Abstract
Objective:
Frailty is an emerging risk factor for adverse outcomes. However, perioperative frailty assessments derived from electronic health records (EHR) have not been studied on a large scale. We aim to estimate the prevalence of frailty and the associated incidence of major adverse cardiovascular events (MACE) among adults hospitalized for non-cardiac surgery.
Methods
Adults aged ≥45 years hospitalized for non-cardiac surgery between 2004–2014 were identified from the National Inpatient Sample. The validated Hospital Frailty Risk Score (HFRS) derived from International Classification of Diseases codes was used to classify patients as low (HFRS <5), medium (5–10), or high (>10) frailty risk. The primary outcome was MACE, defined as myocardial infarction, cardiac arrest, and in-hospital mortality. Multivariable logistic regression was used to estimate the adjusted odds of MACE stratified by age and HFRS.
Results:
A total of 55,349,978 hospitalizations were identified, of which 81.0%, 14.4%, and 4.6% had low, medium, and high HFRS, respectively. Patients with higher HFRS had more cardiovascular risk factors and comorbidities. MACE occurred during 2.5% of surgical hospitalizations and was common among patients with high frailty scores (high HFRS: 9.1%, medium: 6.9%, low: 1.3%, p<0.001). Medium (adjusted odds ratio [aOR] 2.05, 95% CI 2.02 to 2.08) and high (aOR 2.75, 95% CI 2.70 to 2.79) HFRS were associated with greater odds of MACE versus low HFRS, with the greatest odds of MACE observed in younger individuals 45–64 years (interaction p-value <0.001).
Conclusions:
The HFRS may identify frail surgical inpatients at risk for adverse perioperative cardiovascular outcomes.
Keywords: aging, cardiovascular, electronic health records, frailty, mortality, surgery
INTRODUCTION:
Each year, over 17 million non-cardiac surgeries are performed in the United States, and older adults age ≥65 years constitute nearly half of all surgical inpatients.1 Frailty, an aging-related state of decreased functional status and physiological reserve, is associated with poor health outcomes and may be an important risk factor for complications after non-cardiac surgery.2,3 Pre-operative frailty screening tools have been developed to incorporate functional assessments such as gait speed, mobility tests, and cognitive evaluations.4,5 Validated scoring systems can also identify frailty from administrative data in electronic health records (EHR).6 However, EHR-derived frailty scoring tools have not been applied on a large scale to predict surgical outcomes. Most prior studies did not specifically focus on cardiovascular outcomes, did not stratify by age or evaluate frailty in younger cohorts, and were limited to analyses on a single type of surgery.7–9 The objective of this study was to estimate the prevalence of frailty using a scoring tool based on administrative data and determine associations between frailty scores and the incidence of perioperative major adverse cardiovascular events (MACE) among adults undergoing non-cardiac surgery in the United States using a large, nationwide database.
METHODS:
Study Population
We identified hospitalizations from the Agency for Healthcare Research and Quality’s (AHRQ) National Inpatient Sample (NIS), an all-payer administrative database that represents a 20% stratified sample of discharges from participating community hospitals in the United States10. In addition, the database used in this study is naturally de-identified without any patient identifiers, and therefore did not warrant external review from our local ethical committee or Institutional Review Board.
Adults aged 45 years and older who underwent non-cardiac surgery between 2004 and 2014 were included if they had a principal International Classification of Diseases Ninth Revision (ICD-9) procedure code corresponding to a major therapeutic operating room procedures (HCUP Procedure Class 4), as previously described.11 The classification of procedures as either diagnostic/therapeutic and major/minor is assigned by AHRQ based on the level of invasiveness and resource utilization for each procedure. Admissions with a primary procedure code for cardiac procedures, cardiac transplantations, bone marrow transplantations, ophthalmologic surgery, radiation therapy, dental surgery, and non-operating room procedures were excluded. Hospitalizations with a primary procedure code for the following non-cardiac surgeries were included: breast, endocrine, otolaryngology, general, genitourinary, gynecologic, neurosurgery, obstetrics, orthopedic, skin and burn, thoracic, noncardiac transplant, and vascular surgery.
Frailty Assessment
Frailty was estimated using the previously validated Hospital Frailty Risk Score (HFRS).6 This risk score was initially derived using a cluster analysis of patients ≥75 years old discharged from National Health Service hospitals in England between 2013–2015. In this derivation study, patients were clustered by International Classification of Diseases, 10th Revision (ICD-10) codes, length of stay, and hospital costs, and a frail cluster was identified based on the prevalence of pre-established frailty syndromes: cognitive impairment, functional dependence, falls and fractures, anxiety and depression, incontinence, pressure ulcers, and mobility problems (Supplemental Table 1). Next, any ICD-10 diagnosis codes that were at least twice as common in the frail cluster than the remainder of the cohort were identified, and each diagnosis code was assigned a score proportional to how strongly it predicted inclusion in the frail cluster. In a validation cohort, increasing HFRS scores predicted higher 30-day mortality, length of stay and 30-day readmission.6 To adapt this score to our database, we used a standard Center for Medicare and Medicaid Services crosswalk to convert ICD-10 codes associated with frailty to the equivalent International Classification of Diseases, 9th Revision (ICD-9) codes. Each surgical inpatient was then assigned an HFRS based on ICD-9 diagnosis codes (Supplemental Table 2). To characterize the relationship between HFRS and the study outcomes, patients were stratified into three cohorts by HFRS score, indicating low (<5), intermediate (5–10), and high (≥10) risk of frailty. Although the original derivation focused on an older cohort age >75 with high disease prevalence, we applied this frailty score to a broader cohort of patients undergoing non-cardiac surgery to evaluate associations with perioperative outcomes.
Outcomes:
The primary endpoint for this study was perioperative MACE defined as the in-hospital composite of acute myocardial infarction, cardiac arrest, or all-cause mortality during the index surgical hospitalization.12 Secondary endpoints included the composite endpoint of death and acute myocardial infarction, and the individual endpoints of mortality, acute myocardial infarction, and cardiac arrest. Stroke was not included as a study endpoint, as ICD-9 codes reflecting neurological deficits contributing to frailty were included in the HFRS. In-hospital mortality was determined from the NIS discharge disposition. Acute myocardial infarction was defined based on ICD-9 diagnosis code for acute ST-segment elevation myocardial infarction (ICD-9 diagnosis codes 410.01 to 410.61, 410.81, and 410.91) and non-ST-segment elevation myocardial infarction (ICD-9 diagnosis code 410.71), as previously described.13 Cardiac arrest was defined by the ICD-9 diagnosis code 427.5.14 Discharge disposition is systematically reported in the NIS database and was included as another clinically relevant study endpoint.
