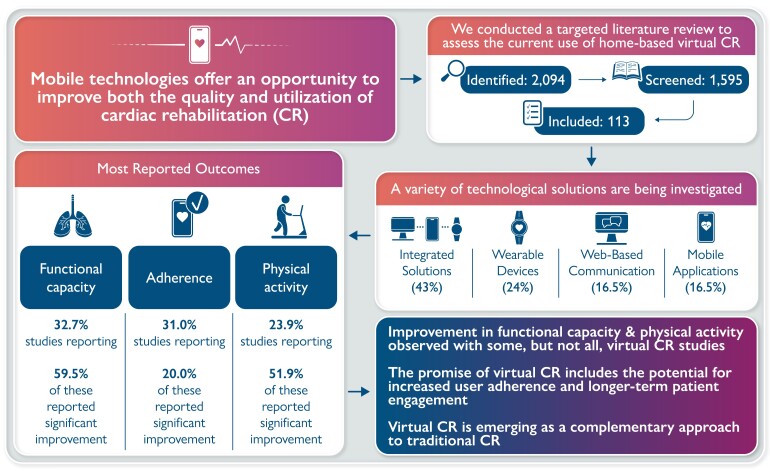
Graphical Abstract
Graphical Abstract.
Adherence to cardiac rehabilitation following a primary event has been demonstrated to improve quality of life, increase functional capacity, and decrease hospitalizations and mortality. Mobile technologies offer an opportunity to improve both the quality and utilization of cardiac rehabilitation, and recent clinical studies investigated this technology. This literature review summarizes the current use of mobile health, wearable activity monitors (WAMs), and other multi-component technologies deployed to support home-based virtual cardiac rehabilitation. The methodology was adapted from the Cochrane Handbook for Systematic Reviews of Interventions. We identified 2094 records, of which 113 were eligible for qualitative analysis. Different virtual cardiac rehabilitation solutions were implemented in the studies: (i) multi-component interventions in 48 studies (42.5%), (ii) WAMs in 27 studies (23.9%), (iii) web-based communications solutions, and (iv) mobile apps, both in 19 studies (16.4%). Functional capacity was the most frequently reported primary outcome (k = 37, 32.7%), followed by user adherence/compliance (k = 35, 31.0%), physical activity (k = 27, 23.9%), and quality of life (k = 14, 12.4%). Studies provided a mixed assessment of the efficacy of virtual cardiac rehabilitation in attaining either significant improvements over baseline or significant improvements in outcomes compared with conventional rehabilitation. Efficacy outcomes with virtual cardiac rehabilitation sometimes improve on the centre-based outcomes; however, superior clinical efficacy may not necessarily be the only outcome of interest. The promise of virtual cardiac rehabilitation includes the potential for increased user adherence and longer-term patient engagement. If these outcomes can be improved, that would be a significant justification for using this technology.
Keywords: Cardiac rehabilitation, Virtual healthcare, Telemedicine, eHealth, patient empowerment, Self-care
Introduction
Cardiovascular disease (CVD) is the leading cause of death globally, and the increasing incidence of CVD constitutes a pandemic that affects populations worldwide.1 Following a primary CVD event [e.g. myocardial infarction (MI), heart failure (HF)], cardiac rehabilitation (CR) programmes may be offered to patients to provide support aimed at secondary prevention.2,3 Many studies have demonstrated the benefits of adherence to CR programmes, leading to improvements in health and quality of life, increased functional capacity, decreased hospitalizations, and mortality (see4,5 for recent reviews). However, one of the more challenging aspects of CR concerns the significant underutilization among eligible patients. In the USA, only 16.3% of Medicare patients and 10.3% of veterans hospitalized for MI, percutaneous coronary intervention, or coronary artery bypass graft surgery between 2007 and 2011 participated in CR.6 In other countries, 20–33% of eligible patients have been reported to enrol in CR.7–10 Despite proven benefits and strong guideline recommendations, less than half of eligible patients with CVD within European Union (EU) countries participate in CR, due to both insufficient referral by medical professionals and suboptimal enrolment of patients who are referred.11
The underutilization of CR is even more pronounced in certain populations, who already have poorer health outcomes across a wide range of indicators. These disparities are evident in CR participation rates among women, individuals from rural communities, and racial and ethnic minorities when compared with the general population.12–14
With the advent of new technologies, smartphones and wearable devices, healthcare professionals and researchers have looked at incorporating these into novel CR programmes in an effort to improve uptake and participation.15 The use of stand-alone devices such as wearable activity monitors (WAMs) or mobile apps running on smartphones and tablets (mHealth) allows the capture of patient performance and exercise information. These interventions allow medical staff to provide regular feedback and guidance to patients.16,17 More sophisticated multi-component interventions with multiple technologies integrated into a comprehensive CR solution are also being investigated.18
Remote home-based virtual CR may provide advantages in removing certain barriers preventing patients from participating in CR, such as transportation issues, time spent travelling, and associated costs.19 In addition to traditional barriers to participation, the COVID-19 pandemic introduced new urgency to remote CR as the lockdowns severely limited access to in-person CR.20 However, given the relative novelty of mobile technologies in CR, most published studies are limited to research settings, usually within academic research centres and funded by external grants. To date, the use of home-based virtual CR has often been in the context of small pilot studies.
