Summary
Background
Screening for islet autoantibodies in children and adolescents identifies individuals who will later develop type 1 diabetes, allowing patient and family education to prevent diabetic ketoacidosis at onset and to enable consideration of preventive therapies. We aimed to assess whether islet autoantibody screening is effective for predicting type 1 diabetes in adolescents aged 10−18 years with an increased risk of developing type 1 diabetes.
Methods
Data were harmonised from prospective studies from Finland (the Diabetes Prediction and Prevention study), Germany (the BABYDIAB study), and the USA (Diabetes Autoimmunity Study in the Young and the Diabetes Evaluation in Washington study). Autoantibodies against insulin, glutamic acid decarboxylase, and insulinoma-associated protein 2 were measured at each follow-up visit. Children who were lost to follow-up or diagnosed with type 1 diabetes before 10 years of age were excluded. Inverse probability censoring weighting was used to include data from remaining participants. Sensitivity and the positive predictive value of these autoantibodies, tested at one or two ages, to predict type 1 diabetes by the age of 18 years were the main outcomes.
Findings
Of 20 303 children with an increased type 1 diabetes risk, 8682 were included for the analysis with inverse probability censoring weighting. 1890 were followed up to 18 years of age or developed type 1 diabetes between the ages of 10 years and 18 years, and their median follow-up was 18·3 years (IQR 14·5–20·3). 442 (23·4%) of 1890 adolescents were positive for at least one islet autoantibody, and 262 (13·9%) developed type 1 diabetes. Time from seroconversion to diabetes diagnosis increased by 0·64 years (95% CI 0·34–0·95) for each 1-year increment of diagnosis age (Pearson’s correlation coefficient 0·88, 95% CI 0·50–0·97, p=0·0020). The median interval between the last prediagnostic sample and diagnosis was 0·3 years (IQR 0·1–1·3) in the 227 participants who were autoantibody positive and 6·8 years (1·6–9·9) for the 35 who were autoantibody negative. Single screening at the age of 10 years was 90% (95% CI 86–95) sensitive, with a positive predictive value of 66% (60–72) for clinical diabetes. Screening at two ages (10 years and 14 years) increased sensitivity to 93% (95% CI 89–97) but lowered the positive predictive value to 55% (49–60).
Interpretation
Screening of adolescents at risk for type 1 diabetes only once at 10 years of age for islet autoantibodies was highly effective to detect type 1 diabetes by the age of 18 years, which in turn could enable prevention of diabetic ketoacidosis and participation in secondary prevention trials.
Funding
JDRF International.
Introduction
Type 1 diabetes is preceded by the appearance of islet autoantibodies, which have been used successfully as biomarkers of disease risk.1 Screening for islet autoimmunity in the general population or in genetically susceptible subpopulations has allowed identification of individuals for type 1 diabetes prevention trials, such as TrialNet,2 GPPAD,3 and INNODIA.4 Follow-up of individuals who have tested positive for one or multiple islet autoantibodies has also helped to prevent the occurrence of diabetic ketoacidosis at the time of diabetes diagnosis.5–9 We have previously analysed optimal screening strategies in young children (aged 1−14 years) in the Type 1 Diabetes Intelligence (T1DI) Consortium, which combined data from multiple birth cohort studies that followed up children throughout adolescence, with regular islet autoantibody measurements to identify individuals with a high risk of developing the disease.10 However, the appearance of autoantibodies and the time from appearance to diagnosis of clinical type 1 diabetes is age dependent.10–13 The youngest children (younger than 5 years) tend to first develop insulin autoantibodies and progress more quickly to clinical diabetes than older children (5 years or older), with insulin autoantibodies as the first appearing autoantibody. Additionally, older children more often develop glutamic acid decarboxylase (GAD) antibodies as the first autoantibody, and their progression to diabetes is less dependent on initial seroconversion age.11 Therefore, we hypothesised that the optimal screening strategy for older children might differ from that for younger children. We aimed to determine the optimal islet autoantibody screening strategy for effective identification of individuals at risk of type 1 diabetes between 10 years and 18 years of age.
Methods
Study design and cohort
The T1DI cohort includes a total of 24 662 participants from five prospective studies: the Type 1 Diabetes Prediction and Prevention (DIPP) study from Finland,14 the Diabetes Prediction in Skåne Study (DiPiS) from Sweden,15 the Diabetes Autoimmunity Study in the Young (DAISY)16 from Colorado, USA, the Diabetes Evaluation in Washington (DEW-IT) study17 from Washington State, USA, and the BABYDIAB study18 from Germany. All participants who were followed up to the age of 18 years or older were included, and data collected from all of their visits from the start of follow-up were considered. When estimating screening performance only information from visits that occurred after 10 years of age was used to simulate screening on individuals aged 10−18 years and without previous information on islet autoimmunity. We excluded individuals who were diagnosed with type 1 diabetes or lost to follow-up before the age of 10 years because there was no information from them between the ages of 10 years and 18 years.
HLA groups A, B, C, and D were defined as previously reported.1,10 On the basis of Type 1 Diabetes Genetics Consortium data, the approximate odds ratios for type 1 diabetes are more than 50 in group A, 11–32 in group B, 4–10 in group C, and 0·1–3·0 in group D.19 Seroconversion was defined as detection of the same islet autoantibody in two consecutive visits, of which the first one was designated as the time of seroconversion. Autoantibodies against insulin, GAD, and insulinoma-associated protein 2 (IA-2) were measured by radiobinding assays validated in international workshops and described elsewhere in detail by the T1DI Study Group.1
Approvals from the local ethics committees were obtained, and written informed consent for all participants were received from the parents, adolescents, or both, as well as adolescent assents when relevant.
Outcomes
The main outcomes were sensitivity and positive predictive value of the presence of any islet autoantibody at a given age or at two ages for type 1 diabetes prediction by the age of 18 years. Specificity and negative predictive values were also calculated.
