Abstract
Progesterone is a sex steroid hormone that plays a critical role in the establishment and maintenance of pregnancy. This hormone drives numerous maternal physiological adaptations to ensure the continuation of pregnancy and facilitate fetal growth, including broad and potent modulation of the maternal immune system to promote maternal-fetal tolerance. In this brief review, we provide an overview of the immunomodulatory functions of progesterone in the decidua, placenta, myometrium, and maternal circulation during pregnancy. Specifically, we summarize current evidence of the regulated functions of innate and adaptive immune cells induced by progesterone and its downstream effector molecules in these compartments, including observations in human pregnancy as well as in animal models. Our review highlights the gaps in knowledge of interactions between progesterone and maternal cellular immunity that may direct future research.
Keywords: Decidua, Dendritic cell, Macrophage, Neutrophil, NK cell, Pregnancy, T cell
1. INTRODUCTION
Progesterone (pregn-4-ene-3,20-dione or P4) is a hormone classified as a natural sex steroid that plays a key role in the maintenance of pregnancy [1–5]. This hormone is produced by the corpus luteum during the latter half of each menstrual cycle (i.e. secretory phase or luteal phase) to transform and prepare the endometrium for implantation [6, 7]. If implantation does not occur, the corpus luteum spontaneously regresses, resulting in progesterone withdrawal and subsequent menstruation [6, 7]. On the other hand, if the embryo implants in the decidualized endometrium (i.e. decidua), human chorionic gonadotropin (hCG) derived from the developing blastocyst maintains the corpus luteum to promote the production of progesterone [8], which is later produced by the placenta [7, 9]. During pregnancy, progesterone drives multiple maternal physiological changes, including the maintenance of myometrial quiescence [10–16], alteration of the cardiovascular system [17], and preparation of mammary glands for lactation [18], among others [19, 20], via the progesterone receptors (PRs) expressed throughout the body [21].
Besides physiological changes, progesterone also exerts potent immunomodulatory functions throughout pregnancy [22–25]. For example, progesterone promotes the expansion or differentiation of regulatory T cells (Tregs) both systemically [26–30] and at the maternal-fetal interface (i.e. the decidua and placenta) [27, 30–32]. Moreover, this hormone dampens the cytotoxic activity and degranulation of natural killer (NK) cells in these compartments [33–38]. In general, progesterone also drives the polarization of circulating and resident immune cells toward an anti-inflammatory phenotype to promote a tolerogenic microenvironment, a process that involves downregulating the release of pro-inflammatory mediators [39–41]. Moreover, progesterone regulates cellular immune processes in the cervix [42–49]. Together, these progesterone-driven immunological changes foster a homeostatic state that ensures a successful pregnancy.
In this brief review, we summarize the known interactions between progesterone and maternal immune cells during pregnancy. In particular, we highlight the specific changes in cellular immune functions that are driven by progesterone in the decidua, placenta, myometrium, and maternal peripheral blood. Given that this hormone exhibits broad functions during pregnancy, we have focused on the direct effects of progesterone or its downstream mediators (e.g. progesterone-induced blocking factor, PIBF) on innate and adaptive immune cell subsets. Importantly, this review also reveals gaps in knowledge of the mechanisms of progesterone-cellular immune interactions that require further investigation.
2.1. DECIDUA
The decidua and its non-pregnant counterpart, the endometrium, are indispensable for successful implantation and pregnancy [50, 51]. During the latter half of the menstrual cycle (i.e. secretory phase or luteal phase), progesterone regulates decidualization, which is the dramatic remodeling of the endometrial tissue in preparation for implantation [7, 52, 53]. This process includes the transformation of glands to the secretory state, leukocyte recruitment, angiogenesis, and mesenchymal-epithelial transition of endometrial stromal cells [54–56]. Once transformation has completed, a constant supply of progesterone is required to maintain the decidua [57, 58]. Furthermore, the decidua hosts a diverse array of immune cells that play a key role in the establishment and maintenance of pregnancy [59, 60]: decidual NK cells promote spiral artery remodeling in early pregnancy [61, 62], homeostatic macrophages act as tissue sentinels and control the decidual microenvironment [63], and Tregs facilitate and maintain maternal tolerance of the semi-allogeneic fetus [64, 65], among others. Given the importance of decidual immune cells in maintaining effective maternal-fetal crosstalk, a large number of studies have focused on the immunological effects of progesterone in this compartment, which are summarized in Figure 1.
Figure 1.
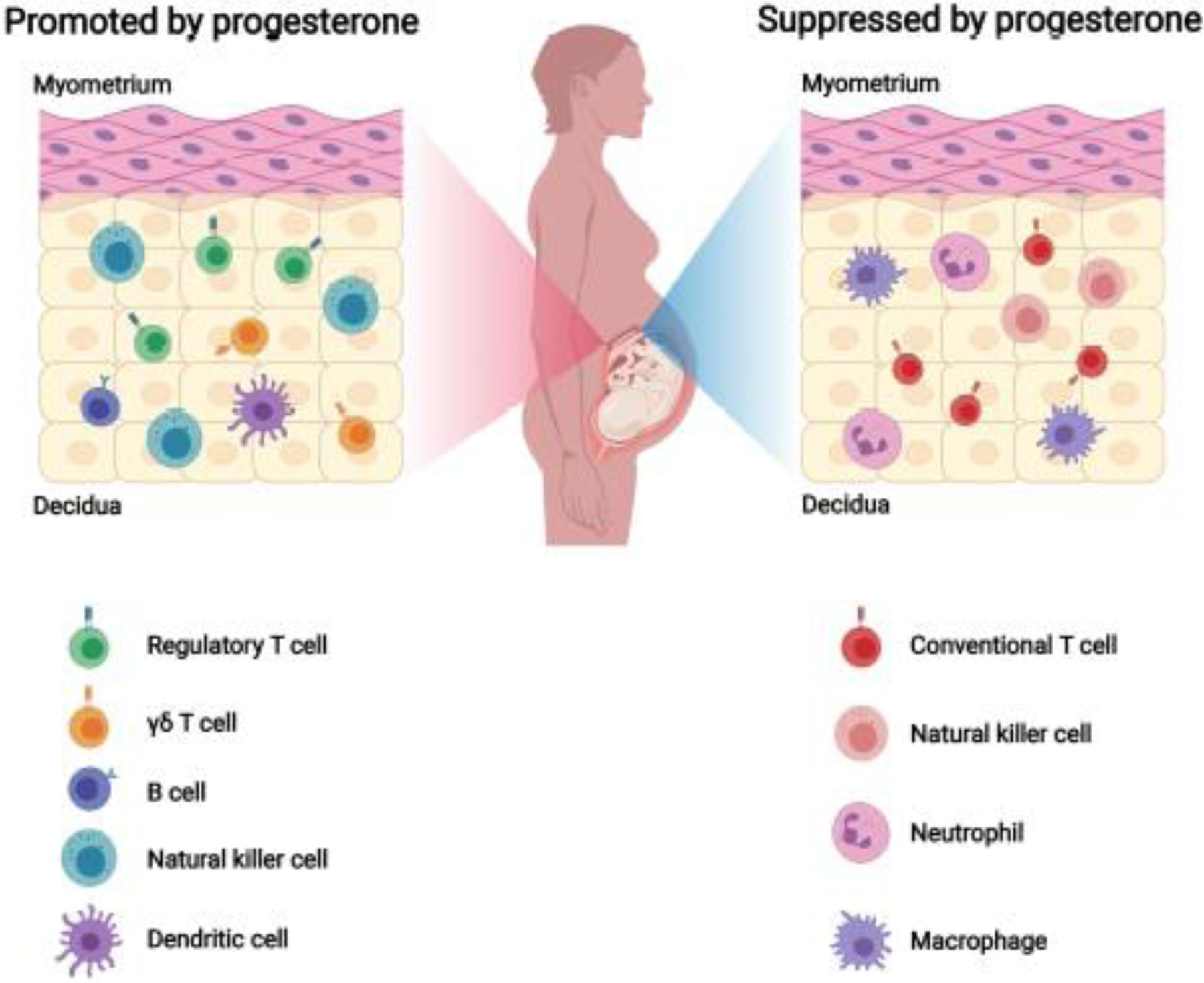
Cellular immune responses altered by progesterone signaling in the decidua. During pregnancy, progesterone favors natural killer (NK)-cell activity that is necessary for successful remodeling and angiogenesis in this tissue. This hormone then fosters a local tolerogenic microenvironment by promoting regulatory T cells, γδ T cells, and B cells as well as controlling the maturation of decidual dendritic cells (DCs). On the other hand, progesterone limits the migration and functions of conventional T cells, cytotoxic NK cells, neutrophils, and macrophages to further maintain decidual homeostasis. Created with BioRender.com.
2.1.1. Decidual effector T cells
Several studies have demonstrated the effects of progesterone on decidual effector T cells. The administration of the PR antagonist RU486 to pregnant mice on 15.5 days post coitum (dpc) led to withdrawal of progesterone action and preterm birth without inducing major changes in the immune cell composition of the decidua [66]. However, progesterone prevented preterm birth induced by activated maternal T cells, and reduced the gene expression of inflammation-associated molecules (Casp11, Ccl22, Icam1, Ctla4, Nod1, and Ccl5) in the decidua, suggesting anti-inflammatory effects of this hormone on T cell-mediated inflammatory processes in this maternal-fetal interface [66]. The alteration of decidual T-cell functions by progesterone is further supported by in vitro studies [35, 67]. Progesterone reduced the perforin production of first-trimester human decidual lymphocytes either directly or indirectly (co-culture of these immune cells with decidual adherent cells), which was potentially mediated by PIBF [35], an immunomodulatory protein produced by activated lymphocytes in the presence of progesterone [68–70]. Moreover, progesterone treatment of CD4+ or CD8+ T cells isolated from the mid-luteal phase human endometrium increased the expression of RANTES [67], a chemokine that has been associated with the induction of immune tolerance at the maternal-fetal interface [67, 71]. Therefore, progesterone appears to preclude excessive T-cell activity in the decidua.
2.1.2. Decidual γδ T cells
Other T-cell subsets are also affected by progesterone. γδ T cells, expressing a T-cell receptor (TCR) composed of a γ chain and δ chain [72], can express PRs [73]. Such cells may participate in the immunomodulatory effects of progesterone in the decidua, mediated in part by their release of PIBF as observed in vitro [74]. Indeed, the frequency of γδ T cells among total T cells was increased in the secretory phase endometrium (high progesterone levels) of non-pregnant women compared to the proliferative phase endometrium (low progesterone levels), and progesterone treatment increased the frequency of γδ T cells during the latter phase [75]. The observed increase in the proportion of γδ T cells was maintained in the early pregnancy decidua [75], supporting the regulation of this T-cell subset by progesterone.
2.1.3. Decidual Tregs
The effects of progestogens on decidual Tregs were also investigated using a clinically-relevant murine model [31]. The vaginal administration of progesterone, which is utilized to treat women who undergo preterm labor with short cervix [76–84], in late pregnancy (days 13 to 17 of gestation) increased the frequency of decidual CD4+ Tregs in mice [31]. By contrast, the systemic administration of 17-alpha-hydroxyprogesterone caproate (17OHP-C) to pregnant mice did not alter Treg proportions in the decidua [31]. Similarly, the systemic administration of progesterone in early pregnancy (days 0, 2, 4, and 6 of gestation) increased the frequency of decidual Tregs by mid-gestation [30]. Therefore, the type of progestogen as well as the timing of administration may be important factors. In addition, progesterone can indirectly modulate the migration of induced Tregs (iTregs) by upregulating RANTES production in endometrial stromal cells under inflammatory conditions, a process mediated by vasoactive intestinal peptide (VIP) [85]. These studies suggest that progesterone exhibits immunosuppressive functions not only through direct effects on decidual immune cells but also by the promotion of Treg expansion or differentiation at the maternal-fetal interface.
2.1.4. Decidual B cells
Although few studies have explored the effects of progesterone on decidual B cells, the treatment of pregnant mice with an anti-PIBF antibody depleted decidual B cells and increased fetal resorption in mice [86]. In this context, a previous study reported that PIBF increased the production of asymmetric antibodies [87], which have a protective role during pregnancy [88]. The above evidence suggests a role for progesterone and PIBF in the maintenance and function of decidual B cells; yet, this concept requires further research.
2.1.5. Decidual NK cells
In vivo studies have explored the relationship between progesterone and uterine and decidual NK cells. The systemic administration of progesterone increased the number of dolichos biflorus agglutinin (DBA) lectin+ uterine NK cells in the endometrium of non-pregnant ovariectomized mice, and this effect was blocked by RU486 [89]. By contrast, treatment with RU486 alone did not decrease the number of decidual NK cells in human first trimester decidua [90]. Serum concentrations of progesterone are also associated with the maintenance of decidual NK cells, as the number of these innate immune cells was retained if progesterone withdrawal was prevented by the systemic administration of this hormone in mice [91]. Consistently, the relationship between the serum concentration of progesterone and frequency of decidual NK cells was also observed after the administration of high doses of dexamethasone in early pregnancy in mice, which decreased both factors [92]. These results suggest the importance of progesterone in the expansion and maintenance of uterine and decidual NK cells.
