Abstract
Background:
Hemorrhage is the leading cause of preventable death after injury. Others have shown delays in massive transfusion cooler arrival increase mortality while prehospital blood product resuscitation can reduce mortality. Our objective was to evaluate if time to resuscitation initiation impacts mortality.
Methods:
We combined data from the PAMPer trial in which patients received prehospital plasma or standard care and the STAAMP trial in which patients received prehospital tranexamic acid (TXA) or placebo. We evaluated the time to early resuscitative intervention (TERI) as time from emergency medical services arrival to packed red blood cells (pRBC), plasma, or TXA initiation in the field or within 90-minutes of trauma center arrival. For patients not receiving an early resuscitative intervention, the TERI was calculated based on trauma center arrival as earliest opportunity to receive a resuscitative intervention, and were propensity matched to those that did to account for selection bias. Mixed-effects logistic regression assessed the association of 30d and 24hr mortality with TERI adjusting for confounders. We also evaluated a subgroup of only patients receiving an early resuscitative intervention as defined above.
Results:
Among the 1504 propensity matched patients, every 1-minute delay in TERI was associated with 2% increase in the odds of 30d mortality (aOR 1.020; 95%CI 1.006–1.033, p<0.01) and 1.5% increase in odds of 24h mortality (aOR 1.015; 95%CI 1.001–1.029, p=0.03). Among the 799 patients receiving an early resuscitative intervention, every 1-minute increase in TERI was associated with a 2% increase in the odds of 30-day mortality (aOR 1.021; 95%CI 1.005–1.038, p=0.01) and 24-hour mortality (aOR 1.023; 95%CI 1.005–1.042, p=0.01).
Conclusions:
TERI is associated with morality in trauma patients with hemorrhagic shock. Bleeding patients need resuscitation initiated early, whether at the trauma center in systems with short prehospital times or in the field when prehospital time is prolonged.
Level of Evidence:
Therapeutic, Level II
Keywords: Emergency Medical Services, Transfusion, Outcome, Blood, Tranexamic Acid
BACKGROUND
Hemorrhage is the leading cause of preventable death in civilian and military trauma.1, 2 The science of resuscitation has made great strides with the advent of damage control resuscitation with emphasis on balanced early blood product resuscitation, minimizing crystalloid, treatment of trauma induced coagulopathy, and early hemorrhage control. Traditionally, this management was only available to bleeding patients after arrival to the trauma center. However, time is critical in the hemorrhaging patient. Deaths from exsanguination occur within a few hours of injury, one third of which occur in the prehospital environment.3
Several studies support this and suggest blood product transfusion and adjuncts such as tranexamic acid (TXA) administered as early as possible in the prehospital setting improve survival.4–11 The Prehospital Air Medical Plasma (PAMPer) trial demonstrated a nearly 10% absolute risk reduction in 30-day mortality for patients receiving prehospital plasma resuscitation compared to standard care.11 However, the Control of Major Bleeding after Trauma (COMBAT) trial showed no difference in 28-day mortality for patients receiving prehospital plasma compared to crystalloid resuscitation.12 Most interestingly, a combined analysis of these two trials showed the mortality benefit for prehospital plasma was present only when prehospital transport times were greater than 20 minutes.13
From these data it appears that early administration of plasma for hemorrhagic shock is the key factor driving the survival benefit for these patients. Further, nearly all data supporting TXA in hemorrhagic shock after trauma shows that earlier administration is associated with improved outcomes, particularly when given within the first hour from injury.6, 9, 10 Taken together, access to early resuscitation techniques, whether at the trauma center with short transport times, or in the field when prehospital times are long, are critical to improved outcomes in hemorrhagic shock patients. However, a close evaluation of the time from injury to receipt of such interventions has not been studied.
Our objective was to evaluate whether the time to availability of resuscitative interventions is associated with mortality among injured patients at risk for hemorrhagic shock. We hypothesize increasing time to availability of packed red blood cells (pRBC), plasma, and/or TXA will be associated with increased mortality.
METHODS
Study Design and Population
We conducted a combined secondary analysis of the PAMPer and Study of Tranexamic Acid during Air and ground Medical Prehospital transport (STAAMP) trials. The details of both trials have been published previously.6, 11, 14, 15 The PAMPer trial was a pragmatic multicenter cluster-randomized trial that enrolled patients at risk for hemorrhagic shock during air medical transport. The intervention was randomized at the level of the air medical base, and patients received initial resuscitation with two units of thawed plasma or standard of care. Standard of care resuscitation included pRBC availability at 13 of 27 participating air medical bases. Inclusion criteria were patients with systolic blood pressure (SBP) of 70–90mmHg plus heart rate (HR)>108bpm, or isolated severe hypotension with SBP<70mmHg at any time prior to arrival at the trauma center.
The STAAMP trial was a pragmatic multicenter double-blind placebo-controlled trial that randomized patients at risk for hemorrhagic shock in the prehospital environment to receive a 1-gram TXA bolus or placebo. Patients receiving TXA in the field were further randomized to three in-hospital TXA dosing regimens. Standard care for these patients also included pRBC availability at 12 of 24 participating emergency medical services (EMS) sites. Inclusion criteria were patients with at least one episode of SBP<90mmHg or HR>110bpm within two hours of injury.
We focused on the early timing of available resuscitative interventions after injury, and therefore excluded patients who underwent interfacility transfer to the trauma center because timing and resuscitation data at the referring hospitals was unknown.
Missing Data
The proportion of missing data for analysis variables was <5% for all variables and given this minimal degree of missingness, we elected not to use imputation methods. We also assessed the distribution of missing data across the trials and did not find substantial differences to suggest systematic bias due to missing data by trial.
Time to Early Resuscitative Intervention
For this study we defined a time interval for each patient, termed the time to early resuscitative intervention (TERI). This time interval started at the time of EMS arrival for each patient and ended with the start of infusion of a resuscitation intervention. We defined resuscitation interventions as administration of pRBC, plasma, and/or TXA. To ensure we captured resuscitative interventions clinically relevant to hemorrhagic shock, we included administration of pRBC, plasma, and/or TXA in the prehospital setting or within the first 90 minutes (median in-hospital emergency department transfusion time) of arrival at the trauma center. For patients not receiving any early resuscitative intervention in the time-frame defined above, the TERI ended at the time of trauma center arrival. This time point was selected as it represented the earliest possible access to a resuscitative intervention for that patient. Figure 1 graphically illustrates the TERI calculation for example patients receiving a prehospital resuscitative intervention, an in-hospital resuscitative intervention, and for those not receiving a resuscitative intervention within 90-minutes of trauma center arrival.