Statistical Analysis
Categorical variables were reported as percentages and compared using chi-square tests. Continuous variables were reported as means and compared using linear regression. Multivariable logistic regression was used to estimate the odds of adverse perioperative cardiovascular events, adjusted for demographics and comorbidities that were not integrated into the frailty scores (Appendix, I). The incidence of perioperative MACE was evaluated in cohorts characterized by low, medium, and high HFRS. Relationships between HFRS and outcomes were also evaluated in cohorts stratified by age (45–64 years, 65–74 years, ≥75 years). National estimates were generated by applying clustering and sampling weights, as per AHRQ guidance. All data are weighted, unless otherwise specified. Statistical analyses were performed using IBM SPSS 27 (IBM, Armonk, NY). All statistical tests are 2-sided, and statistical significance was defined as p<0.05. The authors are prepared to share the raw data from the database that was used all analyses for this study.
Patient and Public Involvement
This study is an analysis using a retrospective database and therefore patients and the public were not directly involved in the study for this research in any way.
RESULTS:
Patient Characteristics:
A total of 11,539,910 hospitalizations for non-cardiac surgery among adults aged ≥45 years were identified, corresponding to 55,349,978 hospitalizations nationwide after applying sampling weights. Of these, patients in 44,818,874 (81.0%) hospitalizations were categorized as low frailty risk, 7,949,829 (14.4%) as medium frailty risk, and 2,562,935 (4.6%) as high frailty risk (Table 1, Figure 1). This corresponds to approximately 950,000 surgical hospitalizations each year associated with medium or high frailty risk in the United States. Patients with the highest-risk frailty scores were older, more frequently female and had a greater burden of comorbidities and cardiovascular diseases, such as hypertension, coronary artery disease, and heart failure (Table 1). Compared to those with low or medium frailty, patients with high frailty scores were more likely to have an urgent surgical admission (elective admissions; high risk: 18.1% vs medium risk: 31.8% vs low risk: 68.1%), and were more likely undergo neurosurgery, orthopedic, or skin/breast surgery (Table 1).
Table 1:
Characteristics of patients undergoing non-cardiac surgery, by frailty risk
| Low Risk (n=44,818,874) | Medium Risk (n=7,949,829) | High Risk (n=2,562,935) | |
|---|---|---|---|
| Age in years, mean (SE) | 64.5 (0.044) | 70.2 (0.065) | 74.6 (0.072) |
| Female Sex | 56.5% (25,272,078) | 55.3% (4,397,000) | 58.0% (1,486,117) |
| Race | |||
| White Non-Hispanic | 65.7% (29,433,388) | 65.9% (5,242,392) | 67.8% (1,738,213) |
| Black Non-Hispanic | 7.5% (3,348,409) | 9.8% (7,773,340) | 10.9% (280,284) |
| Hispanic | 5.6% (2,515,927) | 6.1% (484,913) | 6.5% (165,570) |
| Asian | 3.8% (1,721,173) | 3.9% (310,458) | 4.2% (108,898) |
| Other | 17.4% (7,799,977) | 14.3% (1,134,727) | 10.5% (269,971) |
| Region | |||
| Northeast | 19.3% (8,667,959) | 17.5% (1,387,639) | 16.0% (411,0270 |
| South | 23.7% (10,602,885) | 24.7% (1,962,196) | 25.1% (642,502) |
| West | 37.4% (16,765,305) | 39.4% (3,131,400) | 40.2% (1,029,128) |
| Central | 19.6% (8,782,725) | 18.5% (1,468,594) | 18.7% (480,278) |
| Primary Payer | |||
| Medicare | 49.4% (22,108,150) | 68.6% (5,447,682) | 78.1% (1,999,691) |
| Medicaid | 4.8% (2,156,947) | 6.2% (494,136) | 5.6% (144,587) |
| Private | 39.2% (17,533,032) | 20.0% (1,585,161) | 12.6% (322,787) |
| Self-Pay | 2.5% (1,100,463) | 2.4% (189,153) | 1.7% (42,911) |
| No Charge | 0.3% (147,266) | 0.3% (23,036) | 0.2% (5,031) |
| Hospital Location and Teaching Status | |||
| Rural Non-Teaching | 9.4% (4,207,374) | 9.0% (710,679) | 8.9% (225,983) |
| Rural Teaching | 40.1% (17,902,838) | 39.7% (3,136,392) | 40.1% (1,022,149) |
| Urban Teaching | 50.5% (22,526,391) | 51.4% (4,061,223) | 51.1% (1,302,164) |
| Hospital Size | |||
| Small | 13.0% (178,692) | 11.0% (870,114) | 11.0% (281,691) |
| Medium | 24.1% (10,749,338) | 24.6% (1,943,174) | 25.1% (641,058) |
| Large | 62.9% (28,071,766) | 64.4% (5,095,005) | 63.8% (1,627,548) |
| Elective Surgery | 68.1% (30,419,659) | 31.8% (2,521,893) | 18.1% (461,099) |