Despite the known effects of progesterone on uterine and decidual NK cells, the expression of PRs by such cells is still under debate. One study demonstrated PR expression on a subset of uterine NK cells from pregnant mice, although the PR+ population of this innate immune subset was small [93]. On the other hand, a lack of PRs was reported on NK cells from the non-pregnant human endometrium [94–96], first trimester human decidua [94–96], and rat decidua (days 8 – 12 and 14 – 21 of gestation) [97]. Moreover, progesterone had no direct effects on the in vitro proliferation, cytolytic activity, or cytokine secretion of CD16−CD56bright NK cells isolated from human endometrium [98]. Therefore, it is plausible that progesterone influences uterine NK cells through indirect mechanisms [99], although a possible role for the glucocorticoid receptor in mediating the direct effects of progesterone on NK cells has been reported [100]. Indeed, the co-culture of endometrial stromal cells and lymphocyte fraction from non-pregnant human endometrium with progesterone induced the expansion of CD56+ NK cells [101]. Further, since co-localization of PR-expressing cells and DBA lectin-expressing decidual NK cells in the murine decidua throughout pregnancy was not observed [102], the progesterone-induced effects of decidual or endometrial stromal cells on uterine NK cells may also be mediated by soluble factors.
One of the most important cytokines produced by endometrial or decidual stromal cells in response to progesterone is interleukin (IL)-15, which is essential for NK-cell development and proliferation [103]. IL-15 was detected in human endometrium and decidua, where its expression is increased in the secretory phase endometrium [104, 105] and first trimester decidua [105]. In addition, progesterone increased the production of IL-15 by human first trimester decidual cells [105] and endometrial stromal cells in vitro [104, 106], suggesting that progesterone controls the release of this cytokine in these tissues. Moreover, NK cells isolated from human first trimester decidua expressed the IL-15 receptor [107], which is expected given the key role of this cytokine in the proliferation, chemoattraction, and differentiation of these innate immune cells [98, 107–110]. Other mediators produced by endometrial or decidual stromal cells with progesterone stimulation are also implicated in the chemoattraction and differentiation of uterine NK cells, such as CXCL12, CX3CL1, CXCL10, CXCL14, and osteopontin, among others [111–113]. Progesterone also upregulated the expression of KLRK1 by murine trophoblasts, resulting in increased production of IFNγ by uterine NK cells [114].
Other factors that can mediate the effects of progesterone on uterine NK cells include PIBF. This factor can inhibit the degranulation of NK cells derived from human peripheral blood [36] and reduce the cytotoxicity of NK cells isolated from human first trimester decidua in vitro [115]. In addition, the inhibition of PIBF by a monoclonal anti-PIBF antibody in mice during early pregnancy (on 1.5 and 4.5 dpc) increased the cytotoxicity of decidual NK cells, leading to implantation failure and resorption [86]. Of note, decidual NK cells are not only affected by PIBF, but also may produce this immunomodulatory molecule in mice [86, 116]; yet, the mechanisms by which progesterone induces PIBF production in decidual NK cells remain unclear. Together, these results suggest that progesterone can indirectly regulate decidual and uterine NK cells, thereby modulating the immune milieu of the endometrium and decidua during implantation and gestation.
2.1.6. Decidual macrophages
Animal and human studies have suggested that macrophages in the endometrium, the non-pregnant counterpart of the decidua, are responsive towards progesterone. In the murine endometrium, the number of F4/80+ macrophages increased during estrus [117], and ovariectomy resulted in a significant decrease of this cell population [118]. Using the same ovariectomy model, another study reported that progesterone inhibited the estrogen-induced infiltration of macrophages into the endometrium, a phenomenon that was mediated by PRs as determined using PR-deficient mice [119]. In addition, progesterone withdrawal during the menstrual cycle also increased the number of macrophages in the human endometrium [120], whereas women who received progesterone treatment during the secretory phase (i.e. hormone replacement therapy) showed increased endometrial expression of macrophage chemoattractant protein (MCP)-1 [121]. These studies suggest that endometrial macrophages are at least partially regulated by progesterone and estrogen in the non-pregnant state.
A role for progesterone in regulating decidual macrophages has also been reported. The blockade of PR by RU486 during the first trimester increased the number of macrophages present in the human decidua [90]. Moreover, a clinically-relevant murine model showed that vaginal progesterone decreased the frequency of decidual macrophages, whereas the systemic administration of 17OHP-C did not [31]. In line with this concept, a murine study suggested that the anti-inflammatory effects of progesterone were at least partially due to inhibited production of macrophage chemoattractants [e.g. colony stimulating factor (CSF)-1 and MCP-1] in decidual stromal cells via the nuclear protein high-mobility group box-1 (HMGB-1) [122]. By contrast, the decidual infiltration of macrophages was not increased during RU486-induced preterm birth in mice [66, 123].
Direct effects of progesterone on monocytes/macrophages have been described using in vitro models. Medroxyprogesterone acetate (MPA; a synthetic progesterone analog), but not natural progesterone, triggered the differentiation of the monocyte cell line THP-1 towards a homeostatic (M2-like) phenotype [124]. These MPA-treated THP-1 cells could promote the decidualization of a human endometrial stromal cell line and the invasion of the trophoblast cell line (HTR8/SVneo), suggesting that MPA facilitates the differentiation of peripheral blood monocytes into homeostatic decidual macrophages [124]. Of note, despite the direct effects of MPA on THP-1 cells, there is currently no evidence of PR expression by decidual or endometrial macrophages [94, 95]. Therefore, the indirect effects of progesterone on decidual macrophages should also be considered.
2.1.7. Decidual DCs
Mature dendritic cells (DCs), defined by the maturation markers CD83 and HLA-DR, were present in human first trimester decidua [125]. Progesterone facilitated the differentiation of human peripheral blood mononuclear cell (PBMC)-derived monocytes into CD83+HLA-DR+ mature DCs in vitro, indicating a potential role for this hormone in the maturation of decidual DCs [125]. Moreover, transcriptome analysis of decidual DCs from human term placentas identified higher upregulation of estrogen- and progesterone-modulated genes as well as genes encoding immunomodulatory cytokines compared to monocyte-derived DCs from PBMCs [126], suggesting that such cells undergo additional differentiation within the decidual microenvironment. This concept is further evidenced by the observation that the maturation of decidual DCs in response to bacterial pathogen-associated molecular patterns (PAMPs) or bacteria known for their placental tropism (Coxiella burnetii and Brucella abortus) was lower compared to that of monocyte-derived DCs [126]. However, a causal link between the effects of progesterone and decidual DCs has not been shown, given that progesterone administration in pregnant CBA/J × DBA/2J abortion-prone mice did not increase the number of total or mature DCs in the decidua [30]. Therefore, future studies are needed to clarify the involvement of progesterone in the maturation and differentiation of decidual DCs.
2.1.8. Decidual neutrophils
The effects of progesterone on decidual neutrophils have also been investigated. The administration of RU486 and a prostaglandin E analog increased the number of neutrophils in human first trimester decidua [127], suggesting that blockade of PR promotes the infiltration of neutrophils into this compartment. In line with this concept, progesterone was reported to reduce the expression of IL-8, which is a strong chemoattractant for neutrophils, in human endometrial explants [128] and term decidual tissues in vitro [129]. Progesterone also inhibited the increased expression of IL-8 and neutrophil chemotaxis induced by mechanical stretch in human decidual cells in vitro [130]. Consistently, the withdrawal or inhibition of progesterone increased the expression of IL-8 in the human endometrium during the menstrual cycle and in human first trimester decidual explants [90, 131]. However, the blockade of PR by RU486 in the first trimester did not change the proportion of human decidual neutrophils [90], and the decidual infiltration of neutrophils was not increased during RU486-induced preterm birth in mice [66, 123]. In addition, several studies reported the upregulation of IL-8 by progesterone in the secretory phase human endometrium [121] and by MPA in IL-1α-stimulated human endometrial stromal cells in vitro [132]. Together, these results suggest that progesterone plays a role in the regulation of neutrophil migration in the decidua; yet, further studies are needed to confirm the precise effects and mechanisms that underlie this phenomenon.
2.2. PLACENTA
The placenta is a unique organ that carries out multiple functions. First, the placenta, together with the extraplacental chorioamniotic membranes, acts as a physical barrier to protect the fetus from exposure to harmful microbes acquired by the mother [133–136]. Importantly, the placenta also acts as the lungs, gut, kidneys, and liver of the developing fetus [137, 138]. Besides its role in fetal physiology, the placenta releases numerous mediators, such as hormones, cytokines, and exosomes/microparticles [137, 139–141], that can modulate maternal physiological systems, including the immune response. Indeed, the placenta is a major producer of progesterone during pregnancy [9], and thus multiple studies have investigated how this hormone can affect the immune cells residing in this central organ.
2.2.1. Placental T cells
Several reports have suggested that progesterone influences the activity of placental T cells. The administration of 17OHP-C significantly increased placental T helper 2 (Th2) cells in a rat model of reduced uterine perfusion pressure (RUPP) [37]. Moreover, the administration of progesterone-dependent PIBF resulted in decreased total CD4+ T cells in the placenta while simultaneously elevating circulating Th2 cells and plasma IL-4 concentrations [38]. Such mechanisms could be at least partially driven by the reduced expression of CXCL9 and CXCL10 reported after pretreatment with 17OHP-C in mice with lipopolysaccharide (LPS)-induced intrauterine inflammation [142]. However, micronized progesterone failed to replicate this effect [142]. Regardless, these reports indicate progesterone-dependent mechanisms controlling placental T-cell activity, and highlight the varying immunological effects caused by progestins.
2.2.2. Placental Tregs
Progesterone has been suggested to maintain an immunosuppressive microenvironment in the placenta by promoting Treg development and activity [27, 32]. Foxp3 expression is highest in the murine placenta during midterm pregnancy (10 dpc), and RU486 treatment significantly decreased such expression [27]. As estrogen has previously been shown to induce Treg expansion in vivo [143], both hormones may play a role in the maintenance of placental Tregs throughout pregnancy. Several mechanisms have been suggested whereby progesterone may promote placental Treg functions. The expression of the immunoregulatory glycan-binding protein Galectin-1 (Gal-1) by extravillous cytotrophoblasts in the human first trimester and term placenta appeared to be driven by progesterone [32]. Furthermore, the expression of Gal-1 by the JEG-3 trophoblast cell line promoted the in vitro expansion of Tregs [32]. Progesterone-stimulated Gal-1 may also promote a tolerogenic placental microenvironment by inducing IL-10 secretion from placental cells [27, 32]. Placental Gal-1 expression was unaffected by the selective knockout of PRs on DCs [144], reinforcing the proposed mechanism that tolerogenic DCs function downstream of Gal-1 [144]. Collectively, these studies provide evidence that progesterone encourages pregnancy maintenance by promoting an immunosuppressive environment in the placenta through local expansion of Tregs and stimulation of the Gal-1 pathway.
2.2.3. Placental NK cells
Several studies have demonstrated immunomodulatory effects of progesterone on placental NK cells [37, 38]. The intraperitoneal administration of 17OHP-C to RUPP rats on day 15 of pregnancy significantly decreased both total and cytolytic placental NK cells as well as placental levels of perforin and granzyme B [37]. Similar findings were observed after supplementation with PIBF in RUPP rats also decreased total and cytolytic placental NK cells [38]. These results are consistent with a previous report showing reduced peripheral NK-cell perforin release upon treatment with this factor in vitro [36]. Thus, progesterone and its induced factor PIBF exert specific immunoregulatory effects on placental NK cells.
2.2.4. Placental neutrophils
Progesterone differentially influences the activity of two distinct populations of neutrophils that are found in the placenta [145, 146]. The administration of RU486 at advanced gestation (>160 days) in pregnant cynomolgus monkeys caused a neutrophilic invasion of the placenta, resulting in damage to this organ [145]. In addition, molecular studies noted markedly depressed the levels of serum and placental pregnancy associated plasma protein A (PAPP-A) [145]. Thus, as a consequence of progesterone withdrawal, the loss of human granulocyte elastase inhibition increases neutrophilic infiltration of the placenta, destroying the villous structures and ultimately leading to fetal demise [145].