This narrative literature review summarizes the current state of the art of mHealth, with WAMs, and other multi-component technologies deployed in support of home-based virtual CR. The main objective is to evaluate the efficacy of home-based CR as compared to traditional centre-based CR and consider the strengths and weaknesses of this new approach. Given that the main challenges of CR are recruitment, participation, and long-term adherence, this review will also explore the potential of the new technologies to address these issues.
The mobile and healthcare solutions are also commonly referred to in the literature as ‘virtual healthcare’ and are especially relevant with COVID-19-associated restrictions and limits on direct human-to-human interactions.21 This review generally uses the term virtual cardiac rehabilitation (virtual CR), broadly defined as the remote delivery of cardiac rehabilitation interventions via connected devices, mobile phones or tablets, and related internet technologies. It is recognized that the terms ‘virtual’ and ‘remote’ may be used differently in other CR literature or contexts. Although the methodology of this review follows the recommendations of the Cochrane Handbook, a targeted literature review form was chosen due to significant heterogeneity in the evidence base. This heterogeneity makes it difficult to conduct a systematic review, since both the diverse methodologies of the included trials and the variety of the interventions do not allow for a systematic comparison of the treatment outcomes across the studies. The adopted methodology of targeted literature review is based on a clearly formulated question using explicit methods to identify, select, qualitatively analyse, and interpret key relevant research. As such, it allows us to understand the salient issues relevant to the research question.
Methods
The methodology for this literature review was adapted from published guidance on methods for systematic literature reviews by Cochrane (Cochrane Handbook for Systematic Reviews of Interventions).22 Results were reported following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline.23
Sources of data
The searches were conducted on 24 September 2020, in MEDLINE® and Embase via the Ovid platform. Grey literature searching included manual screening of leading cardiac medical body websites for conference abstracts (2018–20), including the European Society of Cardiology, the American Heart Association, and the American College of Cardiology.
Search strategies
Keywords included the MeSH heading ‘cardiac rehabilitation’ and ‘heart rehabilitation’. For the virtual healthcare solutions, keywords included ‘telehealth’, ‘telecommunications’, and ‘telemedicine’; wearable* or device* or gadget* or sensor* or smart*, among others. No limits on language were set for the search, although titles and abstracts in English only were screened. Details of search strategies are provided in Supplementary material online, Tables S1 and S2.
Study selection
Study selection was carried out by one senior (PhD level) trained methodologist and study eligibility criteria were defined using a PICOS (Population, Intervention, Comparator, Outcomes, Study design) framework as presented in Table 1. Following duplicate removal, all titles and abstracts were screened for potential eligibility according to the prespecified PICOS criteria (Table 1). All studies identified as eligible during abstract screening were then screened at a full-text stage by two investigators. Reasons for inclusion/exclusion were documented.
Table 1.
PICOS eligibility criteria
| PICO item | Inclusion criteria | Exclusion criteria |
|---|---|---|
| Population | Patient engaged in rehabilitation due to underlying cardiovascular disease, including but not limited to myocardial infarction, percutaneous coronary intervention, coronary artery bypass, stable angina, ischaemic heart disease, ischaemic stroke, haemorrhagic stroke, atrial fibrillation, peripheral arterial disease, aortic aneurysm, cardiomyopathy and myocarditis, hypertensive heart disease endocarditis, rheumatic heart disease | N/A |
| Interventions | Virtual or mobile (mHealth) healthcare solutions [defined as a care team + connected devices + a digital solution (e.g. smartphone app) or a platform], which can also include telephonic interventions or infrastructures such as an health care practitioner/nurse call centres | Self-contained devices or apps |
| Comparators | Any or none | N/A |
| Outcomes | Efficacy/effectiveness outcomes: • Improvements in risk factors, exercise capacity, cardiovascular symptoms, blood pressure, metabolic panel, body-mass index (BMI), anxiety/depression, and medication adherence to secondary preventive therapies • Survival after an initial cardiac event • User adherence/compliance Safety outcomes (including but not limited to): • Total adverse events, device-related adverse events, serious adverse events, adverse events leading to discontinuation of study, major adverse cardiac events including not limited to: cardiovascular death, myocardial infarction, stroke, hospitalization (all reasons), • Increase of existing diuretic dose or addition of a new diuretic due to fluid retention, hyperkalaemia, heart failure measures (heart failure progression, B-type natriuretic peptide biomarker change) Health-related quality-of-life outcomes • Patient reported and health related quality of life (including but not limited to EuroQol-5D, EuroQol-Visual Analogue Scale, Heart Quality of Life, MacNew Heart Disease Health-Related Quality of Life Questionnaire, Quality of Life after Myocardial Infarction, The Minnesota Living with Heart Failure Questionnaire) |
N/A |
| Study design | • Randomized Controlled Trials (RCTs) • Non-randomized clinical trials • Observational studies • Cohort studies (retrospective and prospective) • Case-control studies • Cross-sectional studies • Systematic literature reviews |
• Notes • Letters • Editorials • Comments • Case reports/series |
| Additional criteria (limits) | ||
| Timing | None | |
| Setting | None | |
| Publication date limit | None | |
| Language | No limit on language was set for the search. Title and abstracts in English were screened. | |
Data extraction
A standardized data extraction table was generated to define study, patient, and intervention characteristics, and outcomes to be extracted from eligible studies. All relevant data from the final list of included studies were extracted by the same reviewer that carried out the study selection. The following data were extracted where available: (i) study design, study settings, intervention category (mobile application, wearable device, web-based, multiple interventions), country, study duration, and sample size; (ii) mean patient age, cardiac condition; medical history (comorbidities), cardiac procedure; (iii) primary outcome(s), secondary outcome(s), statistical significance of primary outcome(s) (i.e. yes, no, not applicable), primary outcome(s) effect size, and dispersion and aggregate category of primary outcome.
