Abstract
Together, data from brain scanners and smartphones have sufficient coverage of biology, psychology, and environment to articulate between-person differences in the interplay within and across biological, psychological, and environmental systems thought to underlie psychopathology. An important next step is to develop frameworks that combine these two modalities in ways that leverage their coverage across layers of human experience to have maximum impact on our understanding and treatment of psychopathology. We review literature published in the last three years highlighting how scanners and smartphones have been combined to date, outline and discuss the strengths and weaknesses of existing approaches, and sketch a network science framework heretofore underrepresented in work combining scanners and smartphones that can push forward our understanding of health and disease.
Keywords: magnetic resonance imaging, ambulatory assessment, network analysis, experience-sampling
“Bit by bit, putting it together
Piece by piece, only way to make a work of art
Every moment makes a contribution
Every little detail plays a part…
Putting it together
That’s what counts!”
- Stephen Sondheim, Putting it Together
Humans are complex systems, with feelings, thoughts, and actions that are interconnected and that change over time (1–3). Changes that occur in these complex systems are the product of dynamic processes that span multiple levels of analysis: biological, psychological, and environmental. An essential goal of biological psychiatry is to understand how between-person differences in the interplay within and across these levels leads some people to experience chronic difficulties in adaptively changing their behavior to meet life’s changing demands. Two influential methodological approaches have been used to meet this goal. One approach uses brain scanners to primarily capture aspects of the biological and psychological layers of human systems, identifying neural correlates of deviations in cognition, affect, and behavior accompanying clinical disorders. Creative designs incorporate aspects of the environmental layer of human systems into this work with scanners, simulating social exclusion by using ball tossing games in which participants are excluded from play (4), exposing participants to aversive odors while in the scanning environment (5), and using complex media (film, television, podcasts) as stimuli (6), for example. However, situating these data within the sociocultural milieu of human experience to understand how the interplay between biological, psychological, and environmental layers of human experience produces clinical symptoms remains a challenge. A second approach, smartphone-based techniques, captures individuals’ current symptoms, as well as the psychosocial correlates of those experiences, in naturalistic environments (7). Work in this modality has characterized the interplay between psychological and environmental systems but, unlike work with scanners, does not tie these relations back to the biological level of analysis.
Together, scanners and smartphones have sufficient coverage of biology, psychology, and environment to articulate between-person differences in the interplay within and across biological, psychological, and environmental systems thought to underlie psychopathology. An important next step is to develop frameworks that combine these two modalities in ways that leverage their coverage across important layers of human experience to have maximum impact on our understanding and treatment of psychopathology. What might this combination of scanners and smartphones look like? By undertaking a systematic review of literature using data combining brain scanners (inclusive of brain imaging modalities and related methods to record neural processes) and smartphones (inclusive of experience-sampling and related ambulatory assessments delivered via smartphone or other portable digital technologies) published in the last three years, we identified existing approaches to combining smartphones and scanners in psychiatry research (see supplement for details of the systematic review). With these findings from the extant literature in hand, we 1) outline and discuss the strengths and weaknesses of existing approaches and 2) sketch a network science framework heretofore underrepresented in work combining scanners and smartphones that can capture the richness of the multiple interacting units across the biological, psychological, and environmental systems highlighted in theories of psychopathology.
Existing approaches to combining scanners and smartphones
Six approaches to combining scanners and smartphones were identified in the extant literature.
Bivariate associations.
By far the most common way of combining scanners and smartphones was by estimating bivariate associations between indices from scanners and smartphones either via correlation or regression approaches (Fig. 1A). This approach to combination was concerned with the extent to which in-scanner data could predict “real-world” behavior (see (8) for example). The inclusion of smartphone data, often collected as participants went about their daily lives, allowed an assessment of the extent to which scanner data, high in experimental control but low in ecological validity, predicted ecologically valid experiences. When links were observed between scanner and smartphone data, this was sometimes interpreted as evidence for identifying mechanistic insight into real-world behavior. For example, an observed association between the blood oxygen level response (BOLD) response in reward-related regions during reward anticipation and daily reports of motivation and pleasure was taken as evidence that differences observed in the scanner had explanatory power for the differences observed in daily life behavior (9). While such bivariate associations are suggestive, combining scanner and smartphone data in this way remains correlative and should be interpreted cautiously when attempting to make mechanistic rather than solely predictive claims. A less common approach predicted scanner data from smartphone data (10,11). These efforts highlighted the greater feasibility of intensively sampling smartphone data as compared to obtaining longitudinal scanner data and the implications of this feasibility for tracking the course of clinical outcomes. For example, keyboard dynamics emerged as reliable measures that distinguished multiple sclerosis patients from controls, with the potential to be valid surrogate markers for clinical disability in multiple sclerosis than less feasible but common neuroimaging assessments (10).
Figure 1.
