Full text
PDF
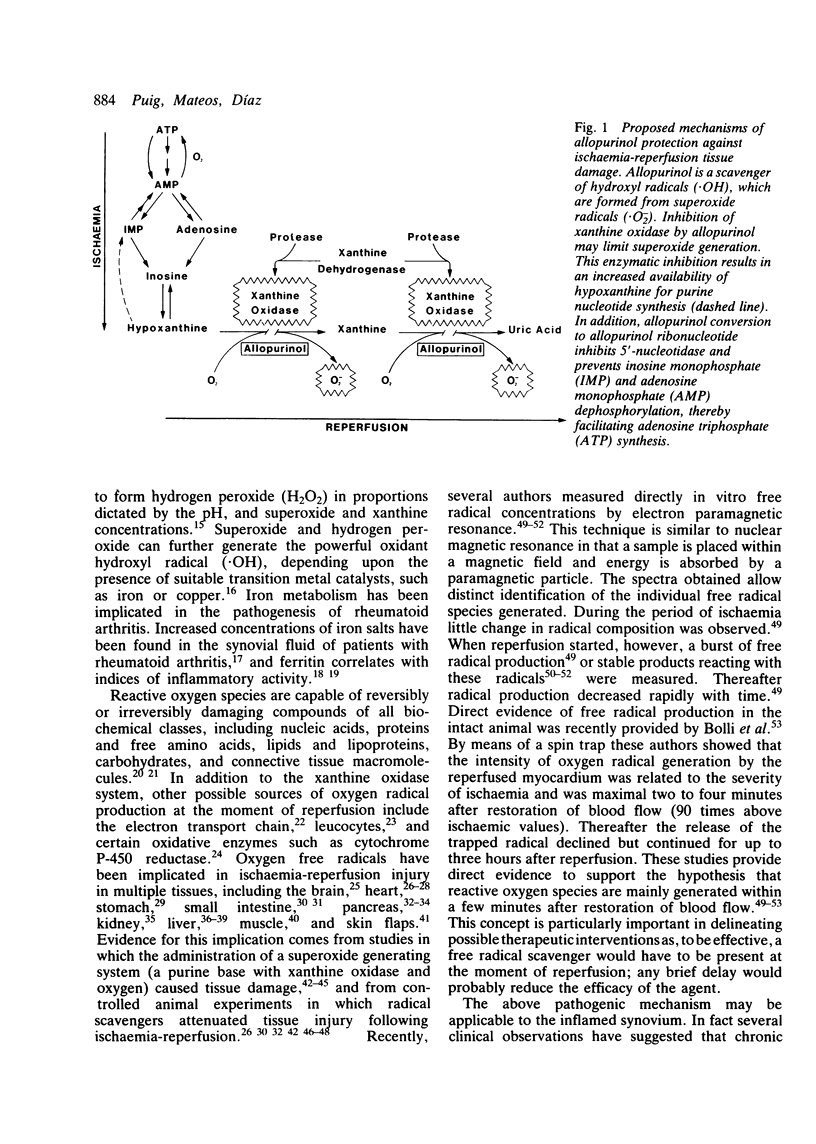




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adkison D., Höllwarth M. E., Benoit J. N., Parks D. A., McCord J. M., Granger D. N. Role of free radicals in ischemia-reperfusion injury to the liver. Acta Physiol Scand Suppl. 1986;548:101–107. [PubMed] [Google Scholar]
- Akizuki S., Yoshida S., Chambers D. E., Eddy L. J., Parmley L. F., Yellon D. M., Downey J. M. Infarct size limitation by the xanthine oxidase inhibitor, allopurinol, in closed-chest dogs with small infarcts. Cardiovasc Res. 1985 Nov;19(11):686–692. doi: 10.1093/cvr/19.11.686. [DOI] [PubMed] [Google Scholar]
- Allen R. E., Outhwaite J. M., Morris C. J., Blake D. R. Xanthine oxidoreductase is present in human synovium. Ann Rheum Dis. 1987 Nov;46(11):843–845. doi: 10.1136/ard.46.11.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arroyo C. M., Kramer J. H., Dickens B. F., Weglicki W. B. Identification of free radicals in myocardial ischemia/reperfusion by spin trapping with nitrone DMPO. FEBS Lett. 1987 Aug 31;221(1):101–104. doi: 10.1016/0014-5793(87)80360-5. [DOI] [PubMed] [Google Scholar]
- Babior B. M. Oxidants from phagocytes: agents of defense and destruction. Blood. 1984 Nov;64(5):959–966. [PubMed] [Google Scholar]
- Blake D. R., Allen R. E., Lunec J. Free radicals in biological systems--a review orientated to inflammatory processes. Br Med Bull. 1987 Apr;43(2):371–385. doi: 10.1093/oxfordjournals.bmb.a072188. [DOI] [PubMed] [Google Scholar]
- Blake D. R., Bacon P. A. Synovial fluid ferritin in rheumatoid arthritis: an index or cause of inflammation? Br Med J (Clin Res Ed) 1981 Jan 17;282(6259):189–189. doi: 10.1136/bmj.282.6259.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake D. R., Gallagher P. J., Potter A. R., Bell M. J., Bacon P. A. The effect of synovial iron on the progression of rheumatoid disease. A histologic assessment of patients with early rheumatoid synovitis. Arthritis Rheum. 1984 May;27(5):495–501. doi: 10.1002/art.1780270503. [DOI] [PubMed] [Google Scholar]
- Blake D. R., Hall N. D., Treby D. A., Halliwell B., Gutteridge J. M. Protection against superoxide and hydrogen peroxide in synovial fluid from rheumatoid patients. Clin Sci (Lond) 1981 Oct;61(4):483–486. doi: 10.1042/cs0610483. [DOI] [PubMed] [Google Scholar]
- Blake D. R., Merry P., Unsworth J., Kidd B. L., Outhwaite J. M., Ballard R., Morris C. J., Gray L., Lunec J. Hypoxic-reperfusion injury in the inflamed human joint. Lancet. 1989 Feb 11;1(8633):289–293. doi: 10.1016/s0140-6736(89)91305-6. [DOI] [PubMed] [Google Scholar]
- Bolli R., Patel B. S., Jeroudi M. O., Lai E. K., McCay P. B. Demonstration of free radical generation in "stunned" myocardium of intact dogs with the use of the spin trap alpha-phenyl N-tert-butyl nitrone. J Clin Invest. 1988 Aug;82(2):476–485. doi: 10.1172/JCI113621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boveris A. Mitochondrial production of superoxide radical and hydrogen peroxide. Adv Exp Med Biol. 1977;78:67–82. doi: 10.1007/978-1-4615-9035-4_5. [DOI] [PubMed] [Google Scholar]
