Graphical Abstract
Summary: Our objective was to determine the effect of inducing an accessory corpus luteum (CL) using gonadotropinreleasing hormone (GnRH) or human chorionic gonadotropin (hCG) on the day of embryo transfer (ET) on pregnancy per ET (P/ET), calving/ET, and pregnancy loss in recipient dairy heifers and lactating cows receiving in vitro-produced (IVP) embryos. Recipients were randomly assigned to receive GnRH, hCG, or no treatment (control) immediately before ET on days 6 to 9 of the estrous cycle. Parity and embryo characteristics were factors that affected the fertility outcomes of GnRH and hCG treatments on the day of ET. Overall, treatments did not have a major impact on P/ET or calving/ET and had no effect on pregnancy loss; however, the high P/ET results in the present study may have reduced any beneficial impact of accessory CL formation.
Highlights
-
•
Parity and embryo characteristics affected the fertility outcomes.
-
•
Treatments did not have a major impact on overall P/ET or calving/ET.
-
•
Accessory CL induced on day of ET tended to improve overall P/ET in heifers.
-
•
hCG reduced calving/ET in primiparous with fresh IVP expanded blastocyst embryos.
-
•
GnRH tended to improve P/ET and calving/ET in transfers using vitrified IVP embryos.
Abstract
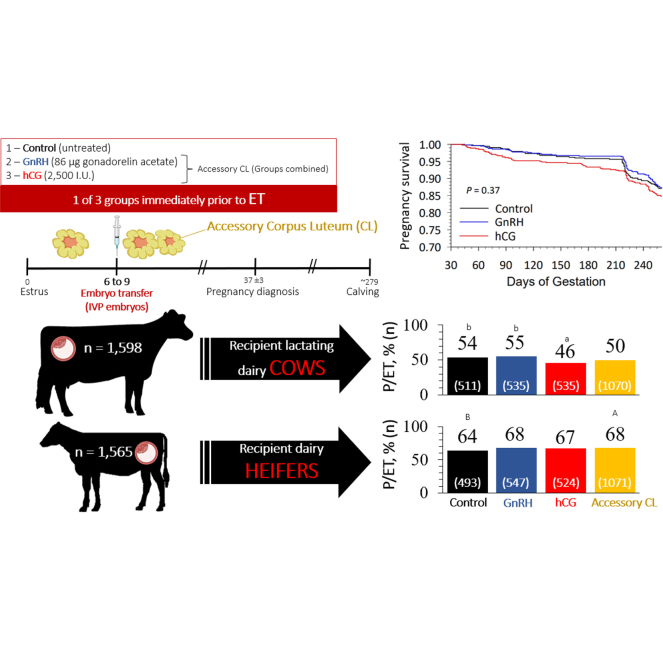
The objective was to determine the effect of inducing an accessory corpus luteum (CL) using GnRH or human chorionic gonadotropin (hCG) on the day of in vitro-produced (IVP) embryo transfer (ET) on pregnancy per ET (P/ET) and calving/ET in dairy heifers and lactating cows. Dairy heifers (11–15 mo of age; n = 1,547) and lactating cows (n = 1,480) detected in estrus by tail chalk (d 0) were used as recipients. Before ET, the presence of a CL was evaluated by transrectal palpation from d 6 to 9 of the estrous cycle. Animals with a CL were randomly assigned to receive 1 of 3 treatments immediately before ET: control (no treatment; n = 1,009), GnRH (86 µg of GnRH; n = 1,085) and hCG (2,500 IU; n = 1,069). Embryos were implanted in the uterine horn ipsilateral to the ovary with a CL (fresh IVP embryos, n = 2,544; vitrified IVP embryos n = 545; slow-freezing IVP embryos, n = 74). Pregnancy diagnosis was performed on d 37 ± 3 of gestation by transrectal palpation. Pregnancy loss data and calving records were collected from the dairy farm management software. Treatment did not affect P/ET, calving/ET, or pregnancy loss either overall or within parity. When treatments inducing CL formation were combined (GnRH + hCG), heifers tended to have greater P/ET than controls (67.7 vs. 63.5%, respectively). Yet, calving/ET were similar. Response variables were also analyzed within embryo type and parity. For heifers receiving stage 6 (blastocyst) fresh IVP embryos, hCG had greater P/ET than controls (74.5 vs. 51.1%, respectively). In addition, GnRH tended to have greater P/ET than controls (67.8 vs. 51.2%, respectively). However, calving/ET in heifers receiving blastocyst fresh IVP embryos was similar among treatments. When only stage 7 (expanded blastocyst) fresh IVP embryos were considered, primiparous GnRH cows had greater P/ET (59.3 vs. 47.1%) and calving/ET (48.6 vs. 38.1%) than hCG. Moreover, hCG showed decreased calving/ET compared with controls in primiparous cows transferred with expanded blastocyst fresh IVP embryos. In summary, the effects of hCG or GnRH at ET on P/ET and calving/ET were inconsistent according to different embryo characteristics (e.g., embryo stage) and parity of recipients. Furthermore, treatment did not improve the overall fertility outcomes for recipient animals receiving IVP embryos.