Figure 1.
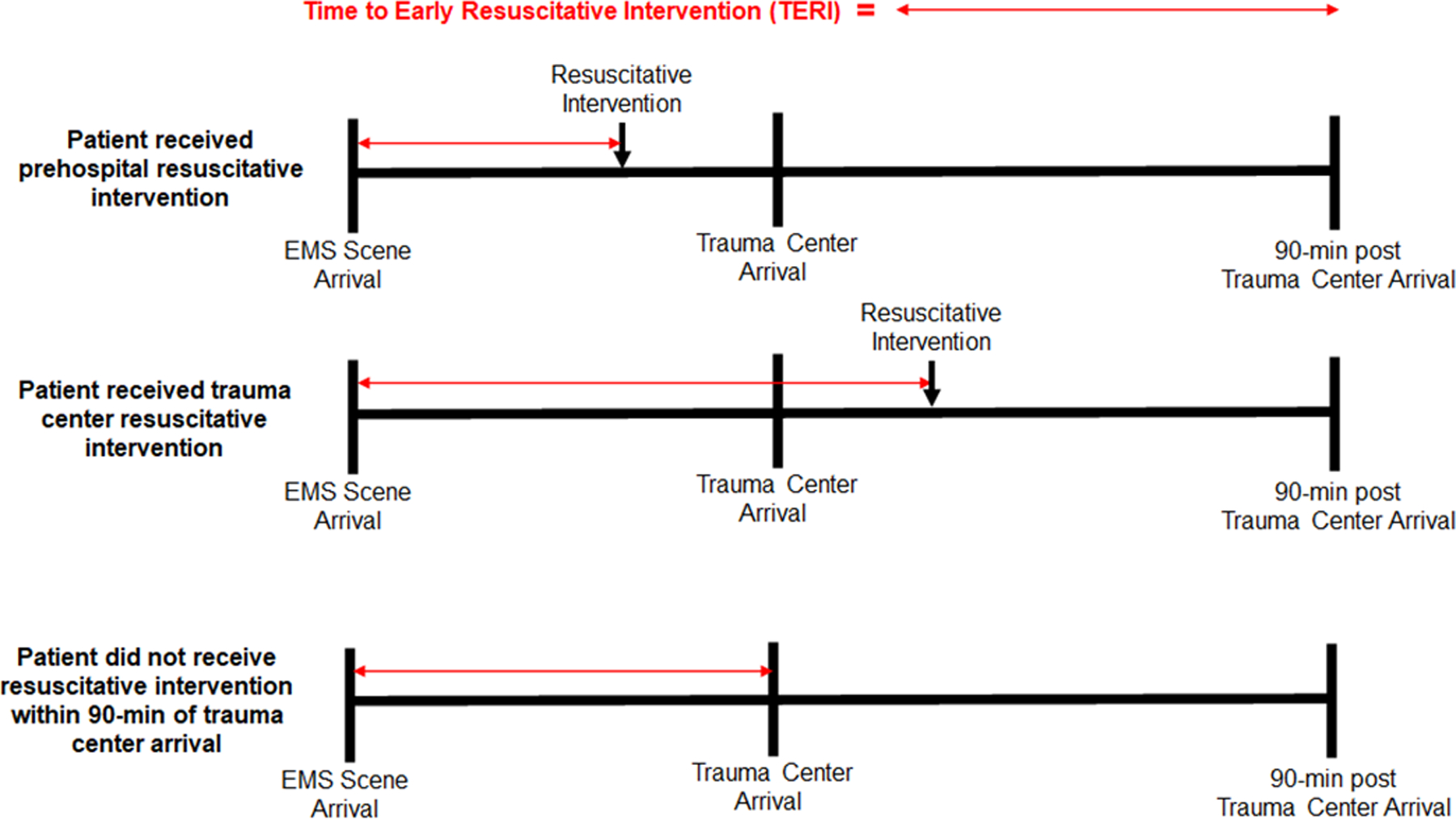
Conceptual diagram of Time to Early Resuscitative Intervention (TERI). calculation in two patients with identical total prehospital times. The time interval begins at the arrival of emergency medical services (EMS) clinicians. The interval ends at the initiation of an early resuscitative intervention either in the prehospital setting (top scenario) or within 90-minutes of trauma center arrival (middle scenario). For patients not receiving an early resuscitative intervention (bottom scenario), the time interval ends at trauma center arrival as the first potential opportunity to receive an early resuscitative intervention. Note this group of patients was only included if matched based on propensity score to receive an early resuscitative intervention.