Progesterone also impacts a more recently described population of homeostatic neutrophils that are functionally dissimilar to polymorphonuclear neutrophils [146]. Possessing anti-inflammatory and quiescent properties, these cells are induced by estrogen and progesterone from immature neutrophils and are proposed to function in placental development and maternal-fetal immune tolerance [146]. Indeed, the co-culture of estrogen- and progesterone-treated neutrophils and T cells resulted in the differentiation of CD4+ T cells displaying a regulatory-like phenotype, termed neutrophil-induced T (niT) cells, which may participate in the process of placentation [146]. The induction of niT cells was shown to rely on the transfer of neutrophil-derived molecules, such as FOXO1, to T cells, and the neutrophil-specific knockdown of FOXO1 resulted in defective placentation and fetal development [146]. Altogether, progesterone plays a dual role in preventing the invasion of peripheral neutrophils to the placenta while inducing pro-angiogenic neutrophils that promote homeostatic placental T cells and thus facilitating proper placental and fetal development.
2.2.5. Placental macrophages
The anti-inflammatory effects of progesterone may also be attributed to its effects on placental macrophage populations [147]. Human placental mesenchymal stem cells (pMSCs) induced a homeostatic M2-like polarization of monocyte-derived macrophages in vitro, which was at least partially driven by progesterone and glucocorticoid receptors expressed by such cells [147]. This mechanism was evidenced by treatment with RU486, which resulted in a shift towards a pro-inflammatory cytokine profile in macrophages [147]. Yet, changes in gene expression were not observed in bone marrow-derived macrophages exposed to progesterone in vitro [148], suggesting that other aspects of the placental microenvironment may also contribute to macrophage phenotypes and activity in this organ. In contrast with the above-mentioned beneficial effects of progesterone on placental macrophages, this hormone has been suggested to increase low-density lipoprotein (LDL) oxidation and cytotoxicity of macrophages and trophoblasts in vitro [149]. Thus, under specific conditions, progesterone could exacerbate placental macrophage-mediated LDL oxidation and acute atherosis; however, this concept requires further investigation.
2.2.6. Placental DCs
Similar to placental macrophages, progesterone signaling of DCs appears to be beneficial for placental development, as impairment of this process resulted in a decreased ratio of placental labyrinth zone area to junctional zone area in mice at 13.5 dpc and 18.5 dpc, although the latter time point was not significantly different from controls [144]. Moreover, loss of progesterone signaling in DCs resulted in decreased placental mRNA expression of Egf and Igf1 [144], both of which have been implicated in placental and fetal development [150, 151]. In addition to enhancing placentation, progesterone may indirectly promote DC-mediated local immune tolerance, as pretreatment of female mice with syngeneic immature DCs prior to allogeneic abortion-prone mating (CBA/J × DBA/2J) resulted in increased numbers of PIBF+ cells in the mid-pregnancy placenta and a reduced rate of abortion [152]. Moreover, in vitro progesterone treatment of pregnant rat-derived placental and embryonic cells reduced indoleamine-2,3-dioxygenase (IDO) expression by DCs [153]. Despite the seemingly counterintuitive nature of this observation, it was speculated that progesterone maintains IDO expression at basal levels to ensure an adequate immune response in the case of infection or other insults [153].
2.2.7. Placental myeloid cells
Lastly, progesterone not only affects the differentiation of myeloid cells in the placenta but can also influence their precursor cells [154], termed immature myeloid cells (IMCs), which are found in this compartment [154, 155]. Murine placental tissues were enriched for granulocytic IMCs, while monocytic IMCs in this tissue were lower compared to subcutaneous murine tumors [154]. In addition, progesterone treatment of bone marrow-derived CD11b+ cells ex vivo resulted in a similar composition of granulocytic and monocytic IMCs [154]. Notably, intraperitoneal LPS injection of pregnant mice (on day 15 and 16) decreased the placental IMC population, and this effect was prevented by progesterone pretreatment [154]. Thus, progesterone may participate in the maintenance of IMCs, and subsequently of mature myeloid cell subsets, in the placenta.
2.3. MYOMETRIUM
The uterus is a primary reproductive organ that undergoes dramatic changes to accommodate the growing fetus and placenta as pregnancy progresses [156]. Such modifications include an increase in uterine size, remodeling of myometrial thickness, and enlargement of the blood vessels, among others [157–161]. The uterus is by nature a contractile organ, which transitions from a state of non-contractility (termed myometrial quiescence) to a state of activation and stimulation that results in myometrial contractions required to effectively deliver the fetus during parturition [16, 162, 163]. This transformation is tightly regulated by complex mechanisms that are still under investigation [10, 12, 164]. Regardless, it is well known that progesterone induces myometrial quiescence through several mechanisms [10–16]. However, the cellular immune responses triggered by this hormone have been less characterized. Indeed, most research has focused on the administration of exogenous progestogens to evaluate such immune responses.
2.3.1. Myometrial T cells
Several reports have indicated effects of progestogens on myometrial T cells. Progesterone receptors were present in pooled CD4+ T cells from the embryo, placenta, and uterus of pregnant rats on 6 dpc [153]. Moreover, the expression of IDO by CD4+ T cells was reduced when cultured with progesterone, which recovered after exposure to RU486 due to its antagonizing effect [153]. In a murine model of abortion induced by stress, treatment with dydrogesterone (a synthetic progestogen [165]) reduced the rate of abortion [166, 167] and restored the physiological proportions of CD8+ T cells in the uterine tissues [167]. However, such an effect was suppressed when CD8+ T cells were depleted by using an anti-CD8 mAb [167]. Furthermore, the injection of dydrogesterone significantly decreased the proportions of TNF- and IFNγ-producing cells while simultaneously increasing the proportion of IL-4-producing cells in the uterus [167]. Importantly, the dydrogesterone-induced downregulation of Th1 cytokines in uterine cells was abolished after CD8+ T-cell depletion [167], suggesting that the protective effects of progestogens against abortion are at least partially CD8+ T cell-dependent. Progesterone also reduced the rate of preterm birth in a model of maternal T-cell activation-induced preterm birth and caused the downregulated myometrial expression of the inflammation associated molecule Il33 [66]. On the other hand, the administration of vaginal progesterone to mice during mid-pregnancy did not alter the proportions of myometrial CD4+ Tregs or CD8+CD25+Foxp3+ T cells [31], suggesting differential effects of progesterone on myometrial T-cell subsets.
2.3.2. Myometrial NK cells
Hormonal replacement with estradiol and/or progesterone in ovariectomized mice induced a marked increase in human CD56bright NK cell adhesion to murine uterine tissues compared to controls, a process that was mediated by L-selectin and α4 integrin [168]. Yet, the proportion of NK cells in the myometrium was reduced in superovulated mice, which have increased serum concentrations of both estradiol and progesterone, suggesting an inverse correlation between myometrial NK cells and systemic concentrations of steroidal hormones [169]. Therefore, further investigation is required to clarify the relationship between myometrial NK cells and both local and systemic progesterone concentrations.
2.3.3. Myometrial neutrophils and macrophages
The proportions of total neutrophils and macrophages in the pregnant murine myometrium were shown to be unaffected by administration of vaginal progesterone [31], potentially due to the capacity of progesterone to block the migration of these innate immune cells to the myometrial tissues [119, 170, 171]. However, vaginal progesterone treatment caused a decrease in the proportions of IFNγ+ neutrophils in the myometrium, highlighting the specific effects of this hormone on inflammatory immune cell subsets [31]. Progesterone signaling of myometrial innate immune cells may differ with gestational age and between species, as the blockade of the PR by RU486 on 15.5 dpc in mice did not increase the infiltration of neutrophils and macrophages into the myometrium [66, 172]. Yet, the sudden blockade of PR by RU486 during late gestation (19.5 dpc) in rats induced an increase in CCL2 gene and protein expression, which was associated with enhanced macrophage infiltration in the myometrial tissues [173]. This is consistent with the evidence showing that there is a reduction in the density of myometrial macrophages after progesterone treatment during late gestation in mice [174]. The cellular composition of the myometrial microenvironment may also drive the effects of progesterone signaling, as this hormone inhibited LPS-induced cytokine secretion in a co-culture of human pregnant myometrial cells and monocytes, but did not demonstrate this effect on each cell type alone [175]. Indeed, one study suggested that myometrial macrophages are dispensable for regulating the proliferative events induced by progesterone during early pregnancy [176]; however, this concept requires further investigation. In contrast with the above study, DCs appear to be responsive to progesterone signaling, given that they expressed PRs [153], and the selective deletion of such receptors on these cells resulted in enhanced DC expression of co-stimulatory molecules, decreased proportions of myometrial Tregs and CD8+CD122+ cells, and reduced fetal weight [144]. Moreover, dydrogesterone-induced Gal-1 promoted the generation of tolerogenic myometrial DCs that could rescue stress-driven fetal loss in mice [177]. Progesterone can therefore exert immunomodulatory effects on DCs that indirectly promote tolerogenic processes in the myometrium during pregnancy.
2.3.4. Myometrial mast cells
Several studies have also reported interactions between progesterone and myometrial mast cells. A pioneer study using rat myometrium demonstrated that the number of mast cells is decreased upon treatment with estrogen, but was unchanged upon treatment with progesterone. Yet, myometrial mast cells significantly increased after treatment with both estrogen and progesterone together, showing a synergistic effect of these hormones [178] that was also demonstrated by the increased expression of iNOS by uterine mast cells [117]. In contrast with this early report, later studies indicated that the number of mast cells in the myometrium of virgin ovariectomized mice was increased after treatment with progesterone [179, 180], as was the maturation and degranulation of such cells [180]. Thus, the impact of progesterone signaling on uterine mast cells requires further research.
2.4. MATERNAL PERIPHERAL BLOOD
Progesterone exhibits strong regulation of the local microenvironment by controlling the infiltration, differentiation, and activity of innate and adaptive immune cells in the decidual, placental, and myometrial tissues. Yet, a large body of evidence supports a role for this hormone in the modulation of systemic maternal immunity as well. Such mechanisms require a state of balance: peripheral tolerance must be maintained to protect the developing fetus while also allowing for a sufficient immune response to protect the mother from any potential insults during pregnancy [181–188]. Consistently, progesterone seems to promote the necessary homeostatic immune profile without completely compromising maternal immune functions.
2.4.1. Peripheral T cells
Adult peripheral T cells have been shown to express the membrane PR (mPR)-α, -β, and -γ [189], which expression can be further modulated by external factors such as vitamin D supplementation [190]. On the other hand, one study reported that glucocorticoid receptors, rather than PRs, mediated progesterone-induced T-cell apoptosis of murine splenocytes [191]. Notwithstanding, progesterone has been reported to diminish the overall activation [189, 192, 193], proliferation [193–196], and cytotoxic activity [197, 198] of peripheral T cells; yet, in vitro treatment with progesterone causes increased intracellular Ca2+ levels [189, 195, 199, 200], suggesting that specific signaling pathways are activated by this hormone. Yet, one study indicated that progesterone may block K+ channels and thus decrease Ca2+ signaling in peripheral T cells, which may promote progesterone-induced T-cell suppression [201]. Progesterone treatment can also regulate peripheral T-cell phenotypes by increasing the expression of immune checkpoint molecules such as CTLA-4 and TIM-3 in patients with a history of recurrent spontaneous abortion [202]. Together with the demonstrated modulation of peripheral T-cell cytokine profiles [195, 196, 203], progesterone seems to exert strong immunoregulatory effects on circulating T-cell responses in the mother.
Several in vitro reports have documented the progesterone-induced release of a blocking factor (PIBF) that may mediate some of the suppressive effects of this hormone. Like progesterone, PIBF prevents the in vitro cytotoxic activity of peripheral T cells [33, 204], and this effect can be blocked by RU486 treatment [33]. PIBF may also carry out some of the immunomodulatory functions of progesterone by preventing the release of pro-inflammatory cytokines such as IL-12 from peripheral lymphocytes [205]. Several animal studies have provided further mechanistic demonstration of PIBF functions in pregnancy [38, 86, 206]. PIBF may be derived from the embryo in response to maternal progesterone signaling, subsequently entering the maternal circulation contained in embryo-derived extracellular vesicles and promoting the production of IL-10 by CD8+ T cells [206]. Indeed, the blockade of this factor in early pregnancy led to impaired implantation and increase resorption rates as well as dysregulation of gene expression in maternal CD4+ and CD8+ T cells [86]. Consistently, the administration of PIBF during late pregnancy in a rat RUPP model increased maternal plasma levels of IL-4 as well as circulating Th2 cells together with reduced IL-6 and IL-17 levels [38]. The above-mentioned studies demonstrate the immunoregulatory effects of progesterone and PIBF on peripheral T-cell activity.
It is worth mentioning that one study specifically investigated the potential interaction between progesterone and peripheral γδ T cells during pregnancy [73]. The authors noted that a substantial proportion of γδ T cells expressed the PR, and that the incubation of peripheral lymphocytes with an anti-γδ TCR antibody decreased their production of PIBF [73]. Thus, peripheral γδ T cells may participate in progesterone-mediated mechanisms of immunoregulation during pregnancy.
