Abstract
Objective
To evaluate and compare clinical efficacy and effect on specific serum biomarker with serial injections of growth factor concentrate (GFC) for knee osteoarthritis (KOA) in a randomized triple blinded placebo controlled interventional study.
Methods
Final assessment was done on 58 patients. Patients with Kellgren-Lawrence grade II, III knee osteoarthritis were administered monthly intraarticular injections(3 injections) of GFC(n = 31) or saline(n = 27) and evaluated clinically with visual analogue scale(VAS) and Western Ontario and McMaster Universities Arthritis Index(WOMAC) at 3,6 and 12 months post therapy. Biochemical analysis was done with serum biomarker of cartilage degeneration, Collagen 2-1 (Coll2-1), estimated at baseline and at final follow up.
Results
Both the groups exhibited statistically significant improvements (P < 0.05) in VAS at 3,6 and 12 months. WOMAC improvement reached statistical significance for GFC group at every evaluation (P < 0.001) but only at 12 months in NS group (P = 0.029). The improvements were clinically meaningful only in GFC group throughout follow up (Minimal clinically important differences >12% of baseline in WOMAC and >2 cm difference in mean for VAS). Intergroup comparison revealed GFC to be much better for both scores at every evaluation (95% CI of 0.2–1.5,[P = 0.008], 1.4–2.9,[P < 0.0001], and 2.7–4.2,[P < 0.0001] for VAS, 7.3–16.0 [P < 0.001], 11.6–21.9 [P < 0.001] and 18.1–31.1[P < 0.001] for WOMAC). Statistically significant decrease in serum Coll2-1 levels were observed for GFC group only. No serious complications were seen.
Conclusion
Serial(three) monthly GFC injections result in clinically meaningful improvement of subjective pain and function outcome scores, sustaining up to 12 months in KOA grade II and III. GFC also lead to significant reduction in serum levels of cartilage degradation biomarker coll2-1.
Keywords: Knee osteoarthritis (KOA), VAS, WOMAC, Serum coll2-1, Growth factor concentrate (GFC)
1. Introduction
Knee is the commonest joint involved in primary osteoarthritis(OA), leading to pain and debility in people aged 50 years or above, severely impairing quality of life. Age, female gender, obesity and genetic susceptibility are some of the known risk factors for knee osteoarthritis(KOA). The basic mechanism of joint damage seems to be an inflammatory process with insufficiency of anabolic factors (like BMPs and IGF-1), combined with an increase in catabolic factors (like TNF alpha and IL-1β), leading to overexpression of matrix degrading proteases causing the sine qua non of OA, that is, cartilage destruction.1 It has now been increasingly recognized that OA is not only a disease of cartilage, but that of the whole joint including the subchondral bone, synovium, menisci and ligaments.
Patients present with pain, limitation of movement, varying degrees of stiffness, occasionally effusion, and deformity in severe grades. Radiographically KOA has 4 grades [kellgren-Lawrence(K-L) classification], based on presence of osteophytes and joint space reduction.2 The management goals in KOA are to keep patient symptom free while maintaining or improving joint function. Non operative treatment in the form of acetaminophen, NSAIDS, physical therapy, are usually the first line of management, but these agents do not alter the basic joint pathology and they need long term consumption which may lead to adverse events. Interventional methods consist of intra articular injections, which avert the systemic side effects of oral medications by direct delivery of the drug at the site of disease. With an aim at reverting or stopping the degenerative process, the recent focus has shifted towards regenerative medicine. Autologous platelet rich plasma(PRP) in different forms has generated a lot of interest with its safety and clinical efficacy reported by many studies, particularly in early grades when compared to hyaluronic acid(HA), steroids or placebo, although its capability to change joint structure is still a matter of debate.3, 4, 5, 6, 7, 8, 9, 10, 11 Plasma rich in growth factors (PRGF) is a low concentrate PRP devoid of cellular components, with proven safety and efficacy.11, 12, 13, 14 It facilitates delivery of growth factors(GFs) (IGF,VEGF, TGF β1,BMPs, vitronectin, fibronectin among others) directly at the target site without need for in vivo activation and may have lesser adverse reactions related to cellular components.11 All these GFs have anabolic, anti-inflammatory, chondroprotective and immunomodulatory properties.15, 16, 17
The lack of fixed protocols, inconsistencies with its preparation and the need for in-vivo activation may result in varying effects. In contrast to PRP which has been compared extensively with HA, placebo and steroids, with conflicting results, there is scarcity of studies employing PRGF for KOA, henceforth we aim to evaluate its clinical efficacy and effect on specific serum OA biomarker of cartilage degradation Collagen 2-1 (Coll2-1) levels in a randomized placebo controlled study.