Propensity Score Matching
We recognize patients that did not receive an early resuscitative intervention in the defined time-frame may have not had an indication for such and likely do not represent the hemorrhagic shock population of interest for the study. Thus, we performed propensity score matching based on the probability of receiving an early resuscitative intervention (prehospital or within 90-minutes of trauma center arrival) to address this selection bias. The propensity score was estimated using age, mechanism of injury, prehospital SBP and HR, prehospital time, prehospital crystalloid volume, prehospital intubation, and trial of enrollment. A 1:1 nearest neighbor matching algorithm was used with replacement and a caliper of 0.05. Replacement allows a control patient to be matched to more than one treated patient, resulting in fewer unique control patients than number of matched pairs. This was done to minimize the difference in propensity score between matched treated and control patients and less bias in our treatment effect estimates while matching most treated patients, given the number of control patients with low propensity scores that did not overlap any treated patients.16 Absolute standardized differences were used to assess the balance of patient characteristics between treatment groups. The standardized difference represents the difference between groups divided by the pooled standard deviation, making it insensitive to large samples sizes, and an absolute standardized difference greater than 0.1 represents significant non-overlap in the distributions of a given variable between groups.17
Statistical Analysis
Our primary outcome was 30-day mortality. Given the potential for in-hospital factors to obscure the impact of TERI on 30-day mortality, we also assessed a secondary outcome of 24-hour mortality, more proximate to the exposure of interest. To ensure 24-hour mortality did not solely drive any association of TERI with 30-day mortality, we repeated all 30-day mortality models excluding patients that died within 24-hours. We constructed a mixed-effects logistic regression model to determine the association between mortality and TERI, analyzed as a continuous variable. Fractional polynomial analysis was performed using first- and second-degree fractional polynomials for the TERI to determine the optimal form and account for the potential of non-linear effects. A two-level nested random effect was incorporated to account for the paired design after matching, which were then clustered within trauma centers. The post-matching models were adjusted for injury severity score (ISS), admission SBR and HR, in-hospital 24-hour crystalloid, 24-hour pRBC and plasma volumes, and head/chest/abdomen abbreviated injury scores (AIS), and the type/setting of early resuscitative intervention received to account for residual imbalance of variables not included in the propensity score. We also included propensity score variables not achieving good balance, if any, in the adjusted models after matching. Given our use of replacement, weight models were employed, with treated patients assigned a weight of 1 and control patients assigned a weight equal to the reciprocal of the number of times the unique control was matched to a different treated patient.18
Data analysis was conducted using Stata v17MP (StataCorp; College Station, TX). Continuous data are presented as median (interquartile range [IQR]). Continuous data were compared using Wilcoxon rank-sum tests, and categorical data compared using Chi-square. Adjusted odds ratios (aOR) and 95% confidence intervals (95%CI) were obtained from regression models. For the treatment effect of TERI, adjusted odds ratios are expressed as the odds per 1-minute increase in TERI. Model discrimination was assessed using the c-statistic, and calibration was graphically assessed using observed versus predicted calibration graphs. Variance inflation factors (VIF) were used to assess for covariate collinearity, and covariates with a VIF>10 were removed from final models. AIS scores were explored as continuous and binary (<3 versus ≥3) variables and models selected based on minimum Akaike and Bayesian Information Criteria. A two-tailed p value ≤0.05 was considered statistically significant. Our Institutional Review Board approved this study. Reporting of this study follows the STROBE guidelines for cohort studies.
Sensitivity Analyses
We performed several sensitivity analyses to assess the robustness of our results. First, we examined the subgroup only receiving an early resuscitative intervention (prehospital or within 90 minutes of trauma center arrival), as patients not receiving an early resuscitative intervention may still be substantively different, despite propensity score matching. We constructed similar mixed-effects logistic regression model to determine the association between mortality and TERI. As this analysis was not propensity matched, the models were adjusted for age, injury severity score (ISS), prehospital and admission SBR and HR, mechanism of injury, prehospital crystalloid volume, prehospital intubation, prehospital time, in-hospital 24-hour crystalloid, 24-hour pRBC and plasma volumes, head/chest/abdomen abbreviated injury scores (AIS), and the type/setting of early resuscitative intervention received. A two-level nested random effect was incorporated to account for clustering by EMS site, which were then clustered within trauma centers.
Second, although the TERI includes three different resuscitative interventions to maximize power, we recognize that different components may not have equivalent effects on mortality. Thus, we independently analyzed patients receiving each of the three resuscitative interventions (pRBC, plasma, TXA) using similar models described above among patients receiving an early resuscitative intervention.
Interaction Testing
We tested two interactions to evaluate whether the effect of TERI on mortality was modified by other variables. First, because prehospital time has been shown to be associated with mortality in severely injured patients and hemorrhagic shock is time-sensitive,19, 20 we tested the interaction between TERI and total prehospital time among patients receiving an early resuscitative intervention.
Second, because some of the resuscitative interventions were previously shown to have benefits in patients with traumatic brain injury (TBI),9, 21 we also tested the interaction between TERI and TBI. Models with significant interactions were stratified across the interaction variable. For prehospital time, we planned stratification of high/low time based on the median value. All interaction testing was performed among patients that received an early resuscitative intervention.
RESULTS
A total of 1187 eligible patients were included; 799 (56%) received an early resuscitative intervention (Fig. 2). Propensity score matching resulted in 752 (94.1%) of treatment patients (receiving early resuscitative intervention) matched to 234 unique control patients, giving 1504 matched pairs due to the matching with replacement design (Table 1). There was good balance among propensity score variables, with all absolute standardized differences <0.1 (eFig. 1). The distribution of TERI in minutes is shown in eFig. 2. When evaluating the possibility of significant non-linear effects of TERI on mortality using fractional polynomials, model comparison likelihood ratio testing did not reject a linear TERI model based on model deviance from a second-order fractional polynomial model (p=0.31). As such we proceeded with outcome models using TERI as a linear continuous variable. Model diagnostics are reported in the Supplementary Results (eResults, eFig. 3, and eFig. 4).
Figure 2.
