Abstract
Immunohistochemical study showed selective localisation of human epidermal growth factor (hEGF) to the synovial lining layer. Although the synovial lining layer of the rheumatoid, osteoarthritic, and traumatic joints was hEGF positive, hEGF staining was especially dense at the rheumatoid synovial lining layer; the staining increasing linearly according to the degree of stratification of the lining layer (r = 1). Human epidermal growth factor was ultrastructurally localised to cytoplasm, especially to rough endoplasmic reticulum, of the synovial lining fibroblast-like (type B) cell. Only the cell surface of macrophage-like (type A) cells was hEGF positive. When different histological variables were compared with each other a positive correlation was found between hEGF staining of the synovial lining layer and the degree of neovascularisation of rheumatoid synovium (r = 0.72). Although some lymphocytes were weakly hEGF positive, neovascularisation did not correlate with the extent of lymphocyte infiltration or of hEGF staining of lymphocytes. Lymphocyte infiltration or hEGF staining of lymphocytes did not correlate with hEGF staining of the synovial lining layer, whereas the lymphocyte infiltration correlated positively with the extent of perivascular accumulation of lymphocytes (r = 0.89). These findings suggest that (a) hEGF is synthesised by and secreted through endoplasmic reticulum and Golgi apparatus from the synovial lining type B cell; (b) hEGF is at least partially responsible for the pathogenesis of stratification of the rheumatoid synovial lining layer, and perhaps of neovascularisation of the rheumatoid synovium, whereas it is not responsible for lymphocyte accumulation to the rheumatoid synovium.
Full text
PDF



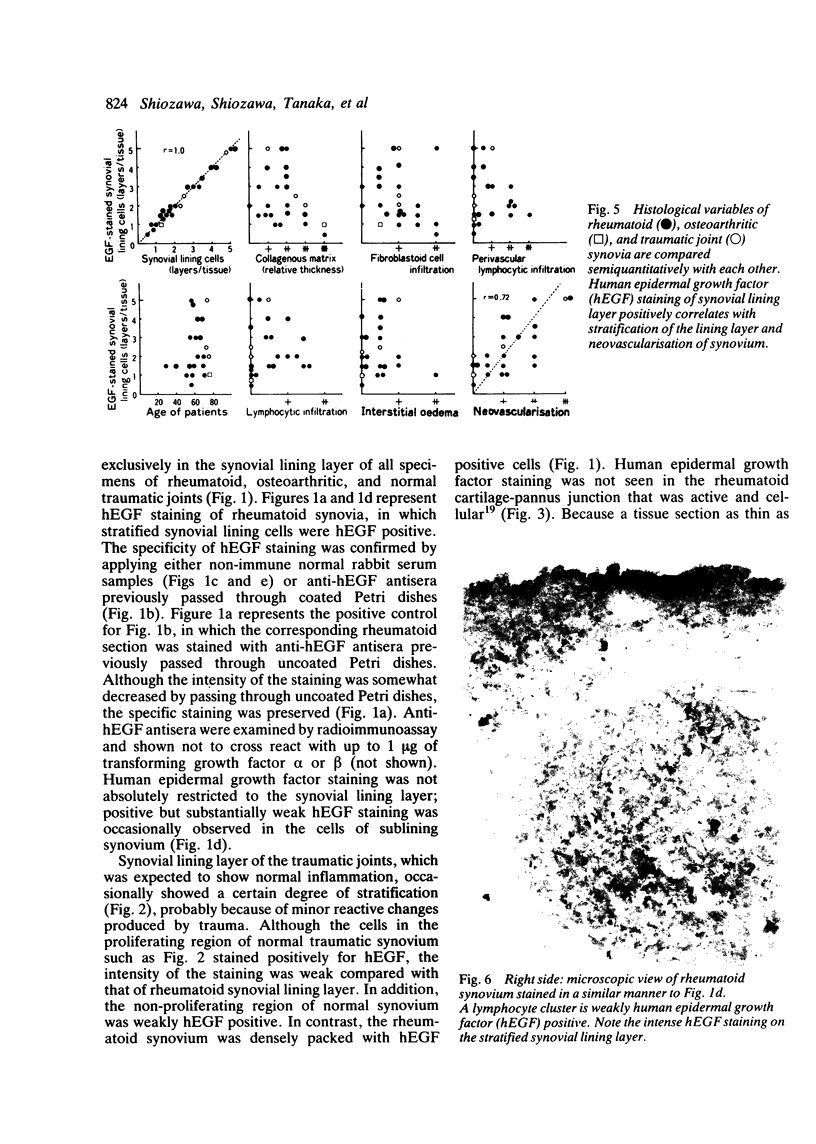
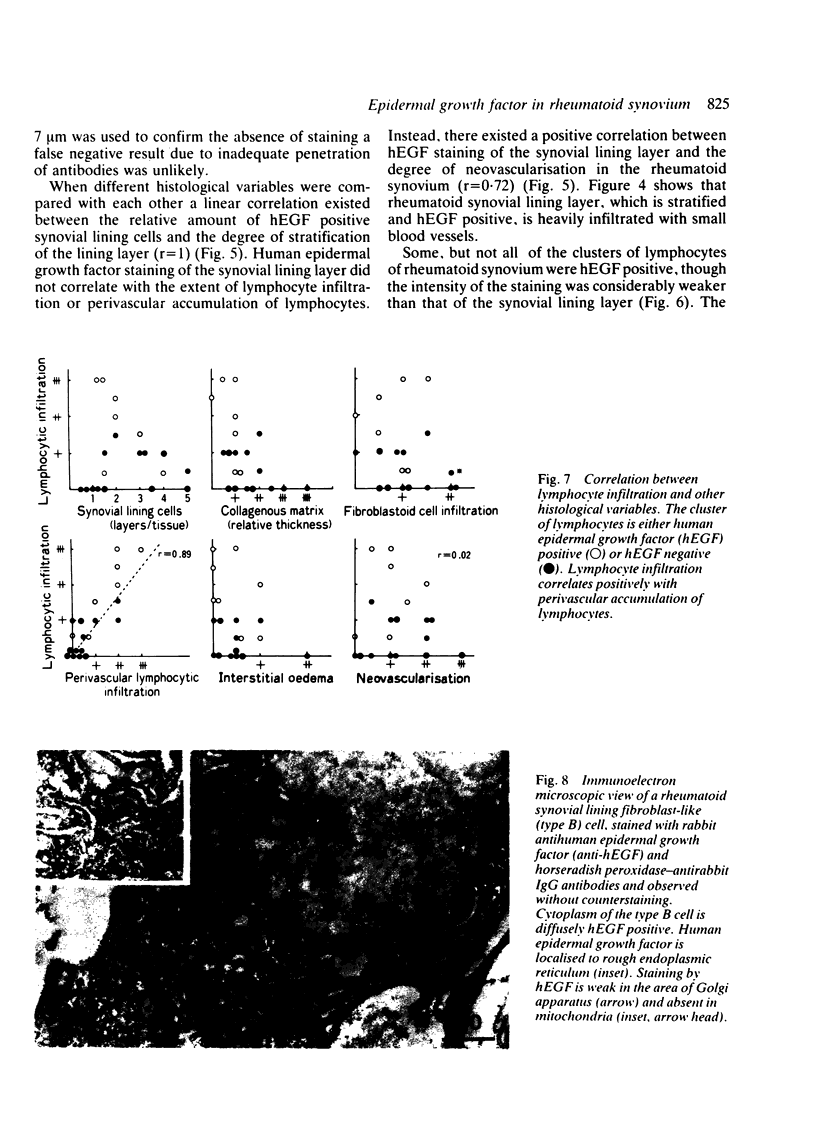



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARLAND P., NOVIKOFF A. B., HAMERMAN D. Electron microscopy of the human synovial membrane. J Cell Biol. 1962 Aug;14:207–220. doi: 10.1083/jcb.14.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BYWATERS E. G. The early radiological signs of rheumatoid arthritis. Bull Rheum Dis. 1960 Nov;11:231–234. [PubMed] [Google Scholar]
- Carpenter G., Cohen S. Epidermal growth factor. Annu Rev Biochem. 1979;48:193–216. doi: 10.1146/annurev.bi.48.070179.001205. [DOI] [PubMed] [Google Scholar]
- Carpenter G., Zendegui J. G. Epidermal growth factor, its receptor, and related proteins. Exp Cell Res. 1986 May;164(1):1–10. doi: 10.1016/0014-4827(86)90449-0. [DOI] [PubMed] [Google Scholar]