The timing of progesterone (P4) increase after ovulation is crucial to the establishment and maintenance of pregnancy (Forde et al., 2011; Lonergan and Sánchez, 2020), promoting a uterine environment appropriate for embryo elongation (Clemente et al., 2009). Progesterone supplementation during early embryo development increases the expression of triglyceride synthesis and glucose transport-associated genes used as embryo energy sources (Forde et al., 2009). Moreover, circulating P4 concentrations >1 ng/mL after the first 5 d of pregnancy in cows submitted to AI and an increase in P4 after d 7 in recipients have been correlated with an increased probability of pregnancy (Starbuck et al., 2001; Kenyon et al., 2013). Additionally, low circulating P4 concentrations are associated with decreased fertility (Lonergan, 2011). However, supplementation of circulating P4 concentrations during embryo development has not resulted in consistent increases in fertility across studies (Monteiro et al., 2015; Steichen and Larson, 2019; García-Guerra et al., 2020).
The induction of an accessory corpus luteum (CL) has been used in the last 2 decades to increase circulating P4 concentrations in the early pregnancy or preimplantation period (Besbaci et al., 2020). The ovulation inducers used for accessory CL formation are GnRH and its analogs, or human chorionic gonadotropin (hCG; Vasconcelos et al., 2011; Nascimento et al., 2013a,b). The GnRH acts on specific sites located in the anterior pituitary, causing a release of an LH surge (Estes et al., 1977), whereas hCG has high homology with LH (Stenman et al., 2006), which makes it a more efficient ovulation inducer in cattle compared with GnRH (Cabrera et al., 2021b). A recent study indicated that cows on d 7 of the estrus cycle had a lower ovulatory response when treated with 100 µg of GnRH (gonadorelin acetate) compared with 2,000, 2,500, or 3,300 IU of hCG (79.0, 88.9, 92.9, and 95.6%, respectively; Cabrera et al., 2021a). In addition to the induction of an accessory CL, heifers and cows receiving hCG still have an increase in the original CL area induced by the luteotropic effect of hCG. Moreover, induction of an accessory CL by hCG during the early luteal phase increases serum P4 concentrations (Cunha et al., 2022; El Azzi et al., 2022).
To the best of our knowledge, no studies have determined the effects of hCG and GnRH on fertility of nulliparous, primiparous, and multiparous recipients receiving in vitro-produced (IVP) embryos using a large sample size. The studies found in the literature determined the effects of either using GnRH or hCG on the day of embryo transfer (ET) or using small sample sizes and the results are controversial, ranging from no difference to positive effects on pregnancy per ET (P/ET; Vasconcelos et al., 2011; Niles et al., 2019; García-Guerra et al., 2020). In addition, Niles et al. (2019) reported that the administration of hCG on the day of ET seems to reduce pregnancy loss in dairy cattle ET programs. These results indicate a need for additional studies with large sample sizes on using GnRH and hCG to improve fertility after ET. Hence, our objective was to determine the effects of using GnRH or hCG immediately before ET on P/ET, pregnancy loss, and calving/ET in a large sample size of recipient dairy heifers and cows receiving IVP embryos. We hypothesized that GnRH or hCG immediately before ET would improve P/ET and calving/ET and decrease pregnancy loss.
All animal handling and experimental procedures were approved by the Animal Care and Use Committee at the University of Wisconsin-Madison. Lactating dairy cows were allocated to freestall barns and dairy heifers to drylots with shade and access to water ad libitum. Cows were fed twice and heifers once daily with a TMR formulated to meet or exceed the nutritional requirements of lactating dairy cows or dairy heifers (NRC, 2001). Treatments and embryo transfers were conducted from July to August 2020 on a large commercial dairy herd in Oregon. Recipient cows' and heifers' ET selection was based on daily detection of estrus by tail chalk. Cows ≥45 DIM and heifers at least 330 d old (~11 mo of age) in estrus were eligible to receive ET. Animals not observed in estrus after 2 wk of daily observation by tail chalk received PGF2α weekly until estrus detection. Animals were eligible for receiving ET for the 2 first services.
Animals were randomly assigned to 1 of 3 groups (control, GnRH, and hCG). Control did not receive any treatment before ET (d 6 of the estrous cycle, n = 2; d 7, n = 824; d 8, n = 176; d 9, n = 7). Animals in the GnRH group received 86 µg of gonadorelin acetate (2 mL of Fertagyl, Merck Animal Health) i.m. immediately before ET (d 6 of estrus, n = 2; d 7, n = 882; d 8, n = 193; d 9, n = 8). Animals in the hCG group received 2,500 IU of hCG (Chorulon, Merck Animal Health) i.m. immediately before ET (d 6 of estrus, n = 1; d 7, n = 886; d 8, n = 177; d 9, n = 5). Embryos were transferred to the ipsilateral uterine horn of the ovary with a CL, as detected by transrectal palpation performed by experienced veterinarians (n = 4). A total of 3,163 ET were performed on 3,027 animals (68% Holstein × Jersey crossbred, 29% Jersey, and 3% Holstein). Of the 3,027 animals, 136 (heifer, n = 18; primiparous, n = 86; multiparous, n = 32) received a second ET during the study after being detected in estrus following the first ET. At transfer, cows (n = 1,480) averaged 89.5 DIM (53–196 DIM), and heifers' (n = 1,547) average age was 357.4 d (331–441 d).
