Introduction: The Clinical Problem
Acute kidney injury (AKI) is a common complication among decompensated patients with cirrhosis and is associated with significant mortality.1 In this setting, the most common phenotypes of AKI are hypovolemic/prerenal, acute tubular injury/necrosis, and hepatorenal syndrome (HRS). In this article, the most recent terminology put forth by the International Club of Ascites is used, HRS-AKI, which replaces the prior term HRS type 1.2 HRS-AKI is a devastating complication of advanced cirrhosis occurring in approximately 17% of AKI cases1 and is associated with a high 90-day mortality rate of 45% to 51%.3,4 The pathophysiology of HRS-AKI is complex.5 Because of portal hypertension, blood pools in the splanchnic circulation, leading to decreased effective arterial blood volume. As a result, endogenous vasoconstricting systems are activated, leading to sodium and water retention and decreased kidney perfusion. Overlapping with this process there also is increased gut permeability, bacterial translocation, and up-regulation of systemic inflammatory mediators, a secondary cascade that contributes further to impaired kidney perfusion and concomitantly leads to the functional kidney injury seen in HRS-AKI. These dynamic pathophysiologic changes could be potentiated further by infections, acute-on-chronic liver failure (ACLF), or variceal bleeding.
With intravenous albumin, terlipressin is recommended as the vasoconstrictor of choice for the management of HRS-AKI.2,5,6 Terlipressin is a vasopressin analogue that acts predominantly on the V1a receptor in the splanchnic arterial vasculature, and, compared with vasopressin or ornipressin, terlipressin has a longer half-life and a more predictable therapeutic window that allows for bolus administration through a peripheral catheter rather than a continuous infusion via central catheter. With the recent Food and Drug Administration approval of terlipressin in the United States, this article reviews the diagnosis of HRS-AKI and provides a clinical guide for terlipressin use in the management of HRS-AKI.
Diagnosis
Given the significant prognostic implications of AKI, timely and accurate differential diagnosis is imperative because management differs distinctly for each AKI phenotype. Therefore, once AKI is recognized in a patient with cirrhosis, clinicians should follow the International Club of Ascites guidelines for the initial management of AKI according to AKI stage.2 Diuretics, vasodilators (including nonselective β-blockers), and nephrotoxins should be discontinued; and precipitating factors should be identified and treated. In the case of AKI stage 2 or higher, volume expansion with 25% intravenous albumin (1 g/kg per day; maximum, 100 g/d) for 2 consecutive days is recommended. Volume expansion with albumin could be considered in patients with AKI stage 1b (serum creatinine [sCr] level, >1.5 mg/dL).6 After 48 hours, if there is an improvement in AKI stage or response to therapy (see Supplementary Table 1 for definitions), the phenotype is suggestive of hypovolemic/prerenal AKI. In the scenario in which there is a partial response to volume expansion, continued volume expansion can be considered, while being mindful to avoid volume overload. It is important to note that guideline-recommended albumin doses are historically and empirically derived, and thus further work is needed to identify an optimal albumin dose for AKI in cirrhosis.
If a patient has not responded to or has AKI progression despite 2 consecutive days of volume expansion with albumin and withdrawal of diuretics, the differential diagnosis in most cases is either HRS-AKI or acute tubular injury/necrosis. To help with timely diagnosis of HRS-AKI, urine analysis and a kidney ultrasound (if not already obtained as part of the initial management of AKI) should be obtained as soon as there is evidence of a null response. The diagnosis of HRS-AKI can be made if all the following criteria are met2:
Increase in sCr of ≥0.3 mg/dL within 48 hours or increase in sCr ≥50% from baseline, which is known or presumed to have occurred within the prior 7 days;
No response to diuretic withdrawal and 2-day volume expansion with albumin (1 g/kg per day; maximum, 100 g/d);
Cirrhosis with ascites;
Absence of shock requiring vasopressors;
No current or recent use of nephrotoxic drugs/contrast dye; and
Absence of proteinuria (<500 mg/d), microhematuria (<50 red blood cells per high-power field), and a normal kidney ultrasound, a spot check for the former two could be used in lieu of a 24-hour urine collection.