Results
Study selection
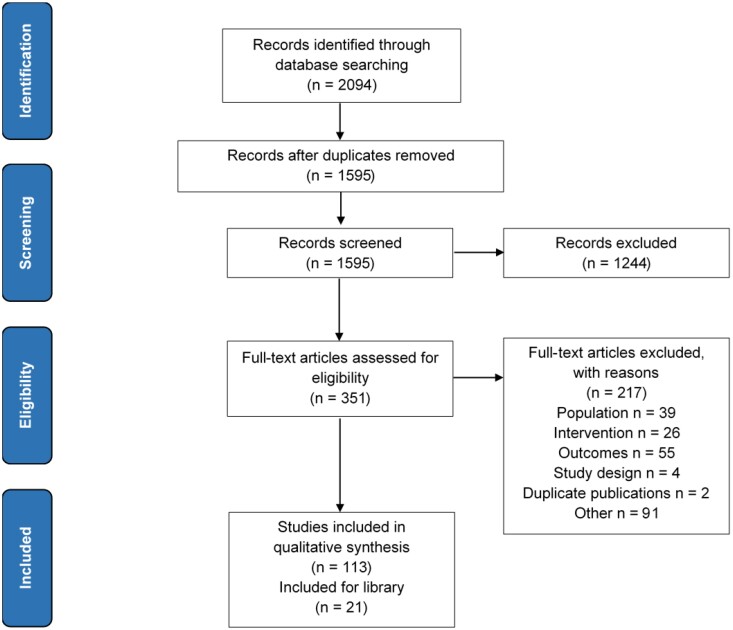
The search identified 2094 records. Title/abstract screening and duplicate removal resulted in the exclusion of 1743 records. Full-text review of the remaining 351 records resulted in the exclusion of 217 records. In total, 134 studies were included comprising of 113 original clinical studies and 21 review articles included for the library. The PRISMA diagram is shown in Figure 1.
Figure 1.
Study selection (PRISMA) diagram.
Study characteristics
Out of 113 included studies, 64 were randomized controlled trials (RCTs), 19 were observational studies, 12 were cross-sectional studies, 8 were single-arm studies, 5 were comparative non-randomized trials, 4 were retrospective studies, and 1 was a prospective cohort study (Table 2). Most study settings included home-based CR (68 out of 113 studies, 60%), followed by studies conducted in academic centres (20/113, 18%) and real-world centres (11/113, 10%). Fourteen studies (12%) were conducted in mixed settings where some of the interventions were home based, and some were centre based. The CR services for the home-based studies in case of interventional studies (57 out of 68 home-based studies were interventional and 11 were observational/retrospective) were provided by the academic centre supervising the study. The intervention group would usually receive additional care in some form of virtual healthcare, whereas the control group (if applicable) would receive the standard level of CR according to usual practice within each institution. For the remaining 11 observational/retrospective studies in home-based settings, the patients were recruited through existing CR programmes.
Table 2.
Number of studies classified by study type and the proportion reporting a significant primary outcome
| Study type | Number of studies (%) | Number of studies with a significant* primary outcome (%) |
|---|---|---|
| Randomized controlled trials (RCT) | 64 (56.6) | 31 (48.4) |
| Observational studies | 19 (16.8) | 4 (21.0) |
| Cross-sectional | 12 (10.6) | 3 (25.0) |
| Single-arm trials | 8 (7.1) | 6 (75) |
| Comparative, non-randomized trials | 5 (4.4) | 2 (40.0) |
| Retrospective | 4 (3.5) | 0 |
| Prospective cohort | 1 (0.88) | 1 (100) |
| Total | 113 | 47 (41.6) |
P < 0.05.
