Abstract
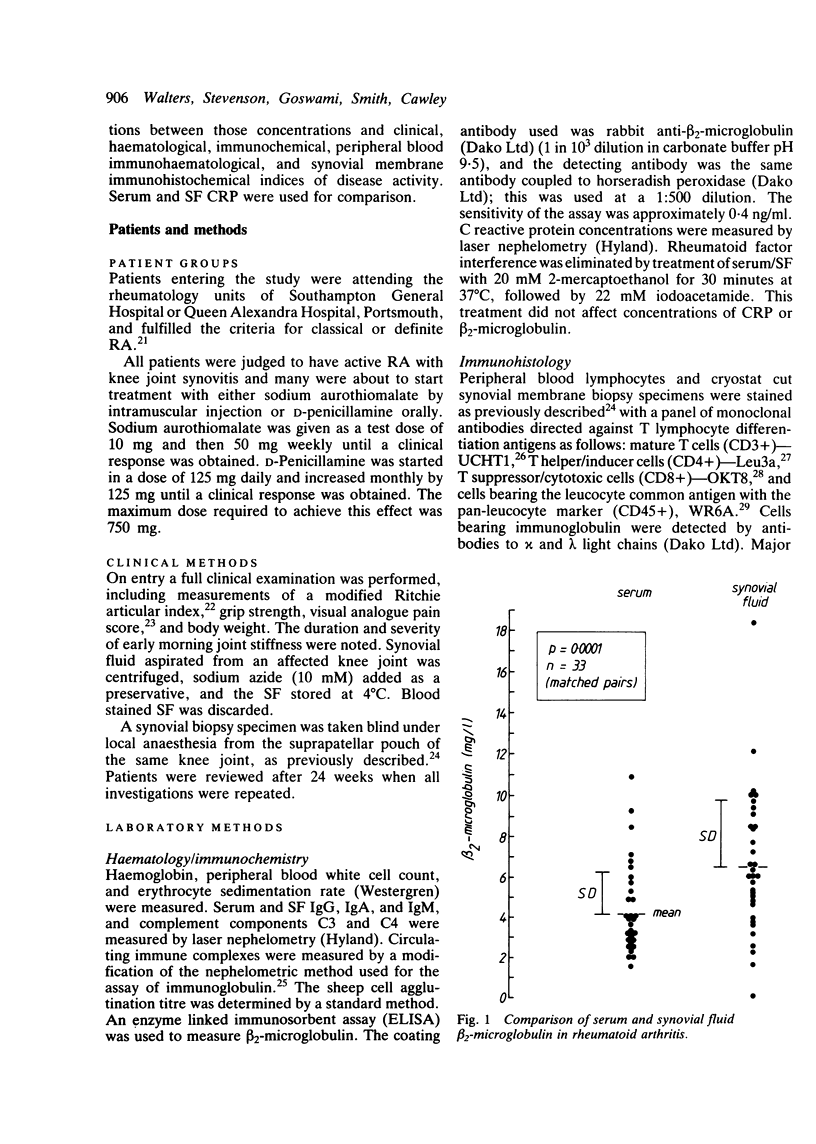
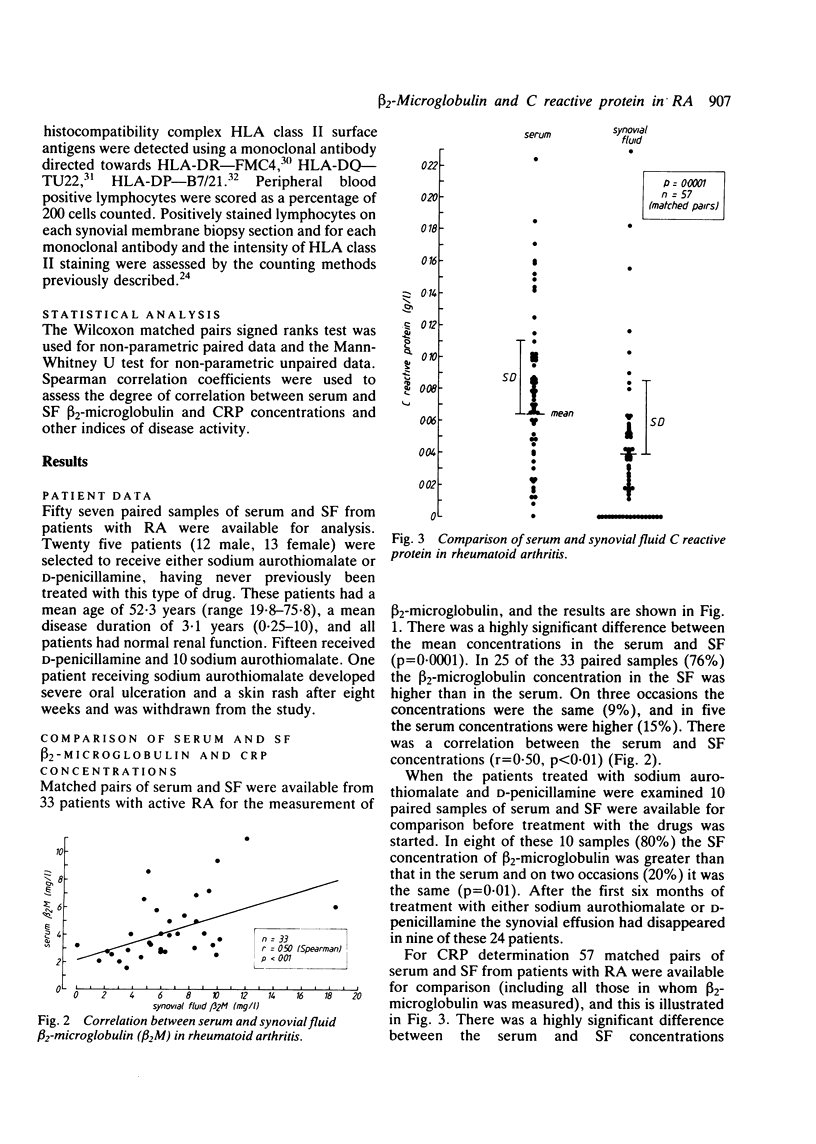
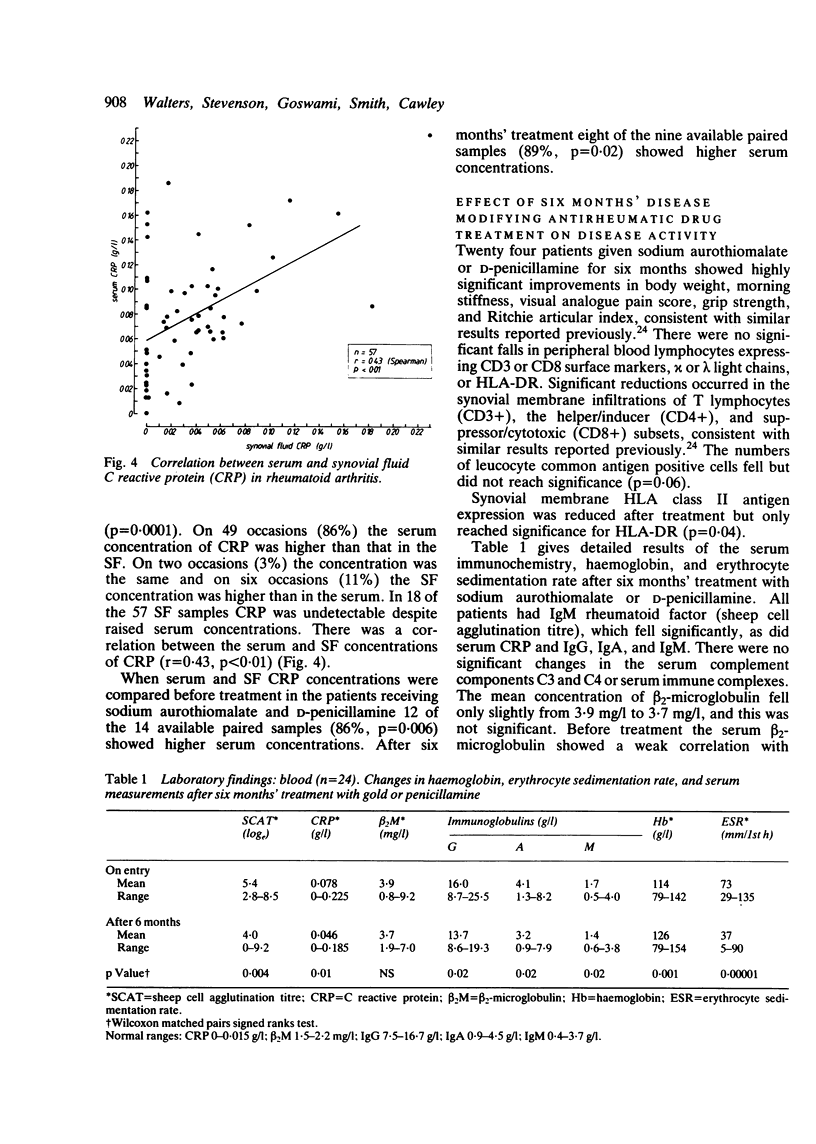
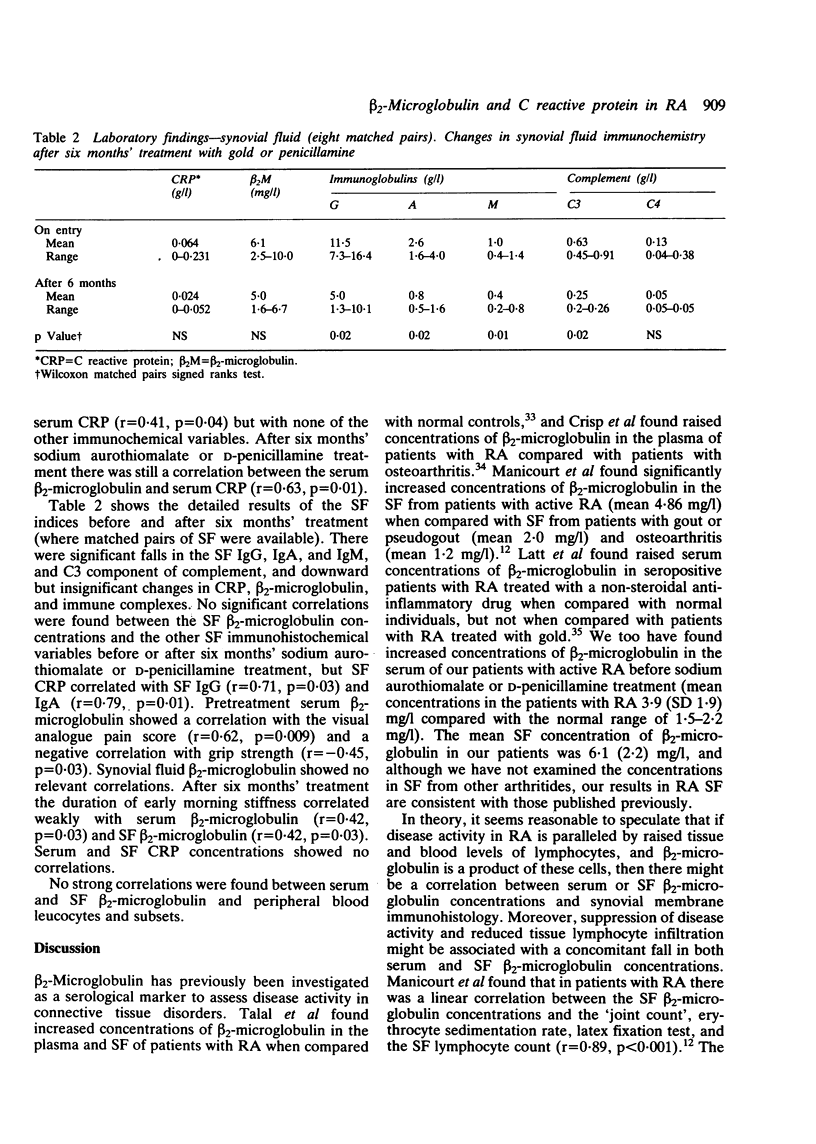
beta 2-Microglobulin and C reactive protein (CPR) were measured in 33 and 57 matched pairs of serum and synovial fluid (SF) respectively, from patients with active rheumatoid arthritis (RA). Serum beta 2-microglobulin concentrations were higher than in normal controls and the SF concentration was higher than the serum concentration on 25 of 33 occasions (76%), suggesting a local production of beta 2-microglobulin within the synovial membrane. There was a correlation between serum and SF concentrations of beta 2-microglobulin (r = 0.50). Patients' serum CRP concentrations in 57 samples were higher than in normal controls and were greater than in the matched SFs on 49 of the 57 paired samples (86%). In 18 samples CRP was absent in the SF, suggesting a local consumption or binding within the synovial membrane. Twenty four patients with RA given either sodium aurothiomalate or D-penicillamine for six months showed highly significant clinical improvements accompanied by reductions in serum and SF immunoglobulin concentrations and knee joint suprapatellar pouch synovial membrane T lymphocyte infiltrates. In this group of patients serum CRP, but not beta 2-microglobulin, fell significantly, but there were no significant changes in SF beta 2-microglobulin or CRP. These data suggest that serum and SF beta 2-microglobulin concentrations are not a useful index for determining the therapeutic response to sodium aurothiomalate and D-penicillamine and that serum rather than SF CRP concentrations are more helpful. The persistent raised serum and SF concentrations of beta 2-microglobulin probably reflect synovial inflammatory infiltrates, which are still considerable despite apparent clinical remission.