Additional findings that may support the diagnosis of HRS-AKI include a fractional exertion of sodium of 0.1% or less,7 and the absence of granular/epithelial casts (muddy brown casts), which can be suggestive of tubular injury.8 However, the latter could be seen in patients with advanced cirrhosis and hyperbilirubinemia1 and its presence should not rule out HRS-AKI if the patient otherwise meets HRS-AKI criteria. In addition, fractional exertion of sodium can be increased in the setting of recent diuretic use, of which the fractional excretion of urea, which is not impacted by diuretic use, could be helpful (optimal cut-off value, <28.16%).9 Urine neutrophil gelatinase-associated lipocalin (NGAL), a tubular injury biomarker, also could aid in the differential diagnosis of AKI in cirrhosis.7,10,11 However, a cut-off value that discriminates between AKI phenotypes is not clearly defined and urine NGAL is not widely available for clinical use in the United States.
Management
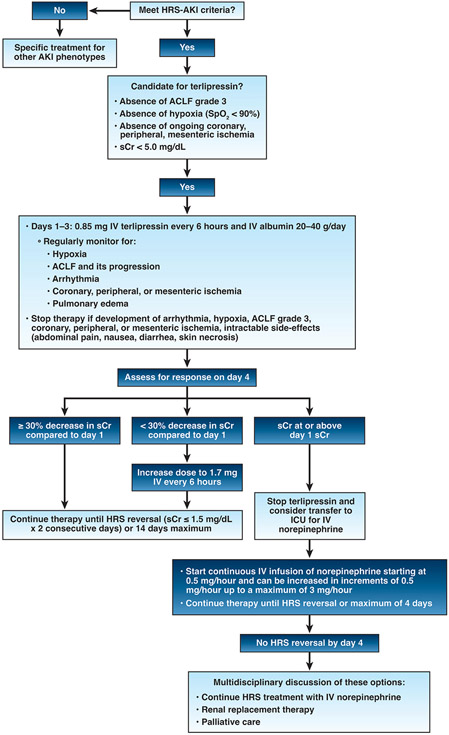
Once the diagnosis of HRS-AKI is made, patients should receive vasoconstrictors promptly in concert with albumin (20–40 g/d). As mentioned previously, the vasoconstrictor of choice recommended by international and US guidelines is terlipressin.2,5,6 Given its eventual place for the management of HRS-AKI in the United States, a management algorithm with terlipressin can be found in Figure 1. Terlipressin is administered by intravenous bolus at a starting dose of 0.85 mg given every 6 hours, with a maximum dose of 1.7 mg every 6 hours12 or continuous infusion (starting dose, 2 mg/24 hours; maximum dose, 12 mg/d). The latter is not Food and Drug Administration approved in the United States.
Figure 1.
Management of HRS-AKI with terlipressin. AKI, acute kidney injury; ACLF, acute-on-chronic liver failure; HRS, hepatorenal syndrome; IV, intravenous; sCr, serum creatinine; SpO2, oxygen saturation.
Before starting terlipressin, patients should be admitted to closely monitored units with telemetry and nurses experienced in taking care of patients with decompensated cirrhosis. Treating physicians and nurses should monitor vital signs every 6 hours and pulse-oximetry (oxygen saturation) should be monitored continuously. Patients also should be assessed for ACLF (defined by European Association for the Study of Chronic Liver Failure6) and pulmonary edema. Given that the risk for respiratory failure was the highest in terlipressin-treated patients with ACLF grade 3 or hypoxia (oxygen saturation, <90%) in A Multi-Center, Randomized, Placebo Controlled, Double-Blind Study to Confirm Efficacy and Safety of Terlipressin in Subjects With Hepatorenal Syndrome Type 1 (CONFIRM) study,13 therapy with terlipressin should be avoided until there is an improvement in ACLF grade or oxygenation, respectively. In addition, terlipressin should be avoided in patients with sCr level greater than 5 mg/dL because a response to therapy is unlikely.3 Terlipressin is contra-indicated in patients with ongoing hypoxia or coronary, peripheral, or mesenteric ischemia.12
Criteria for Stopping Terlipressin
During therapy, terlipressin should be discontinued if a patient develops arrhythmia; hypoxia; coronary, peripheral, mesenteric ischemia; or intractable side effects such as abdominal pain, nausea, diarrhea, or skin necrosis. Moreover, given the high risk for respiratory failure in patients who progress to ACLF grade 3, terlipressin discontinuation can be considered and potentially resumed when there is improvement in ACLF grade. In patients with pulmonary edema, albumin could be discontinued temporarily, or dose reduced, and loop diuretics considered.