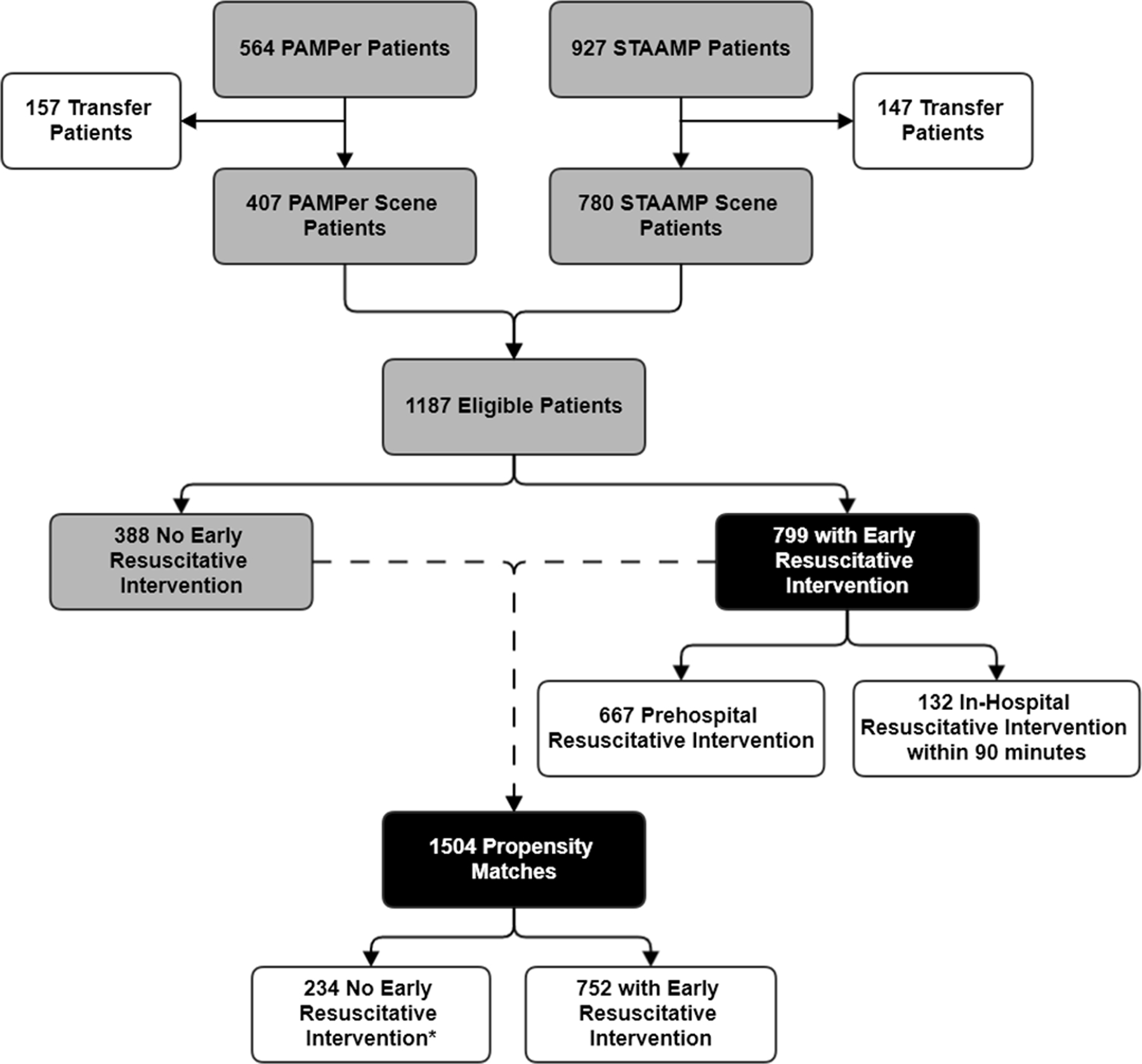
Patient flow diagram for enrollment from the Prehospital Air Medical Plasma (PAMPer) and Study of Tranexamic Acid in Air and ground medical Prehospital transport (STAAMP) trials.
Table 1.
Study population characteristics after propensity score matching.
| No Early Resuscitative Intervention | Early Resuscitative Intervention | Absolute Standardized Difference | |
|---|---|---|---|
| N | 752* | 752 | - |
| Age, median (IQR) | 38 (26, 57) | 40 (27, 56) | 0.036 |
| Gender, N (%) | 0.150 | ||
| Male | 584 (77.7%) | 535 (71.1%) | |
| Female | 168 (22.3%) | 217 (28.9%) | |
| Mechanism (% Blunt) | 655 (87.1%) | 651 (86.6%) | 0.016 |
| Prehospital time, median (IQR) | 39 (32, 49) | 39 (30, 49) | 0.009 |
| TERI, median (IQR) | 39 (32, 49) | 22 (14, 35) | 0.751 |
| Prehospital SBP, median (IQR) | 86 (69.5, 128) | 85 (69, 126.5) | 0.025 |
| Prehospital HR, median (IQR) | 116.5 (109.5, 128) | 118 (110, 128) | 0.065 |
| Prehospital crystalloid, median (IQR) | 500 (0, 1050) | 500 (0, 1100) | 0.001 |
| Prehospital intubation | 328 (43.6%) | 314 (41.8%) | 0.038 |
| Admission SBP, median (IQR) | 118 (100, 140) | 110 (87, 132) | 0.288 |
| Admission HR, median (IQR) | 106 (92, 121) | 107 (91, 121) | 0.053 |
| ISS, median (IQR) | 17 (9, 24) | 17 (9, 29) | 0.271 |
| Head AIS, median (IQR) | 2 (0, 3) | 1 (0, 3) | 0.080 |
| pRBC 24hr, median (IQR) | 0 (0, 0) | 2 (0, 6) | 0.686 |
| Plasma 24hr, median (IQR) | 0 (0, 0) | 0 (0, 2) | 0.456 |
| Crystalloid 24hr, median (IQR) | 3047 (1453, 4725) | 4060 (1968, 6610) | 0.318 |
| 24hr Mortality, N (%) | 83 (11.0%) | 92 (12.2%) | 0.037 |
| 30d Mortality, N (%) | 163 (21.7%) | 146 (19.4%) | 0.056 |
752 matches among 234 unique patients due to replacement matching technique that allows control patients to be matched more than once to different treatment patients
IQR, interquartile range; TERI, time to early resuscitative intervention; SBP, systolic blood pressure; HR, heart rate; RR, respiratory rate; GCS, Glasgow Coma Scale; ISS, injury severity score; AIS, abbreviate injury scale; pRBC, packed red blood cells,
In the propensity matched cohort, every 1-minute increase in TERI was associated with a 2% increase in the odds of 30-day mortality (aOR 1.020; 95%CI 1.006–1.033, p<0.01, Table 2) and a 1.5% increase in odds of 24-hour mortality (aOR 1.015; 95%CI 1.001–1.029, p=0.04 Table 3). Similar results for the association between TERI and odds of 30-day mortality were seen when excluding deaths within 24-hours (aOR 1.016; 95%CI 1.004–1.027, p=0.01)
Table 2.