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- 1958 REVISION of diagnostic criteria for rheumatoid arthritis. Arthritis Rheum. 1959 Feb;2(1):16–20. doi: 10.1002/1529-0131(195902)2:1<16::aid-art1780020104>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Amos R. S., Constable T. J., Crockson R. A., Crockson A. P., McConkey B. Rheumatoid arthritis: relation of serum C-reactive protein and erythrocyte sedimentation rates to radiographic changes. Br Med J. 1977 Jan 22;1(6055):195–197. doi: 10.1136/bmj.1.6055.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach M. L., Huang S. W., Hong R., Poulik M. D. Beta 2-microglobulin: association with lymphocyte receptors. Science. 1973 Dec 28;182(4119):1350–1352. doi: 10.1126/science.182.4119.1350. [DOI] [PubMed] [Google Scholar]
- Beckman I. G., Bradley J., Brooks D. A., Kupa A., McNamara P. J., Thomas M. E., Zola H. Human lymphocyte markers defined by antibodies derived from somatic cell hybrids. II. A hybridoma secreting antibody against an antigen expressed by human B and null lymphocytes. Clin Exp Immunol. 1980 Jun;40(3):593–601. [PMC free article] [PubMed] [Google Scholar]
- Bernier G. M., Fanger M. W. Synthesis of 2 -microglobulin by stimulated lymphocytes. J Immunol. 1972 Aug;109(2):407–409. [PubMed] [Google Scholar]
- Beverley P. C., Callard R. E. Distinctive functional characteristics of human "T" lymphocytes defined by E rosetting or a monoclonal anti-T cell antibody. Eur J Immunol. 1981 Apr;11(4):329–334. doi: 10.1002/eji.1830110412. [DOI] [PubMed] [Google Scholar]