2. Materials and methods
2.1. Study design
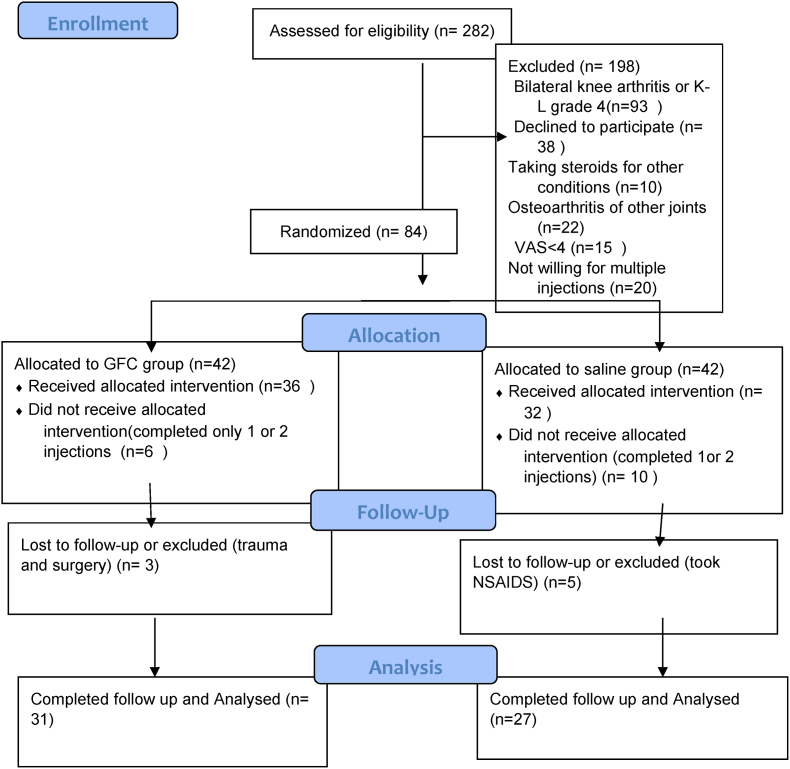
This prospective interventional study was a single centre, randomized, triple blinded, placebo controlled trial conducted in a tertiary referral centre (Fig. 1). The protocol of study and the amendments were reviewed and approved by institutional review board and ethics committee. An informed consent was taken from all patients for intervention and publication of data.
Fig. 1.
Flow diagram of the study.
2.2. Study participants
Patients aged >18 years with clinical features of KOA and showing K-L grade II, III on standing anteroposterior and lateral knee radiographs with moderate pain on VAS(>4) were included. Patients were excluded if they had bilateral KOA, received previous injections in the same knee for last 6 months, patients on steroids or having multiple joint OA, previous knee surgery, secondary knee arthritis(including inflammatory arthritis), presence of systemic illness or local infection, uncontrolled diabetes, malignancy, genu varum or valgum of more than 10°, coagulopathy, NSAID intake in previous 3 weeks, platelet count of less than 1.5 lakh/μl.
2.3. Randomization and blinding
A computer generated sequence was used to randomize patients in a 1:1 ratio by using website randomization.com, where they were distributed in 16 blocks with 6 cases in each block. Principal investigators had no access to allocation sequence, it was restricted to a third party. Eligibility was determined by coordinating physician based on inclusion and exclusion criteria and were allocated to one of the two groups, growth factor concentrate (GFC) and saline. To ensure proper blinding of the injector and patients, the syringes with final product to be injected were loaded from vacutainer by a resident not involved in study and covered with opaque sterile marking tape(sterilization indicator tape). The accessor was also blinded to the group allocation.
2.4. PRGF preparation and procedure
In order to maintain proper blinding, blood was collected from every included patient as routine blood investigations (Cell count,ESR,CRP, blood Sugar,PT-INR, clotting and bleeding time) were done in all. We have used commercially available PRGF, “OSSINEXT GFC therapy kit” (Wockhardt ltd).
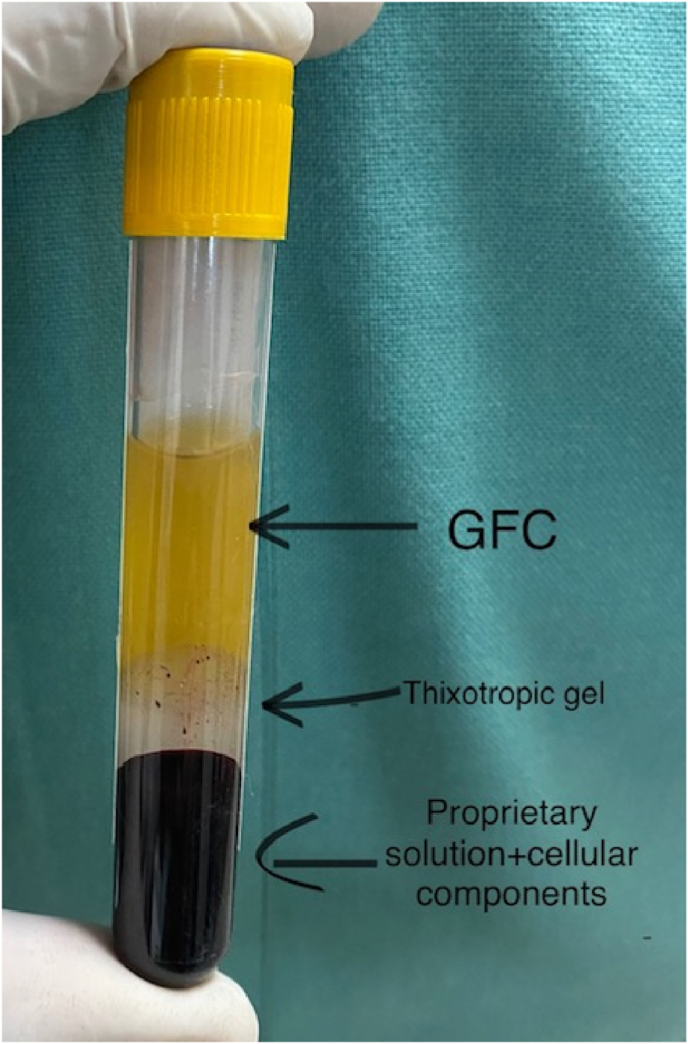
6-8 ml of the patient's blood was collected in the given vacutainer containing Wockhardt's proprietary solution which was mixed with this solution by gently inverting it few times and then kept it upright for half hour (Fig. 2). This results in platelet activation and release of GFs from platelet granules. This solution was centrifuged at 3400 rpm for 10mins in the centrifuge machine available in the institute blood bank. This centrifugation leads to GFC separation from rest of blood components. The tube final components are the propriety solution + cellular components at bottom with GFC (yellowish fluid) at the top and thixotropic gel in between them (Fig. 3). This GFC was transferred to another sterile syringe. 3 ml of GFC concentrate was obtained which was administered within half hour.