Studies included in this review provided an overall mixed assessment of the efficacy of virtual CR either in attaining significant improvements over baseline or in attaining significant improvements in outcomes compared with conventional hospital-based cardiac rehabilitation. Less than half of the included studies—47 out of 113 (41.6%)—reported a significant result for a primary outcome (Table 2). It should be noted that this ratio refers to the significance of the result for any primary outcome, combining both within-group differences (i.e. baseline vs. elapsed time interval) and between-group differences [i.e. baseline vs. elapsed time interval but comparing usual centre-based CR (CBCR) vs. virtual CR]. The latter comparative outcome was reported in 16 studies, and these results will be described in a subsequent section.
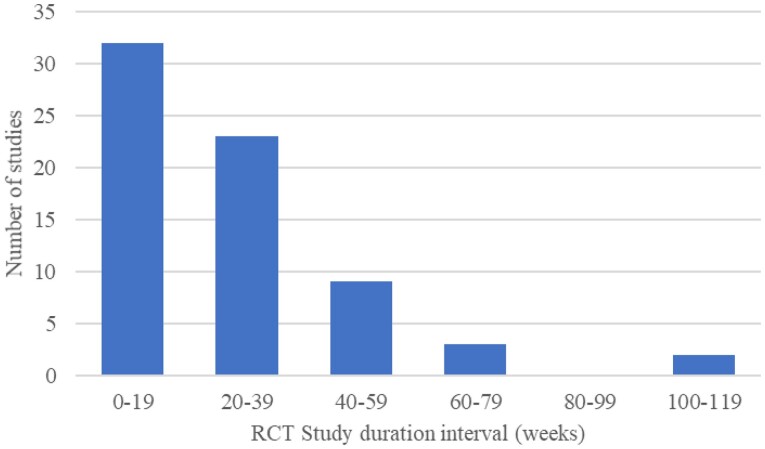
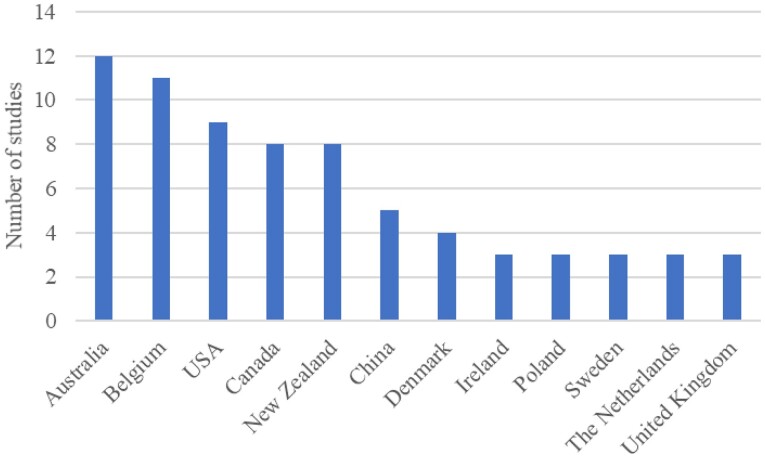
The distribution of study duration in the RCTs included is shown in Figure 2. The study duration of RCTs ranged between 2 weeks and 104 weeks, with mean and median durations of 26.5 weeks and 24 weeks, respectively. Patient populations in included RCTs ranged between 15 and 731, with total, mean, and median populations of 8446, 120, and 102, respectively. The follow-up duration of the majority of RCTs was <39 weeks (Figure 2), corresponding to Phases II and III of a CR programme. The studies were conducted in 23 different countries shown in Figure 3.
Figure 2.
Study distribution of RCT duration. RCT, randomized controlled trial.
Figure 3.
Number of studies in different countries (Top 12).
Patient characteristics
The baseline demographics of the patients were typical for patients enrolled in CR programmes. The mean age of patients across the 101 studies reporting this information ranged from 49.224 to 80.225 with a median reported mean age of 60.9 and a weighted (by sample size) mean average age of 61.4 years. A total of 11 541 patients were included among the studies reporting mean age and sample size. In terms of sex, among 105 studies reporting the breakdown, the proportion of female participants ranged from 0 to 68.8%, with a mean of 23.4% and a median of 19%. Among 18 studies reporting the racial composition of the patient populations, the proportion of white participants ranged from 55.4 to 98.8%, with a mean of 80.4% and a median of 85%.
A total of 70 studies reported on the urban/rural divide: 54 studies enrolled participants from urban centres, 8 studies enrolled participants from both urban and rural settings, and the remaining 8 studies included participants from rural areas only.