- Crisp A. J., Coughlan R. J., Mackintosh D., Clark B., Panayi G. S. beta 2 microglobulin plasma levels reflect disease activity in rheumatoid arthritis. J Rheumatol. 1983 Dec;10(6):954–956. [PubMed] [Google Scholar]
- Dixon J. S., Bird H. A., Sitton N. G., Pickup M. E., Wright V. C-reactive protein in the serial assessment of disease activity in rheumatoid arthritis. Scand J Rheumatol. 1984;13(1):39–44. doi: 10.3109/03009748409102666. [DOI] [PubMed] [Google Scholar]
- Engleman E. G., Benike C. J., Glickman E., Evans R. L. Antibodies to membrane structures that distinguish suppressor/cytotoxic and helper T lymphocyte subpopulations block the mixed leukocyte reaction in man. J Exp Med. 1981 Jul 1;154(1):193–198. doi: 10.1084/jem.154.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evrin P. E., Wibell L. The serum levels and urinary excretion of 2 -microglobulin in apparently healthy subjects. Scand J Clin Lab Invest. 1972 Feb;29(1):69–74. doi: 10.3109/00365517209081057. [DOI] [PubMed] [Google Scholar]
- Gewurz H. Biology of C-reactive protein and the acute phase response. Hosp Pract (Hosp Ed) 1982 Jun;17(6):67–81. doi: 10.1080/21548331.1982.11702332. [DOI] [PubMed] [Google Scholar]
- Grey H. M., Kubo R. T., Colon S. M., Poulik M. D., Cresswell P., Springer T., Turner M., Strominger J. L. The small subunit of HL-A antigens is beta 2-microglobulin. J Exp Med. 1973 Dec 1;138(6):1608–1612. doi: 10.1084/jem.138.6.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdstock G., Hammond P. G., Iles S., Smith J. L., Tanner A. R., Wright R. Activation of monocytes by portal serum and its relationship to immunoglobulin, immune complex and endotoxin content. Liver. 1982 Sep;2(3):222–229. doi: 10.1111/j.1600-0676.1982.tb00200.x. [DOI] [PubMed] [Google Scholar]