2.4.2. Peripheral Tregs
Similar to conventional T cells, peripheral Tregs from pregnant women have also been reported to express PRs, such as mPRα [207], that likely mediate the increased expression of CTLA-4, TIM-3, and PD-1 observed on Tregs treated with this hormone in vitro [202]. Indeed, the culture of murine splenic CD4+ T cells with progesterone induced their differentiation into Tregs, which was driven in part by nuclear PR (nPR)-A and nPR-B, and can be blocked by RU486 [27]. Several studies using murine models indicated that systemic progesterone treatment results in at least a modest increase in systemic Treg proportions [26, 27, 30], although it has been suggested that physiological changes in systemic progesterone levels during pregnancy are not directly correlated with the expansion of Tregs that takes place in early gestation [208]. Nonetheless, clinical studies have reported that pregnant women with cervical incompetence [28] or preterm labor [29] that received vaginal progesterone displayed higher peripheral Treg counts after treatment [28, 29], providing further evidence of a relationship between progesterone and the development of immunoregulatory T cell subsets during pregnancy. Of note, one study reported that progesterone expanded murine splenocyte-derived Tregs via the glucocorticoid receptor [209].
2.4.3. Peripheral NK cells
Expression of both PR isoforms has been noted on peripheral NK cells from non-pregnant adults [210]. The expression of PR-A and PR-B seems to be limited to mature KIR+ NK cells, and treatment of such cells with progesterone suppressed IFNγ secretion and caused apoptosis in vitro that could be reversed by anti-progestins such as ZK98.299 or RU486 [210]. Progesterone seemingly induced the expansion of local endometrial CD56+ NK cells, but not those derived from the circulation [101]; yet, this hormone may also promote the migration of peripheral NK cells to the myometrium and decidua tissues in early pregnancy by upregulating the expression of integrins in these tissues, thereby enhancing NK-cell adhesion [168]. Yet, in vivo and in vitro observations suggest that luteinizing hormone (LH) and estradiol may also exert some influence on NK-cell adhesion/migration [211]. Consistent with the elevated expression of integrins at the maternal-fetal interface, peripheral NK cells derived from pregnant women show higher migratory capacity compared to non-pregnant or male-derived cells, and progesterone upregulated decidual stromal cell chemokine expression that facilitated NK cell migration [111]. After cell-cell contact with decidual stromal cells, peripheral NK cells acquired a chemokine receptor profile that is described on decidual NK cells, providing further evidence that peripheral NK cells are recruited to the decidua as part of their accumulation in early pregnancy [111].
Progesterone was indicated to prevent NK-cell cytotoxicity promoted by RU486 [34]. This hormone can also modulate the functions of peripheral NK cells by blocking perforin action [35], likely through PIBF-mediated inhibition of degranulation-related processes as observed in vitro [33, 36]. Dysregulation of this mechanism may have translational implications, as PIBF+ lymphocytes were found to be more prevalent in women with healthy pregnancies compared to those at risk of preterm birth [69]. The blockade of PIBF in early pregnancy resulted in reduced numbers of decidual PIBF+ NK cells, increased peripheral NK cell activity, and pregnancy loss, emphasizing the importance of this progesterone-induced factor [86]. Indeed, the administration of PIBF to a rat RUPP model resulted in reduction of circulating NK cell numbers as well as reduced plasma IL-17 and IL-6 levels [38].
More recently, the expression of the inhibitory checkpoint marker Tim3 was also described on a subset of peripheral NK cells during pregnancy [212]. The expression of Tim3 was upregulated by physiological progesterone concentrations, and peripheral Tim3+ NK cells were induced by progesterone treatment in an abortion-prone mouse model [212]. Interestingly, such Tim3+ NK cells may also exert immunosuppressive functions and displayed potential to induce Tregs [212]. Thus, progesterone modulates peripheral NK-cell phenotypes and function to promote systemic immune regulation during pregnancy.
2.4.4. Peripheral neutrophils
Neutrophils represent a major component of circulating leukocytes, and thus any potential effects of progesterone on these immune cells could have major implications on maternal systemic immunity. A clinical study of women with twin pregnancies revealed that vaginal progesterone treatment between 24 – 34 weeks of gestation resulted in increased numbers of peripheral neutrophils [213]. Such neutrophils displayed reduced CD11b and total peripheral leukocytes tended to have lower mRNA expression of pro-inflammatory cytokines together with higher IL-10 [213]; moreover, progesterone was shown to prevent NETosis (i.e. the release of neutrophil extracellular traps or NETs) of peripheral neutrophils [214]. Such findings are consistent with in vitro observations that peripheral neutrophils from male donors display an anti-inflammatory/quiescent phenotype after culture with progesterone [146], and such cells induced the differentiation of T cells to a regulatory-like phenotype in vitro that may further promote pregnancy-specific immune tolerance [146]. Thus, progesterone exerts immunoregulatory effects on peripheral neutrophils that can indirectly impact other immune cell subsets as well as placental and fetal development.
2.4.5. Peripheral DCs and monocytes
Several investigations have pointed to DCs and monocytes as being receptive to progesterone signaling. Peripheral monocytes derived from non-pregnant adults showed reduced mRNA and/or protein expression of several key molecules such as TLR2, CD14, and COX2 in response to in vitro progesterone treatment [215]. Moreover, progesterone inhibited LPS-induced NF-κB signaling by upregulating IκBα expression in peripheral monocytes [215] and reduced the mRNA expression of IL-1β [216]. Such functions may rely on the regulation of monocyte miRNAs, as progesterone suppressed TLR-driven monocyte responses by inhibiting miR-155 and thereby reducing IL-6 and IFN-β [217]. Bone marrow-derived DCs also showed increased in vitro differentiation in response to progesterone, and expressed higher levels of co-stimulatory and activation markers such as MHC-II, CD40, CD54, and CD86 [218]. Notably, progesterone seems to promote a DC cytokine profile that favors production of IL-10 [218–220] and other homeostatic cytokines [220] together with downregulation of pro-inflammatory mediators such as TNF [218]. Yet, in vitro progesterone treatment did not alter mature DC phenotype or capacity for stimulating T-cell proliferation [221], potentially indicating a diminished effect of progesterone on fully matured DCs. Progesterone may have differential effects on distinct DC subsets, as peripheral plasmacytoid DCs were negatively correlated with the increasing progesterone concentrations observed in normal pregnancy [222]. However, this observation has not been supported mechanistically, and may not indicate a causal relationship.
2.5. PROGESTERONE RECEPTORS
The progesterone receptor (PR) belongs to the nuclear receptor superfamily of transcription factors, and was the first receptor to be characterized as having multiple true isoforms [15, 223]. The two best-characterized isoforms are termed PR-A and PR-B, which despite their high similarity (PR-A is simply a truncated PR-B) regulated different target genes [223]. Important for reproductive immunology, the anti-inflammatory effects of progesterone signaling are thus at least partially reliant on the relative expression levels of PR-A and PR-B within a specific tissue or cell type [15, 224]. A subsequent investigation reported that the PR-C isoform [225] is upregulated in the laboring myometrium resulting from activation of the NF-κB pathway [226], favoring the withdrawal of progesterone action. While multiple other isoforms and splice variants have been reported in the literature [223], few follow-up investigations have been carried out and thus their relevance for pregnancy remains unclear. A mitochondrial PR (PR-M) was also described [227], which regulates cellular respiration [228]. In addition, animal studies have led to the discovery of multiple membrane progesterone receptors (mPRs) and progesterone receptor membrane components (PGRMCs), which were subsequently identified in humans [229–231]. Furthermore, it has also been demonstrated that progesterone can bind the nuclear glucocorticoid receptor (nGR), whereby it may exert some of its anti-inflammatory effects [25, 209]. Thus, given the potential signaling pathways by which progesterone can influence immune function, it is unsurprising that there remains a significant gap in knowledge of this area, particularly in regards to pregnancy and maternal-fetal tolerance. There is accumulating evidence that maternal peripheral T cells express mPRs and PGRMCs during pregnancy [25, 196, 207], and studies of other disease contexts have shown that progesterone signaling modulates the TCR as well as transcription factors such as NF-κB, AP-1, and NFAT [25, 196, 201, 232–234]; yet, the expression of PRs and GRs by other immune cells is less firmly established. Nonetheless, herein we have summarized the current data supporting the presence or absence of PR and/or GR by individual immune cell subsets during pregnancy, a topic that requires continued investigation.
3. CONCLUSION
Herein, we have summarized the importance of progesterone signaling for regulating cellular immune functions in the maternal periphery as well as in the decidual, placental, and myometrial tissues. Current evidence indicates specific interactions between progesterone and individual immune cell subsets that depend on the localization of these cells in addition to other pregnancy-specific variables such as gestational age. Despite a strong body of evidence for such progesterone-driven mechanisms, additional investigation is required to further unravel the specific immunomodulatory functions of this important hormone throughout pregnancy.
HIGHLIGHTS.
Progesterone has specific effects on local and systemic cellular immunity
Progesterone promotes maternal-fetal tolerance by expanding regulatory T cells
Progesterone regulates the infiltration and activity of inflammatory immune cells
ACKNOWLEDGEMENTS
We regret our inability to cite all relevant studies due to the length of this brief review.
This research was supported, in part, by the Perinatology Research Branch, Division of Obstetrics and Maternal-Fetal Medicine, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, U.S. Department of Health and Human Services (NICHD/NIH/DHHS); and, in part, with Federal funds from NICHD/NIH/DHHS under Contract No. HHSN275201300006C. Dr. Romero has contributed to this work as part of his official duties as an employee of the United States Federal Government. This research was also supported by the Wayne State University Perinatal Initiative in Maternal, Perinatal and Child Health. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Abbreviations:
- 17OHP-C
17-alpha-hydroxyprogesterone caproate
- CSF-1
colony stimulating factor-1
- DBA
dolichos biflorus agglutinin
- DC
dendritic cell
- dpc
days post coitum
- Gal-1
Galectin-1
- GR
glucocorticoid receptor
- hCG
human chorionic gonadotropin
- HMGB1
nuclear protein high-mobility group box-1
- IDO
indoleamine-2,3-dioxygenase
- IL
interleukin
- IMC
immature myeloid cell
- iTreg
induced regulatory T cell
- LDL
low-density lipoprotein
- LH
luteinizing hormone
- LPS
lipopolysaccharide
- MCP-1
macrophage chemoattractant protein-1
- MPA
medroxyprogesterone acetate
- mPR
membrane progesterone receptor
- NET
neutrophil extracellular trap
- niT cell
neutrophil-induced T cell
- NK cell
natural killer cell
- nPR
nuclear progesterone receptor
- P4
progesterone
- PAMP
pathogen-associated molecular pattern
- PAPP-A
pregnancy associated plasma protein A
- PBMC
peripheral blood mononuclear cell
- PIBF
progesterone-induced blocking factor
- PGRMC
progesterone receptor membrane component
- pMSC
placental mesenchymal stem cell
- PR
progesterone receptor
- RUPP
reduced uterine perfusion pressure
- TCR
T-cell receptor
- Th
T helper cell
- Treg
regulatory T cell
- VIP
vasoactive intestinal peptide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DECLARATION OF INTEREST
The authors declare no potential conflicts of interest.