| Surgery Type | |||
| General | 22.0% (9,864,343) | 19.7% (1,562,635) | 15.0% (384,125) |
| Endocrine | 1.3% (588,007) | 0.3% (26,208) | 0.2% (6,058) |
| Genitourinary | 7.3% (3,291,950) | 6.3% (504,084) | 6.8% (173,055) |
| Gynecological | 6.6% (2,943,820) | 1.7% (136,296) | 0.6% (14,142) |
| Neurosurgery | 5.7% (2,549857) | 5.3% (422,423) | 6.8% (173,606) |
| Obstetrics | 0.1% (44,644) | 0.0% (189) | 0.0% (10*) |
| Orthopedics | 40.3% (18,042,009) | 42.9% (3,413,212) | 47.2% (1,209,538) |
| Otolaryngology | 0.8% (353,445) | 0.6% (45,823) | 0.4% (11,355) |
| Skin/Breast | 3.7% (1,638,594) | 6.5% (514,751) | 7.4% 9189,245) |
| Thoracic | 2.1% (952,095) | 2.7% (212,032) | 2.1% (54,816) |
| Transplant | 0.3% (116,833) | 0.6% (46,249) | 0.3% (7,513) |
| Vascular | 9.9% (4,433,279) | 13.4% (1,065,928) | 13.2% (339,473) |
| Tobacco Use | 21.0% (9,396,928) | 25.0% (1,986,434) | 21.1% (540,095) |
| Obesity | 11.8% (5,273,011) | 10.9% (866,429) | 8.5% (218,704) |
| Hypertension | 56.5% (25,317,435) | 66.0% (5,248,762) | 68.9% (1,764,882) |
| Hyperlipidemia | 28.9% (12,960,985) | 31.3% (2,488,172) | 30.8% (790,631) |
| Diabetes (total) | 21.2% (9,523,847) | 31.9% (2,535,590) | 32.0% (819,248) |
| Coronary Artery Disease | 16.3% (7,314,865) | 23.8% (1,894,514) | 25.4% (652,246) |
| Prior Percutaneous Coronary Intervention | 3.7% (1,662,025) | 4.2% (332,389) | 3.6% (92,319) |
| Prior Coronary Artery Bypass Grafting | 4.5% (2,009,293) | 5.5% (438,962) | 5.0% (127,839) |
| Atrial Fibrillation | 6.7% (3,017,831) | 15.7% (1,249,556) | 20.1% (516,206) |
| History of Venous Thromboembolism | 2.4% (1,084,084) | 3.4% (269,543) | 3.6% (92,539) |
| Prior Stroke | 2.3% (1,045,772) | 4.4% (350,166) | 5.8% (148,277) |
| Chronic Kidney Disease | 4.6% (2,046,008) | 20.5% (1,625,766) | 27.2% (697,507) |
| End Stage Renal Disease | 1.6% (696,856) | 6.3% (501,401) | 5.6% (143,098) |
| Congestive Heart Failure | 4.5% (1,991,270) | 14.8% (1,175,972) | 18.8% (481,416) |
| Chronic Pulmonary Disease | 15.7% (7,041,853) | 22.3% (1,769,798) | 21.8% (558,878) |
| Peripheral Vascular Disease | 5.9% (2,665,861) | 12.6% (998,360) | 14.0% (358,764) |
| Valvular Disease | 3.6% (1,633,184) | 6.6% (522,635) | 7.7% (198,519) |
| Any Malignancy | 5.1% (2,279,196) | 7.4% (587,606) | 6.7% (170,708) |
| Any Anemia | 11.5% (5,165,927) | 29.8% (2,369,645) | 37.7% (966,768) |
| Drug Abuse | 0.8% (357,515) | 1.7% (134,586) | 1.6% (40,185) |
| Alcohol Abuse | 1.6% (735,252) | 4.3% (345,394) | 4.3% (110,961) |
| Coagulopathy | 2.1% (919,340) | 7.3% (580,340) | 9.4% (240,427) |
| Rheumatoid Arthritis | 2.7% (1,203,424) | 3.6% (287,447) | 3.3% (84,845) |
| Autoimmune Deficiency Syndrome | 0.1% (41,679) | 0.2% (16,143) | 0.1% (435) |
| Iron Deficiency Anemia | 10.2% (4,563,179) | 27.5% (2,187,237) | 35.2% (901,477) |
| Blood Loss Anemia | 1.4% (643,738) | 2.6% (208,570) | 3.0% (78,019) |
| Depression | 8.8% (3,937,325) | 13.0% (1,033,370) | 15.1% (387,592) |
| Diabetes, Uncomplicated | 17.9% (8,017,048) | 20.2% (1,604,236) | 20.6% (527,791) |
| Diabetes, Chronic Comp | 2.9% (1,301,197) | 10.3% (820,256) | 10.9% (278,176) |
| Hypertension | 55.8% (25,030,637) | 64.8% (5,152,107) | 68.2% (1,746,941) |
| Hypothyroidism | 10.9% (4,905,501) | 13.7% (1,086,695) | 15.8% (403,728) |
| Liver Disease | 1.8% (818,476) | 3.2% (257,038) | 2.8% (71,894) |
| Lymphoma | 0.4% (199,973) | 0.8% (66,203) | 0.8% (20,466) |
| Fluid/Elec Disorder | 7.3% (3,284,980) | 46.1% (3,663,044) | 59.9% (1,534,831) |
| Metastatic Cancer | 3.0% (1,332,513) | 3.9% (312,685) | 3.1% (80,018) |
| Other neurological Disorder | 2.6% (1,177,076) | 9.9% (789,145) | 33.3% (853,535) |
| Obesity | 11.0% (4,917,723) | 11.5% (913,150) | 9.9% (254,917) |
| Paralysis | 0.6% (282,787) | 5.3% (419,270) | 12.2% (312,987) |
| Psychoses | 1.9% (831,660) | 3.9% (311,321) | 5.0% (129,404) |
| Pulmonary Circulation Disease | 1.0% (459,170) | 3.7% (294,005) | 5.1% (131,676) |
| Renal Failure | 4.8% (2,170,405) | 20.9% (1,658,617) | 27.4% (702,398) |
| Solid tumor without metastasis | 1.7% (758,860) | 2.7% (213,051) | 2.8% (71,627) |
| Peptic Ulcer | 0.0% (15,843) | 0.1% (5,103) | 0.1% (1,517) |
| Weight Loss | 1.8% (789,737) | 9.9% (784,832) | 15.7% (402,848) |
all variables with p<0.05
Figure 1: Incidence of MACE after non-cardiac surgery, by frailty score.