Animals received fresh IVP embryos (IVP-F; n = 2,544), vitrified IVP embryos (VIT; n = 545), or slow-freezing IVP embryos (n = 74). All embryos were fertilized with frozen-thawed sex-sorted semen, except for 14 embryos, in which conventional semen-fertilized fresh blastocysts were used (control, n = 5; GnRH, n = 7; hCG, n = 2). The fresh embryos consisted of 29 early blastocysts, 335 blastocysts, 2,105 expanded blastocysts, 56 hatched blastocysts, and 19 expanded hatched blastocysts. The frozen and VIT embryos consisted of blastocyst-stage embryos.
Eighteen animals were culled before pregnancy diagnosis and were excluded from the analysis. Pregnancy was diagnosed on d 37 ± 3 of gestation by transrectal palpation. Animals observed in estrus after ET were also considered not pregnant and received insemination or a subsequent ET (n = 136). Animals previously diagnosed as pregnant at d 37 ± 3 of gestation with signs of abortion, or detected in estrus by tail chalk during gestation, were considered to undergo pregnancy loss after confirmation by transrectal palpation. Pregnancy was reconfirmed before dry-off (~60 d before the due date) by transrectal palpation. Pregnancy loss was only considered in gestations with <260 d. Data related to recipients (e.g., breed, age, parity), embryo transfers (e.g., estrus date, transfer date, technician, embryo used), pregnancy diagnoses, cullings, abortions, and calvings were retrieved from the farm management software (BoviSync, https://bovisync.com/).
All statistical analyses were performed using SAS software, version 9.4 (SAS Institute Inc.). Binomial outcomes such as P/ET, calving/ET, and pregnancy loss were analyzed by logistic regression using the GLIMMIX procedure. Nulliparous (n = 1), primiparous (n = 6), and multiparous (n = 11) animals without pregnancy diagnoses were excluded from all analyses. The initial statistical model included the effect of treatment, parity (nulliparous, primiparous, multiparous), technician, embryo type (IVP-F, VIT, slow-freezing), or embryo developmental stage (early blastocyst, hatched blastocyst, blastocyst, expanded blastocyst, expanded hatched blastocyst), day of ET (6 and 7 or 8 and 9), and 2-way interactions with treatment. Non-significant variables (P > 0.20) and interactions (P > 0.20), as well as parity × treatment, were removed from the model using backward stepwise elimination. The final model consisted of treatment, parity, and parity × treatment. The final model was also used to analyze the effect of treatment within 3 different embryo categories separately (IVP-F blastocyst, IVP-F expanded blastocyst embryos, and VIT).
The time to pregnancy loss detection was analyzed by Cox's proportional hazards regression using the PHREG procedure. The model included treatment and parity (nulliparous, primiparous, and multiparous). The HAZARDRATIO statement with confidence interval (CI) option was used to calculate the hazard ratio (HR) differences among parities, treatments, and treatments within parities and the 95% CI to the pregnancy loss. Graphs of the probability of pregnancy survival after the first pregnancy diagnosis were generated by the LogRank Survival Analysis in the Kaplan-Meier option of Sigma-Plot (version 13.0, Systat Software Inc.). Differences among treatments were considered significant when P ≤ 0.05, whereas P > 0.05 and P ≤ 0.10 were considered a tendency.
The overall P/ET at 37 ± 3 d of gestation in our study was 56.5%. Treatment did not affect overall P/ET and calving/ET (Table 1). The overall P/ET in the present study was considered high compared with previous studies that have induced accessory CL in recipient dairy heifers or cows using GnRH or hCG (Vasconcelos et al., 2011; Niles et al., 2019). Vasconcelos et al. (2011) investigated the effect of GnRH or hCG either 7 d after timed AI or on the day of ET (d7) in lactating synchronized Holstein cows. In that study, overall P/ET on d 28 and 60 were 45.2% and 37.4%, respectively. The authors performed AI and ET throughout the year on a Brazilian dairy farm with mean temperatures ranging from 11.7°C to 30.3°C with cows housed in ventilated freestall barns. In their study, induction of accessory CL with GnRH or hCG improved d 28 and 60 P/ET compared with controls (no treatment), but treatment did not affect cows receiving timed AI. In contrast, our study was conducted only during the warm season (July and August) in Oregon, and animals were housed in unventilated barns. However, our overall 37-d P/ET in cows was slightly higher (49.8%) compared with their 28-d P/ET.
Table 1.