- Hurlimann J., Thorbecke G. J., Hochwald G. M. The liver as the site of C-reactive protein formation. J Exp Med. 1966 Feb 1;123(2):365–378. doi: 10.1084/jem.123.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner I., Gewurz H., Benson M. D. C-reactive protein and the acute-phase response. J Lab Clin Med. 1981 Jun;97(6):739–749. [PubMed] [Google Scholar]
- Latt D., Weiss J. B., Jayson M. I. beta 2-Microglobulin levels in serum and urine of rheumatoid arthritis patients on gold therapy. Ann Rheum Dis. 1981 Apr;40(2):157–160. doi: 10.1136/ard.40.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallya R. K., Vergani D., Tee D. E., Bevis L., de Beer F. C., Berry H., Hamilton E. D., Mace B. E., Pepys M. B. Correlation in rheumatoid arthritis of concentrations of plasma C3d, serum rheumatoid factor, immune complexes and C-reactive protein with each other and with clinical features of disease activity. Clin Exp Immunol. 1982 Jun;48(3):747–753. [PMC free article] [PubMed] [Google Scholar]
- Manicourt D., Brauman H., Orloff S. Plasma and urinary levels of beta2 microglobulin in rheumatoid arthritis. Ann Rheum Dis. 1978 Aug;37(4):328–332. doi: 10.1136/ard.37.4.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manicourt D., Brauman H., Orloff S. Synovial fluid beta 2 microglobulin and hydroxyproline fractions in rheumatoid arthritis and nonautoimmune arthropathies. Ann Rheum Dis. 1980 Jun;39(3):207–216. doi: 10.1136/ard.39.3.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCalmon R. T., Kubo R. T., Grey H. M. Effect of anti-beta2-microglobulin on antigen and allogeneic lymphocyte-induced proliferation of human lymphocytes. J Immunol. 1975 Jun;114(6):1766–1770. [PubMed] [Google Scholar]