REFERENCES
- 1.Csapo AI, et al. , Peripheral plasma progesterone levels during human pregnancy and labor. Am J Obstet Gynecol, 1971. 110(5): p. 630–2. [DOI] [PubMed] [Google Scholar]
- 2.Csapo AI, et al. , The significance of the human corpus luteum in pregnancy maintenance. I. Preliminary studies. Am J Obstet Gynecol, 1972. 112(8): p. 1061–7. [DOI] [PubMed] [Google Scholar]
- 3.Csapo AI, Pulkkinen MO, and Kaihola HL, The effect of luteectomy-induced progesterone-withdrawal on the oxytocin and prostaglandin response of the first trimester pregnant human uterus. Prostaglandins, 1973. 4(3): p. 421–9. [DOI] [PubMed] [Google Scholar]
- 4.Csapo AI and Erdos T, The critical control of progesterone levels and pregnancy by antiprogesterone. Am J Obstet Gynecol, 1976. 126(5): p. 598–601. [DOI] [PubMed] [Google Scholar]
- 5.Csapo AI and Erdos T, Prevention of the abortifacient action of antiprogesterone serum by progesterone. Am J Obstet Gynecol, 1977. 128(2): p. 212–4. [DOI] [PubMed] [Google Scholar]
- 6.Stocco C, Telleria C, and Gibori G, The molecular control of corpus luteum formation, function, and regression. Endocr Rev, 2007. 28(1): p. 117–49. [DOI] [PubMed] [Google Scholar]
- 7.Critchley HOD, et al. , Physiology of the Endometrium and Regulation of Menstruation. Physiol Rev, 2020. 100(3): p. 1149–1179. [DOI] [PubMed] [Google Scholar]
- 8.Cole LA, Biological functions of hCG and hCG-related molecules. Reprod Biol Endocrinol, 2010. 8: p. 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tuckey RC, Progesterone synthesis by the human placenta. Placenta, 2005. 26(4): p. 273–81. [DOI] [PubMed] [Google Scholar]
- 10.Norwitz ER, Robinson JN, and Challis JR, The control of labor. N Engl J Med, 1999. 341(9): p. 660–6. [DOI] [PubMed] [Google Scholar]
- 11.Mesiano S, Myometrial progesterone responsiveness. Semin Reprod Med, 2007. 25(1): p. 5–13. [DOI] [PubMed] [Google Scholar]
- 12.Smith R, Parturition. N Engl J Med, 2007. 356(3): p. 271–83. [DOI] [PubMed] [Google Scholar]
- 13.Zakar T and Mesiano S, How does progesterone relax the uterus in pregnancy? N Engl J Med, 2011. 364(10): p. 972–3. [DOI] [PubMed] [Google Scholar]
- 14.Romero R, Dey SK, and Fisher SJ, Preterm labor: one syndrome, many causes. Science, 2014. 345(6198): p. 760–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel B, et al. , Role of nuclear progesterone receptor isoforms in uterine pathophysiology. Hum Reprod Update, 2015. 21(2): p. 155–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mendelson CR, Gao L, and Montalbano AP, Multifactorial Regulation of Myometrial Contractility During Pregnancy and Parturition. Front Endocrinol (Lausanne), 2019. 10: p. 714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kodogo V, Azibani F, and Sliwa K, Role of pregnancy hormones and hormonal interaction on the maternal cardiovascular system: a literature review. Clin Res Cardiol, 2019. 108(8): p. 831–846. [DOI] [PubMed] [Google Scholar]
- 18.Obr AE and Edwards DP, The biology of progesterone receptor in the normal mammary gland and in breast cancer. Mol Cell Endocrinol, 2012. 357(1–2): p. 4–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Graham JD and Clarke CL, Physiological action of progesterone in target tissues. Endocr Rev, 1997. 18(4): p. 502–19. [DOI] [PubMed] [Google Scholar]
- 20.Gellersen B, Fernandes MS, and Brosens JJ, Non-genomic progesterone actions in female reproduction. Hum Reprod Update, 2009. 15(1): p. 119–38. [DOI] [PubMed] [Google Scholar]
- 21.Asavasupreechar T, et al. , Systemic distribution of progesterone receptor subtypes in human tissues. J Steroid Biochem Mol Biol, 2020. 199: p. 105599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mendelson CR, Minireview: fetal-maternal hormonal signaling in pregnancy and labor. Mol Endocrinol, 2009. 23(7): p. 947–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schumacher A, Costa SD, and Zenclussen AC, Endocrine factors modulating immune responses in pregnancy. Front Immunol, 2014. 5: p. 196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Solano ME and Arck PC, Steroids, Pregnancy and Fetal Development. Front Immunol, 2019. 10: p. 3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shah NM, et al. , Progesterone-Related Immune Modulation of Pregnancy and Labor. Front Endocrinol (Lausanne), 2019. 10: p. 198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao JX, Zeng YY, and Liu Y, Fetal alloantigen is responsible for the expansion of the CD4(+)CD25(+) regulatory T cell pool during pregnancy. J Reprod Immunol, 2007. 75(2): p. 71–81. [DOI] [PubMed] [Google Scholar]
- 27.Mao G, et al. , Progesterone increases systemic and local uterine proportions of CD4+CD25+ Treg cells during midterm pregnancy in mice. Endocrinology, 2010. 151(11): p. 5477–88. [DOI] [PubMed] [Google Scholar]
- 28.Koucky M, et al. , Low levels of circulating T-regulatory lymphocytes and short cervical length are associated with preterm labor. J Reprod Immunol, 2014. 106: p. 110–7. [DOI] [PubMed] [Google Scholar]
- 29.Areia AL, et al. , Does progesterone administration in preterm labor influence Treg cells? J Perinat Med, 2016. 44(6): p. 605–11. [DOI] [PubMed] [Google Scholar]
- 30.Schumacher A, Dauven D, and Zenclussen AC, Progesterone-driven local regulatory T cell induction does not prevent fetal loss in the CBA/JxDBA/2J abortion-prone model. Am J Reprod Immunol, 2017. 77(3). [DOI] [PubMed] [Google Scholar]
- 31.Furcron AE, et al. , Vaginal progesterone, but not 17alpha-hydroxyprogesterone caproate, has antiinflammatory effects at the murine maternal-fetal interface. Am J Obstet Gynecol, 2015. 213(6): p. 846 e1–846 e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramhorst RE, et al. , Galectin-1 confers immune privilege to human trophoblast: implications in recurrent fetal loss. Glycobiology, 2012. 22(10): p. 1374–86. [DOI] [PubMed] [Google Scholar]
- 33.Szekeres-Bartho J, et al. , Immunoregulatory effects of a suppressor factor from healthy pregnant women’s lymphocytes after progesterone induction. Cell Immunol, 1989. 122(2): p. 281–94. [DOI] [PubMed] [Google Scholar]
- 34.Hansen KA, et al. , Natural killer cell activity from pregnant subjects is modulated by RU 486. Am J Obstet Gynecol, 1992. 166(1 Pt 1): p. 87–90. [DOI] [PubMed] [Google Scholar]
- 35.Laskarin G, et al. , Progesterone directly and indirectly affects perforin expression in cytolytic cells. Am J Reprod Immunol, 1999. 42(5): p. 312–20. [DOI] [PubMed] [Google Scholar]
- 36.Faust Z, et al. , Progesterone-induced blocking factor inhibits degranulation of natural killer cells. Am J Reprod Immunol, 1999. 42(2): p. 71–5. [PubMed] [Google Scholar]
- 37.Elfarra JT, et al. , 17-Hydroxyprogesterone caproate improves T cells and NK cells in response to placental ischemia; new mechanisms of action for an old drug. Pregnancy Hypertens, 2020. 19: p. 226–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cottrell JN, et al. , Progesterone induced blocking factor improves blood pressure, inflammation and pup weight in response to reduced uterine perfusion pressure (RUPP). Am J Physiol Regul Integr Comp Physiol, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hamano N, et al. , Effect of female hormones on the production of IL-4 and IL-13 from peripheral blood mononuclear cells. Acta Otolaryngol Suppl, 1998. 537: p. 27–31. [PubMed] [Google Scholar]
- 40.Choi BC, et al. , Progesterone inhibits in-vitro embryotoxic Th1 cytokine production to trophoblast in women with recurrent pregnancy loss. Hum Reprod, 2000. 15 Suppl 1: p. 46–59. [DOI] [PubMed] [Google Scholar]
- 41.Raghupathy R, et al. , Progesterone-induced blocking factor (PIBF) modulates cytokine production by lymphocytes from women with recurrent miscarriage or preterm delivery. J Reprod Immunol, 2009. 80(1–2): p. 91–9. [DOI] [PubMed] [Google Scholar]
- 42.Word RA, et al. , Transgene insertion on mouse chromosome 6 impairs function of the uterine cervix and causes failure of parturition. Biol Reprod, 2005. 73(5): p. 1046–56. [DOI] [PubMed] [Google Scholar]
- 43.Timmons BC and Mahendroo MS, Timing of neutrophil activation and expression of proinflammatory markers do not support a role for neutrophils in cervical ripening in the mouse. Biol Reprod, 2006. 74(2): p. 236–45. [DOI] [PubMed] [Google Scholar]
- 44.Timmons BC, Fairhurst AM, and Mahendroo MS, Temporal changes in myeloid cells in the cervix during pregnancy and parturition. J Immunol, 2009. 182(5): p. 2700–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yellon SM, Ebner CA, and Elovitz MA, Medroxyprogesterone acetate modulates remodeling, immune cell census, and nerve fibers in the cervix of a mouse model for inflammation-induced preterm birth. Reprod Sci, 2009. 16(3): p. 257–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Timmons B, Akins M, and Mahendroo M, Cervical remodeling during pregnancy and parturition. Trends Endocrinol Metab, 2010. 21(6): p. 353–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Holt R, et al. , The molecular mechanisms of cervical ripening differ between term and preterm birth. Endocrinology, 2011. 152(3): p. 1036–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mahendroo M, Cervical remodeling in term and preterm birth: insights from an animal model. Reproduction, 2012. 143(4): p. 429–38. [DOI] [PubMed] [Google Scholar]
- 49.Yellon SM, Immunobiology of Cervix Ripening. Front Immunol, 2019. 10: p. 3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chaouat G, Kolb JP, and Wegmann TG, The murine placenta as an immunological barrier between the mother and the fetus. Immunol Rev, 1983. 75: p. 31–60. [DOI] [PubMed] [Google Scholar]
- 51.Mori M, et al. , The decidua-the maternal bed embracing the embryo-maintains the pregnancy. Semin Immunopathol, 2016. 38(6): p. 635–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wetendorf M and DeMayo FJ, The progesterone receptor regulates implantation, decidualization, and glandular development via a complex paracrine signaling network. Mol Cell Endocrinol, 2012. 357(1–2): p. 108–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Szwarc MM, et al. , Early growth response 1 transcriptionally primes the human endometrial stromal cell for decidualization. J Steroid Biochem Mol Biol, 2019. 189: p. 283–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Simon L, et al. , Stromal progesterone receptors mediate induction of Indian Hedgehog (IHH) in uterine epithelium and its downstream targets in uterine stroma. Endocrinology, 2009. 150(8): p. 3871–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gellersen B and Brosens JJ, Cyclic decidualization of the human endometrium in reproductive health and failure. Endocr Rev, 2014. 35(6): p. 851–905. [DOI] [PubMed] [Google Scholar]
- 56.Muter J, et al. , Progesterone-Dependent Induction of Phospholipase C-Related Catalytically Inactive Protein 1 (PRIP-1) in Decidualizing Human Endometrial Stromal Cells. Endocrinology, 2016. 157(7): p. 2883–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brosens JJ, Hayashi N, and White JO, Progesterone receptor regulates decidual prolactin expression in differentiating human endometrial stromal cells. Endocrinology, 1999. 140(10): p. 4809–20. [DOI] [PubMed] [Google Scholar]
- 58.Blanks AM and Brosens JJ, Progesterone action in the myometrium and decidua in preterm birth. Facts Views Vis Obgyn, 2012. 4(3): p. 33–43. [PMC free article] [PubMed] [Google Scholar]
- 59.Arck PC and Hecher K, Fetomaternal immune cross-talk and its consequences for maternal and offspring’s health. Nat Med, 2013. 19(5): p. 548–56. [DOI] [PubMed] [Google Scholar]
- 60.Erlebacher A, Immunology of the maternal-fetal interface. Annu Rev Immunol, 2013. 31: p. 387–411. [DOI] [PubMed] [Google Scholar]
- 61.Murphy SP, et al. , Interferon gamma in successful pregnancies. Biol Reprod, 2009. 80(5): p. 848–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moffett A and Colucci F, Uterine NK cells: active regulators at the maternal-fetal interface. J Clin Invest, 2014. 124(5): p. 1872–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nagamatsu T and Schust DJ, The immunomodulatory roles of macrophages at the maternal-fetal interface. Reprod Sci, 2010. 17(3): p. 209–18. [DOI] [PubMed] [Google Scholar]
