | 0.10 |
| Change from baseline in VALS | 18.6 | 15.6 | 21.5 | 23.4 | 17.7 | 29.1 | 17.2 | 13.0 | 21.5 | 0.14 | 0.61 | 0.09 |
| CST | 300 | 285 | 315 | 257 | 227 | 287 | 303 | 281 | 325 | 0.01 | 0.81 | 0.02 |
| Change from baseline in CST | −397 | −415 | −380 | −443 | −477 | −409 | −394 | −419 | −369 | 0.02 | 0.81 | 0.02 |
Abbreviations: LS=Least Square; CL=Confidence Limits; VALS-Visual Acuity Letter Score; CST=Central Subfield Thickness.
Figure 1:
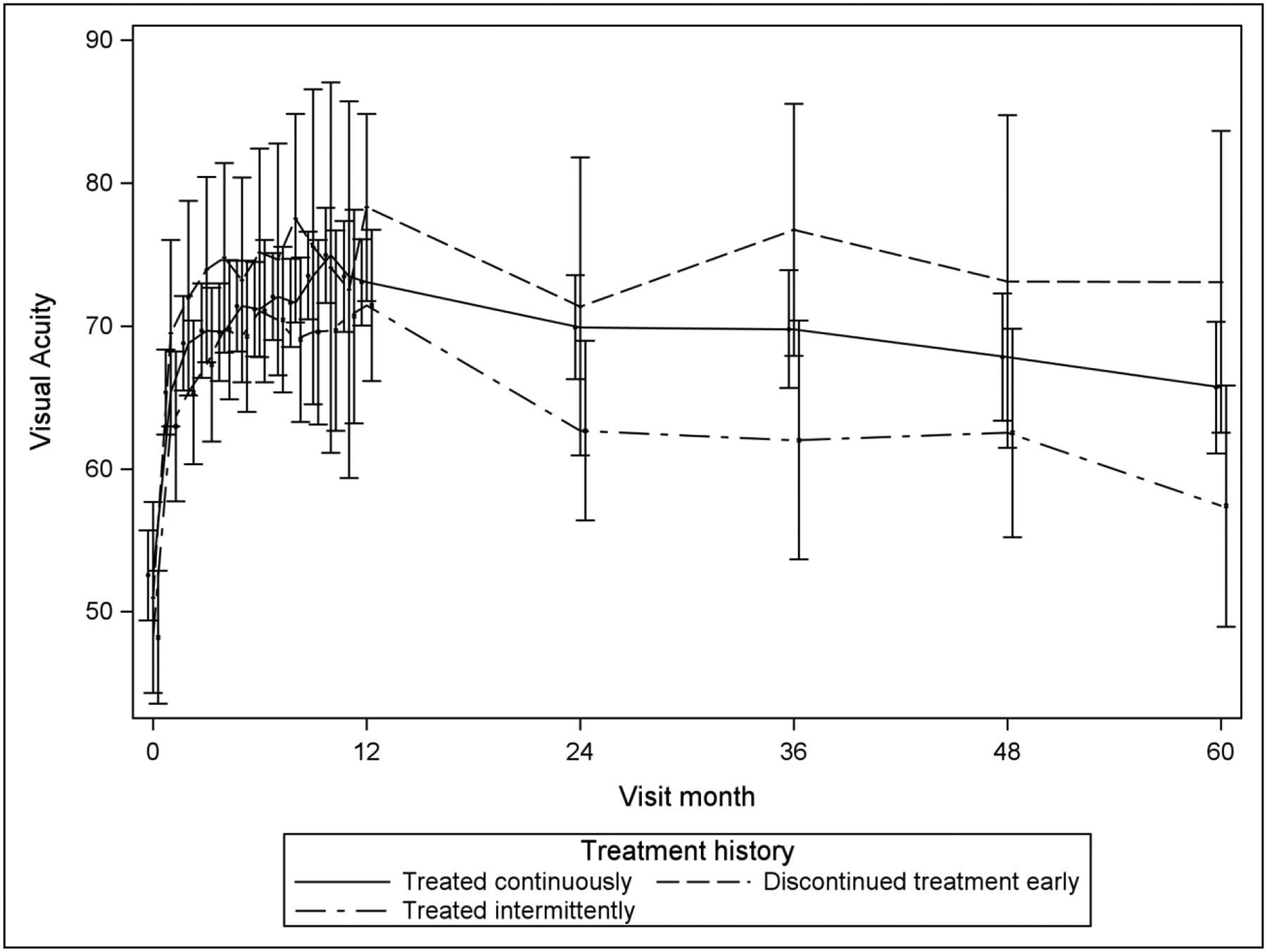
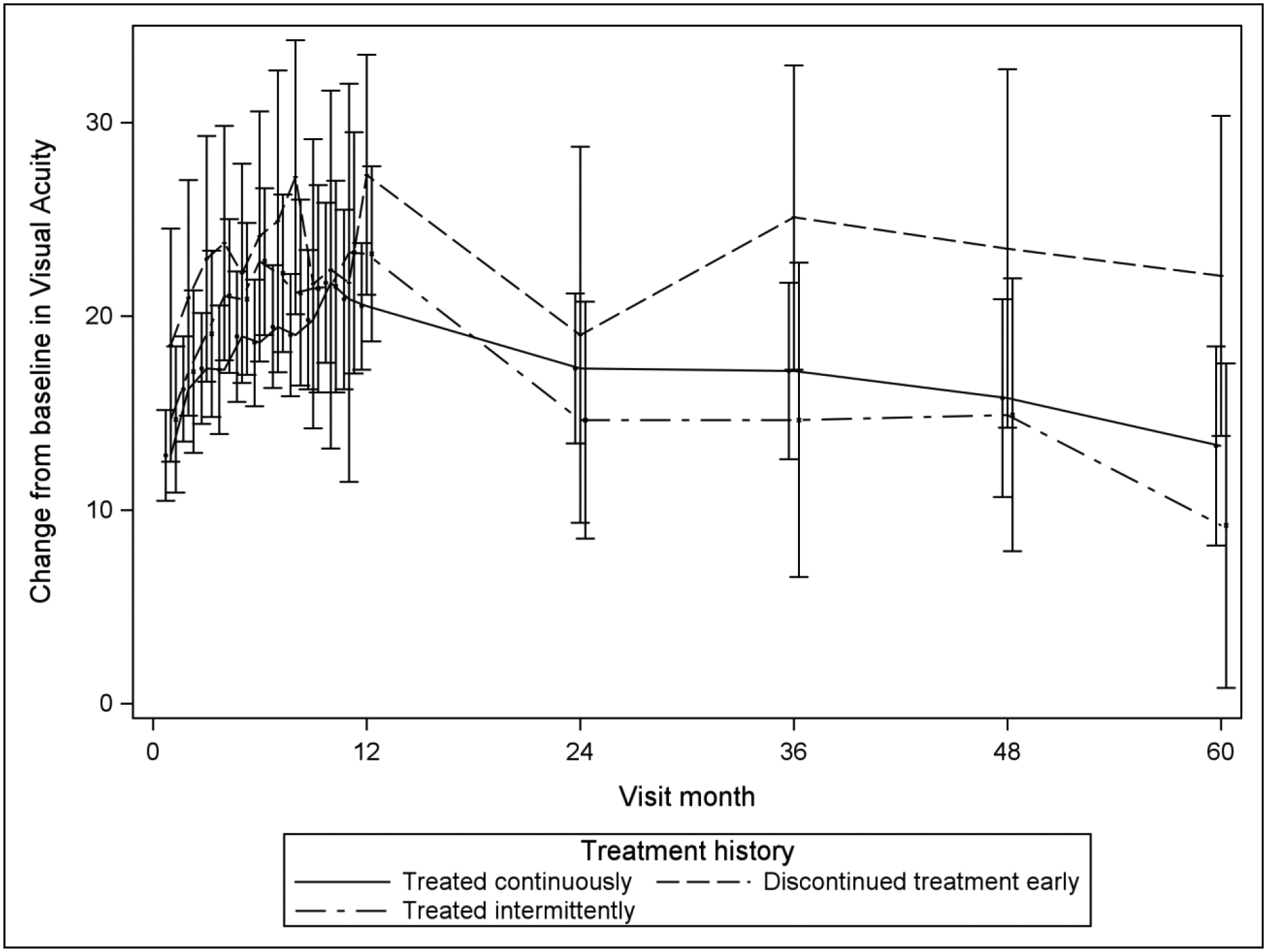
Mean Visual Acuity and Change from Baseline in Visual Acuity over Time, by Treatment Group Classification
Figure 2:
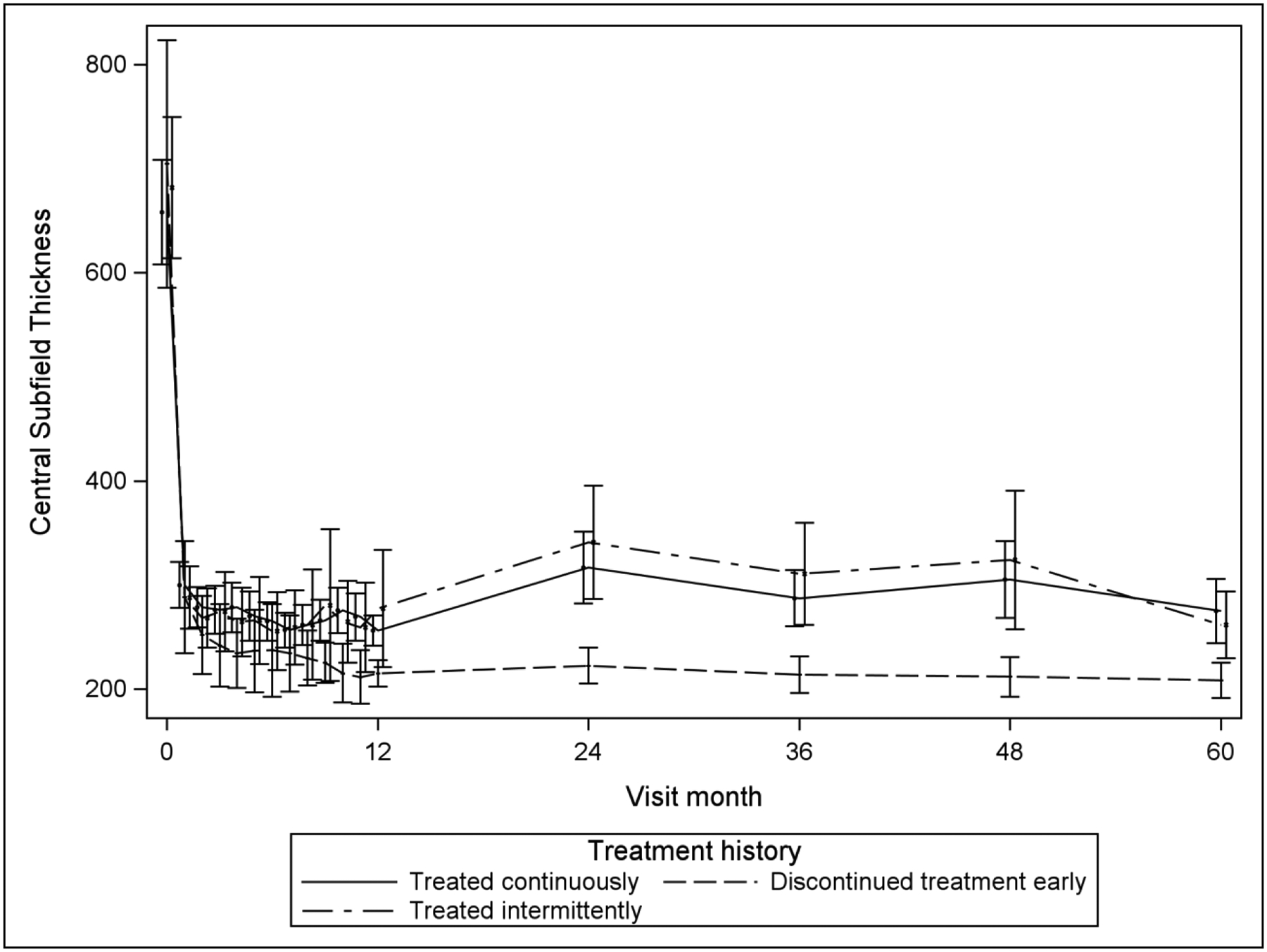
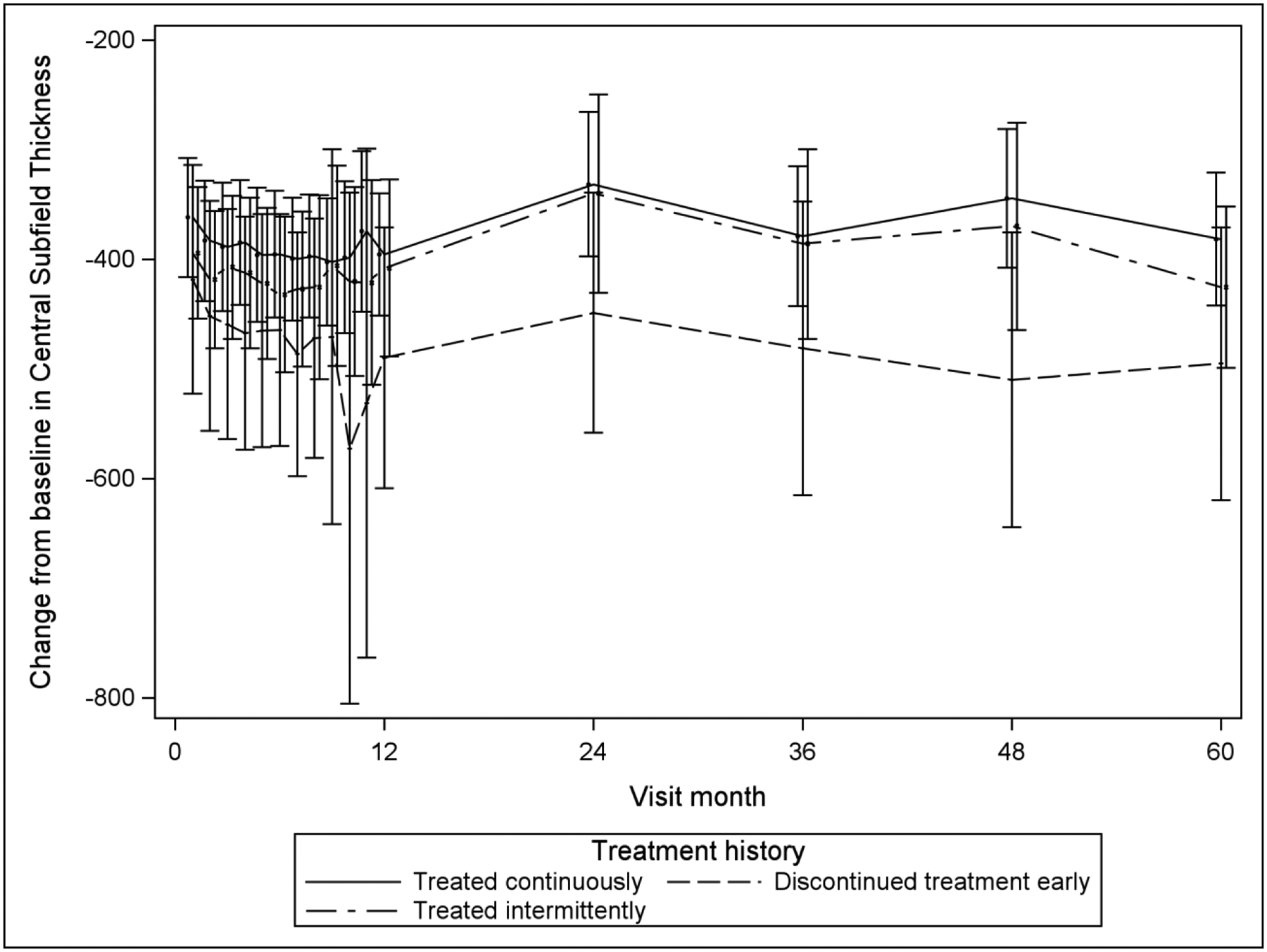
Mean CST and Mean Change from Baseline in CST over Time, by Treatment Group Classification
Discussion
Analysis of Month 60 outcomes of SCORE2 demonstrated that, among patients initially randomized to intravitreal aflibercept or bevacizumab for the treatment of macular