Effect of treatment [control = untreated; or treatment with GnRH or human chorionic gonadotropin (hCG) immediately before embryo transfer (ET)] or ovulation inducers (treatments combined, GnRH+hCG) on pregnancy per ET (P/ET), calving/ET, and pregnancy loss in recipient dairy heifers and lactating cows receiving fresh, frozen, or vitrified in vitro-produced embryos
| Item | Treatment |
Ovulation inducers (GnRH+hCG) |
P-value1 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | GnRH | hCG | Treatment | Ovulation inducers | Parity | Treatment × parity | Ovulation inducers × parity | ||
| P/ET 37 d, % (n) | |||||||||
| Overall | 57.4 (1,004) | 59.6 (1,082) | 57.1 (1,059) | 58.4 (2,141) | 0.68 | 0.98 | <0.001 | 0.39 | 0.23 |
| Nulliparous | 63.5 (493) | 68.2 (547) | 67.2 (524) | 67.7 (1,071) | 0.25 | 0.10 | — | — | — |
| Primiparous | 53.0(362) | 54.2 (349) | 48.5 (342) | 51.4 (691) | 0.29 | 0.61 | — | — | — |
| Multiparous | 47.7 (149) | 44.6 (186) | 45.1 (193) | 44.9 (379) | 0.84 | 0.56 | — | — | — |
| Calving/ET,2 % (n) | |||||||||
| Overall | 47.4 (966) | 49.2 (1,022) | 45.7 (1,004) | 47.5 (2026) | 0.38 | 0.70 | <0.001 | 0.64 | 0.32 |
| Nulliparous | 52.7 (476) | 57.6 (514) | 54.5 (492) | 56.1 (1,006) | 0.33 | 0.23 | — | — | — |
| Primiparous | 44.0 (348) | 43.5 (333) | 39.4 (325) | 41.5 (658) | 0.41 | 0.45 | — | — | — |
| Multiparous | 38.0 (142) | 36.0 (175) | 33.7 (187) | 34.1 (362) | 0.70 | 0.50 | — | — | — |
| Pregnancy loss,2 % (n) | |||||||||
| Overall | 14.8 (538) | 13.9 (585) | 16.6 (550) | 15.2 (1,135) | 0.38 | 0.82 | 0.82 | 0.55 | 0.88 |
| Nulliparous | 15.2 (296) | 12.5 (340) | 16.3(320) | 14.6 (660) | 0.47 | 0.77 | — | — | — |
| Primiparous | 15.6 (178) | 16.2 (173) | 14.1 (149) | 15.2 (322) | 0.82 | 0.99 | — | — | — |
| Multiparous | 14.1 (64) | 12.5 (72) | 22.2 (81) | 17.7 (153) | 0.27 | 0.67 | — | — | — |
Variables were considered significant when P ≤ 0.05, whereas P > 0.05 and P ≤ 0.10 were considered a tendency.
Pregnant culled animals (n = 153: sold for dairy n = 15; sold for beef n = 70; died n = 68).
In the present study, we did not evaluate ovulatory response to GnRH or hCG, nor number of CL at time of transfer and later. Thus, we did not assess the effect of the interaction treatment by accessory CL formation on P/ET. Still, we speculate that most heifers and cows likely ovulated to either treatment and formed an accessory CL, since most recipients were on d 7 (82%) or 8 (17%) of the estrous cycle at the day of ET (Vasconcelos et al., 1999). Therefore, we combined both GnRH and hCG treatments to evaluate the effects of ovulation inducers on the outcomes. When treatments were combined, ovulation inducers tended (P = 0.10) to affect P/ET compared with control only in heifers. This difference was due to a greater (P = 0.02) and a tendency (P = 0.08) for greater P/ET in heifers treated (GnRH + hCG) and transferred with IVP-F and VIT embryos (Table 2), respectively. Nevertheless, P/ET was not different between treatments in heifers receiving expanded blastocyst IVP-F embryos, which accounted for most transfers in the study (Table 2).
Table 2.
Effect of treatment [control = untreated; or treatment with GnRH, or human chorionic gonadotropin (hCG) immediately before embryo transfer (ET)] or ovulation inducers (treatments combined; GnRH+hCG) on pregnancy per ET (P/ET), calving/ET, and pregnancy loss in recipient dairy heifers and lactating cows receiving stage 6 (blastocyst) and stage 7 (expanded blastocyst) fresh in vitro-produced (IVP) embryos or vitrified IVP embryos
| Item | Treatment |
Ovulation inducers (GnRH+hCG) |
P-value |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | GnRH | hCG | Treatment | Ovulation inducers | Parity | Treatment × parity | Ovulation inducers × parity | ||
| Fresh IVP blastocyst embryos | |||||||||
| P/ET 37 d, % (n) | |||||||||
| Overall | 46.4 (112) | 54.1 (114) | 60.2 (108) | 58.1 (222) | 0.31 | 0.16 | 0.002 | 0.61 | 0.30 |