Fig. 2.
6–8 ml of blood collected in the vacutainer.
Fig. 3.
The final product after single centrifugation.(GFC-Growth factor concentrate).
The knee to be injected was cleaned and painted properly with betadine. Each patient received 3 ml of either GFC or normal saline 0.9%(NS) into the knee joint via the anterolateral approach under sterile conditions. All injections were given without local anaesthsia.
2.5. Post procedure protocol
Patients were observed in a separate room for 60 mins and sent home on oral tramadol (maximum up to 72 h). They were instructed to continue with their routine daily activities and to avoid co-intervention with NSAIDS, steroids, chondoprotective medications and other anti-inflammatory drugs. They were permitted to take oral tramadol for pain (maximum up to 50 mg/day) and were asked to note down the day tramadol was taken on a medication diary. Counselling regarding lifestyle modification and exercise was given to all patients consistent with standard KOA care. 3 injections one month apart were given in each patients. Patients were called on their cellphone numbers every two weeks and enquired about any complications or tramadol intake.
Patients, accessor and physician performing follow up assessment were kept blinded to group allocation.
2.6. Outcomes
The primary outcome measures were visual analogue scale (VAS), Western Ontario and McMaster Universities Arthritis Index(WOMAC) and serum coll2-1 estimation. VAS (Minimal clinically important difference[MCID] >2 point difference of mean) was measured on a 10cm line (0-no pain,10-worst pain, 4-6 moderate pain). WOMAC (higher scores indicated worse symptoms and function,MCID >12% of baseline). VAS and WOMAC were calculated at baseline, just before each injection and then at 3,6 and 12 months of last injection. Serum coll2-1 estimation was done at baseline and then at final follow up interval of 12 months post intervention. Cusabio Technology LLC's (Houston USA) human Coll2-1 ELISA kits were used for detection of this biomarker. This test utilizes the sandwich ELISA test technique for quantitative analysis.
Secondary objective was to assess the average intake of drug (tramadol) for pain.
Sample size calculation: Based on the prevalence of KOA(22%),18 with assumed power of study to be 80%(β = 0.2),a false positive rate of 5%(α = 0.05), the minimum sample size required in the present study was 66. 68 patients received the allocated intervention in total.
Statistical analysis: SPSS version 22 was opted for data analysis. Independent t-test was used for comparison of means across groups, and to assess inter and intragroup changes, ANOVA was used. Post hoc analysis was also done. A 2 sided P < 0.05 was considered as statistically significant.
3. Results
Data from 58 patients (27 in NS, 31 in GFC group) in total was available for assessment at final follow up (10 lost to follow up).
Majority of the patients were female. Mean BMI (kg/m2) was higher in the GFC group(28.52 vs 26.57, P = 0.042). Rest of the baseline characteristics; number of patients, mean age, K-L grade(II and III) were similar in both the groups. There was no statistical difference with regard to baseline VAS, WOMAC and Coll2-1 levels among the two groups(P > 0.05). (Table 1).
Table 1.
Baseline parameters.
| GFC group(n = 31) | SALINE group(n = 27) | P-value | |
|---|---|---|---|
| Age (year) | 57.45 ± 11.43 | 55.85 ± 7.50 | 0.54 |
| Sex-F/M, N,% | 25/6,80.6/19.4 | 15/12, 51.7/48.4 | 0.039 |
| BMI(Kg/m2) | 28.52 ± 2.35 | 26.57 ± 4.58 | 0.042 |
| K-L Grade(N) | 0.44 | ||
| 2 | 7 (22.6) | 4 (14.8) | |
| 3 | 24 (77.4) | 23 (85.2) | |
| WOMAC baseline(mean) | 71.77 ± 7.76 | 67 ± 9.36 | 0.08 |
| VAS baseline(mean) | 7.87 ± 0.76 | 7.89 ± 0.97 | 0.94 |
3.1. Outcome measures
VAS: Mean VAS scores improved to 5.26+-1.18, 4.29 +-1.42 and 2.94+-1.46 at 3,6 and 12 months respectively, in the GFC group. This improvement from baseline (7.87 +-0.76) was statistically significant (P < 0.001). In saline group, there was a significant mean VAS change from 7.89+-0.97 at baseline to 6.11+-1.16, 6.48+-1.34 and 6.37+-1.39 at 3,6 and 12 months respectively. This improvement was statistically significant (P < 0.05). (Table 2).
Table 2.