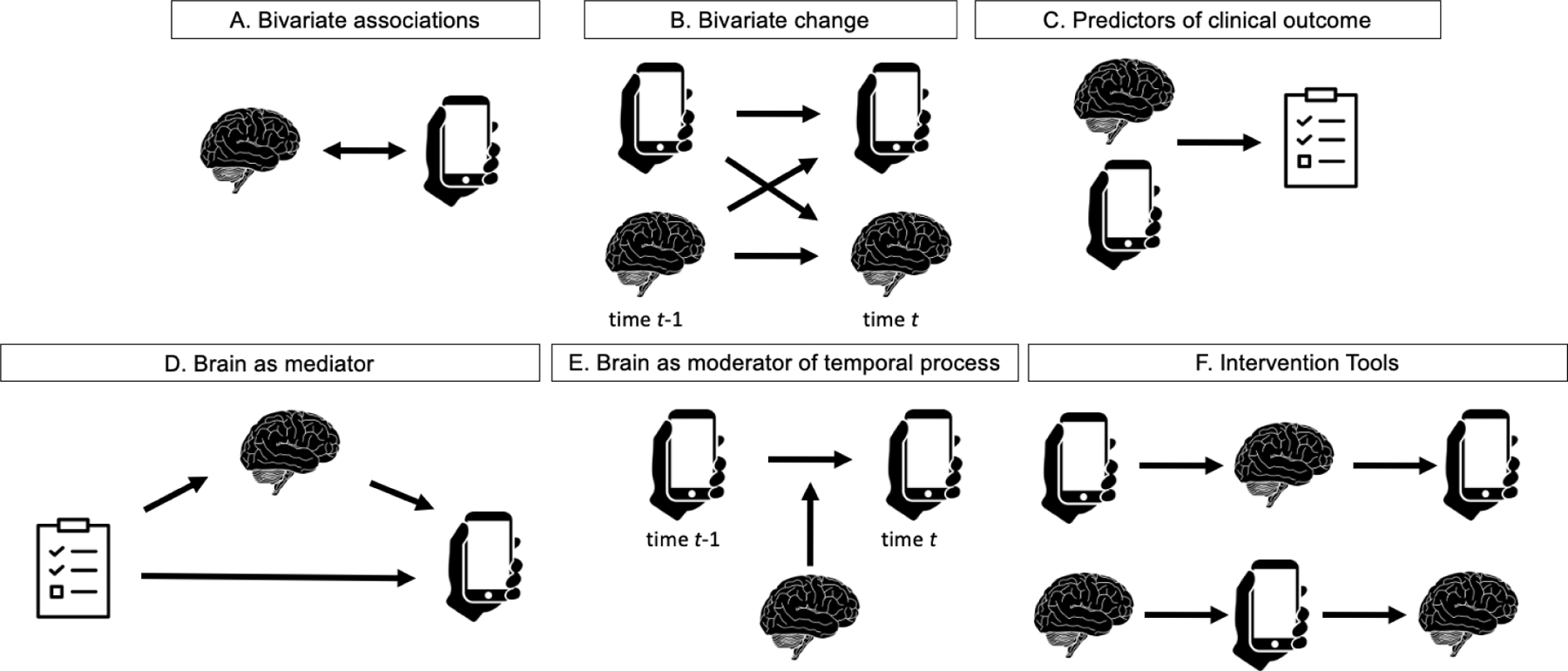
Existing approaches to combining scanners and smartphones emerging from systematic review of the literature combining scanners and smartphones.
The smartphone data in work estimating bivariate associations between scanners and smartphones generally consisted of static indices: proportion of positive experiences with classmates (12), average subjective stress (13), and average momentary subclinical psychosis (14). Thus, their inclusion, as compared to less burdensome retrospective survey reports, increased ecological validity and reduced retrospective biases often introduced in questionnaires that ask participants to recall and aggregate information about longer periods of time (e.g., previous 30 days (15)). However, only a few studies made use of one of the features unique to smartphone data over traditional survey measures: the ability to capture dynamics. Through repeated assessment of participants as they go about their daily lives, smartphone data collection results in rich time series data that can capture moment-to-moment or day-to-day fluctuations in clinical symptoms (16) social experiences (17) and changes in the environment (18,19). Biological psychiatry has a keen understanding that it is not simply the presence or absence of symptoms that characterizes clinical disorders. Instead, the temporal characteristics of symptoms across time are key considerations. For example, affective lability (i.e., intense, frequent, and reactive shifts in affect) is commonly observed in borderline personality disorder (20) while a diagnostic marker of depression is sustained depressed mood nearly every day (21). Capturing this lability or lack of change requires the ability to intensively sample affect over time, a task for which smartphone-based approaches are exquisitely suited.
The use of dynamic indices from smartphone data, particularly of affective experiences, is beginning to emerge in studies combining scanners and smartphones with bivariate correlations or regression (22–24). Even less common in the reviewed papers than temporal smartphone features were temporal scanner features (see for exception (24)). Although it is common to aggregate BOLD data across the entire duration of a scanning session, the brain exhibits dynamics over many timescales, from the sub-second to the lifespan (25,26). Just as biological psychiatry recognizes the importance of dynamics in behavior and symptoms as important for the understanding of psychopathology, the time-varying organization of functional brain systems in depression and schizophrenia deviates from healthy controls, for example (27,28). Attention can be directed to brain dynamics by, for example, taking a sliding window approach, subdividing data from a neuroimaging scan into smaller windows of time and computing functional connectivity indices within each window (29). Alternative, model-based approaches are also possible, computing the dynamics that a brain is capable of given its network structure and assumptions of how activity travels along that structure (30).
Studying brain dynamics has advantages, capturing temporal information about functional connectivity that predicts psychiatric states and conditions, often above and beyond status functional connectivity (31,32). However, in parallel to these affordances to studying brain dynamics are several limitations. For instance, the field lacks consistent analytical approaches, resulting in inconsistent treatment of time and consideration of temporal ordering in analytical pipelines. Further, there is little consensus surrounding appropriate null models for evaluating aspects of time-varying functional connectivity. It also remains an open question what nonneural factors drive changes in resting time-varying functional connectivity (see (33) for a review on questions and controversies in the study of time-varying functional connectivity).
Bivariate change.
The second way of combining scanner and smartphone data overcomes some of the limitations of cross-sectional, bivariate combinations by collecting scanner and smartphone data at multiple assessment periods and calculating bivariate change (Fig. 1B). For example, change in keystroke dynamics were associated with changes in disease activity as assessed by scanners (34,35). The temporal precedence afforded by multiple timepoints of data provides stronger evidence that the bivariate association between brain and smartphone index represents a causal association. Perhaps surprisingly, given the key role for dynamics in psychiatric disorders, collection of even two timepoints of smartphone and scanner data was not common in the reviewed papers. By combining longitudinal scanners and smartphone data, researchers could determine how fluctuations in behavior in real-world contexts both influence and are influenced by changes in brain function. In the ideal case, these data would take the form of intensive sampling of both brain and behavior, facilitating an examination of how naturalistic day-to-day fluctuations in experience (e.g., fluctuations in positive mood) are associated with fluctuations in aspects of functional brain architecture (e.g., brain network flexibility (36)). Such intensive sampling (encompassing multiple laboratory visits) could prove prohibitive, especially when aiming to reduce burdens placed on individuals experiencing psychopathology. However, creative approaches can be used. One such example that emerged in the review captured cognitive performance and neural correlates associated with naturalistic fluctuations in daily stress (37). To reduce the number of laboratory visits necessary to capture within-person differences in stress, participants provided stress ratings three times per day for two weeks, allowing investigators to identify a high-stress and a low-stress day, during which participants were brought to the laboratory to undergo scanning.
Predictors of clinical outcomes.