Odds ratios and 95% confidence intervals for 30-day mortality from multivariable multi-level logistic regression in propensity matched cohort
| Odds Ratio | 95% CI | p-value | |
|---|---|---|---|
| TERI per 1-minute increase | 1.020 | 1.006 – 1.033 | 0.004 |
| ISS | 1.052 | 1.021 – 1.084 | <0.001 |
| 24hr Crystalloid Volume | 1.000 | 1.000 – 1.000 | <0.001 |
| Admission SBP | 0.981 | 0.974 – 0.987 | 0.000 |
| Admission HR | 0.989 | 0.982 – 0.997 | 0.006 |
| 24hr Plasma Volume | 0.960 | 0.881 – 1.046 | 0.351 |
| 24hr pRBC Volume | 1.168 | 1.091 – 1.249 | <0.001 |
| Head AIS | 1.295 | 1.091 – 1.537 | <0.001 |
| Chest AIS | 1.229 | 1.097 – 1.376 | 0.003 |
| Abdomen AIS | 1.130 | 1.007 – 1.269 | 0.038 |
| Lower Extremity AIS | 0.896 | 0.787 – 1.019 | 0.094 |
| Resuscitative Intervention Setting | |||
| No ERI | 1.000 | ||
| Prehospital | 0.365 | 0.227 – 0.576 | <0.001 |
| In-hospital | 0.419 | 0.192 – 0.915 | 0.002 |
OR, odds ratio; 95%CI, 95% confidence interval; TERI, time to early resuscitative intervention; ISS, injury severity score; SBP, systolic blood pressure; HR, heart rate; pRBC, packed red blood cells; AIS, abbreviated injury scale
Table 3.
Odds ratios and 95% confidence intervals for 24-hour mortality from multivariable multi-level logistic regression in propensity matched cohort
| Odds Ratio | 95% CI | p-value | |
|---|---|---|---|
| TERI per 1-minute increase | 1.015 | 1.001 – 1.029 | 0.039 |
| ISS | 1.058 | 1.030 – 1.086 | <0.001 |
| 24hr Crystalloid Volume | 0.999 | 0.999 – 1.000 | <0.001 |
| Admission SBP | 0.967 | 0.9547– 0.976 | <0.001 |
| Admission HR | 0.991 | 0.981 – 1.001 | 0.068 |
| 24hr Plasma Volume | 0.926 | 0.836 – 1.026 | 0.142 |
| 24hr pRBC Volume | 1.242 | 1.142 – 1.350 | <0.001 |
| Head AIS | 1.307 | 1.017 – 1.680 | 0.036 |
| Chest AIS | 1.258 | 1.083 – 1.460 | 0.003 |
| Abdomen AIS | 1.125 | 0.968 – 1.307 | 0.124 |
| Lower Extremity AIS | 0.775 | 0.651 – 0.924 | 0.005 |
| Resuscitative Intervention Setting | |||
| No ERI | 1.000 | ||
| Prehospital | 0.423 | 0.217 – 0.836 | 0.028 |
| In-hospital | 0.588 | 0.197 – 1.753 | 0.341 |
TERI, time to early resuscitative intervention; ISS, injury severity score; SBP, systolic blood pressure; HR, heart rate; pRBC, packed red blood cells; AIS, abbreviated injury scale
When evaluating only the 799 patients that underwent an early resuscitative intervention, the distribution of TERI in minutes is shown in eFig. 5. In this group, every 1-minute increase in TERI was associated with a 2% increase in the odds of 30-day mortality (aOR 1.021; 95%CI 1.005–1.038, p=0.01, eTable 1) and 24-hour mortality (aOR 1.023; 95%CI 1.005–1.042, p=0.01, eTable 2). Again, similar results for the association between TERI and odds of 30-day mortality were seen when excluding deaths within 24-hours (aOR 1.019; 95%CI 1.007–1.032, p<0.01). In sensitivity analysis of individual resuscitative intervention components among patients receiving an early resuscitative intervention, increasing TERI remained associated with increased odds of 30-day and 24-hour mortality (Table 4).
Table 4.
Association of Time to Early Resuscitative Intervention (TERI) on mortality among individual resuscitative intervention components among patients receiving an early resuscitative intervention.
| aOR* | 95%CI | p-value | |
|---|---|---|---|
| pRBC | |||
| 30-day mortality | 1.015 | 1.001–1.029 | 0.04 |
| 24-hour mortality | 1.047 | 1.001–1.086 | 0.02 |
| Plasma | |||
| 30-day mortality | 1.021 | 1.001–1.042 | 0.04 |
| 24-hour mortality | 1.044 | 1.007–1.083 | 0.02 |
| TXA † | |||
| 30-day mortality | 1.042 | 1.003–1.083 | 0.04 |
| 24-hour mortality | 1.073 | 1.013–1.137 | 0.02 |
adjusted odd per 1-minute increase in TERI
only available as prehospital intervention
aOR, adjusted odds ratio; 95%CI, 95% confidence interval; pRBC, packed red blood cells; TXA, tranexamic acid
Prehospital time did not significantly modify the effect of TERI on 30-day mortality as observed in our interaction testing (p=0.73). In the 30-day mortality model, total prehospital time was not significantly associated with mortality (aOR 0.988; 95%CI 0.958–1.019, p=0.44), yet TERI remained associated with 30-day mortality (aOR 1.033; 95%CI 1.002–1.067, p=0.04). Similarly, the interaction between total prehospital time and 24-hour mortality was not significant (p=0.20); however, increasing total prehospital was associated with an increase in the odds of 24-hour mortality (aOR 1.046; 1.001–1.093, p=0.04), as was TERI (aOR 1.055; 95%CI 1.001–1.102, p=0.02). The interaction between TERI and severe TBI was not significant for 30-day (p=0.63) or 24-hour mortality (p=0.78). Both increasing TERI and severe TBI were associated with increased 30-day and 24-hour mortality (p<0.05).
DISCUSSION
We demonstrate time to early resuscitative intervention is associated with both 30-day and 24-hour mortality among patients at risk for hemorrhagic shock. Total prehospital time did not modify the effect of TERI on 30-day or 24-hour mortality, suggesting these are distinct factors to be considered independently in the prehospital care of these patients. Both total prehospital time and TERI were associated with 24-hour mortality, yet TERI alone was associated with 30-day mortality, implying that TERI may be more important than total prehospital time for this population of injured patients. Severe TBI did not modify the effect of TERI on mortality, indicating TERI is an important factor for 24-hour and 30-day mortality in patients at risk for hemorrhage, both with and without TBI. A similar effect of TERI on mortality was observed for the individual components of early resuscitative interventions (pRBC, plasma, TXA), suggesting the effect observed is not driven by a singular resuscitative component.
