Abstract
Functional properties caused by TP53 mutations are involved in cancer development and progression. Although most of the mutations lose normal p53 functions, some of them, gain-of-function (GOF) mutations, exhibiting novel oncogenic functions. No reports have analyzed the impact of TP53 mutations on the gene expression profile of the p53 signaling pathway across cancer types. This study is a cross-cancer type analysis of the effects of TP53 mutations on gene expression. A hierarchical cluster analysis of the expression profile of the p53 signaling pathway classified 21 cancer types into two clusters (A1 and A2). Changes in the expression of cell cycle-related genes and MKI67 by TP53 mutations were greater in cluster A1 than in cluster A2. There was no distinct difference in the effects between GOF and non-GOF mutations on the gene expression profile of the p53 signaling pathway.
Subject terms: Gene expression, Gene regulation, Cancer genomics
Introduction
TP53, encoding p53, is the most frequently mutated gene in human tumors1–3, and it is estimated that > 50% of all human cancers show mutations2,4. p53 regulates the expression of downstream genes as a transcriptional activating factor through specific DNA binding sites5. Its most well-known functions include anti-proliferative effects, such as cell cycle arrest6–8, DNA repair9,10, apoptosis11, and cellular senescence1–3. Additionally, p53 induces cell death by apoptosis for irreparable DNA damage11. During DNA damage, the activated p53 promotes DNA repair by inhibiting cell proliferation and arresting the cell cycle. It also prevents the accumulation of genetic mutations by eliminating unrepairable cells. Therefore, p53 is called “the guardian of the genome”11. p53 reportedly suppresses the IGF-1/mTOR pathway12 and enhances antitumor effects through exosome secretion13.
The category classifications and gene lists based on each function of p53 can be reviewed in the “Kyoto Encyclopedia of Genes and Genomes” (KEGG, http://www.genome.jp/kegg/). KEGG is an integrated bioinformatics database that links genomes with various functional information including pathways (interaction networks between molecules in metabolic and signaling pathways), and continues to be manually developed and updated based on literature information14–16. Information on various KEGG pathways, including the p53 signaling pathway, may be used for pathway analyses and enrichment analyses. In fact, as of February 1, 2023, a PubMed search for “kegg” and “pathway analysis” yielded 3,561 reports from 2005 to 2023 (2,007 reports from 2020 to 2023), and the search for “kegg” and “enrichment analysis” yielded 5,725 reports from 2006 to 2023 (4,370 reports from 2020 to 2023). This indicates that KEGG pathway database is recognized as an important database even after 2020. The p53 signaling pathway in the KEGG pathway database (https://www.genome.jp/kegg/pathway.html) lists 67 genes as genes on the p53 signaling pathway in September 2021. In the category classification, the genes are first classified into categories of genes upstream and downstream of the p53 signaling pathway. The downstream gene categories are further classified by their functions into apoptosis-, cell cycle-, DNA repair and damage prevention-, exosome secretion-, angiogenesis and metastasis formation suppression-, IGF-1/mTOR pathway suppression-, and p53 negative feedback-related categories.p21 is required for examining the functional activity of p53. p21 is a protein downstream of the p53 signaling pathway and is involved in cell cycle inhibition and cellular senescence through p53-dependent and -independent pathways17,18. Since p21 activity is greatly affected by the regulation of its expression by p537, it is considered a useful surrogate marker when examining the functional activity of p53. The International Agency for Research on Cancer (IARC, https://www.iarc.who.int/) database has published sequence-specific transcriptional activities of mutant p53 of all 2341 variants of mutant p53 with one amino acid substitution for p21, MDM2, BAX, 14-3-3σ, AIP1, GADD45, NOXA and p53R219. The TP53 database was transferred from IARC to US National Cancer Institute on October 25, 2021. They are now available on https://tp53.isb-cgc.org/.
The mutations in the TP53 gene result in loss of normal p53 functions such as senescence and apoptosis, and affect the carcinogenic process20. TP53 mutations have been reported as a poor prognostic factor in breast cancer21, head and neck cancer22, and hematologic malignancies23. Contrarily, there are many reports showing no association between TP53 mutations and prognosis in cancers, such as colon24, lung25, and bladder cancers26. Several reports suggest that TP53 mutations are a favorable prognostic factor in brain tumors27 and ovarian cancer28; thus, the prognostic impact of TP53 mutations may differ by cancer type29. Additionally, TP53 mutations have been reported to be useful as a marker of response to chemotherapy in lung30 and breast cancers31. Contrarily, they have also been found to be a marker of refractory to chemotherapy in head and neck cancers32, suggesting that the effects of TP53 mutations on drug sensitivity may also differ by cancer type29. However, no report to date has analyzed these points across different cancer types.
The majority of TP53 mutations are missense mutations. Some studies have shown that some missense mutations in p53 not only deactivate the tumor suppressor function of p53, but also acquire novel oncogenic functions such as tumor cell proliferation, antiapoptotic effects, and promotion of angiogenesis and metastasis formation. These are termed gain-of-function (GOF) mutations, a subtype of TP53 mutations4,33–36. The concept of GOF mutations is well established, but no clear definition has been established so far37. Since GOF is a mutation that gains a new function as described above in addition to the loss of p53 function, it is not a simple counterpart of a loss-of-function mutation37. GOF mutations are more likely to accumulate p53 in the nucleus than non-gain-of-function (non-GOF) mutations, and they induce extensive loss of transcriptional function. Contrarily, many non-GOF mutations have been reported to preserve a certain level of transcriptional function and to maintain normal activation of p53-regulated genes to some degrees37,38. Furthermore, GOF mutations have been reported to be a possible poor prognostic factor in glioblastomas39 and T-cell lymphomas40. The differences in TP53 mutation subtypes (e.g., GOF vs. non-GOF mutations) may have different effects on other gene expressions in different cancer types41,42. However, there have been no reports comparing these differences across cancer types.
Based on this background, we hypothesized that the changes in the gene expression profile of the p53 signaling pathway caused by TP53 mutations differ among cancer types. We also hypothesized that the effects of different TP53 mutation subtypes (GOF vs. non-GOF mutations) on the gene expression profile of the p53 signaling pathway would differ by cancer type. Then, we verified these hypotheses by comparing the changes in the gene expression profile of the p53 signaling pathway caused by TP53 mutations across cancer types.
Results
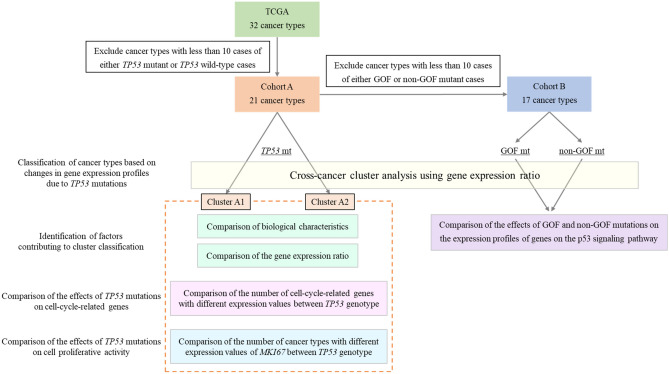
The study workflow is shown in Fig. 1.
Figure 1.
The workflow of analyses. TCGA, The Cancer Genome Atlas; TP53 mt, TP53 mutation; TP53 wt, TP53 wild-type; GOF mt, TP53 gain-of-function mutation; non-GOF mt, TP53 non-gain-of-function mutation.
Comparison of p53 transcriptional activity
The transcriptional activity of mutant p53 on p21 was significantly correlated with that of mutant p53 on all other target genes (all P < 0.0001, r = 0.70–0.83, Supplementary Tables S1 and S2).
Cancer types for analyses
The cases of all 32 cancer types listed in The Cancer Genome Atlas (TCGA, https://portal.gdc.cancer.gov/) database were classified into two groups according to the presence or absence of TP53 mutations (Table 1). As a result, 21 cancer types (adrenocortical carcinoma, bladder urothelial carcinoma, breast invasive carcinoma, cervical squamous cell carcinoma, colon adenocarcinoma, esophageal carcinoma, glioblastoma multiforme, head and neck squamous cell carcinoma, kidney chromophobe, brain lower grade glioma, liver hepatocellular carcinoma, lung adenocarcinoma, lung squamous cell carcinoma, mesothelioma, ovarian serous cystadenocarcinoma, pancreatic adenocarcinoma, prostate adenocarcinoma, rectum adenocarcinoma, sarcoma, stomach adenocarcinoma, and uterine corpus endometrial carcinoma) contained 10 or more cases each of TP53 mutant and wild-type cases. This cohort of cancer types was defined as “Cohort A.” In cohort A, the number of TP53 mutant cases was 2,735, whereas the number of TP53 wild-type cases was 3,618. The TP53 mutations identified in this study consisted of 2257 (71.8%) missense, 452 (14.4%) nonsense, and 433 (13.8%) frameshift mutations. For each cancer type in cohort A, TP53 mutant cases were further classified into GOF and non-GOF mutant cases (Supplementary Table S3). There were 1,169 (37.2%) GOF and 1973 (62.8%) non-GOF mutations (Supplementary Table S4). As a result, 17 cancer types (bladder urothelial carcinoma, breast invasive carcinoma, cervical squamous cell carcinoma, colon adenocarcinoma, esophageal carcinoma, glioblastoma multiforme, head and neck squamous cell carcinoma, brain lower grade glioma, liver hepatocellular carcinoma, lung adenocarcinoma, lung squamous cell carcinoma, ovarian serous cystadenocarcinoma, pancreatic adenocarcinoma, prostate adenocarcinoma, rectum adenocarcinoma, sarcoma, and stomach adenocarcinoma) contained 10 or more GOF mutant and non-GOF mutant cases each, and we defined this cohort of cancer types as “Cohort B.”
