Abstract
Hyperphosphorylated tau in the locus coeruleus (LC) is ubiquitous in prodromal Alzheimer’s disease (AD), and LC neurons degenerate as AD progresses. Hyperphosphorylated tau alters firing rates in other brain regions, but its effects on LC neurons are unknown. We assessed single unit LC activity in anesthetized wild-type (WT) and TgF344-AD rats at 6 months, which represents a prodromal stage when LC neurons are the only cells containing hyperphosphorylated tau in TgF344-AD animals, and at 15 months when β-amyloid (Aβ) and tau pathology are both abundant in the forebrain. At baseline, LC neurons from TgF344-AD rats were hypoactive at both ages compared to WT littermates but showed elevated spontaneous bursting properties. Differences in footshock-evoked LC firing depended on age, with 6-month TgF344-AD rats demonstrating aspects of hyperactivity, and 15-month transgenic rats showing hypoactivity. Early LC hyperactivity is consistent with appearance of prodromal neuropsychiatric symptoms and is followed by LC hypoactivity which contributes to cognitive impairment. These results support further investigation into disease stage-dependent noradrenergic interventions for AD.
Keywords: Locus Coeruleus, Tau, Electrophysiology, Alzheimer’s disease, TgF344-AD, Aging
1. Introduction
Accumulation of hyperphosphorylated tau within subcortical nuclei and subsequent dysfunction of these neurons is a nearly ubiquitous feature along Alzheimer’s disease (AD) progression (Ehrenberg et al., 2017; Theofilas et al., 2015). A seminal report from Braak and colleagues (Braak et al., 2011), independently replicated by other groups (Elobeid et al., 2012; Pletnikova et al., 2018; Theofilas et al., 2017), positions the noradrenergic locus coeruleus (LC) as the earliest site of pathological tau deposition, well before cortical β-amyloid (Aβ) plaque accumulation or the onset of diagnostic cognitive deficits. During prodromal phases of AD, non-cognitive symptoms consistent with noradrenergic hyperactivity, including sleep disturbances, agitation, and anxiety, emerge coincident with the appearance of hyperphosphorylated tau in the LC (Ehrenberg et al., 2018; Johansson et al., 2021; Kelberman et al., 2022; Pentkowski et al., 2018; Weinshenker, 2018). Cerebrospinal fluid norepinephrine (NE) levels and its turnover are elevated in early AD (Elrod et al., 1997; Henjum et al., 2022; Hoogendijk et al., 1999; Palmer et al., 1987), and a recent neuroimaging study demonstrated that higher LC signal on a neuromelanin-sensitive MRI was predictive of neuropsychiatric symptom severity in AD patients (Cassidy et al., 2022), further supporting the theory of LC hyperactivity during initial stages of disease. At the same time, numerous studies have linked the deterioration of LC integrity to cognitive and structural decline in aging and AD (Bachman et al., 2021; Jacobs et al., 2021; Kelly et al., 2017; Prokopiou et al., 2022; van Hooren et al., 2021; Wilson et al., 2013), suggestive of reduced LC-NE transmission during later stages of the disease.
While neurochemical, neuropathological, and behavioral results are consistent with disease stage-specific alterations in LC activity, direct evidence for changes in LC firing is mostly lacking. Aβ pathology often induces neural hyperactivity (Busche and Hyman, 2020), including in the LC (Kelly et al., 2021), whereas tau pathology typically induces neuronal hypoactivity (Busche et al., 2019). However, there are other reports of tau-mediated hyperactivity (Holth et al., 2013; Huijbers et al., 2019; Shimojo et al., 2020), suggesting region and/or cell-type specific effects. Given that Aβ only accumulates in the LC during late stages of AD (Cole et al., 1993; Kelly et al., 2021), the dysregulation of LC circuits at the level of the cell bodies is likely dominated by the early accumulation of hyperphosphorylated tau. Therefore, understanding the impact of aberrant tau on LC neural activity is critical for determining the neurobiological underpinnings of prodromal AD symptoms and progression to cognitive impairment. This information could then inform rational development of early biomarkers and therapeutic interventions at various disease stages.
The objective of this study was to delineate the effects of AD-like hyperphosphorylated tau on LC firing rates. Though some studies have begun to track LC activity in humans using functional MRI (Prokopiou et al., 2022), these techniques lack spatial and target specificity for small regions like the LC (Kelberman et al., 2020). We therefore employed the TgF344-AD rat model, which expresses mutant human amyloid precursor protein and presenilin-1 (APP/PS1) that cause autosomal dominant, early-onset AD (Cohen et al., 2013). This model possesses several benefits for our study. These rats demonstrate many of the same behavioral phenotypes that are observed in AD that are influenced by LC activity. These include early anxiety-like behaviors followed later by cognitive impairment that can be reversed by LC activation (Cohen et al., 2013; Kelberman et al., 2022; Pentkowski et al., 2018; Rorabaugh et al., 2017). In addition, TgF344-AD rats, unlike their APP/PS1 transgenic mouse counterparts, develop endogenous tau pathology that first appears in the LC (Rorabaugh et al., 2017). This tau deposition is coincident with the appearance of non-cognitive behavioral abnormalities but prior to tau or Aβ pathology elsewhere in the brain, reminiscent of human disease progression. In the current study, we recorded single unit LC activity from anesthetized TgF344-AD rats and wild-type (WT) littermates at baseline and in response to footshock at 6 months, an age when anxiety-like behavior emerges and hyperphosphorylated tau in the LC is the only detectable AD-like neuropathology, as well as 15 months, when brain-wide tau and Aβ pathology are evident in combination with deficits in learning and memory.
2. Methods
2.1. Animals
This study used a total of 42 TgF344-AD rats and WT littermates on a Fischer background aged 6 or 15 months. TgF344-AD rats were hemizygous for the APPsw/PS1ΔE9 transgene that contains mutations causative of autosomal dominant early-onset AD (Cohen et al., 2013). Rats were housed in groups of 2–3 on a 12-h light/dark cycle (lights on at 7:00 am) with food and water available ad libitum.
All experiments were conducted in accordance with the Institutional Animal Care and Use Committee at Emory University. Male and female rats were assigned to sex-balanced experimental groups, given the lack of prominent sex differences noted in this strain (Cohen et al., 2013; Kelberman et al., 2022; Sare et al., 2020).
2.2. Surgery
At two months of age, all rats to be used for electrophysiology underwent stereotaxic surgery. Rats were anesthetized with 5% isoflurane and maintained at 2% throughout surgery. Prior to incision, rats were given ketoprofen (5 mg/kg, s.c.). An AAV9-PRSx8-mCherry-WPRE-rBG virus was infused bilaterally targeting the LC (AP: −3.8 mm, ML: +/− 1.2 mm, DV: −7.0 mm from lambda with the head tilted 15 degrees downward). The injection syringe was left in place for 5 min following the infusion prior to being moved dorsally 1 mm and waiting an additional 2 min to ensure diffusion of virus at the site of injection. This tau-free virus was to be used as a control infusion for a planned experiment that we did not end up pursuing, and nothing further was done with it in this study. Electrophysiology recordings were performed approximately 4 or 13 months following surgery.
2.3. Electrophysiology
At 6 or 15 months, rats were anesthetized with chloral hydrate (400 mg/kg, i.p.) and secured in a stereotaxic frame. An incision was made to expose the skull, which was leveled based on measurements made at bregma and lambda. A 15° head tilt was employed to avoid the sagittal sinus, and bilateral burr holes were drilled over the approximate location of the LC (AP: 3.8–4.0 mm, ML: 0.9–1.3 mm from lambda). 16-channel silicone probes (V1×16-Poly2-10mm-50s-177-V16_100-50, NeuroNexus; Ann Arbor, MI) were connected to a u-series Cereplex headstage (Blackrock Neurotech; Salt Lake City, UT). A 16-channel Cereplex Direct System was used to acquire digitized signals with a 250 Hz-5 kHz bandpass filter and 10 kS/s sampling rate. During electrophysiological recordings, LC units were identified based on field standard criteria, including stereotaxic coordinates, location adjacent to the mesencephalic trigeminal nucleus (Me5), biphasic response to footpinch/footshock, and reduction/cessation of spontaneous activity following injection of the selective α2-adrenergic receptor agonist clonidine (0.1 mg/kg i.p.) (Hirata and Aston-Jones, 1994; Kalwani et al., 2014; Totah et al., 2018; Vazey and Aston-Jones, 2014; West et al., 2015). Each recording began with a 5-min baseline period, which was immediately followed by 10 applications of a contralateral footpinch for LC verification, each separated by 10 s, as described previously (West et al., 2015). Afterwards, 0.5 ms 10 mA footshocks (each separated by 10 s for 5.5 min) were applied to the contralateral hindpaw, followed by the same pattern using 5 ms 10 mA footshocks. Footshocks were delivered by an ISO-Flex stimulus isolator and controlled by a Master-8 (A.M.P. Instruments; Jerusalem, Israel). LC spikes were manually sorted using Blackrock Offline Spike Sorting Software.
Electrophysiology analysis was performed in NeuroExplorer v5 (Nex Technologies; Colorado Springs, CO) or Matlab (R2019a; Mathworks; Natick, MA). To ensure that single units were being analyzed, neurons with >2% of recorded spikes within a predefined 3 ms refractory period were eliminated (Kalwani et al., 2014). The 5-min baseline recording served as the basal firing rate and to calculate the interspike interval for each single unit. Spontaneous bursting properties (number of bursts, percentage of spikes within a burst, burst duration, spikes per burst, interspike interval within a burst, burst rate, and interburst interval) of LC neurons during baseline recordings were also quantified. Spontaneous bursts were defined as two spikes with an interspike interval of <0.08 s and terminated with an interspike interval >0.16s, as previously described (Grace and Bunney, 1983; Iro et al., 2021). Footshock was used to ascertain changes to sensory evoked LC activity. LC response to footshock was divided into three response categories: immediate (0–60 ms), intermediate (60–100 ms), and late (200–400 ms). These periods were based on a previous report demonstrating that altering the length (0.5, 2, and 5 ms) of footshock could elicit both a standard immediate and long latency LC response (Hirata and Aston-Jones, 1994).