A third approach combined data from scanners and smartphones by treating them as features that could independently predict clinically-relevant outcomes (Fig. 1C). For example, the average feeling of peer connectedness across a 10-day daily diary and the BOLD response to positive peer feedback during an in-scanner social incentive delay task were used as predictors of suicidal idea in a regression analysis (38). These efforts build on brain-as-predictor applications that show that neuroimaging indices are often predictive of health-relevant behaviors above and beyond self-reports, explaining previously unaccounted for variance in behavioral outcomes (39,40). In the examples of this approach in the reviewed manuscripts, smartphone data consisted of aggregated data across the data collection period. Thus, although these smartphone data are high on ecological validity, their predictive capacity could be improved by creating dynamic features from the intensive longitudinal data (41).
Brain as mediator.
A fourth approach treated data from scanners or smartphones as mediators, or explanatory variables in a causal chain (Fig. 1D). Two examples emerged. The first tested the extent to which gender’s association with a greater proportion of positive experiences with peers as assessed via smartphones was mediated by greater nucleus accumbens-precuneus functional connectivity (12). The second tested the extent to which negative affect inertia mediated the association between default mode system efficiency and depression (42). Although both cross-sectional mediation analyses lack the longitudinal data required to test the causal process unfolding over time that mediation analyses implicitly endorse (43), combining scanners and smartphones in this way revealed theoretical positions whereby between-person differences in brain organization are thought to be causally implicated in between-person differences in real-world behaviors associated with psychopathology.
Brain as moderator of temporal process.
A fifth approach made use of the temporal richness of experience-sampling data to examine dynamic processes and tested the extent to which the brain might moderate these processes (Fig. 1E). For example, one study assessed repetitive negative thinking and sadness 10 times per day over 4 consecutive days (44). These dense repeated measures, coupled with appropriate analytic techniques (see (45) for review), facilitated a focus on between-person differences in how moments of increased sadness at one moment led to increases in repetitive negative thinking at the next moment. Including functional connectivity indices associated with cognitive flexibility as moderators of this sadness to repetitive negative thinking link allowed tests for the role of large-scale functional brain network activity as a moderator of real-world, dynamic cognitive-affective processes.
Intervention tools.
A final approach treated scanners and smartphones as intervention tools (Fig. 1F). For example, one study delivered a daily compassion meditation intervention to participants via smartphone over a four-week period (46). By bookending this smartphone intervention with functional brain scans, this design facilitated testing of the extent to which changes induced by the smartphone intervention became codified in the brain. In an example where the scanner was used as the intervention tool, one study examined the ability for in-scanner neurofeedback to change the extent of affective instability, as assessed by smartphone before and after neurofeedback training in patients with borderline personality disorder (47).
Advantages and opportunities for advancement.
By reviewing existing approaches to combining scanners and smartphones, we find that the field of biological psychiatry is making use of several advantages that stem from the unique combination of scanners and smartphones to provide insight into clinical outcomes. These advantages include increasing the ecological validity of behaviors being predicted by neuroimaging assessments, leveraging the different facets of human functioning captured by scanners and smartphones to improve prediction of clinical outcomes, and providing insight into dynamic processes and their neural correlates. There remain exciting opportunities for combining scanners and smartphones, especially by focusing on the unique opportunities afforded by intensive repeated measures available through both scanners and smartphones in the field of psychiatry. One particularly difficult challenge revealed by the current review is the implicit assumption that fluctuations in behavior are more substantial than fluctuations in brain function and organization. This assumption can be seen in the intensive sampling of behavior from moment-to-moment and day-to-day in most smartphone studies reviewed as compared to the often static, within-scanner assessments. Presumably, fluctuations in behavior observed in smartphone assessments derive, at least partially, from fluctuations in brain function, necessitating methodologies capable of more directly matching fluctuations in brain to fluctuations in behavior than is currently represented in the literature combining scanners and smartphones. A key methodological development to overcome this conceptual limitation is leveraging emerging brain modalities that can now more easily be deployed outside the lab (e.g., portable eye-tracking, fNIRS, and mobile EEG (48–50)) and assess fluctuations in brain function concurrent with fluctuations in behavior.
One specific way forward we highlight in the rest of the manuscript is a dynamic network approach that more directly connects the combination of these data modalities with theoretical perspectives that highlight that humans are complex systems made of many interacting components within and across biological, psychological, and environmental systems.
Ways forward: Networks and bringing together facets of human experience
Network science has emerged as a framework with the potential to characterize the complex interactions occurring across biological, psychological, and environmental systems (51). Advances in neuroimaging (52,53) and the development of appropriate tools to describe and model the parts and pathways for communication of the brain (54) have resulted in the booming field of network neuroscience, a field mapping, recording, analyzing, and modeling the elements and interactions of neural systems (51). As the name suggests, network neuroscience relies on formal representations of the brain as a network to capture the parts (nodes) and interrelationships of these parts (edges). From neuroimaging data, one can construct a graph, a simple mathematical representation of a network composed of nodes representing system elements and edges representing element relations or interactions. Nodes are typically parcels of gray matter voxels, ranging from single voxels to larger clusters of voxels. Associations among nodes (edges) are established in several ways, taking the form of either structural or functional connectivity. Structural connectivity describes anatomical, or physical connections between nodes or neural elements (55). With magnetic resonance imaging data, anatomical connections usually refer to white-matter fiber tracts that physically link brain regions and are derived from applying tractography algorithms to diffusion images. Functional connectivity, by contrast, represents communication or coordination between nodes and edges are defined based on statistical similarities in the time series of nodes (56).
Seven studies in our review applied network neuroscience approaches to scanner data (14,24,42,44,57–59). The benefits of network science are not specific to data from scanners. Although no studies in our review used networks to analyze smartphone data, the network perspective so relevant for the brain can be extended to behavior, emotion, cognitive, and environmental systems more broadly. There is a burgeoning literature, for example, that takes a network perspective of mental disorders (60). This network perspective conceives of mental disorders as a complex system of mutually reinforcing and interacting symptoms (61,62). In these networks, symptoms make up nodes of the networks. Unlike structural brain networks, there are no physical edges connecting emotions, thoughts, or actions. Instead, the collection of multiple reports of the intensity of certain emotions, thoughts, and actions via smartphones facilitates the estimation of edges in a way analogous to the construction of edges in functional networks of the brain: inferring coordination or causal associations among symptoms across time by estimating correlations, partial correlations, or regression coefficients characterizing both time-lagged and contemporaneous associations among nodes (63).