Intergroup and intragroup Comparison of VAS among the study groups at different intervals.
| Interval | GFC Group |
Saline Group |
t-test | p value | ||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| Baseline | 7.87 | 0.76 | 7.89 | 0.97 | 0.006 | 0.94 |
| 3 months | 5.26 | 1.18 | 6.11 | 1.16 | 7.68 | 0.008* |
| 6 Months | 4.29 | 1.42 | 6.48 | 1.34 | 36.22 | <0.001* |
| 12 Months | 2.94 | 1.46 | 6.37 | 1.39 | 83.52 | <0.001* |
| Post Hoc Analysis | p value | |
|---|---|---|
| Baseline vs 3 months | 0.003* | 0.009* |
| Baseline vs 6 Months | <0.001* | 0.012* |
| Baseline vs 12 Months | <0.001* | 0.011* |
(*statistically significant).
WOMAC: Baseline mean WOMAC was 71.77+-7.6, in the GFC group which improved to 52.32+-8.95, 46+-11.90 and 34.84+-13.63 at 3,6 and 12 months respectively. This improvement was statistically significant (P < 0.001). Similarly in NS group, mean baseline WOMAC was 67+-9.36, which improved to 64+-7.33,62.78+-6.70 and 58.93+-8.06 at 3,6,12 months respectively. This was statistically significant only at 12 months (P = 0.029). (Table 3).
Table 3.
Intergroup and intragroup Comparison of WOMAC among the study groups at different intervals.
| Interval | GFC Group |
Saline Group |
t-test | p value | ||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| Baseline | 71.77 | 7.76 | 67 | 9.36 | 2.94 | 0.08 |
| 3 months | 52.32 | 8.95 | 64 | 7.33 | 28.98 | <0.001* |
| 6 Months | 46 | 11.90 | 62.78 | 6.70 | 41.97 | <0.001* |
| 12 Months | 34.84 | 13.63 | 58.93 | 8.06 | 64.56 | <0.001* |
| Post Hoc Analysis | p value | |
|---|---|---|
| Baseline vs 3 months | <0.001* | 0.41 |
| Baseline vs 6 Months | <0.001* | 0.24 |
| Baseline vs 12 Months | <0.001* | 0.029* |
(*statistically significant).
3.2. Intergroup comparison
Intergroup comparison revealed VAS was significantly better in GFC group at every evaluation, difference = 0.85,95% CI of 0.2–1.5,(P = 0.008) at 3 months, difference = 2.2,95% CI of 1.4–2.9,(P < 0.0001) at 6 months, and difference = 3.43,95% CI of 2.7–4.2,(P < 0.0001) at 12 months (Table 2).
Similarly WOMAC was also significantly better in the GFC group at all follow intervals, difference = 11.68,95% CI of 7.3–16.0 [P < 0.001], difference = 16.78,95% CI of 11.6–21.9 [P < 0.001] and difference = 24.09,95% CI of 18.1–31.1[P < 0.001] at 3,6 and 12 months respectively (Table 3).
Baseline Coll2-1 levels were 1407.87+-404.29 pg/ml in the GFC group, and 1461.44+-369.23 pg/ml in the NS group. Only GFC group showed statistical significant decrease in Coll2-1 at 12 months (1000.93+-373.65) (P = 0.007). (Table 4).
Table 4.
Serum Coll2-1 level (picogram/ml) comparison between the two groups.
| Interval | Group GFC |
Group Saline |
t-test | p value | ||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| Baseline | 1407.87 | 404.29 | 1461.44 | 369.23 | 0.28 | 0.60 |
| 12 Months | 1000.90 | 373.65 | 1311.30 | 375.89 | 9.90 | 0.003* |
| t-test | 6.19 | 1.81 | ||||
| p value | 0.007* | 0.29 | ||||
(*statistically significant).
Average number of days of analgesic (tramadol) intake was 1.39 ± 1.45 in GFC group and 10.33 ± 2.79 in saline group (P < 0.001).
4. Discussion
The findings of interest in this study are that serial injections of GFC if given intra-articularly at monthly intervals provide profound relief of pain (VAS) and improves function (WOMAC), lasting up to 1 year in grade II and III of patients with KOA compared to placebo. Biomarker estimation with serum cartilage degradation marker (Coll2-1) pre and post therapy demonstrated that patients receiving GFC had a significant decrease in serum levels of this specific biomarker at 12 months, which may point towards a protective effect of GFs on articular cartilage. The interesting finding to note was that the patients in the saline group also had statistically significant improvement in VAS. WOMAC also showed significant change at 12 months, but both the scores failed to reach MCIDs for this group at any time point. There was no significant change in Coll2-1 levels in this group. The mechanism of how a placebo effect occurs is still a matter of controversy and debate. Multiple sources have suggested a neurobiological phenomenon to be the cause of analgesic effect, which leads to increase in endogenous opioid system specifically its activation of μ-receptors.19,20 The gold standard for interventional studies are placebo controlled trials and use of normal saline as a ‘placebo control’ is widespread. There is evidence of clinically significant short term(<3months) and long term(6–12 months) improvements in patient reported outcomes with intraarticular NS when provided during comparison studies and hence the use of saline as a placebo has been challenged by many studies as it may have active analgesic effect in addition to analgesia because of placebo effect (approximately 30% pain reduction).21,22 However, another study revealed that the effects attributed to NS may be procedural rather than physiological as its comparison to sham(dry needling) demonstrated equivalent subjective outcomes at all time intervals through 6 months.23 We also observed that NS may have some analgesic effect but fails to provide clinically meaningful symptom relief.