| Nulliparous | 51.2 (43)a,b, A,B | 67.8 (59)a,b, A,B | 74.5 (51)b | 70.9 (110) | 0.06 | 0.02 | — | — | — |
| Primiparous | 44.7 (47) | 43.3 (30) | 48.7 (37) | 46.3 (67) | 0.90 | 0.87 | — | — | — |
| Multiparous | 40.9 (22) | 44.0 (25) | 45.0 (20) | 44.4 (45) | 0.96 | 0.78 | — | — | — |
| Calving/ET,1 % (n) | |||||||||
| Overall | 37.4 (107) | 42.1 (107) | 48.1 (102) | 45.0 (209) | 0.37 | 0.30 | 0.13 | 0.90 | 0.95 |
| Nulliparous | 42.5 (40) | 51.0 (53) | 52.2 (46) | 51.2 (99) | 0.63 | 0.34 | — | — | — |
| Primiparous | 35.6 (45) | 31.0 (29) | 47.2 (36) | 40.0 (65) | 0.37 | 0.64 | — | — | — |
| Multiparous | 31.8 (22) | 36.0 (25) | 40.0 (20) | 37.8 (45) | 0.86 | 0.63 | — | — | — |
| Pregnancy loss,1 % (n) | |||||||||
| Overall | 14.9 (47) | 21.0 (57) | 17.0 (59) | 19.0 (116) | 0.80 | 0.66 | 0.98 | 0.80 | 0.21 |
| Nulliparous | 10.5 (19) | 21.0 (34) | 27.3 (33) | 23.9 (67) | 0.38 | 0.11 | — | — | — |
| Primiparous | 15.8 (19) | 25.0 (12) | 0.0 (17) | 10.3 (29) | 0.82 | 0.58 | — | — | — |
| Multiparous | 22.2 (9) | 18.2 (11) | 11.1 (9) | 15.0 (20) | 0.82 | 0.64 | — | — | — |
| Fresh IVP expanded blastocyst embryos | |||||||||
| P/ET 37 d, % (n) | |||||||||
| Overall | 60.7 (631)B | 60.9 (723)B | 56.2 (726)A | 58.2 (1,449) | 0.14 | 0.28 | <0.001 | 0.39 | 0.72 |
| Nulliparous | 65.9 (355) | 65.8 (404) | 64.8 (400) | 65.3 (804) | 0.93 | 0.84 | — | — | — |
| Primiparous | 55.2 (203)a,b, A,B | 59.3 (216)b | 47.1 (208)a,b, A,B | 53.3 (424) | 0.04 | 0.66 | — | — | — |
| Multiparous | 50.7 (73) | 44.7 (103) | 43.2 (118) | 43.9 (221) | 0.59 | 0.31 | — | — | — |
| Calving/ET,1 % (n) | |||||||||
| Overall | 50.0 (608)b | 50.3 (684)a,b, A,B | 45.0 (689)a,b, A,B | 47.6 (1,373) | 0.08 | 0.16 | <0.001 | 0.42 | 0.41 |
| Nulliparous | 54.2 (343) | 55.4 (381) | 53.3 (377) | 54.4 (758) | 0.88 | 0.97 | — | — | — |
| Primiparous | 45.6 (195)ab | 48.6 (208)b | 37.6 (197)a | 43.2 (405) | 0.07 | 0.57 | — | — | — |
| Multiparous | 41.4 (70) | 33.7 (95) | 30.4 (115) | 31.9 (210) | 0.23 | 0.15 | — | — | — |
| Pregnancy loss,1 % (n) | |||||||||
| Overall | 15.6 (360) | 14.2 (401) | 16.4(371) | 15.3 (772) | 0.50 | 0.55 | 0.56 | 0.60 | 0.49 |
| Nulliparous | 16.2 (222) | 13.2 (243) | 14.8 (236) | 14.0 (479) | 0.65 | 0.44 | — | — | — |
| Primiparous | 14.4 (104) | 15.8 (120) | 14.9 (87) | 15.5 (207) | 0.96 | 0.81 | — | — | — |
| Multiparous | 14.7 (34) | 15.8 (38) | 27.1 (48) | 22.1 (86) | 0.29 | 0.37 | — | — | — |
| Vitrified IVP | |||||||||
| P/ET 37 d, % (n) | |||||||||
| Overall | 51.8 (197)A | 59.9 (177)B | 57.8 (161) | 58.9 (338) | 0.20 | 0.08 | <0.001 | 0.74 | 0.47 |
| Nulliparous | 62.3 (69)A | 76.9 (65)B | 71.4 (56) | 74.4 (121) | 0.18 | 0.08 | — | — | — |
| Primiparous | 48.3 (87) | 48.6 (72) | 50.8 (65) | 49.6 (137) | 0.95 | 0.84 | — | — | — |
| Multiparous | 41.5 (41) | 52.5 (40) | 50.0 (40) | 51.3 (80) | 0.5 | 0.31 | — | — | — |
| Calving/ET,1 % (n) | |||||||||
| Overall | 43.2 (190)A | 53.0 (166)B | 45.7 (151) | 49.5 (317) | 0.15 | 0.13 | <0.001 | 0.42 | 0.27 |
| Nulliparous | 52.9 (68)a | 73.0 (63)b | 59.6 (52) | 67.0 (115) | 0.06 | 0.06 | — | — | — |
| Primiparous | 39.3 (84) | 36.9 (65) | 37.7 (61) | 37.3 (126) | 0.95 | 0.77 | — | — | — |
| Multiparous | 34.2 (38) | 47.4 (38) | 39.5 (38) | 43.4 (76) | 0.50 | 0.35 | — | — | — |
| Pregnancy loss,1 % (n) | |||||||||
| Overall | 13.7 (95) | 7.4 (95) | 16.9 (83) | 11.8 (178) | 0.18 | 0.99 | 0.24 | 0.78 | 0.56 |
| Nulliparous | 14.3 (42) | 4.2 (48) | 13.9 (36) | 8.3 (84) | 0.25 | 0.31 | — | — | — |
| Primiparous | 15.4 (39) | 14.3 (48) | 20.7 (29) | 17.5 (57) | 0.78 | 0.78 | — | — | — |
| Multiparous | 7.1 (14) | 5.3 (19) | 16.7 (18) | 10.8 (37) | 0.50 | 0.70 | — | — | — |
Means within a row with different lowercase superscripts differ (P < 0.05).
Means within a row with different uppercase superscripts tend to differ (P ≤ 0.10).
Pregnant culled animals (n = 153: sold for dairy n = 15; sold for beef n = 70; died n = 68).