- Burton K. P., McCord J. M., Ghai G. Myocardial alterations due to free-radical generation. Am J Physiol. 1984 Jun;246(6 Pt 2):H776–H783. doi: 10.1152/ajpheart.1984.246.6.H776. [DOI] [PubMed] [Google Scholar]
- Cochran T., Stefanko J., Moore C., Saik R. Dimethylsulfoxide protection against gastric stress ulceration. Curr Surg. 1983 Nov-Dec;40(6):435–437. [PubMed] [Google Scholar]
- Corte E. D., Stirpe F. The regulation of rat liver xanthine oxidase. Involvement of thiol groups in the conversion of the enzyme activity from dehydrogenase (type D) into oxidase (type O) and purification of the enzyme. Biochem J. 1972 Feb;126(3):739–745. doi: 10.1042/bj1260739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross C. E., Halliwell B., Borish E. T., Pryor W. A., Ames B. N., Saul R. L., McCord J. M., Harman D. Oxygen radicals and human disease. Ann Intern Med. 1987 Oct;107(4):526–545. doi: 10.7326/0003-4819-107-4-526. [DOI] [PubMed] [Google Scholar]
- Cunningham S. K., Keaveny T. V., Fitzgerald P. Effect of allopurinol on tissue ATP, ADP and AMP concentrations in renal ischaemia. Br J Surg. 1974 Jul;61(7):562–565. doi: 10.1002/bjs.1800610716. [DOI] [PubMed] [Google Scholar]
- DeConti R. C., Calabresi P. Use of allopurinol for prevention and control of hyperuricemia in patients with neoplastic disease. N Engl J Med. 1966 Mar 3;274(9):481–486. doi: 10.1056/NEJM196603032740902. [DOI] [PubMed] [Google Scholar]
- DeWall R. A., Vasko K. A., Stanley E. L., Kezdi P. Responses of the ischemic myocardium to allopurinol. Am Heart J. 1971 Sep;82(3):362–370. doi: 10.1016/0002-8703(71)90302-4. [DOI] [PubMed] [Google Scholar]
- Del Maestro R. F. An approach to free radicals in medicine and biology. Acta Physiol Scand Suppl. 1980;492:153–168. [PubMed] [Google Scholar]
- Demopoulos H. B., Flamm E. S., Pietronigro D. D., Seligman M. L. The free radical pathology and the microcirculation in the major central nervous system disorders. Acta Physiol Scand Suppl. 1980;492:91–119. [PubMed] [Google Scholar]
- Edwards N. L., Recker D., Airozo D., Fox I. H. Enhanced purine salvage during allopurinol therapy: an important pharmacologic property in humans. J Lab Clin Med. 1981 Nov;98(5):673–683. [PubMed] [Google Scholar]
- Ettinger B., Tang A., Citron J. T., Livermore B., Williams T. Randomized trial of allopurinol in the prevention of calcium oxalate calculi. N Engl J Med. 1986 Nov 27;315(22):1386–1389. doi: 10.1056/NEJM198611273152204. [DOI] [PubMed] [Google Scholar]
- Fox I. H., Marchant P. J. Purine catabolism in man: inhibition of 5'-phosphomonesterase activities from placental microsomes. Can J Biochem. 1976 Dec;54(12):1055–1060. doi: 10.1139/o76-154. [DOI] [PubMed] [Google Scholar]
- Garlick P. B., Davies M. J., Hearse D. J., Slater T. F. Direct detection of free radicals in the reperfused rat heart using electron spin resonance spectroscopy. Circ Res. 1987 Nov;61(5):757–760. doi: 10.1161/01.res.61.5.757. [DOI] [PubMed] [Google Scholar]
- Godin D. V., Bhimji S. Effects of allopurinol on myocardial ischemic injury induced by coronary artery ligation and reperfusion. Biochem Pharmacol. 1987 Jul 1;36(13):2101–2107. doi: 10.1016/0006-2952(87)90137-7. [DOI] [PubMed] [Google Scholar]
