Abstract
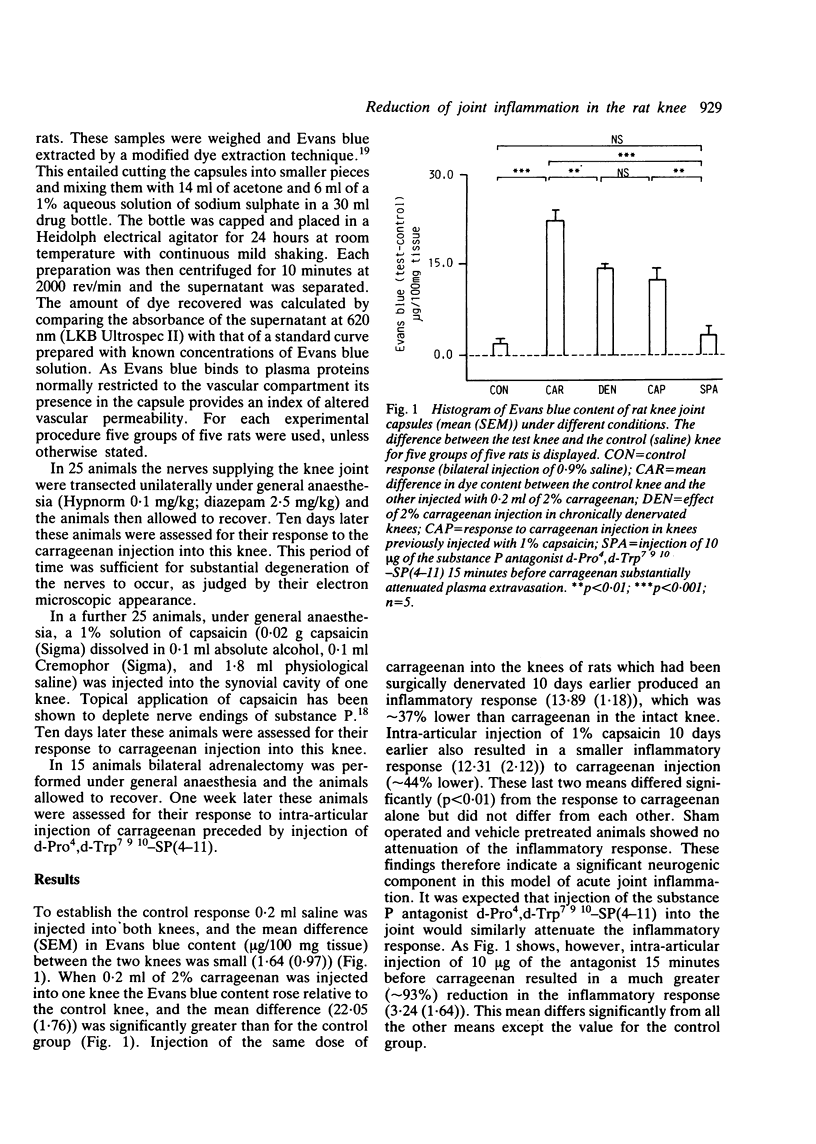
The pathophysiology of acute joint inflammation remains unclear. Evidence is available to suggest a neurally mediated component to the inflammatory process. Acute joint inflammation in the rat knee, induced by intra-articular injection of 2% carrageenan, was reduced by 44% in animals whose knee had previously been injected with 1% capsaicin, while chronic joint denervation produced a 37% reduction. These results indicate a significant neurogenic component in this model of acute joint inflammation. Substance P may be the mediator of this response as intra-articular injection of this agent provoked an acute inflammatory response. Pretreatment of the test knee with the substance P antagonist d-Pro4,d-Trp7 9 10-SP(4-11), however, resulted in a 93% reduction of the inflammatory response to carrageenan. This unexpectedly large effect suggests that this substance P antagonist blocks both neurogenic and non-neurogenic mediators of inflammation. Sympathetic efferent fibres innervating the knee joint were not found to contribute to the neurogenic component of the inflammatory process.