García-Guerra et al. (2020) reported no effect of GnRH administered on d 5 of the estrous cycle on P/ET in heifers transferred fresh IVP blastocysts and expanded blastocysts. Still, GnRH on d 5 decreased pregnancy losses between d 33 and 60 of gestation compared with untreated heifers when transferred with expanded blastocysts (15.2 vs. 27.1%, respectively). Niles et al. (2019) also reported a reduction in pregnancy losses of recipient heifers treated with hCG compared with controls (10 vs. 22%, respectively). However, the limited number of pregnant heifers in that study (n = 131) did not provide enough statistical power for a sound evaluation of the effects of hCG on pregnancy losses. In our study, treatment did not affect heifers' overall calving/ET and pregnancy losses (Table 1). Still, in heifers receiving VIT embryos, GnRH tended to increase calving/ET compared with controls (Table 2).
On the contrary, in primiparous cows receiving expanded blastocyst IVP-F embryos, hCG decreased (P = 0.01) and tended to decrease P/ET (P = 0.10) compared with GnRH and control, respectively (Table 2). This result is opposed to our hypothesis that hCG would increase P/ET. In addition, previous studies using 2,000 or 3,300 IU of hCG on d 5 after timed AI increased or tended to increase P/AI only for primiparous cows (Nascimento et al., 2013a; Zolini et al., 2019). A recent meta-analysis, including 107 trials from 52 publications, evaluated the association of GnRH or hCG between d 4 and 15 after AI with P/AI in dairy cattle (nulliparous, primiparous, and multiparous; Besbaci et al., 2020). Their results suggested that GnRH and hCG only provided P/AI improvements in primiparous cows. Moreover, the benefits of GnRH or hCG treatments were seen only in studies with very poor (<30%) or poor P/AI (30.1 to 45%), whereas ovulation inducers did not benefit cows with good P/AI (>60.1%). The high P/ET for heifers and cows in the present study may have contributed to not finding major differences among treatments.
Parity had an effect (P < 0.001) on overall pregnancy outcomes, with greater P/ET and calving/ET in nulliparous (66.4 and 55.0%, respectively) than in primiparous (52.0 and 42.4%, respectively) and multiparous cows (45.6 and 35.7%, respectively). Primiparous cows had greater (P = 0.02) P/ET and calving/ET than multiparous. A similar negative direct effect of parity number on P/ET using IVP embryos has been reported by Ferraz et al. (2016). This decrease in P/ET as recipient parity number increases appears to be related to the uterine environment or to a systemic effect rather than to an oocyte or embryo effect. Calving problems and metritis have been associated with decrease in P/ET in recipient cows (Ferraz et al., 2016), which appears to contribute to the reduction in P/ET for cows compared with heifer recipients. As cows experience more parturitions, they may be more likely to have experienced calving problems and uterine diseases throughout their lives (Dohoo and Martin, 1984). However, we did not find studies indicating whether calving problems or uterine diseases occurring in previous parities would affect fertility in subsequent parities. Although parity affected P/ET and calving/ET, the proportion of pregnancy loss did not differ among parities (Table 1). These results indicate that parity effects on fertility after ET are most likely related to the pregnancy development during the first 37 d of gestation.
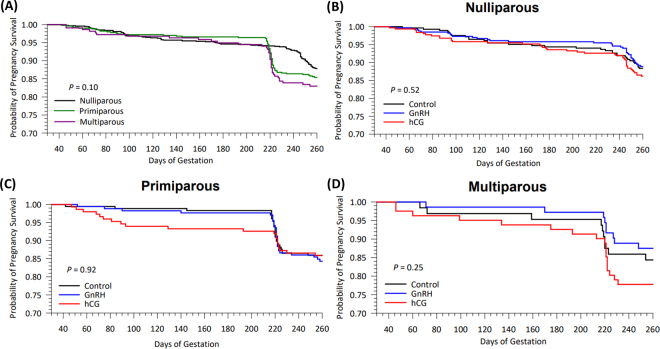
Time to pregnancy loss identification tended to differ (P = 0.10) among parities. The hazard to pregnancy loss detection was greater (P = 0.05) for multiparous compared with nulliparous (HR = 1.43; CI = 1.00–2.10), indicating that multiparous cows had earlier pregnancy loss compared with nulliparous (Figure 1). Hazard to pregnancy loss did not differ for nulliparous versus primiparous (P = 0.16) or multiparous versus primiparous (P = 0.43). A limitation of our study was that time of pregnancy loss was determined by signs of abortion or identification of estrus after the first pregnancy diagnosis at 37 ± 3 d of gestation, or only at a second pregnancy diagnosis before dry-off (~60 d before the due date). Most animals in the study (34.5%) were identified with pregnancy loss at the second pregnancy diagnosis from 210 and 230 d of gestation, which may not be precise. Thus, time to pregnancy loss results in our study should be interpreted with caution because these animals may have failed to show signs of estrus or were not detected in estrus after an earlier pregnancy loss. Future research with more frequent pregnancy confirmations throughout gestation after ET using IVP embryos is required to identify a more accurate time of pregnancy loss.
Figure 1.
Kaplan-Meier survival curves for the probability of pregnancy survival after pregnancy diagnosis at 37 ± 3 d of gestation in different parities (A; n = 1,644) or only in heifers (B; n = 928), primiparous (C; n = 499), or multiparous (D; n = 217) cows not treated (control) or treated with GnRH or human chorionic gonadotropin (hCG) immediately before embryo transfer.