Statistical analysis
We used islet autoantibody test results of samples measured within a window of 6 months before and after the indicated participant age. If several samples were analysed within the time window of 12 months, the result closest to the indicated age was considered. Participants were then designated as positive for at least one islet autoantibody (against insulin, GAD, or IA-2), negative for all three autoantibodies, or without a test result if no measurement was available within the target window. We considered screening at a single age or two ages from 10 years to 18 years, and each screening test was evaluated using time-dependent metrics for sensitivity and positive predictive value.20 We calculated cumulative sensitivity20 whereby diagnosed participants with missing tests were included in the denominator when calculating sensitivity to allow comparison of all age pairs (referred to as comparative sensitivity). The optimum screening ages from the comparative sensitivity calculation were then evaluated among children completing testing and observation (referred to as observed sensitivity). To account for right censored participants who were lost from follow-up before the age of 18 years, we used inverse probability censoring weighting.21 The 95% CIs for sensitivities and positive predictive values at various screening ages were computed as the mean plus or minus 1·96 multiplied by the SD from 10 000 bootstrap samples with replacement. Pearson’s correlation was used to calculate the possible correlation between time from seroconversion to diagnosis of type 1 diabetes and age at diabetes diagnosis. SciPy (version 1.3.3) was used for statistical analyses.
Role of the funding source
The funder of the study supported the creation of the harmonised T1DI dataset and the data analyses. The funder had no role in study design and writing of the report. Two JDRF employees worked in the team that analysed and interpreted the data and developed the report.
Results
Of the 24 662 participants in the T1DI cohort, all 4359 participants from the DiPiS were excluded from the current analysis because little follow-up data on them were available for the age range considered. Inverse probability censoring weighting was used to include data from 8682 participants. 1890 participants were identified as having been followed up to the age of 18 years or diagnosed with type 1 diabetes between the ages of 10 years and 18 years (appendix p 3). Their median follow-up time from the age of 10 years until the end of follow-up or diagnosis of diabetes was 9·9 years (IQR 8·1–11·4). In the cohort of 1890 participants, a median of 16 (IQR 10–21) samples per participant were analysed for islet autoantibodies. The median duration of prospective follow-up was 18·3 years (IQR 14·5–20·3), and the median age at type 1 diabetes diagnosis was 12·6 years (11·2–15·0; table). 1844 (97·5%) participants were followed up from 0−9 years to 18 years, and those who seroconverted did so at a median age of 7·1 years (IQR 4·0–10·5; table). 1413 (74·8%) participants did not seroconvert and did not develop type 1 diabetes (table; figure 1). 215 (11·4%) participants seroconverted but did not progress to type 1 diabetes within the follow-up period (table; figure 1). 227 (12·0%) had a seroconversion at a median age of 5·0 years (IQR 2·9–7·9) and developed type 1 diabetes at a median age of 12·7 years (11·2–14·9; figure 1; table). 35 (1·9%) participants developed type 1 diabetes without identified seroconversion (table; figure 1). The median interval between the last prediagnostic sample and diagnosis was 0·3 years (IQR 0·1–1·3) in the 227 participants who were autoantibody positive, whereas it was 6·8 years (1·6–9·9) for the 35 who were autoantibody negative.
Table:
Description of participants
| Whole cohort (n=l890) | Seroconversion and type 1 diabetes (n=227) | No seroconversion but type 1 diabetes (n=35) | Seroconversion but no type 1 diabetes (n=215) | No seroconversion and no type 1 diabetes (n=1413) | |
|---|---|---|---|---|---|
| Data source | |||||
| DIPP study | 203 (10·7%) | 116 (51·1%) | 9 (25·7%) | 45 (20·9%) | 33 (2·3%) |
| DAISY | 599 (31·7%) | 46 (20·3%) | 12 (34·3%) | 50 (23·3%) | 491 (34·7%) |
| DEW-IT study | 304 (16·1%) | 27 (11·9%) | 8 (22·9%) | 50 (23·3%) | 219 (15·5%) |
| BABYDIAB study | 784 (41·5%) | 38 (16·7%) | 6 (17·1%) | 70 (32·6%) | 670 (47·4%) |
| Sex | |||||
| Female | 918 (48·6%) | 84 (37·0%) | 13 (37·1%) | 106 (49·3%) | 715 (50·6%) |
| Male | 972 (51·4%) | 143 (63·0%) | 22 (62·9%) | 109 (50·7%) | 698 (49·4%) |
| Family history of type 1 diabetes | |||||
| Yes | 1119 (59·2%) | 79 (34·8%) | 16 (45·7%) | 105 (48·8%) | 919 (65·0%) |
| No | 568 (30·1%) | 32 (14·1%) | 10 (28·6%) | 65 (30·2%) | 461 (32·6%) |
| Unknown | 203 (10·7%) | 116 (51·1%) | 9 (25·7%) | 45 (20·9%) | 33 (2·3%) |
| HLA group* | |||||
| A | 348 (18·4%) | 83 (36·6%) | 14 (40·0%) | 44 (20·5%) | 207 (14·6%) |
| B | 675 (35·7%) | 105 (46·3%) | 13 (37·1%) | 86 (40·0%) | 471 (33·3%) |
| C | 372 (19·7%) | 27 (11·9%) | 4 (11·4%) | 38 (17·7%) | 303 (21·4%) |
| D | 488 (25·8%) | 12 (5·3%) | 2 (5·7%) | 46 (21·4%) | 428 (30·3%) |
| Missing | 7 | 0 | 2 | 1 | 4 |
| Age at the last follow-up, years | 19·8 (18·0–21·3) | 12·2 (10·5–14·4) | 11·9 (10·6–13·4) | 20·3 (18·6–22·5) | 20·1 (18·4–21·6) |
| Age at seroconversion, years | 7·1 (4·0–10·5) | 5·0 (2·9–7·9) | ·· | 9·5 (6·6–13·6) | ·· |
| Age at diagnosis, years | 12·6 (11·2–15·0) | 12·7 (11·2–14·9) | 12·4 (11·3–16·1) | ·· | ·· |
Data are n (%) or median (IQR). Percentages might not sum to 100 as a result of rounding. Description of the cohort of participants followed up between 10 years and at least 18 years of age or diagnosed with type 1 diabetes between the ages of 10 years and 18 years. DAISY=Diabetes Autoimmunity Study in the Young. DEW-IT=Diabetes Evaluation in Washington. DIPP=Diabetes Prediction and Prevention.