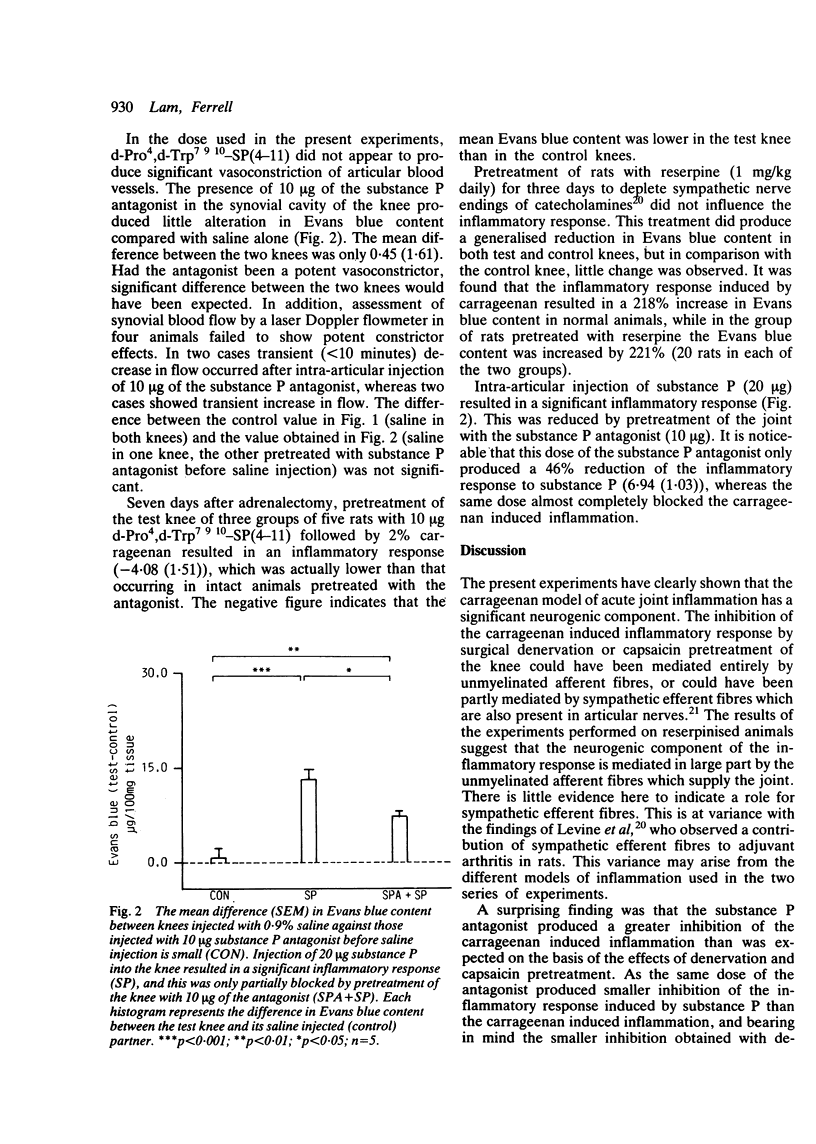
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballard D. R., Abboud F. M., Mayer H. E. Release of a humoral vasodilator substance during neurogenic vasodilatation. Am J Physiol. 1970 Nov;219(5):1451–1457. doi: 10.1152/ajplegacy.1970.219.5.1451. [DOI] [PubMed] [Google Scholar]
- Bar-Shavit Z., Goldman R., Stabinsky Y., Gottlieb P., Fridkin M., Teichberg V. I., Blumberg S. Enhancement of phagocytosis - a newly found activity of substance P residing in its N-terminal tetrapeptide sequence. Biochem Biophys Res Commun. 1980 Jun 30;94(4):1445–1451. doi: 10.1016/0006-291x(80)90581-1. [DOI] [PubMed] [Google Scholar]
- Bayliss W. M. On the origin from the spinal cord of the vaso-dilator fibres of the hind-limb, and on the nature of these fibres. J Physiol. 1901 Feb 28;26(3-4):173–209. doi: 10.1113/jphysiol.1901.sp000831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colpaert F. C., Donnerer J., Lembeck F. Effects of capsaicin on inflammation and on the substance P content of nervous tissues in rats with adjuvant arthritis. Life Sci. 1983 Apr 18;32(16):1827–1834. doi: 10.1016/0024-3205(83)90060-7. [DOI] [PubMed] [Google Scholar]
- Cox B. F., Schelper R. L., Faraci F. M., Brody M. J. Autonomic, sensory, and motor dysfunction following intrathecal administration of three substance P antagonists. Exp Brain Res. 1988;70(1):61–72. doi: 10.1007/BF00271848. [DOI] [PubMed] [Google Scholar]
- Ferrell W. R., Russell N. J. Extravasation in the knee induced by antidromic stimulation of articular C fibre afferents of the anaesthetized cat. J Physiol. 1986 Oct;379:407–416. doi: 10.1113/jphysiol.1986.sp016260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamse R., Holzer P., Lembeck F. Decrease of substance P in primary afferent neurones and impairment of neurogenic plasma extravasation by capsaicin. Br J Pharmacol. 1980 Feb;68(2):207–213. doi: 10.1111/j.1476-5381.1980.tb10409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLTON F. A., HOLTON P. The capillary dilator substances in dry powders of spinal roots; a possible role of adenosine triphosphate in chemical transmission from nerve endings. J Physiol. 1954 Oct 28;126(1):124–140. doi: 10.1113/jphysiol.1954.sp005198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLTON P., PERRY W. L. M. On the transmitter responsible for antidromic vasodilatation in the rabbit's ear. J Physiol. 1951 Jun;114(1-2):240–251. doi: 10.1113/jphysiol.1951.sp004615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada M., Takeuchi M., Fukao T., Katagiri K. A simple method for the quantitative extraction of dye extravasated into the skin. J Pharm Pharmacol. 1971 Mar;23(3):218–219. doi: 10.1111/j.2042-7158.1971.tb08647.x. [DOI] [PubMed] [Google Scholar]