In conclusion, parity and embryo characteristics were factors that influenced the fertility outcomes of GnRH and hCG treatments on day of ET. The interaction of these factors should be determined in future research, to allow clear interpretation of the effects of treatments on fertility of recipient dairy heifers and cows. Overall, treatments did not have a major effect on P/ET or calving/ET and had no effect on pregnancy loss; however, the high P/ET results in the present study may have reduced any beneficial effect of GnRH or hCG on day of ET. Using different herds with a variation in fertility may elucidate this premise.
Notes
We thank Merck Animal Health (Rahway, NJ) for donating Fertagyl (GnRH) and Chorulon (hCG) used in this study. Marcelo Siqueira El Azzi was partially financed by CAPES (Brazilian Coordination of Improvement of Higher Education Personnel, Brazil) – Finance Code 001.
The authors have not stated any conflicts of interest.
References
- Besbaci M., Abdelli A., Minviel J.J., Belabdi I., Kaidi R., Raboisson D. Association of pregnancy per artificial insemination with gonadotropin-releasing hormone and human chorionic gonadotropin administered during the luteal phase after artificial insemination in dairy cows: A meta-analysis. J. Dairy Sci. 2020;103:2006–2018. doi: 10.3168/jds.2019-16439. 31785879. [DOI] [PubMed] [Google Scholar]
- Cabrera E.M., Lauber M.R., Peralta E.M., Bilby T.R., Fricke P.M. Human chorionic gonadotropin dose response for induction of ovulation 7 days after a synchronized ovulation in lactating Holstein cows. JDS Commun. 2021;2:35–40. doi: 10.3168/jdsc.2020-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera E.M., Lauber M.R., Valdes-Arciniega T., El Azzi M.S., Martins J.P.N., Bilby T.R., Fricke P.M. Replacing the first gonadotropin-releasing hormone treatment in an Ovsynch protocol with human chorionic gonadotropin decreased pregnancies per artificial insemination in lactating dairy cows. J. Dairy Sci. 2021;104:8290–8300. doi: 10.3168/jds.2021-20274. 33838886. [DOI] [PubMed] [Google Scholar]
- Clemente M., De La Fuente J., Fair T., Al Naib A., Gutierrez-Adan A., Roche J.F., Rizos D., Lonergan P. Progesterone and conceptus elongation in cattle: A direct effect on the embryo or an indirect effect via the endometrium? Reproduction. 2009;138:507–517. doi: 10.1530/REP-09-0152. 19556439. [DOI] [PubMed] [Google Scholar]
- Cunha T.O., Statz L.R., Domingues R.R., Andrade J.P.N., Wiltbank M.C., Martins J.P.N. Accessory corpus luteum induced by human chorionic gonadotropin on day 7 or days 7 and 13 of the estrous cycle affected follicular and luteal dynamics and luteolysis in lactating Holstein cows. J. Dairy Sci. 2022;105:2631–2650. doi: 10.3168/jds.2021-20619. 34955260. [DOI] [PubMed] [Google Scholar]
- Dohoo I.R., Martin S.W. Disease, production and culling in Holstein-Friesian cows. IV. Effects of disease on production. Prev. Vet. Med. 1984;2:755–770. doi: 10.1016/0167-5877(84)90031-X. [DOI] [Google Scholar]
- El Azzi M.S., Valdes-Arciniega T., Anta-Galvan E., Leão I.M.R., Sala R.V., Fosado M., de Souza J.C., Martins J.P.N. Effects of human chorionic gonadotropin on the last day of a 5-day CIDR Synch protocol and 5 days later on circulating progesterone concentrations and luteal area in Holstein heifers. JDS Commun. 2022;3:368–372. doi: 10.3168/jdsc.2022-0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes K.S., Padmanabhan V., Convey E.M. Localization of gonadotropin within releasing hormone (GnRH) within the bovine hypothalamus. Biol. Reprod. 1977;17:706–711. doi: 10.1095/biolreprod17.5.706. 339966. [DOI] [PubMed] [Google Scholar]
- Ferraz P.A., Burnley C., Karanja J., Viera-Neto A., Santos J.E.P., Chebel R.C., Galvão K.N. Factors affecting the success of a large embryo transfer program in Holstein cattle in a commercial herd in the southeast region of the United States. Theriogenology. 2016;86:1834–1841. doi: 10.1016/j.theriogenology.2016.05.032. 27364084. [DOI] [PubMed] [Google Scholar]
- Forde N., Beltman M.E., Duffy G.B., Duffy P., Mehta J.P., Ó'Gaora P., Roche J.F., Lonergan P., Crowe M.A. Changes in the endometrial transcriptome during the bovine estrous cycle: Effect of low circulating progesterone and consequences for conceptus elongation. Biol. Reprod. 2011;84:266–278. doi: 10.1095/biolreprod.110.085910. 20881316. [DOI] [PubMed] [Google Scholar]
- Forde N., Carter F., Fair T., Crowe M.A., Evans A.C.O., Spencer T.E., Bazer F.W., McBride R., Boland M.P., O'Gaora P., Lonergan P., Roche J.F. Progesterone-regulated changes in endometrial gene expression contribute to advanced conceptus development in cattle. Biol. Reprod. 2009;81:784–794. doi: 10.1095/biolreprod.108.074336. 19553605. [DOI] [PubMed] [Google Scholar]