Note that seven (0·4%) of 1890 participants are not included in the HLA groups due to indeterminate typing results.
Figure 1: Follow-up times for participants.
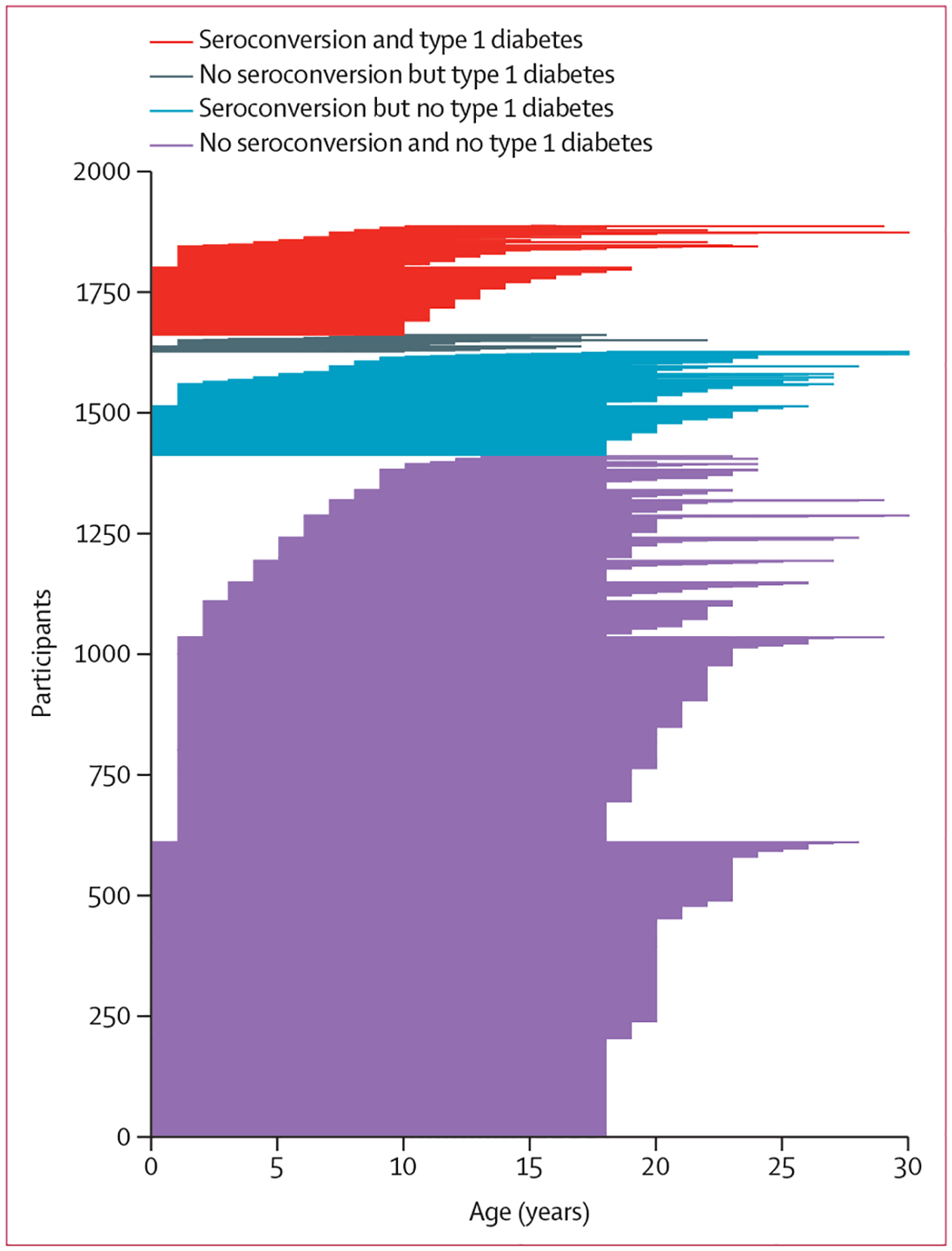
Each horizontal line represents the timeline for each participant; the start of the line is the age at which follow-up started, and the end of the line is the age at which the participant was diagnosed with type 1 diabetes (red and grey lines) or lost to follow-up (blue and purple lines).
The DEW-IT study included mostly older children than the three other studies, whereas the number of children in the DIPP study declined towards the end of the observation period because many children were diagnosed before the age of 10 years and the follow-up of autoantibody-negative children ended at the age of 15 years (figure 2A). Most participants who seroconverted did so before the age of 10 years and, in the DIPP study, even before 6 years of age (figure 2B). The cumulative number of islet autoantibody-positive children in the follow-up period increased steadily until 12 years of age, after which the number declined due to onset of type 1 diabetes and the end of autoantibody screening at the age of 15 years in some cohorts (figure 2C). The number of participants with newly diagnosed type 1 diabetes was highest between the ages of 10 years and 12 years (figure 2D). The median time from seroconversion to diagnosis of type 1 diabetes increased by age at diagnosis, although individual variation remained wide (figure 3). The median time between seroconversion and diagnosis increased by 0·64 years (95% CI 0·34–0·95) for each 1-year increase in diagnosis age (Pearson’s correlation coefficient 0·88, 95% CI 0·50–0·97, p=0·0020).
Figure 2: Number of all participants, those who newly seroconverted, those who were islet autoantibody positive, and those diagnosed with type 1 diabetes.