Physiologic perturbations and resultant trauma induced coagulopathy occur within minutes of injury for patients at risk of hemorrhagic shock.22, 23 Left unchecked, this sets the patient on a very early and ominous trajectory. One-third of exsanguinating deaths after trauma occur in the prehospital setting, underscoring the need for early resuscitation initiation especially in rural settings.3 Given this, we are increasingly seeing the tenants of damage control necessarily pushed into the prehospital phase of trauma care. Of note we found that shorter times to early resuscitative intervention was associated with improved 30-day mortality even when excluding 24-hour deaths. Thus, the early introduction of blood products and/or TXA may mitigate the early immune and inflammatory effects of hemorrhagic shock including endotheliopathy that may lead to longer term improved outcomes.24–27
Indirect evidence of the benefits of earlier resuscitation in hemorrhagic shock after injury exists. In a secondary analysis of a randomized trial, reducing the time to massive transfusion protocol activation at the trauma center was associated with lower 24-hour and 30-day mortality, with a 5% increase for every 1-minute delay in the arrival of the first cooler of blood products.28 Early resuscitation initiation in the form of prehospital blood product transfusion has a growing body of evidence suggesting outcome benefits. Initially shown in military settings,29, 30 civilian data corroborated a reduction in mortality at 24-hours and 30-days as well as improvements in coagulation profiles.4, 5, 7 The PAMPer trial demonstrated a 10% absolute reduction in 30-day mortality for patients administered thawed plasma as compared to the standard care arm for patients with evidence of hemorrhagic shock.11 Importantly, separation in the survival curve occurred as early as three hours from injury in favor of plasma, underscoring the importance of early resuscitation. In a secondary analysis of PAMPer, patients receiving combined prehospital pRBC and plasma experienced the greatest mortality benefit after severe injury.7
TXA inhibits fibrinolysis and studies to date that demonstrate a benefit all indicate a time-dependence with early administration showing improved survival.6, 9, 10 CRASH-2 demonstrated a reduction in mortality from exsanguination with TXA administration within three hours, and the greatest effect was observed for those with receipt within one hour of injury.10 CRASH-3 evaluated TBI patients and also showed improved mortality with early TXA administration.31 Rowell and colleagues demonstrated prehospital TXA reduced mortality among patients with intra-cranial hemorrhage.9 Data contributing to the present study from the STAAMP trial demonstrated a mortality benefit for those that received TXA ≤ 1 hour, a 3-gram total dose, and those with severe hypotension.6 This benefit of early TXA intervention was corroborated by a secondary analysis of this RCT for patients at greatest risk of hemorrhage.32 Another analysis of STAAMP data reported potential synergy of pRBC and TXA administration associated with a reduction in mortality.33
The importance of time to resuscitation in these patients is particularly reinforced by a post-hoc analysis of PAMPer and COMBAT by Pusateri et al.13 They sought to examine the disparate outcomes between these trials with seemingly similar interventions (prehospital plasma first resuscitation), with PAMPer showing a mortality benefit while COMAT showed to effect on mortality. By combining trial participants, they first found the survival benefit of plasma was among patients with transport times >20 minutes but not patients with transport times ≤20 minutes. They then analyzed plasma and standard care patients separately, comparing mortality stratified by a 20-minute transport time. Transport time >20 minutes was associated with increased mortality in the standard care patients, but no difference was observed in those who received plasma. This suggests prehospital plasma may mitigate the increased mortality associated with prolonged prehospital time. Considered with our current results, we posit that early resuscitation is critical either at the trauma center with short prehospital times or in the field with prolonged prehospital times.
In the present study we quantify the association between the TERI and mortality and highlight the impact of delays in advanced resuscitation that occur on the order of minutes for patients with hemorrhagic shock. The practical implications of our findings are dependent on individual EMS and trauma system resources. To decrease the time to initiate resuscitative interventions, storing blood products and resuscitation adjuncts in the emergency department are a common strategy at many trauma centers, as well as well-defined massive transfusion protocols with documented activation criteria.28 Additionally, as noted above, making blood products and TXA available in the prehospital setting is a growing strategy for EMS systems. Blood products are not usually available from ground EMS agencies, although TXA is increasingly available. Urban systems with short transport times should minimize out of hospital time, rather than push prehospital resuscitative interventions, which may actually prolong prehospital time and come at the expense of fundamental interventions such as hemorrhage control. Our data should not be misconstrued; definitive hemorrhage control remains imperative and adding resuscitative interventions that prolong definitive management is not warranted. However, in rural areas, long transport times are inevitable, and waiting to initiate resuscitation with blood products or adjuncts is not ideal for bleeding patients. These systems more frequently utilize air medical transport for severely injured patients, which often have greater early resuscitation capabilities.
EMS clinicians should consider initiating these resuscitative interventions when transport times are long or when there are delays in prehospital time such as with prolonged extrication. They should also consider activating air medical transport to ensure earlier initiation of resuscitative interventions for the patient in these circumstances. At a system level, support should focus on increased availability of blood products and TXA in areas with prolonged transport times as robust logistics are necessary for a successful prehospital blood product program.34 Finally, the TERI represents an important conceptual construct for future research as well as a potential quality metric to consider in EMS and trauma systems for patients with hemorrhage.
We have important limitations to acknowledge. First, our data was derived from the combination of two trials, each with different hypotheses from each other and the present study. Additionally, the present results may not be generalizable to all severely injured patients given the predominance of blunt injuries and relatively long prehospital times in our study. However, we believe the transport times are important to consider in the treatment algorithms—delayed times should necessitate early resuscitative interventions while with anticipated short prehospital times, the focus should be on immediate transport to the trauma center. We do not have time of injury, thus differential time prior to EMS activation and arrival may impact our results. Our propensity matched analysis cannot control for unobserved factors and thus residual unobserved confounders may still impact those that did and did not receive an early resuscitative intervention differently. We do not have in-hospital TXA time; however, all patients getting in-hospital TXA would likely be getting at least one transfusion which would be captured. Further, TXA is unlikely to be the first advanced resuscitation intervention for bleeding patients in-hospital given current damage control resuscitation strategies. Finally, we explored identifying a threshold of times where availability of early resuscitative interventions made a difference in mortality; however, small sample sizes across the distribution of the early time period we focused on precluded us from drawing meaningful conclusions. This is an important next step for larger studies to investigate.