Extending this network perspective to smartphone data in studies combining scanners and smartphones will align the analytic treatment of smartphone data with theoretical notions of humans as complex systems. It will also expand the feature space used in existing work using smartphone data to predict clinical outcomes (e.g., Fig. 1C). But perhaps the most exciting potentiality of constructing both brain and behavior networks in work using scanners and smartphones is the avenues that would open for new ways of combining scanner and smartphone data. Networks need not be limited to one level or layer of the complex biological, psychological, and environmental components implicated in psychopathology. Instead, multilayer network approaches allow the combination of scanner and smartphone networks (64–70). In multilayer networks, each layer constitutes a different network. For example, a network constructed from a different participant, patient group, experimental condition, time point, or data modality. A node can exist in all layers or in a subset of layers and may be linked throughout layers by an edge representing the node’s identity. Multiple types of edges can link nodes within and between layers to represent different types of associations between network elements. Multilayer network approaches have successfully been applied to a diverse range of fields, including neuroscience, ecology, public health, biology, and political science among others (64,71–74).
Recent multilayer applications provide insight into why they may be useful for connecting scanner and smartphone networks. A recent study built a network of networks in which the cognitive nodes were scores from multiple cognitive tasks, including matrix reasoning and digit recall, and neural nodes were region-based cortical volume of several brain regions and fractional anisotropy (proxy for white matter integrity) of several brain regions (75). Partial correlation networks were estimated such that the edges represented conditional dependencies among the cognitive and neural variables. The resulting network was a complex, multi-layer structure of interdependent facets of brain and behavior. There are several benefits to this multilayer approach. First, the ability to combine different layers of human systems within the same overall multilayer network allows the application of network statistics to be applied to one object. This directly addresses the inherent dependency between biological, psychological, and environmental layers of human systems. Second, multilayer frameworks open the ability to probe and predict how perturbations at one node in one network layer (e.g., brain) might impact another node in another network layer (e.g., behavior), helping to guide where it may be best to intervene. Placing brain and behavior in the same analytic paradigm also avoids a hierarchical prioritization of brain or behavior, as observed in mediational approaches which posited a more central role for neuroimaging facets as causes for clinical outcomes than behavior (e.g., Fig. 1D), which reflects a reductionist thinking that does not reflect the interdependent nature of biology, psychology, and environment (76). And, perhaps most importantly, it provides a framework to test for between-person differences in the interplay within and across biological, psychological, and social levels of analysis which may provide fruitful for understanding clinical disorders.
Although a variety of neuroimaging modalities were used in the studies we reviewed (i.e., functional connectivity, structural, resting state, and lesion), in principle graph theoretical network analysis can be used for any imaging modality. In the typical application of network analysis, brain regions are represented as nodes and the connections between brain regions are represented as edges. The nature of the edges differs across imaging modalities. For example, in functional networks, edges typically represent statistical similarities in the BOLD times series of nodes while, in structural networks, edges are often estimated by reconstructing the trajectory of axonal tracts using indices of the diffusion of water molecules within fibers (77,78). Despite each of these approaches resulting in a network, each modality captures a different spatial and temporal scale of the multilevel brain (25, 51) such that the choice of modality will be driven by the researcher’s specific question. Where needed for the question at hand, a multilayer perspective allows multiple network layers from different modalities, capturing aspects of brain network organization at various temporal and spatial levels, to be considered in-tandem, each modality making up a layer (e.g., 79,80).
Thus, the groundwork has been laid to combine multimodal data from scanners and smartphones into a multilayer human system network (see Figure 3) that is capable of integrating the many facets of human experience (25,81–83). Of course, incorporating multilayer networks into biological psychiatry work incorporating scanners and smartphones will not be without difficulty. An important challenge researchers are currently tackling (see (84) for example) is precisely how to best connect distinct layers with one another to form a multilayer structure, especially in a way that maintains the within-person temporal associations among facets of experience that can be estimated from smartphone time series data in a way that cannot be achieved using traditional, retrospective survey measures.
Despite the strengths of multilayer network approaches, namely the flexibility to integrate different types of high dimensional data, there are some cautions that warrant further discussion. On the side of feasibility, there is inherent difficulty in collecting the types of high dimensional data ripe for multiplex network analysis (e.g., electronic health records, connected wearable gadgets, brain scanners, and smartphones). As the number of available measures increases, the choices to examine similarities across the layers also increases, and these indices may largely be based on what researchers are interested in, which can have a large influence on the results. For example, estimating the correlations in a multilayer network requires estimating all the edges across layers and not just the correlations among emotions in the emotion layer (Figure 2). In this way, communities in multilayer networks can occur within and between layers (70), nodes can have relations (edges) within and across layers, and nodes can communicate with one another even if they do not have direct edges between them across layers (85). Some research questions may necessitate collapsing multiple layers into a single layer describing the clustering of patient health states (e.g., cardiovascular disease, affective disorders, cerebrovascular disease (86)) whereas other questions may seek to incorporate multiple layers to uncover the combined impacts of genetics and lifestyle factors on disease to build comorbidity clinical profiles (87). These example applications of multilayer networks highlight the critical consideration that the relevant layers need to be measured with enough samples to achieve statistical power, which may require extra effort especially when collecting multimodal data. Consequently, researchers may seek to maximize efficiency by performing many analyses on the data, making multiple correction and preregistration especially relevant when using multilayer networks. Confronting such challenges is inherent to multidimensional systems modeling.
Figure 2.