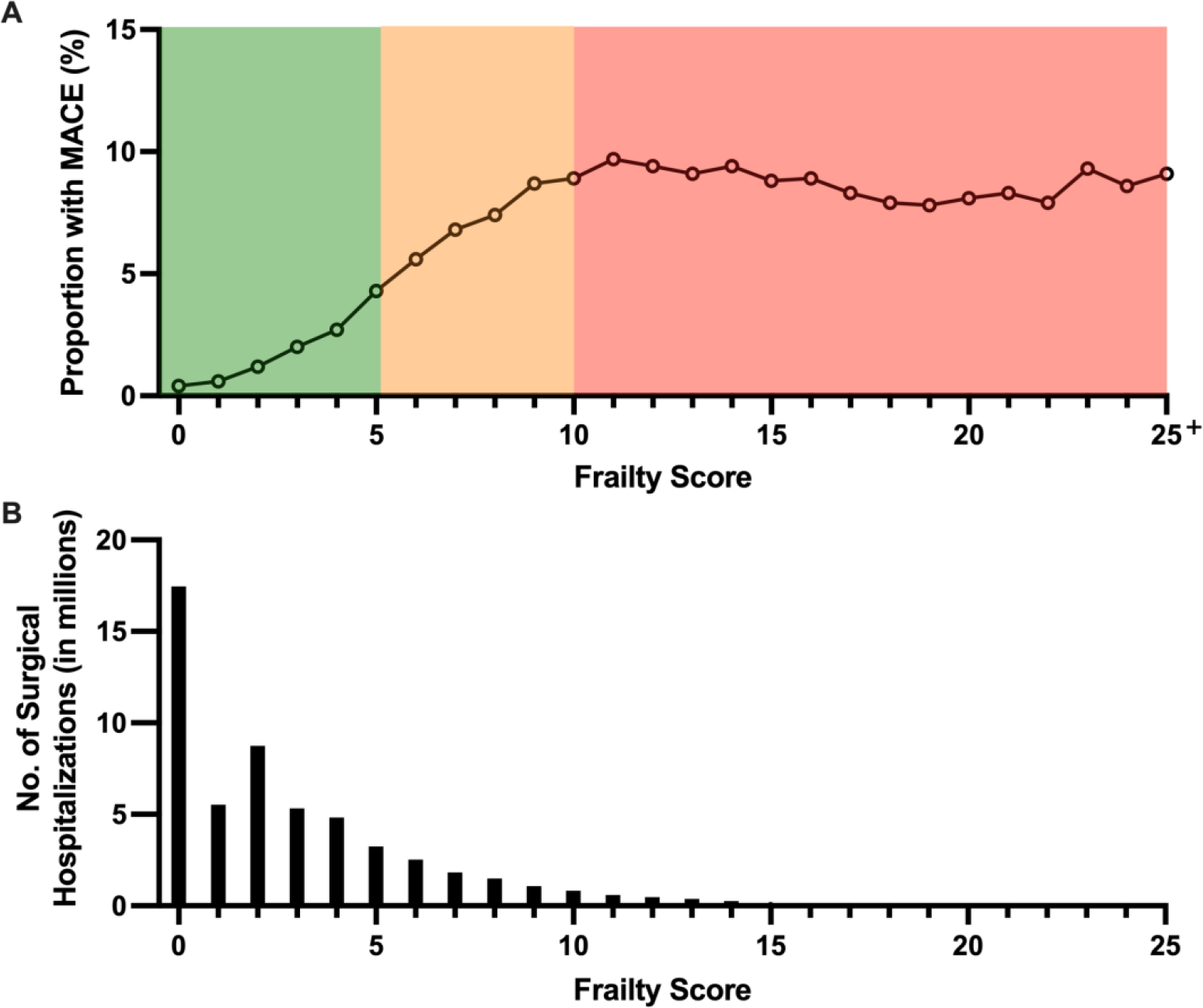
The incidence of major adverse cardiovascular events (MACE) in patients undergoing non-cardiac surgery (Panel A) and the total number of surgical hospitalizations (Panel B) by frailty score as a continuous variable. The frailty risk score categories are highlighted in Panel A as low (green), medium (yellow), and high (red) risk. In Panel A, the proportion of patients with MACE after non-cardiac surgery increased with increasing frailty scores, but plateaus in the high frailty risk group. In Panel B, it is shown that most patients undergoing non-cardiac surgery had low frailty risk scores.
In-Hospital Outcomes and Discharge Disposition
Perioperative MACE occurred during 2.5% of hospitalizations for non-cardiac surgery overall, with a higher incidence in patients with high (9.1%) and medium (6.9%) HFRS compared to those with low HFRS (1.3%, p<0.001) (Table 2, Figure 1). After adjustment for demographics and clinical covariates (Appendix), medium HFRS (aOR 2.05, 95% CI 2.02–2.08) and high HFRS (aOR 2.75, 95% CI 2.70–2.80) were associated with increased odds for perioperative MACE compared to those with low HFRS. Associations between HFRS and perioperative MACE were observed across age groups (Figure 2, Figure 3, Supplemental Table 3). The association between high HFRS and MACE was most striking in younger individuals (45–64 years: aOR 4.39, 95% CI 4.23–4.56; 65–74 years: aOR 3.15, 95% CI 3.06–3.23; ≥75 years: aOR 3.15, 95% CI 3.08–3.22; interaction p-value <0.001) (Figure 2). Associations between HFRS and perioperative MACE were also observed in cohorts stratified by sex, diabetes mellitus, coronary artery disease, non-cardiac surgery subtype, and elective or urgent surgical hospitalization (Supplemental Figures 1–4).
Table 2:
Outcomes and Discharge Disposition after non-cardiac surgery, by frailty score
| Low Risk (n=44,818,874) | Medium Risk (n=7,949,829) | High Risk (n=2,562,935) | p-value | |
|---|---|---|---|---|
| In Hospital Outcomes | ||||
| MACE (Death, MI, or Arrest) | 1.3% (575,166) | 6.9% (548,442) | 9.1% (234,262) | p<0.001 |
| Death or MI | 1.2% (549,370) | 6.6% (521,274) | 8.6% (220,985) | p<0.001 |
| All-Cause Mortality | 0.8% (357,940) | 4.8% (380,481) | 6.3% (161,053) | p<0.001 |
| Myocardial Infarction | 0.5% (226,451) | 2.3% (178,909) | 2.9% (74,823) | p<0.001 |
| Cardiac Arrest | 0.2% (77,248) | 0.9% (74,104) | 1.2% (31,707) | p<0.001 |
| Discharge Disposition | ||||
| Routine Discharge Home | 62.9% (28,177,146) | 30.4% (2,411,227) | 14.1% (360,291) | p<0.001 |
| Short Term Hospital | 0.6% (289,317) | 1.7% (137,721) | 2.0% (50,049) | p<0.001 |
| Skilled nursing facility/Intermediate care facility | 17.6% (7,862,504) | 44.5% (3,532,288) | 63.5% (1,627,428) | p<0.001 |
| Home Health Care | 18.0% (8,052,951) | 18.4% (1,461,675) | 13.9% (356,614) | p<0.001 |
| Unknown | 0.0% (6,987) | 0.1% (4,512) | 0.1% (2,334) | p<0.001 |
Figure 2: Adjusted odd ratios for risk of MACE, stratified by frailty and age.