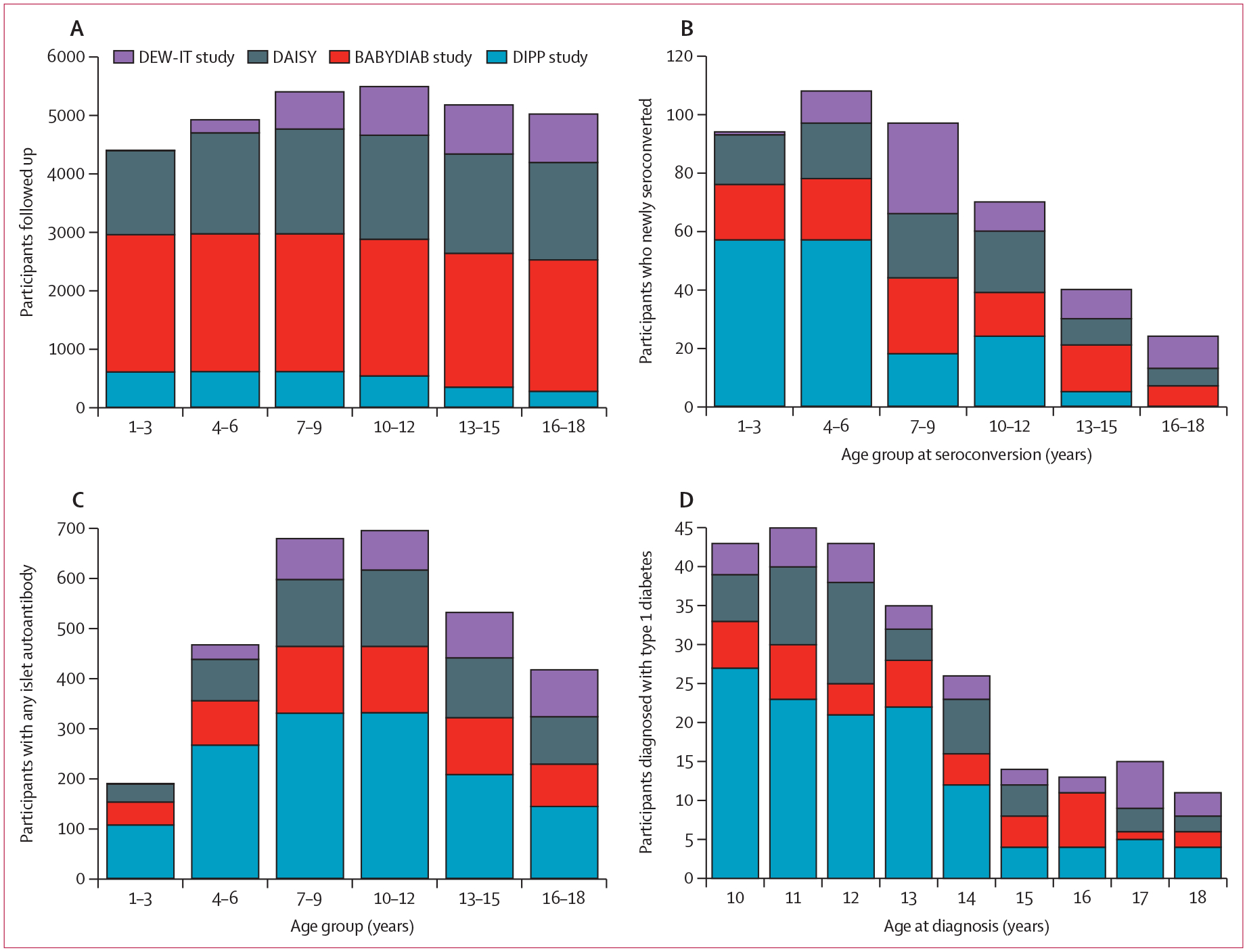
The number of participants at each age range (or age) includes those within a window from 6 months before to 6 months after the specified age (or age range). (A) Number of participants who had at least one follow-up visit between the age of 10 years and 18 years, followed up through each age range for each study site. (B) Number of participants who newly seroconverted at each age range (islet autoantibody incidence). (C) Number of participants with any islet autoantibody at each age range (islet autoantibody prevalence). (D) Number of participants diagnosed with type 1 diabetes at each age. DAISY=Diabetes Autoimmunity Study in the Young. DEW-IT=Diabetes Evaluation in Washington. DIPP=Diabetes Prediction and Prevention.
Figure 3: Median time from seroconversion to diagnosis of type 1 diabetes.
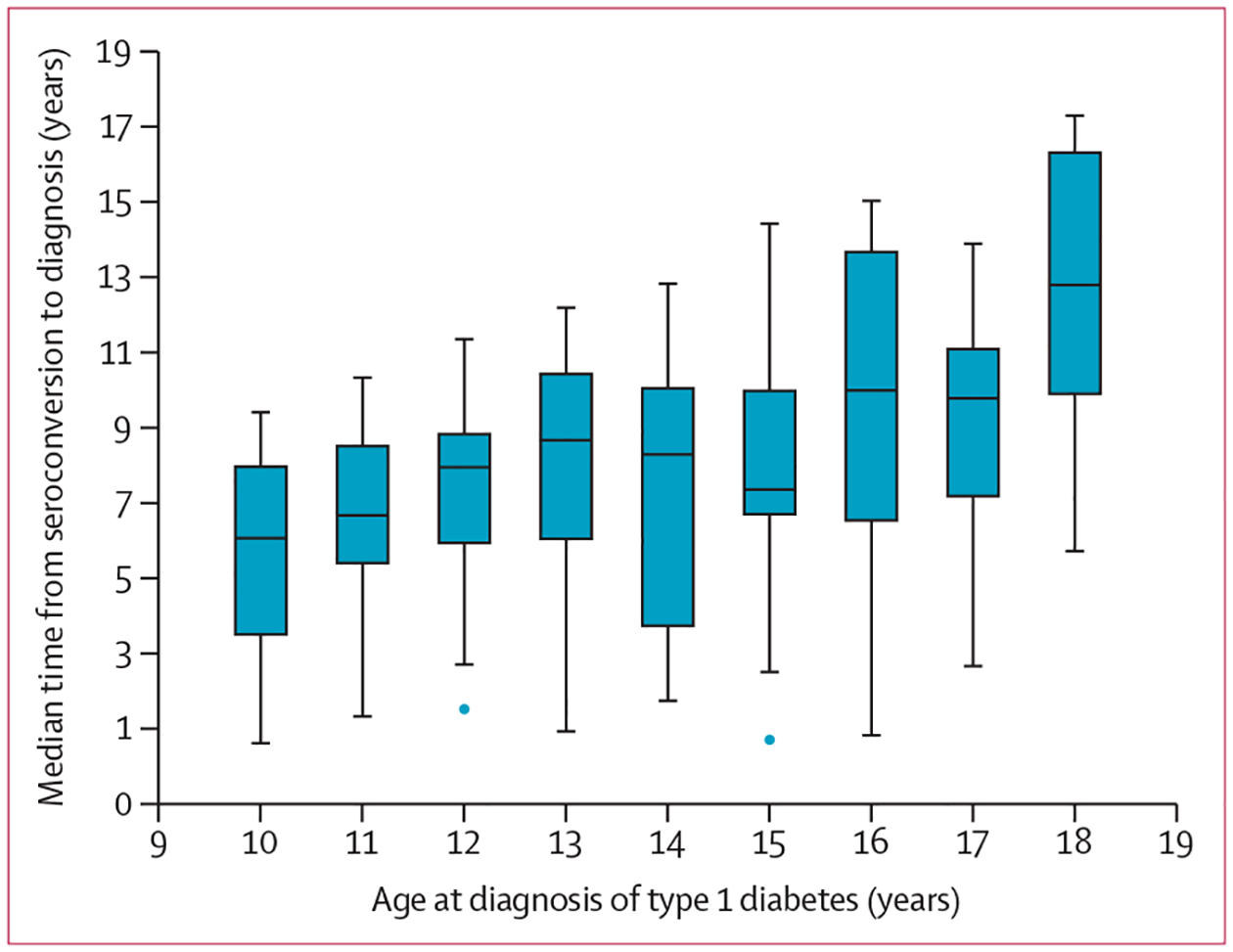
The median time from seroconversion to diagnosis of type 1 diabetes by age at diagnosis in the 227 participants followed up in the complete cohort. The horizontal line represents the median; the box extends from the 25th to 75th percentile; and the upper whisker extends to the last datum less than Q3 + 1·5 × IQR, and the lower whisker extends to the last datum greater than Q1 − 1·5 × IQR. Blue circles beyond the whiskers represent outliers.