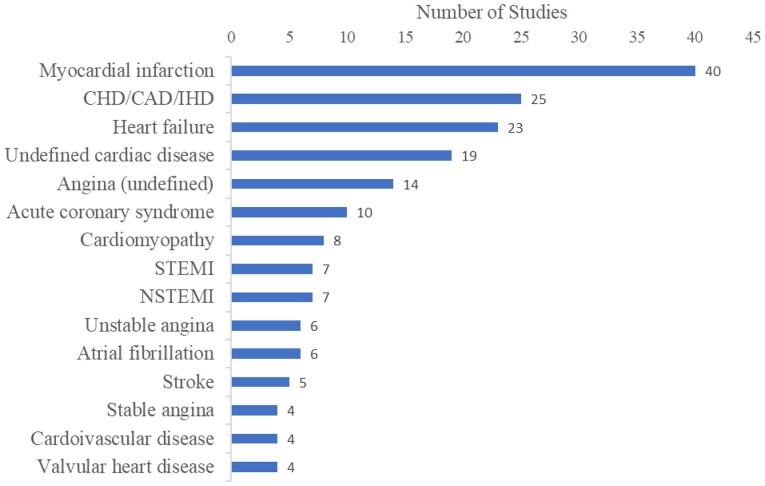
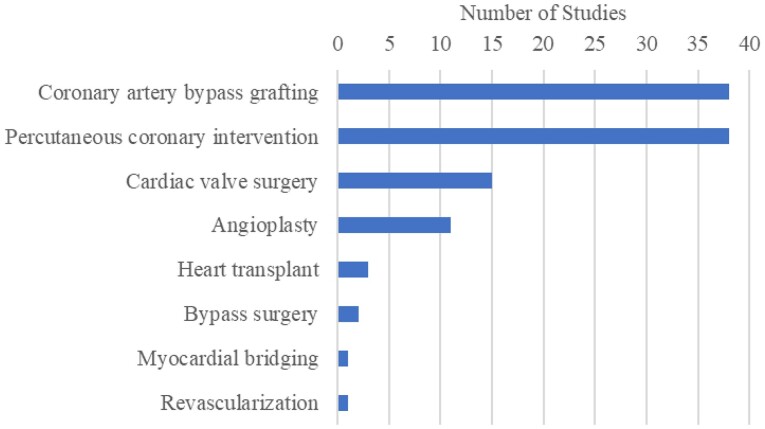
A wide variety of cardiovascular conditions were represented. There were 34 distinct conditions identified and the 15 most frequently noted are shown in Figure 4 (some studies included mixed patient populations with multiple conditions). The most commonly reported invasive cardiac procedures are listed in Figure 5.
Figure 4.
Number of studies reporting on disease/condition (Top 15). CAD, coronary artery disease; CHD, coronary heart disease; IHD, ischaemic heart disease; NSTEMI, non-ST-Elevation Myocardial Infarction; STEMI, ST-Elevation Myocardial Infarction.
Figure 5.
Number of studies reporting on invasive cardiac procedures. Myocardial bridging refers to myocardial bridge unroofing surgery.
Enrolment in virtual CR was restricted to patients recovering after the procedure without excessive cardiac risk (patients in Phase II—IV of CR).
Interventions
A wide variety of virtual CR solutions were implemented in the 113 included studies to engage with patients and facilitate behaviour modification to promote cardiac rehabilitation. These technological interventions were classified into four categories for the purposes of this review:
Multi-component interventions with multiple technologies integrated into a comprehensive CR solution in 48 studies (42.5%). An example of this approach is shown in a paper by Avila et al.26 on the results of the TeleRehabilitation in Coronary Heart disease (TRiCH) study. The virtual intervention consisted of an individualised exercise prescription for home-based exercise for 3 months. Patients were asked to log all exercise data and to upload the data on the online web application for review by the investigators. Based on these data, an individualised exercise prescription was created, recommending patients exercise for at least 150 min a week at a target heart rate of 70–80% of heart rate reserve (HRR) at home for 3 months. Once a week, patients received feedback by phone or e-mail.
WAMs (e.g. Fitbit, Actigraph, Apple Watch) in 27 studies (23.9%). An example of this category is a study by Batalik et al.27 which enrolled 56 cardiac rehabilitation patients and randomized them into a 12-week regular outpatient training group and interventional home-based telerehabilitation group. For both groups, the intensity of the training was prescribed to be performed at 70–80% of heart rate reserve for 60 min, three times a week. The interventional home-based patients started their training with a wrist-worn heart rate monitor in their home environment. These patients received feedback once a week, reflecting data uploaded on the internet application. Training adherence in both groups was determined and compared. The results showed similar outcomes for both groups, suggesting that telerehabilitation via wrist-worn heart rate monitor could become an alternative kind of cardiac rehabilitation which deserves attention and further analysis.