CONCLUSION
Increasing time to early resuscitative intervention is associated with 24-hour and 30-day mortality in patients at risk for hemorrhagic shock after injury. TERI may be more important in this population than total prehospital time for long term outcomes. Taken together, our results suggest that TERI is a crucial interval and bleeding patients need resuscitation initiated as early as possible, whether at the trauma center in systems with short prehospital transport times or in the field when prehospital time is prolonged. EMS clinicians should consider initiating available resuscitative interventions in the field when arrival at the trauma center may be delayed for patients with suspected hemorrhagic shock. Future efforts to evaluate TERI in the context of other life-saving interventions available in the field is needed. Individual trauma and EMS systems should weigh the costs and logistics with the potential benefit for deployment of emergency department and prehospital blood products and TXA to reduce the TERI, particularly in rural communities in order to optimize outcomes for patients in hemorrhagic shock after injury.
Supplementary Material
SDC 2. STROBE Checklist.
SDC 1. Supplemental Results, Supplemental Tables, and Supplemental Figures.
Funding:
The original trials were funded by grants W81XWH-12-2-0023 and W81XWH 13-2-0080 from the US Army Medical Research and Material Command. No funding or support was directly received to perform the current study.
Footnotes
Conflicts: There are no conflicts of interest for the current study.
This paper was presented as a podium presentation at the American Association for the Surgery of Trauma’s Eighty-first Annual Meeting in Chicago, IL, September 21st – 24th, 2022.
Contributor Information
Andrew-Paul Deeb, Division of Trauma and General Surgery, Department of Surgery, University of Pittsburgh, Pittsburgh, Pennsylvania, Pittsburgh, Pennsylvania.
Francis X. Guyette, Department of Emergency Medicine, University of Pittsburgh, Pittsburgh, Pennsylvania.
Brian J. Daley, Department of Surgery, University of Tennessee Health Science Center, Knoxville, Tennessee.
Richard S. Miller, Department of Surgery, John Peter Smith Health Network, Fort Worth, Texas.
Brian G. Harbrecht, Department of Surgery, University of Louisville, Louisville, Kentucky.
Jeffrey A. Claridge, Department of Surgery, MetroHealth Medical Center/Case Western Reserve University, Cleveland, Ohio.
Herb A. Phelan, Department of Surgery, Louisiana State University Health Sciences Center – New Orleans, New Orleans, LA.
Brian J. Eastridge, Department of Surgery, University of Texas Health San Antonio, San Antonio, TX.
Bellal Joseph, Department of Surgery, University of Arizona, Tucson, AZ.
Raminder Nirula, Department of Surgery, University of Utah, Salt Lake City, UT.
Gary A. Vercruysse, Department of Surgery, University of Arizona, Tucson, AZ.
Jason L. Sperry, Division of Trauma and General Surgery, Department of Surgery, University of Pittsburgh, Pittsburgh, Pennsylvania.
Joshua B. Brown, Division of Trauma and General Surgery, Department of Surgery, University of Pittsburgh, Pittsburgh, Pennsylvania, Pittsburgh, Pennsylvania.
REFERENCES
- 1.Davis JS, Satahoo SS, Butler FK, Dermer H, Naranjo D, Julien K, et al. An analysis of prehospital deaths: Who can we save? J Trauma Acute Care Surg 2014;77:213–218. [DOI] [PubMed] [Google Scholar]
- 2.Eastridge BJ, Hardin M, Cantrell J, Oetjen-Gerdes L, Zubko T, Mallak C, et al. Died of wounds on the battlefield: causation and implications for improving combat casualty care. J Trauma 2011;71:S4–8. [DOI] [PubMed] [Google Scholar]
- 3.Drake SA, Holcomb JB, Yang Y, Thetford C, Myers L, Brock M, et al. Establishing a Regional Trauma Preventable/Potentially Preventable Death Rate. Ann Surg 2018. [DOI] [PubMed] [Google Scholar]
- 4.Brown JB, Cohen MJ, Minei JP, Maier RV, West MA, Billiar TR, et al. Pretrauma Center Red Blood Cell Transfusion Is Associated With Reduced Mortality and Coagulopathy in Severely Injured Patients With Blunt Trauma. Ann Surg 2014;261:997–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown JB, Sperry JL, Fombona A, Billiar TR, Peitzman AB, Guyette FX. Pre-Trauma Center Red Blood Cell Transfusion Is Associated with Improved Early Outcomes in Air Medical Trauma Patients. J Am Coll Surg 2015:797–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guyette FX, Brown JB, Zenati MS, Early-Young BJ, Adams PW, Eastridge BJ, et al. Tranexamic Acid During Prehospital Transport in Patients at Risk for Hemorrhage After Injury: A Double-blind, Placebo-Controlled, Randomized Clinical Trial. JAMA Surg 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guyette FX, Sperry JL, Peitzman AB, Billiar TR, Daley BJ, Miller RS, et al. Prehospital Blood Product and Crystalloid Resuscitation in the Severely Injured Patient: A Secondary Analysis of the Prehospital Air Medical Plasma Trial. Ann Surg 2019. [DOI] [PubMed] [Google Scholar]
- 8.Holcomb JB, Donathan DP, Cotton BA, Del Junco DJ, Brown G, Wenckstern TV, et al. Prehospital Transfusion of Plasma and Red Blood Cells in Trauma Patients. Prehosp Emerg Care 2014;19:1–9. [DOI] [PubMed] [Google Scholar]
- 9.Rowell SE, Meier EN, McKnight B, Kannas D, May S, Sheehan K, et al. Effect of Out-of-Hospital Tranexamic Acid vs Placebo on 6-Month Functional Neurologic Outcomes in Patients With Moderate or Severe Traumatic Brain Injury. JAMA 2020;324:961–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shakur H, Roberts I, Bautista R, Caballero J, Coats T, Dewan Y, et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet 2010;376:23–32. [DOI] [PubMed] [Google Scholar]
- 11.Sperry JL, Guyette FX, Brown JB, Yazer MH, Triulzi DJ, Early-Young BJ, et al. Prehospital Plasma during Air Medical Transport in Trauma Patients at Risk for Hemorrhagic Shock. N Engl J Med 2018;379:315–326. [DOI] [PubMed] [Google Scholar]
