Abstract
Marijuana is one of the most commonly abused substances during pregnancy. Synthetic cannabinoids (SCBs) are a group of heterogeneous compounds that are 40- to 600-fold more potent than Δ9-tetrahydrocannabinol, the major psychoactive component of marijuana. With SCBs being legally available for purchase and the prevalence of unplanned pregnancies, the possibility of prenatal exposure to SCBs is high. However, the effects of prenatal SCB exposure on embryonic brain development are not well understood. In this study, we use complex correlation mapping optical coherence angiography to evaluate changes in murine fetal brain vasculature in utero, minutes after maternal exposure to an SCB, CP-55940. Results showed a significant decrease (P < 0.05) in fetal brain vessel diameter, length fraction and area density when compared to the sham group. This preliminary study shows that acute prenatal exposure to an SCB resulted in significant fetal brain vasoconstriction during the peak period for brain development.
Keywords: brain vasculature, murine embryos, optical coherence tomography, prenatal cannabinoid exposure, synthetic cannabinoids
Graphical Abstract

1 |. INTRODUCTION
Prenatal substance abuse is a major public health concern [1]. Marijuana is commonly used during pregnancy [2, 3], and has become a target of renewed research interest due to its legalization in several states in the United States. Synthetic cannabinoids (SCBs) are a group of heterogeneous compounds that have been developed to understand the endogenous cannabinoid system and for use as potential therapeutics [4, 5]. However, several variations of SCBs are commercially produced and are marketed as herbal mixtures under brand names such as “Spice” and “K2.” In addition to providing similar psychoactive effects to that of natural cannabinoids, the popularity of SCB usage has increased due to the availability of ready-to-use formulations, easy availability over the internet and in specialty shops, and because SCBs are undetectable in routine drug screenings [6, 7].
Although Δ9-tetrahydrocannabinol (Δ9-THC), the major psychoactive component of marijuana, and SCBs target the same receptors, they have several important differences that result in increased potency of SCBs [7]. For example, SCBs are direct agonists of the cannabinoid receptors whereas Δ9-THC is only a partial agonist [8–10]. Moreover, variations in chemical structure [11] and concentration [12] in different SCB products, or sometimes even within the same product, can mean that SCBs are 40- to 600-fold more potent than Δ9-THC and may have higher binding affinities for cannabinoid receptors [4]. Hence, SCBs have greater toxicity and exhibit more prominent harmful effects as compared to Δ9-THC.
Recent studies have reported an increase in natural and SCB use in women of reproductive age [13–17], which when combined with the prevalence of unplanned pregnancies, increases the likelihood of prenatal cannabinoid exposure. Δ9-THC, is capable of crossing the fetoplacental barrier due to its liphophilic nature [18, 19] and is secreted through breast milk [20, 21]. Thus, research studying the effects of prenatal and neonatal exposure to cannabinoids on embryonic development is needed but is sparse.
Prenatal cannabinoid exposure during the first trimester is known to have extremely detrimental effects on the developing embryo, including an increased incidence of fetal reabsorption, edema, phocomelia, omphalocele, spina bifida, exencephaly and myelocele [22, 23]. In addition, several studies have focused on the effects of SCB exposure during early stages (first trimester) of embryonic development [24], since research has shown that the endogenous cannabinoid system starts developing very early in vertebrates [25–27]. Nevertheless, the effects of second trimester exposure have not been well documented.
The second trimester marks the peak period for fetal neurogenesis and angiogenesis [28], and endocannabinoid signaling plays a pivotal role in brain development [29]. The developing microvasculature supports nutritional needs [30], provides endocrine control of fetal growth [31], and promotes neural development [32] in the embryo. Thus, there is a need to study the effects of prenatal cannabinoid exposure during the second trimester on fetal brain development and microvasculature.
Histological staining has been the gold standard for studying brain development. However, due to its invasive and time-consuming nature and need for tissue fixation, which alters the gross morphology of the tissue [33, 34], histological techniques are unsuitable for live embryonic imaging. Other noninvasive imaging modalities such as ultrasound bio-microscopy, micromagnetic resonance imaging and microcomputed tomography have been used to study brain development [35]. However, due to trade-offs between the imaging depth and resolution, long acquisition times, and the use of external contrast agents and ionizing radiation, these methods are not well-suited for live embryonic imaging of brain microvasculature. On the other hand, optical coherence tomography (OCT) [36] is a well-developed optical imaging modality that has been used to image embryonic development over the past decade. Due to its noninvasive nature, ability to provide cross-sectional images of live embryos, relatively high spatial and temporal resolution, and no need for external contrast agents, OCT has been preferred over other imaging modalities to image small animal embryos [37, 38]. We have used OCT in our previous work to image mouse and rat embryonic development, thus demonstrating the capabilities of OCT to image small mammal embryos [39–44].