2.4. Tissue Preparation and Immunohistochemistry
Following completion of electrophysiological recordings, rats were overdosed with isoflurane and perfused with potassium phosphate-buffered saline followed by 4% paraformaldehyde (PFA). Brains were removed and stored in 4% PFA overnight then transferred to 30% sucrose until sectioning. Brain sections containing the LC were sliced at 30 um and either dry mounted or stored in cryoprotectant until being processing for immunohistochemistry.
Dry mounted sections were counterstained with neutral red. Slides were submerged in increasing concentrations of ethanol (70%, 95%, and 100%) for 1 min each, followed by 10 min in neutral red, dunked 3–5 times in increasing concentrations of ethanol (70%, 95%, and 100%), and finally in xylene for 2 min. Slides were coverslipped with permount and imaged on a Keyence BZ-X700 microscope (KEYENCE; Osaka, Japan).
To confirm that α2-adrenergic receptors (Adra2a) are specific to noradrenergic neurons in the LC region and thus the only cells that could respond to clonidine during our recordings, we performed fluorescent in situ hybridization using the RNAscope Multiplex Fluorescent V2 Assay (Advanced Cell Diagnostics, Newark, CA, USA) on brainstem sections containing the LC. One 6-month WT male was lightly anesthetized, and the brain was quickly removed and flash frozen in isopentane on dry ice. The brain was stored at −80C until sectioning on a cryostat at 16μm. The RNAscope assay was performed following the manufacturer’s protocol, multiplexing tyrosine hydroxylase (Th) and Adra2a. Images were taken in a 1μm pitch z-stack (10um total) on a Keyence BZ-X700 microscope at 20x and 40x. A representative 2x section was also captured to visualize most of the coronal section containing the LC.
2.5. Statistical Analysis:
Figures were made and statistical analyses were performed using GraphPad Prism (v. 9.2.0; San Diego, CA) with the mean ± SEM. A two-way ANOVA was used to identify main effects of age, genotype, or their interaction. When applicable, post-hoc tests were performed across genotypes within an age group using the Holm-Sidak correction. For all analyses, statistical significance was set at α = 0.05. Supplemental figures are presented as raincloud plots consisting of a density plot, box-and-whiskers plot, and individual data points that were created in R using modified code from open-source platforms and from (Allen et al., 2019; Kay, 2022; Wickham, 2016; Xu et al., 2021). All code is available upon reasonable request.
3. Results
3.1. LC Neural Recording Verification
We used neuroanatomical, sensory, electrophysiological, and pharmacological methods to verify that recordings were from bona fide LC neurons. LC units were located at a similar depth but medial to Me5, which was identified by jaw deflection (Figure 1A). Putative LC units displayed a canonical biphasic response to footpinch/footshock, and as previously reported, a subset of these neurons demonstrated a late phase (200–400 ms) response to 5 ms footshock that was not observed in response to 0.5 ms footshock (Figure 1B). LC cells, unlike other adjacent nuclei, express high levels of Adra2a and are inhibited by the α2-adrenergic receptor agonist clonidine (McCune et al., 1993) (Figure 1C, D). Finally, we verified that electrode tracks were in the LC post-recording using a neutral red counterstain (Figure 1E).
Figure 1. Multimodal confirmation of single unit recordings from LC neurons.
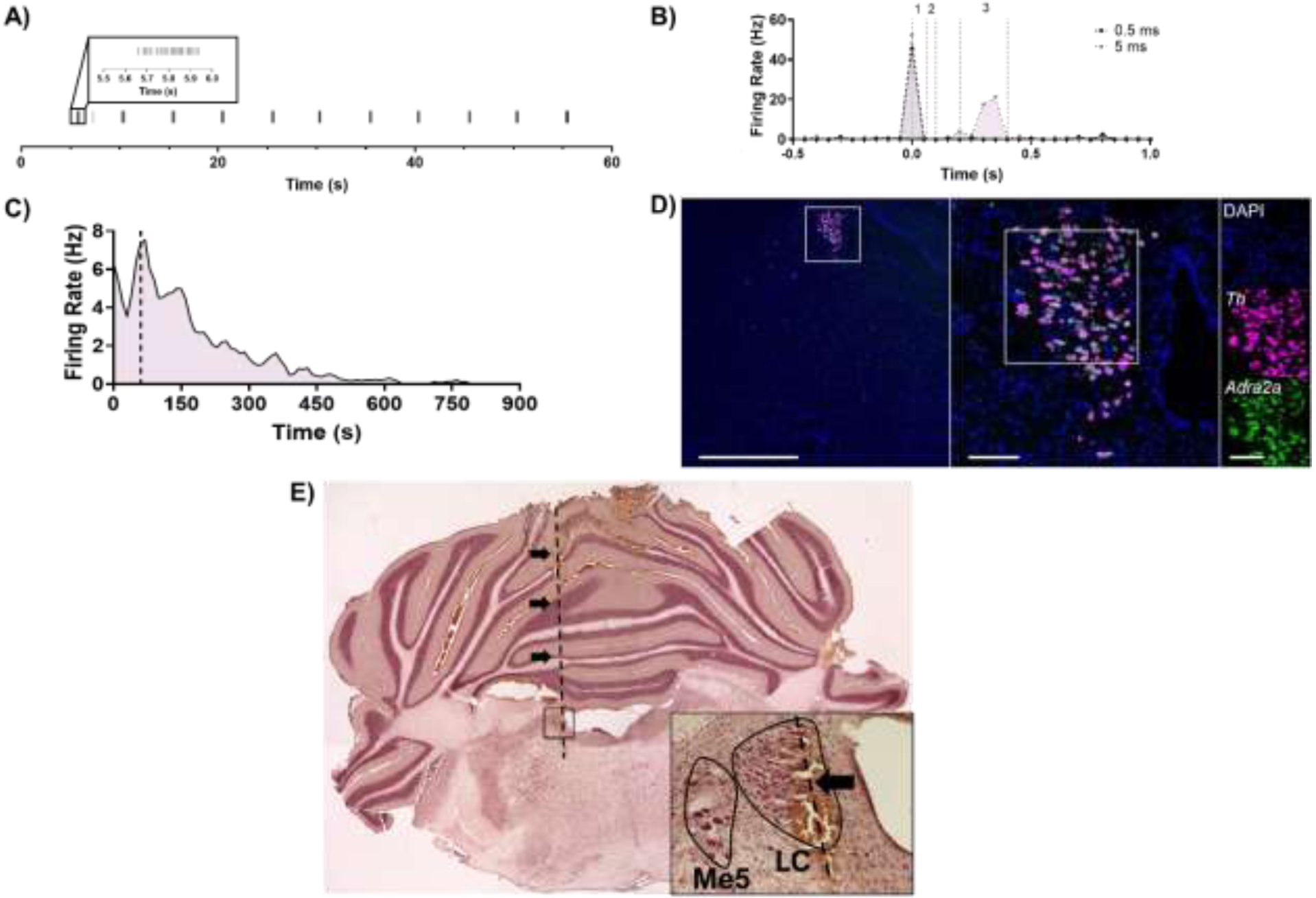
A) The approximate depth of the LC was estimated by locating Me5 via jaw deflection every 5 s during 1-min long recording. B) Putative LC units displayed a biphasic response to footshock (gray, 0.5 ms; purple, 5 ms; 0.05 ms bins around footshock at time 0 s). Unit responses were also quantified between 0–60 ms (1), 60–100 ms (2), and 200–400 ms (3) following footshock. C) Noradrenergic LC neuron activity is inhibited by the α2-adrenergic receptor clonidine (0.1 mg/kg i.p.; dashed line), and D) RNAscope performed at the level of the LC confirmed that expression of α2-adrenergic receptors (green) is limited to TH-expressing (magenta) LC neurons in this part of the brain. Scale bars: 1 mm at 2x, 100 μm at 20x, and 100 μm at 40x magnification. E) Localization of electrode tracks (arrows and dashed line) in the LC core using neutral red counterstaining. Main image stitched from four images at 2x magnification. Inset taken at 20x magnification with the LC outlined in black and the electrode track marked by an arrow.
3.2. Alteration of Pacemaker-like LC Firing in TgF344-AD Rats
LC neurons fire with regular pacemaker activity between 0.5–2 Hz under normal conditions that maintains baseline levels of arousal, attention, and noradrenergic tone (Aston-Jones and Cohen, 2005; Poe et al., 2020). To assess tau pathology- and age-induced changes in tonic firing, we recorded periods of spontaneous activity from 75–120 isolated LC neurons per group (N=8–11 animals). We graphed our data in the main figures using traditional bar graphs which highlight group level differences in firing rates as well as in supplemental figures using raincloud plots to highlight the variability and patterns that are not captured by more traditional visualization methods. There was a significant main effect of genotype (F1, 385 = 4.35, p = 0.04) on baseline firing rates, such that LC neurons of TgF344-AD rats were less active than those from WT littermates (Figure 2A; Supplemental Figure 1A). There was no effect of age (F1, 385 = 0.11, p = 0.74) or an age × genotype interaction (F1, 385 = 0.78, p = 0.38). We also quantified interspike interval for units with two or more spikes (N=74–119 neurons/group), defined as the time between successive action potentials (Figure 2B; Supplemental Figure 1B). There was a trend towards a main effect of genotype (F1, 379 = 3.55, p = 0.06), where TgF344-AD LC neurons had a shorter time between successive spikes, which was surprising given that tonic firing was lower in the TgF344-AD rats. However, LC neurons can also transiently fire in brief bursts, even in the absence of external stimuli (Akaike, 1982; Aston-Jones and Bloom, 1981a; Finlayson and Marshall, 1988; Iro et al., 2021; Safaai et al., 2015). Therefore, we quantified the spontaneous bursting properties of LC neurons from TgF344-AD and WT rats. Of the neurons exhibiting spontaneous bursts (N=48–88 neurons/group), there was a main effect of genotype on firing rate within a burst (F1, 267 = 6.57, p = 0.01) and on interspike interval within a burst (F1, 267 = 8.02, p < 0.01). During spontaneous bursts, firing rates were higher in TgF344-AD rats and interspike intervals were lower (Figures 2C, D; Supplemental Figure 1C, D). There were no alterations in other aspects of spontaneous bursts (Table 1). Overall, these changes indicate lower basal firing rates but elevated bursting properties of LC neurons in TgF344-AD rats.