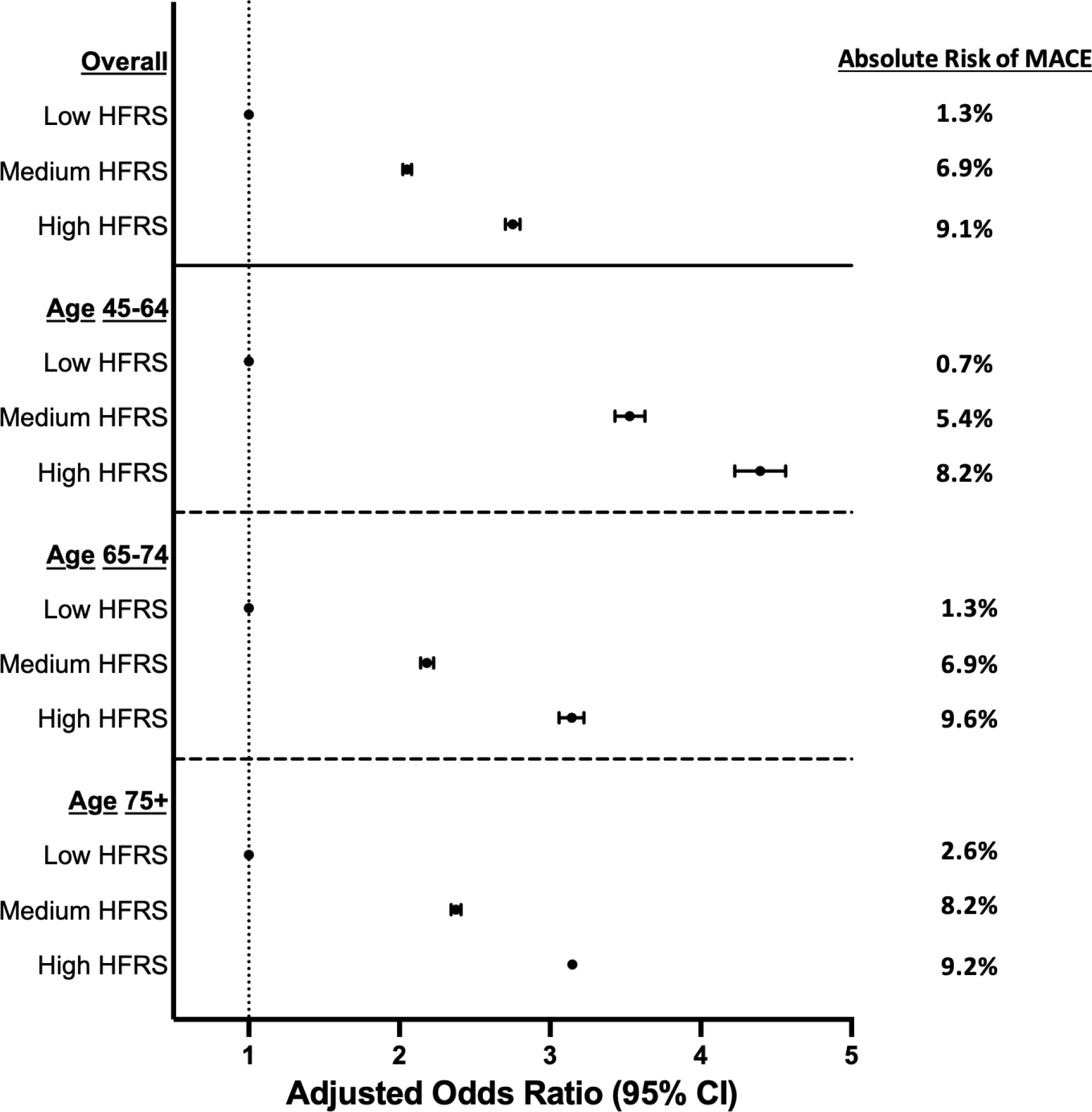
Plot of adjusted odds ratios for the risk of post-operative major adverse cardiovascular events (MACE) in patients undergoing non-cardiac surgery by frailty classification, overall and stratified by age. The analysis was adjusted for age, sex, race, and multiple comorbidities that are listed in Supplemental Table 1. In both the overall and aged-stratified analysis, higher frailty risk scores were associated with an increasing risk for post-operative MACE. The highest adjusted odds ratio for the risk of post-operative MACE were found in patients aged 45 to 64.
Figure 3: Prevalence of MACE by frailty risk, stratified by age.
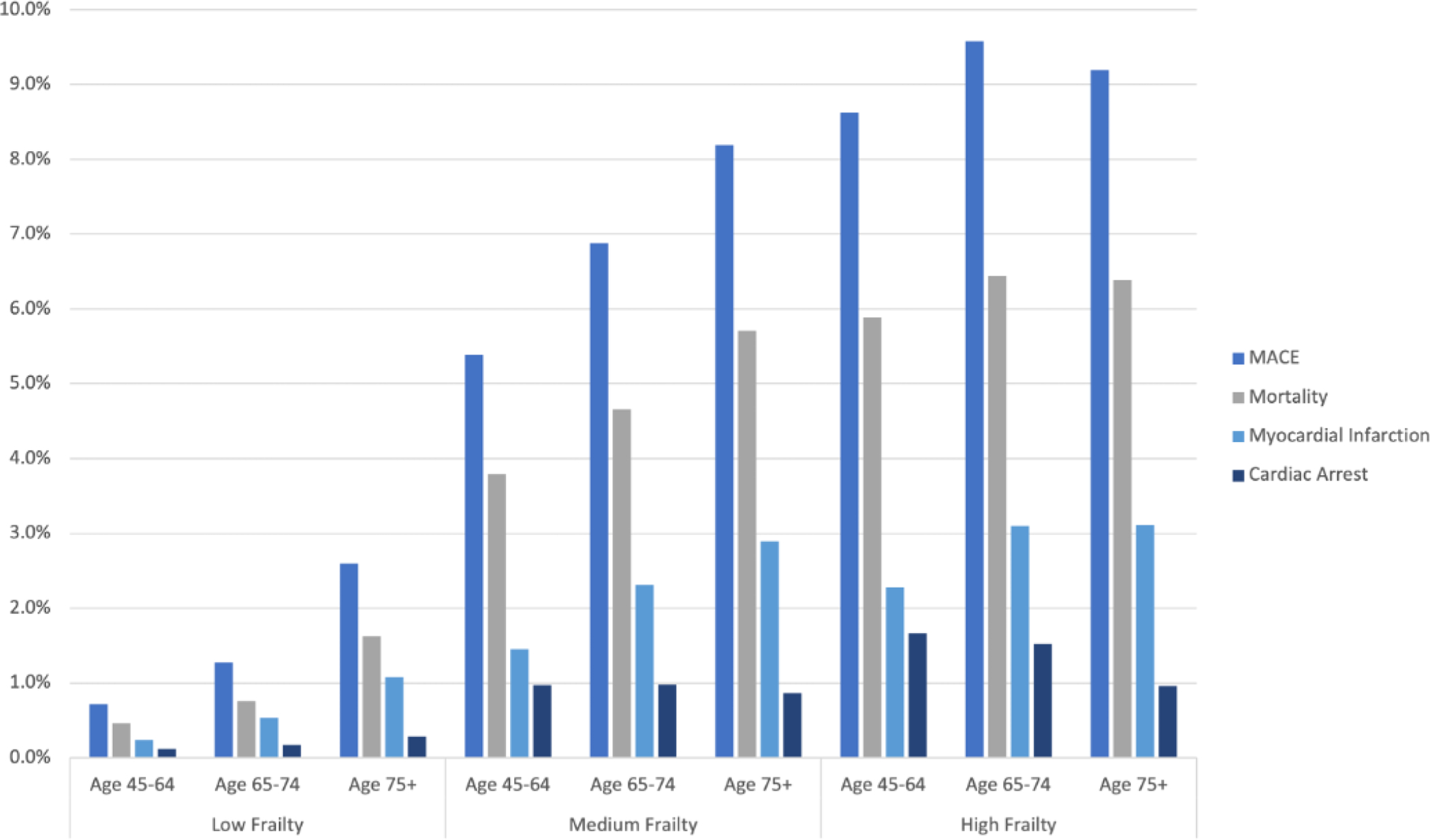
The prevalence of MACE and its individual components (death, acute myocardial infarction, and cardiac arrest) in patients undergoing non-cardiac surgery, are shown stratified by frailty score and age. The incidence of MACE and its individual components increased substantially in the medium and high-risk frailty score groups. The prevalence of these outcomes also increased with age within the low and medium frailty risk groups, but not within the high frailty risk score group.