Doppler OCT (DOCT) [45, 46], speckle variance OCT (SVOCT) [47–49], intensity-based correlation mapping OCT (IB-cmOCT) [50] and optical microangiography (OMAG) [51–53] are examples of functional extensions of OCT that have been most commonly used to image microvasculature and measure blood flow velocities. DOCT detects the Doppler shift created by dynamic particles in vasculature to quantify flow velocities. However, this technique is Doppler angle dependent and is not sensitive to flow in the direction perpendicular to the scanning beam. SVOCT and IB-cmOCT [54], on the other hand, are intensity-based techniques that use the variance and decorrelation of the signal intensity over time to image vasculature. Although there is no angular dependence, the processing kernel size and the intensity-based detection paradigm may limit the vascular imaging resolution and sensitivity to small vessels. OMAG, an angiography technique based on the complex OCT signal, is sensitive to the capillary-level blood vessels. However, the need for extensive post-processing to compensate for the motion artifacts makes it complicated and cumbersome [55, 56]. Recently, a technique called correlation mapping optical coherence angiography (cm-OCA) has been demonstrated to achieve higher signal-to-noise ratio (SNR) of motion contrast than IB-cmOCT and less sensitivity to global phase fluctuations [57]. The reported decorrelation artifacts at low SNR regions can be compensated by using the temporal variance of the background noise [58].
In this study, we use cm-OCA [58] to evaluate the changes in murine fetal brain vasculature at 14.5 days post-coitum (DPC) minutes after maternal SCB exposure. Pregnant mice were intraperitoneally exposed to CP-55940, a well characterized compound in SCB research, and cm-OCA was used to visualize the fetal brain vasculature every 5 minutes for 45 minutes post-exposure. Our results show a drastic decrease in fetal brain vasculature as compared to the sham group, similar to results obtained when pregnant mice were administered a binge-like bolus of ethanol [59].
2 |. MATERIALS AND METHODS
2.1 |. OCT imaging
A phase stabilized swept source OCT system (PhS-SSOCT) was used for imaging the embryonic brain, as shown in Figure 1. The system consisted of a broadband swept source laser, with a central wavelength of 1310 nm, scan range of 150 nm, scan rate of 30 kHz, axial resolution of 11 μm in air, lateral resolution of 27.8 μm, and an output power of 39 mW. A balanced photodetector recorded the interference pattern, which was digitized by a high-speed analog to digital converter. After resampling the raw interference pattern into linear k-space, a fast Fourier transform was performed on the spectral interference pattern to obtain a one-dimensional (1D) depth profile also known as an A-scan. A two-dimensional (2D) cross-sectional image, also known as a B-scan, was obtained using a scanning galvanometer-mounted mirror. Three-dimensional (3D) volumes were formed from multiple B-scans. More information on this system can be found in our previous work [60].
FIGURE 1.

Experimental schematic, including the OCT system. ADC - analog to digital converter; DAC - digital to analog converter
2.2 |. Animal protocols
All procedures were performed under an approved protocol by the University of Houston Institutional Animal Care and Use Committee. Timed overnight matings of CD-1 mice (Charles River Laboratories, Inc. Wilmington, Massachusetts) were set up, and the presence of a vaginal plug was considered 0.5 DPC. On 14.5 DPC, a period corresponding to the transition between the first and the second trimester equivalent period of brain development, pregnant female mice were anesthetized by isoflurane inhalation. The anesthetized mice were weighed and placed on a heated platform to maintain body temperature throughout the entire procedure. Abdominal fur was removed, and a small incision was made in the abdomen exposing the uterine horn for imaging. The selected embryo was safely secured using forceps to minimize bulk motion due to maternal respiration and heartbeat. Initial OCT images were recorded of the embryonic brain. After initial measurements, the liver of the pregnant mother was sprayed with CP-55940, at a dose of 2 mg/kg, which was suspended in a vehicle of dimethyl sulfoxide (DMSO): Alkamuls El620 (Rhodia, NJ): lactated Ringer’s solution at a ratio of 1:1:18. Subsequent measurements were taken every 5 minutes for a 45-minute period. For the sham group, an equivalent volume of only the vehicle solution was delivered onto the peritoneal cavity. After completing all measurements, the animal was euthanized by an isoflurane overdose followed by cervical dislocation.