Figure 2. Basal firing properties of LC neurons from 6- and 15-month TgF344-AD rats and WT littermates.
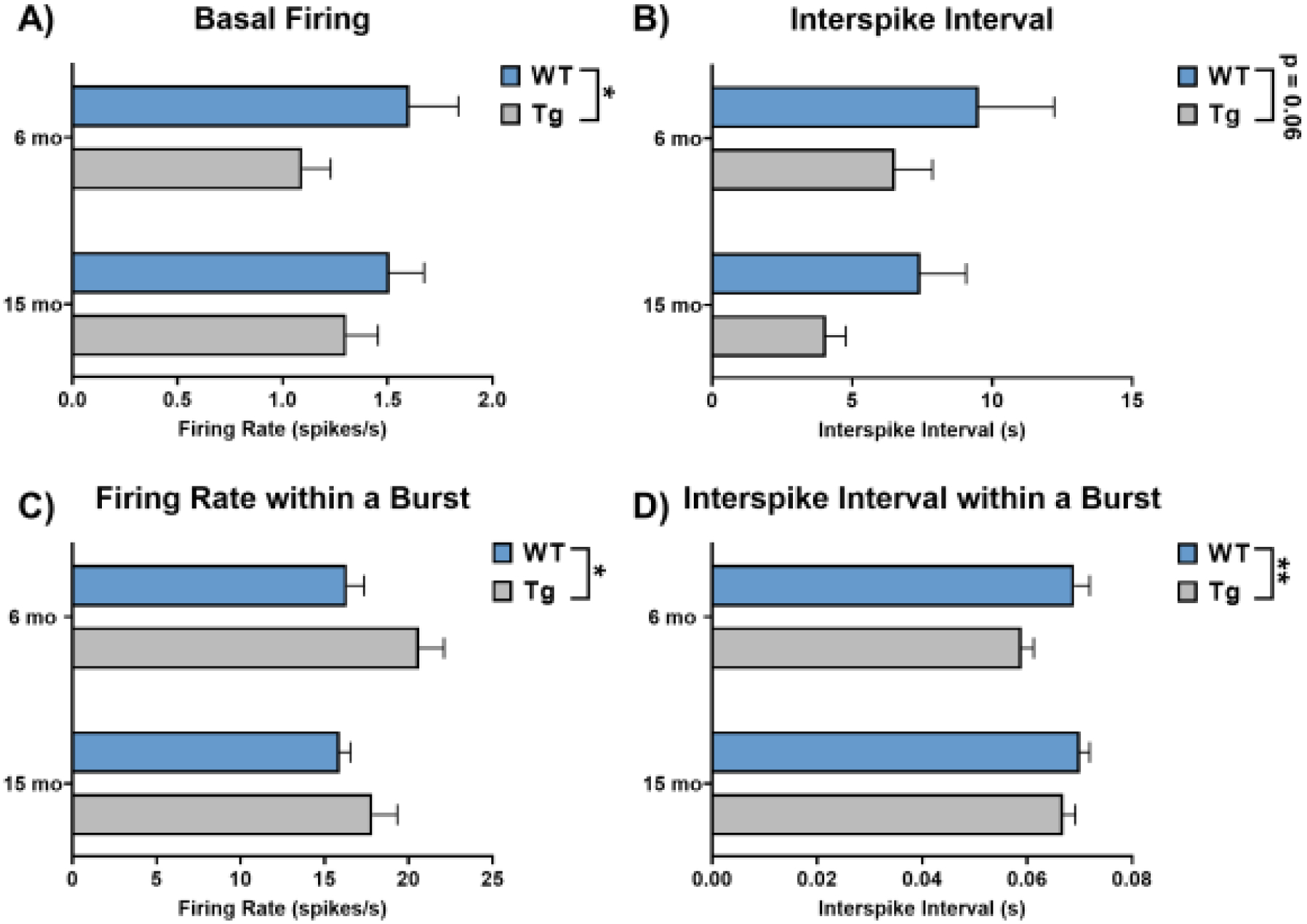
A) Baseline firing was lower in TgF344-AD rats at both ages. B) There was a trend towards decreased interspike interval in TgF344-AD rats. C) Firing rate during spontaneous bursts was higher in TgF344-AD rats. D) Interspike intervals within a burst were lower in TgF344-AD rats, and there was an additional effect of age where younger rats had lower interspike intervals. N=74–120 neurons/group (N=8–11 animals/group) for A and B. N=48–88 neurons/group (N=8–11 animals/group) for C and D. *p<0.05, **p<0.01.
Table 1.
Spontaneous bursting properties of LC neurons.
| Measure | Main Effect | F Statistic | p-value | Summary Values [Mean +/− SEM (N)] | ||
|---|---|---|---|---|---|---|
| Number of Bursts | Age | F1,385 = 0.002712 | 0.9585 | WT | Tg | |
| Genotype | F1,385 = 3.258 | 0.0719 | 6 month | 26.89 ± 6.00 (75) | 16.43 ± 3.29 (107) | |
| Interaction | F1,385 = 0.6039 | 0.4376 | 15 month | 23.53 ± 3.58 (120) | 19.37 ± 3.45 (87) | |
| Percent of Spikes in a Burst | Age | F1,385 = 1.649 | 0.1999 | WT | Tg | |
| Genotype | F1,385 = 0.1737 | 0.6771 | 6 month | 18.41 ± 2.75 (75) | 19.64 ± 2.37 (107) | |
| Interaction | F1,385 = 0.8379 | 0.3606 | 15 month | 23.84 ± 2.23 (120) | 20.55 ± 2.44 (87) | |
| Burst Duration | Age | F1,267 = 0.5341 | 0.4655 | WT | Tg | |
| Genotype | F1,267 = 2.652 | 0.1046 | 6 month | 0.36 ± 0.07 (48) | 0.32 ± 0.04 (75) | |
| Interaction | F1,267 = 0.5149 | 0.4737 | 15 month | 0.44 ± 0.06 (88) | 0.32 ± 0.02 (60) | |
| Spikes Per Burst | Age | F1,267 = 0.9097 | 0.3411 | WT | Tg | |
| Genotype | F1,267 = 0.06193 | 0.8037 | 6 month | 7.46 ± 1.41 (48) | 8.61 ± 1.56 (75) | |
| Interaction | F1,267 = 1.356 | 0.2453 | 15 month | 7.72 ± 1.08 (88) | 5.94 ± 0.34 (60) | |
| Interburst Interval | Age | F1,226 = 0.01437 | 0.9047 | WT | Tg | |
| Genotype | F1,226 = 2.222 | 0.1375 | 6 month | 16.27 ± 3.67 (37) | 37.83 ± 8.13 (60) | |
| Interaction | F1,226 = 3.516 | 0.0621 | 15 month | 27.51 ± 5.21 (75) | 25.05 ± 5.42 (58) | |
3.3. Dysregulated LC Response to Footshock in TgF344-AD Rats
LC neurons fire in transient bursts in awake animals to a variety of salient stimuli, including novelty, tones, pain, and stress (Aston-Jones and Bloom, 1981b; Uematsu et al., 2017; Vankov et al., 1995). We characterized the responsiveness of LC neurons 0–60, 60–100, and 200–400 ms following footshock (10 mA, 0.5 or 5 ms), which maintains its ability to trigger LC bursting under anesthesia, as described (Hirata and Aston-Jones, 1994). For the immediate response phase (Figure 3A, 3B; Supplemental Figure 2A, B), there was a reduction of LC activity in TgF344-AD rats that was specific to the 0.5 ms footshock (F1, 433 = 6.06, p = 0.01). There was a significant age × genotype interaction on LC firing rates in the mid-phase response to both 0.5 ms (F1, 433 = 6.26, p = 0.01) and 5 ms (F1, 452 = 9.56, p < 0.01) footshock (Figure 3C, 3D; Supplemental Figure 2C, D). LC neurons from TgF344-AD rats were hyperactive at 6 months following the 5 ms footshock (t452 = 2.36, p = 0.04), but hypoactive at 15 months following the 0.5 (t433 = 2.16, p = 0.03) and 5 ms footshock (t452 = 2.03, p = 0.04), compared to age-matched WT littermates. We observed no main effects on late phase response to either 0.5 ms or 5 ms footshock (Figure 3 E, F; Supplemental Figure 2E, F).
Figure 3: Footshock response of LC neurons from 6- and 15-month TgF344-AD rats and WT littermates.
