Abstract
Managing coronary saphenous vein graft failure has remained an unmet need since the inception of interventional cardiology. The present case constitutes an opportunity to revisit percutaneous strategies to treat saphenous vein graft failure, providing a travel though interventional strategies and showing a contemporary approach to this problem. (Level of Difficulty: Intermediate.)
Key Words: chronic total occlusion, percutaneous coronary intervention, saphenous vein graft failure
Central Illustration
Past Medical History
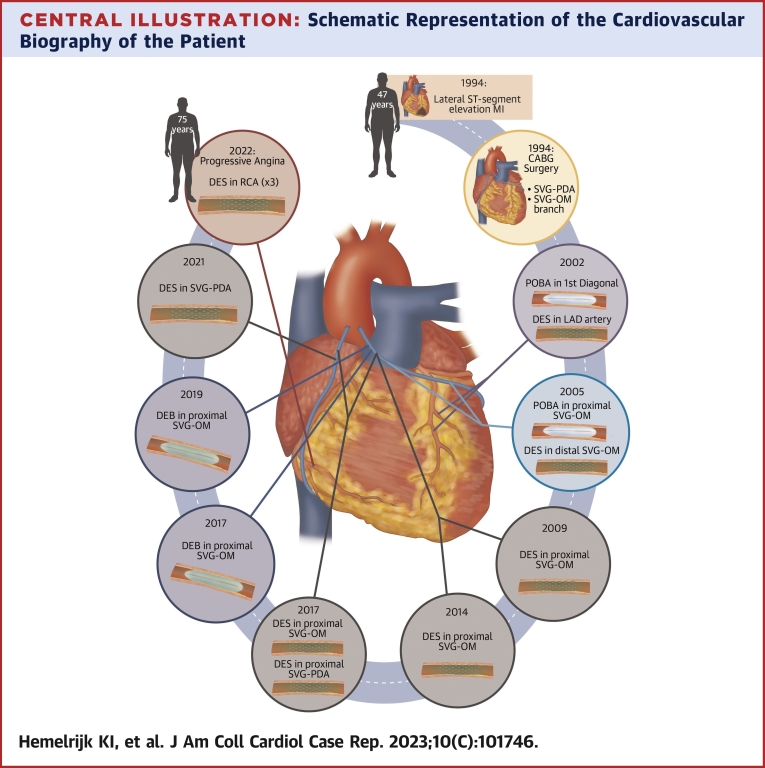
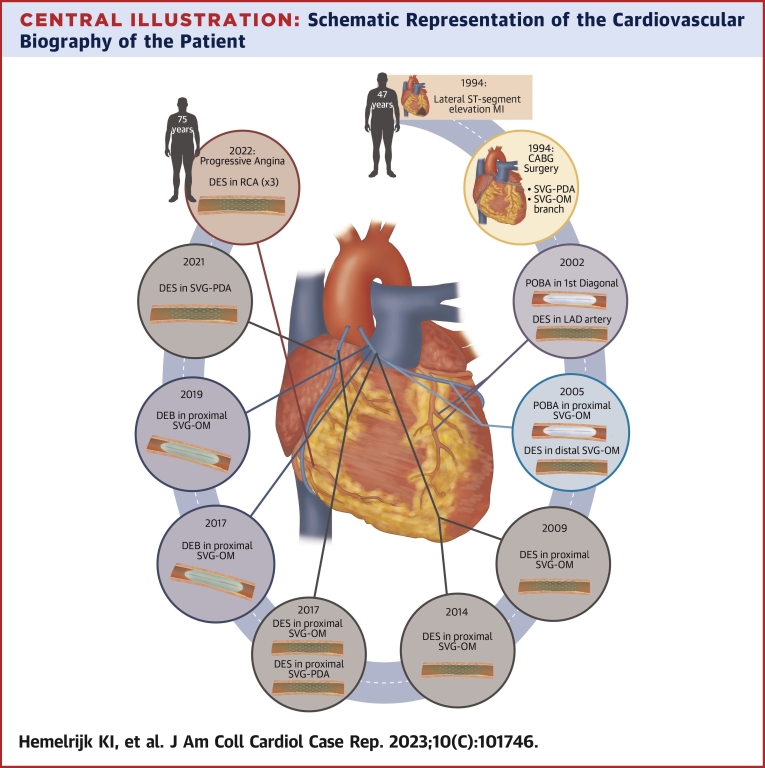
We revisit the cardiovascular biography of a 75-year-old man (Figure 1). In 1994, when he was 47 years old, he had a lateral ST-segment elevation myocardial infarction (MI), which was managed conservatively. He was again admitted for unstable angina (UA) months later, having 2 angiographically severe stenoses in the left circumflex artery and right coronary artery (RCA), treated surgically with 2 saphenous vein grafts (SVGs) to the obtuse marginal (OM) branch and posterior descending artery (PDA). The reasons for avoiding arterial grafts at the time is unknown.