2.3 |. Cm-OCA imaging, data processing, and statistical analysis
The OCT beam was scanned to image an area of 6.0 × 6.2 mm2. The 3D OCT images consisted of 600 B-scans per volume, with each B-scan consisting of 600 A-scans. Five repeated B-scans were recorded at each spatial position. The time for each B-scan was 20 ms and the total acquisition time was 84 seconds including the galvanometer flyback time between B-scans. A discrete Fourier transform-based sub-pixel registration technique [61] was used to correct the axial shift (bulk motion) between each pair of 5 B-scans that were recorded at each position. The average temporal correlation between these 5 B-scans, in pairs, was calculated to obtain the correlation coefficient, where SNR-dependent artifacts were corrected by using the temporal variance of the background noise as a function of imaging depth [58]. Angiograms with a global correlation value below a threshold (the difference between the mean and the SD) were discarded. The spatial distribution of the temporal correlation coefficients of the entire 3D image yielded the 3D cm-OCA image. The en face image of the fetal brain vasculature was obtained by taking a maximum intensity projection (MIP) along the axial direction of the 3D cm-OCA image. Finally, a frequency rejection filter [62] was applied to the 2D MIP to remove bulk motion artifacts due to maternal respiration and heartbeat. Final 2D MIP images were formed using Amira software (EFI Co., Portland, Oregon).
A hessian filter-based approach was used to enhance the contrast and connectivity of the blood vessels in the 2D projection (Hessian based Frangi Vesselness filter, Dirk-Jan Kroon, MathWorks File Exchange, Mathworks, Natick, Massachusetts). ImageJ was used to calculate the vessel area density (VAD) (from the binarized MIP) and vessel length fraction (VLF) (from the skeletonized binary MIP), while Matlab (Mathworks, Natick, Massachusetts) was used to calculate the vessel diameter (VD). VAD is defined as the area of the image that corresponds to vasculature, Avessel, divided by the total area of the image, Aimage, (VAD = Avessel/Aimage), and VLF is defined as the total length of the vessels, Lvessel, divided by the total area of the image (VLF = Lvessel/Aimage).
Only a portion of the 2D projection that included the main vessel under investigation was selected for the quantifications. A nonparametric Kruskal-Wallis ANOVA was performed on the VD data of each sample to assess if there was a statistically significant change over time. A 2-sided Mann-Whitney U test was performed to test for statistically significant changes between both groups, for all quantification parameters, at 45-minute post-exposure.
3 |. RESULTS
A total of 8 fetuses, one from each pregnant mouse were imaged, 3 in the cannabinoid group and 5 in the sham (vehicle) group. Figure 2A and B show the MIP of the cm-OCA images of the fetal brain vasculature of one embryo from the cannabinoid group before and 45 minutes after exposure to the SCB, respectively.