- McConkey B., Davies P., Crockson R. A., Crockson A. P., Butler M., Constable T. J., Amos R. S. Effects of gold, dapsone, and prednisone on serum C-reactive protein and haptoglobin and the erythrocyte sedimentation rate in rheumatoid arthritis. Ann Rheum Dis. 1979 Apr;38(2):141–144. doi: 10.1136/ard.38.2.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore G. E., Gerner R. E., Franklin H. A. Culture of normal human leukocytes. JAMA. 1967 Feb 20;199(8):519–524. [PubMed] [Google Scholar]
- Peterson P. A., Cunningham B. A., Berggård I., Edelman G. M. 2 -Microglobulin--a free immunoglobulin domain. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1697–1701. doi: 10.1073/pnas.69.7.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulik M. D., Bernoco M., Bernoco D., Ceppellinni R. Aggregation of HL-A Antigens at the Lymphocyte Surface Induced by Antiserum to beta2-Microglobulin. Science. 1973 Dec 28;182(4119):1352–1355. doi: 10.1126/science.182.4119.1352. [DOI] [PubMed] [Google Scholar]
- Poulik M. D., Bloom A. D. Beta 2 -microglobulin production and secretion by lymphocytes in culture. J Immunol. 1973 May;110(5):1430–1433. [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. A monoclonal antibody reactive with the human cytotoxic/suppressor T cell subset previously defined by a heteroantiserum termed TH2. J Immunol. 1980 Mar;124(3):1301–1307. [PubMed] [Google Scholar]
- Ritchie D. M., Boyle J. A., McInnes J. M., Jasani M. K., Dalakos T. G., Grieveson P., Buchanan W. W. Clinical studies with an articular index for the assessment of joint tenderness in patients with rheumatoid arthritis. Q J Med. 1968 Jul;37(147):393–406. [PubMed] [Google Scholar]