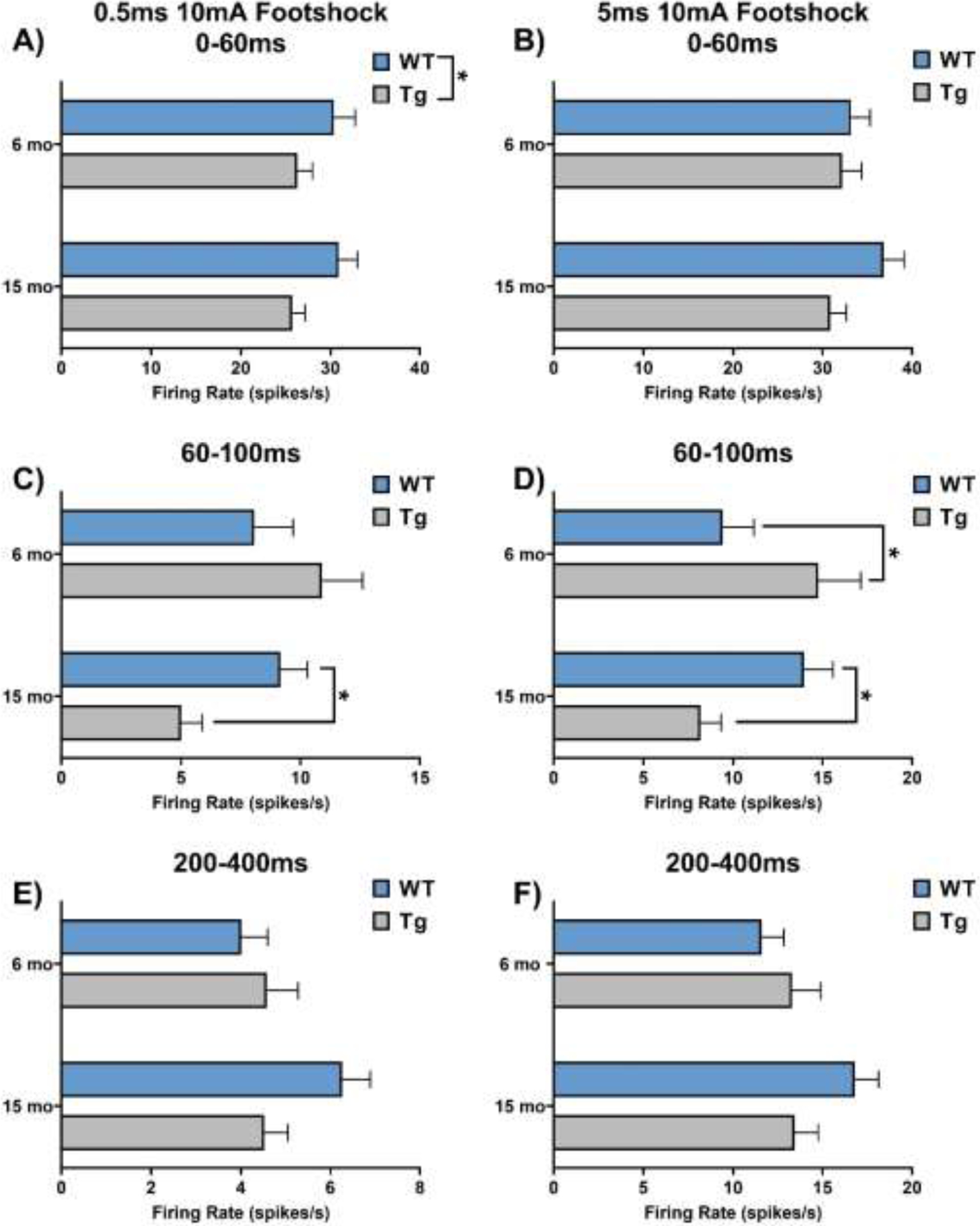
A & B) LC neuron firing rate was lower in TgF344-AD rats during the immediate phase in response to 0.5 ms footshock, but this effect was not seen in response to 5 ms footshock. C & D) There was an age × genotype interaction in the intermediate phase of response to both 0.5 and 5 ms footshock. Post-hoc analysis revealed that young TgF344-AD animals demonstrated hyperactivity in response to 5 ms footshock, while aged TgF344-AD animals showed hypoactivity in response to both 0.5 and 5 ms footshock. E & F) There were no differences in late phase response to footshock. N=84–128 neurons/group (N=8–11 animals/group) for A, C, and E. N=94–126 neurons/group (N=8–11 animals/group) for B, D, and F. *p<0.05.
4. Discussion
4.1. Overview of changes in LC firing and associations with symptoms of AD
The LC is one of the earliest regions to develop AD-related neuropathology in the form of hyperphosphorylated tau and undergoes frank cell death in mid- to late-stage disease (Braak et al., 2011; Busch et al., 1997; Elobeid et al., 2012; Pletnikova et al., 2018; Theofilas et al., 2017). The appearance of hyperphosphorylated tau in the LC is coincident with the emergence of prodromal symptoms of AD such as sleep disturbances, agitation, dysregulated mood, and increased anxiety, which are suggestive of LC hyperactivity (Ehrenberg et al., 2018; Johansson et al., 2021; Kelberman et al., 2022; Pentkowski et al., 2018; Weinshenker, 2018). Early LC hyperactivity is further supported by increases in cerebrospinal fluid NE levels and turnover (Elrod et al., 1997; Henjum et al., 2022; Hoogendijk et al., 1999; Palmer et al., 1987), axonal sprouting and elevated receptor density (Szot et al., 2006, 2007), and adrenergic receptor hypersensitivity (Goodman et al., 2021). By contrast, late-stage AD is characterized by phenotypes consistent with NE deficiency such as apathy and cognitive impairment that are correlated with loss of LC integrity (David et al., 2022; Hezemans et al., 2022; Kelly et al., 2017; Matthews et al., 2002; Ye et al., 2022). These data suggest that early hyperphosphorylated tau triggers compensatory mechanisms that increase LC-NE transmission and maintain function, which eventually fail as LC neurons succumb to more advanced pathology (Goodman et al., 2021; Szot et al., 2006, 2007). The divergent phenotypes across the progression of AD could be explained, at least in part, by changes in LC firing rates.
Prior to our study, only two investigations tracked changes in LC activity using rodent AD models, one with Aβ and one with mutant P301S tau pathology (Downs et al., 2022; Kelly et al., 2021). Mislocalization of GABA receptors associated with soluble Aβ oligomers in the LC led to hyperactivity, whereas effects of P301S tau expression were minimal. There are limitations in these studies that should be noted. In both cases, only one aspect of AD-like pathology was incorporated, the transgenes were driven by ubiquitous promoters, and LC Aβ and P301S tau are more reminiscent of late-stage AD and frontotemporal dementia, respectively. It is therefore unsurprising that we uncovered different dysregulated tonic and phasic LC firing patterns using an AD model that develops both Aβ and tau pathology (Figure 4), the latter of which is comprised of endogenous wild-type tau and isolated to the LC at early ages.
Figure 4: Summary of dysregulated LC firing patterns as a function of age in TgF344-AD rats.
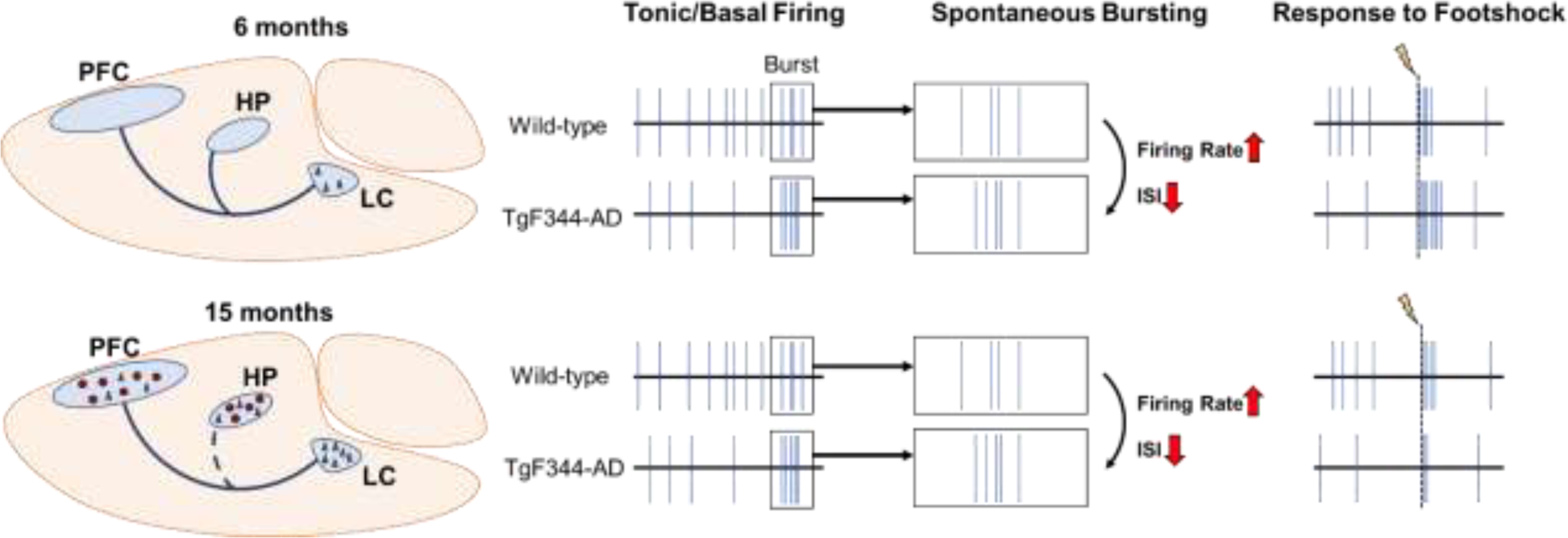
At 6 months, the LC is the only brain region that displays AD-like neuropathology in the form of hyperphosphorylated tau (black triangles). LC neurons show tonic hypoactivity, but elevated firing rate and shorter interspike intervals during periods of spontaneous bursts. LC response to footshock (lightning bolt/dashed line) at this age is also elevated in TgF344-AD rats. At 15 months, hyperphosphorylated tau pathology in the LC worsens, and tau and Aβ (red circles) pathology are present throughout the forebrain. There is also evidence of noradrenergic denervation, specifically to the hippocampus (dashed blue line). Similar to 6-month TgF344-AD rats, LC neurons again show tonic hypoactivity, and elevated firing rate and lower interspike intervals during periods of spontaneous bursts. However, in contrast to younger rats, 15-month TgF344-AD rats have an impaired, rather than elevated, footshock response.