FIGURE 2.
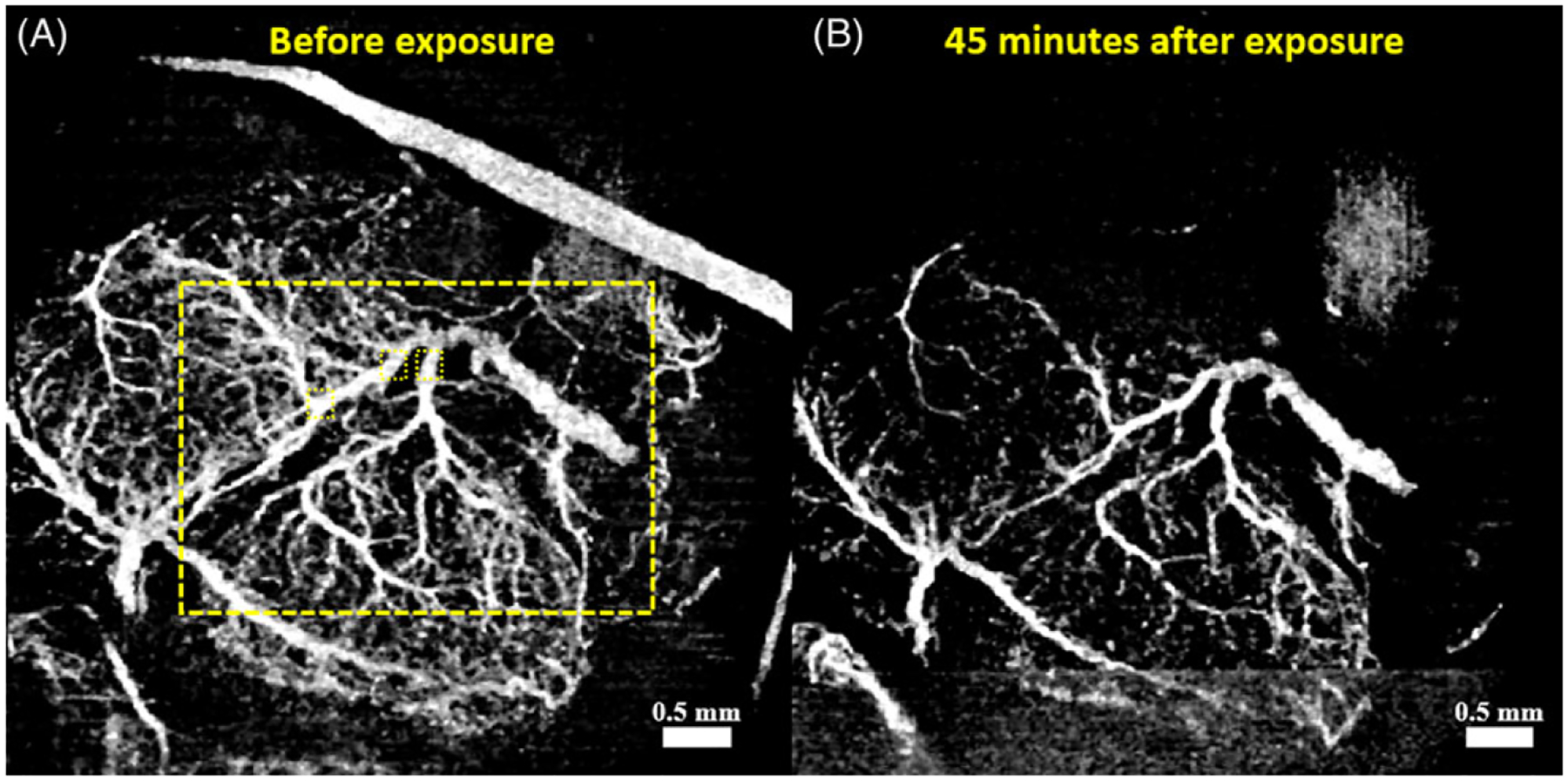
Cm-OCA images of one typical sample from the cannabinoid group. (A) 2D MIP of the cm-OCA image before maternal cannabinoid exposure. The large dashed rectangle depicts the region of VLD and VAD quantifications, and the small dashed squares are the regions used for VD quantifications. (B) 2D MIP of the cm-OCA image 45 minutes after maternal cannabinoid exposure
Figure 3A and B depict the MIP of the cm-OCA images of the fetal brain vasculature of one embryo from the cannabinoid group before and 45 minutes after exposure to the vehicle solution, respectively
FIGURE 3.

Cm-OCA images of one typical sample from the sham group. (A) 2D MIP of the cm-OCA image before maternal vehicle exposure. The large dashed rectangle depicts the region of VLD and VAD quantifications, and the small dashed squares are the regions used for VD quantifications. (B) 2D MIP of the cm-OCA image 45 minutes after maternal vehicle exposure
As seen from Figures 2 and 3, there is a large decrease in vasculature after cannabinoid exposure, which is not seen in the case of the vehicle. Conversely, a slight increase in vasculature is observed 45 minutes after exposure to the vehicle solution. VAD and VLF calculations were all performed in the area within the large dashed yellow rectangles shown in Figures 2A and 3A, which included the main vessel under investigation. VD quantifications were performed in three locations, depicted by the three small dotted yellow boxes, to ensure consistency in the results.
Figures 4A–C plot quantified VAD, VLF and VD, respectively, for each of the samples shown in Figures 2 and 3. The plots show the percentage change in all three parameters over a 45-minute period at intervals of 5 minutes as compared to the initial measurement before the exposure to the cannabinoid or sham. All three parameters decreased for the sample exposed to the cannabinoid, while all three parameters increased for the sample exposed to the vehicle (sham). The VAD, VLF and VD decreased by approximately 40%, 56%, and 33%, respectively, in the cannabinoids group, whereas it increased by approximately 35%, 26% and 17%, respectively, in the sham group.
FIGURE 4.

Quantifications of one sample from the sham and cannabinoid groups. Percentage change in (A) VAD, (B) VLF, (C) VD after exposure, every 5 minutes for 45 minutes. The error bars represent the standard deviation
A Kruskal-Wallis ANOVA was performed on the VD data of all samples, as three positions in the vessel were measured at every time point. All samples in the cannabinoid group showed a statistically significant decrease (P < 0.001) in diameter over time, whereas all the samples from the sham group showed a statistically significant increase (P = 0.028) in vessel diameter over time.