Blood derived products in different forms (PRP or autologous conditioned plasma[ACP], autologous conditioned Serum[ACS], concentrated growth factor[CGF], homologous platelet lysate[PL]) have been used for treatment of KOA for few decades now. They differ in method of preparation mainly and are reported to have different concentration of cells, cytokines and growth factors. PRP or ACP is obtained from whole blood collected in anticoagulant containing tubes which are subjected to differential centrifugation, the final product yields a plasma which is rich in platelets.24 ACS(sanakin,orthokine) was developed in the mid 90's, with the idea of obtaining an injectable material enriched mainly with IL-1Ra by incubating venous blood at 37° for hours in a specially designed syringe containing medical grade glass beads, which leads to dose-dependent production of anti-inflammatory cytokine IL-1Ra by white blood cells.25 Post incubation, syringe is centrifuged, and the serum supernatant (ACS) is filtered. CGF is similar to Platelet Rich Fibrin(PRF, involves a different centrifuge speed), but different from PRP as it does not require use of any anticoagulant or thrombin, only centrifuged autologous blood and in comparison to PRF, CGF is reported to contain a denser and richer GF‒fibrin matrix with a 3D fibrin network in which growth factors are closely bound to one another.26 PL is prepared by activation with human thrombin or by the addition of calcium salts to counterbalance the anticoagulant added for blood collection.27 Thrombin and calcium ions activate platelets and convert fibrinogen into fibrin, resulting in the formation of a platelet gel and a liquid PL. Autologous or single-donor PLs can also be prepared by a freeze–thaw process (−80/37 °C), with centrifugation at 2600 rpm×g for 30 mins and storage at −80 °C until use.27 PL preparations are reported to be acellular, thereby reducing concerns over immunogenicity, and contains high concentrations of growth factors and cytokines.28 In addition, platelet lysate preparations can be filtered to remove immunoglobulins and can be stored for longer periods of time and made available for future use.
Numerous studies have reported PRP to be better than placebo/HA/steroids in improving long term (up to 1 year) patient reported outcomes,3, 4, 5, 6, 7, 8, 9, 10, 11 with a tendency towards better efficacy in early grades but positive clinical outcomes can be seen in advanced grades as well.8, 9, 10, 11,29,30 In a placebo controlled trial of 610 participants by Chu et al.,31 patients receiving 3 weekly injections of PRP or sham saline, reported significantly better WOMAC pain and function scores in the PRP group at 12 months and the deterioration in tibiofemoral cartilage volume as well as synovial fluid TNF alpha,IL-1β levels were lower in PRP group over 60 months. Lacko M.32 and colleagues found that there was a significant elevation in serum levels of anabolic and anti-inflammatory biomarkers accompanied by reduced levels of pro-inflammatory biomarkers, 3 months after three intra-articular applications of PRP.
Despite the abundance of evidence to support the clinical use of PRP in KOA, there are studies which have reported its inefficiency to provide significant symptom relief or bring about any change in joint structure.33,34 The contrasting reports may be due to several factors related to PRP; amount, frequency, duration, concentration, type(Leukocyte poor or rich,activated,inactivated) as well as patient factors(native platelet count, BMI, age, gender, grade). GFC is an activated platelet concentrate, devoid of RBCs and WBCs (hence the minimal or no pro-inflammatory activity), requires single centrifugation and small volume of patient's blood(4–8ml). According to one argument, PRP activation prior to application ensures GFs are released optimally, while the contrasting school of thought argues that appropriate GF release will occur in response to in vivo environment.35 Weibrich et al.36 reported a 5-fold increase in GF level in activated PRP than inactivated PRP and Lee et al.37 reported only a 1.06-fold increase. However, studies using PRGF are few. Reissadat et al.11 reported that 2-injections of PRGF, 3-weeks apart resulted in better pain and functional outcomes(global, pain, and ADL score of Lequesne) than 3 weekly injections of HA, through a 48 week period. Similar results were seen by vaquerizo et al.14 with superiority in improvement of Lequesne index, WOMAC, and OMERACT-OARSI responders at 24–48 weeks of PRGF-Endoret therapy over HA. In another RCT of 200 patients comparing the results of HA, ozone, PRP and PRGF, the authors reported PRP and PGRF demonstrated better therapeutic effects at 12 months.38 Multiple monthly injections of activated PRP may reduce the articular cartilage damage evident on MRI as soon as 6 months in grade 2,3 KOA as reported by Lisi C et al.39 In a systemic review of 14 trials, the authors concluded that activated PRP may result in better clinical outcomes than inactivated PRP.40 Inactivated PRP depends on the patient's body for activation, thus the effects may vary. In order to remove this uncertainty we have used GFC. Our results are in accordance with the above studies, with serial GFC injections demonstrating significantly improved pain and function outcomes through a 12 month period.
Similar to Kon et al.41 who reported PRP to be more effective in younger patients and in contrast to results observed by Patel5 and Reissadeit6 et al. (age not to be a significant factor in the outcome scores), we observed younger patients performed better. Sex and BMI did not have any influence on outcomes according to multiple studies despite having a greater proportion of female patients3, 4, 5, 6,29 while as others41 have observed better results in individuals with low BMI. Male patients performed better and BMI had no effect on our results.
Coll2-1 is a cleavage product of type 2 collagen, the predominant component of cartilage ECM, specific for hyaline cartilage.42 Cartilage degradation is the hallmark of osteoarthritis and Coll2-1 levels have seen to be elevated in OA and hence it can be used as a marker of disease progression43 as well as a predictor for response to therapy.42,44 Apart from being a biomarker, Coll2-1 is an active contributor to the disease process and can be used as a therapeutic target.16 Fawyzy et al.44 reported the decrease in serum levels of this biomarker 3 months post intervention with single injection of PRP was significant, but this study lacked a comparator group. Accordingly, our estimation of serum Coll2-1 showed a significant decrease in its levels at 1 year after GFC therapy and no significant change following NS injections, indicating GFC may have some chondroprotective effect.