- García-Guerra A., Sala R.V., Carrenho-Sala L., Baez G.M., Motta J.C.L., Fosado M., Moreno J.F., Wiltbank M.C. Postovulatory treatment with GnRH on day 5 reduces pregnancy loss in recipients receiving an in vitro produced expanded blastocyst. Theriogenology. 2020;141:202–210. doi: 10.1016/j.theriogenology.2019.05.010. 31606718. [DOI] [PubMed] [Google Scholar]
- Kenyon A.G., Mendonça L.G.D., Lopes G., Jr., Lima J.R., Santos J.E.P., Chebel R.C. Minimal progesterone concentration required for embryo survival after embryo transfer in lactating Holstein cows. Anim. Reprod. Sci. 2013;136:223–230. doi: 10.1016/j.anireprosci.2012.10.014. 23199583. [DOI] [PubMed] [Google Scholar]
- Lonergan P. Influence of progesterone on oocyte quality and embryo development in cows. Theriogenology. 2011;76:1594–1601. doi: 10.1016/j.theriogenology.2011.06.012. [DOI] [PubMed] [Google Scholar]
- Lonergan P., Sánchez J.M. Symposium review: Progesterone effects on early embryo development in cattle. J. Dairy Sci. 2020;103:8698–8707. doi: 10.3168/jds.2020-18583. [DOI] [PubMed] [Google Scholar]
- Monteiro P.L.J., Jr., Nascimento A.B., Pontes G.C.S., Fernandes G.O., Melo L.F., Wiltbank M.C., Sartori R. Progesterone supplementation after ovulation: Effects on corpus luteum function and on fertility of dairy cows subjected to AI or ET. Theriogenology. 2015;84:1215–1224. doi: 10.1016/j.theriogenology.2015.06.023. 26255222. [DOI] [PubMed] [Google Scholar]
- Nascimento A.B., Bender R.W., Souza A.H., Ayres H., Araujo R.R., Guenther J.N., Sartori R., Wiltbank M.C. Effect of treatment with human chorionic gonadotropin on day 5 after timed artificial insemination on fertility of lactating dairy cows. J. Dairy Sci. 2013;96:2873–2882. doi: 10.3168/jds.2012-5895. 23453519. [DOI] [PubMed] [Google Scholar]
- Nascimento A.B., Souza A.H., Guenther J.N., Costa F.P.D., Sartori R., Wiltbank M.C. Effects of treatment with human chorionic gonadotrophin or intravaginal progesterone-releasing device after AI on circulating progesterone concentrations in lactating dairy cows. Reprod. Fertil. Dev. 2013;25:818–824. doi: 10.1071/RD12104. 23058209. [DOI] [PubMed] [Google Scholar]
- Niles A.M., Fricke H.P., Carvalho P.D., Wiltbank M.C., Hernandez L.L., Fricke P.M. Effect of treatment with human chorionic gonadotropin 7 days after artificial insemination or at the time of embryo transfer on reproductive outcomes in nulliparous Holstein heifers. J. Dairy Sci. 2019;102:2593–2606. doi: 10.3168/jds.2018-15588. 30692012. [DOI] [PubMed] [Google Scholar]
- NRC . Seventh rev. ed. National Research Council; 2001. Nutrient Requirements of Dairy Cattle. [Google Scholar]
- Starbuck G.R., Darwash A.O., Mann G.E., Lamming G.E. The detection and treatment of post insemination progesterone insufficiency in dairy cows. BSAP Occas. Publ. 2001;26:447–450. doi: 10.1017/S0263967X00034091. [DOI] [Google Scholar]
- Steichen M.M., Larson J.E. Effects of supplemental progesterone using a CIDR insert on pregnancy per embryo transfer of dairy heifer recipients of embryos produced in vitro. Anim. Reprod. Sci. 2019;203:45–51. doi: 10.1016/j.anireprosci.2019.02.006. 30799028. [DOI] [PubMed] [Google Scholar]
- Stenman U.H., Tiitinen A., Alfthan H., Valmu L. The classification, functions and clinical use of different isoforms of HCG. Hum. Reprod. Update. 2006;12:769–784. doi: 10.1093/humupd/dml029. 16877746. [DOI] [PubMed] [Google Scholar]
- Vasconcelos J.L.M., Sá Filho O.G., Justolin P.L.T., Morelli P., Aragon F.L., Veras M.B., Soriano S. Effects of postbreeding gonadotropin treatments on conception rates of lactating dairy cows subjected to timed artificial insemination or embryo transfer in a tropical environment. J. Dairy Sci. 2011;94:223–234. doi: 10.3168/jds.2010-3462. 21183033. [DOI] [PubMed] [Google Scholar]
- Vasconcelos J.L.M., Silcox R., Rosa G., Pursley J., Wiltbank M. Synchronization rate, size of the ovulatory follicle, and pregnancy rate after synchronization of ovulation beginning on different days of the estrous cycle in lactating dairy cows. Theriogenology. 1999;52:1067–1078. doi: 10.1016/S0093-691X(99)00195-8. [DOI] [PubMed] [Google Scholar]
- Zolini A.M., Ortiz W.G., Estrada-Cortes E., Ortega M.S., Dikmen S., Sosa F., Giordano J.O., Hansen P.J. Interactions of human chorionic gonadotropin with genotype and parity on fertility responses of lactating dairy cows. J. Dairy Sci. 2019;102:846–856. doi: 10.3168/jds.2018-15358. 30447974. [DOI] [PubMed] [Google Scholar]