- 12.Moore HB, Moore EE, Chapman MP, McVaney K, Bryskiewicz G, Blechar R, et al. Plasma-first resuscitation to treat haemorrhagic shock during emergency ground transportation in an urban area: a randomised trial. The Lancet 2018;392:283–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pusateri AE, Moore EE, Moore HB, Le TD, Guyette FX, Chapman MP, et al. Association of Prehospital Plasma Transfusion With Survival in Trauma Patients With Hemorrhagic Shock When Transport Times Are Longer Than 20 Minutes: A Post Hoc Analysis of the PAMPer and COMBAT Clinical Trials. JAMA Surg 2020;155:e195085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown JB, Guyette FX, Neal MD, Claridge JA, Daley BJ, Harbrecht BG, et al. Taking the Blood Bank to the Field: The Design and Rationale of the Prehospital Air Medical Plasma (PAMPer) Trial. Prehosp Emerg Care 2015;19:343–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown JB, Neal MD, Guyette FX, Peitzman AB, Billiar TR, Zuckerbraun BS, et al. Design of the Study of Tranexamic Acid during Air Medical Prehospital Transport (STAAMP) Trial: Addressing the Knowledge Gaps. Prehosp Emerg Care 2015;19:79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bottigliengo D, Baldi I, Lanera C, Lorenzoni G, Bejko J, Bottio T, et al. Oversampling and replacement strategies in propensity score matching: a critical review focused on small sample size in clinical settings. BMC Med Res Methodol 2021;21:256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Austin PC. Using the Standardized Difference to Compare the Prevalence of a Binary Variable Between Two Groups in Observational Research. Communications in Statistics - Simulation and Computation 2009;38:1228–1234. [Google Scholar]
- 18.Stuart EA. Matching methods for causal inference: A review and a look forward. Stat Sci 2010;25:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown JB, Rosengart MR, Forsythe RM, Reynolds BR, Gestring ML, Hallinan WM, et al. Not all prehospital time is equal: Influence of scene time on mortality. J Trauma Acute Care Surg 2016;81:93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen X, Guyette FX, Peitzman AB, Billiar TR, Sperry JL, Brown JB. Identifying patients with time-sensitive injuries: Association of mortality with increasing prehospital time. J Trauma Acute Care Surg 2019;86:1015–1022. [DOI] [PubMed] [Google Scholar]
- 21.Gruen DS, Guyette FX, Brown JB, Okonkwo DO, Puccio AM, Campwala IK, et al. Association of Prehospital Plasma With Survival in Patients With Traumatic Brain Injury: A Secondary Analysis of the PAMPer Cluster Randomized Clinical Trial. JAMA Netw Open 2020;3:e2016869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Floccard B, Rugeri L, Faure A, Saint Denis M, Boyle EM, Peguet O, et al. Early coagulopathy in trauma patients: an on-scene and hospital admission study. Injury 2012;43:26–32. [DOI] [PubMed] [Google Scholar]
- 23.Spielmann S, Kerner T, Ahlers O, Keh D, Gerlach M, Gerlach H. Early detection of increased tumour necrosis factor alpha (TNFα) and soluble TNF receptor protein plasma levels after trauma reveals associations with the clinical course. Acta Anaesthesiologica Scandinavica 2001;45:364–370. [DOI] [PubMed] [Google Scholar]
- 24.Burk AM, Martin M, Flierl MA, Rittirsch D, Helm M, Lampl L, et al. Early complementopathy after multiple injuries in humans. Shock 2012;37:348–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gruen DS, Brown JB, Guyette FX, Vodovotz Y, Johansson PI, Stensballe J, et al. Prehospital plasma is associated with distinct biomarker expression following injury. JCI Insight 2020;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jimenez JJ, Iribarren JL, Lorente L, Rodriguez JM, Hernandez D, Nassar I, et al. Tranexamic acid attenuates inflammatory response in cardiopulmonary bypass surgery through blockade of fibrinolysis: a case control study followed by a randomized double-blind controlled trial. Crit Care 2007;11:R117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu J, Moheimani H, Li S, Kar UK, Bonaroti J, Miller RS, et al. High Dimensional Multiomics Reveals Unique Characteristics of Early Plasma Administration in Polytrauma Patients With TBI. Ann Surg 2022;276:673–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meyer DE, Vincent LE, Fox EE, OʼKeeffe T, Inaba K, Bulger E, et al. Every minute counts: Time to delivery of initial massive transfusion cooler and its impact on mortality. J Trauma Acute Care Surg 2017;83:19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Apodaca A, Olson CM, Jr., Bailey J, Butler F, Eastridge BJ, Kuncir E. Performance improvement evaluation of forward aeromedical evacuation platforms in Operation Enduring Freedom. J Trauma Acute Care Surg 2013;75:S157–163. [DOI] [PubMed] [Google Scholar]
- 30.Morrison JJ, Oh J, DuBose JJ, O’Reilly DJ, Russell RJ, Blackbourne LH, et al. En-route care capability from point of injury impacts mortality after severe wartime injury. Ann Surg 2013;257:330–334. [DOI] [PubMed] [Google Scholar]
- 31.collaborators C-t. Effects of tranexamic acid on death, disability, vascular occlusive events and other morbidities in patients with acute traumatic brain injury (CRASH-3): a randomised, placebo-controlled trial. Lancet 2019;394:1713–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li SR, Guyette F, Brown J, Zenati M, Reitz KM, Eastridge B, et al. Early Prehospital Tranexamic Acid Following Injury Is Associated With a 30-day Survival Benefit: A Secondary Analysis of a Randomized Clinical Trial. Ann Surg 2021;274:419–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deeb AP, Hoteit L, Li S, Guyette FX, Eastridge BJ, Nirula R, et al. Prehospital synergy: Tranexamic acid and blood transfusion in patients at risk for hemorrhage. J Trauma Acute Care Surg 2022;93:52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deeb AP, Brown JB. Prehospital Resuscitation. In: Moore HB, Moore EE, Neal MD, eds. Trauma Induced Coagulopathy Cham, Switzerland: Springer, 2021:495–514. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SDC 2. STROBE Checklist.
SDC 1. Supplemental Results, Supplemental Tables, and Supplemental Figures.