None of our patients had any complications except for injection related pain which got relieved in few hours.
Limitations: Single centre study with low sample size which can be explained by the fact that we have included patients with unilateral KOA only. We have presumed that the product produced by this particular kit is concentrated growth factors and due to financial constraints, growth factor estimation was done for few cases only. Further large studies with both specific serum and synovial biomarker analysis along with modern highly sensitive imaging are needed.
5. Conclusion
Serial(three) monthly intraarticular GFC injections result in clinically meaningful improvements of patient reported pain and function outcome scores, with benefits sustaining up to 12 months, in KOA grade II and III. Biochemical analysis revealed a significant reduction in serum levels of cartilage degradation biomarker Coll2-1.
Funding/sponsorship
None received.
Informed consent
Taken from all participants for study and data use.
Institutional ethics committee approval
Taken prior to start of study.
Author contributions
Concept-A.S.,A.H.,S.B.; Design-A.S.,A.H.,S.B.; Supervision-A.S.,A.H.,S.B.; Resources- H.H.,A.S.,A.H.; Materials -H.H.,A.S.,A.H.,S.B.; Data processing-H.H.,A.S.,A.H.,A.G; Literature search-H.H.,A.S.,A.H.,A.G.; Writing manuscript- A.H.,A.S.,S.B.; Critical review A.S.,S.B.
Declaration of competing interest
None to declare.
Acknowledgement
We thank the technical manager in our Hospital Laboratory as well as laboratory hematologist for their cooperation and for providing us with laboratory equipment.
Contributor Information
Amit Saraf, Email: amitsaraf_75@yahoo.com.
Altaf Hussain, Email: khan.altaf7@gmail.com.
Sandeep Bishnoi, Email: sandeepbishnoi.bishnoi@gmail.com.
Hamza Habib, Email: hamzahabib@ymail.com.
Abhishek Garg, Email: Abhishekgarg1307@ymail.com.
References
- 1.Primorac D., Molnar V., Rod E., et al. Knee osteoarthritis: a review of pathogenesis and state-of-the-art non-operative therapeutic considerations. Genes. 2020 Jul 26;11(8):854. doi: 10.3390/genes11080854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kellgren J., Lawrence J. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957 Dec;16(4):494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith P.A. Intra-articular autologous conditioned plasma injections provide safe and efficacious treatment for knee osteoarthritis: an FDA-sanctioned, randomized, double-blind, placebo-controlled clinical trial. Am J Sports Med. 2016 Apr;44(4):884–891. doi: 10.1177/0363546515624678. [DOI] [PubMed] [Google Scholar]
- 4.Cerza F., Carni S., Carcangiu A., et al. Comparison between hyaluronic acid and platelet-rich plasma, intra-articular infiltration in the treatment of gonarthrosis. Am J Sports Med. 2012 Dec;40(12):2822–2827. doi: 10.1177/0363546512461902. [DOI] [PubMed] [Google Scholar]
- 5.Patel S., Dhillon M.S., Aggarwal S., Marwaha N., Jain A. Treatment with platelet-rich plasma is more effective than placebo for knee osteoarthritis: a prospective, double-blind, randomized trial. Am J Sports Med. 2013 Feb;41(2):356–364. doi: 10.1177/0363546512471299. [DOI] [PubMed] [Google Scholar]
- 6.Raeissadat S.A., Rayegani S.M., Hassanabadi H., et al. Knee osteoarthritis injection choices: platelet-rich plasma (PRP) versus hyaluronic acid (a one-year randomized clinical trial) Clin Med Insights Arthritis Musculoskelet Disord. 2015 Jan 7;8:1–8. doi: 10.4137/CMAMD.S17894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanchez M., Fiz N., Azofra J., et al. A randomized clinical trial evaluating plasma rich in growth factors (PRGF-Endoret) versus hyaluronic acid in the short-term treatment of symptomatic knee osteoarthritis. Arthroscopy. 2012 Aug;28(8):1070–1078. doi: 10.1016/j.arthro.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 8.Cook C.S., Smith P.A. Clinical update: why PRP should Be your first choice for injection therapy in treating osteoarthritis of the knee. Curr Rev Musculoskelet Med. 2018 Dec;11(4):583–592. doi: 10.1007/s12178-018-9524-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McLarnon M., Heron N. Intra-articular platelet-rich plasma injections versus intra-articular corticosteroid injections for symptomatic management of knee osteoarthritis: systematic review and meta-analysis. BMC Muscoskel Disord. 2021 Jun;22(1):550. doi: 10.1186/s12891-021-04308-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Migliorini F., Driessen A., Quack V., et al. Comparison between intra-articular infiltrations of placebo, steroids, hyaluronic and PRP for knee osteoarthritis: a Bayesian network meta-analysis. Arch Orthop Trauma Surg. 2021 Sep;141(9):1473–1490. doi: 10.1007/s00402-020-03551-y. [DOI] [PubMed] [Google Scholar]