Learning Objectives
-
•
To understand the challenges posed by SVG attrition to percutaneous interventions.
-
•
To revisit percutaneous intervention strategies that have been used over time to treat SVG attrition.
-
•
To envisage the value of CTO PCI on the native vessel as an alternate, potentially more durable therapeutic solution to patients with SVG attrition.
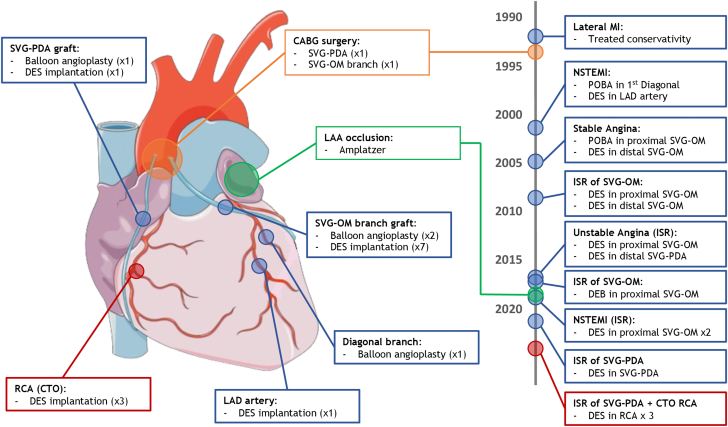
Figure 1.
Cardiovascular Intervention Timeline
(Left) Illustrative anatomic representation of the total cumulative interventions by location. (Right) Chronological timeline order (by year) of the respective interventions. CABG= coronary artery bypass graft; CTO = chronic total occlusion; DEB = drug-eluting balloon; DES = drug-eluting stent; ISR = in-stent restenosis; LAA = left atrial appendage; LAD = left anterior descending; MI = myocardial infarction; NSTEMI = non–ST-segment elevation myocardial infarction; OM = obtuse marginal; PDA = posterior descending artery; POBA = plain old balloon angioplasty; RCA = right coronary artery; SVG = saphenous vein graft.
Eight years later (2002), the patient underwent left anterior descending artery stenting and plain old balloon angioplasty (POBA) of the first diagonal branch after being admitted for MI. In 2005, he had occlusion of both the mid-RCA and distal circumflex artery. The SVG-OM graft had a severe proximal lesion, treated by POBA, and there was another in the distal segment, treated with drug-eluting stent (DES) implantation. In 2009, because of restenosis of the proximal SVG-OM graft lesion, another DES was implanted.
In 2014, the patient was once more admitted with UA with a severe proximal PDA graft lesion, treated with DES implantation. This relieved symptoms until 2017, when he was once more admitted for UA and showed severe stenosis in the proximal SVG-PDA graft and proximal SVG-OM graft, both of which were treated with DES implantation. In-stent restenosis of the SVG-OM graft was diagnosed 3 months later, and treated with a drug-eluting balloon.
In 2019, the patient presented again with MI caused by a second in-stent restenosis in the proximal SVG-OM graft, treated with another drug-eluting balloon. The SVG-PDA graft had its second in-stent restenosis in 2021, this time treated with DES implantation.
Current Presentation
Almost 22 years after coronary artery bypass graft (CABG) and after 9 percutaneous coronary interventions (PCIs) addressing SVG failure, the patient presented again with progressive angina. A new coronary angiogram was performed (Figure 2), which showed the chronic total occlusion (CTO) in the proximal RCA previously described (Rentrop grade 1; Japanese Chronic Total Occlusion score: 3; Werner classification: C2) and severe lesions in the ostial, medial, and distal segments of SVG-PDA graft.
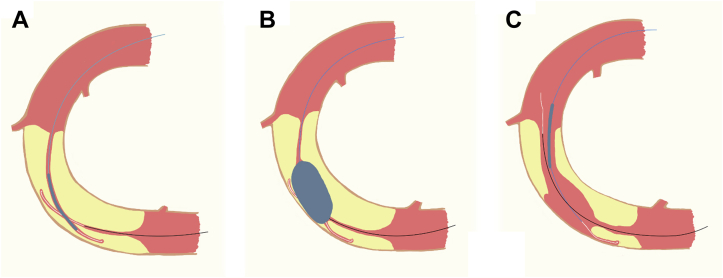
Figure 2.