- Rowe I. F., Sheldon J., Riches P. G., Keat A. C. Comparative studies of serum and synovial fluid C reactive protein concentrations. Ann Rheum Dis. 1987 Oct;46(10):721–726. doi: 10.1136/ard.46.10.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J., Huskisson E. C. Graphic representation of pain. Pain. 1976 Jun;2(2):175–184. [PubMed] [Google Scholar]
- Sjöblom K. G., Saxne T., Wollheim F. A. Plasma levels of beta 2-microglobulin in rheumatoid arthritis. Ann Rheum Dis. 1980 Aug;39(4):333–339. doi: 10.1136/ard.39.4.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ström T., Evrin P. E. Beta-2-microglobulin in RA. Ann Rheum Dis. 1981 Apr;40(2):211–213. doi: 10.1136/ard.40.2.211-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talal N., Grey H. M., Zvaifler N., Michalski J. P., Daniels T. E. Elevated salivary and synovial fluid beta2-microglobulin in Sjogren's syndrome and rheumatoid arthritis. Science. 1975 Mar 28;187(4182):1196–1198. doi: 10.1126/science.46621. [DOI] [PubMed] [Google Scholar]
- Tamao Y., Blakley R. L. Direct spectrophotometric observation of an intermediate formed from deoxyadenosylcobalamin in ribonucleotide reduction. Biochemistry. 1973 Jan 2;12(1):24–34. doi: 10.1021/bi00725a005. [DOI] [PubMed] [Google Scholar]
- Walters M. T., Smith J. L., Moore K., Evans P. R., Cawley M. I. An investigation of the action of disease modifying antirheumatic drugs on the rheumatoid synovial membrane: reduction in T lymphocyte subpopulations and HLA-DP and DQ antigen expression after gold or penicillamine therapy. Ann Rheum Dis. 1987 Jan;46(1):7–16. doi: 10.1136/ard.46.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson A. J., DeMars R., Trowbridge I. S., Bach F. H. Detection of a novel human class II HLA antigen. 1983 Jul 28-Aug 3Nature. 304(5924):358–361. doi: 10.1038/304358a0. [DOI] [PubMed] [Google Scholar]