When screening at a single age was considered, screening at 10 years showed the highest comparative sensitivity of 63% (95% CI 56–71; figure 4A). The corresponding positive predictive value was 38% (95% CI 32–46; figure 4B). Specificity was 38% (95% CI 36–40; appendix p 4). When screening was performed twice, at 10 years and 14 years, comparative sensitivity improved to 72% (95% CI 65–78), but the positive predictive value decreased to 29% (24–34). Specificity was 63% (95% CI 61–66). The negative predictive value was 99–100% for all single-age and pairwise screening timepoints (appendix p 5). Figure 4 summarises the sensitivities and positive predictive values of islet autoantibody screening at all different single ages between 10 years and 17 years and with different pairwise combinations of two screening timepoints. Sex-specific comparative sensitivities were relatively similar for males and females, but the positive predictive values were slightly higher for the males (appendix p 6).
Figure 4: Comparative sensitivity and positive predictive value of screening for any islet autoantibody.
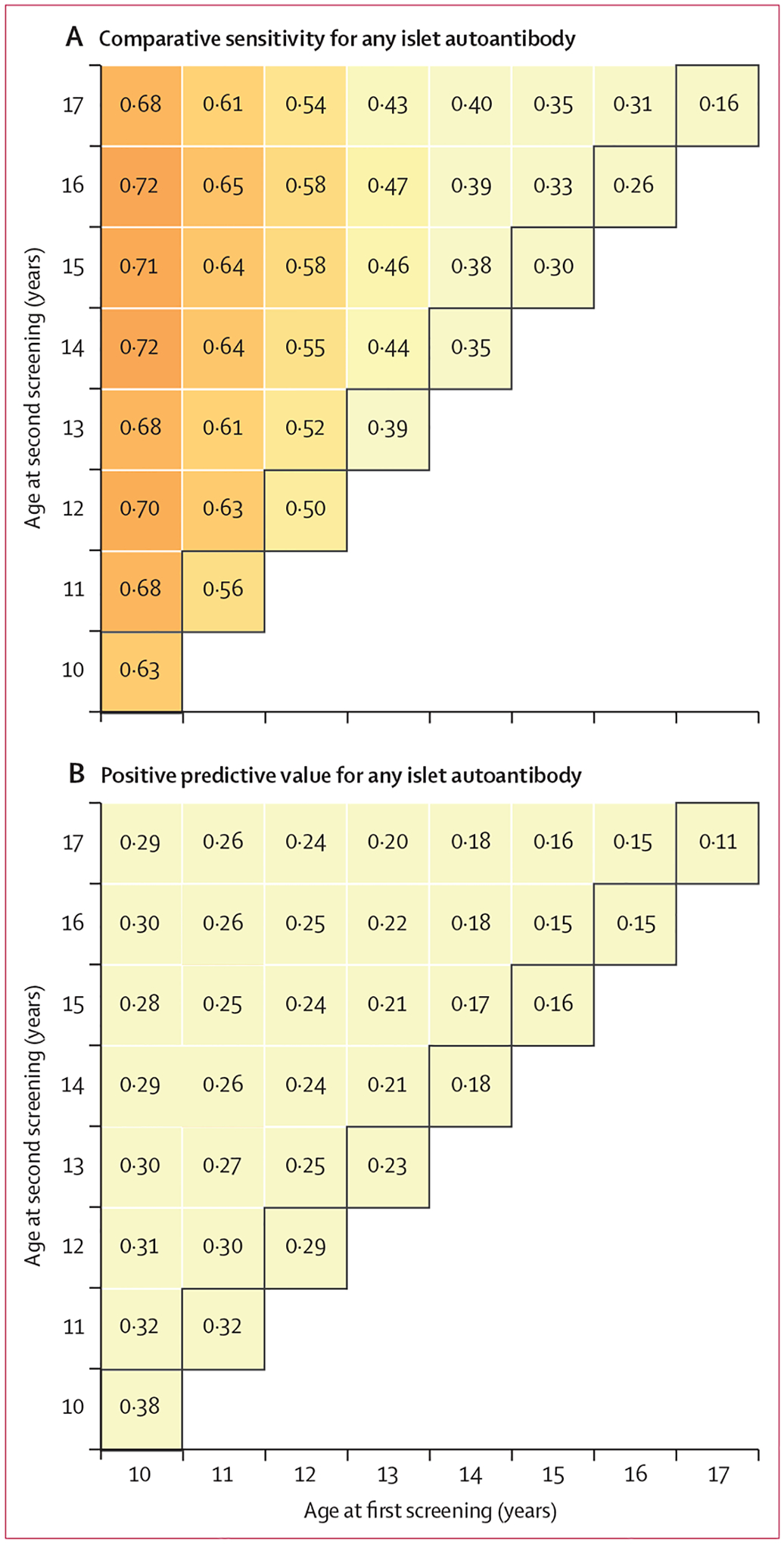
Comparative sensitivity (A) and positive predictive value (B) from screening any islet autoantibody at single ages (diagonal numbers highlighted within black squares) and at all combinations of two ages between 10 years and 17 years for risk of type 1 diabetes in participants followed up beyond the age of 10 years. The positive predictive value at a single age or age pair indicates the likelihood that autoantibody-positive participants will develop type 1 diabetes during follow-up.