Representation of the Reverse CART Technique
(A) Two guidewires are positioned and overlapped inside the CTO. (B) A balloon is advanced over the anterograde guidewire and inflated after crossing the proximal cap. (C) After balloon inflation, a new pathway is formed that allows retrograde progression of the distal guidewire. CART = combined antegrade and retrograde subintimal tracking; CTO = chronic total occlusion.
After heart team discussion, it was decided to avoid further interventions on the SVG-PDA graft and focus on the RCA CTO. After detailed angiography analysis, a retrograde approach was chosen as the initial strategy, using the PDA graft as the conduit to access the vessel distal to the CTO (reverse combined antegrade and retrograde subintimal tracking technique) (Figure 2).
Procedure Description
Because of severe bilateral distal arterial disease, the right humeral artery was chosen for primary access. The right humeral and the right femoral arteries were canulated with 7-F and 6-F arterial sheets, respectively. The PDA was approached with a 7-F Amplatz right 2 and the RCA with a 6-F Amplatz left 2 guide catheter.
A standard workhorse guidewire was advanced through the PDA, reaching the distal RCA, and was then switched to a Fielder XTA wire (Asahi Intecc Medical) using a microcatheter and retrogradely advanced to the distal CTO cap. Guidewire escalation to a Miracle 12 wire (Asahi Intecc Medical) was unable to completely cross the lesion. Anterograde guidewire escalation to a Confianza Pro wire (Asahi Intecc Medical) was pursued and advanced until obtaining adequate guidewire overlap inside the CTO.
An 1.1-mm CTO balloon was advanced anterogradely and inflated inside, followed by a 2.5-mm noncompliant balloon, which then allowed the Miracle 12 guidewire to retrogradely cross the lesion and reach the proximal RCA. This wire was then changed to an RG3 guidewire (Asahi Intecc Medical), which was advanced to the proximal humeral arterial sheet. After predilating the all RCA segments with a 2.0-mm balloon, intravascular ultrasound was performed, showing diffuse fibrolipidic plaque throughout all segments, with moderate calcification and negative remodeling in the previous CTO location. A total of 3 DESs were implanted with good angiographic results and adequate stent apposition confirmed by intravascular ultrasound (Figure 3). At the 3-month follow-up, the patient is asymptomatic.
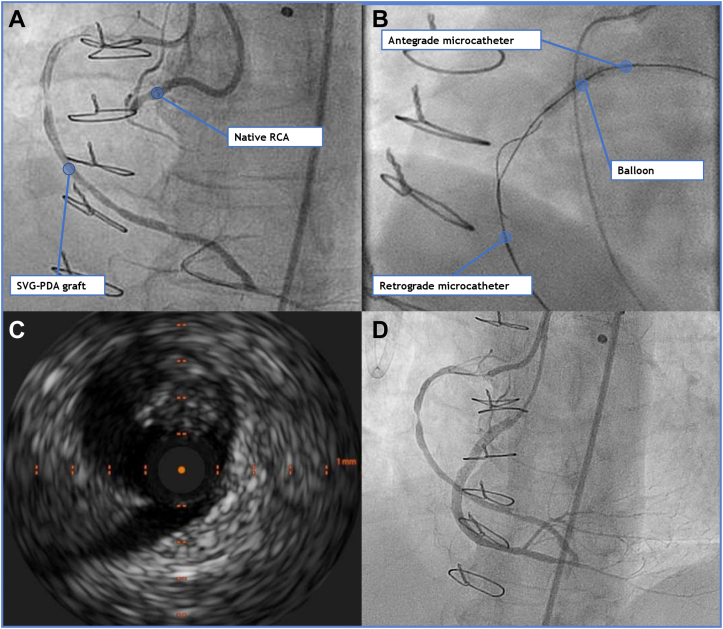
Figure 3.
Right Coronary Artery Total Occlusion and Angioplasty
(A) Bilateral injection showing 2 severe restenotic lesions (middle and distal segments) of the PDA graft and (B) CTO of the proximal RCA. (C) After retrograde wiring using the reverse CART technique, intravascular ultrasound of the RCA was performed, showing diffuse disease with mild parietal calcium and negative remodeling. (D) The final result after implanting 3 drug-eluting stents (distal, middle, and proximal segments). CART = combined antegrade and retrograde subintimal tracking; CTO = chronic total occlusion; PDA = posterior descending artery; RCA = right coronary artery; SVG = saphenous vein graft.