edema associated with CRVO or HRVO, VALS remained markedly improved over baseline through Month 60, and that while most participants received treatment during each of the 5 study years, there was a wide range in the number of treatments participants received per year after Month 12, with about one-third of participants receiving no treatment in year 5.3 These findings led to the aims of the current paper, which are to characterize those participants who did not receive continued treatment and investigate whether their VALS and CST outcomes differed from those participants who received treatment throughout the 5 years of SCORE2 follow-up.
In both univariate and multivariate analyses, younger age was associated with treatment discontinuation. This may be because younger patients are more likely to improve with anti-VEGF therapy and, therefore, may be less likely to require continued treatment. This hypothesis is consistent with the finding in both SCORE2 and the Standard Care vs COrticosteroid for REtinal vein Occlusion (SCORE)-CRVO trial (which compared intravitreal triamcinolone with observation for macular edema secondary to CRVO) that younger age is a significant baseline factor predictive of better 6-month visual acuity outcomes.6,7 This may be due to an enhanced resilience of younger patients’ photoreceptors that may facilitate retinal recovery after an acute insult such as retinal vein occlusion compared with photoreceptors of older individuals. Among patients treated with grid photocoagulation for macular edema in the Central Vein Occlusion Study, visual acuity outcome tended to be better in younger patients, although the interaction between treatment effect and age was not statistically significant, possibly due to the limited sample size.8
Black race was also significantly associated with treatment discontinuation in both univariate and multivariate analysis. This may be because black patients are more likely to improve with anti-VEGF therapy and, therefore, may be less