Table 1.
Histological type, developmental origin, TP53 mutation rate, GOF mutation rate, and cluster classification for each cancer type in cohort A.
| Cancer type | Histlogical type | Developmental origin | Ratio of TP53 mt (%) | Ratio of GOF (%) | Cluster classification |
|---|---|---|---|---|---|
| ACC | Non-adenocarcinoma | Mesoderm | 15.9 | 9.1 | A1 |
| BLCA | Non-adenocarcinoma | Endoderm | 47.0 | 41.3 | A1 |
| BRCA | Adenocarcinoma | Ectoderm | 31.0 | 34.2 | A1 |
| ESCA | Adenocarcinoma | Endoderm | 86.1 | 46.6 | A1 |
| GBM | Non-adenocarcinoma | Ectoderm | 31.4 | 53.5 | A1 |
| KICH | Non-adenocarcinoma | Mesoderm | 24.6 | 21.4 | A1 |
| LGG | Non-adenocarcinoma | Ectoderm | 46.4 | 61.1 | A1 |
| LIHC | Non-adenocarcinoma | Endoderm | 27.5 | 42.2 | A1 |
| LUAD | Adenocarcinoma | Endoderm | 44.7 | 23.2 | A1 |
| LUSC | Non-adenocarcinoma | Endoderm | 83.0 | 32.6 | A1 |
| MESO | Non-adenocarcinoma | Mesoderm | 15.1 | 23.1 | A1 |
| OV | Adenocarcinoma | Mesoderm | 94.1 | 35.9 | A1 |
| PAAD | Non-adenocarcinoma | Endoderm | 49.6 | 42.9 | A1 |
| PRAD | Adenocarcinoma | Mesoderm | 9.5 | 32.6 | A1 |
| SARC | Non-adenocarcinoma | Mesoderm | 32.5 | 32.8 | A1 |
| STAD | Adenocarcinoma | Endoderm | 43.7 | 38.3 | A1 |
| UCEC | Adenocarcinoma | Mesoderm | 35.1 | 49.7 | A1 |
| CESC | Non-adenocarcinoma | Mesoderm | 7.7 | 19.0 | A2 |
| COAD | Adenocarcinoma | Endoderm | 52.0 | 48.5 | A2 |
| HNSC | Non-adenocarcinoma | Ectoderm | 66.3 | 11.2 | A2 |
| READ | Adenocarcinoma | Endoderm | 71.4 | 54.4 | A2 |
Genes on the p53 signaling pathway for analyses
The 67 genes registered as genes on the p53 signaling pathway and category classification defined by KEGG for each gene are listed in Supplementary Table S5. For these genes, the median expression of each gene was calculated for each cancer type. The median expression of each gene and the number of cancer types for which the median expression was less than the expression threshold (Transcripts Per Million (TPM) value 1.043) are shown in Supplementary Table S6. Genes whose median expression was below the threshold in more than half of the cancer types were common in cohorts A and B (ADGRB1, IGF1, RPRM, TP53AIP1). Therefore, 63 genes were selected for analyses in this study.
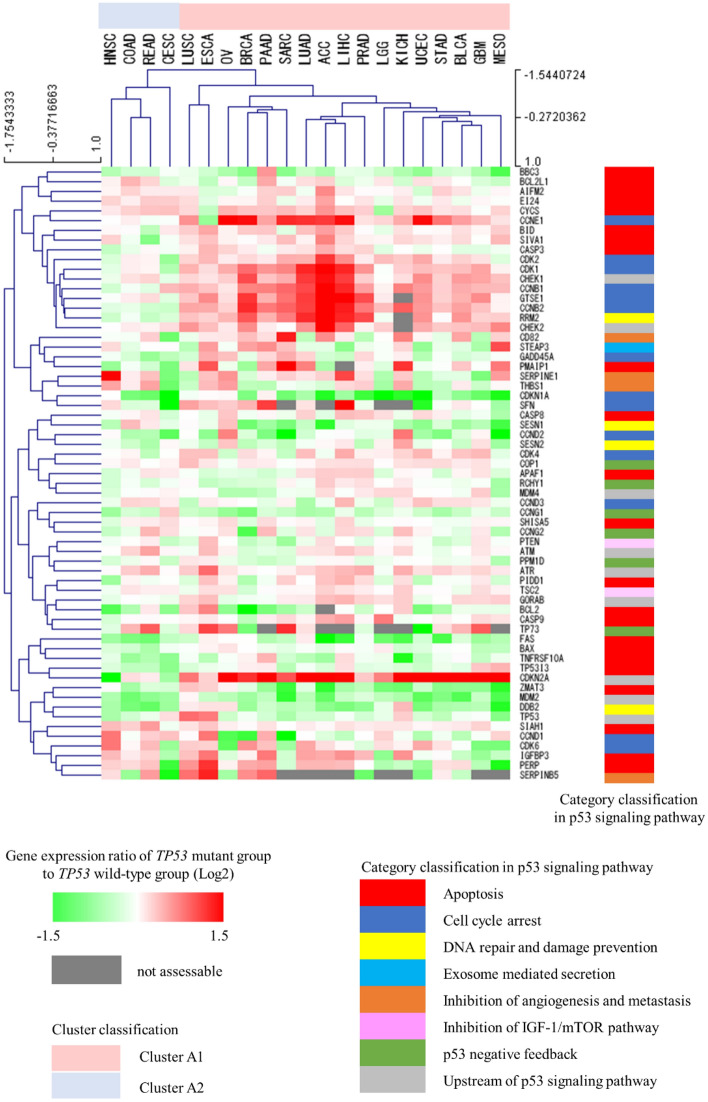
A cross-cancer unsupervised hierarchical cluster analysis based on changes in the gene expression profile of the p53 signaling pathway
To examine whether the changes in the gene expression profile of the p53 signaling pathway caused by TP53 mutations differ among cancer types, we performed a cross-cancer unsupervised hierarchical cluster analysis using the gene expression ratios of the p53 signaling pathway in cohort A. As a result, the 21 cancer types in cohort A were classified into clusters comprising 17 (cluster A1) and 4 (cluster A2) cancer types. Cluster A1 included adrenocortical carcinoma, bladder urothelial carcinoma, breast invasive carcinoma, esophageal carcinoma, glioblastoma multiforme, kidney chromophobe, brain lower grade glioma, liver hepatocellular carcinoma, lung adenocarcinoma, lung squamous cell carcinoma, mesothelioma, ovarian serous cystadenocarcinoma, pancreatic adenocarcinoma, prostate adenocarcinoma, sarcoma, stomach adenocarcinoma, and uterine corpus endometrial carcinoma, whereas cluster A2 included cervical squamous cell carcinoma, colon adenocarcinoma, head and neck squamous cell carcinoma, and rectum adenocarcinoma (Fig. 2).
Figure 2.
Unsupervised cross-cancer hierarchical clustering. This cluster analysis was performed using the complete linkage method with Pearson correlation distance. ACC Adrenocortical carcinoma, BLCA Bladder urothelial carcinoma, BRCA Breast invasive carcinoma, CESC Cervical squamous cell carcinoma, COAD Colon adenocarcinoma, ESCA Esophageal carcinoma, GBM Glioblastoma multiforme, HNSC Head and neck squamous cell carcinoma, KICH Kidney chromophobe, LGG Brain lower grade glioma, LIHC Liver hepatocellular carcinoma, LUAD Lung adenocarcinoma, LUSC Lung squamous cell carcinoma, MESO Mesothelioma, OV Ovarian serous cystadenocarcinoma, PAAD Pancreatic adenocarcinoma, PRAD Prostate adenocarcinoma, READ Rectum adenocarcinoma, SARC Sarcoma, STAD Stomach adenocarcinoma, UCEC Uterine corpus endometrial carcinoma.
Identification of factors contributing to cluster A1 and A2 classifications
We explored the factors contributing to the classification of clusters A1 and A2. The histological type, developmental origin, TP53 mutation rate, GOF mutation rate, and cluster classification for each cancer type are shown in Table 1. An examination of the histological bias between the two clusters revealed no statistically significant difference in the proportion of adenocarcinoma and non-adenocarcinoma (P = 1.0000, Supplementary Table S7). The same study of developmental origin bias found no significant difference between the two clusters (P = 1.0000, Supplementary Table S8). We also examined whether there was any bias in the proportion of TP53 genotype between the two clusters and found no statistically significant differences in either the proportion of TP53 (P = 0.4737) or GOF (P = 0.7055) mutant cases. We additionally examined whether the proportion of hotspot mutation differed between clusters A1 and A2, but there were no significant differences (P = 0.6945, Supplementary Table S9).