- 11.Raeissadat S.A., Gharooee Ahangar A., Rayegani S.M., Minator Sajjadi M., Ebrahimpour A., Yavari P. Platelet-rich plasma-derived growth factor vs hyaluronic acid injection in the individuals with knee osteoarthritis: a one year randomized clinical trial. J Pain Res. 2020 Jul 8;13:1699–1711. doi: 10.2147/JPR.S210715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anitua E., Sánchez M., Orive G., Padilla S. A biological therapy to osteoarthritis treatment using platelet-rich plasma. Expet Opin Biol Ther. 2013;13(8):1161–1172. doi: 10.1517/14712598.2013.801450. [DOI] [PubMed] [Google Scholar]
- 13.Sánchez M., Beitia M., Pompei O., et al. Regenerative Medicine. IntechOpen; London, UK: 2020. Isolation,Activation, and mechanism of action of platelet-rich plasma and its applications for joint repair. [DOI] [Google Scholar]
- 14.Vaquerizo V., Plasencia M.A., Arribas I., et al. Comparison of intraarticular injections of plasma rich in growth factors (PRGF-Endoret) versus durolane hyaluronic acid in the treatment of patients with symptomatic osteoarthritis: a randomized controlled trial. Arthroscopy. 2013 Oct;29(10):1635–1643. doi: 10.1016/j.arthro.2013.07.264. [DOI] [PubMed] [Google Scholar]
- 15.Bendinelli P., Matteucci E., Dogliotti G., et al. Molecular basis of anti-inflammatory action of platelet-rich plasma on human chondrocytes: mechanisms of NF-κB inhibition via HGF. J Cell Physiol. 2010;225(3):757–766. doi: 10.1002/jcp.22274. [DOI] [PubMed] [Google Scholar]
- 16.Montaseri A., Busch F., Mobasheri A., et al. IGF-1 and PDGF-bb suppress IL-1β-induced cartilage degradation through down-regulation of NF-κB signaling: involvement of Src/PI-3K/AKT pathway. PLoS One. 2011;6(12) doi: 10.1371/journal.pone.002866344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sakata R., McNary S.M., Miyatake K., et al. Stimulation of the superficial zone protein and lubrication in the articular cartilage by human platelet-rich plasma. Am J Sports Med. 2015;43(6):1467–1473. doi: 10.1177/0363546515575023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pal C.P., Singh P., Chaturvedi S., Pruthi K.K., Vij A. Epidemiology of knee osteoarthritis in India and related factors. Indian J Orthop. 2016 Sep;50(5):518–522. doi: 10.4103/0019-5413.189608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kienle G.S., Kiene H. The powerful placebo effect: fact or fiction? J Clin Epidemiol. 1997 Dec;50(12):1311–1318. doi: 10.1016/s0895-4356(97)00203-5. [DOI] [PubMed] [Google Scholar]
- 20.Finniss D.G., Kaptchuk T.J., Miller F., Benedetti F. Biological, clinical, and ethical advances of placebo effects. Lancet. 2010 Feb 20;375(9715):686–695. doi: 10.1016/S0140-6736(09)61706-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saltzman B.M., Leroux T., Meyer M.A., et al. The therapeutic effect of intra-articular normal saline injections for knee osteoarthritis: a meta-analysis of evidence level 1 studies. Am J Sports Med. 2017;45(11):2647–2653. doi: 10.1177/0363546516680607. [DOI] [PubMed] [Google Scholar]
- 22.Altman R.D., Devji T., Bhandari M., Fierlinger A., Niazi F., Christensen R. Clinical benefit of intra-articular saline as a comparator in clinical trials of knee osteoarthritis treatments: a systematic review and meta-analysis of randomized trials. Semin Arthritis Rheum. 2016 Oct;46(2):151–159. doi: 10.1016/j.semarthrit.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 23.Tambiah J.R.S., Simsek I., Swearingen C.J., et al. Comparing patient-reported outcomes from sham and saline-based placebo injections for knee osteoarthritis: data from a randomized clinical trial of lorecivivint. Am J Sports Med. 2022 Mar;50(3):630–636. doi: 10.1177/03635465211067201. [DOI] [PubMed] [Google Scholar]
- 24.Dhurat R., Sukesh M. Principles and methods of preparation of platelet-rich plasma: a review and author's perspective. J Cutan Aesthetic Surg. 2014 Oct-Dec;7(4):189–197. doi: 10.4103/0974-2077.150734. PMID: 25722595; PMCID: PMC4338460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Evans C.H., Chevalier X., Wehling P. Autologous conditioned serum. Phys Med Rehabil Clin. 2016 Nov;27(4):893–908. doi: 10.1016/j.pmr.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 26.Mijiritsky E., Assaf H.D., Peleg O., Shacham M., Cerroni L., Mangani L. Use of PRP, PRF and CGF in periodontal regeneration and facial rejuvenation-A narrative review. Biology. 2021 Apr 10;10(4):317. doi: 10.3390/biology10040317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shih D.T., Burnouf T. Preparation, quality criteria, and properties of human blood platelet lysate supplements for ex vivo stem cell expansion. N Biotech. 2015 Jan 25;32(1):199–211. doi: 10.1016/j.nbt.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Da Fonseca L., Santos G.S., Huber S.C., Setti T.M., Setti T., Lana J.F. Human platelet lysate-A potent (and overlooked) orthobiologic. J Clin Orthop Trauma. 2021 Jul 28;21 doi: 10.1016/j.jcot.2021.101534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Joshi Jubert N., Rodríguez L., Reverté-Vinaixa M.M., Navarro A. Platelet-rich plasma injections for advanced knee osteoarthritis: a prospective, randomized, double-blinded clinical trial. Orthop J Sports Med. 2017 Feb 13;(2):5. doi: 10.1177/2325967116689386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saraf A., Hussain A., Bishnoi S., Azam G., Habib H. Serial platelet-rich plasma intra-articular injections in kellgren and lawrence grade IV knee joint osteoarthritis: a prospective blinded placebo-controlled interventional study. Indian J Orthop. 