In our study, both young and old TgF344-AD animals showed tonic LC hypoactivity but elevated spontaneous bursting properties. Moreover, LC activity in response to footshock stress was elevated in 6-month TgF344-AD rats. Importantly, we and others have reported behavioral abnormalities in young TgF344-AD reminiscent of neuropsychiatric symptoms in prodromal AD (Ehrenberg et al., 2018; Johansson et al., 2021; Kelberman et al., 2022; Pentkowski et al., 2018; Wu et al., 2020). At first glance, reduced pacemaker LC firing is inconsistent with evidence that excessive NE transmission contributes to comorbidities seen in prodromal phases of AD (Weinshenker, 2018). Specifically, elevations in tonic, but not phasic, LC activity are associated with stress and anxiety (Curtis et al., 2012; McCall et al., 2015; Valentino and Foote, 1988). On the other hand, increased phasic LC activity driven by spontaneous bursting and/or environmental stimuli could promote improper transitions through different stages of arousal (Aston-Jones and Bloom, 1981a; Carter et al., 2010; Takahashi et al., 2010), resulting in fractured sleep/wake cycles that are common in AD (Ehrenberg et al., 2018; Weinshenker, 2018). The importance of distinguishing between different patterns of LC firing across disease stages is highlighted by a recent report that enhancement of phasic LC firing protects LC neurons from deleterious forms of tau, whereas high tonic firing results in AD-associated psychiatric symptoms and worsened neuronal health (Omoluabi et al., 2021). Together, these data are consistent with the idea that a combination of homeostatic mechanisms to maintain LC function in the presence of pathology and damage (e.g. altered LC firing, increased NE turnover, elevated adrenergic receptor sensitivity and density, and axonal sprouting) contributes to prodromal behaviors. These homeostatic/compensatory mechanisms ultimately fail in later disease, as indicated by reduced NE tissue levels, noradrenergic denervation, and frank LC neuron loss (Goodman et al., 2021; Kelberman et al., 2022; Matthews et al., 2002; Nazarali and Reynolds, 1992; Reinikainen et al., 1988; Rorabaugh et al., 2017), and our results showing tonic LC hypoactivity and blunted response to footshock in 15-month TgF344-AD rats. This culminates in a loss of LC-NE transmission and cognitive impairment, which our lab has successfully rescued by selectively augmenting tonic LC firing (Armbruster et al., 2007; Rorabaugh et al., 2017; Vazey and Aston-Jones, 2014).
4.2. Potential mechanisms underlying changes in LC firing rates
While the neurobiological mechanisms underlying the changes in LC firing properties we observed remain to be determined, the nature of those changes offers clues. Reductions in tonic firing rates and hypoactivity in aged TgF344-AD rats are unlikely due to cell death because there is no LC neuron loss at this age (Rorabaugh et al., 2017). However, we know that β-adrenergic receptor function in the dentate gyrus is heightened in young TgF344-AD rats, indicating that adrenergic receptor plasticity exists in this model (Goodman et al., 2021). In the pons, α2-adrenergic receptors are abundantly expressed within the LC itself (Figure 1) and regulate its activity as inhibitory autoreceptors. Changes in the density or sensitivity of these receptors, which have been noted in the human condition with other subtypes of adrenergic receptors (Goodman et al., 2021; Szot et al., 2006, 2007), could alter LC neuron firing properties. Furthermore, elevated phasic activity may result in greater NE release compared to tonic activity (Florin-Lechner et al., 1996). A surplus of NE at the level of LC cell bodies would culminate in augmented inhibition from neighboring LC neurons, also potentially leading to tonic LC hypoactivity.
Besides its intrinsic pacemaker activity, the LC also receives extensive excitatory, inhibitory, and modulatory (serotonin (5-HT), hypocretin/orexin, corticotropin releasing hormone) input from various cortical and subcortical regions that could contribute to altered firing rates in TgF344-AD rats (Benarroch, 2009; Delaville et al., 2011). GABAergic inputs arising from the ventrolateral preoptic area or the peri-LC region modulate basal noradrenergic tone and have been mainly studied in the context of arousal (Breton-Provencher and Sur, 2019; Lu et al., 2002; Nitz and Siegel, 1997). In addition, given that the LC and peri-LC GABAergic neurons receive a set of non-overlapping innervations (Breton-Provencher and Sur, 2019), it is possible that changes in firing rates are the result of a dysregulated, multi-synaptic pathway. For example, systemic application of a 5-HT receptor agonist lowers tonic but enhances phasic firing of the LC in a GABA-dependent manner (Chiang and Aston-Jones, 1993a). Similarly, 5-HT dampens sensitivity of LC neurons to glutamatergic inputs, which originate from the cortex, lateral habenula, and paragigantocellularis nucleus (Aston-Jones et al., 1991; Chiang and Aston-Jones, 1993b; Ennis and Aston-Jones, 1988; Herkenham and Nauta, 1979; Jodo and Aston-Jones, 1997). Thus, altered serotonergic signaling to the LC represents a singular mechanism that could influence both altered tonic and phasic firing patterns. With respect to footshock-evoked LC activity, the immediate and middle phase responses are also mediated, in part, by excitatory amino acids (Chiang and Aston-Jones, 1993b; Ennis and Aston-Jones, 1988). Changes in excitatory amino acid transmission, receptor expression, or localization could be responsible for various aspects of dysregulated LC firing. Other neuropeptides, such as hypocretin/orexin and corticotropin releasing factor powerfully activate LC neurons and are both associated with elevations of tonic LC firing and the expression of anxiety-like phenotypes (Curtis et al., 2012; Horvath et al., 1999; McCall et al., 2015; Sears et al., 2013; Valentino and Foote, 1988). The TgF344-AD rats represent an excellent model to further evaluate AD pathology-associated changes in these systems and their influence on LC activity.
4.3. Clinical Implications
We have previously posited that LC/NE-based therapies for AD should be guided by disease stage (Weinshenker, 2018). The dysregulated/hyperactive NE transmission that is produced by early AD pathology (e.g. hyperphosphorylated tau) and promotes prodromal symptoms may be best alleviated by therapies that decrease LC firing and/or NE signaling, while reduced LC-NE transmission resulting from advanced pathology and deterioration of LC neurons would benefit from LC-NE stimulation. Our current findings largely support this model. At 6-months of age, TgF344-AD rats have hyperphosphorylated tau in the LC but no degeneration and display phenotypes relevant to neuropsychiatric disorders such as anxiety, and they primarily present with LC hyperactivity (increased firing during bursts and in responses to footshock stress). Meanwhile 15-month transgenic animal have increased tau pathology, some loss of LC fibers/terminals, cognitive impairment, and display LC hypoactivity (reduced baseline and footshock-induced firing). Moreover, we previously reported that deficits in reversal learning in 15-month TgF344-AD rats can be rescued by chemogenetic LC stimulation (Rorabaugh et al., 2017), and we predict that drugs that suppress LC activity or antagonism of adrenergic receptors would be effective at alleviating prodromal phenotypes in young TgF344-AD rats (Weinshenker, 2018). These results provide a foundation for translation to the clinical setting, where promising data already exist. For example, adrenergic antagonists have been reported to reduce agitation/aggression and anxiety in subjects with probable or possible AD (Peskind et al., 2005; Wang et al., 2009), and our recent phase II study showed beneficial effects of the NE transporter inhibitor atomoxetine, which increases extracellular NE levels, on AD biomarkers in mild cognitive impairment (Levey et al., 2022). Atomoxetine also improves response inhibition in Parkinson’s disease patients, with the greatest benefits observed in those with lower LC integrity (O’Callaghan et al., 2021). Whether this is also true in AD has not been determined but will be important for guiding the use of personalized noradrenergic-based interventions.
4.4. Limitations
There are several caveats to the current study that are worth discussing. Given the extracellular in vivo nature of our recordings, we were unable to determine whether the neurons recorded from TgF344-AD rats were tau positive or negative. However, our previous report demonstrated that essentially all LC neurons stain positive for the CP13 antibody at 6 months of age (Rorabaugh et al., 2017). This staining becomes more intense by 15 months, but does not stain for more mature versions of hyperphosphorylated/aggregated tau (i.e. AT8, MC1, PHF1) (Rorabaugh et al., 2017). Given the extensive gap junctions between LC neurons (Ishimatsu and Williams, 1996; Rash et al., 2007), it is also possible that even the rare tau-negative neurons are dysfunctional in TgF344-AD rats. Furthermore, there is evidence of LC heterogeneity, including different firing rates that are dependent upon projection targets, which may have influenced our results (Chandler et al., 2014). However, this is unlikely given that we recorded LC neurons from a range of coordinates. Follow-up studies using slice electrophysiology coupled with retrograde tracers and tau antibody staining would help determine whether differences in LC firing rates are tau-dependent and/or projection specific, with the latter being particularly interesting given the selective vulnerability of forebrain-projecting LC neurons (Kelberman et al., 2020; Schwarz and Luo, 2015; Weinshenker, 2018). It is possible that Aβ pathology, and not just aberrant tau, contributed to the changes in LC firing rates in TgF344-AD rats, as a previous report identified the presence of soluble Aβ pathology in LC neurons of postmortem human AD brains, as well as in APP/PS1 transgenic mice that was associated with LC hyperactivity (Kelly et al., 2021). Aβ pathology has not been examined in the LC of TgF344-AD rats, but one caveat is that commonly used antibodies (i.e. 4G8) have off-target binding to the amyloid precursor protein (Kelly et al., 2021), which is ubiquitously overexpressed in these animals (Cohen et al., 2013). In TgF344-AD rats, plaques are minimal in forebrain regions at 6 months of age, evident at 12 months of age, and ubiquitous at 16 months of age (Cohen et al., 2013; Kelberman et al., 2022; Rorabaugh et al., 2017). Therefore, we speculate that early alterations to LC firing rates are likely dominated by the effects of hyperphosphorylated tau, while interactions between tau and Aβ are more likely during late stages of disease. Finally, there may be some concern that viral expression of the fluorescent marker mCherry altered LC firing rates in the current study. However, because all animals in the current report received viral infusions, the effects of mCherry expression would be equivalent across groups. Moreover, the WT rats in our study demonstrated LC firing rates consistent with non-manipulated rats based on previous literature (Aston-Jones and Bloom, 1981a, b; Chandler et al., 2014; Hirata and Aston-Jones, 1994; Totah et al., 2018; Vazey and Aston-Jones, 2014; West et al., 2015), indicating that any effects of the virus were negligible.
5. Conclusions
We have identified disease stage-dependent changes in LC firing patterns in a rat model of AD that accumulates hyperphosphorylated tau in the LC prior to forebrain pathology. These data further support the notion that early LC hyperactivity and late LC hypoactivity contribute to prodromal symptoms and cognitive/memory impairments of AD, respectively. These insights should prove useful for developing noradrenergic-based therapeutics for AD.
Supplementary Material
Supplemental Figure 1. Basal firing properties of LC neurons from 6- and 15-month TgF344-AD rats and WT littermates. A) Baseline firing was lower in TgF344-AD rats at both ages. B) There was a trend towards decreased interspike interval in TgF344-AD rats. C) Firing rate during spontaneous bursts was higher in TgF344-AD rats. D) Interspike intervals within a burst were lower in TgF344-AD rats, and there was an additional effect of age where younger rats had lower interspike intervals. N=74–120 neurons/group (N=8–11 animals/group) for A and B. N=48–88 neurons/group (N=8–11 animals/group) for C and D. *p<0.05, **p<0.01.