Figure 5 shows a summary of all the quantifications performed on all samples from both groups. The data is represented as the mean percentage change in each of the quantification parameters at 45-minute post-maternal exposure as compared to the initial measurements before the exposure to the cannabinoid or vehicle. Figures 5A–C depicts the percentage changes in the VAD, VLF, and VD respectively, for all samples in the vehicle and cannabinoid groups. A Mann-Whitney U test showed that at 45-minutes post-exposure, the vehicle and the cannabinoid groups showed statistically significant differences in all three parameters. Table 1 shows the results of the Mann-Whitney U test for each of the parameters used to quantify vasculature.
FIGURE 5.

Percentage change in (A) VAD, (B) VLF, and (C) VD 45-minute post-maternal exposure. The asterisk indicates statistical significance (P < 0.05 by a 2-sided Mann-Whitney U test)
TABLE 1.
Results of the Mann-Whitney U test
| VAD | VLF | VD | |
|---|---|---|---|
| NVehicle | 5 | 5 | 15 |
| NCannabinoid | 3 | 3 | 9 |
| U | 15 | 15 | 135 |
| P | 0.0369 | 0.0369 | <0.001 |
4 |. DISCUSSION
Brain vasculature that develops during the second trimester is known to be particularly vulnerable to teratogens, as it is considered the peak period for fetal neurogenesis and angiogenesis [63]. Hence, identifying changes in normal vasculature development during this period is of immense importance. In this study, in utero cm-OCA was used to evaluate the changes in murine embryonic brain vasculature caused by prenatal exposure to CP-55940, a SCB. The results showed a significant decrease in vasculature within 45 minutes of maternal exposure when compared to the sham group.
All the VAD and VLF calculations were performed over a certain area depicted by the dashed yellow rectangles in Figures 2 and 3 to avoid external influence in the quantifications. For instance, because we do not have control over the region of the uterus through which we image the brain, blood vessels from the uterus might be a part of the MIP as seen on the top right corner of Figure 2A. Because we clamp the uterus using forceps to reduce bulk motion, this blood vessel eventually disappears over time and is not seen in Figure 2B, which shows the vasculature 45-minute post-maternal exposure. This clamping technique was uniformly used across all embryos (including sham group) and seems to not introduce significant alternations to maternal/embryonic nutrients exchange, as confirmed by post-experimental visual examination of the embryos.
CP-55940 was administered as a single intraperitoneal exposure at a dose of 2 mg/kg. We followed a similar protocol to Gilbert et al [64]. However, in our work, the SCB was dissolved in DMSO instead of ethanol because our previous work has shown that ethanol causes significant acute fetal brain vasoconstriction [59]. It can be seen from Figure 3 that there is a slight increase in vasculature in the sham group. This change could possibly be due to the DMSO in the vehicle solution. DMSO is known to possess histamine releasing properties, which has been shown to cause vasodilation [65, 66]. This explains the results from the Kruskal-Wallis ANOVA. Both the sham and the cannabinoid groups showed statistically significant changes in VD over time. However, the sham group had a positive linearly fitted slope, and the cannabinoid group had a negative slope, showing the increase and decrease in VD, respectively.
Our previous work evaluating the effects of maternal alcohol exposure on fetal brain vasculature showed vasoconstriction within 45 minutes of exposure to a binge-like bolus of ethanol, which is similar to our results from this study. Although fetal alcohol syndrome-like features have been reported due to prenatal cannabis exposure in human cannabinoid developmental toxicology studies [67], several animal-based studies have not corroborated these results. This area of research is still nascent and requires more attention. Meticulous dose-response studies, research covering a range of gestational periods of exposure, as well as multiple exposure experiments are needed to better understand the complex process of cannabinoid exposure to the fetal brain vasculature. Correspondingly, our research is focused on developing such studies in our future work.
One important limitation of our work is the inability to quantify blood flow. As a part of our future work, we plan to introduce DOCT to help with blood flow quantifications. We plan to use DOCT to calculate blood flow rates and correlate them with the changes in the developing vasculature. The flow rate quantifications will also be used to calculate other parameters such as velocity time integral, acceleration and Pourcelot resistive index, which can be used to assess cardiac output, cardiac stroke volume and vascular resistance, respectively. DOCT will also provide the direction of flow, that will help us to identify differences between the changes in arteries and veins, if any, caused due to prenatal SCB exposure.