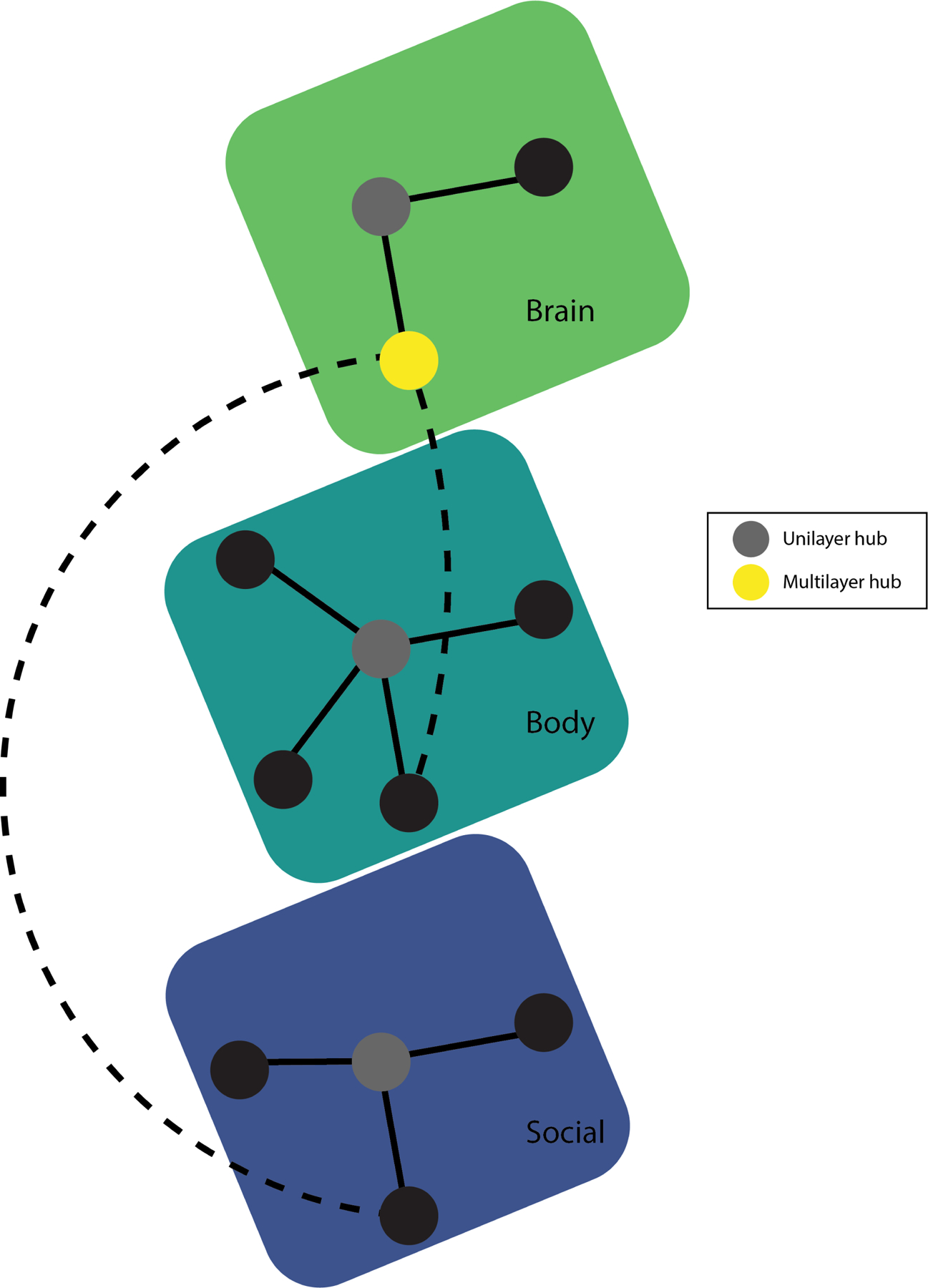
Example of a multilayer human system network, encoding information about psychology (symptom network), biology (brain network), and environment (social network), and the interlayer links between them. Figure adapted from (88). A multilayer network framework that incorporates possible mechanisms beyond symptoms may offer additional explanatory insight in biological psychiatry. For example, densely connected symptom networks is associated with greater vulnerability to develop psychopathology (89,90) than less-dense networks. Similarly, many psychiatric disorders share brain network alterations in functional connectivity (e.g., altered functional connectivity in the default mode network has been observed in Alzheimer disease, autism, schizophrenia, depression, and epilepsy (91,92). Considering the social network layer, living alone and away from family have been observed in alcohol dependence (93) and low and high depressive symptoms have been strongly correlated with such scores in friends and neighbors (94). A multilayer network approach that integrates psychological, biological, and environmental can offer insight into shared and distinct features in each layer across psychiatric disorders. In this way, a multilayer network framework can identify common targets for intervention, be it in symptom, brain, or social networks as well as offer insight into explanations for psychiatric comorbidity.
Conclusion
Our review of recently published literature combining scanners and smartphones indicates that the field of biological psychiatry is making use of several advantages that stem from the unique combination of scanners and smartphones to provide insight into clinical outcomes. There remains room to grow in the ways scanners and smartphones are combined that will more directly connect the combination of these data modalities with theoretical perspectives that highlight that humans are complex systems made of many interacting components within and across biological, psychological, and environmental systems. In particular, network perspectives, especially with reference to smartphone data, were not represented in the reviewed work, highlighting a key gap to be filled. We look forward to continued work with scanners and smartphones and the potential this work holds for characterizing how between-person differences in the interplay within and across biological, psychological, and environmental levels leads some people to experience chronic difficulties in adaptively changing their behavior to meet life’s changing demands.
Supplementary Material
Acknowledgements
The authors acknowledge support from the Army Research Office under Grant Number W911NF-18-1-0244, the National Institute on Drug Abuse (K01 DA047417), the Brain & Behavior Research Foundation, the John D. and Catherine T. MacArthur Foundation, the Swartz Foundation, the Paul G. Allen Family Foundation, the Alfred P. Sloan Foundation and the NSF (IIS-1926757). The views and conclusions contained in this document are those of the authors and should not be interpreted as representing the official policies, either expressed or implied, of the Army Research Office or the U.S. Government. The U.S. Government is authorized to reproduce and distribute reprints for Government purposes notwithstanding any copyright notation herein.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Baltes PB, Lindenberger U, Staudinger UM (2006). Lifespan theory in developmental psychology In Lerner RM (Ed.), Handbook of child psychology. Vol. 1: Theoretical models of human development (6th ed, pp. 569–664). New York, NY: Wiley. [Google Scholar]
- 2.Ford DH, Lerner RM (1992). Developmental systems theory: An integrative approach Newbury Park, CA: Sage. [Google Scholar]
- 3.Magnusson D, Cairns RB (1996). Developmental science: Toward a unified framework. Developmental Science Cambridge University Press, pp. 7–30. [Google Scholar]
- 4.Eisenberger NI., Lieberman MD, Williams KD (2003): Does rejection hurt? An fMRI study of social exclusion. Science 302: 290–292. [DOI] [PubMed] [Google Scholar]
- 5.Weigard A, Wilson SJ, Shapiro Z, Galloway-Long H, Huang-Pollock C (2021): Neural correlates of working memory’s suppression of aversive olfactory distraction effects. Brain Imaging and Behavior 15: 2254–2268. [DOI] [PubMed] [Google Scholar]
- 6.Grall C, Finn ES (2022): Leveraging the power of media to drive cognition: a media-informed approach to naturalistic neuroscience. Social Cognitive and Affective Neuroscience 17: 598–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Santangelo PS, Ebner-Priemer UW, Trull TJ (2013): Experience sampling methods in clinical psychology. The Oxford handbook of research strategies for clinical psychology Oxford University Press, pp. 188–210. [Google Scholar]
- 8.Krönke KM, Wolff M, Mohr H, Kräplin A, Smolka MN, Bühringer G, Goschke T (2020): Predicting real-life self-control from brain activity encoding the value of anticipated future outcomes. Psychological Science 31: 268–279. [DOI] [PubMed] [Google Scholar]
- 9.Moran EK, Culbreth AJ, Kandala S, Barch DM (2019): From neuroimaging to daily functioning: A multimethod analysis of reward anticipation in people with schizophrenia. Journal of Abnormal Psychology 128: 723–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lam KH, Meijer KA, Loonstra FC, Coerver EME, Twose J, Redeman E, et al. (2021): Real-world keystroke dynamics are a potentially valid biomarker for clinical disability in multiple sclerosis. Multiple Sclerosis Journal 27: 1421–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Obuchi M, Huckins JF, Wang W, daSilva A, Rogers C, Murphy E, et al. (2020): Predicting brain functional connectivity using mobile sensing. Proceedings of the ACM on Interactive, Mobile, Wearable and Ubiquitous Technologies 4: 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alarcón G, Morgan JK, Allen NB, Sheeber L, Silk JS, Forbes EE (2020): Adolescent gender differences in neural reactivity to a friend’s positive affect and real-world positive experiences in social