- Cohen S., Carpenter G. Human epidermal growth factor: isolation and chemical and biological properties. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1317–1321. doi: 10.1073/pnas.72.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreher R. Origin of synovial type A cells during inflammation. An experimental approach. Immunobiology. 1982 Apr;161(3-4):232–245. doi: 10.1016/S0171-2985(82)80079-X. [DOI] [PubMed] [Google Scholar]
- Edwards J. C., Willoughby D. A. Demonstration of bone marrow derived cells in synovial lining by means of giant intracellular granules as genetic markers. Ann Rheum Dis. 1982 Apr;41(2):177–182. doi: 10.1136/ard.41.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassbender H. G., Simmling-Annefeld M., Stofft E. Transformation der Synovialzellen bei rheumatoider Arthritis. Verh Dtsch Ges Pathol. 1980;64:193–212. [PubMed] [Google Scholar]
- Førre O., Thoen J., Lea T., Dobloug J. H., Mellbye O. J., Natvig J. B., Pahle J., Solheim B. G. In situ characterization of mononuclear cells in rheumatoid tissues, using monoclonal antibodies. No reduction of T8-positive cells or augmentation in T4-positive cells. Scand J Immunol. 1982 Oct;16(4):315–319. doi: 10.1111/j.1365-3083.1982.tb00729.x. [DOI] [PubMed] [Google Scholar]
- HIROHATA K., KOBAYASHI I. FINE STRUCTURES OF THE SYNOVIAL TISSUES IN RHEUMATOID ARTHRITIS. Kobe J Med Sci. 1964 Dec;10:195–225. [PubMed] [Google Scholar]
- Hirata Y., Moore G. W., Bertagna C., Orth D. N. Plasma concentrations of immunoreactive human epidermal growth factor (urogastrone) in man. J Clin Endocrinol Metab. 1980 Mar;50(3):440–444. doi: 10.1210/jcem-50-3-440. [DOI] [PubMed] [Google Scholar]
- Hirata Y., Uchihashi M., Nakashima H., Fujita T., Matsukura S., Matsui K. Specific receptors for epidermal growth factor in human bone tumour cells and its effect on synthesis of prostaglandin E2 by cultured osteosarcoma cell line. Acta Endocrinol (Copenh) 1984 Sep;107(1):125–130. doi: 10.1530/acta.0.1070125. [DOI] [PubMed] [Google Scholar]
- Hogg N., Palmer D. G., Revell P. A. Mononuclear phagocytes of normal and rheumatoid synovial membrane identified by monoclonal antibodies. Immunology. 1985 Dec;56(4):673–681. [PMC free article] [PubMed] [Google Scholar]
- Iguchi T., Kurosaka M., Ziff M. Electron microscopic study of HLA-DR and monocyte/macrophage staining cells in the rheumatoid synovial membrane. Arthritis Rheum. 1986 May;29(5):600–613. doi: 10.1002/art.1780290504. [DOI] [PubMed] [Google Scholar]
- KULKA J. P., BOCKING D., ROPES M. W., BAUER W. Early joint lesions of rheumatoid arthritis; report of eight cases, with knee biopsies of lesions of less than one year's duration. AMA Arch Pathol. 1955 Feb;59(2):129–150. [PubMed] [Google Scholar]
- Kasselberg A. G., Orth D. N., Gray M. E., Stahlman M. T. Immunocytochemical localization of human epidermal growth factor/urogastrone in several human tissues. J Histochem Cytochem. 1985 Apr;33(4):315–322. doi: 10.1177/33.4.3884705. [DOI] [PubMed] [Google Scholar]