- Granger D. N., Höllwarth M. E., Parks D. A. Ischemia-reperfusion injury: role of oxygen-derived free radicals. Acta Physiol Scand Suppl. 1986;548:47–63. [PubMed] [Google Scholar]
- Granger D. N., Rutili G., McCord J. M. Superoxide radicals in feline intestinal ischemia. Gastroenterology. 1981 Jul;81(1):22–29. [PubMed] [Google Scholar]
- Green C. J., Healing G., Lunec J., Fuller B. J., Simpkin S. Evidence of free-radical-induced damage in rabbit kidneys after simple hypothermic preservation and autotransplantation. Transplantation. 1986 Feb;41(2):161–165. doi: 10.1097/00007890-198602000-00005. [DOI] [PubMed] [Google Scholar]
- Grum C. M., Ketai L. H., Myers C. L., Shlafer M. Purine efflux after cardiac ischemia: relevance to allopurinol cardioprotection. Am J Physiol. 1987 Feb;252(2 Pt 2):H368–H373. doi: 10.1152/ajpheart.1987.252.2.H368. [DOI] [PubMed] [Google Scholar]
- Grøgaard B., Parks D. A., Granger D. N., McCord J. M., Forsberg J. O. Effects of ischemia and oxygen radicals on mucosal albumin clearance in intestine. Am J Physiol. 1982 May;242(5):G448–G454. doi: 10.1152/ajpgi.1982.242.5.G448. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J. 1984 Apr 1;219(1):1–14. doi: 10.1042/bj2190001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearse D. J., Manning A. S., Downey J. M., Yellon D. M. Xanthine oxidase: a critical mediator of myocardial injury during ischemia and reperfusion? Acta Physiol Scand Suppl. 1986;548:65–78. [PubMed] [Google Scholar]
- Im M. J., Shen W. H., Pak C. J., Manson P. N., Bulkley G. B., Hoopes J. E. Effect of allopurinol on the survival of hyperemic island skin flaps. Plast Reconstr Surg. 1984 Feb;73(2):276–278. doi: 10.1097/00006534-198402000-00023. [DOI] [PubMed] [Google Scholar]
- Jones C. E., Crowell J. W., Smith E. E. Significance of increased blood uric acid following extensive hemorrhage. Am J Physiol. 1968 Jun;214(6):1374–1377. doi: 10.1152/ajplegacy.1968.214.6.1374. [DOI] [PubMed] [Google Scholar]
- Kamiike W., Burdelski M., Steinhoff G., Ringe B., Lauchart W., Pichlmayr R. Adenine nucleotide metabolism and its relation to organ viability in human liver transplantation. Transplantation. 1988 Jan;45(1):138–143. doi: 10.1097/00007890-198801000-00030. [DOI] [PubMed] [Google Scholar]
- Kamiike W., Watanabe F., Hashimoto T., Tagawa K., Ikeda Y., Nakao K., Kawashima Y. Changes in cellular levels of ATP and its catabolites in ischemic rat liver. J Biochem. 1982 Apr;91(4):1349–1356. doi: 10.1093/oxfordjournals.jbchem.a133822. [DOI] [PubMed] [Google Scholar]
- Korthuis R. J., Granger D. N., Townsley M. I., Taylor A. E. The role of oxygen-derived free radicals in ischemia-induced increases in canine skeletal muscle vascular permeability. Circ Res. 1985 Oct;57(4):599–609. doi: 10.1161/01.res.57.4.599. [DOI] [PubMed] [Google Scholar]
- Kugler G. Myocardial release of inosine, hypoxanthine and lactate during pacing-induced angina in humans with coronary artery disease. Eur J Cardiol. 1979 Mar;9(3):227–240. [PubMed] [Google Scholar]