- Jancsó N., Jancsó-Gábor A., Szolcsányi J. Direct evidence for neurogenic inflammation and its prevention by denervation and by pretreatment with capsaicin. Br J Pharmacol Chemother. 1967 Sep;31(1):138–151. doi: 10.1111/j.1476-5381.1967.tb01984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimball E. S., Persico F. J., Vaught J. L. Substance P, neurokinin A, and neurokinin B induce generation of IL-1-like activity in P388D1 cells. Possible relevance to arthritic disease. J Immunol. 1988 Nov 15;141(10):3564–3569. [PubMed] [Google Scholar]
- Langford L. A., Schmidt R. F. Afferent and efferent axons in the medial and posterior articular nerves of the cat. Anat Rec. 1983 May;206(1):71–78. doi: 10.1002/ar.1092060109. [DOI] [PubMed] [Google Scholar]
- Langley J. N. Antidromic action: Part I. J Physiol. 1923 Aug 16;57(6):428–446. doi: 10.1113/jphysiol.1923.sp002081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lembeck F., Donnerer J., Barthó L. Inhibition of neurogenic vasodilation and plasma extravasation by substance P antagonists, somatostatin and [D-Met2, Pro5]enkephalinamide. Eur J Pharmacol. 1982 Nov 19;85(2):171–176. doi: 10.1016/0014-2999(82)90462-9. [DOI] [PubMed] [Google Scholar]
- Lembeck F., Holzer P. Substance P as neurogenic mediator of antidromic vasodilation and neurogenic plasma extravasation. Naunyn Schmiedebergs Arch Pharmacol. 1979 Dec;310(2):175–183. doi: 10.1007/BF00500282. [DOI] [PubMed] [Google Scholar]
- Levine J. D., Dardick S. J., Roizen M. F., Helms C., Basbaum A. I. Contribution of sensory afferents and sympathetic efferents to joint injury in experimental arthritis. J Neurosci. 1986 Dec;6(12):3423–3429. doi: 10.1523/JNEUROSCI.06-12-03423.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotz M., Carson D. A., Vaughan J. H. Substance P activation of rheumatoid synoviocytes: neural pathway in pathogenesis of arthritis. Science. 1987 Feb 20;235(4791):893–895. doi: 10.1126/science.2433770. [DOI] [PubMed] [Google Scholar]
- Lundberg J. M., Brodin E., Hua X., Saria A. Vascular permeability changes and smooth muscle contraction in relation to capsaicin-sensitive substance P afferents in the guinea-pig. Acta Physiol Scand. 1984 Feb;120(2):217–227. doi: 10.1111/j.1748-1716.1984.tb00127.x. [DOI] [PubMed] [Google Scholar]
- Marasco W. A., Showell H. J., Becker E. L. Substance P binds to the formylpeptide chemotaxis receptor on the rabbit neutrophil. Biochem Biophys Res Commun. 1981 Apr 30;99(4):1065–1072. doi: 10.1016/0006-291x(81)90727-0. [DOI] [PubMed] [Google Scholar]
- Mazurek N., Pecht I., Teichberg V. I., Blumberg S. The role of the N-terminal tetrapeptide in the histamine releasing action of substance P. Neuropharmacology. 1981 Nov;20(11):1025–1027. doi: 10.1016/0028-3908(81)90091-5. [DOI] [PubMed] [Google Scholar]
- Nilsson J., von Euler A. M., Dalsgaard C. J. Stimulation of connective tissue cell growth by substance P and substance K. Nature. 1985 May 2;315(6014):61–63. doi: 10.1038/315061a0. [DOI] [PubMed] [Google Scholar]