Discussion
CABG surgery is the recommended revascularization modality in many patients with multivessel coronary disease,1,2 SVG attrition causes accelerated atherosclerosis by different mechanisms than the ones described for native vessel disease3,4 and accounts for approximately 50% to 60% graft occlusions.3,5 Modification of coronary hemorheology by CABG is associated with progression of stenosis severity in grafted vessels,6,7 which may explain the progression of the initial RCA stenosis to CTO over time.
Looking back into the cardiovascular biography of our patient (Central Illustration), it is interesting to see how operators focused their treatment on SVG attrition using techniques that were initially developed and tested in native coronary atherosclerotic lesions, including POBA, DES, and drug-eluting balloon. SVG interventions are not seldomly performed,5 have periprocedural risk of distal embolization and no reflow, and have high rates of restenosis.3,5 This accounts for the worse prognosis when compared with native coronary artery PCI.3 Despite the Class IIa recommendation to consider PCI of the native coronary artery over the SVG,8 graft intervention is still commonly chosen over native CTO PCI because of lower technical difficulty.
Central Illustration.
Schematic Representation of the Cardiovascular Biography of the Patient
A different strategy was pursued for our patient after recurrent failure of PCI strategies for SVG. Of note, this approach was not commonly pursued during the time period when our patient’s journey began: in making the choice to treat the SVG and not the native vessel, it should be kept in mind that PCI success in CTO lesions has remained very low for many years. However, in the last developing decade, contemporary success rates of CTO PCI (85%-94%)9 justify a paradigm shift in the management of patients with SVG attrition after CABG.
Conclusions
Our case illustrates the long struggle held by interventional cardiologists in treating SVG attrition. Contemporary CTO techniques may be more effective in treating patients with SVG failure and should be primarily considered.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Daemen J., Boersma E., Flather M., et al. Long-term safety and efficacy of percutaneous coronary intervention with stenting and coronary artery bypass surgery for multivessel coronary artery disease: a meta-analysis with 5-year patient-level data from the ARTS, ERACI-II, MASS-II, and SoS trials. Circulation. 2008;118:1146–1154. doi: 10.1161/CIRCULATIONAHA.107.752147. [DOI] [PubMed] [Google Scholar]
- 2.Hlatky M.A., Boothroyd D.B., Bravata D.M., et al. Coronary artery bypass surgery compared with percutaneous coronary interventions for multivessel disease: a collaborative analysis of individual patient data from ten randomised trials. Lancet. 2009;373:1190–1197. doi: 10.1016/S0140-6736(09)60552-3. [DOI] [PubMed] [Google Scholar]
- 3.Xenogiannis I., Zenati M., Bhatt D., et al. Saphenous vein graft failure: from pathophysiology to prevention and treatment strategies. Circulation. 2021;144(9):728–745. doi: 10.1161/CIRCULATIONAHA.120.052163. [DOI] [PubMed] [Google Scholar]
- 4.Kwiecinski J., Tzolos E., Fletcher A., et al. Bypass grafting and native coronary artery disease activity. J Am Coll Cardiol Img. 2022;15(5):875–887. doi: 10.1016/j.jcmg.2021.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Broughton S., Aranda-Michel E., Sezer A., et al. Long-term outcomes of percutaneous coronary intervention in patients with prior coronary artery bypass grafting. Heart Surg Forum. 2022;25(2):232–240. doi: 10.1532/hsf.4457. [DOI] [PubMed] [Google Scholar]
- 6.Pereg D., Fefer P., Samuel M., et al. Native coronary artery patency after coronary artery bypass surgery. J Am Coll Cardiol Intv. 2014:761–767. doi: 10.1016/j.jcin.2014.01.164. [DOI] [PubMed] [Google Scholar]
- 7.Dautov R., Nguyen C., Altisent O., et al. Recanalization of chronic total occlusions in patients with previous coronary bypass surgery and consideration of retrograde access via saphenous vein grafts. Circ Cardiovasc Interv. 2016;9:1–12. doi: 10.1161/CIRCINTERVENTIONS.115.003515. [DOI] [PubMed] [Google Scholar]
- 8.Neumann F., Sousa-Uva M., Ahlsson A., et al. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. 2019;40(2):87–165. doi: 10.1093/eurheartj/ehy855. [DOI] [PubMed] [Google Scholar]
- 9.Wu E., Brilakis E., Mashayekhi K., et al. Global chronic total occlusion crossing algorithm: JACC state-of-the-art review. J Am Coll Cardiol. 2021;78(8):840–853. doi: 10.1016/j.jacc.2021.05.055. [DOI] [PubMed] [Google Scholar]