- 64.Aluvihare VR, Kallikourdis M, and Betz AG, Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol, 2004. 5(3): p. 266–71. [DOI] [PubMed] [Google Scholar]
- 65.Samstein RM, et al. , Extrathymic generation of regulatory T cells in placental mammals mitigates maternal-fetal conflict. Cell, 2012. 150(1): p. 29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Arenas-Hernandez M, et al. , Effector and Activated T Cells Induce Preterm Labor and Birth That Is Prevented by Treatment with Progesterone. J Immunol, 2019. 202(9): p. 2585–2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ramhorst R, et al. , Induction of maternal tolerance to fetal alloantigens by RANTES production. Am J Reprod Immunol, 2006. 56(5–6): p. 302–11. [DOI] [PubMed] [Google Scholar]
- 68.Szekeres-Bartho J, et al. , The mechanism of the inhibitory effect of progesterone on lymphocyte cytotoxicity: I. Progesterone-treated lymphocytes release a substance inhibiting cytotoxicity and prostaglandin synthesis. Am J Reprod Immunol Microbiol, 1985. 9(1): p. 15–8. [DOI] [PubMed] [Google Scholar]
- 69.Szekeres-Bartho J, Faust Z, and Varga P, The expression of a progesterone-induced immunomodulatory protein in pregnancy lymphocytes. Am J Reprod Immunol, 1995. 34(6): p. 342–8. [DOI] [PubMed] [Google Scholar]
- 70.Szekeres-Bartho J, Sucurovic S, and Mulac-Jericevic B, The Role of Extracellular Vesicles and PIBF in Embryo-Maternal Immune-Interactions. Front Immunol, 2018. 9: p. 2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ramhorst RE, et al. , Identification of RANTES as a novel immunomodulator of the maternal allogeneic response. Clin Immunol, 2004. 110(1): p. 71–80. [DOI] [PubMed] [Google Scholar]
- 72.Hayday AC, gammadelta T Cell Update: Adaptate Orchestrators of Immune Surveillance. J Immunol, 2019. 203(2): p. 311–320. [DOI] [PubMed] [Google Scholar]
- 73.Polgar B, et al. , The role of gamma/delta T cell receptor positive cells in pregnancy. Am J Reprod Immunol, 1999. 41(4): p. 239–44. [DOI] [PubMed] [Google Scholar]
- 74.Liang Q, et al. , Correlations of the expression of gammadelta T cells and their co-stimulatory molecules TIGIT, PD-1, ICOS and BTLA with PR and PIBF in the peripheral blood and decidual tissues of women with unexplained recurrent spontaneous abortion. Clin Exp Immunol, 2021. 203(1): p. 55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cai D, Tang Y, and Yao X, Changes of gammadeltaT cell subtypes during pregnancy and their influences in spontaneous abortion. J Reprod Immunol, 2019. 131:p. 57–62. [DOI] [PubMed] [Google Scholar]
- 76.Fonseca EB, et al. ,Progesterone and the risk of preterm birth among women with a short cervix. N Engl J Med, 2007. 357(5): p. 462–9. [DOI] [PubMed] [Google Scholar]
- 77.DeFranco EA, et al. , Vaginal progesterone is associated with a decrease in risk for early preterm birth and improved neonatal outcome in women with a short cervix: a secondary analysis from a randomized, double-blind, placebo-controlled trial. Ultrasound Obstet Gynecol, 2007. 30(5): p. 697–705. [DOI] [PubMed] [Google Scholar]
- 78.O’Brien JM, et al. , Effect of progesterone on cervical shortening in women at risk for preterm birth: secondary analysis from a multinational, randomized, double-blind, placebo-controlled trial. Ultrasound Obstet Gynecol, 2009. 34(6): p. 653–9. [DOI] [PubMed] [Google Scholar]
- 79.Hassan SS, et al. , Vaginal progesterone reduces the rate of preterm birth in women with a sonographic short cervix: a multicenter, randomized, double-blind, placebo-controlled trial. Ultrasound Obstet Gynecol, 2011. 38(1): p. 18–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Romero R, et al. , Vaginal progesterone in women with an asymptomatic sonographic short cervix in the midtrimester decreases preterm delivery and neonatal morbidity: a systematic review and metaanalysis of individual patient data. Am J Obstet Gynecol, 2012. 206(2): p. 124 e1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Romero R, et al. , A blueprint for the prevention of preterm birth: vaginal progesterone in women with a short cervix. J Perinat Med, 2013. 41(1): p. 27–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Conde-Agudelo A, et al. , Vaginal progesterone vs. cervical cerclage for the prevention of preterm birth in women with a sonographic short cervix, previous preterm birth, and singleton gestation: a systematic review and indirect comparison metaanalysis. Am J Obstet Gynecol, 2013. 208(1): p. 42 e1–42 e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Romero R, et al. , Vaginal progesterone decreases preterm birth </= 34 weeks of gestation in women with a singleton pregnancy and a short cervix: an updated meta-analysis including data from the OPPTIMUM study. Ultrasound Obstet Gynecol, 2016. 48(3): p. 308–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Romero R, et al. , Vaginal progesterone for preventing preterm birth and adverse perinatal outcomes in singleton gestations with a short cervix: a meta-analysis of individual patient data. Am J Obstet Gynecol, 2018. 218(2): p. 161–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Grasso E, et al. , VIP contribution to the decidualization program: regulatory T cell recruitment. J Endocrinol, 2014. 221(1): p. 121–31. [DOI] [PubMed] [Google Scholar]
- 86.Csabai T, et al. , Altered Immune Response and Implantation Failure in Progesterone-Induced Blocking Factor-Deficient Mice. Front Immunol, 2020. 11: p. 349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kelemen K, et al. , A progesterone-induced protein increases the synthesis of asymmetric antibodies. Cell Immunol, 1996. 167(1): p. 129–34. [DOI] [PubMed] [Google Scholar]
- 88.Zenclussen AC, et al. , Asymmetric antibodies and pregnancy. Am J Reprod Immunol, 2001. 45(5): p. 289–94. [DOI] [PubMed] [Google Scholar]
- 89.Kuang H, et al. , Hormonal regulation of uterine natural killer cells in mouse preimplantation uterus. J Mol Histol, 2010. 41(1): p. 1–7. [DOI] [PubMed] [Google Scholar]
- 90.Critchley HO, et al. , Sex steroid regulation of leukocyte traffic in human decidua. Hum Reprod, 1996. 11(10): p. 2257–62. [DOI] [PubMed] [Google Scholar]
- 91.Kokubu K, et al. , Differentiation and elimination of uterine natural killer cells in delayed implantation and parturition mice. J Reprod Dev, 2005. 51(6): p. 773–6. [DOI] [PubMed] [Google Scholar]
- 92.Namdar Ahmadabad H, et al. , Pregnancy outcomes following the administration of high doses of dexamethasone in early pregnancy. Clin Exp Reprod Med, 2016. 43(1): p. 15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.van den Heuvel M, et al. , An analysis of the uterine lymphocyte-derived hybridoma cell line GWM 1–2 for expression of receptors for estrogen, progesterone and interleukin 2. J Reprod Immunol, 1996. 31(1–2): p. 37–50. [DOI] [PubMed] [Google Scholar]
- 94.King A, Gardner L, and Loke YW, Evaluation of oestrogen and progesterone receptor expression in uterine mucosal lymphocytes. Hum Reprod, 1996. 11(5): p. 1079–82. [DOI] [PubMed] [Google Scholar]
- 95.Stewart JA, Bulmer JN, and Murdoch AP, Endometrial leucocytes: expression of steroid hormone receptors. J Clin Pathol, 1998. 51(2): p. 121–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Henderson TA, et al. , Steroid receptor expression in uterine natural killer cells. J Clin Endocrinol Metab, 2003. 88(1): p. 440–9. [DOI] [PubMed] [Google Scholar]
- 97.Ogle TF, et al. , Stromal cell progesterone and estrogen receptors during proliferation and regression of the decidua basalis in the pregnant rat. Biol Reprod, 1997. 57(3): p. 495–506. [DOI] [PubMed] [Google Scholar]
- 98.Kitaya K, et al. , Effect of female sex steroids on human endometrial CD16neg CD56bright natural killer cells. Fertil Steril, 2003. 79 Suppl 1: p. 730–4. [DOI] [PubMed] [Google Scholar]
- 99.van den Heuvel M, et al. , Trafficking of peripheral blood CD56(bright) cells to the decidualizing uterus--new tricks for old dogmas? J Reprod Immunol, 2005. 67(1–2): p. 21–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Guo W, et al. , Glucocorticoid receptor mediates the effect of progesterone on uterine natural killer cells. Am J Reprod Immunol, 2012. 67(6): p. 463–73. [DOI] [PubMed] [Google Scholar]
- 101.Inoue T, et al. , Progesterone stimulates the induction of human endometrial CD56+ lymphocytes in an in vitro culture system. J Clin Endocrinol Metab, 1996. 81(4): p. 1502–7. [DOI] [PubMed] [Google Scholar]
- 102.Oh MJ and Croy BA, A map of relationships between uterine natural killer cells and progesterone receptor expressing cells during mouse pregnancy. Placenta, 2008. 29(4): p. 317–23. [DOI] [PubMed] [Google Scholar]
- 103.Fehniger TA and Caligiuri MA, Interleukin 15: biology and relevance to human disease. Blood, 2001. 97(1): p. 14–32. [DOI] [PubMed] [Google Scholar]
- 104.Okada S, et al. , Expression of interleukin-15 in human endometrium and decidua. Mol Hum Reprod, 2000. 6(1): p. 75–80. [DOI] [PubMed] [Google Scholar]
- 105.Kitaya K, et al. , IL-15 expression at human endometrium and decidua. Biol Reprod, 2000. 63(3): p. 683–7. [DOI] [PubMed] [Google Scholar]
- 106.Okada H, et al. , Progesterone enhances interleukin-15 production in human endometrial stromal cells in vitro. J Clin Endocrinol Metab, 2000. 85(12): p. 4765–70. [DOI] [PubMed] [Google Scholar]
- 107.Verma S, et al. , Human decidual natural killer cells express the receptor for and respond to the cytokine interleukin 15. Biol Reprod, 2000. 62(4): p. 959–68. [DOI] [PubMed] [Google Scholar]
- 108.Croy BA, et al. , Update on pathways regulating the activation of uterine Natural Killer cells, their interactions with decidual spiral arteries and homing of their precursors to the uterus. J Reprod Immunol, 2003. 59(2): p. 175–91. [DOI] [PubMed] [Google Scholar]
- 109.Marinic M, et al. , Evolutionary transcriptomics implicates HAND2 in the origins of implantation and regulation of gestation length. Elife, 2021. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Strunz B, et al. , Continuous human uterine NK cell differentiation in response to endometrial regeneration and pregnancy. Sci Immunol, 2021. 6(56). [DOI] [PubMed] [Google Scholar]
- 111.Carlino C, et al. , Recruitment of circulating NK cells through decidual tissues: a possible mechanism controlling NK cell accumulation in the uterus during early pregnancy. Blood, 2008. 111(6): p. 3108–15. [DOI] [PubMed] [Google Scholar]
- 112.Qu X, et al. , Osteopontin expression in human decidua is associated with decidual natural killer cells recruitment and regulated by progesterone. In Vivo, 2008. 22(1): p. 55–61. [PubMed] [Google Scholar]
- 113.Mokhtar NM, et al. , Progestin regulates chemokine (C-X-C motif) ligand 14 transcript level in human endometrium. Mol Hum Reprod, 2010. 16(3): p. 170–7. [DOI] [PubMed] [Google Scholar]
- 114.Carayannopoulos LN, et al. , Murine trophoblast cells induce NK cell interferon-gamma production through KLRK1. Biol Reprod, 2010. 83(3): p. 404–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Laskarin G, et al. , Progesterone induced blocking factor (PIBF) mediates progesterone induced suppression of decidual lymphocyte cytotoxicity. Am J Reprod Immunol, 2002. 48(4): p. 201–9. [DOI] [PubMed] [Google Scholar]
- 116.Bogdan A, Berta G, and Szekeres-Bartho J, PIBF positive uterine NK cells in the mouse decidua. J Reprod Immunol, 2017. 119: p. 38–43. [DOI] [PubMed] [Google Scholar]
- 117.Huang J, et al. , Cellular localization and hormonal regulation of inducible nitric oxide synthase in cycling mouse uterus. J Leukoc Biol, 1995. 57(1): p. 27–35. [DOI] [PubMed] [Google Scholar]
- 118.De M and Wood GW, Influence of oestrogen and progesterone on macrophage distribution in the mouse uterus. J Endocrinol, 1990. 126(3): p. 417–24. [DOI] [PubMed] [Google Scholar]
- 119.Tibbetts TA, Conneely OM, and O’Malley BW, Progesterone via its receptor antagonizes the pro-inflammatory activity of estrogen in the mouse uterus. Biol Reprod, 1999. 60(5): p. 1158–65. [DOI] [PubMed] [Google Scholar]
- 120.Critchley HO, et al. , Role of inflammatory mediators in human endometrium during progesterone withdrawal and early pregnancy. J Clin Endocrinol Metab, 1999. 84(1): p. 240–8. [DOI] [PubMed] [Google Scholar]