The expression ratios of each gene on the p53 signaling pathway were compared between clusters A1 and A2. Fifteen genes had significantly different gene expression ratios between the two clusters (Supplementary Table S10). These extracted genes were classified by using the KEGG category: six genes were constitutive for the cell cycle-related category, and four genes each were constitutive for the category upstream of the p53 signaling pathway and for apoptosis-related category. In each of these categories, the extracted genes accounted for 42.9% (6/14) of the genes related to the cell cycle, 44.4% (4/9) of the genes upstream of the p53 signaling pathway, and 17.3% (4/23) of the genes related to apoptosis (Table 2).
Table 2.
Results of comparison of gene expression between cluster A1 and A2 (counted for each category classification).
| Category classification in the KEGG p53 pathway | Number of component genes | Number of genes with significant differences between cluster A1 and A2 (%) |
|---|---|---|
| Cell cycle arrest | 14 | 6 (42.9) |
| Upstream of p53 signaling pathway | 9 | 4 (44.4) |
| Apoptosis | 23 | 4 (17.3) |
| DNA repair and damage prevention | 4 | 1 (25.0) |
| Inhibition of angiogenesis and metastasis | 4 | 0 (0) |
| Inhibition of IGF-1/mTOR pathway | 2 | 0 (0) |
| p53 negative feedback | 6 | 0 (0) |
| Exosome mediated secretion | 1 | 0 (0) |
| Total | 63 | 15 |
Comparison of expression values of cell cycle-related genes in each cancer type in cohort A
Based on the results obtained from the analyses described so far, it was suggested that cell proliferative activity might be important as a phenotypic difference between clusters A1 and A2 caused by TP53 mutations. We, therefore, compared the expression values of 14 cell cycle-related genes on the p53 signaling pathway between the TP53 mutant and TP53 wild-type groups for each cancer type. The number of genes with significantly different expression values between the two groups was significantly higher in the 17 cancer types in cluster A1 than in the four cancer types in cluster A2 (P = 0.0429, Supplementary Table S11).
Comparison of MKI67 expression values in each cancer type in cohort A
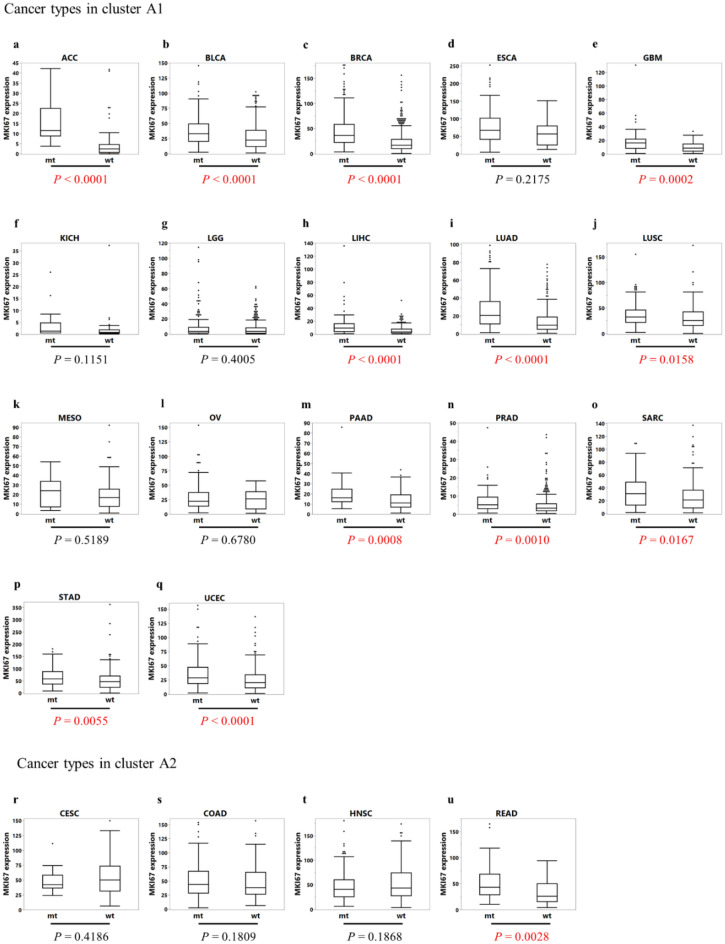
The gene expression value of MKI67, a marker reflecting cell proliferative activity44,45, was compared between the TP53 mutant and TP53 wild-type groups in each cancer type (Fig. 3a–u). The number of cancer types with significantly upregulated MKI67 expression levels in the TP53 mutant group in comparison to the TP53 wild-type group was 12/17 (70.6%) in cluster A1 and 1/4 (25.0%) in cluster A2 (P = 0.2528, Table 3).
Figure 3.
Box plot showing a comparison of the MKI67 expression values between the TP53 mutant and TP53 wild-type groups for each cancer type in cohort A. Each P-value was calculated using the Wilcoxon rank sum test. (a) ACC Adrenocortical carcinoma, (b) BLCA Bladder urothelial carcinoma, (c) BRCA Breast invasive carcinoma; (d) ESCA Esophageal carcinoma, (e) GBM Glioblastoma multiforme, (f) KICH Kidney chromophobe, (g) LGG Brain lower grade glioma, (h) LIHC Liver hepatocellular carcinoma, (i) LUAD Lung adenocarcinoma, (j) LUSC Lung squamous cell carcinoma, (k) MESO Mesothelioma, (l) OV Ovarian serous cystadenocarcinoma, (m) PAAD Pancreatic adenocarcinoma, (n) PRAD Prostate adenocarcinoma, (o) SARC Sarcoma, (p) STAD Stomach adenocarcinoma, (q) UCEC Uterine corpus endometrial carcinoma, (r) CESC Cervical squamous cell carcinoma, (s) COAD Colon adenocarcinoma, (t) HNSC Head and neck squamous cell carcinoma, (u) READ Rectum adenocarcinoma, mt TP53 mutant group, wt TP53 wild-type group.
Table 3.
Comparison of the number of cancer types with a significant difference in MKI67 expression by TP53 genotype between cluster A1 and A2.
| Is there a significant difference in MKI67 expression between TP53 mt and wt? | ||||
|---|---|---|---|---|
| Number of applicable cancer types (%) | Total | P value | ||
| Yes | No | |||
| Cluster A1 | 12 (70.6) | 5 (29.4) | 17 | 0.2528 |
| Cluster A2 | 1 (25.0) | 3 (75.0) | 4 | |
| Total | 13 (61.9) | 2 (38.1) | 21 | |
A cross-cancer unsupervised hierarchical cluster analysis based on the changes in the gene expression profile of the p53 signaling pathway caused by TP53 mutation subtypes
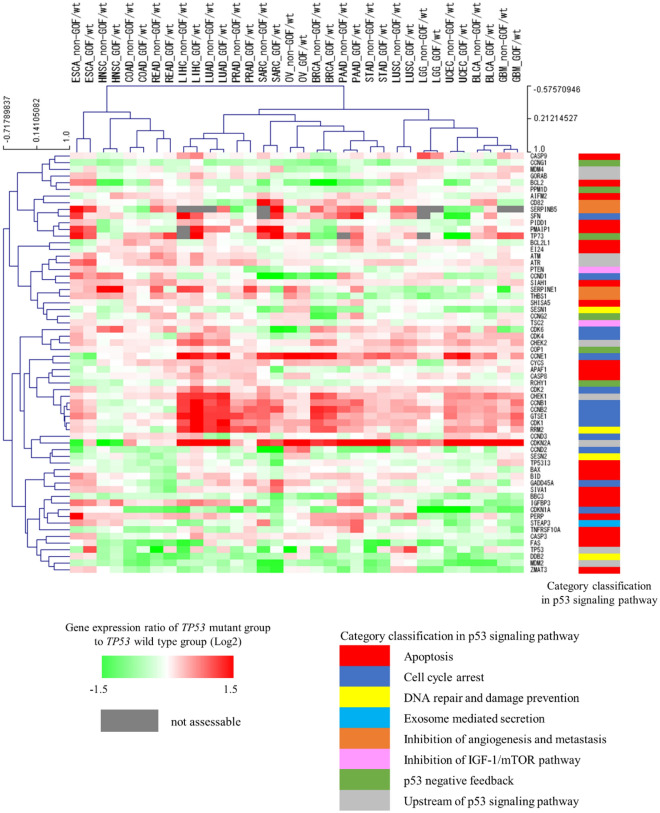
To examine whether GOF and non-GOF mutations have different effects on the gene expression profile of the p53 signaling pathway, the gene expression ratios of the GOF mutant group to the TP53 wild-type group and the non-GOF mutant group to the TP53 wild-type group were calculated for each gene by cancer types in cohort B. Then, we performed a cross-cancer unsupervised hierarchical cluster analysis using the calculated gene expression ratios. The results showed that GOF and non-GOF mutations of the same cancer type were placed nearest to each other in all 17 cancer types (Fig. 4).