Another limitation to the current technique is the system sensitivity and sensitivity roll-off, which affect phase stability [68] and subsequently determine the quality of the cm-OCA map. For a curved structure like that of an embryo, the vessels in deeper regions might be difficult to visualize due to low SNR at greater depths. As this was a concern, during imaging, the embryonic brains were oriented in a way that there was good visibility of the dorsal vessels of the brain. However, shadowing artifacts are still a concern. To address this issue and image deeper vessels, a projection resolved algorithm [69] will be covered in our future work. In addition to this, applying a phase correction scheme [58, 70] and a 2D Gabor wavelet filter [71] in OCTA has been shown useful to enhance the vessel contrast and connectivity. Thus, both algorithms, will be also implemented in our future work to image the microvasculature. Moreover, we will evaluate the effectiveness of other angiographic techniques, such as split spectrum imaging in order to further improve the SNR [72].
5 |. CONCLUSION
This study evaluated acute vasculature changes in the fetal brain due to prenatal exposure to the SCB CP-55940 using in utero cm-OCA. The results showed a drastic decrease in vessel area density (approximately 53%), vessel length fraction (approximately 65%), and vessel diameter (approximately 35%) over a period of 45 minutes after maternal cannabinoid exposure, which was not seen in the sham group. Similar to prenatal exposure to ethanol in our previous work, this study demonstrates that the SCB, CP-55940, acts as a vasoconstrictor on the fetal brain during the peak period of angiogenesis and neurogenesis in a mouse model.
ACKNOWLEDGMENTS
The authors would like to thank Ms. Connie Yan, Ms. Noemi Bustamante, Mr. Achuth Nair, and Mr. Justin Rippy at the University of Houston for their technical assistance. This work was supported by the National Institute of Health grants R01HL120140, R01HD086765, and R01HD095520.
Footnotes
AUTHOR BIOGRAPHIES
Please see Supporting Information online.
REFERENCES
- [1].Forray A F1000Res. 2016, 5, F1000 Faculty Rev-1887. [Google Scholar]
- [2].McCabe JE, Arndt S, Matern Child Health J 2012, 16, 1696. [DOI] [PubMed] [Google Scholar]
- [3].Ebrahim SH, Gfroerer J, Obstet Gynecol. 2003, 101, 374. [DOI] [PubMed] [Google Scholar]
- [4].Castaneto MS, Gorelick DA, Desrosiers NA, Hartman RL, Pirard S, Huestis MA, Drug Alcohol Depend. 2014, 144, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Tai S, Fantegrossi WE, Curr. Addict. Rep 2014, 1, 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Gunderson EW, Haughey HM, Ait-Daoud N, Joshi AS, Hart CL, Am. J. Addict 2012, 21, 320. [DOI] [PubMed] [Google Scholar]
- [7].Mills B, Yepes A, Nugent K, Am. J. Med. Sci 2015, 350, 59. [DOI] [PubMed] [Google Scholar]
- [8].Breivogel CS, Childers SR, J. Pharmacol. Exp. Ther 2000, 295, 328. [PubMed] [Google Scholar]
- [9].Sim LJ, Hampson RE, Deadwyler SA, Childers SR, J. Neurosci 1996, 16, 8057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Tao Q, Abood ME, J. Pharmacol. Exp. Ther 1998, 285, 651. [PubMed] [Google Scholar]
- [11].Seely KA, Prather PL, James LP, Moran JH, Mol Interv. 2011, 11, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Fattore L, Fratta W, Front Behav. Neurosci 2011, 5, 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Papaseit E, Farre M, Schifano F, Torrens M, Curr Opin Psychiatry. 2014, 27, 243. [DOI] [PubMed] [Google Scholar]
- [14].Schneir AB, Cullen J, Ly BT, J. Emerg. Med 2011, 40, 296. [DOI] [PubMed] [Google Scholar]
- [15].Brown HL, Graves CR, Clin Obstet Gynecol. 2013, 56, 107. [DOI] [PubMed] [Google Scholar]