2022 Aug 30;56(10):1722–1728. doi: 10.1007/s43465-022-00730-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chu J., Duan W., Yu Z., et al. Intra-articular injections of platelet-rich plasma decrease pain and improve functional outcomes than sham saline in patients with knee osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2022 Dec;30(12):4063–4071. doi: 10.1007/s00167-022-06887-7. [DOI] [PubMed] [Google Scholar]
- 32.Lacko M., Harvanová D., Slovinská L., et al. Effect of intra-articular injection of platelet-rich plasma on the serum levels of osteoarthritic biomarkers in patients with unilateral knee osteoarthritis. J Clin Med. 2021 Dec 11;10(24):5801. doi: 10.3390/jcm10245801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dório M., Pereira R.M.R., Luz A.G.B., Deveza L.A., de Oliveira R.M., Fuller R. Efficacy of platelet-rich plasma and plasma for symptomatic treatment of knee osteoarthritis: a double-blinded placebo-controlled randomized clinical trial. BMC Muscoskel Disord. 2021 Sep 24;22(1):822. doi: 10.1186/s12891-021-04706-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bennell K.L., et al. Effect of intra-articular platelet-rich plasma vs placebo injection on pain and medial tibial cartilage volume in patients with knee osteoarthritis: the RESTORE randomized clinical trial. JAMA. 2021 Nov 23;326(20):2021–2030. doi: 10.1001/jama.2021.19415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hamilton B., Tol J.L., Knez W., Chalabi H. Exercise and the platelet activator calcium chloride both influence the growth factor content of platelet-rich plasma (PRP): overlooked biochemical factors that could influence PRP treatment. Br J Sports Med. 2015 Jul;49(14):957–960. doi: 10.1136/bjsports-2012-091916. Epub 2013 Jun 15. [DOI] [PubMed] [Google Scholar]
- 36.Weibrich G., Kleis W.K., Buch R., Hitzler W.E., Hafner G. The Harvest Smart PRePTM system versus the Friadent-Schütze platelet-rich plasma kit. Clin Oral Implants Res. 2003 Apr;14(2):233–239. doi: 10.1034/j.1600-0501.2003. [DOI] [PubMed] [Google Scholar]
- 37.Lee J.W., et al. Platelet-rich plasma: quantitative assessment of growth factor levels and comparative analysis of activated and inactivated groups. Arch Plast Surg. 2013 Sep;40(5):530–535. doi: 10.5999/aps.2013.40.5.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raeissadat S.A., Ghazi Hosseini P., Bahrami M.H., et al. The comparison effects of intra-articular injection of Platelet Rich Plasma (PRP), Plasma Rich in Growth Factor (PRGF), Hyaluronic Acid (HA), and ozone in knee osteoarthritis; a one year randomized clinical trial. BMC Muscoskel Disord. 2021 Feb 3;22(1):134. doi: 10.1186/s12891-021-04017-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lisi C., Perotti C., Scudeller L., et al. Treatment of knee osteoarthritis: platelet-derived growth factors vs. hyaluronic acid. A randomized controlled trial. Clin Rehabil. 2018 Mar;32(3):330–339. doi: 10.1177/0269215517724193.Epub.2017.Aug.8. PMID: 28783969. [DOI] [PubMed] [Google Scholar]
- 40.Simental-Mendía M., Ortega-Mata D., Tamez-Mata Y., Olivo C.A.A., Vilchez-Cavazos F. Comparison of the clinical effectiveness of activated and non-activated platelet-rich plasma in the treatment of knee osteoarthritis: a systematic review and meta-analysis. Clin Rheumatol. 2022 Dec 11 doi: 10.1007/s10067-022-06463-x. [DOI] [PubMed] [Google Scholar]
- 41.Kon E., Buda R., Filardo G., et al. Platelet-rich plasma: intra-articular knee injections produced favorable results on degenerative cartilage lesions. Knee Surg Sports Traumatol Arthrosc. 2010 Apr;18(4):472–479. doi: 10.1007/s00167-009-0940-8. [DOI] [PubMed] [Google Scholar]
- 42.Deberg M., et al. New serum biochemical markers (Coll 2-1 and Coll 2-1 NO2) for studying oxidative-related type II collagen network degradation in patients with osteoarthritis and rheumatoid arthritis. Osteoarthritis Cartilage. 2005 Mar;13(3):258–265. doi: 10.1016/j.joca.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 43.Hick A.-C., Malaise M., Loeuille D., et al. Cartilage biomarkers coll2-1 and coll2-1NO2 are associated with knee OA MRI features and are helpful in identifying patients at risk of disease worsening. CARTILAGE. 2021;13(1_suppl):1637S–1647S. doi: 10.1177/19476035211021892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fawzy R.M., Hashaad N.I., Mansour A.I. Decrease of serum biomarker of type II Collagen degradation (Coll2-1) by intra-articular injection of an autologous plasma-rich-platelet in patients with unilateral primary knee osteoarthritis. Eur J Rheumatol. 2017 Jun;4(2):93–97. doi: 10.5152/eurjrheum.2017.160076. [DOI] [PMC free article] [PubMed] [Google Scholar]