Figure 4.
Unsupervised cross-cancer hierarchical clustering. This cluster analysis was performed using the complete linkage method with Pearson correlation distance. BLCA Bladder urothelial carcinoma, BRCA Breast invasive carcinoma, COAD Colon adenocarcinoma, ESCA Esophageal carcinoma, GBM Glioblastoma multiforme, HNSC Head and neck squamous cell carcinoma, LGG Brain lower grade glioma, LIHC Liver hepatocellular carcinoma, LUAD Lung adenocarcinoma, LUSC Lung squamous cell carcinoma, OV Ovarian serous cystadenocarcinoma, PAAD Pancreatic adenocarcinoma, PRAD Prostate adenocarcinoma, READ Rectum adenocarcinoma, SARC Sarcoma, STAD Stomach adenocarcinoma, UCEC Uterine corpus endometrial carcinoma, GOF/wt Gene expression ratio (Log2) of TP53 gain-of-function mutant group to TP53 wild-type group, non-GOF/wt Gene expression ratio (Log2) of TP53 non-gain-of-function mutant group to TP53 wild-type group.
Discussion
In this study, we examined the effects of TP53 mutations on the gene expression profile of the p53 signaling pathway and cell proliferative activity across cancer types. The results indicated that the cancer types can be classified into two major groups based on the magnitude of gene expression changes related to the cell cycle and cell proliferative activity caused by TP53 mutations. Furthermore, there was no distinct difference in the effects of GOF and non-GOF mutations on the gene expression profile of the p53 signaling pathway.
Because mutant p53 transcriptional activity on p21 correlates very highly with the transcriptional activity of mutant p53 on each of the other genes, the transcriptional activity on p21 was used to define the TP53 genotype. Genes in the p53 signaling pathway listed in KEGG do not include all genes associated with p53; however, because this gene set contains the primary functions of p53 and the major genes involved in this pathway, we believe that it is reasonable to use them for assessing gene expression changes caused by TP53 gene mutations. In addition, the use of an existing gene set eliminates selection bias during the selection of genes for analysis. Furthermore, the phenotypic changes caused by TP53 mutations are not solely defined by the expression of genes directly downstream of TP53 gene, but are also likely to be affected by the expression levels of genes further downstream. This is also applicable to upstream genes that receive negative feedback from p53 and their related genes. Moreover, changes in the expression of the TP53 gene itself caused by mutations may differ among the various cancer types. For these reasons, we considered it necessary to include not only the genes directly regulated by p53 but also downstream and related genes in our analysis, which resulted in the use of the entire KEGG gene set for the p53 signaling pathway. A cross-cancer unsupervised hierarchical cluster analysis was performed to determine whether the changes in the gene expression profile of the p53 signaling pathway due to TP53 mutations differ among cancer types. We found that the 21 cancer types in cohort A could be classified into two major clusters (A1 and A2) based on the differential effects of TP53 mutations on the gene expression profile of the p53 signaling pathway. These two clusters were not characterized by the differences in histological type, developmental origin, TP53 genotype proportion, the proportion of hotspot mutations. There have been several reports on the relationship between the type of GOF and the tumor spectrum in which they occur46,47. The results of the present study were consistent with previous reports, with a higher percentage of GOF with hotspot mutations, R249 in LIHC48,49 and R273 in LGG50,51. As genes for which gene expression changes due to TP53 mutations contribute to cluster A1 and A2 classifications, 15 genes were extracted, consisting mainly of cell cycle-related genes and genes upstream of the p53 signaling pathway. Among the 15 genes, cell cycle-related genes accounted for the largest number (6 genes), suggesting that they might have an important role on the classification of the two clusters. The four genes upstream of the p53 signaling pathway were CDKN2A, CHEK1, CHEK2, and GORAB. Although CDKN2A regulates p53 expression via MDM2, it receives repression feedback from p53 and an inverse correlation between CDKN2A expression and p53 function has been reported in human tumor cell lines52. CHEK1 phosphorylates p53 at multiple sites, while also receiving feedback from p53 to repress its expression53. Furthermore, GORAB inhibits p53 ubiquitination via MDM2 and receives feedback from p53 to repress its expression54. Based on these reports, we hypothesized that this feedback was the basis for why upstream genes of p53 were extracted in this study. CDKN2A encodes two transcripts (p16INK4a and p14ARF) with different transcription start sites55. p16INK4a inhibits cell cycle progression by directly blocking the interaction between CDK4/6 and cyclin D56. p14ARF inhibits p53 degradation through direct binding to MDM257. Both CHK1 and CHK2 encoded by CHEK1 and CHEK2, respectively, inhibit the activation of CDC25, an important factor in G2/M phase progression58. Furthermore, SCYL1BP1, encoded by GORAB, promotes MDM2 degradation and inhibits cell cycle progression in the G1/S phase59. Therefore, all of the four abovementioned genes have inhibitory functions on the cell cycle, suggesting that the cell cycle might have a particularly large impact on the classification of the two clusters among the functions of p53 signaling pathway. Additionally, TP53 mutations reportedly accelerate cell cycle pathways in breast cancer and lung adenocarcinoma, which were classified as cluster A1 in this study60. Contrarily, the acceleration of cell cycle pathways by TP53 mutations was not observed in colon cancer and squamous cell carcinoma of the head and neck, which were classified as cluster A260. These reports support the validity of the results obtained in this study. Based on the abovementioned points, we examined the changes in the expression of cell cycle-related genes on the p53 signaling pathway due to TP53 mutations between clusters A1 and A2. Altered expression levels were observed more in the cell cycle-related genes in cluster A2 than in cluster A1, suggesting that the changes in cell proliferative activity might be particularly important as a phenotypic difference caused by TP53 mutations between clusters A1 and A2.
Ki-67 has been reported as a marker of cell proliferative activity in various cancer types61–63. MKI67, the gene encoding Ki-67, is located on chromosome 10q25. MKI67 is expressed throughout the cell cycle except during the G0 phase64. The expression of MKI67, as well as Ki-67, is known as a cell proliferative marker in various cancer types44,45. Our results showed that, in many cancer types of cluster A1, the expression level of MKI67 was significantly higher in the TP53 mutant group than in the TP53 wild-type group. Contrarily, in most cancer types in cluster A2, the expression of MKI67 did not differ between the TP53 mutant and TP53 wild-type groups. From this result, the cell proliferative activity in the TP53 mutant group might be increased as compared to that of the TP53 wild-type group in cluster A1, which had relatively large changes in expression of cell cycle-related genes on the p53 signaling pathway due to the TP53 mutations. Contrarily, the cell proliferative activity in the TP53 mutant group might be less changed compared to the TP53 wild-type group in cluster A2, which had relatively small changes in the expression of cell cycle-related genes due to the TP53 mutations.
It has been reported that the expression status of MKI67 positively correlates with tumor growth and malignancy65. Several retrospective analyses have examined the prognostic significance of MKI67 expression status and reported that a high expression of MKI67 in cancer tissues was associated with early recurrence after radical resection and high malignancy of the tumor66–68. MKI67 expression level correlated with the sensitivity to anticancer drugs in several cancer types, such as breast69 and ovarian cancers70. Based on the results of this study and these findings, we hypothesized that TP53 mutations in cancer types of cluster A1 increase tumor malignancy and shorten the prognosis but they increase sensitivity to anticancer drugs compared to cancer types of cluster A2. We tried to validate the association between TP53 mutations and prognosis, including response to chemotherapy, in the TCGA cohort, but the validation was difficult because of the insufficient prognostic follow-up period, the extremely small number of cases in many cancer types when matched for case background (age, sex, stage, etc.) between patients with wild-type and mutant TP53, and the lack of treatment data (treatment line, drugs, etc.). Instead, we found some reports that supported our findings of the prognosis, such as reports of poor prognosis for the TP53 mutation group in breast cancer21,71 and bladder cancer72, and no prognostic impact of TP53 status in colon cancer and rectal cancer24,73. Moreover, we found some reports that supported our findings of the response to chemotherapy, such as reports of TP53 mutation as a marker of chemotherapy response in breast cancer31and bladder cancer74, and as a marker of resistance to chemotherapy in head and neck cancer32,75. Data from clinical sequencing and the efficacy of chemotherapy and prognosis are now being collected in Japan. We are planning additional studies using these data to validate our hypothesis. The results of this study suggest that the cascade mediated by TP53 mutations may be responsible for the poor prognosis in cancer types of cluster A1. Therefore, we expect that molecularly targeted therapies reactivating mutant p53 inactivation, such as eprenetapopt for myelodysplastic syndromes and COTI-2 for triple negative breast cancer, may be more sensitive to the cancers in cluster A1 than in cluster A2.Therefore, our study findings were considered to be of great significance in the context of expanding personalized precision medicine based on the information of genetic mutations, including TP53 genotype, through the introduction of cancer gene panel examinations into clinical practice.