- [16].Holbrook BD, Rayburn WF, Obstet. Gynecol. Clin. North Am 2014, 41, 229. [DOI] [PubMed] [Google Scholar]
- [17].Calvigioni D, Hurd YL, Harkany T, Keimpema E, Eur. Child Adolesc. Psychiatry 2014, 23, 931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hutchings DE, Martin BR, Gamagaris Z, Miller N, Fico T, Life Sci. 1989, 44, 697. [DOI] [PubMed] [Google Scholar]
- [19].Bailey JR, Cunny HC, Paule MG, Slikker W Jr.., Toxicol. Appl. Pharmacol 1987, 90, 315. [DOI] [PubMed] [Google Scholar]
- [20].Perez-Reyes M, Wall ME, Engl N. J. Med 1982, 307, 819. [DOI] [PubMed] [Google Scholar]
- [21].Garry A, Rigourd V, Amirouche A, Fauroux V, Aubry S, Serreau R, J. Toxicol 2009, 2009, 596149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Geber WF, Schramm LC, Toxicol. Appl. Pharmacol 1969, 14, 276. [DOI] [PubMed] [Google Scholar]
- [23].Persaud TV, Ellington AC, Lancet 1967, 2, 1306. [DOI] [PubMed] [Google Scholar]
- [24].Psychoyos D, Hungund B, Cooper T, Finnell RH, Birth Defects Res B Dev Reprod Toxicol. 2008, 83, 477. [DOI] [PubMed] [Google Scholar]
- [25].Psychoyos D, Vinod KY, Cao J, Xie S, Hyson RL, Wlodarczyk B, He W, Cooper TB, Hungund BL, Finnell RH, Birth Defects Res B Dev Reprod Toxicol. 2012, 95, 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Begbie J, Doherty P, Graham A, J. Anat 2004, 205, 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Buckley NE, Hansson S, Harta G, Mezey E, Neuroscience. 1998, 82, 1131–1149. [DOI] [PubMed] [Google Scholar]
- [28].Workman AD, Charvet CJ, Clancy B, Darlington RB, Finlay BL, J. Neurosci 2013, 33, 7368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Harkany T, Guzman M, Galve-Roperh I, Berghuis P, Devi LA, Mackie K, Trends Pharmacol. Sci 2007, 28, 83. [DOI] [PubMed] [Google Scholar]
- [30].Norman MG, O’Kusky JR, J. Neuropathol. Exp. Neurol 1986, 45, 222. [PubMed] [Google Scholar]
- [31].Fowden AL, Forhead AJ, Horm. Res 2009, 72, 257. [DOI] [PubMed] [Google Scholar]
- [32].Tam SJ, Watts RJ, Annu Rev Neurosci. 2010, 33, 379. [DOI] [PubMed] [Google Scholar]
- [33].Sharpe J, Ahlgren U, Perry P, Hill B, Ross A, Hecksher-Sorensen J, Baldock R, Davidson D, Science 2002, 296, 541. [DOI] [PubMed] [Google Scholar]
- [34].Walls JR, Coultas L, Rossant J, Henkelman RM, PLoS One. 2008, 3, e2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Dickinson ME, Dev Dyn. 2006, 235, 2386. [DOI] [PubMed] [Google Scholar]
- [36].Huang D, Swanson EA, Lin CP, Schuman JS, Stinson WG, Chang W, Hee MR, Flotte T, Gregory K, Puliafito CA, et al. , Science 1991, 254, 1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Raghunathan R, Singh M, Dickinson ME, Larin KV J. Biomed. Opt 2016, 21, 50902, 050902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Men J, Huang Y, Solanki J, Zeng X, Alex A, Jerwick J, Zhang Z, Tanzi RE, Li A, Zhou C, IEEE J Sel Top Quantum Electron. 2016, 22, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Wang S, Garcia MD, Lopez AL 3rd., Overbeek PA, Larin KV, Larina IV, Biomed Opt Express. 2017, 8, 407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Wu C, Sudheendran N, Singh M, Larina IV, Dickinson ME, Larin KV J. Biomed. Opt 2016, 21, 26002, 026002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Singh M, Raghunathan R, Piazza V, Davis-Loiacono AM, Cable A, Vedakkan TJ, Janecek T, Frazier MV, Nair A, Wu C, Larina IV, Dickinson ME, Larin KV, Biomed. Opt. Express 2016, 7, 2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Wang S, Burton JC, Behringer RR, Larina IV, Sci. Rep 2015, 5, 13216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Larina IV, Syed SH, Sudheendran N, Overbeek PA, Dickinson ME, Larin KV, J. Biomed. Opt 2012, 17, 081410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Larina IV, Sudheendran N, Ghosn M, Jiang J, Cable A, Larin KV, Dickinson ME, J. Biomed. Opt 2008, 13, 060506. [DOI] [PubMed] [Google Scholar]