contexts. Developmental cognitive neuroscience 43: 100779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin-Soelch C, Guillod M, Gaillard C, Recabarren RE, Federspiel A, Mueller-Pfeiffer C, et al. (2021): Increased Reward-Related Activation in the Ventral Striatum During Stress Exposure Associated With Positive Affect in the Daily Life of Young Adults With a Family History of Depression. Preliminary Findings. Frontiers in Psychiatry 11: 563475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Michielse S, Lange I, Bakker J, Goossens L, Verhagen S, Wichers M, et al. (2020): White matter microstructure and network-connectivity in emerging adults with subclinical psychotic experiences. Brain Imaging and Behavior 14: 1876–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwarz N (2007): Retrospective and concurrent self-reports: the rationale for real-time data capture. The science of real-time data capture: self-reports in health research Oxford University Press, 11–26. [Google Scholar]
- 16.Lydon-Staley DM, Leventhal AM, Piper ME, Schnoll RA, Bassett DS (2021): Temporal networks of tobacco withdrawal symptoms during smoking cessation treatment. Journal of Abnormal Psychology 130: 89–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang X, Ram N, Gest SD, Lydon-Staley DM, Conroy DE, Pincus AL, Molenaar P (2018): Socioemotional Dynamics of Emotion Regulation and Depressive Symptoms: A Person-Specific Network Approach. Complexity 2018. [DOI] [PMC free article] [PubMed]
- 18.Epstein DH, Tyburski M, Craig IM, Phillips KA, Jobes ML, Vahabzadeh M, et al. (2014): Real-time tracking of neighborhood surroundings and mood in urban drug misusers: application of a new method to study behavior in its geographical context. Drug and Alcohol Dependence 134: 22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Müller SR, Peters H, Matz SC, Wang W, Harari GM (2020): Investigating the relationships between mobility behaviours and indicators of subjective well–being using smartphone–based experience sampling and GPS tracking. European Journal of Personality 34: 714–732. [Google Scholar]
- 20.Nica EI, Links PS (2009): Affective instability in borderline personality disorder: Experience sampling findings. Current Psychiatry Reports 11: 74–81. [DOI] [PubMed] [Google Scholar]
- 21.American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders: DSM-5
- 22.Hua JP, Trull TJ, Merrill AM, McCarty RM, Straub KT, Kerns JG (2020): Daily-life affective instability in emotional distress disorders is associated with function and structure of posterior parietal cortex. Psychiatry Research: Neuroimaging 296: 111028. [DOI] [PubMed] [Google Scholar]
- 23.Kaiser RH, Peterson E, Kang MS, Van Der Feen J, Aguirre B, Clegg R, et al. (2019): Frontoinsular network markers of current and future adolescent mood health. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging 4: 715–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McGowan AL, Parkes L, He X, Stanoi O, Kang Y, Lomax S, et al. (2021): Controllability of structural brain networks and the waxing and waning of negative affect in daily life. Biological Psychiatry Global Open Science [DOI] [PMC free article] [PubMed]
- 25.Betzel RF, Bassett DS (2017): Multi-scale brain networks. Neuroimage 160: 73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kopell NJ, Gritton HJ, Whittington MA, Kramer MA (2014): Beyond the connectome: the dynome. Neuron 83: 1319–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li C, Dong M, Womer FY, Han S, Yin Y, Jiang X, et al. (2021): Transdiagnostic time-varying dysconnectivity across major psychiatric disorders. Human Brain Mapping 42: 1182–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lydon-Staley DM, Bassett DS (2018): Network neuroscience: A framework for developing biomarkers in psychiatry. Current topics in behavioral neurosciences: Biomarkers in psychiatry Springer, pp. 79–109. [DOI] [PubMed] [Google Scholar]
- 29.Allen EA, Damaraju E, Plis SM, Erhardt EB, Eichele T, Calhoun VD (2014): Tracking whole-brain connectivity dynamics in the resting state. Cerebral Cortex 24: 663–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gu S, Pasqualetti F, Cieslak M, Telesford QK, Yu AB, Kahn AE, et al. (2015): Controllability of structural brain networks. Nature Communications 6: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jin C, Jia H, Lanka P, Rangaprakash D, Li L, Liu T, et al. (2017): Dynamic brain connectivity is a better predictor of PTSD than static connectivity. Human Brain Mapping 38: 4479–4496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liegeois R, Li J, Kong R, Orban C, Van De Ville D, Ge T, et al. (2019): Resting brain dynamics at different timescales capture distinct aspects of human behavior. Nature Communications 10: 2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lurie DJ, Kessler D, Bassett DS, Betzel RF, Breakspear M, Kheilholz S, et al. (2020). Questions and controversies in the study of time-varying functional connectivity in resting fMRI. Network Neuroscience 4: 30–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lam KH, Twose J, McConchie H, Licitra G, Meijer K, de Ruiter L, et al. (2022): Smartphone‐derived keystroke dynamics are sensitive to relevant changes in multiple sclerosis. European Journal of Neurology 29: 522–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Twose J, Licitra G, McConchie H, Lam KH, Killestein J (2020): Early-warning signals for disease activity in patients diagnosed with multiple sclerosis based on keystroke dynamics. Chaos: An Interdisciplinary Journal of Nonlinear Science 30: 113133. [DOI] [PubMed] [Google Scholar]
- 36.Betzel RF, Satterthwaite TD, Gold JI, Bassett DS (2017): Positive affect, surprise, and fatigue are correlates of network flexibility. Scientific Reports 7: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rahdar A, Galván A (2014): The cognitive and neurobiological effects of daily stress in adolescents. NeuroImage 92: 267–273. [DOI] [PubMed] [Google Scholar]