GOF and non-GOF mutations are known to result in loss of function for downstream genes. However, in the first part of this study, we showed that the impact of TP53 mutations on gene expression in the p53 signaling pathway listed in KEGG among various cancer types, despite the fact that TP53 mutations (GOF + non-GOF mutations) exhibit loss of function. This suggests that the effects of GOF and non-GOF mutations on the genes of the p53 signaling pathway listed in KEGG may also differ across cancer types; thus, we conducted the latter part of the analysis to examine this point. To date, there have been no reports comparing and validating whether there are differences in effects on the overall p53 pathway between GOFs and non-GOFs across various cancers. This is the first report to examine the impact of distinct TP53 mutation subtypes (GOF and non-GOF mutations) on the gene expression profile of the p53 signaling pathway through a cross-cancer expression analysis. We found that there was no clear difference between them. The effects of GOF mutations on the expression of genes other than TP53 have been reported, such as the upregulation of EGFR in lung cancer cell lines76 and of HSPG2 in mouse models of pancreatic cancer77. Furthermore, genes reported to be associated with GOF, such as EGF36, PDGF78, and VEGF79, which are genes involved in growth factor signaling, and KLF1780, a negative regulator of metastasis and the epithelial-mesenchymal transition, are not included in the p53 pathway listed in KEGG. This suggests that GOF may have a significant impact on genes other than the classical p53 signaling pathway of KEGG. In addition, GOF and non-GOF mutations reportedly have different phenotypic outcomes. For example, prostate cell lines with GOF mutations have higher cell proliferative activity under androgen deprivation than those with non-GOF mutations42. GOF and non-GOF mutations also have different prognostic effects depending on the primary site of colorectal cancer41. Our study results showed no distinct difference between GOF and non-GOF mutations in their effects on the changes in the gene expression profile of the p53 signaling pathway. To clarify the mechanism by which different TP53 mutation subtypes have different phenotypic effects, further studies that combine approaches based on a comparative analysis of comprehensive gene expression profiles, genetic changes due to mutations in tumor suppressor genes and oncogenes other than TP53, and epigenetic changes including DNA methylation abnormalities should be required. Furthermore, temperature-sensitive TP53 mutations have been reported81, and an important relationship between TP53 mutations and host immune responses was found previously82. Thus, it will be necessary to study the phenotypic effects of these mutations in the future.
This study has several limitations. First, the case backgrounds, such as sex, age, and tumor stage, were not standardized across cancer types because of the retrospective design of the present study. Second, the use of the median gene expression value for each cancer type enabled a cross-cancer analysis, but the small sample size (21 cancer types in cohort A and 17 cancer types in cohort B) provides insufficient power for statistical analyses. Third, since some cancer types were excluded from this study, we cannot rule out the possibility that some cancer types do not belong to either cluster A1 or A2 obtained in this study. Fourth, although the GOF and non-GOF mutations were defined based on the IARC mutation data, some TP53 mutations have not yet been considered in terms the GOF mutation characteristics. Therefore, some of the mutations defined as non-GOF mutations in this study may possibly include mutations that are actually GOF mutations. Fifth, other various biological factors may influence the changes in the gene expression profile of the p53 signaling pathway, such as the differences in TP53 mutation patterns, coexistence of other genetic mutations, and epigenetic changes. And the expression of TP53 gene itself is also affected by nonsense-mediated mRNA decay (NMD)83,84 and loss-of-heterozygosity (LOH)85,86 as well as by TP53 mutations. To conduct these integrated analyses, it is necessary to construct a more large-scale database. Currently, the National Cancer Center and Cancer Genome Information Management Center (C-CAT; Center for Cancer Genomics and Advanced Therapeutics) are continuously collecting cancer genome and clinical information of a vast number of cancer patients. We believe that such a database will enable the integrated analyses described above and will further our understanding of the molecular biological abnormalities of cancers caused by TP53 mutations.
In conclusion, our findings revealed that the cancer types can be classified into two major groups based on the magnitude of gene expression changes related to the cell cycle and cell proliferative activity caused by TP53 mutations. Furthermore, there was no distinct difference in the effects of GOF and non-GOF mutations on the gene expression profile of the p53 signaling pathway.
Methods
Cases and cancer types
All the data in this study are publicly accessible at the TCGA dataset. This study included all 32 cancer types at the TCGA database (adrenocortical carcinoma, bladder urothelial carcinoma, breast invasive carcinoma, cervical squamous cell carcinoma, colon adenocarcinoma, esophageal carcinoma, glioblastoma multiforme, head and neck squamous cell carcinoma, kidney chromophobe, brain lower grade glioma, liver hepatocellular carcinoma, lung adenocarcinoma, lung squamous cell carcinoma, mesothelioma, ovarian serous cystadenocarcinoma, pancreatic adenocarcinoma, prostate adenocarcinoma, rectum adenocarcinoma, sarcoma, stomach adenocarcinoma, uterine corpus endometrial carcinoma, uterine carcinosarcoma, skin cutaneous melanoma, cholangiocarcinoma, diffuse large B-cell lymphoma, thymoma, kidney renal papillary cell carcinoma, kidney renal clear cell carcinoma, testicular germ cell tumors, thyroid carcinoma, pheochromocytoma and paraganglioma, and uveal melanoma). We collected the gene expression data, clinical information, and TP53 genotype of all 32 cancer types from cBioportal (https://www.cbioportal.org/). This study was conducted in accordance with the Declaration of Helsinki.
Comparison of p53 transcriptional activity
IARC database has reported all transcriptional activities of 2,314 different mutant p53 relative to p21, MDM2, BAX, 14-3-3σ, AIP1, GADD45, NOXA and p53R19. For p21 and each other target gene, we calculated the correlation coefficients for the transcriptional activity of 2,314 different mutant p53.
Definition of TP53 genotype
As of September 2021, we obtained TP53 mutation data by Whole Exome Sequencing from cBioportal. Among theTP53 gene mutations, only mutations with an allele frequency of > 20% were recognized as mutations87. We defined “TP53 mutation” as nonsense and frameshift mutations of TP53 gene. Missense mutations that were reported to reduce the transcriptional activity of p53 on p21 in the IARC database were also defined as “TP53 mutation,” and cases with the other missense mutations were excluded from this study. The absence of mutations in the TP53 gene was defined as “TP53 wild-type.” Among TP53 mutations, we defined “hotspot mutation” as those that corresponded to six hot spot locations (R175, G245, R248, R249, R273 and R282)4. The proportion of hotspot mutation is the ratio of the number of hotspot mutations in the total number of mutations. IARC defines GOF mutation as a functional property that mutant p53 but not wild-type p53 exhibits by measuring the activity of proteins overexpressed in human and yeast cells. Among the TP53 mutations, the mutations reported as GOF TP53 mutations in the IARC database were defined as “GOF mutation” and the mutations other than GOF mutations were defined as “non-GOF mutation.” All variants of TP53 mutation were included in the comparing GOF and non-GOF mutations.
Cohort for analyses
From 32 cancer types, the group of cancer types contained 10 or more cases each with TP53 mutations, and TP53 wild-type cases were categorized into “cohort A.” Among the cancer types in cohort A, the group of cancer types containing 10 or more cases of GOF and non-GOF mutations each was defined as “cohort B”.
Genes on the p53 signaling pathway
Genes on the p53 signaling pathway in KEGG were used for this study. Gene expression data registered with TCGA were obtained by RNA sequencing. As of September 2021, Fragments Per Kilobase of exon per Million mapped reads (FPKM) normalized data were obtained from the Genomic Data Commons Data Portal (GDC Portal, https://portal.gdc.cancer.gov/) and converted into TPM for analysis. The median expression levels of each gene on the p53 pathway were calculated for each cancer type. Genes with the median expression value of < 1.043 in more than half of the cases in cohort A or B were excluded from further analyses.
Cross-cancer unsupervised hierarchical cluster analyses based on gene expression profile changes
The effects of TP53 mutations on the gene expression profile of the p53 signaling pathway can be expressed as the gene expression ratio of the median expression value of TP53 mutant group to that of the TP53 wild-type group. Therefore, in cohort A, we used the logarithm of the expression ratio of each gene with a base of 2 (Log2 [median gene expression of TP53 mutant group]/[median gene expression of TP53 wild-type group]) for a cross-cancer unsupervised hierarchical cluster analysis. This cluster analysis was performed using the complete linkage method with Pearson correlation distance by using the Multiple Experiment Viewer (MeV 4.9.0, National Library of Medicine of the US National Institute of Health, http://mev.tm4.org/). Similarly, in cohort B, the logarithm of the expression ratio of GOF to TP53 wild-type for each gene with a base of 2 (Log2 [median gene expression of GOF mutant group]/[median gene expression of TP53 wild-type group]) and of non-GOF to TP53 wild-type (Log2 [median gene expression of non-GOF mutant group]/[median gene expression of TP53 wild-type group]) were used for a cross-cancer unsupervised hierarchical cluster analysis.