Supplemental Figure 2: Footshock response of LC neurons from 6- and 15-month TgF344-AD rats and WT littermates. A & B) LC neuron firing rate was lower in TgF344-AD rats during the immediate phase in response to 0.5 ms footshock, but this effect was not seen in response to 5 ms footshock. C & D) There was an age × genotype interaction in the intermediate phase of response to both 0.5 and 5 ms footshock. Post-hoc analysis revealed that young TgF344-AD animals demonstrated hyperactivity in response to 5 ms footshock, while aged TgF344-AD animals showed hypoactivity in response to both 0.5 and 5 ms footshock. E & F) There were no differences in late phase response to footshock. N=84–128 neurons/group (N=8–11 animals/group) for A, C, and E. N=94–126 neurons/group (N=8–11 animals/group) for B, D, and F. *p<0.05.
Highlights.
Recorded locus coeruleus (LC) neurons in a rat model of Alzheimer’s disease (AD)
TgF344-AD rats develop early endogenous LC tau pathology akin to human AD
6- and 15-month TgF344-AD rats had reduced tonic LC firing
LC neurons from 6-month TgF344-AD rats were hyperactive in response to footshock
LC neuron dysfunction may contribute to AD symptoms
Funding and Acknowledgements
This work was supported by funding from the National Institutes of Aging (AG062581 to DW, AG069502 to MAK), the National Institute of Neurological Disorders and Stroke (MS96050 to MAK), and the Eli Lilly Innovation Fellowship Award (JMR and CRA).
We would like to thank A Korukonda for acquiring the image for Figure 1E and CH West for helping establish the electrophysiological methods used in this study.
This research project was supported in part by the Viral Vector Core of the Emory Center for Neurodegenerative Disease Core Facilities and the Vector Core of the University of Pennsylvania.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement
The authors have nothing to disclose.
CRediT authorship contribution statement
Michael A. Kelberman: Conceptualization, Methodology, Software, Validation, Formal analysis, Investigation, Resources, Data Curation, Writing – Original Draft, Writing – Review & Editing, Visualization, Funding Acquisition. Jacki M. Rorabaugh: Conceptualization, Methodology, Investigation, Writing – Review & Editing, Funding Acquisition. Claire R. Anderson: Conceptualization, Methodology, Investigation, Writing – Review & Editing, Funding Acquisition. Alexia Marriott: Investigation, Writing – Review & Editing. Seth D. DePuy: Methodology, Writing – Review & Editing. Kurt Rasmussen: Methodology, Writing – Review & Editing. Katharine E. McCann: Writing – Review & Editing, Software, Visualization. Jay M. Wiess: Conceptualization, Methodology, Writing – Review & Editing, Resources, Supervision, Funding Acquisition. David Weinshenker: Conceptualization, Methodology, Validation, Formal analysis, Resources, Writing – Original Draft, Writing – Review & Editing, Visualization, Supervision, Funding Acquisition.
References
- Akaike T, 1982. Periodic bursting activities of locus coerulleus neurons in the rat. Brain Res 239(2), 629–633. [DOI] [PubMed] [Google Scholar]
- Allen M, Poggiali D, Whitaker K, Marshall TR, Kievit RA, 2019. Raincloud plots: a multi-platform tool for robust data visualization. Wellcome Open Res 4, 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armbruster BN, Li X, Pausch MH, Herlitze S, Roth BL, 2007. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc Natl Acad Sci U S A 104(12), 5163–5168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Akaoka H, Charlety P, Chouvet G, 1991. Serotonin selectively attenuates glutamate-evoked activation of noradrenergic locus coeruleus neurons. J Neurosci 11(3), 760–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Bloom FE, 1981a. Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J Neurosci 1(8), 876–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Bloom FE, 1981b. Norepinephrine-containing locus coeruleus neurons in behaving rats exhibit pronounced responses to non-noxious environmental stimuli. J Neurosci 1(8), 887–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD, 2005. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci 28, 403–450. [DOI] [PubMed] [Google Scholar]
- Bachman SL, Dahl MJ, Werkle-Bergner M, Duzel S, Forlim CG, Lindenberger U, Kuhn S, Mather M, 2021. Locus coeruleus MRI contrast is associated with cortical thickness in older adults. Neurobiol Aging 100, 72–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benarroch EE, 2009. The locus ceruleus norepinephrine system: functional organization and potential clinical significance. Neurology 73(20), 1699–1704. [DOI] [PubMed] [Google Scholar]
- Braak H, Thal DR, Ghebremedhin E, Del Tredici K, 2011. Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J Neuropathol Exp Neurol 70(11), 960–969. [DOI] [PubMed] [Google Scholar]
- Breton-Provencher V, Sur M, 2019. Active control of arousal by a locus coeruleus GABAergic circuit. Nat Neurosci 22(2), 218–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch C, Bohl J, Ohm TG, 1997. Spatial, temporal and numeric analysis of Alzheimer changes in the nucleus coeruleus. Neurobiol Aging 18(4), 401–406. [DOI] [PubMed] [Google Scholar]
- Busche MA, Hyman BT, 2020. Synergy between amyloid-beta and tau in Alzheimer’s disease. Nat Neurosci 23(10), 1183–1193. [DOI] [PubMed] [Google Scholar]
- Busche MA, Wegmann S, Dujardin S, Commins C, Schiantarelli J, Klickstein N, Kamath TV, Carlson GA, Nelken I, Hyman BT, 2019. Tau impairs neural circuits, dominating amyloid-beta effects, in Alzheimer models in vivo. Nat Neurosci 22(1), 57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter ME, Yizhar O, Chikahisa S, Nguyen H, Adamantidis A, Nishino S, Deisseroth K, de Lecea L, 2010. Tuning arousal with optogenetic modulation of locus coeruleus neurons. Nat Neurosci 13(12), 1526–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy CM, Therriault J, Pascoal TA, Cheung V, Savard M, Tuominen L, Chamoun M, McCall A, Celebi S, Lussier F, Massarweh G, Soucy JP, Weinshenker D, Tardif C, Ismail Z, Gauthier S, Rosa-Neto P, 2022. Association of locus coeruleus integrity with Braak stage and neuropsychiatric symptom severity in Alzheimer’s disease. Neuropsychopharmacology 47(5), 1128–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler DJ, Gao WJ, Waterhouse BD, 2014. Heterogeneous organization of the locus coeruleus projections to prefrontal and motor cortices. Proc Natl Acad Sci U S A 111(18), 6816–6821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang C, Aston-Jones G, 1993a. A 5-hydroxytryptamine2 agonist augments gamma-aminobutyric acid and excitatory amino acid inputs to noradrenergic locus coeruleus neurons. Neuroscience 54(2), 409–420. [DOI] [PubMed] [Google Scholar]
- Chiang C, Aston-Jones G, 1993b. Response of locus coeruleus neurons to footshock stimulation is mediated by neurons in the rostral ventral medulla. Neuroscience 53(3), 705–715. [DOI] [PubMed] [Google Scholar]
- Cohen RM, Rezai-Zadeh K, Weitz TM, Rentsendorj A, Gate D, Spivak I, Bholat Y, Vasilevko V, Glabe CG, Breunig JJ, Rakic P, Davtyan H, Agadjanyan MG, Kepe V, Barrio JR, Bannykh S, Szekely CA, Pechnick RN, Town T, 2013. A transgenic Alzheimer rat with plaques, tau pathology, behavioral impairment, oligomeric abeta, and frank neuronal loss. J Neurosci 33(15), 6245–6256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole G, Neal JW, Singhrao SK, Jasani B, Newman GR, 1993. The distribution of amyloid plaques in the cerebellum and brain stem in Down’s syndrome and Alzheimer’s disease: a light microscopical analysis. Acta Neuropathol 85(5), 542–552. [DOI] [PubMed] [Google Scholar]
- Curtis AL, Leiser SC, Snyder K, Valentino RJ, 2012. Predator stress engages corticotropin-releasing factor and opioid systems to alter the operating mode of locus coeruleus norepinephrine neurons. Neuropharmacology 62(4), 1737–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David MCB, Del Giovane M, Liu KY, Gostick B, Rowe JB, Oboh I, Howard R, Malhotra PA, 2022. Cognitive and neuropsychiatric effects of noradrenergic treatment in Alzheimer’s disease: systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaville C, Deurwaerdere PD, Benazzouz A, 2011. Noradrenaline and Parkinson’s disease. Front Syst Neurosci 5, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs AM, Catavero CM, Kasten MR, McElligott ZA, 2022. Tauopathy and alcohol consumption interact to alter locus coeruleus excitatory transmission and excitability in male and female mice. Alcohol. [DOI] [PubMed] [Google Scholar]
- Ehrenberg AJ, Nguy AK, Theofilas P, Dunlop S, Suemoto CK, Di Lorenzo Alho AT, Leite RP, Diehl Rodriguez R, Mejia MB, Rub U, Farfel JM, de Lucena Ferretti-Rebustini RE, Nascimento CF, Nitrini R, Pasquallucci CA, Jacob-Filho W, Miller B, Seeley WW, Heinsen H, Grinberg LT, 2017. Quantifying the accretion of hyperphosphorylated tau in the locus coeruleus and dorsal raphe nucleus: the pathological building blocks of early Alzheimer’s disease. Neuropathol Appl Neurobiol 43(5), 393–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenberg AJ, Suemoto CK, Franca Resende EP, Petersen C, Leite REP, Rodriguez RD, Ferretti-Rebustini REL, You M, Oh J, Nitrini R, Pasqualucci CA, Jacob-Filho W, Kramer JH, Gatchel JR, Grinberg LT, 2018. Neuropathologic Correlates of Psychiatric Symptoms in Alzheimer’s Disease. J Alzheimers Dis 66(1), 115–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elobeid A, Soininen H, Alafuzoff I, 2012. Hyperphosphorylated tau in young and middle-aged subjects. Acta Neuropathol 123(1), 97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elrod R, Peskind ER, DiGiacomo L, Brodkin KI, Veith RC, Raskind MA, 1997. Effects of Alzheimer’s disease severity on cerebrospinal fluid norepinephrine concentration. Am J Psychiatry 154(1), 25–30. [DOI] [PubMed] [Google Scholar]