- 38.Hutchinson EA, Sequeira SL, Silk JS, Jones NP, Oppenheimer C, Scott L, Ladouceur CD (2021): Peer Connectedness and Pre‐Existing Social Reward Processing Predicts US Adolescent Girls’ Suicidal Ideation During COVID‐19. Journal of Research on Adolescence 31: 703–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Falk EB, O’Donnell MB, Tompson S, Gonzalez R, Dal Cin S, Strecher V, et al. (2016): Functional brain imaging predicts public health campaign success. Social Cognitive and Affective Neuroscience 11: 204–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lydon‐Staley DM, MacLean RR, Falk EB, Bassett DS, Wilson SJ (2021): The feasibility of an in‐scanner smoking lapse paradigm to examine the neural correlates of lapses. Addiction Biology 26: e13001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shin KE, Newman MG, Jacobson NC (2022): Emotion network density is a potential clinical marker for anxiety and depression: Comparison of ecological momentary assessment and daily diary. British Journal of Clinical Psychology 61: 31–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Provenzano J, Fossati P, Dejonckheere E, Verduyn P, Kuppens P (2021): Inflexibly sustained negative affect and rumination independently link default mode network efficiency to subclinical depressive symptoms. Journal of Affective Disorders 293: 347–354. [DOI] [PubMed] [Google Scholar]
- 43.Maxwell SE, Cole DA (2007): Bias in cross-sectional analyses of longitudinal mediation. Psychological Methods 12: 23–44. [DOI] [PubMed] [Google Scholar]
- 44.Lydon-Staley DM, Kuehner C, Zamoscik V, Huffziger S, Kirsch P, Bassett DS (2019): Repetitive negative thinking in daily life and functional connectivity among default mode, fronto-parietal, and salience networks. Translational Psychiatry 9: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brinberg M, Lydon-Staley DM (2022): Conceptualizing and Examining Change in Communication Research. MediArXiv Preprints: 10.33767/osf.io/v2fq4 [DOI] [PMC free article] [PubMed]
- 46.Janes AC, Datko M, Roy A, Barton B, Druker S, Neal C, et al. (2019): Quitting starts in the brain: a randomized controlled trial of app-based mindfulness shows decreases in neural responses to smoking cues that predict reductions in smoking. Neuropsychopharmacology 44: 1631–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zaehringer J, Ende G, Santangelo P, Kleindienst N, Ruf M, Bertsch K, et al. (2019): Improved emotion regulation after neurofeedback: A single-arm trial in patients with borderline personality disorder. NeuroImage: Clinical 24: 102032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Piñeyro Salvidegoitia M, Jacobsen N, Bauer AKR, Griffiths B, Hanslmayr S, & Debener S (2019): Out and about: Subsequent memory effect captured in a natural outdoor environment with smartphone EEG. Psychophysiology, 56(5), e13331. [DOI] [PubMed] [Google Scholar]
- 49.Kim J, Kim H, Kim DH, Lee SK, Roh JY, Kim CH, et al. (2021): Effects of cranial electrotherapy stimulation with novel in-ear electrodes on anxiety and resting-state brain activity: A randomized double-blind placebo-controlled trial. Journal of Affective Disorders 295: 856–864. [DOI] [PubMed] [Google Scholar]
- 50.Tseng VWS, Valliappan N, Ramachandran V, Choudhury T, Navalpakkam V (2021): Digital biomarker of mental fatigue. NPJ Digital Medicine 4: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bassett DS, Sporns O (2017): Network neuroscience. Nature Neuroscience 20: 353–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jeurissen B, Descoteaux M, Mori S, Leemans A (2019): Diffusion MRI fiber tractography of the brain. NMR in Biomedicine 32: e3785. [DOI] [PubMed] [Google Scholar]
- 53.Sotiropoulos SN, Jbabdi S, Xu J, Andersson JL, Moeller S, Auerbach EJ, et al. (2013): Advances in diffusion MRI acquisition and processing in the Human Connectome Project. Neuroimage 80: 125–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bassett DS, Zurn P, Gold JI (2018): On the nature and use of models in network neuroscience. Nature Reviews Neuroscience 19: 566–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sporns O (2011): Networks of the brain. Cambridge MA: MIT Press. [Google Scholar]
- 56.Friston KJ (2011): Functional and effective connectivity: a review. Brain connectivity 1: 13–36. [DOI] [PubMed] [Google Scholar]
- 57.Krönke K-M, Wolff M, Shi Y, Kräplin A, Smolka MN, Bühringer G, Goschke T (2020): Functional connectivity in a triple-network saliency model is associated with real-life self-control. Neuropsychologia 149: 107667. [DOI] [PubMed] [Google Scholar]
- 58.Newbold DJ, Laumann TO, Hoyt CR, Hampton JM, Montez DF, Raut RV, et al. (2020): Plasticity and spontaneous activity pulses in disused human brain circuits. Neuron 107: 580–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dupuy M, Abdallah M, Swendsen J, N’Kaoua B, Chanraud S, Schweitzer P, et al. (2022): Real-time cognitive performance and positive symptom expression in schizophrenia. European Archives of Psychiatry and Clinical Neuroscience 272: 415–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Borsboom D (2017): A network theory of mental disorders. World Psychiatry 16: 5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Borsboom D, Cramer AO (2013): Network analysis: an integrative approach to the structure of psychopathology. Annual Review of Clinical Psychology 9: 91–121. [DOI] [PubMed] [Google Scholar]
- 62.Robinaugh DJ, Hoekstra RH, Toner ER, Borsboom D (2020): The network approach to psychopathology: a review of the literature 2008–2018 and an agenda for future research. Psychological Medicine 50: 353–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Epskamp S, Waldorp LJ, Mõttus R, Borsboom D (2018): The Gaussian graphical model in cross-sectional and time-series data. Multivariate Behavioral Research 53: 453–480. [DOI] [PubMed] [Google Scholar]
- 64.Bassett DS, Wymbs NF, Porter MA, Mucha PJ, Carlson JM, Grafton ST (2011): Dynamic reconfiguration of human brain networks during learning. Proceedings of the National Academy of Sciences 108: 7641–7646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kivelä M, Arenas A, Barthelemy M, Gleeson JP, Moreno Y, Porter MA (2014): Multilayer networks. Journal of Complex Networks 2: 203–271. [Google Scholar]