Identification of factors contributing to clustering
When comparing the characteristics between the clusters in the cohort A, histologic types were classified into adenocarcinoma and non-adenocarcinoma, and the developmental origin into endoderm, mesoderm, and ectoderm, based on a previous report88, and their proportions were compared.
Additionally, the proportion of TP53 and GOF mutant cases for each cancer type were compared between the clusters. The genes with significantly different expression ratios (P < 0.05 by Wilcoxon rank sum test) between the clusters were also extracted.
Statistical analysis
Statistical analysis was performed using R’s ExactRankTests package or JMP Pro16® (SAS Institute, Cary, NC). The Pearson correlation coefficient was used to calculate the correlation coefficient. Wilcoxon rank sum tests were used to examine the differences in the proportion of cases between the two groups, comparison of gene expression values, extraction of genes with differential expression values, and comparison of the number of genes extracted. Fisher’s exact test was used to compare the characteristics of cancer types among the clusters and the number of applicable cancer types among the clusters. The significance levels were set at P < 0.05.
Supplementary Information
Acknowledgements
The authors thank associate professor Tomohiro Nakamura for guidance on statistical analyses. The authors would like to thank Enago (www.enago.jp) for the English language review.
Author contributions
K.S., S.T., K.O., Y.O., and C.I. designed the study. K.S. performed the major parts of the study. K.S., S.T., K.O., and Y.O. performed statistical analyses. K.S., S.T., K.O., Y.O., S.W., and C.I. interpreted the results. K.S. drafted the manuscript. S.T., K.O., Y.O., S.W., and C.I. revised the manuscript critically for intellectual content. All authors approved the final version of the manuscript.
Data availability
Gene expression and TP53 mutation data of each patient used in this study can be retrieved from the public GDC Portal (https://portal.gdc.cancer.gov/) and cBioportal (https://www.cbioportal.org/), respectively.
Competing interests
Professor Chikashi Ishioka received research funding from Hitachi, Chugai, Taiho, Takeda, Daiichi Sankyo, Asahi Kasei, and Ono; and honoraria from Chugai and Daiichi Sankyo. All remaining authors have declared no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-32092-8.
References
- 1.Levine AJ, Oren M. The first 30 years of p53: Growing ever more complex. Nat Rev Cancer. 2009;9:749–758. doi: 10.1038/nrc2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vousden KH, Prives C. Blinded by the light: The growing complexity of p53. Cell. 2009;137:413–431. doi: 10.1016/j.cell.2009.04.037. [DOI] [PubMed] [Google Scholar]
- 3.Levine AJ, Hu W, Feng Z. The P53 pathway: What questions remain to be explored? Cell Death Differ. 2006;13:1027–1036. doi: 10.1038/sj.cdd.4401910. [DOI] [PubMed] [Google Scholar]
- 4.Muller PA, Vousden KH. p53 mutations in cancer. Nat. Cell Biol. 2013;15:2–8. doi: 10.1038/ncb2641. [DOI] [PubMed] [Google Scholar]
- 5.Laptenko O, Prives C. Transcriptional regulation by p53: One protein, many possibilities. Cell Death Differ. 2006;13:951–961. doi: 10.1038/sj.cdd.4401916. [DOI] [PubMed] [Google Scholar]
- 6.Harper JW, Adami GR, Wei N, Keyomarsi K, Elledge SJ. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993;75:805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- 7.El-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, et al. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75:817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- 8.Martín-Caballero J, Flores JM, García-Palencia P, Serrano M. Tumor susceptibility of p21(Waf1/Cip1)-deficient mice. Cancer Res. 2001;61:6234–6238. [PubMed] [Google Scholar]
- 9.Sengupta S, Harris CC. p53: Traffic cop at the crossroads of DNA repair and recombination. Nat. Rev. Mol. Cell Biol. 2005;6:44–55. doi: 10.1038/nrm1546. [DOI] [PubMed] [Google Scholar]
- 10.Bertrand P, Saintigny Y, Lopez BS. p53’s double life: Transactivation-independent repression of homologous recombination. Trends Genet. 2004;20:235–243. doi: 10.1016/j.tig.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 11.Lane DP. Cancer. p53, guardian of the genome. Nature. 1992;358:15–16. doi: 10.1038/358015a0. [DOI] [PubMed] [Google Scholar]
- 12.Feng Z, Levine AJ. The regulation of energy metabolism and the IGF-1/mTOR pathways by the p53 protein. Trends Cell Biol. 2010;20:427–434. doi: 10.1016/j.tcb.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lespagnol A, Duflaut D, Beekman C, Blanc L, Fiucci G, Marine JC, et al. Exosome secretion, including the DNA damage-induced p53-dependent secretory pathway, is severely compromised in TSAP6/Steap3-null mice. Cell Death Differ. 2008;15:1723–1733. doi: 10.1038/cdd.2008.104. [DOI] [PubMed] [Google Scholar]
- 14.Kanehisa M, Furumichi M, Sato Y, Kawashima M, Ishiguro-Watanabe M. KEGG for taxonomy-based analysis of pathways and genomes. Nucleic Acids Res. 2023;51:D587–d592. doi: 10.1093/nar/gkac963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kanehisa M. Toward understanding the origin and evolution of cellular organisms. Protein Sci. 2019;28:1947–1951. doi: 10.1002/pro.3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanehisa M, Goto S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28:27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qian Y, Chen X. Tumor suppression by p53: Making cells senescent. Histol. Histopathol. 2010;25:515–526. doi: 10.14670/hh-25.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abbas T, Dutta A. p21 in cancer: Intricate networks and multiple activities. Nat. Rev. Cancer. 2009;9:400–414. doi: 10.1038/nrc2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kato S, Han SY, Liu W, Otsuka K, Shibata H, Kanamaru R, et al. Understanding the function-structure and function-mutation relationships of p53 tumor suppressor protein by high-resolution missense mutation analysis. Proc. Natl. Acad. Sci. U. S. A. 2003;100:8424–8429. doi: 10.1073/pnas.1431692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mantovani F, Collavin L, Del Sal G. Mutant p53 as a guardian of the cancer cell. Cell Death Differ. 2019;26:199–212. doi: 10.1038/s41418-018-0246-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boersma BJ, Howe TM, Goodman JE, Yfantis HG, Lee DH, Chanock SJ, et al. Association of breast cancer outcome with status of p53 and MDM2 SNP309. J. Natl. Cancer. Inst. 2006;98:911–919. doi: 10.1093/jnci/djj245. [DOI] [PubMed] [Google Scholar]
- 22.Yamazaki Y, Chiba I, Hirai A, Sugiura C, Notani K, Kashiwazaki H, et al. Specific p53 mutations predict poor prognosis in oral squamous cell carcinoma. Oral Oncol. 2003;39:163–169. doi: 10.1016/s1368-8375(02)00064-7. [DOI] [PubMed] [Google Scholar]
- 23.Dicker F, Herholz H, Schnittger S, Nakao A, Patten N, Wu L, et al. The detection of TP53 mutations in chronic lymphocytic leukemia independently predicts rapid disease progression and is highly correlated with a complex aberrant karyotype. Leukemia. 2009;23:117–124. doi: 10.1038/leu.2008.274. [DOI] [PubMed] [Google Scholar]
- 24.Ince WL, Jubb AM, Holden SN, Holmgren EB, Tobin P, Sridhar M, et al. Association of k-ras, b-raf, and p53 status with the treatment effect of bevacizumab. J. Natl. Cancer Inst. 2005;97:981–989. doi: 10.1093/jnci/dji174. [DOI] [PubMed] [Google Scholar]
- 25.Szymanowska A, Jassem E, Dziadziuszko R, Skrzypski M, Kobierska-Gulida G, Holm K, et al. Analysis of prognostic value of TP53 gene mutations in non-small cell lung cancer. Pneumonol. Alergol. Pol. 2005;73:264–269. [PubMed] [Google Scholar]
- 26.Hernández S, López-Knowles E, Lloreta J, Kogevinas M, Jaramillo R, Amorós A, et al. FGFR3 and Tp53 mutations in T1G3 transitional bladder carcinomas: Independent distribution and lack of association with prognosis. Clin. Cancer Res. 2005;11:5444–5450. doi: 10.1158/1078-0432.Ccr-05-0122. [DOI] [PubMed] [Google Scholar]
- 27.Smith JS, Tachibana I, Passe SM, Huntley BK, Borell TJ, Iturria N, et al. PTEN mutation, EGFR amplification, and outcome in patients with anaplastic astrocytoma and glioblastoma multiforme. J. Natl. Cancer Inst. 2001;93:1246–1256. doi: 10.1093/jnci/93.16.1246. [DOI] [PubMed] [Google Scholar]