- Ennis M, Aston-Jones G, 1988. Activation of locus coeruleus from nucleus paragigantocellularis: a new excitatory amino acid pathway in brain. J Neurosci 8(10), 3644–3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlayson PG, Marshall KC, 1988. Synchronous bursting of locus coeruleus neurons in tissue culture. Neuroscience 24(1), 217–225. [DOI] [PubMed] [Google Scholar]
- Florin-Lechner SM, Druhan JP, Aston-Jones G, Valentino RJ, 1996. Enhanced norepinephrine release in prefrontal cortex with burst stimulation of the locus coeruleus. Brain Res 742(1–2), 89–97. [DOI] [PubMed] [Google Scholar]
- Goodman AM, Langner BM, Jackson N, Alex C, McMahon LL, 2021. Heightened Hippocampal beta-Adrenergic Receptor Function Drives Synaptic Potentiation and Supports Learning and Memory in the TgF344-AD Rat Model during Prodromal Alzheimer’s Disease. J Neurosci 41(26), 5747–5761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA, Bunney BS, 1983. Intracellular and extracellular electrophysiology of nigral dopaminergic neurons--3. Evidence for electrotonic coupling. Neuroscience 10(2), 333–348. [DOI] [PubMed] [Google Scholar]
- Henjum K, Watne LO, Godang K, Halaas NB, Eldholm RS, Blennow K, Zetterberg H, Saltvedt I, Bollerslev J, Knapskog AB, 2022. Cerebrospinal fluid catecholamines in Alzheimer’s disease patients with and without biological disease. Transl Psychiatry 12(1), 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M, Nauta WJ, 1979. Efferent connections of the habenular nuclei in the rat. J Comp Neurol 187(1), 19–47. [DOI] [PubMed] [Google Scholar]
- Hezemans FH, Wolpe N, O’Callaghan C, Ye R, Rua C, Jones PS, Murley AG, Holland N, Regenthal R, Tsvetanov KA, Barker RA, Williams-Gray CH, Robbins TW, Passamonti L, Rowe JB, 2022. Noradrenergic deficits contribute to apathy in Parkinson’s disease through the precision of expected outcomes. PLoS Comput Biol 18(5), e1010079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata H, Aston-Jones G, 1994. A novel long-latency response of locus coeruleus neurons to noxious stimuli: mediation by peripheral C-fibers. J Neurophysiol 71(5), 1752–1761. [DOI] [PubMed] [Google Scholar]
- Holth JK, Bomben VC, Reed JG, Inoue T, Younkin L, Younkin SG, Pautler RG, Botas J, Noebels JL, 2013. Tau loss attenuates neuronal network hyperexcitability in mouse and Drosophila genetic models of epilepsy. J Neurosci 33(4), 1651–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogendijk WJ, Feenstra MG, Botterblom MH, Gilhuis J, Sommer IE, Kamphorst W, Eikelenboom P, Swaab DF, 1999. Increased activity of surviving locus ceruleus neurons in Alzheimer’s disease. Ann Neurol 45(1), 82–91. [DOI] [PubMed] [Google Scholar]
- Horvath TL, Peyron C, Diano S, Ivanov A, Aston-Jones G, Kilduff TS, van Den Pol AN, 1999. Hypocretin (orexin) activation and synaptic innervation of the locus coeruleus noradrenergic system. J Comp Neurol 415(2), 145–159. [PubMed] [Google Scholar]
- Huijbers W, Schultz AP, Papp KV, LaPoint MR, Hanseeuw B, Chhatwal JP, Hedden T, Johnson KA, Sperling RA, 2019. Tau Accumulation in Clinically Normal Older Adults Is Associated with Hippocampal Hyperactivity. J Neurosci 39(3), 548–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iro CM, Hamati R, El Mansari M, Blier P, 2021. Repeated but Not Single Administration of Ketamine Prolongs Increases of the Firing Activity of Norepinephrine and Dopamine Neurons. Int J Neuropsychopharmacol 24(7), 570–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimatsu M, Williams JT, 1996. Synchronous activity in locus coeruleus results from dendritic interactions in pericoerulear regions. J Neurosci 16(16), 5196–5204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs HIL, Becker JA, Kwong K, Engels-Dominguez N, Prokopiou PC, Papp KV, Properzi M, Hampton OL, d’Oleire Uquillas F, Sanchez JS, Rentz DM, El Fakhri G, Normandin MD, Price JC, Bennett DA, Sperling RA, Johnson KA, 2021. In vivo and neuropathology data support locus coeruleus integrity as indicator of Alzheimer’s disease pathology and cognitive decline. Sci Transl Med 13(612), eabj2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jodo E, Aston-Jones G, 1997. Activation of locus coeruleus by prefrontal cortex is mediated by excitatory amino acid inputs. Brain Res 768(1–2), 327–332. [DOI] [PubMed] [Google Scholar]
- Johansson M, Stomrud E, Insel PS, Leuzy A, Johansson PM, Smith R, Ismail Z, Janelidze S, Palmqvist S, van Westen D, Mattsson-Carlgren N, Hansson O, 2021. Mild behavioral impairment and its relation to tau pathology in preclinical Alzheimer’s disease. Transl Psychiatry 11(1), 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalwani RM, Joshi S, Gold JI, 2014. Phasic activation of individual neurons in the locus ceruleus/subceruleus complex of monkeys reflects rewarded decisions to go but not stop. J Neurosci 34(41), 13656–13669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay M, 2022. ggdist: Visualizations of distributions and uncertainty. 10.5281/zenodo.6862765 [DOI] [PubMed] [Google Scholar]
- Kelberman M, Keilholz S, Weinshenker D, 2020. What’s That (Blue) Spot on my MRI? Multimodal Neuroimaging of the Locus Coeruleus in Neurodegenerative Disease. Front Neurosci 14, 583421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelberman MA, Anderson CR, Chlan E, Rorabaugh JM, McCann KE, Weinshenker D, 2022. Consequences of Hyperphosphorylated Tau in the Locus Coeruleus on Behavior and Cognition in a Rat Model of Alzheimer’s Disease. J Alzheimers Dis 86(3), 1037–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly L, Seifi M, Ma R, Mitchell SJ, Rudolph U, Viola KL, Klein WL, Lambert JJ, Swinny JD, 2021. Identification of intraneuronal amyloid beta oligomers in locus coeruleus neurons of Alzheimer’s patients and their potential impact on inhibitory neurotransmitter receptors and neuronal excitability. Neuropathol Appl Neurobiol 47(4), 488–505. [DOI] [PubMed] [Google Scholar]
- Kelly SC, He B, Perez SE, Ginsberg SD, Mufson EJ, Counts SE, 2017. Locus coeruleus cellular and molecular pathology during the progression of Alzheimer’s disease. Acta Neuropathol Commun 5(1), 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levey AI, Qiu D, Zhao L, Hu WT, Duong DM, Higginbotham L, Dammer EB, Seyfried NT, Wingo TS, Hales CM, Gamez Tansey M, Goldstein DS, Abrol A, Calhoun VD, Goldstein FC, Hajjar I, Fagan AM, Galasko D, Edland SD, Hanfelt J, Lah JJ, Weinshenker D, 2022. A phase II study repurposing atomoxetine for neuroprotection in mild cognitive impairment. Brain 145(6), 1924–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Bjorkum AA, Xu M, Gaus SE, Shiromani PJ, Saper CB, 2002. Selective activation of the extended ventrolateral preoptic nucleus during rapid eye movement sleep. J Neurosci 22(11), 4568–4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews KL, Chen CP, Esiri MM, Keene J, Minger SL, Francis PT, 2002. Noradrenergic changes, aggressive behavior, and cognition in patients with dementia. Biol Psychiatry 51(5), 407–416. [DOI] [PubMed] [Google Scholar]
- McCall JG, Al-Hasani R, Siuda ER, Hong DY, Norris AJ, Ford CP, Bruchas MR, 2015. CRH Engagement of the Locus Coeruleus Noradrenergic System Mediates Stress-Induced Anxiety. Neuron 87(3), 605–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCune SK, Voigt MM, Hill JM, 1993. Expression of multiple alpha adrenergic receptor subtype messenger RNAs in the adult rat brain. Neuroscience 57(1), 143–151. [DOI] [PubMed] [Google Scholar]
- Nazarali AJ, Reynolds GP, 1992. Monoamine neurotransmitters and their metabolites in brain regions in Alzheimer’s disease: a postmortem study. Cell Mol Neurobiol 12(6), 581–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitz D, Siegel JM, 1997. GABA release in the locus coeruleus as a function of sleep/wake state. Neuroscience 78(3), 795–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Callaghan C, Hezemans FH, Ye R, Rua C, Jones PS, Murley AG, Holland N, Regenthal R, Tsvetanov KA, Wolpe N, Barker RA, Williams-Gray CH, Robbins TW, Passamonti L, Rowe JB, 2021. Locus coeruleus integrity and the effect of atomoxetine on response inhibition in Parkinson’s disease. Brain 144(8), 2513–2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omoluabi T, Torraville SE, Maziar A, Ghosh A, Power KD, Reinhardt C, Harley CW, Yuan Q, 2021. Novelty-like activation of locus coeruleus protects against deleterious human pretangle tau effects while stress-inducing activation worsens its effects. Alzheimers Dement (N Y) 7(1), e12231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer AM, Francis PT, Bowen DM, Benton JS, Neary D, Mann DM, Snowden JS, 1987. Catecholaminergic neurones assessed ante-mortem in Alzheimer’s disease. Brain Res 414(2), 365–375. [DOI] [PubMed] [Google Scholar]
- Pentkowski NS, Berkowitz LE, Thompson SM, Drake EN, Olguin CR, Clark BJ, 2018. Anxiety-like behavior as an early endophenotype in the TgF344-AD rat model of Alzheimer’s disease. Neurobiol Aging 61, 169–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peskind ER, Tsuang DW, Bonner LT, Pascualy M, Riekse RG, Snowden MB, Thomas R, Raskind MA, 2005. Propranolol for disruptive behaviors in nursing home residents with probable or possible Alzheimer disease: a placebo-controlled study. Alzheimer Dis Assoc Disord 19(1), 23–28. [DOI] [PubMed] [Google Scholar]