Web-based communications solutions: This category was selected to separate the studies where the only means of communication between the patient and the healthcare professional was a personal computer with internet access. An example is a study by Duan et al.24 aiming to evaluate the effect of an 8-week Web-based intervention in terms of physical activity (PA), fruit and vegetable consumption (FVC), lifestyle changes, social-cognitive outcomes, and health outcomes compared with a waiting control group in Chinese cardiac patients. The web intervention content was designed based on the Health Action Process Approach theory. Based on the collected data, two types of feedback were provided: (i) individualized feedback on patient self-reported behaviour performance 4 weeks ago, 3 weeks ago, 2 weeks ago, and 1 week ago, and (ii) criterion-based feedback (e.g. accumulated at least 150 min with moderate intensity of PA per week and five portions of FVC per day).
Mobile apps running on smartphones and tablets (mHealth), in 19 studies (16.4%) for each device. An example is a paper by Rosario et al.28 showing the results of a pilot study aiming to determine if a smartphone-based adjunct to standard care could increase the completion rate of a cardiac rehabilitation programme (CRP). Sixty-six participants who were about to commence a hospital-based CRP were randomized so that half received three devices embedded with near-field communication, namely a smartphone [pre-installed with an application (app) designed specifically for cardiac rehabilitation], portable blood pressure monitor, and weight scale whilst completing the CRP. All patient measurements (i.e. activity, questionnaire responses, blood pressure, and weight) were stored securely on the smartphone before being re-transmitted to centre’s remote telehealth platform. During the intervention, patients continued their CRP programme as scheduled.
A summary of the overall results separated by the four categories is presented in Table 3. A majority of multi-component intervention studies were RCTs (k = 36, 75.0%) and just over half of multi-component studies reported a significant result for improvement in the primary outcome (k = 27, 56.3%) (Table 3). Over half of mHealth studies were also RCTs (k = 11, 57.9%); however, just under half of mHealth studies reported significant improvements in primary outcomes (k = 9, 47.4%) (Table 3). The proportion of RCTs and significant improvements in primary outcomes in studies utilizing WAMs and web-based communications were all <50% (Table 3).
Table 3.
Number of studies utilizing different technologies, number of corresponding RCTs, and studies with significant primary outcomes
| Technology Number of studies (% of total) | Number of RCTs (% of technology studies) | Studies with a significant* primary outcome (% of technology studies) | Studies with significant* improvements in virtual CR vs. usual CR patient groupsa |
|---|---|---|---|
| Multi-component interventions 48 (42.5) | 36 (75.0) | 27 (56.3) | 9 |
| WAMs 27 (23.9) | 9 (33.3) | 10 (37.0) | 1 |
| Web-based communication 19 (16.8) | 8 (42.1) | 1 (5.3) | 0 |
| mHealth 19 (16.8) | 11 (57.9) | 9 (47.4) | 6 |
| Total: 113 | 64 (56.6) | 47 (41.6) | 16 |
RCT, randomized controlled trial; mHealth, mobile apps; WAM, wearable activity monitor.
Note that comparative studies were limited to virtual cardiac rehabilitation vs. control groups with hospital-based cardiac rehabilitation.
P < 0.05.
A total of 16 studies reported significant between-group improvements in primary outcomes of virtual CR vs. usual hospital-based CR; 9 of these were studies utilizing multi-component interventions, 6 used mHealth, and 1 reported on the use of WAMs (Tables 3 and 4). These are described in more detail in the next sections on specific interventions.
Table 4.
Studies reporting significant differences in primary outcomes between virtual cardiac rehabilitation and routine hospital-based cardiac rehabilitation
| Short reference | Technology | Primary aggregate outcome | Study design | Patient cardiac conditions | Country |
|---|---|---|---|---|---|
| Avila 201826 | Multiple interventions (web-based communication; WAM) | Functional capacity | RCT | CABG, PCI | Belgium |
| Bernocchi 201829 | Multiple interventions (web-based communication; WAM) | Functional capacity | RCT | CHF + COPD | NRa |
| Frederix 201530 | Multiple interventions (web-based communication; WAM) | Functional capacity | RCT | CABG, PCI | Belgium |
| Frederix 201731 | Multiple interventions (web-based communication; WAM) | Functional capacity | RCT | STEMI, NSTEMI, unstable angina, stable angina | Belgium |
| Skobel 201732 | Multiple interventions (web-based communication; WAM) | Functional capacity | RCT | CAD | Germany; UK; Spain |
| Pfaeffli Dale 201533 | Multiple interventions (mobile application; web-based communication) | Patient adherence/compliance | RCT | MI, unstable angina, angina | New Zealand |
| Rosario 201828 | Multiple interventions (NR) | Patient adherence/compliance | RCT | Undefined cardiac disease | Australia |
| Varnfield 201434 | Multiple interventions (WAM; mHealth; web-based Communication) | Patient adherence/compliance | RCT | STEMI, NSTEMI, HF, angina, stroke, bypass surgery, angioplasty/stent, heart valve problems | Australia |
| Claes 202035 | Multiple Interventions (Web-based communication; WAM) | Physical activity | RCT | PCI, CABG, valve repair | Belgium; Ireland |
| Eyles 201736 | mHealth | Quality of Life | RCT | Cardiovascular disease | New Zealand |
| Sjolin 201937 | mHealth | Quality of Life | RCT | AMI | NRb |
| Widmer 201438 | mHealth | Quality of Life | Observational | ACS | USA |
| Cai 201939 | mHealth | Functional capacity | RCT | AF | NRc |
| Yudi 202140 | mHealth | Functional capacity | RCT | STEMI, NSTEMI, unstable angina | Australia |
| Ding 201241 | mHealth | Patient adherence/compliance | RCT | Undefined cardiac disease | Australia |
| Izawa 201242 | WAM | Physical activity | RCT | Undefined cardiac disease | Japan |
ACS, acute coronary syndrome; AF, atrial fibrillation; AMI, acute myocardial infarction; CABG, coronary artery bypass graft; CAD, coronary artery disease; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; HF, heart failure; MI, myocardial infarction; mHealth, mobile health apps; NSTEMI, ST-Elevation Myocardial Infarction; PCI, percutaneous intervention; STEMI, non-ST-Elevation Myocardial Infarction.