Consistent with package labeling12 and the CONFIRM trial protocol,3 response to terlipressin should be assessed on day 4 of therapy (Figure 1). If sCr level has decreased by 30% or more from baseline, treatment should be continued until HRS reversal (sCr ≤1.5 mg/dL on 2 consecutive days) is achieved or for a maximum of 14 days. If the sCr level has not decreased by 30% or more, the dose of terlipressin can be doubled (1.7 mg) and therapy can continue until HRS reversal is achieved or for a maximum of 14 days. Abdominal pain, diarrhea, or signs/symptoms of mesenteric ischemia should be actively monitored because these side effects are dose dependent. Continuous infusion of terlipressin has been associated with a lower rate of side effects than intravenous boluses, but it has not yet been approved in the United States. In the CONFIRM study, HRS reversal was achieved in 32% of patients.3 Predictors of HRS reversal can be found in Table 1,3,4,11,14 which include a lower sCr level and lower severity of liver disease or ACLF at the time of terlipressin initiation. Hence, higher rates of HRS reversal could be achieved by early initiation of terlipressin, which can be accomplished by timely diagnosis of HRS-AKI. Lastly, in patients whose day 4 sCr level is unchanged or worsened compared with day 1 sCr, terlipressin should be discontinued. Second-line therapy with continuous norepinephrine could be considered in this clinical scenario (or if terlipressin is not available/contraindicated) but would be off-label and requires further study. Norepinephrine has performed similarly when compared against terlipressin,5 but requires central access and intensive care unit monitoring. Historically, oral midodrine and subcutaneous octreotide has been used in combination as another off-label option when terlipressin is not available. This combination therapy is ineffective5 and its use should be discouraged when other vasoconstrictors are available. In all patients, liver transplantation evaluation should be strongly considered because liver transplantation is the definitive treatment of HRS-AKI.
Table 1.
Baseline Predictors of Response to Terlipressin
| Predictor | Commentary |
|---|---|
| Lower sCr3,4 | Reflective of the timing of initiation (earlier in the natural history of HRS-AKI; ie, at lower stages of AKI), the greater the likelihood of response to therapy |
| Lower MAP4 | Linear correlation between increases in MAP from baseline and response Although a target MAP is not clear, a sustained increase of 5–10 mm Hg has been associated with response |
| Severity of liver disease3,4,14 | Higher MELD score and severity of ACLF at baseline have been associated with decreased response |
| Presence of SIRS and alcohol-associated hepatitis3 | Presence of SIRS and alcohol-associated hepatitis are positive predictors Mechanisms are unclear |
| Urine NGAL11 | Urine NGAL <220 ng/mL at baseline has been associated with response |
ACLF, acute on chronic liver failure; MAP, mean arterial pressure; MELD, Model for End-stage Liver Disease; NGAL, neutrophil gelatinase-associated lipocalin; sCr, serum creatinine; SIRS, systemic inflammatory response syndrome.
Take Home Message
HRS-AKI is a rare but serious cause of AKI in patients with decompensated cirrhosis and is associated with high mortality. Timely diagnosis and thus early initiation of terlipressin and albumin for the management of HRS-AKI improves the likelihood of HRS reversal, which is a vital part of supportive care for decompensated cirrhosis. Terlipressin should be avoided in patients with ACLF grade 3; hypoxia; ongoing coronary, peripheral, and mesenteric ischemia; and sCr greater than 5 mg/dL. Further studies investigating the role of biomarkers (ie, urine NGAL) for the diagnosis of HRS-AKI and predicting treatment response are highly needed, as are studies investigating continuous infusion of terlipressin and mean arterial pressure vs sCr-based titration of terlipressin for improving HRS reversal rates and survival. Nevertheless, as clinicians treating patients with HRS-AKI, terlipressin is a much-needed intervention for the early and sustained reversal of this deadly syndrome.