When evaluating only children completing both testing and observation, the observed sensitivity for screening at a single age of 10 years was very high at 90% (95% CI 86–95), with a positive predictive value of 66% (60–72). For screening at the two ages of 10 years and 14 years, observed sensitivity increased to 93% (95% CI 89–97), but the positive predictive value decreased to 55% (49–60).
Discussion
To our knowledge, there are few published studies focusing on islet autoantibody screening in adolescents.22 The large multinational T1DI cohort made it possible to analyse different screening strategies in a cohort in which many participants were followed up until at least 18 years of age. Our analysis shows that single islet autoantibody screening at 10 years of age is an effective way to identify adolescents at high risk of developing type 1 diabetes. Screening twice at the ages of 10 years and 14 years improves sensitivity only marginally. Given that the cost of two-age screening is twice that of a single screening, we consider a single screening at 10 years of age a good choice. However, the acceptability of islet autoantibody screening by families, adolescents, and clinicians is important to consider when planning screening programmes for islet autoimmunity. Single testing might be more economical and more acceptable, but this needs to be evaluated in the setting of each specific country. The cost of islet autoantibody screening might also be affected by the possibility to integrate it with ongoing paediatric health surveillance programmes. Future studies are needed to clarify these questions. Furthermore, combining a genetic risk score including non-HLA risk variants with islet autoantibody screening could be an even more effective way to identify most individuals who will develop type 1 diabetes in the future.23
When considering the entirety of childhood, initiation of islet autoimmunity peaks at an early age of 1–2 years. Individuals with early seroconversion tend to develop diabetes faster than those with later appearing autoimmunity.24,25 We previously showed that screening of children at 2 years and 6 years of age effectively identifies most individuals who develop type 1 diabetes before 15 years of age.10 Our analysis provides a framework for screening of older children and adolescents, which is meaningful for several important reasons. First, establishing the safety of immuno-therapeutic drugs to prevent or delay clinical type 1 diabetes necessarily involves testing in adults, then adolescents, and then younger children, so identifying relevant cohorts of adolescents is important for the middle stage of this process.26 It has been challenging to find participants for such trials.27 Second, the American Academy of Pediatrics recommends paediatric screening for hypercholesterolaemia between the ages of 9 years and 11 years, and this represents one of the few childhood ages where a routine blood sample is likely to be collected.28 Third, it is noteworthy that diabetic ketoacidosis at the time of diagnosis of type 1 diabetes occurs most often in two high-risk age groups, toddlers and adolescents.29 The presence of a family member with type 1 diabetes is known to reduce the risk of diabetic ketoacidosis at diagnosis.30 Screening for islet autoimmunity helps to direct education and glycaemic monitoring to the group with the highest risk of developing type 1 diabetes, which is likely to further reduce the incidence of ketoacidosis.7,8,31
To compare performance of various two-age screening pairs in a dataset with variable numbers of participants tested at each specified age, comparative sensitivities using the cumulative sensitivity method20 were calculated by also including cases with missing tests in the denominator. This was necessary to allow comparison of screening results from all age pairs. However, it led to lower sensitivity estimates than the observed sensitivity, which considered only participants actually tested at the specified ages. Therefore, once the optimum strategy was identified by the comparative method, the actual observed sensitivity was determined using participants tested at the optimum timepoint (or timepoints) and observed during the follow-up period. A limitation of this approach might be that we cannot rule out some bias originating from the distribution of non-tested cases within the cumulative sensitivity calculation, but this was the best available method to compare age pairs and revealed high sensitivity and positive predictive value in direct observation.
In the past, it has been shown that learning about a child’s increased genetic risk of type 1 diabetes induces only mild anxiety in most parents.32 A cohort study by Johnson and colleagues showed that any islet autoantibody positivity in children increased parental anxiety, but anxiety decreased constantly during the 4-year follow-up.33 However, a significant proportion of up to 43% of the parents were still reporting high scores of anxiety after 3 years of follow-up.33 In another study, other family life stressors were found to be more substantial than becoming aware of autoantibody positivity.34 It remains to be investigated whether awareness of autoantibody positivity affects the general wellbeing and development of adolescents themselves.
Strengths of our study include a large dataset of adolescents from three different countries, systematic screening for islet autoantibodies, and follow-up of autoantibody-positive participants up to the age of 18 years. Data from all participants with any visit after 10 years of age were used by applying the inverse probability censoring weighting, thereby reducing the potential bias caused by non-random loss to follow-up. Our study participants represent a wide variation of class II HLA genotypes more broadly representative of the full spectrum of type 1 diabetes than many similar studies.1 However, a limitation is that up to 59% of the participants were first-degree relatives of patients with type 1 diabetes. Therefore, these results should be replicated in an unselected adolescent population because the best age for screening in the general population could be different from what is shown in the population in this study. The ultimate aim should be to establish a global screening strategy for the risk of type 1 diabetes. Childhood type 1 diabetes is most prevalent in northern European populations, and the current results can first be applied in these countries. Although the incidence of type 1 diabetes is lower elsewhere, the much larger childhood populations in these settings mean that screening for islet autoimmunity could save larger numbers of children from diabetic ketoacidosis. This necessitates new studies in populations of non-European ancestries. Autoantibodies against zinc transporter 8 were not included because they were not measured systematically in the original cohorts, and future studies should also include them. Another limitation is the short follow-up period beyond the age of 18 years, which was reflected by modest positive predictive values for the screening, particularly when islet autoantibody screening of the older adolescents was considered. If the follow-up were longer, the positive predictive values would certainly increase because of the accumulating number of type 1 diabetes diagnoses in adulthood, albeit slowly. The original studies focused on early ages because they were performed in the framework of paediatric and adolescent medicine. Since most new cases of type 1 diabetes are actually diagnosed at adult age,35 future research should include extended follow-up after adolescent screening to include more complete outcome data. However, for the time being, this report provides the best estimate for optimal screening of islet autoantibodies in adolescents.