- Klareskog L., Forsum U., Kabelitz D., Plöen L., Sundström C., Nilsson K., Wigren A., Wigzell H. Immune functions of human synovial cells. Phenotypic and T cell regulatory properties of macrophage-like cells that express HLA-DR. Arthritis Rheum. 1982 May;25(5):488–501. doi: 10.1002/art.1780250502. [DOI] [PubMed] [Google Scholar]
- Massagué J. Epidermal growth factor-like transforming growth factor. II. Interaction with epidermal growth factor receptors in human placenta membranes and A431 cells. J Biol Chem. 1983 Nov 25;258(22):13614–13620. [PubMed] [Google Scholar]
- McLean I. W., Nakane P. K. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J Histochem Cytochem. 1974 Dec;22(12):1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- Oka Y., Orth D. N. Human plasma epidermal growth factor/beta-urogastrone is associated with blood platelets. J Clin Invest. 1983 Jul;72(1):249–259. doi: 10.1172/JCI110964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y., Nakanishi I., Kajikawa K. Repair of the mouse synovial membrane after chemical synovectomy with osmium tetroxide. Acta Pathol Jpn. 1984 Jul;34(4):705–714. doi: 10.1111/j.1440-1827.1984.tb07599.x. [DOI] [PubMed] [Google Scholar]
- Okada Y., Nakanishi I., Kajikawa K. Ultrastructure of the mouse synovial membrane. Development and organization of the extracellular matrix. Arthritis Rheum. 1981 Jun;24(6):835–843. doi: 10.1002/art.1780240611. [DOI] [PubMed] [Google Scholar]
- Palade G. Intracellular aspects of the process of protein synthesis. Science. 1975 Aug 1;189(4200):347–358. doi: 10.1126/science.1096303. [DOI] [PubMed] [Google Scholar]
- Palmer D. G., Selvendran Y., Allen C., Revell P. A., Hogg N. Features of synovial membrane identified with monoclonal antibodies. Clin Exp Immunol. 1985 Mar;59(3):529–538. [PMC free article] [PubMed] [Google Scholar]
- Poulter L. W., Duke O., Hobbs S., Janossy G., Panayi G. Histochemical discrimination of HLA-DR positive cell populations in the normal and arthritic synovial lining. Clin Exp Immunol. 1982 May;48(2):381–388. [PMC free article] [PubMed] [Google Scholar]
- ROPES M. W., BENNETT G. A., COBB S., JACOX R., JESSAR R. A. 1958 Revision of diagnostic criteria for rheumatoid arthritis. Bull Rheum Dis. 1958 Dec;9(4):175–176. [PubMed] [Google Scholar]
- Schreiber A. B., Winkler M. E., Derynck R. Transforming growth factor-alpha: a more potent angiogenic mediator than epidermal growth factor. Science. 1986 Jun 6;232(4755):1250–1253. doi: 10.1126/science.2422759. [DOI] [PubMed] [Google Scholar]
- Schumacher H. R., Kitridou R. C. Synovitis of recent onset. A clinicopathologic study during the first month of disease. Arthritis Rheum. 1972 Sep-Oct;15(5):465–485. doi: 10.1002/art.1780150502. [DOI] [PubMed] [Google Scholar]