- Kuthan H., Ullrich V. Oxidase and oxygenase function of the microsomal cytochrome P450 monooxygenase system. Eur J Biochem. 1982 Sep 1;126(3):583–588. doi: 10.1111/j.1432-1033.1982.tb06820.x. [DOI] [PubMed] [Google Scholar]
- Lasley R. D., Ely S. W., Berne R. M., Mentzer R. M., Jr Allopurinol enhanced adenine nucleotide repletion after myocardial ischemia in the isolated rat heart. J Clin Invest. 1988 Jan;81(1):16–20. doi: 10.1172/JCI113288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunec J., Blake D. R., McCleary S. J., Brailsford S., Bacon P. A. Self-perpetuating mechanisms of immunoglobulin G aggregation in rheumatoid inflammation. J Clin Invest. 1985 Dec;76(6):2084–2090. doi: 10.1172/JCI112212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manson P. N., Anthenelli R. M., Im M. J., Bulkley G. B., Hoopes J. E. The role of oxygen-free radicals in ischemic tissue injury in island skin flaps. Ann Surg. 1983 Jul;198(1):87–90. doi: 10.1097/00000658-198307000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marubayashi S., Dohi K., Yamada K., Kawasaki T. Changes in the levels of endogenous coenzyme Q homologs, alpha-tocopherol, and glutathione in rat liver after hepatic ischemia and reperfusion, and the effect of pretreatment with coenzyme Q10. Biochim Biophys Acta. 1984 Jan 24;797(1):1–9. [PubMed] [Google Scholar]
- Marubayashi S., Takenaka M., Dohi K., Ezaki H., Kawasaki T. Adenine nucleotide metabolism during hepatic ischemia and subsequent blood reflow periods and its relation to organ viability. Transplantation. 1980 Oct;30(4):294–296. doi: 10.1097/00007890-198010000-00011. [DOI] [PubMed] [Google Scholar]
- Mateos F. A., Puig J. G., Jiménez M. L., Fox I. H. Hereditary xanthinuria. Evidence for enhanced hypoxanthine salvage. J Clin Invest. 1987 Mar;79(3):847–852. doi: 10.1172/JCI112893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. The reduction of cytochrome c by milk xanthine oxidase. J Biol Chem. 1968 Nov 10;243(21):5753–5760. [PubMed] [Google Scholar]
- McCord J. M. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985 Jan 17;312(3):159–163. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- Moorhouse P. C., Grootveld M., Halliwell B., Quinlan J. G., Gutteridge J. M. Allopurinol and oxypurinol are hydroxyl radical scavengers. FEBS Lett. 1987 Mar 9;213(1):23–28. doi: 10.1016/0014-5793(87)81458-8. [DOI] [PubMed] [Google Scholar]
- Nelson D. J., Buggé C. J., Krasny H. C., Elion G. B. Formation of nucleotides of (6-14C)allopurinol and (6-14C)oxipurinol in rat tissues and effects on uridine nucleotide pools. Biochem Pharmacol. 1973 Aug 15;22(16):2003–2022. doi: 10.1016/0006-2952(73)90082-8. [DOI] [PubMed] [Google Scholar]
- Nordström G., Seeman T., Hasselgren P. O. Beneficial effect of allopurinol in liver ischemia. Surgery. 1985 Jun;97(6):679–684. [PubMed] [Google Scholar]
- Owens M. L., Lazarus H. M., Wolcott M. W., Maxwell J. G., Taylor J. B. Allopurinol and hypoxanthine pretreatment of canine kidney donors. Transplantation. 1974 Apr;17(4):424–427. doi: 10.1097/00007890-197404000-00015. [DOI] [PubMed] [Google Scholar]