- 121.Caballero-Campo P, et al. , Hormonal and embryonic regulation of chemokines IL-8, MCP-1 and RANTES in the human endometrium during the window of implantation. Mol Hum Reprod, 2002. 8(4): p. 375–84. [DOI] [PubMed] [Google Scholar]
- 122.Aikawa S, et al. , Uterine deficiency of high-mobility group box-1 (HMGB1) protein causes implantation defects and adverse pregnancy outcomes. Cell Death Differ, 2020. 27(5): p. 1489–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Shynlova O, et al. , Infiltration of myeloid cells into decidua is a critical early event in the labour cascade and post-partum uterine remodelling. J Cell Mol Med, 2013. 17(2): p. 311–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tsai YC, et al. , Medroxyprogesterone acetate drives M2 macrophage differentiation toward a phenotype of decidual macrophage. Mol Cell Endocrinol, 2017. 452: p. 74–83. [DOI] [PubMed] [Google Scholar]
- 125.Ivanova E, et al. , CD83 monocyte-derived dendritic cells are present in human decidua and progesterone induces their differentiation in vitro. Am J Reprod Immunol, 2005. 53(4): p. 199–205. [DOI] [PubMed] [Google Scholar]
- 126.Gorvel L, et al. , Myeloid decidual dendritic cells and immunoregulation of pregnancy: defective responsiveness to Coxiella burnetii and Brucella abortus. Front Cell Infect Microbiol, 2014. 4: p. 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Milne SA, et al. , Leukocyte populations and steroid receptor expression in human first-trimester decidua; regulation by antiprogestin and prostaglandin E analog. J Clin Endocrinol Metab, 2005. 90(7): p. 4315–21. [DOI] [PubMed] [Google Scholar]
- 128.Kelly RW, et al. , Progesterone control of interleukin-8 production in endometrium and chorio-decidual cells underlines the role of the neutrophil in menstruation and parturition. Hum Reprod, 1994. 9(2): p. 253–8. [DOI] [PubMed] [Google Scholar]
- 129.Kelly RW, Leask R, and Calder AA, Choriodecidual production of interleukin-8 and mechanism of parturition. Lancet, 1992. 339(8796): p. 776–7. [DOI] [PubMed] [Google Scholar]
- 130.Zhao Y, et al. , Cyclic stretch augments production of neutrophil chemokines and matrix metalloproteinases-1 (MMP-1) from human decidual cells, and the production was reduced by progesterone. Am J Reprod Immunol, 2013. 69(5): p. 454–62. [DOI] [PubMed] [Google Scholar]
- 131.Milne SA, et al. , Perivascular interleukin-8 messenger ribonucleic acid expression in human endometrium varies across the menstrual cycle and in early pregnancy decidua. J Clin Endocrinol Metab, 1999. 84(7): p. 2563–7. [DOI] [PubMed] [Google Scholar]
- 132.Arici A, MacDonald PC, and Casey ML, Progestin regulation of interleukin-8 mRNA levels and protein synthesis in human endometrial stromal cells. J Steroid Biochem Mol Biol, 1996. 58(1): p. 71–6. [DOI] [PubMed] [Google Scholar]
- 133.Kwon JY, Romero R, and Mor G, New insights into the relationship between viral infection and pregnancy complications. Am J Reprod Immunol, 2014. 71(5): p. 387–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Delorme-Axford E, Sadovsky Y, and Coyne CB, The Placenta as a Barrier to Viral Infections. Annu Rev Virol, 2014. 1(1): p. 133–46. [DOI] [PubMed] [Google Scholar]
- 135.Ander SE, Diamond MS, and Coyne CB, Immune responses at the maternal-fetal interface. Sci Immunol, 2019. 4(31). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kreis NN, et al. , A Message from the Human Placenta: Structural and Immunomodulatory Defense against SARS-CoV-2. Cells, 2020. 9(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Burton GJ and Jauniaux E, What is the placenta? Am J Obstet Gynecol, 2015. 213(4 Suppl): p. S6.e1, S6–8. [DOI] [PubMed] [Google Scholar]
- 138.Maltepe E and Fisher SJ, Placenta: the forgotten organ. Annu Rev Cell Dev Biol, 2015. 31: p. 523–52. [DOI] [PubMed] [Google Scholar]
- 139.Sasaki K and Norwitz ER, Gonadotropin-releasing hormone/gonadotropin-releasing hormone receptor signaling in the placenta. Curr Opin Endocrinol Diabetes Obes, 2011. 18(6): p. 401–8. [DOI] [PubMed] [Google Scholar]
- 140.Taglauer ES, Wilkins-Haug L, and Bianchi DW, Review: cell-free fetal DNA in the maternal circulation as an indication of placental health and disease. Placenta, 2014. 35 Suppl(Suppl): p. S64–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Fitzgerald W, et al. , Extracellular vesicles generated by placental tissues ex vivo: A transport system for immune mediators and growth factors. Am J Reprod Immunol, 2018. 80(1): p. e12860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Novak CM, et al. , Progesterone improves perinatal neuromotor outcomes in a mouse model of intrauterine inflammation via immunomodulation of the placenta. Am J Reprod Immunol, 2018. 79(5): p. e12842. [DOI] [PubMed] [Google Scholar]
- 143.Polanczyk MJ, et al. , Cutting edge: estrogen drives expansion of the CD4+CD25+ regulatory T cell compartment. J Immunol, 2004. 173(4): p. 2227–30. [DOI] [PubMed] [Google Scholar]
- 144.Thiele K, et al. , Impaired Progesterone-Responsiveness of CD11c(+) Dendritic Cells Affects the Generation of CD4(+) Regulatory T Cells and Is Associated With Intrauterine Growth Restriction in Mice. Front Endocrinol (Lausanne), 2019. 10: p. 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sinosich MJ, et al. , RU 486 mediated leukocytic inflammatory reaction at the utero-placental interface. Asia Oceania J Obstet Gynaecol, 1989. 15(4): p. 375–81. [DOI] [PubMed] [Google Scholar]
- 146.Nadkarni S, et al. , Neutrophils induce proangiogenic T cells with a regulatory phenotype in pregnancy. Proc Natl Acad Sci U S A, 2016. 113(52): p. E8415–E8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Abumaree MH, et al. , Human placental mesenchymal stem cells (pMSCs) play a role as immune suppressive cells by shifting macrophage differentiation from inflammatory M1 to anti-inflammatory M2 macrophages. Stem Cell Rev Rep, 2013. 9(5): p. 620–41. [DOI] [PubMed] [Google Scholar]
- 148.Zhao H, et al. , Hypoxia regulates placental angiogenesis via alternatively activated macrophages. Am J Reprod Immunol, 2018. 80(3): p. e12989. [DOI] [PubMed] [Google Scholar]
- 149.Zhu XD, Bonet B, and Knopp RH, 17beta-Estradiol, progesterone, and testosterone inversely modulate low-density lipoprotein oxidation and cytotoxicity in cultured placental trophoblast and macrophages. Am J Obstet Gynecol, 1997. 177(1): p. 196–209. [DOI] [PubMed] [Google Scholar]
- 150.Rab A, et al. , Placental gene expression patterns of epidermal growth factor in intrauterine growth restriction. Eur J Obstet Gynecol Reprod Biol, 2013. 170(1): p. 96–9. [DOI] [PubMed] [Google Scholar]
- 151.Nawathe AR, et al. , Insulin-like growth factor axis in pregnancies affected by fetal growth disorders. Clin Epigenetics, 2016. 8: p. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Miranda S, et al. , Dendritic cells therapy confers a protective microenvironment in murine pregnancy. Scand J Immunol, 2006. 64(5): p. 493–9. [DOI] [PubMed] [Google Scholar]
- 153.Bianchi P, et al. , Progesterone Decreases in vitro Indoleamine 2, 3-dioxygenase Expression in Dendritic and CD4(+) Cells from Maternal-Fetal Interface of Rats. Immunol Invest, 2017. 46(5): p. 447–459. [DOI] [PubMed] [Google Scholar]
- 154.Gutzeit O, et al. , Progesterone treatment enhances the expansion of placental immature myeloid cells in a mouse model of premature labor. J Reprod Immunol, 2019. 131: p. 7–12. [DOI] [PubMed] [Google Scholar]
- 155.Mei-Dan E, et al. , Proangiogenic immature myeloid cells populate the human placenta and their presence correlates with placental and birthweight. Am J Obstet Gynecol, 2012. 207(2): p. 141 e1–5. [DOI] [PubMed] [Google Scholar]
- 156.Cunningham FG, et al. , Maternal physiology. 25 ed. Williams Obstetrics. 2018, New York: McGraw-Hill. 49–78. [Google Scholar]
- 157.Csapo AI, et al. , Volume and activity of the pregnant human uterus. Am J Obstet Gynecol, 1963. 85: p. 819–35. [DOI] [PubMed] [Google Scholar]
- 158.Geirsson RT, Intrauterine volume in pregnancy. Acta Obstet Gynecol Scand Suppl, 1986. 136: p. 1–74. [PubMed] [Google Scholar]
- 159.Degani S, et al. , Myometrial thickness in pregnancy: longitudinal sonographic study. J Ultrasound Med, 1998. 17(10): p. 661–5. [DOI] [PubMed] [Google Scholar]
- 160.Riemer RK and Heymann MA, Regulation of uterine smooth muscle function during gestation. Pediatr Res, 1998. 44(5): p. 615–27. [DOI] [PubMed] [Google Scholar]
- 161.Osol G and Moore LG, Maternal uterine vascular remodeling during pregnancy. Microcirculation, 2014. 21(1): p. 38–47. [DOI] [PubMed] [Google Scholar]
- 162.López Bernal A, et al. , Parturition: activation of stimulatory pathways or loss of uterine quiescence? Adv Exp Med Biol, 1995. 395: p. 435–51. [PubMed] [Google Scholar]
- 163.Shynlova O, et al. , Myometrial activation: Novel concepts underlying labor. Placenta, 2020. 92: p. 28–36. [DOI] [PubMed] [Google Scholar]
- 164.Menon R, et al. , Novel concepts on pregnancy clocks and alarms: redundancy and synergy in human parturition. Hum Reprod Update, 2016. 22(5): p. 535–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Griesinger G, et al. , Dydrogesterone: pharmacological profile and mechanism of action as luteal phase support in assisted reproduction. Reprod Biomed Online, 2019. 38(2): p. 249–259. [DOI] [PubMed] [Google Scholar]
- 166.Joachim R, et al. , The progesterone derivative dydrogesterone abrogates murine stress-triggered abortion by inducing a Th2 biased local immune response. Steroids, 2003. 68(10–13): p. 931–40. [DOI] [PubMed] [Google Scholar]
- 167.Blois SM, et al. , Depletion of CD8+ cells abolishes the pregnancy protective effect of progesterone substitution with dydrogesterone in mice by altering the Th1/Th2 cytokine profile. J Immunol, 2004. 172(10): p. 5893–9. [DOI] [PubMed] [Google Scholar]
- 168.Chantakru S, et al. , Coordinate regulation of lymphocyte-endothelial interactions by pregnancy-associated hormones. J Immunol, 2003. 171(8): p. 4011–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Dorfeshan P, Salehnia M, and Moazzeni SM, Ovarian stimulation affects the population of mouse uterine NK cells at early pregnancy. Biomed Res Int, 2013. 2013: p. 182531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Zhao Y, et al. , Cyclic stretch augments production of neutrophil chemokines and matrix metalloproteinase-1 in human uterine smooth muscle cells. Am J Reprod Immunol, 2013. 69(3): p. 240–7. [DOI] [PubMed] [Google Scholar]
- 171.Gomez-Lopez N, et al. , Maternal circulating leukocytes display early chemotactic responsiveness during late gestation. BMC Pregnancy Childbirth, 2013. 13 Suppl 1: p. S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Shynlova O, et al. , Myometrial immune cells contribute to term parturition, preterm labour and post-partum involution in mice. J Cell Mol Med, 2013. 17(1): p. 90–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Shynlova O, et al. , Monocyte chemoattractant protein-1 (CCL-2) integrates mechanical and endocrine signals that mediate term and preterm labor. J Immunol, 2008. 181(2): p. 1470–9. [DOI] [PubMed] [Google Scholar]
- 174.Edey LF, et al. , Progesterone, the maternal immune system and the onset of parturition in the mouse. Biol Reprod, 2018. 98(3): p. 376–395. [DOI] [PubMed] [Google Scholar]
- 175.Rajagopal SP, et al. , Crosstalk between monocytes and myometrial smooth muscle in culture generates synergistic pro-inflammatory cytokine production and enhances myocyte contraction, with effects opposed by progesterone. Mol Hum Reprod, 2015. 21(8): p. 672–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Care AS, et al. , Ovarian steroid hormone-regulated uterine remodeling occurs independently of macrophages in mice. Biol Reprod, 2014. 91(3): p. 60. [DOI] [PubMed] [Google Scholar]
- 177.Blois SM, et al. , A pivotal role for galectin-1 in fetomaternal tolerance. Nat Med, 2007. 13(12): p. 1450–7. [DOI] [PubMed] [Google Scholar]
- 178.Maraspin LE and Bo WJ, Effects of hormones, pregnancy and pseudopregnancy on the mast cell count in the rat uterus. Life Sci I, 1971. 10(2): p. 111–20. [DOI] [PubMed] [Google Scholar]