- Shimizu S., Shiozawa S., Shiozawa K., Imura S., Fujita T. Quantitative histologic studies on the pathogenesis of periarticular osteoporosis in rheumatoid arthritis. Arthritis Rheum. 1985 Jan;28(1):25–31. doi: 10.1002/art.1780280105. [DOI] [PubMed] [Google Scholar]
- Shimuzu S., Shiozawa S., Shiozawa K., Imura S., Ishikawa H., Hirohata K., Fujita T. The restoration of proliferation and differentiation of peripheral blood mononuclear non-adherent cells into immunoglobulin-secreting cells by autologous synovial adherent cells from patients with rheumatoid arthritis. Virchows Arch B Cell Pathol Incl Mol Pathol. 1988;54(6):350–356. doi: 10.1007/BF02899233. [DOI] [PubMed] [Google Scholar]
- Shiozawa S., Jasin H. E., Ziff M. Absence of immunoglobulins in rheumatoid cartilage-pannus junctions. Arthritis Rheum. 1980 Jul;23(7):816–821. doi: 10.1002/art.1780230707. [DOI] [PubMed] [Google Scholar]
- Shiozawa S., Shiozawa K., Fujita T. Morphologic observations in the early phase of the cartilage-pannus junction. Light and electron microscopic studies of active cellular pannus. Arthritis Rheum. 1983 Apr;26(4):472–478. doi: 10.1002/art.1780260404. [DOI] [PubMed] [Google Scholar]
- Shiozawa S., Shiozawa K., Fujita T. Presence of HLA-DR antigen on synovial type A and B cells: an immunoelectron microscopic study in rheumatoid arthritis, osteoarthritis and normal traumatic joints. Immunology. 1983 Dec;50(4):587–594. [PMC free article] [PubMed] [Google Scholar]
- Shiozawa S., Williams R. C., Jr, Ziff M. Immunoelectron microscopic demonstration of prostaglandin E in rheumatoid synovium. Arthritis Rheum. 1982 Jun;25(6):685–693. doi: 10.1002/art.1780250612. [DOI] [PubMed] [Google Scholar]
- Shupnik M. A., Ip N. Y., Tashjian A. H., Jr Characterization and regulation of receptors for epidermal growth factor in mouse calvaria. Endocrinology. 1980 Dec;107(6):1738–1746. doi: 10.1210/endo-107-6-1738. [DOI] [PubMed] [Google Scholar]
- Shupnik M. A., Tashjian A. H., Jr Functional receptors for epidermal growth factor on human osteosarcoma cells. J Cell Physiol. 1981 Dec;109(3):403–410. doi: 10.1002/jcp.1041090305. [DOI] [PubMed] [Google Scholar]
- Tam J. P., Marquardt H., Rosberger D. F., Wong T. W., Todaro G. J. Synthesis of biologically active rat transforming growth factor I. Nature. 1984 May 24;309(5966):376–378. doi: 10.1038/309376a0. [DOI] [PubMed] [Google Scholar]
- Tashjian A. H., Jr, Levine L. Epidermal growth factor stimulates prostaglandin production and bone resorption in cultured mouse calvaria. Biochem Biophys Res Commun. 1978 Dec 14;85(3):966–975. doi: 10.1016/0006-291x(78)90638-1. [DOI] [PubMed] [Google Scholar]
- Tiku M. L., Teodorescu M., Skosey J. L. Immunobiological function of normal rabbit synovial cells. Cell Immunol. 1985 Apr 1;91(2):415–424. doi: 10.1016/0008-8749(85)90239-4. [DOI] [PubMed] [Google Scholar]