likely to require continued treatment. Alternatively, black patients may be more likely than white patients to discontinue treatment because of access to care issues.
Longitudinally, there is no difference among the status of treatment discontinuation with respect to VALS, but there is for CST, with treatment discontinuation significantly associated with a lower CST. Further, study participants with treatment discontinuation were significantly more likely than participants treated continuously or intermittently to have complete resolution of macular edema at Month 60. These findings are not surprising, since OCT evidence of macular edema is commonly considered by retina specialists when deciding whether or not to treat patients with CRVO or HRVO with anti-VEGF therapy.
To our knowledge, and based on a computerized search of the PubMed database, this is the first study to report factors associated with treatment discontinuation among patients treated for macular edema associated with retinal vein occlusion. A limitation of our analysis is that we study here only Month 60 completers, comprising less than half the SCORE2 study population. Other limitations include that we are unable to determine whether treatment discontinuation is indicative of an absence of a need for treatment, patient preference, or physician behavior. At Month 60 and longitudinally, the discontinued early group has significantly better CST and a higher proportion of participants with complete resolution of macular edema, but not significantly better VALS, than the treated continuously and treated intermittently groups, suggesting that treatment may be discontinued when no further structural improvement is needed. Finally, this analysis is exploratory and without adjustment for multiple testing, rather than being driven by a priori hypotheses. Study results support the need for continued monitoring and individualized treatment for patients treated with anti-VEGF therapy for macular edema due to CRVO or HRVO.