- 28.Ueno Y, Enomoto T, Otsuki Y, Sugita N, Nakashima R, Yoshino K, et al. Prognostic significance of p53 mutation in suboptimally resected advanced ovarian carcinoma treated with the combination chemotherapy of paclitaxel and carboplatin. Cancer Lett. 2006;241:289–300. doi: 10.1016/j.canlet.2005.10.035. [DOI] [PubMed] [Google Scholar]
- 29.Brosh R, Rotter V. When mutants gain new powers: News from the mutant p53 field. Nat. Rev. Cancer. 2009;9:701–713. doi: 10.1038/nrc2693. [DOI] [PubMed] [Google Scholar]
- 30.Tsao MS, Aviel-Ronen S, Ding K, Lau D, Liu N, Sakurada A, et al. Prognostic and predictive importance of p53 and RAS for adjuvant chemotherapy in non small-cell lung cancer. J. Clin. Oncol. 2007;25:5240–5247. doi: 10.1200/jco.2007.12.6953. [DOI] [PubMed] [Google Scholar]
- 31.Bertheau P, Plassa F, Espié M, Turpin E, de Roquancourt A, Marty M, et al. Effect of mutated TP53 on response of advanced breast cancers to high-dose chemotherapy. Lancet. 2002;360:852–854. doi: 10.1016/s0140-6736(02)09969-5. [DOI] [PubMed] [Google Scholar]
- 32.Fouret P, Temam S, Charlotte F, Lacau-St-Guily J. Tumour stage, node stage, p53 gene status, and bcl-2 protein expression as predictors of tumour response to platin-fluorouracil chemotherapy in patients with squamous-cell carcinoma of the head and neck. Br. J. Cancer. 2002;87:1390–1395. doi: 10.1038/sj.bjc.6600648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yue X, Zhao Y, Xu Y, Zheng M, Feng Z, Hu W. Mutant p53 in cancer: Accumulation, gain-of-function, and therapy. J. Mol. Biol. 2017;429:1595–1606. doi: 10.1016/j.jmb.2017.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu J, Zhang C, Feng Z. Tumor suppressor p53 and its gain-of-function mutants in cancer. Acta Biochim. Biophys. Sin. (Shanghai) 2014;46:170–179. doi: 10.1093/abbs/gmt144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blandino G, Deppert W, Hainaut P, Levine A, Lozano G, Olivier M, et al. Mutant p53 protein, master regulator of human malignancies: A report on the Fifth Mutant p53 Workshop. Cell Death Differ. 2012;19:180–183. doi: 10.1038/cdd.2011.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muller PA, Caswell PT, Doyle B, Iwanicki MP, Tan EH, Karim S, et al. Mutant p53 drives invasion by promoting integrin recycling. Cell. 2009;139:1327–1341. doi: 10.1016/j.cell.2009.11.026. [DOI] [PubMed] [Google Scholar]
- 37.Oren M, Rotter V. Mutant p53 gain-of-function in cancer. Cold Spring Harb. Perspect. Biol. 2010;2:a001107. doi: 10.1101/cshperspect.a001107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sande CM, Chang B, Monga V, Bossler AD, Ma D. Biallelic TP53 gain of function mutations in rapidly progressing solid tumors. Cancer Genet. 2018;222–223:20–24. doi: 10.1016/j.cancergen.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 39.Ham SW, Jeon HY, Jin X, Kim EJ, Kim JK, Shin YJ, et al. TP53 gain-of-function mutation promotes inflammation in glioblastoma. Cell Death Differ. 2019;26:409–425. doi: 10.1038/s41418-018-0126-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alexandrova EM, Yallowitz AR, Li D, Xu S, Schulz R, Proia DA, et al. Improving survival by exploiting tumour dependence on stabilized mutant p53 for treatment. Nature. 2015;523:352–356. doi: 10.1038/nature14430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pan M, Jiang C, Tse P, Achacoso N, Alexeeff S, Solorzano AV, et al. TP53 gain-of-function and non-gain-of-function mutations are differentially associated with sidedness-dependent prognosis in metastatic colorectal cancer. J. Clin. Oncol. 2022;40:171–179. doi: 10.1200/jco.21.02014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nesslinger NJ, Shi XB, de Vere White RW. Androgen-independent growth of LNCaP prostate cancer cells is mediated by gain-of-function mutant p53. Cancer Res. 2003;63:2228–2233. [PubMed] [Google Scholar]
- 43.Jehl F, Degalez F, Bernard M, Lecerf F, Lagoutte L, Désert C, et al. RNA-seq data for reliable SNP detection and genotype calling: Interest for coding variant characterization and cis-regulation analysis by allele-specific expression in livestock species. Front. Genet. 2021;12:655707. doi: 10.3389/fgene.2021.655707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sinn HP, Schneeweiss A, Keller M, Schlombs K, Laible M, Seitz J, et al. Comparison of immunohistochemistry with PCR for assessment of ER, PR, and Ki-67 and prediction of pathological complete response in breast cancer. BMC Cancer. 2017;17:124. doi: 10.1186/s12885-017-3111-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patel A, Oshi M, Yan L, Matsuyama R, Endo I, Takabe K. The unfolded protein response is associated with cancer proliferation and worse survival in hepatocellular carcinoma. Cancers (Basel) 2021 doi: 10.3390/cancers13174443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xiong S, Chachad D, Zhang Y, Gencel-Augusto J, Sirito M, Pant V, et al. Differential gain-of-function activity of three p53 hotspot mutants in vivo. Cancer Res. 2022;82:1926–1936. doi: 10.1158/0008-5472.Can-21-3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Olive KP, Tuveson DA, Ruhe ZC, Yin B, Willis NA, Bronson RT, et al. Mutant p53 gain of function in two mouse models of Li-Fraumeni syndrome. Cell. 2004;119:847–860. doi: 10.1016/j.cell.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 48.Wang H, Liao P, Zeng SX, Lu H. It takes a team: A gain-of-function story of p53-R249S. J. Mol. Cell Biol. 2019;11:277–283. doi: 10.1093/jmcb/mjy086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu X, Tian W, Cheng J, Li D, Liu T, Zhang L. Microsecond molecular dynamics simulations reveal the allosteric regulatory mechanism of p53 R249S mutation in p53-associated liver cancer. Comput. Biol. Chem. 2020;84:107194. doi: 10.1016/j.compbiolchem.2019.107194. [DOI] [PubMed] [Google Scholar]
- 50.Brázdová M, Quante T, Tögel L, Walter K, Loscher C, Tichý V, et al. Modulation of gene expression in U251 glioblastoma cells by binding of mutant p53 R273H to intronic and intergenic sequences. Nucleic Acids Res. 2009;37:1486–1500. doi: 10.1093/nar/gkn1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Salnikova LE. Clinicopathologic characteristics of brain tumors are associated with the presence and patterns of TP53 mutations: Evidence from the IARC TP53 database. Neuromol. Med. 2014;16:431–447. doi: 10.1007/s12017-014-8290-1. [DOI] [PubMed] [Google Scholar]
- 52.Stott FJ, Bates S, James MC, McConnell BB, Starborg M, Brookes S, et al. The alternative product from the human CDKN2A locus, p14(ARF), participates in a regulatory feedback loop with p53 and MDM2. Embo J. 1998;17:5001–5014. doi: 10.1093/emboj/17.17.5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cheung KJ, Jr, Li G. Tissue-specific regulation of Chk1 expression by p53. Exp. Mol. Pathol. 2001;71:156–160. doi: 10.1006/exmp.2001.2398. [DOI] [PubMed] [Google Scholar]
- 54.Yan J, Di Y, Shi H, Rao H, Huo K. Overexpression of SCYL1-BP1 stabilizes functional p53 by suppressing MDM2-mediated ubiquitination. FEBS Lett. 2010;584:4319–4324. doi: 10.1016/j.febslet.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Quelle DE, Zindy F, Ashmun RA, Sherr CJ. Alternative reading frames of the INK4a tumor suppressor gene encode two unrelated proteins capable of inducing cell cycle arrest. Cell. 1995;83:993–1000. doi: 10.1016/0092-8674(95)90214-7. [DOI] [PubMed] [Google Scholar]
- 56.Serrano M, Hannon GJ, Beach D. A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature. 1993;366:704–707. doi: 10.1038/366704a0. [DOI] [PubMed] [Google Scholar]
- 57.Zhang Y, Xiong Y, Yarbrough WG. ARF promotes MDM2 degradation and stabilizes p53: ARF-INK4a locus deletion impairs both the Rb and p53 tumor suppression pathways. Cell. 1998;92:725–734. doi: 10.1016/s0092-8674(00)81401-4. [DOI] [PubMed] [Google Scholar]
- 58.Thanasoula M, Escandell JM, Suwaki N, Tarsounas M. ATM/ATR checkpoint activation downregulates CDC25C to prevent mitotic entry with uncapped telomeres. Embo J. 2012;31:3398–3410. doi: 10.1038/emboj.2012.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang ZP, Xie YH, Ling DY, Li JR, Jiang J, Fan YH, et al. SCYL1BP1 has tumor-suppressive functions in human lung squamous carcinoma cells by regulating degradation of MDM2. Asian Pac. J. Cancer Prev. 2014;15:7467–7471. doi: 10.7314/apjcp.2014.15.17.7467. [DOI] [PubMed] [Google Scholar]