The incidence of the individual endpoints of the composite of MACE, including myocardial infarction, cardiac arrest, and all-cause mortality, were higher in patients with elevated HFRS (Table 2, Figure 3, Supplemental Figure 5). Patients with a high HFRS had the greatest odds for mortality (aOR 2.98, 95% CI 2.92 – 3.04), myocardial infarction (aOR 2.12, 95% CI 2.07 – 2.16), and cardiac arrest (aOR 3.05, 95% CI 2.97 – 3.13) when compared to those with low HFRS (Supplemental Figure 6, Panels A–C). Associations between HFRS and perioperative mortality, myocardial infarction, and cardiac arrest were also observed in cohorts stratified by age.
Discharge disposition after hospitalization varied significantly by HFRS. Surgical hospitalizations with the highest HFRS were least likely result in a discharge to home (high frailty: 14.1%; medium frailty: 30.4%; low frailty: 62.9%, p<0.001) and were more likely to be discharged to a skilled nursing (SNF) or intermediate care facility (ICF) (high frailty: 63.5%; medium frailty: 44.5%; low frailty: 17.6%, p<0.001) than those with lower frailty scores (Table 2). In all frailty groups, older adults were more likely to be discharged to a SNF or ICF than younger individuals (Supplemental Figure 7).
DISCUSSION:
In this analysis of a large, nationwide database of United States surgical hospitalizations, nearly one-fifth of all patients undergoing non-cardiac surgery were identified as frail, and those with higher frailty risk scores had higher risk for perioperative MACE, including myocardial infarction, cardiac arrest, and all-cause mortality. Frail patients were less likely to be discharged home after surgery and were more likely to require post-operative care in a skilled nursing or intermediate care facility. The findings from our study add to the growing body of literature that highlights the impact of frailty on perioperative outcomes, morbidity, and mortality. The HFRS provides a convenient, standardized approach to systematically identify frail surgical inpatients at risk of adverse outcomes, and has the potential to be an important tool for frailty assessments in the inpatient setting.
In our study, patients with high frailty scores were older and had a higher burden of comorbidities than those with lower scores. Still, frailty was independently associated with MACE after adjustment for relevant demographics and other comorbidities, in the overall cohort and across age strata. These findings suggest that frailty offers a unique measure of physiological capacity that is not captured by age alone. Although the comorbidity profiles of frail patients may differ with age, high frailty scores were associated with adverse events in all age groups. In fact, among younger patients in whom operative risks may be underestimated, frailty assessments may have particularly important prognostic value. Furthermore, recent studies have suggested that frailty may be superior to age when predicting adverse outcomes in patients undergoing non-cardiac surgeries.15,16 Therefore, assessment of frailty is an emerging component of the pre-operative evaluation for adults across the age spectrum, and based on this and other studies, the HFRS may serve as a valuable tool in addition to other frailty assessments for risk stratification, regardless of age.
Frailty has traditionally been defined as a state of functional decline from both aging and disease-related changes over time. Recent studies suggest that frailty may represent a chronic disease state associated with systemic inflammation, leading to worsening morbidity among affected individuals.17,18 Several of the inflammatory markers that are elevated in frail patients are also closely linked to the development of cardiovascular disease.19,20 Frail patients are also more likely to exhibit medication non-compliance, have poor dietary habits, and sedentary lifestyles.21,22 Therefore, frailty may both directly contribute to and result from cardiovascular disease. Frailty is also associated with multi-morbidity, and the development of frailty in patients with multiple chronic diseases may also lead to significant disabilities. Although high frailty risk scores were associated with an increased number of comorbidities in our study, frailty was associated with MACE even after robust covariate adjustment.
While the value of frailty assessment in pre-operative risk stratification has been demonstrated, implementation challenges remain. Some frailty assessments are based on the phenotypic measures of frailty, such as weakness, weight loss, exhaustion, low physical activity, and slowed walking speed.23 In contrast, inpatient-based frailty assessments, such as the Identification Seniors at Risk and Triage Risk Stratification tools, often focus on comorbidity burdens associated with declines in physiologic capability.24–26 Other popular frailty scoring tools, such as the Clinical Frailty Scale, combine clinical judgement with accumulation of comorbidities and may be used in inpatient and outpatient settings. The Frailty Index by Rockwood et. al. is based on deficit accumulation model.27 Scoring models derived from electronic health record diagnoses can offer algorithmic approaches to frailty assessment during inpatient hospitalization.28,29 The HFRS provides a standardized approach to this type of frailty assessment by utilizing a widely used administrative coding system.9,25,30 Integration of frailty assessments, like the HFRS, with other traditional risk stratification tools may optimize the approach to pre-operative risk assessment in patients planned for non-cardiac surgery.31
Beyond in-hospital cardiovascular complications of surgery, perioperative frailty assessments may also provide insights into the likely discharge disposition after non-cardiac surgery.23,32,33 In this analysis, nearly 64% of all patients with high frailty risk scores required discharge to short-term acute care, skilled nursing, or intermediate care facilities, versus less than 18% of patients with low frailty scores. The dramatic differences in the need for placement in a skilled nursing or subacute rehabilitation center at discharge were observed by frailty risk score across all age strata. Ultimately, early interventions, such as pre-habilitation, physical therapy, and rehabilitation in the perioperative period, may mitigate post-operative care needs. However, the efficacy of interventions targeted at patients with evidence of frailty prior to surgery requires additional investigation in prospective trials.