First author affiliation is located in Italy.
First author affiliation is located in Sweden.
First author affiliation is located in China.
Wearable activity monitors
In studies with WAMs as the only virtual CR intervention used (k = 27), 10 (37.0%) reported significant effects on a primary outcome associated with cardiac rehabilitation.42–51 All primary patient outcomes in these studies concerned either the amount and intensity of physical activity and/or heart rate monitoring. Two randomized controlled trials provided evidence supporting the direct positive impact and improvement in physical activity for patients fitted with an accelerometer to monitor physical activity42 and a wireless electrocardiogram (ECG) device to monitor heart rate49 compared with their respective control groups; however, the latter study did not provide CR to the control group, whereas the former study did (Tables 3 and 4).
Mobile apps (mHealth)
Nineteen studies used a mobile app as the only virtual CR intervention. The studies included in this category are distinct from the studies using mobile apps integrated into a comprehensive CR solution, which are reported under the ‘Multi-component interventions’ category. Six studies utilizing mobile app-based virtual CR reported significantly improved outcomes in the intervention arm compared with control CR, five of these were RCTs36,37,39–41 with one observational study (Tables 3 and 4).38 Specific primary outcomes in these RCTs included VO2 peak,39 6MWT,40 diet (specifically reduced salt intake),36 cardiac risk profile,37 and patient adherence/compliance.41 The observational study compared reductions in blood pressure.38
Web-based interventions
This literature review identified 19 studies where a web-based intervention was the only virtual CR intervention. Eight studies were RCTs and one of these reported significant improvements in physical activity and FVC; however, this result is tempered by the lack of CR in the control arm (Table 3).24
Multi-component interventions
By far, the largest number of included studies used a combination of technologies to achieve the CR goals. Forty-eight studies used multi-component interventions for providing virtual CR solutions. Out of those, 28 (54.9%) reported significant effects in the primary outcome (Tables 3 and 4).26,28–35,52–68 Nine of these studies reported significant improvements in the virtual CR arm compared with usual CR, and all nine were RCTs (Tables 3 and 4).26,28–31,33–35,69 The primary outcome was VO2 peak in four studies,26,30–32 patient adherence/compliance in three,28,33,34 and one each with 6MWT29 and physical activity.35
Outcomes
Specific primary outcomes in included studies were grouped together in aggregate categories as presented in Table 5. Functional capacity was the most frequently reported aggregate primary outcome category (k = 37, 32.7%), followed by user adherence/compliance (k = 35, 31.0%), physical activity (k = 27, 23.9%), and quality of life (k = 14, 12.4%). RCTs comprised the majority of study types in the functional capacity category (k = 28, 75.7%) and over half of the functional capacity studies reported significant results for improvements in primary outcomes (both within-group and between-group comparisons) (k = 22, 59.5%). Almost half of the studies in the physical activity outcomes category comprised RCTs (k = 13, 48.1%) and just over half reported significant improvements in outcomes (k = 14, 51.9%).
Table 5.