- 179.Padilla L, et al. , Histamine content and mast cells distribution in mouse uterus: the effect of sexual hormones, gestation and labor. Cell Mol Biol, 1990. 36(1): p. 93–100. [PubMed] [Google Scholar]
- 180.Jensen F, et al. , Estradiol and progesterone regulate the migration of mast cells from the periphery to the uterus and induce their maturation and degranulation. PLoS One, 2010. 5(12): p. e14409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Lin H, et al. , Synthesis of T helper 2-type cytokines at the maternal-fetal interface. J Immunol, 1993. 151(9): p. 4562–73. [PubMed] [Google Scholar]
- 182.Tafuri A, et al. , T cell awareness of paternal alloantigens during pregnancy. Science, 1995. 270(5236): p. 630–3. [DOI] [PubMed] [Google Scholar]
- 183.Marzi M, et al. , Characterization of type 1 and type 2 cytokine production profile in physiologic and pathologic human pregnancy. Clin Exp Immunol, 1996. 106(1): p. 127–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ekerfelt C, et al. , Paternal leukocytes selectively increase secretion of IL-4 in peripheral blood during normal pregnancies: demonstrated by a novel one-way MLC measuring cytokine secretion. Am J Reprod Immunol, 1997. 38(5): p. 320–6. [DOI] [PubMed] [Google Scholar]
- 185.Ekerfelt C, et al. , Th2-deviation of fetus-specific T cells. Immunol Today, 1999. 20(11): p. 534. [DOI] [PubMed] [Google Scholar]
- 186.Sacks G, Sargent I, and Redman C, An innate view of human pregnancy. Immunol Today, 1999. 20(3): p. 114–8. [DOI] [PubMed] [Google Scholar]
- 187.Sacks G, Sargent I, and Redman C, Innate immunity in pregnancy. Immunol Today, 2000. 21(4): p. 200–1. [DOI] [PubMed] [Google Scholar]
- 188.Gomez-Lopez N, et al. , The Cellular Transcriptome in the Maternal Circulation During Normal Pregnancy: A Longitudinal Study. Front Immunol, 2019. 10: p. 2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Chien CH, et al. , Mifepristone acts as progesterone antagonist of non-genomic responses but inhibits phytohemagglutinin-induced proliferation in human T cells. Hum Reprod, 2009. 24(8): p. 1968–75. [DOI] [PubMed] [Google Scholar]
- 190.Rafiee M, et al. , Vitamin D3 induces the expression of membrane progesterone receptors (mPRs) on naive CD4(+) T lymphocyte cells in women of reproductive age. Int Immunopharmacol, 2019. 72: p. 55–61. [DOI] [PubMed] [Google Scholar]
- 191.Hierweger AM, et al. , Progesterone modulates the T-cell response via glucocorticoid receptor-dependent pathways. Am J Reprod Immunol, 2019. 81(2): p. e13084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Clemens LE, Siiteri PK, and Stites DP, Mechanism of immunosuppression of progesterone on maternal lymphocyte activation during pregnancy. J Immunol, 1979. 122(5): p. 1978–85. [PubMed] [Google Scholar]
- 193.Stites DP, Bugbee S, and Siiteri PK, Differential actions of progesterone and cortisol on lymphocyte and monocyte interaction during lymphocyte activation--relevance to immunosuppression in pregnancy. J Reprod Immunol, 1983. 5(4): p. 215–28. [DOI] [PubMed] [Google Scholar]
- 194.Fujisaki S, et al. , Synergistic effect of progesterone on prostaglandin E modulation of the mitogenic response of human peripheral lymphocytes. J Reprod Immunol, 1985. 7(1): p. 15–26. [DOI] [PubMed] [Google Scholar]
- 195.Chien EJ, et al. , Non-genomic immunosuppressive actions of progesterone inhibits PHA-induced alkalinization and activation in T cells. J Cell Biochem, 2006. 99(1): p. 292–304. [DOI] [PubMed] [Google Scholar]
- 196.Lissauer D, et al. , Progesterone promotes maternal-fetal tolerance by reducing human maternal T-cell polyfunctionality and inducing a specific cytokine profile. Eur J Immunol, 2015. 45(10): p. 2858–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Feinberg BB, et al. , Increased progesterone concentrations are necessary to suppress interleukin-2-activated human mononuclear cell cytotoxicity. Am J Obstet Gynecol, 1991. 165(6 Pt 1): p. 1872–6. [DOI] [PubMed] [Google Scholar]
- 198.Solano ME, et al. , Progesterone and HMOX-1 promote fetal growth by CD8+ T cell modulation. J Clin Invest, 2015. 125(4): p. 1726–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Lai JN, et al. , The non-genomic rapid acidification in peripheral T cells by progesterone depends on intracellular calcium increase and not on Na+/H+-exchange inhibition. Steroids, 2012. 77(10): p. 1017–24. [DOI] [PubMed] [Google Scholar]
- 200.Lin VH, et al. , The rapid immunosuppression in phytohemagglutinin-activated human T cells is inhibited by the proliferative Ca(2+) influx induced by progesterone and analogs. Steroids, 2016. 111: p. 71–78. [DOI] [PubMed] [Google Scholar]
- 201.Ehring GR, et al. , A nongenomic mechanism for progesterone-mediated immunosuppression: inhibition of K+ channels, Ca2+ signaling, and gene expression in T lymphocytes. J Exp Med, 1998. 188(9): p. 1593–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Wang S, et al. , Th17/Treg-cell balance in the peripheral blood of pregnant females with a history of recurrent spontaneous abortion receiving progesterone or cyclosporine A. Exp Ther Med, 2021. 21(1): p. 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Piccinni MP, et al. , Defective production of both leukemia inhibitory factor and type 2 T-helper cytokines by decidual T cells in unexplained recurrent abortions. Nat Med, 1998. 4(9): p. 1020–4. [DOI] [PubMed] [Google Scholar]
- 204.Szekeres-Bartho J, et al. , The mechanism of the inhibitory effect of progesterone on lymphocyte cytotoxicity: II. Relationship between cytotoxicity and the cyclooxygenase pathway of arachidonic acid metabolism. Am J Reprod Immunol Microbiol, 1985. 9(1): p. 19–22. [DOI] [PubMed] [Google Scholar]
- 205.Par G, Bartok B, and Szekeres-Bartho J, Cyclooxygenase is involved in the effects of progesterone-induced blocking factor on the production of interleukin 12. Am J Obstet Gynecol, 2000. 183(1): p. 126–30. [DOI] [PubMed] [Google Scholar]
- 206.Pallinger E, et al. , PIBF+ extracellular vesicles from mouse embryos affect IL-10 production by CD8+ cells. Sci Rep, 2018. 8(1): p. 4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Areia A, et al. , Membrane progesterone receptors in human regulatory T cells: a reality in pregnancy. BJOG, 2015. 122(11): p. 1544–50. [DOI] [PubMed] [Google Scholar]
- 208.Thuere C, et al. , Kinetics of regulatory T cells during murine pregnancy. Am J Reprod Immunol, 2007. 58(6): p. 514–23. [DOI] [PubMed] [Google Scholar]
- 209.Engler JB, et al. , Glucocorticoid receptor in T cells mediates protection from autoimmunity in pregnancy. Proc Natl Acad Sci U S A, 2017. 114(2): p. E181–E190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Arruvito L, et al. , NK cells expressing a progesterone receptor are susceptible to progesterone-induced apoptosis. J Immunol, 2008. 180(8): p. 5746–53. [DOI] [PubMed] [Google Scholar]
- 211.van den Heuvel MJ, et al. , Menstrual cycle hormones induce changes in functional interactions between lymphocytes and decidual vascular endothelial cells. J Clin Endocrinol Metab, 2005. 90(5): p. 2835–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Li Y, et al. , Tim-3 signaling in peripheral NK cells promotes maternal-fetal immune tolerance and alleviates pregnancy loss. Sci Signal, 2017. 10(498). [DOI] [PubMed] [Google Scholar]
- 213.Norman JE, et al. , Effect of prolonged in vivo administration of progesterone in pregnancy on myometrial gene expression, peripheral blood leukocyte activation, and circulating steroid hormone levels. Reprod Sci, 2011. 18(5): p. 435–46. [DOI] [PubMed] [Google Scholar]
- 214.Giaglis S, et al. , Multimodal Regulation of NET Formation in Pregnancy: Progesterone Antagonizes the Pro-NETotic Effect of Estrogen and G-CSF. Front Immunol, 2016. 7: p. 565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Jitprasertwong P, et al. , Female sex hormones modulate Porphyromonas gingivalis lipopolysaccharide-induced Toll-like receptor signaling in primary human monocytes. J Periodontal Res, 2016. 51(3): p. 395–406. [DOI] [PubMed] [Google Scholar]
- 216.Polan ML, et al. , Progesterone and estradiol modulate interleukin-1 beta messenger ribonucleic acid levels in cultured human peripheral monocytes. J Clin Endocrinol Metab, 1989. 69(6): p. 1200–6. [DOI] [PubMed] [Google Scholar]
- 217.Sun Y, et al. , miR-155 mediates suppressive effect of progesterone on TLR3, TLR4-triggered immune response. Immunol Lett, 2012. 146(1–2): p. 25–30. [DOI] [PubMed] [Google Scholar]
- 218.Liang J, et al. , Progesterone regulates mouse dendritic cells differentiation and maturation. Int Immunopharmacol, 2006. 6(5): p. 830–8. [DOI] [PubMed] [Google Scholar]
- 219.Huck B, et al. , Pregnancy associated hormones modulate the cytokine production but not the phenotype of PBMC-derived human dendritic cells. Eur J Obstet Gynecol Reprod Biol, 2005. 122(1): p. 85–94. [DOI] [PubMed] [Google Scholar]
- 220.Kyurkchiev D, et al. , Female sex steroid hormones modify some regulatory properties of monocyte-derived dendritic cells. Am J Reprod Immunol, 2007. 58(5): p. 425–33. [DOI] [PubMed] [Google Scholar]
- 221.Segerer SE, et al. , Impact of female sex hormones on the maturation and function of human dendritic cells. Am J Reprod Immunol, 2009. 62(3): p. 165–73. [DOI] [PubMed] [Google Scholar]
- 222.Yang M, et al. , Decline of Plasmacytoid Dendritic Cells and Their Subsets in Normal Pregnancy Are Related with Hormones. J Reprod Med, 2015. 60(9–10): p. 423–9. [PubMed] [Google Scholar]
- 223.Azeez JM, et al. , New insights into the functions of progesterone receptor (PR) isoforms and progesterone signaling. Am J Cancer Res, 2021. 11(11): p. 5214–5232. [PMC free article] [PubMed] [Google Scholar]
- 224.Tan H, et al. , Progesterone receptor-A and -B have opposite effects on proinflammatory gene expression in human myometrial cells: implications for progesterone actions in human pregnancy and parturition. J Clin Endocrinol Metab, 2012. 97(5): p. E719–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Wei LL, et al. , 5’-Heterogeneity in human progesterone receptor transcripts predicts a new amino-terminal truncated “C”-receptor and unique A-receptor messages. Mol Endocrinol, 1990. 4(12): p. 1833–40. [DOI] [PubMed] [Google Scholar]
- 226.Condon JC, et al. , Up-regulation of the progesterone receptor (PR)-C isoform in laboring myometrium by activation of nuclear factor-kappaB may contribute to the onset of labor through inhibition of PR function. Mol Endocrinol, 2006. 20(4): p. 764–75. [DOI] [PubMed] [Google Scholar]
- 227.Saner KJ, et al. , Cloning and expression of a novel, truncated, progesterone receptor. Mol Cell Endocrinol, 2003. 200(1–2): p. 155–63. [DOI] [PubMed] [Google Scholar]
- 228.Price TM and Dai Q, The Role of a Mitochondrial Progesterone Receptor (PR-M) in Progesterone Action. Semin Reprod Med, 2015. 33(3): p. 185–94. [DOI] [PubMed] [Google Scholar]
- 229.Zhu Y, Bond J, and Thomas P, Identification, classification, and partial characterization of genes in humans and other vertebrates homologous to a fish membrane progestin receptor. Proc Natl Acad Sci U S A, 2003. 100(5): p. 2237–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Valadez-Cosmes P, et al. , Membrane progesterone receptors in reproduction and cancer. Mol Cell Endocrinol, 2016. 434: p. 166–75. [DOI] [PubMed] [Google Scholar]
- 231.Cahill MA, Progesterone receptor membrane component 1: an integrative review. J Steroid Biochem Mol Biol, 2007. 105(1–5): p. 16–36. [DOI] [PubMed] [Google Scholar]
- 232.Dosiou C, et al. , Expression of membrane progesterone receptors on human T lymphocytes and Jurkat cells and activation of G-proteins by progesterone. J Endocrinol, 2008. 196(1): p. 67–77. [DOI] [PubMed] [Google Scholar]
- 233.Ndiaye K, et al. , Progesterone effects on lymphocytes may be mediated by membrane progesterone receptors. J Reprod Immunol, 2012. 95(1–2): p. 15–26. [DOI] [PubMed] [Google Scholar]
- 234.Kuwabara T, et al. , Regulation of T-Cell Signaling by Post-Translational Modifications in Autoimmune Disease. Int J Mol Sci, 2018. 19(3). [DOI] [PMC free article] [PubMed] [Google Scholar]