- [45].Chen Z, Milner TE, Srinivas S, Wang X, Malekafzali A, van Gemert MJ, Nelson JS, Opt. Lett 1997, 22, 1119. [DOI] [PubMed] [Google Scholar]
- [46].Izatt JA, Kulkarni MD, Yazdanfar S, Barton JK, Welch AJ, Opt. Lett 1997, 22, 1439. [DOI] [PubMed] [Google Scholar]
- [47].Barton JK, Stromski S, Optics Express. 2005, 13, 5234. [DOI] [PubMed] [Google Scholar]
- [48].Mahmud MS, Cadotte DW, Vuong B, Sun C, Luk TW, Mariampillai A, Yang VX, J. Biomed. Opt 2013, 18, 50901. [DOI] [PubMed] [Google Scholar]
- [49].Mariampillai A, Standish BA, Moriyama EH, Khurana M, Munce NR, Leung MK, Jiang J, Cable A, Wilson BC, Vitkin IA, Yang VX, Opt. Lett 2008, 33, 1530. [DOI] [PubMed] [Google Scholar]
- [50].Jonathan E, Enfield J, Leahy MJ, J. Biophotonics 2011, 4, 583. [DOI] [PubMed] [Google Scholar]
- [51].Wang RK, Jacques SL, Ma Z, Hurst S, Hanson SR, Gruber A, Opt. Express 2007, 15, 4083. [DOI] [PubMed] [Google Scholar]
- [52].Wang RK, An L, Opt Express. 2009, 17, 8926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].An L, Qin J, Wang RK, Opt Express. 2010, 18, 8220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Lal C, Subhash HM, Alexandrov S, Leahy MJ, Appl Opt. 2018, 57, E224. [DOI] [PubMed] [Google Scholar]
- [55].Jingjiang X, Shaozhen S, Yuandong L, Ruikang KW, Phys Med Biol. 2018, 63, 015023. [Google Scholar]
- [56].An L, Shen T, Wang RK Using ultrahigh sensitive optical microangiography to achieve comprehensive depth resolved microvasculature mapping for human retina, Vol. 16, SPIE, City, 2011, pp.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Guo L, Li P, Pan C, Liao R, Cheng Y, Hu W, Chen Z, Ding Z, Li P, Journal of Optics. 2015, 18, 025301. [Google Scholar]
- [58].Makita S, Kurokawa K, Hong Y-J, Miura M, Yasuno Biomed Y, Opt. Express 2016, 7, 1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Raghunathan R, Wu C, Singh M, Liu CH, Miranda RC, Larin KV, J. Biophotonics 2018, 11, e201700238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Manapuram RK, Manne VGR, Larin KV, Laser Physics. 2008, 18, 1080. [Google Scholar]
- [61].Guizar-Sicairos M, Thurman ST, Fienup JR, Opt. Lett 2008, 33, 156. [DOI] [PubMed] [Google Scholar]
- [62].Liu G, Chin RW, Opt. Lett 2013, 11, 031701. [Google Scholar]
- [63].Bake S, Tingling JD, Miranda RC, Alcohol. Clin. Exp. Res 2012, 36, 748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Gilbert MT, Sulik KK, Fish EW, Baker LK, Dehart DB, S. E. Parnell Neurotoxicol Teratol 2016, 58, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Kaneda T, Sasaki N, Urakawa N, Shimizu Pharmacology. 2016, 97, 171. [DOI] [PubMed] [Google Scholar]
- [66].Wong LK, Reinertson Iowa EL, State University Veterinarian. 1984, 46, 2. [Google Scholar]
- [67].Gibson GT, Baghurst PA, Colley DP Aust N Z J Obstet Gynaecol. 1983, 23, 15. [DOI] [PubMed] [Google Scholar]
- [68].Hyle Park B, Pierce MC, Cense B, Yun S-H, Mujat M, Tearney GJ, Bouma BE, Boer J. F. d., Opt. Express 2005, 13, 3931. [DOI] [PubMed] [Google Scholar]
- [69].Zhang M, Hwang TS, Campbell JP, Bailey ST, Wilson DJ, Huang D, Biomed. Opt. Express 2016, 7, 816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].An L, Shen TT, Wang RK, J. Biomed. Opt 2011, 16, 106013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Hendargo HC, Estrada R, Chiu SJ, Tomasi C, Farsiu S, Izatt JA, Biomed. Opt. Express 2013, 4, 803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Jia Y, Tan O, Tokayer J, Potsaid B, Wang Y, Liu JJ, Kraus MF, Subhash H, Fujimoto JG, Hornegger J, Huang Opt Express. 2012, 20, 4710. [DOI] [PMC free article] [PubMed] [Google Scholar]