- Paller M. S., Hoidal J. R., Ferris T. F. Oxygen free radicals in ischemic acute renal failure in the rat. J Clin Invest. 1984 Oct;74(4):1156–1164. doi: 10.1172/JCI111524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks D. A., Bulkley G. B., Granger D. N. Role of oxygen free radicals in shock, ischemia, and organ preservation. Surgery. 1983 Sep;94(3):428–432. [PubMed] [Google Scholar]
- Parks D. A., Bulkley G. B., Granger D. N. Role of oxygen-derived free radicals in digestive tract diseases. Surgery. 1983 Sep;94(3):415–422. [PubMed] [Google Scholar]
- Parks D. A., Granger D. N. Ischemia-induced vascular changes: role of xanthine oxidase and hydroxyl radicals. Am J Physiol. 1983 Aug;245(2):G285–G289. doi: 10.1152/ajpgi.1983.245.2.G285. [DOI] [PubMed] [Google Scholar]
- Parks D. A., Granger D. N. Xanthine oxidase: biochemistry, distribution and physiology. Acta Physiol Scand Suppl. 1986;548:87–99. [PubMed] [Google Scholar]
- Peterson D. A., Asinger R. W., Elsperger K. J., Homans D. C., Eaton J. W. Reactive oxygen species may cause myocardial reperfusion injury. Biochem Biophys Res Commun. 1985 Feb 28;127(1):87–93. doi: 10.1016/s0006-291x(85)80129-7. [DOI] [PubMed] [Google Scholar]
- Puig J. G., Jiménez M. L., Mateos F. A., Fox I. H. Adenine nucleotide turnover in hypoxanthine-guanine phosphoribosyl-transferase deficiency: evidence for an increased contribution of purine biosynthesis de novo. Metabolism. 1989 May;38(5):410–418. doi: 10.1016/0026-0495(89)90189-3. [DOI] [PubMed] [Google Scholar]
- Reimer K. A., Hill M. L., Jennings R. B. Prolonged depletion of ATP and of the adenine nucleotide pool due to delayed resynthesis of adenine nucleotides following reversible myocardial ischemic injury in dogs. J Mol Cell Cardiol. 1981 Feb;13(2):229–239. doi: 10.1016/0022-2828(81)90219-4. [DOI] [PubMed] [Google Scholar]
- Rundles R. W. The development of allopurinol. Arch Intern Med. 1985 Aug;145(8):1492–1503. [PubMed] [Google Scholar]
- Sanfey H., Bulkley G. B., Cameron J. L. The role of oxygen-derived free radicals in the pathogenesis of acute pancreatitis. Ann Surg. 1984 Oct;200(4):405–413. doi: 10.1097/00000658-198410000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanfey H., Sarr M. G., Bulkley G. B., Cameron J. L. Oxygen-derived free radicals and acute pancreatitis: a review. Acta Physiol Scand Suppl. 1986;548:109–118. [PubMed] [Google Scholar]
- Saugstad O. D., Hallman M., Abraham J. L., Epstein B., Cochrane C., Gluck L. Hypoxanthine and oxygen induced lung injury: a possible basic mechanism of tissue damage? Pediatr Res. 1984 Jun;18(6):501–504. doi: 10.1203/00006450-198406000-00002. [DOI] [PubMed] [Google Scholar]
- Shlafer M., Kane P. F., Kirsh M. M. Superoxide dismutase plus catalase enhances the efficacy of hypothermic cardioplegia to protect the globally ischemic, reperfused heart. J Thorac Cardiovasc Surg. 1982 Jun;83(6):830–839. [PubMed] [Google Scholar]
- Siems W., Mielke B., Müller M., Heumann C., Räder L., Gerber G. Status of glutathione in the rat liver. Enhanced formation of oxygen radicals at low oxygen tension. Biomed Biochim Acta. 1983;42(9):1079–1089. [PubMed] [Google Scholar]