There are a few limitations in our analysis. First, the NIS is a large administrative inpatient database that is subject to errors in coding and misclassification. Second, the NIS lacks discrete clinical data such as physical examination findings, vital signs, body mass index, 6-minute walk tests, measures of grip strength, and laboratory values. Third, although the original HFRS score was described using ICD-10 codes, for the purpose of these analyses, ICD-10 codes were translated to corresponding ICD-9 codes. Not all ICD-10 codes had an equivalent ICD-9 codes. Fourth, we did not include stroke in the primary composite endpoint for MACE, as the some of the ICD-9 codes used for stroke were also included in the HFRS to reflect neurological deficits contributing to frailty, and the acuity or chronicity of neurologic findings could not be determined from diagnosis codes. Fifth, the threshold values for risk categories used in our analysis differ slightly from those originally described for the HFRS. However, the lower risk thresholds descried herein better fit the distribution of frailty in our large, nationwide cohort of individuals age ≥45 years undergoing noncardiac surgery. Sixth, our analysis was limited to cases from 2004–2014, which may not capture contemporary practices in post-surgical care or outcomes. Seventh, although we report discharge disposition, we do not have data on the pre-hospital residential status, and therefore changes in residential status (for example new admission to a nursing facility) could not be determined. Finally, the NIS does not distinguish between outpatient and inpatient diagnoses or present on admission status. While many of the diagnoses relevant to the HFRS were likely made prior to the surgical hospitalization, we are unable to exclude the possibility that some diagnoses were established after an adverse cardiovascular event. We are therefore unable to exclude associations by reverse causality using this dataset. Prospective studies are needed to evaluate the HFRS calculated from pre-admission diagnoses at the time of presentation to confirm its potential utility as a pre-operative risk stratification tool embedded in the EHR.
In this analysis of a large US database, nearly one-fifth of all patients undergoing non-cardiac surgery had a combination of diagnoses indicative of at least moderate frailty defined by the HFRS. Higher frailty scores were associated with increased risks of perioperative MACE, myocardial infarction, cardiac arrest, and all-cause mortality independent of age and other comorbidities. Patients with high versus low HFRS were more likely to be discharged to acute care, skilled nursing, or intermediate care facilities after hospital discharge. While additional prospective studies are needed to validate these results, our findings suggest that the HFRS may identify frail surgical inpatients at risk for adverse perioperative cardiovascular outcomes.
Supplementary Material
Highlights.
A validated risk score for frailty is associated with an increased risk for the composite of in-hospital mortality, acute myocardial infarction, or cardiac arrest after non-cardiac surgery.
The association between high frailty and surgical outcomes were observed across age groups, with greater odds of cardiac events observed in younger individuals.
High frailty scores are associated with an increased likelihood of non-home discharge after non-cardiac surgery.
ABBREVIATIONS AND ACRONYMS
- EHR
electronic health record
- MACE
major adverse cardiovascular events
- AHRQ
Agency for Healthcare Research and Quality
- NIS
National Inpatient Sample
- ICD
International Classification of Disease
- HFRS
hospital frailty risk score
Footnotes
Declaration of Interest:
Dr. Smilowitz is supported, in part, by the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Number K23HL150315
Emaad Siddiqui: Methodology, Formal Analysis, Investigation, Writing – Original Draft, Visualization. Darcy Banco: Conceptualization, Writing – Review & Editing. Jefferey Berger: Conceptualization, Writing – Review & Editing, Supervision. Nathaniel Smilowitz: Conceptualization, Investigation, Formal Analysis, Writing – Review & Editing, Supervision, Project Administration
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES:
- 1.Steiner CA, Karaca Z, Moore BJ, Imshaug MC, Pickens G. Surgeries in Hospital-Based Ambulatory Surgery and Hospital Inpatient Settings, 2014: Statistical Brief #223. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. 2006. [PubMed] [Google Scholar]
- 2.Xue QL. The frailty syndrome: definition and natural history. Clin Geriatr Med. Feb 2011;27(1):1–15. doi: 10.1016/j.cger.2010.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Basic D, Shanley C. Frailty in an older inpatient population: using the clinical frailty scale to predict patient outcomes. J Aging Health. Jun 2015;27(4):670–85. doi: 10.1177/0898264314558202 [DOI] [PubMed] [Google Scholar]
- 4.Subramaniam S, Aalberg JJ, Soriano RP, Divino CM. New 5-Factor Modified Frailty Index Using American College of Surgeons NSQIP Data. J Am Coll Surg. Feb 2018;226(2):173–181 e8. doi: 10.1016/j.jamcollsurg.2017.11.005 [DOI] [PubMed] [Google Scholar]
- 5.Shahrokni A, Tin A, Alexander K, et al. Development and Evaluation of a New Frailty Index for Older Surgical Patients With Cancer. JAMA Netw Open. May 3 2019;2(5):e193545. doi: 10.1001/jamanetworkopen.2019.3545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gilbert T, Neuburger J, Kraindler J, et al. Development and validation of a Hospital Frailty Risk Score focusing on older people in acute care settings using electronic hospital records: an observational study. Lancet. May 5 2018;391(10132):1775–1782. doi: 10.1016/S0140-6736(18)30668-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sepehri A, Beggs T, Hassan A, et al. The impact of frailty on outcomes after cardiac surgery: a systematic review. J Thorac Cardiovasc Surg. Dec 2014;148(6):3110–7. doi: 10.1016/j.jtcvs.2014.07.087 [DOI] [PubMed] [Google Scholar]