Supplementary Material
Funding
Supported by grant K23 DK128567 from the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health and institutional research support from Mallinckrodt Pharmaceuticals (A.S.A.); and by grant K23 DK131278 from the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health and institutional research support from Mallinckrodt Pharmaceuticals (G.C.).
Abbreviations used in this paper:
- ACLF
acute-on-chronic liver failure
- AKI
acute kidney injury
- CONFIRM
A Multi-Center, Randomized, Placebo Controlled, Double-Blind Study to Confirm Efficacy and Safety of Terlipressin in Subjects With Hepatorenal Syndrome Type 1
- HRS
hepatorenal syndrome
- NGAL
neutrophil gelatinase associated lipocalin
- sCr
serum creatinine
Footnotes
Conflicts of interest
These authors disclose the following: Salvatore Piano served on the Mallinckrodt Advisory Board and has received consultant fees from Plasma Protein Therapeutics Association and Resolution Therapeutics; Justin M. Belcher has received consultant fees from Mallinckrodt Pharmaceuticals; and Andrew S. Allegretti has received consulting fees from Mallinckrodt Pharmaceuticals, Ocelot Bio, and Cymabay Therapeutics. The remaining authors disclose no conflicts.
References
- 1.Garcia-Tsao G, Parikh CR, Viola A. Acute kidney injury in cirrhosis. Hepatology 2008;48:2064–2077. [DOI] [PubMed] [Google Scholar]
- 2.Angeli P, Gines P, Wong F, et al. Diagnosis and management of acute kidney injury in patients with cirrhosis: revised consensus recommendations of the International Club of Ascites. Gut 2015;64:531–537. [DOI] [PubMed] [Google Scholar]
- 3.Wong F, Pappas SC, Curry MP, et al. Terlipressin plus albumin for the treatment of type 1 hepatorenal syndrome. N Engl J Med 2021;384:818–828. [DOI] [PubMed] [Google Scholar]
- 4.Sanyal AJ, Boyer TD, Frederick RT, et al. Reversal of hepatorenal syndrome type 1 with terlipressin plus albumin vs. placebo plus albumin in a pooled analysis of the OT-0401 and REVERSE randomised clinical studies. Aliment Pharmacol Ther 2017;45:1390–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biggins SW, Angeli P, Garcia-Tsao G, et al. Diagnosis, evaluation, and management of ascites, spontaneous bacterial peritonitis and hepatorenal syndrome: 2021 practice guidance by the American Association for the Study of Liver Diseases. Hepatology 2021;74:1014–1048. [DOI] [PubMed] [Google Scholar]
- 6.EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol 2018;69:406–460. [DOI] [PubMed] [Google Scholar]
- 7.Belcher JM, Sanyal AJ, Peixoto AJ, et al. Kidney biomarkers and differential diagnosis of patients with cirrhosis and acute kidney injury. Hepatology 2014;60:622–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perazella MA, Coca SG, Kanbay M, et al. Diagnostic value of urine microscopy for differential diagnosis of acute kidney injury in hospitalized patients. Clin J Am Soc Nephrol 2008;3:1615–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patidar KR, Kang L, Bajaj JS, et al. Fractional excretion of urea: a simple tool for the differential diagnosis of acute kidney injury in cirrhosis. Hepatology 2018;68:224–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allegretti AS, Parada XV, Endres P, et al. Urinary NGAL as a diagnostic and prognostic marker for acute kidney injury in cirrhosis: a prospective study. Clin Transl Gastroenterol 2021;12:e00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gambino C, Piano S, Stenico M, et al. Diagnostic and prognostic performance of urinary neutrophil gelatinase-associated lipocalin in patients with cirrhosis and acute kidney injury. Hepatology. Published online September 20, 2022. 10.1002/hep.32799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong F, Pappas SC, Reddy KR, et al. Terlipressin use and respiratory failure in patients with hepatorenal syndrome type 1 and severe acute-on-chronic liver failure. Aliment Pharmacol Ther 2022;56:1284–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Terlipressin prescribing information [FDA]. 2022. Accessed October 30, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/022231s000lbl.pdf [Google Scholar]
- 14.Piano S, Schmidt HH, Ariza X, et al. Association Between grade of acute on chronic liver failure and response to terlipressin and albumin in patients with hepatorenal syndrome. Clin Gastroenterol Hepatol 2018;16:1792–1800.e3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.