- Simpson P. J., Lucchesi B. R. Free radicals and myocardial ischemia and reperfusion injury. J Lab Clin Med. 1987 Jul;110(1):13–30. [PubMed] [Google Scholar]
- Singer J. Z., Wallace S. L. The allopurinol hypersensitivity syndrome. Unnecessary morbidity and mortality. Arthritis Rheum. 1986 Jan;29(1):82–87. doi: 10.1002/art.1780290111. [DOI] [PubMed] [Google Scholar]
- Southorn P. A., Powis G. Free radicals in medicine. II. Involvement in human disease. Mayo Clin Proc. 1988 Apr;63(4):390–408. doi: 10.1016/s0025-6196(12)64862-9. [DOI] [PubMed] [Google Scholar]
- Swain J. L., Sabina R. L., McHale P. A., Greenfield J. C., Jr, Holmes E. W. Prolonged myocardial nucleotide depletion after brief ischemia in the open-chest dog. Am J Physiol. 1982 May;242(5):H818–H826. doi: 10.1152/ajpheart.1982.242.5.H818. [DOI] [PubMed] [Google Scholar]
- Toledo-Pereyra L. H., Simmons R. L., Najarian J. S. Effect of allopurinol on the preservation of ischemic kidneys perfused with plasma or plasma substitutes. Ann Surg. 1974 Nov;180(5):780–782. doi: 10.1097/00000658-197411000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo-Pereyra L. H., Simmons R. L., Najarian J. S. Factors determining successful liver preservation for transplantation. Ann Surg. 1975 Mar;181(3):289–298. doi: 10.1097/00000658-197503000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vary T. C., Angelakos E. T., Schaffer S. W. Relationship between adenine nucleotide metabolism and irreversible ischemic tissue damage in isolated perfused rat heart. Circ Res. 1979 Aug;45(2):218–225. doi: 10.1161/01.res.45.2.218. [DOI] [PubMed] [Google Scholar]
- Werns S. W., Shea M. J., Mitsos S. E., Dysko R. C., Fantone J. C., Schork M. A., Abrams G. D., Pitt B., Lucchesi B. R. Reduction of the size of infarction by allopurinol in the ischemic-reperfused canine heart. Circulation. 1986 Mar;73(3):518–524. doi: 10.1161/01.cir.73.3.518. [DOI] [PubMed] [Google Scholar]
- Wisner J., Green D., Ferrell L., Renner I. Evidence for a role of oxygen derived free radicals in the pathogenesis of caerulein induced acute pancreatitis in rats. Gut. 1988 Nov;29(11):1516–1523. doi: 10.1136/gut.29.11.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff T., Blake D. R., Freeman J., Andrews F. J., Salt P., Lunec J. Is chronic synovitis an example of reperfusion injury? Ann Rheum Dis. 1986 Jul;45(7):608–611. doi: 10.1136/ard.45.7.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweier J. L., Flaherty J. T., Weisfeldt M. L. Direct measurement of free radical generation following reperfusion of ischemic myocardium. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1404–1407. doi: 10.1073/pnas.84.5.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweier J. L., Kuppusamy P., Lutty G. A. Measurement of endothelial cell free radical generation: evidence for a central mechanism of free radical injury in postischemic tissues. Proc Natl Acad Sci U S A. 1988 Jun;85(11):4046–4050. doi: 10.1073/pnas.85.11.4046. [DOI] [PMC free article] [PubMed] [Google Scholar]