Acknowledgements
Funding:
Supported by the National Eye Institute (National Institutes of Health, Department of Health and Human Services) grants U10EY023529, U10EY023533, and U10EY023521. Support also provided in part by Regeneron, Inc and Allergan, Inc through donation of investigational drug. This work was supported in part by an unrestricted grant from Research to Prevent Blindness, Inc. to the University of Wisconsin Madison Department of Ophthalmology and Visual Sciences and to the Jules Stein Eye Institute and Doheny Eye Institute, Department of Ophthalmology at the University of California Los Angeles, CA.
Financial Disclosures:
Dr. Scott serves as Principal Investigator and Chair of SCORE2, which is funded by the National Eye Institute, and has served as a consultant for Regeneron (Tarrytown, NJ) and F. Hoffmann-La Roche AG (Basel, Switzerland) and on the Data and Safety Monitoring and Safety Review Committees of clinical trials sponsored by Novartis (Basel, Switzerland). Dr. Ip reports receiving consultant fees from Clearside, Boehringer Ingelheim, OccuRx, Genentech, Astellas, Allergan, Amgen, Outlook, Novartis, and Regeneron and research support from Astellas, Biogen, Lineage Cell Therapeutics, Novartis, and RegenexBio.
The SCORE2 Study Data Coordinating Center Principal Investigator, Paul VanVeldhuisen, PhD, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Scott IU, VanVeldhuisen PC, Ip MS, et al. , for the SCORE2 Investigator Group. Effect of bevacizumab vs aflibercept on visual acuity among patients with macular edema due to central retinal vein occlusion. The SCORE2 randomized clinical trial. JAMA. 2017;317(20):2072–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scott IU, Oden NL, VanVeldhuisen PC, et al. , for the SCORE2 Investigator Group. Month 24 outcomes after treatment initiation with anti-vascular endothelial growth factor therapy for macular edema due to central retinal or hemiretinal vein occlusion. SCORE2 report 10: a secondary analysis of the SCORE2 randomized clinical trial. JAMA Ophthalmol 2019;137(12):1389–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scott IU, VanVeldhuisen PC, Oden NL, Ip MS, Blodi BA, on behalf of the SCORE2 Investigator Group. AOS Thesis. Month 60 outcomes after treatment initiation with anti-VEGF therapy for macular edema due to central retinal or hemiretinal vein occlusion. SCORE2 Report 19. Am J Ophthalmol 2022;240:330–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194. [DOI] [PubMed] [Google Scholar]
- 5.Scott IU, VanVeldhuisen PC, Ip MS, et al. SCORE2 Report 2: Study Design and Baseline Characteristics. Ophthalmology. 2017;124(2):245–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scott IU, VanVeldhuisen PC, Ip MS, et al. Baseline factors associated with 6-month visual acuity and retinal thickness outcomes in patients with macular edema secondary to central retinal vein occlusion or hemiretinal vein occlusion: SCORE2 Study report 4. JAMA Ophthalmol 2017;135(6):639–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scott IU, VanVeldhuisen PC, Oden NL, et al. Baseline predictors of visual acuity and retinal thickness outcomes in patients with retinal vein occlusion. Standard Care versus Corticosteroid for Retinal Vein Occlusion Study report 10. Ophthalmology 2011;118:345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Central Vein Occlusion Study Group. Evaluation of grid pattern photocoagulation for macular edema in central vein occlusion: the Central Vein Occlusion Study Group M report. Ophthalmology 1995;102(10):1425–1433. [DOI] [PubMed] [Google Scholar]