- 60.Li L, Li M, Wang X. Cancer type-dependent correlations between TP53 mutations and antitumor immunity. DNA Repair (Amst.) 2020;88:102785. doi: 10.1016/j.dnarep.2020.102785. [DOI] [PubMed] [Google Scholar]
- 61.Scholzen T, Gerdes J. The Ki-67 protein: From the known and the unknown. J. Cell Physiol. 2000;182:311–322. doi: 10.1002/(sici)1097-4652(200003)182:3<311::Aid-jcp1>3.0.Co;2-9. [DOI] [PubMed] [Google Scholar]
- 62.Kriegsmann M, Warth A. What is better/reliable, mitosis counting or Ki67/MIB1 staining? Transl. Lung Cancer Res. 2016;5:543–546. doi: 10.21037/tlcr.2016.10.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thotakura M, Tirumalasetti N, Krishna R. Role of Ki-67 labeling index as an adjunct to the histopathological diagnosis and grading of astrocytomas. J. Cancer Res. Ther. 2014;10:641–645. doi: 10.4103/0973-1482.139154. [DOI] [PubMed] [Google Scholar]
- 64.du Manoir S, Guillaud P, Camus E, Seigneurin D, Brugal G. Ki-67 labeling in postmitotic cells defines different Ki-67 pathways within the 2c compartment. Cytometry. 1991;12:455–463. doi: 10.1002/cyto.990120511. [DOI] [PubMed] [Google Scholar]
- 65.Oshi M, Patel A, Le L, Tokumaru Y, Yan L, Matsuyama R, et al. G2M checkpoint pathway alone is associated with drug response and survival among cell proliferation-related pathways in pancreatic cancer. Am. J. Cancer Res. 2021;11:3070–3084. [PMC free article] [PubMed] [Google Scholar]
- 66.Nakanishi K, Sakamoto M, Yamasaki S, Todo S, Hirohashi S. Akt phosphorylation is a risk factor for early disease recurrence and poor prognosis in hepatocellular carcinoma. Cancer. 2005;103:307–312. doi: 10.1002/cncr.20774. [DOI] [PubMed] [Google Scholar]
- 67.Wu SY, Liao P, Yan LY, Zhao QY, Xie ZY, Dong J, et al. Correlation of MKI67 with prognosis, immune infiltration, and T cell exhaustion in hepatocellular carcinoma. BMC Gastroenterol. 2021;21:416. doi: 10.1186/s12876-021-01984-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Geyer FC, Rodrigues DN, Weigelt B, Reis-Filho JS. Molecular classification of estrogen receptor-positive/luminal breast cancers. Adv. Anat. Pathol. 2012;19:39–53. doi: 10.1097/PAP.0b013e31823fafa0. [DOI] [PubMed] [Google Scholar]
- 69.Kolacinska A, Fendler W, Szemraj J, Szymanska B, Borowska-Garganisz E, Nowik M, et al. Gene expression and pathologic response to neoadjuvant chemotherapy in breast cancer. Mol. Biol. Rep. 2012;39:7435–7441. doi: 10.1007/s11033-012-1576-1. [DOI] [PubMed] [Google Scholar]
- 70.Grabowski JP, Martinez Vila C, Richter R, Taube E, Plett H, Braicu E, et al. Ki67 expression as a predictor of chemotherapy outcome in low-grade serous ovarian cancer. Int. J. Gynecol. Cancer. 2020;30:498–503. doi: 10.1136/ijgc-2019-000976. [DOI] [PubMed] [Google Scholar]
- 71.Alsner J, Jensen V, Kyndi M, Offersen BV, Vu P, Børresen-Dale AL, et al. A comparison between p53 accumulation determined by immunohistochemistry and TP53 mutations as prognostic variables in tumours from breast cancer patients. Acta Oncol. 2008;47:600–607. doi: 10.1080/02841860802047411. [DOI] [PubMed] [Google Scholar]
- 72.Chi Q, Xu H, Song D, Wang Z, Wang Z, Ma G. α-E-catenin (CTNNA1) inhibits cell proliferation, invasion and EMT of bladder cancer. Cancer Manag. Res. 2020;12:12747–12758. doi: 10.2147/cmar.S259269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Conlin A, Smith G, Carey FA, Wolf CR, Steele RJ. The prognostic significance of K-ras, p53, and APC mutations in colorectal carcinoma. Gut. 2005;54:1283–1286. doi: 10.1136/gut.2005.066514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lyu Q, Lin A, Cao M, Xu A, Luo P, Zhang J. Alterations in TP53 are a potential biomarker of bladder cancer patients who benefit from immune checkpoint inhibition. Cancer Control. 2020;27:1073274820976665. doi: 10.1177/1073274820976665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cabelguenne A, Blons H, de Waziers I, Carnot F, Houllier AM, Soussi T, et al. p53 alterations predict tumor response to neoadjuvant chemotherapy in head and neck squamous cell carcinoma: A prospective series. J. Clin. Oncol. 2000;18:1465–1473. doi: 10.1200/jco.2000.18.7.1465. [DOI] [PubMed] [Google Scholar]
- 76.Vaughan CA, Pearsall I, Singh S, Windle B, Deb SP, Grossman SR, et al. Addiction of lung cancer cells to GOF p53 is promoted by up-regulation of epidermal growth factor receptor through multiple contacts with p53 transactivation domain and promoter. Oncotarget. 2016;7:12426–12446. doi: 10.18632/oncotarget.6998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vennin C, Mélénec P, Rouet R, Nobis M, Cazet AS, Murphy KJ, et al. CAF hierarchy driven by pancreatic cancer cell p53-status creates a pro-metastatic and chemoresistant environment via perlecan. Nat. Commun. 2019;10:3637. doi: 10.1038/s41467-019-10968-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Weissmueller S, Manchado E, Saborowski M, Morris JPT, Wagenblast E, Davis CA, et al. Mutant p53 drives pancreatic cancer metastasis through cell-autonomous PDGF receptor β signaling. Cell. 2014;157:382–394. doi: 10.1016/j.cell.2014.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pfister NT, Fomin V, Regunath K, Zhou JY, Zhou W, Silwal-Pandit L, et al. Mutant p53 cooperates with the SWI/SNF chromatin remodeling complex to regulate VEGFR2 in breast cancer cells. Genes Dev. 2015;29:1298–1315. doi: 10.1101/gad.263202.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ali A, Shah AS, Ahmad A. Gain-of-function of mutant p53: Mutant p53 enhances cancer progression by inhibiting KLF17 expression in invasive breast carcinoma cells. Cancer Lett. 2014;354:87–96. doi: 10.1016/j.canlet.2014.07.045. [DOI] [PubMed] [Google Scholar]
- 81.Shiraishi K, Kato S, Han SY, Liu W, Otsuka K, Sakayori M, et al. Isolation of temperature-sensitive p53 mutations from a comprehensive missense mutation library. J. Biol. Chem. 2004;279:348–355. doi: 10.1074/jbc.M310815200. [DOI] [PubMed] [Google Scholar]
- 82.Cui Y, Guo G. Immunomodulatory function of the tumor suppressor p53 in host immune response and the tumor microenvironment. Int. J. Mol. Sci. 2016 doi: 10.3390/ijms17111942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gudikote JP, Cascone T, Poteete A, Sitthideatphaiboon P, Wu Q, Morikawa N, et al. Inhibition of nonsense-mediated decay rescues p53β/γ isoform expression and activates the p53 pathway in MDM2-overexpressing and select p53-mutant cancers. J. Biol. Chem. 2021;297:101163. doi: 10.1016/j.jbc.2021.101163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cowen LE, Tang Y. Identification of nonsense-mediated mRNA decay pathway as a critical regulator of p53 isoform β. Sci. Rep. 2017;7:17535. doi: 10.1038/s41598-017-17283-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.McCann JJ, Vasilevskaya IA, McNair C, Gallagher P, Neupane NP, de Leeuw R, et al. Mutant p53 elicits context-dependent pro-tumorigenic phenotypes. Oncogene. 2022;41:444–458. doi: 10.1038/s41388-021-01903-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Choi WH, Lee S, Cho S. Microsatellite alterations and protein expression of 5 major tumor suppressor genes in gastric adenocarcinomas. Transl. Oncol. 2018;11:43–55. doi: 10.1016/j.tranon.2017.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Uji K, Naoi Y, Kagara N, Shimoda M, Shimomura A, Maruyama N, et al. Significance of TP53 mutations determined by next-generation “deep” sequencing in prognosis of estrogen receptor-positive breast cancer. Cancer Lett. 2014;342:19–26. doi: 10.1016/j.canlet.2013.08.028. [DOI] [PubMed] [Google Scholar]
- 88.Gao X, Cui X, Zhang X, Zhao C, Zhang N, Zhao Y, et al. Differential genetic mutations of ectoderm, mesoderm, and endoderm-derived tumors in TCGA database. Cancer Cell Int. 2020;20:595. doi: 10.1186/s12935-020-01678-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Gene expression and TP53 mutation data of each patient used in this study can be retrieved from the public GDC Portal (https://portal.gdc.cancer.gov/) and cBioportal (https://www.cbioportal.org/), respectively.