- Pletnikova O, Kageyama Y, Rudow G, LaClair KD, Albert M, Crain BJ, Tian J, Fowler D, Troncoso JC, 2018. The spectrum of preclinical Alzheimer’s disease pathology and its modulation by ApoE genotype. Neurobiol Aging 71, 72–80. [DOI] [PubMed] [Google Scholar]
- Poe GR, Foote S, Eschenko O, Johansen JP, Bouret S, Aston-Jones G, Harley CW, Manahan-Vaughan D, Weinshenker D, Valentino R, Berridge C, Chandler DJ, Waterhouse B, Sara SJ, 2020. Locus coeruleus: a new look at the blue spot. Nat Rev Neurosci 21(11), 644–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokopiou PC, Engels-Dominguez N, Papp KV, Scott MR, Schultz AP, Schneider C, Farrell ME, Buckley RF, Quiroz YT, El Fakhri G, Rentz DM, Sperling RA, Johnson KA, Jacobs HIL, 2022. Lower novelty-related locus coeruleus function is associated with Abeta-related cognitive decline in clinically healthy individuals. Nat Commun 13(1), 1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rash JE, Olson CO, Davidson KG, Yasumura T, Kamasawa N, Nagy JI, 2007. Identification of connexin36 in gap junctions between neurons in rodent locus coeruleus. Neuroscience 147(4), 938–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinikainen KJ, Paljarvi L, Huuskonen M, Soininen H, Laakso M, Riekkinen PJ, 1988. A post-mortem study of noradrenergic, serotonergic and GABAergic neurons in Alzheimer’s disease. J Neurol Sci 84(1), 101–116. [DOI] [PubMed] [Google Scholar]
- Rorabaugh JM, Chalermpalanupap T, Botz-Zapp CA, Fu VM, Lembeck NA, Cohen RM, Weinshenker D, 2017. Chemogenetic locus coeruleus activation restores reversal learning in a rat model of Alzheimer’s disease. Brain 140(11), 3023–3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safaai H, Neves R, Eschenko O, Logothetis NK, Panzeri S, 2015. Modeling the effect of locus coeruleus firing on cortical state dynamics and single-trial sensory processing. Proc Natl Acad Sci U S A 112(41), 12834–12839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sare RM, Cooke SK, Krych L, Zerfas PM, Cohen RM, Smith CB, 2020. Behavioral Phenotype in the TgF344-AD Rat Model of Alzheimer’s Disease. Front Neurosci 14, 601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz LA, Luo L, 2015. Organization of the locus coeruleus-norepinephrine system. Curr Biol 25(21), R1051–R1056. [DOI] [PubMed] [Google Scholar]
- Sears RM, Fink AE, Wigestrand MB, Farb CR, de Lecea L, Ledoux JE, 2013. Orexin/hypocretin system modulates amygdala-dependent threat learning through the locus coeruleus. Proc Natl Acad Sci U S A 110(50), 20260–20265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimojo M, Takuwa H, Takado Y, Tokunaga M, Tsukamoto S, Minatohara K, Ono M, Seki C, Maeda J, Urushihata T, Minamihisamatsu T, Aoki I, Kawamura K, Zhang MR, Suhara T, Sahara N, Higuchi M, 2020. Selective Disruption of Inhibitory Synapses Leading to Neuronal Hyperexcitability at an Early Stage of Tau Pathogenesis in a Mouse Model. J Neurosci 40(17), 3491–3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szot P, White SS, Greenup JL, Leverenz JB, Peskind ER, Raskind MA, 2006. Compensatory changes in the noradrenergic nervous system in the locus ceruleus and hippocampus of postmortem subjects with Alzheimer’s disease and dementia with Lewy bodies. J Neurosci 26(2), 467–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szot P, White SS, Greenup JL, Leverenz JB, Peskind ER, Raskind MA, 2007. Changes in adrenoreceptors in the prefrontal cortex of subjects with dementia: evidence of compensatory changes. Neuroscience 146(1), 471–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Kayama Y, Lin JS, Sakai K, 2010. Locus coeruleus neuronal activity during the sleep-waking cycle in mice. Neuroscience 169(3), 1115–1126. [DOI] [PubMed] [Google Scholar]
- Theofilas P, Dunlop S, Heinsen H, Grinberg LT, 2015. Turning on the Light Within: Subcortical Nuclei of the Isodentritic Core and their Role in Alzheimer’s Disease Pathogenesis. J Alzheimers Dis 46(1), 17–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theofilas P, Ehrenberg AJ, Dunlop S, Di Lorenzo Alho AT, Nguy A, Leite REP, Rodriguez RD, Mejia MB, Suemoto CK, Ferretti-Rebustini REL, Polichiso L, Nascimento CF, Seeley WW, Nitrini R, Pasqualucci CA, Jacob Filho W, Rueb U, Neuhaus J, Heinsen H, Grinberg LT, 2017. Locus coeruleus volume and cell population changes during Alzheimer’s disease progression: A stereological study in human postmortem brains with potential implication for early-stage biomarker discovery. Alzheimers Dement 13(3), 236–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totah NK, Neves RM, Panzeri S, Logothetis NK, Eschenko O, 2018. The Locus Coeruleus Is a Complex and Differentiated Neuromodulatory System. Neuron 99(5), 1055–1068 e1056. [DOI] [PubMed] [Google Scholar]
- Uematsu A, Tan BZ, Ycu EA, Cuevas JS, Koivumaa J, Junyent F, Kremer EJ, Witten IB, Deisseroth K, Johansen JP, 2017. Modular organization of the brainstem noradrenaline system coordinates opposing learning states. Nat Neurosci 20(11), 1602–1611. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Foote SL, 1988. Corticotropin-releasing hormone increases tonic but not sensory-evoked activity of noradrenergic locus coeruleus neurons in unanesthetized rats. J Neurosci 8(3), 1016–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hooren RWE, Verhey FRJ, Ramakers I, Jansen WJ, Jacobs HIL, 2021. Elevated norepinephrine metabolism is linked to cortical thickness in the context of Alzheimer’s disease pathology. Neurobiol Aging 102, 17–22. [DOI] [PubMed] [Google Scholar]
- Vankov A, Herve-Minvielle A, Sara SJ, 1995. Response to novelty and its rapid habituation in locus coeruleus neurons of the freely exploring rat. Eur J Neurosci 7(6), 1180–1187. [DOI] [PubMed] [Google Scholar]
- Vazey EM, Aston-Jones G, 2014. Designer receptor manipulations reveal a role of the locus coeruleus noradrenergic system in isoflurane general anesthesia. Proc Natl Acad Sci U S A 111(10), 3859–3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LY, Shofer JB, Rohde K, Hart KL, Hoff DJ, McFall YH, Raskind MA, Peskind ER, 2009. Prazosin for the treatment of behavioral symptoms in patients with Alzheimer disease with agitation and aggression. Am J Geriatr Psychiatry 17(9), 744–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinshenker D, 2018. Long Road to Ruin: Noradrenergic Dysfunction in Neurodegenerative Disease. Trends Neurosci 41(4), 211–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West CH, Boss-Williams KA, Ritchie JC, Weiss JM, 2015. Locus coeruleus neuronal activity determines proclivity to consume alcohol in a selectively-bred line of rats that readily consumes alcohol. Alcohol 49(7), 691–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham H, 2016. ggplot2 Elegant Graphics for Data Analysis. Springer-Verlag; New York. ISBN 978-3-319-24277-4. [Google Scholar]
- Wilson RS, Nag S, Boyle PA, Hizel LP, Yu L, Buchman AS, Schneider JA, Bennett DA, 2013. Neural reserve, neuronal density in the locus ceruleus, and cognitive decline. Neurology 80(13), 1202–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, Yang L, Li Y, Dong Y, Yang B, Tucker LD, Zong X, Zhang Q, 2020. Effects of Exercise Training on Anxious-Depressive-like Behavior in Alzheimer Rat. Med Sci Sports Exerc 52(7), 1456–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S, Chen M, Feng T, Zhan L, Zhou L, Yu G, 2021. Use ggbreak to Effectively Utilize Plotting Space to Deal With Large Datasets and Outliers. Front Genet 12, 774846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye R, O’Callaghan C, Rua C, Hezemans FH, Holland N, Malpetti M, Jones PS, Barker RA, Williams-Gray CH, Robbins TW, Passamonti L, Rowe J, 2022. Locus Coeruleus Integrity from 7 T MRI Relates to Apathy and Cognition in Parkinsonian Disorders. Mov Disord 37(8), 1663–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Basal firing properties of LC neurons from 6- and 15-month TgF344-AD rats and WT littermates. A) Baseline firing was lower in TgF344-AD rats at both ages. B) There was a trend towards decreased interspike interval in TgF344-AD rats. C) Firing rate during spontaneous bursts was higher in TgF344-AD rats. D) Interspike intervals within a burst were lower in TgF344-AD rats, and there was an additional effect of age where younger rats had lower interspike intervals. N=74–120 neurons/group (N=8–11 animals/group) for A and B. N=48–88 neurons/group (N=8–11 animals/group) for C and D. *p<0.05, **p<0.01.
Supplemental Figure 2: Footshock response of LC neurons from 6- and 15-month TgF344-AD rats and WT littermates. A & B) LC neuron firing rate was lower in TgF344-AD rats during the immediate phase in response to 0.5 ms footshock, but this effect was not seen in response to 5 ms footshock. C & D) There was an age × genotype interaction in the intermediate phase of response to both 0.5 and 5 ms footshock. Post-hoc analysis revealed that young TgF344-AD animals demonstrated hyperactivity in response to 5 ms footshock, while aged TgF344-AD animals showed hypoactivity in response to both 0.5 and 5 ms footshock. E & F) There were no differences in late phase response to footshock. N=84–128 neurons/group (N=8–11 animals/group) for A, C, and E. N=94–126 neurons/group (N=8–11 animals/group) for B, D, and F. *p<0.05.