- 8.Green P, Woglom AE, Genereux P, et al. The impact of frailty status on survival after transcatheter aortic valve replacement in older adults with severe aortic stenosis: a single-center experience. JACC Cardiovasc Interv. Sep 2012;5(9):974–81. doi: 10.1016/j.jcin.2012.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tjeertes EKM, van Fessem JMK, Mattace-Raso FUS, Hoofwijk AGM, Stolker RJ, Hoeks SE. Influence of Frailty on Outcome in Older Patients Undergoing Non-Cardiac Surgery - A Systematic Review and Meta-Analysis. Aging Dis. Oct 2020;11(5):1276–1290. doi: 10.14336/AD.2019.1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.(HCUP) HUP. National Inpatient Sample. Agency for Healthcare Research and Quality. 2004–2014; [Google Scholar]
- 11.Smilowitz NR, Gupta N, Ramakrishna H, Guo Y, Berger JS, Bangalore S. Perioperative Major Adverse Cardiovascular and Cerebrovascular Events Associated With Noncardiac Surgery. JAMA Cardiol. Feb 01 2017;2(2):181–187. doi: 10.1001/jamacardio.2016.4792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gupta PK, Gupta H, Sundaram A, et al. Development and validation of a risk calculator for prediction of cardiac risk after surgery. Circulation. Jul 26 2011;124(4):381–7. doi: 10.1161/CIRCULATIONAHA.110.015701 [DOI] [PubMed] [Google Scholar]
- 13.Alqahtani F, Aljohani S, Tarabishy A, Busu T, Adcock A, Alkhouli M. Incidence and Outcomes of Myocardial Infarction in Patients Admitted With Acute Ischemic Stroke. Stroke. Nov 2017;48(11):2931–2938. doi: 10.1161/STROKEAHA.117.018408 [DOI] [PubMed] [Google Scholar]
- 14.Fugate JE, Brinjikji W, Mandrekar JN, et al. Post-cardiac arrest mortality is declining: a study of the US National Inpatient Sample 2001 to 2009. Circulation. Jul 31 2012;126(5):546–50. doi: 10.1161/CIRCULATIONAHA.111.088807 [DOI] [PubMed] [Google Scholar]
- 15.Sundermann SH, Dademasch A, Seifert B, et al. Frailty is a predictor of short- and mid-term mortality after elective cardiac surgery independently of age. Interact Cardiovasc Thorac Surg. May 2014;18(5):580–5. doi: 10.1093/icvts/ivu006 [DOI] [PubMed] [Google Scholar]
- 16.Hewitt J, Carter B, McCarthy K, et al. Frailty predicts mortality in all emergency surgical admissions regardless of age. An observational study. Age Ageing. May 1 2019;48(3):388–394. doi: 10.1093/ageing/afy217 [DOI] [PubMed] [Google Scholar]
- 17.McAdams-DeMarco MA, Ying H, Thomas AG, et al. Frailty, Inflammatory Markers, and Waitlist Mortality Among Patients With End-stage Renal Disease in a Prospective Cohort Study. Transplantation. Oct 2018;102(10):1740–1746. doi: 10.1097/TP.0000000000002213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soysal P, Stubbs B, Lucato P, et al. Inflammation and frailty in the elderly: A systematic review and meta-analysis. Ageing Res Rev. Nov 2016;31:1–8. doi: 10.1016/j.arr.2016.08.006 [DOI] [PubMed] [Google Scholar]
- 19.Ferrucci L, Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol. Sep 2018;15(9):505–522. doi: 10.1038/s41569-018-0064-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Phan HM, Alpert JS, Fain M. Frailty, inflammation, and cardiovascular disease: evidence of a connection. Am J Geriatr Cardiol. Mar-Apr 2008;17(2):101–7. [PubMed] [Google Scholar]
- 21.Jankowska-Polanska B, Dudek K, Szymanska-Chabowska A, Uchmanowicz I. The influence of frailty syndrome on medication adherence among elderly patients with hypertension. Clin Interv Aging. 2016;11:1781–1790. doi: 10.2147/CIA.S113994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kehler DS, Hay JL, Stammers AN, et al. A systematic review of the association between sedentary behaviors with frailty. Exp Gerontol. Dec 2018;114:1–12. doi: 10.1016/j.exger.2018.10.010 [DOI] [PubMed] [Google Scholar]
- 23.Makary MA, Segev DL, Pronovost PJ, et al. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg. Jun 2010;210(6):901–8. doi: 10.1016/j.jamcollsurg.2010.01.028 [DOI] [PubMed] [Google Scholar]
- 24.Cesari M, Gambassi G, van Kan GA, Vellas B. The frailty phenotype and the frailty index: different instruments for different purposes. Age Ageing. Jan 2014;43(1):10–2. doi: 10.1093/ageing/aft160 [DOI] [PubMed] [Google Scholar]
- 25.Warnier RM, van Rossum E, van Velthuijsen E, Mulder WJ, Schols JM, Kempen GI. Validity, Reliability and Feasibility of Tools to Identify Frail Older Patients in Inpatient Hospital Care: A Systematic Review. J Nutr Health Aging. Feb 2016;20(2):218–30. doi: 10.1007/s12603-015-0567-z [DOI] [PubMed] [Google Scholar]
- 26.McCusker J, Bellavance F, Cardin S, Trepanier S, Verdon J, Ardman O. Detection of older people at increased risk of adverse health outcomes after an emergency visit: the ISAR screening tool. J Am Geriatr Soc. Oct 1999;47(10):1229–37. doi: 10.1111/j.1532-5415.1999.tb05204.x [DOI] [PubMed] [Google Scholar]
- 27.Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. Aug 30 2005;173(5):489–95. doi: 10.1503/cmaj.050051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rockwood K, Mitnitski A. Frailty defined by deficit accumulation and geriatric medicine defined by frailty. Clin Geriatr Med. Feb 2011;27(1):17–26. doi: 10.1016/j.cger.2010.08.008 [DOI] [PubMed] [Google Scholar]
- 29.Clegg A, Bates C, Young J, et al. Development and validation of an electronic frailty index using routine primary care electronic health record data. Age Ageing. May 2016;45(3):353–60. doi: 10.1093/ageing/afw039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Belga S, Majumdar SR, Kahlon S, et al. Comparing three different measures of frailty in medical inpatients: Multicenter prospective cohort study examining 30-day risk of readmission or death. J Hosp Med. Aug 2016;11(8):556–62. doi: 10.1002/jhm.2607 [DOI] [PubMed] [Google Scholar]
- 31.Hall DE, Arya S, Schmid KK, et al. Development and Initial Validation of the Risk Analysis Index for Measuring Frailty in Surgical Populations. JAMA Surg. Feb 1 2017;152(2):175–182. doi: 10.1001/jamasurg.2016.4202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ekerstad N, Swahn E, Janzon M, et al. Frailty is independently associated with 1-year mortality for elderly patients with non-ST-segment elevation myocardial infarction. Eur J Prev Cardiol. Oct 2014;21(10):1216–24. doi: 10.1177/2047487313490257 [DOI] [PubMed] [Google Scholar]
- 33.Curtis E, Romanowski K, Sen S, Hill A, Cocanour C. Frailty score on admission predicts mortality and discharge disposition in elderly trauma patients over the age of 65 y. J Surg Res. Oct 2018;230:13–19. doi: 10.1016/j.jss.2018.04.017 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