- 66.De Domenico M, Porter MA, Arenas A (2015): MuxViz: a tool for multilayer analysis and visualization of networks. Journal of Complex Networks 3: 159–176. [Google Scholar]
- 67.Boccaletti S, Bianconi G, Criado R, Del Genio CI, Gómez-Gardenes J, Romance M, et al. (2014): The structure and dynamics of multilayer networks. Physics Reports 544: 1–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Battiston F, Nicosia V, Latora V (2017): The new challenges of multiplex networks: Measures and models. The European Physical Journal Special Topics 226: 401–416. [Google Scholar]
- 69.Aleta A, Moreno Y (2019): Multilayer networks in a nutshell. Annual Review of Condensed Matter Physics 10: 45–62. [Google Scholar]
- 70.Mucha PJ, Richardson T, Macon K, Porter MA, Onnela J-P (2010): Community structure in time-dependent, multiscale, and multiplex networks. Science 328: 876–878. [DOI] [PubMed] [Google Scholar]
- 71.Granell C, Gómez S, Arenas A (2014): Competing spreading processes on multiplex networks: awareness and epidemics. Physical Review E 90: 012808. [DOI] [PubMed] [Google Scholar]
- 72.Gallotti R, Barthelemy M (2014): Anatomy and efficiency of urban multimodal mobility. Scientific Reports 4: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pilosof S, Porter MA, Pascual M, Kéfi S (2017): The multilayer nature of ecological networks. Nature Ecology & Evolution 1: 1–9. [DOI] [PubMed] [Google Scholar]
- 74.Beardsley K, Liu H, Mucha PJ, Siegel DA, Tellez JF (2020): Hierarchy and the Provision of Order in International Politics. The Journal of Politics 82: 731–746. [Google Scholar]
- 75.Simpson-Kent IL, Fried EI, Akarca D, Mareva S, Bullmore ET, CALM Team, et al. (2021): Bridging brain and cognition: A multilayer network analysis of brain structural covariance and general intelligence in a developmental sample of struggling learners. Journal of Intelligence 9: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Borsboom D, Cramer AO, Kalis A (2019): Brain disorders? Not really: Why network structures block reductionism in psychopathology research. Behavioral and Brain Sciences 42: e2. [DOI] [PubMed] [Google Scholar]
- 77.Li J, Shi Y, Toga AW (2016): Mapping brain anatomical connectivity using diffusion magnetic resonance imaging: Structural connectivity of the human brain. IEEE signal processing magazine 33: 36–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mukherjee P, Berman JI, Chung SW, Hess CP, Henry RG (2008): Diffusion tensor MR imaging and fiber tractography: theoretic underpinnings. American Journal of Neuroradiology 29: 632–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Crofts JJ, Forrester M, O’Dea RD (2016): Structure-function clustering in multiplex brain networks. EPL (Europhysics Letters) 116: 18003. [Google Scholar]
- 80.Vaiana M, Muldoon SF (2020): Multilayer brain networks. Journal of Nonlinear Science 30: 2147–2169. [Google Scholar]
- 81.Zhang X, Man Y, Zhuang X, Shen J, Zhang Y, Cui Y, et al. (2021): Plant multiscale networks: charting plant connectivity by multi-level analysis and imaging techniques. Science China Life Science 64: 1392–1422. [DOI] [PubMed] [Google Scholar]
- 82.Li M, Chen S, Zhao Y, Zhang Y, Wang Y, Tian Q (2021): Multiscale Spatio-Temporal Graph Neural Networks for 3D Skeleton-Based Motion Prediction. IEEE Transactions on Image Processing 30: 7760–7775. [DOI] [PubMed] [Google Scholar]
- 83.Ren Y, Zhao W, You W, Zhai K (2021): Multiscale and partial correlation networks analysis of risk connectedness in global equity markets. Physica A: Statistical Mechanics and its Applications 573: 125911. [Google Scholar]
- 84.Falk EB, Bassett DS (2017): Brain and social networks: fundamental building blocks of human experience. Trends in Cognitive Sciences 21: 674–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu W, Suzumura T, Ji H, Hu G (2018): Finding overlapping communities in multilayer networks. PloS one 13: e0188747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Haug N, Deischinger C, Gyimesi M, Kautzky-Willer A, Thurner S, Klimek P (2020): High-risk multimorbidity patterns on the road to cardiovascular mortality. BMC Medicine 18: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Moni MA, Liò P (2015): How to build personalized multi-omics comorbidity profiles. Frontiers in Cell and Developmental Biology 3: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Breedt LC, Santos FA, Hillebrand A, Reneman L, van Rootselaar A- F, Schoonheim MM, et al. (2021): Multimodal multilayer network centrality relates to executive functioning. bioRxiv [DOI] [PMC free article] [PubMed]
- 89.Hasmi L, Drukker M, Guloksuz S, Menne-Lothmann S, Decoster J, van Winkel R, et al. (2017): Network approach to understanding emotion dynamics in relation to childhood trauma and genetic liability to psychopathology: replication of a prospective experience sampling analysis. Frontiers in Psychology 8: 1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Klippel A, Viechtbauer W, Reininghaus U, Wigman J, van Borkulo C, et al. (2017): The cascade of stress: a network approach to explore differential dynamics in populations varying in risk for psychosis. Schizophrenia Bulletin 44: 328–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.van den Heuvel MP, Sporns O (2019): A cross-disorder connectome landscape of brain dysconnectivity. Nature Reviews Neuroscience 20: 435–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.van den Heuvel MP, Scholtens LH, Kahn RS (2019): Multiscale neuroscience of psychiatric disorders. Biological Psychiatry 86: 512–522. [DOI] [PubMed] [Google Scholar]
- 93.McCrady BS (2004): To have but one true friend: implications for practice of research on alcohol use disorders and social network. Psychology of Addictive Behaviors 18: 113–21. [DOI] [PubMed] [Google Scholar]
- 94.Rosenquist JN, Fowler JH, Christakis NA (2011): Social network determinants of depression. Molecular Psychiatry 16: 273–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