In conclusion, this study shows the importance of screening for islet autoantibodies at least once at the age of 10 years to predict future type 1 diabetes. Since individuals with an increased genetic or familial risk are known to have a higher future risk of type 1 diabetes, screening might also be particularly important in these groups during adolescence. We believe that these results provide a pragmatic, efficient strategy for islet autoantibody screening that would facilitate prevention of diabetic ketoacidosis and recruitment of young people into new prevention trials. Future studies should identify the optimal screening strategy for the general adolescent population.
Supplementary Material
Research in context.
Evidence before this study
We searched PubMed with the search terms “screening”, “autoantibod*”, “prediction”, “type 1 diabetes”, and “child*” for articles published in English up to July 5, 2022. Several prospective studies have followed up children who are genetically susceptible to type 1 diabetes from birth or young individuals who have a family member with type 1 diabetes for the development of islet autoantibodies. Confirmed positivity for multiple islet autoantibodies predicts almost inevitable progression to clinical diabetes within 15 years from seroconversion; however, the lag time from islet autoimmunity to diabetes diagnosis is highly variable. Young age at seroconversion is associated with a faster progression rate than is older age. Furthermore, the annual incidence of islet autoantibodies declines as the child gets older, at least in individuals with high HLA-conferred susceptibility for type 1 diabetes. Recent analyses from prospective follow-up studies of children at an increased genetic risk of type 1 diabetes indicate that screening at two ages, at 2 years and 5–7 years, effectively identifies individuals who later develop childhood type 1 diabetes. However, no published data exist about the optimal screening strategy for islet autoimmunity in adolescents aged 10–18 years.
Added value of this study
In this prospective cohort study, we observed that double screening at the ages of 10 years and 14 years, or even single screening at 10 years, was highly sensitive in detecting adolescents who will develop type 1 diabetes. By contrast, almost no one who remained islet autoantibody negative at these screening ages developed type 1 diabetes.
Implications of all the available evidence
Islet autoantibody screening in adolescents effectively identifies individuals with an increased risk of developing type 1 diabetes and, therefore, might allow prevention of diabetic ketoacidosis. These young people might also be able to take part in secondary prevention trials. Furthermore, these data will be important for the ongoing islet autoantibody screening programmes in the general population. Future research should validate these optimal screening age (or ages) in other unselected adolescent populations and potentially also adult populations.
Acknowledgments
We thank the participating children and families, and the personnel of the study sites. This work was supported by funding from JDRF (IBM: 1-RSC-2017-368-I-X, 1-IND-2019-717-I-X, DAISY: 1-SRA-2019-722-I-X, 1-RSC-2017-517-I-X, 5-ECR-2017-388-A-N, DIPP: 1-RSC-2018-555-I-X, 1-SRA-2019-721-I-X, and DEW-IT: 1-SRA-2019-719-I-X, 1-RSC-2017-516-I-X).
Footnotes
See Online for appendix
Declaration of interests
MG and VA are employees of IBM. FM and OL performed this work as employees of JDRF. All other authors declare no competing interests.
Data sharing
The data that support the findings of this study are available through the Type 1 Diabetes Intelligence Consortium upon request to the corresponding author. The data are not publicly available due to privacy regulations.
References
- 1.Anand V, Li Y, Liu B, et al. Islet autoimmunity and HLA markers of presymptomatic and clinical type 1 diabetes: joint analyses of prospective cohort studies in Finland, Germany, Sweden, and the U.S. Diabetes Care 2021; 44: 2269–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Battaglia M, Anderson MS, Buckner JH, et al. Understanding and preventing type 1 diabetes through the unique working model of TrialNet. Diabetologia 2017; 60: 2139–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Winkler C, Haupt F, Heigermoser M, et al. Identification of infants with increased type 1 diabetes genetic risk for enrollment into primary prevention trials—GPPAD-02 study design and first results. Pediatr Diabetes 2019; 20: 720–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dunger DB, Bruggraber SFA, Mander AP, et al. INNODIA master protocol for the evaluation of investigational medicinal products in children, adolescents and adults with newly diagnosed type 1 diabetes. Trials 2022; 23: 414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barker JM, Barriga KJ, Yu L, et al. Prediction of autoantibody positivity and progression to type 1 diabetes: Diabetes Autoimmunity Study in the Young (DAISY). J Clin Endocrinol Metab 2004; 89: 3896–902. [DOI] [PubMed] [Google Scholar]
- 6.Winkler C, Schober E, Ziegler A-G, Holl RW. Markedly reduced rate of diabetic ketoacidosis at onset of type 1 diabetes in relatives screened for islet autoantibodies. Pediatr Diabetes 2012; 13: 308–13. [DOI] [PubMed] [Google Scholar]
- 7.Elding Larsson H, Vehik K, Gesualdo P, et al. Children followed in the TEDDY study are diagnosed with type 1 diabetes at an early stage of disease. Pediatr Diabetes 2014; 15: 118–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hekkala AM, Ilonen J, Toppari J, Knip M, Veijola R. Ketoacidosis at diagnosis of type 1 diabetes: effect of prospective studies with newborn genetic screening and follow up of risk children. Pediatr Diabetes 2018; 19: 314–19. [DOI] [PubMed] [Google Scholar]
- 9.Nakhla M, Cuthbertson D, Becker DJ, et al. Diabetic ketoacidosis at the time of diagnosis of type 1 diabetes in children: insights from TRIGR. JAMA Pediatr 2021; 175: 518–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghalwash M, Dunne JL, Lundgren M, et al. Two-age islet-autoantibody screening for childhood type 1 diabetes: a prospective cohort study. Lancet Diabetes Endocrinol 2022; 10: 589–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bauer W, Veijola R, Lempainen J, et al. Age at seroconversion, HLA genotype, and specificity of autoantibodies in progression of islet autoimmunity in childhood. J Clin Endocrinol Metab 2019; 104: 4521–30. [DOI] [PubMed] [Google Scholar]
- 12.Krischer JP, Liu X, Lernmark Å, et al. Characteristics of children diagnosed with type 1 diabetes before vs after 6 years of age in the TEDDY cohort study. Diabetologia 2021; 64: 2247–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kwon BC, Anand V, Achenbach P, et al. Progression of type 1 diabetes from latency to symptomatic disease is predicted by distinct autoimmune trajectories. Nat Commun 2022; 13: 1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kupila A, Muona P, Simell T, et al. Feasibility of genetic and immunological prediction of type I diabetes in a population-based birth cohort. Diabetologia 2001; 44: 290–97. [DOI] [PubMed] [Google Scholar]
- 15.Elding Larsson H A Swedish approach to the prevention of type 1 diabetes. Pediatr Diabetes 2016; 17 (suppl 22): 73–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rewers M, Bugawan TL, Norris JM, et al. Newborn screening for HLA markers associated with IDDM: Diabetes Autoimmunity Study in the Young (DAISY). Diabetologia 1996; 39: 807–12. [DOI] [PubMed] [Google Scholar]
- 17.Wion E, Brantley M, Stevens J, et al. Population-wide infant screening for HLA-based type 1 diabetes risk via dried blood spots from the public health infrastructure. Ann NY Acad Sci 2003; 1005: 400–03. [DOI] [PubMed] [Google Scholar]
- 18.Ziegler AG, Hummel M, Schenker M, Bonifacio E. Autoantibody appearance and risk for development of childhood diabetes in offspring of parents with type 1 diabetes: the 2-year analysis of the German BABYDIAB study. Diabetes 1999; 48: 460–68. [DOI] [PubMed] [Google Scholar]
- 19.Sharp SA, Rich SS, Wood AR, et al. Development and standardization of an improved type 1 diabetes genetic risk score for use in newborn screening and incident diagnosis. Diabetes Care 2019; 42: 200–07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kamarudin AN, Cox T, Kolamunnage-Dona R. Time-dependent ROC curve analysis in medical research: current methods and applications. BMC Med Res Methodol 2017; 17: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vock DM, Wolfson J, Bandyopadhyay S, et al. Adapting machine learning techniques to censored time-to-event health record data: a general-purpose approach using inverse probability of censoring weighting. J Biomed Inform 2016; 61: 119–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knip M, Korhonen S, Kulmala P, et al. Prediction of type 1 diabetes in the general population. Diabetes Care 2010; 33: 1206–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferrat LA, Vehik K, Sharp SA, et al. A combined risk score enhances prediction of type 1 diabetes among susceptible children. Nat Med 2020; 26: 1247–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parikka V, Näntö-Salonen K, Saarinen M, et al. Early seroconversion and rapidly increasing autoantibody concentrations predict prepubertal manifestation of type 1 diabetes in children at genetic risk. Diabetologia 2012; 55: 1926–36. [DOI] [PubMed] [Google Scholar]
- 25.Krischer JP, Lynch KF, Schatz DA, et al. The 6 year incidence of diabetes-associated autoantibodies in genetically at-risk children: the TEDDY study. Diabetologia 2015; 58: 980–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dayan CM, Besser REJ, Oram RA, et al. Preventing type 1 diabetes in childhood. Science 2021; 373: 506–10. [DOI] [PubMed] [Google Scholar]
- 27.Herold KC, Bundy BN, Long SA, et al. An anti-CD3 antibody, teplizumab, in relatives at risk for type 1 diabetes. N Engl J Med 2019; 381: 603–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Ferranti SD, Rodday AM, Parsons SK, et al. Cholesterol screening and treatment practices and preferences: a survey of United States pediatricians. J Pediatr 2017; 185: 99–105.e2. [DOI] [PubMed] [Google Scholar]
- 29.Hekkala A, Reunanen A, Koski M, Knip M, Veijola R. Age-related differences in the frequency of ketoacidosis at diagnosis of type 1 diabetes in children and adolescents. Diabetes Care 2010; 33: 1500–02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karges B, Prinz N, Placzek K, et al. A comparison of familial and sporadic type 1 diabetes among young patients. Diabetes Care 2021; 44: 1116–24. [DOI] [PubMed] [Google Scholar]
- 31.Steck AK, Vehik K, Bonifacio E, et al. Predictors of progression from the appearance of islet autoantibodies to early childhood diabetes: The Environmental Determinants of Diabetes in the Young (TEDDY). Diabetes Care 2015; 38: 808–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simonen P, Korhonen T, Simell T, et al. Parental reactions to information about increased genetic risk of type 1 diabetes mellitus in infants. Arch Pediatr Adolesc Med 2006; 160: 1131–36. [DOI] [PubMed] [Google Scholar]
- 33.Johnson SB, Lynch KF, Roth R, Schatz D. My child is islet autoantibody positive: impact on parental anxiety. Diabetes Care 2017; 40: 1167–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goldstein E, Hermann R, Renfors TJ, et al. From genetic risk awareness to overt type 1 diabetes: parental stress in a placebo-controlled prevention trial. Diabetes Care 2009; 32: 2181–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thomas NJ, Jones SE, Weedon MN, Shields BM, Oram RA, Hattersley AT. Frequency and phenotype of type 1 diabetes in the first six decades of life: a cross-sectional, genetically stratified survival analysis from UK Biobank. Lancet Diabetes Endocrinol 2018; 6: 122–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available through the Type 1 Diabetes Intelligence Consortium upon request to the corresponding author. The data are not publicly available due to privacy regulations.