Number of studies reporting on aggregate primary outcomes and proportions of RCTs and studies with a significant primary outcome
| Aggregate primary outcome category (APOC) number of studies (% of total) | Specific primary outcomes | Number of RCTs (% of APOC) | Studies with a significant primary outcome* (% of APOC) |
|---|---|---|---|
| Functional capacity 37 (32.7) | • VO2 peak • 6MWT • Exercise capacity • Functional capacity • Heart rate |
28 (75.7) | 22 (59.5) |
| Patient adherence/compliance 35 (31.0) | • User adherence • User compliance • Patient engagement • User experience • Device utilization |
13 (37.1) | 7 (20.0) |
| Physical activity 27 (23.9) | • Number of steps • Daily step count • Exercise time • Sedentary time |
13 (48.1) | 14 (51.9) |
| Quality of life 14 (12.4) | • Quality of life • Blood pressure • Weight loss • Salt intake • Cardiac risk profile change • Anxiety/depression |
10 (71.4) | 4 (28.6) |
| Total: 113 | 64 (56.6) | 47 (41.6) |
6MWT, 6-minute walk test; APOC, aggregate primary outcome category; RCT, randomized clinical trial; VO2, oxygen uptake.
P < 0.05.
Out of 35 studies reporting on patient adherence/compliance, only 7 (20.0%) reported a significant improvement in primary outcomes; 6 used multiple interventions,28,33,34,54,58,61 while 1 used a mobile application.41 Many of the studies reporting on patient adherence/compliance were non-comparative and, therefore, not designed to capture relative adherence rates between virtual and traditional CR. However, of the seven studies reporting a significant primary outcome under the category of user adherence/compliance, four were RCTs showing significantly better outcomes in the intervention arm28,33,34,41 and one was a non-randomized controlled trial similarly reporting significantly better patient adherence/compliance in the intervention arm.61 The two other studies were single-arm, reporting significantly improved user adherence/compliance at 4 weeks over baseline in heart-healthy eating,54 and higher adherence to exercise recommendations at 12 weeks compared with baseline.58
Most studies did not report results separated by sex. The studies generally balanced the male/female ratio across the intervention groups and reported only the overall results per group. There were three studies that did stratify by sex but only one found statistically significant differences in the outcomes.70 In a study investigating correlates of objectively measured physical activity, sex was a significant factor in moderate-to-vigorous physical activity.70 Similarly, the studies did not tend to stratify results by age. Out of the six studies that considered age as a factor, two reported significant differences in outcomes across the age groups. A study investigating mobile technology use across age groups found significant differences in the overall use and confidence in the technology.71 A study examining attitudes, perceptions, and behavioural intentions towards remote digital CR found differences both in attitudes towards healthy lifestyles through mobile phones and in acceptance rates of virtual CR classes.72
No intervention-related adverse events were reported in the included studies. The adverse events reported were related to the medical condition of the patients and, when compared, the frequency of adverse events did not differ between the intervention and comparator groups.
Discussion
The aim of this literature review was to provide a ‘state of the art’ update on the growing body of evidence relating the use of different virtual CR solutions available to address the significant underutilization of cardiac rehabilitation. The virtual CR solutions described are available for clinicians and healthcare practitioners to monitor patient progress, intervene when necessary, and to reinforce desirable healthy habits and lifestyle choices. As with any new intervention modality, the question of comparative efficacy is at the forefront of clinical investigations. In this respect, the results for virtual CR compared to traditional centre-based CR are mixed at best (see Table 2). This is not a surprise; other recently published reviews found a similar result.18 This may not be a major drawback, however. The main challenge for CR in general is to engage as many patients needing this intervention as possible and keep them engaged over an extended period. Virtual CR may be the tool that can help accomplish those goals.
When it comes to efficacy, this review attempted to find the intervention domains in which virtual CR is most efficacious based on the comparative results of the included studies. Given the variety of interventions and methodologies, the goal was to identify the underlying efficacy patterns by grouping the interventions into functionally similar categories. In addition to the efficacy, patient enrolment and adherence to the treatment regimen are the main challenges facing CR. Studies reporting on adherence and patient satisfaction reported encouraging results, albeit the potential of virtual CR seems to be still unfulfilled. One limitation of the published studies is a relatively restricted demographic pool from which the studies are recruiting patients. Increasing diversity and extending virtual CR into the real-world settings are important challenges that are yet to be addressed. Finally, COVID-19 lockdowns and restrictions brought the importance of virtual healthcare to a broader audience. CR patients were one of the most vulnerable populations with respect to this virus and the lessons from the pandemic are still being debated.
Virtual cardiac rehabilitation efficacy
In the current evidence base, the most investigated outcomes were related to exercise and physical activity, cardiac risk factors, user adherence, as well as patient experience and preferences. Overall, this review shows that virtual and mobile technologies can help to enable effective home-based cardiac rehabilitation (HBCR), providing a viable complement to the traditional centre-based CR (CBCR). Outcomes with virtual CR from the studies surveyed were comparable to those with CBCR. In some cases, outcomes with virtual CR were statistically significantly better than those using the traditional approach, notably in nine RCTs using multiple interventions to assess: functional capacity,26,29–32 patient adherence/compliance,28,33,34 and physical activity.35 Significant outcomes were also observed in six studies using mHealth interventions: two of these were RCTs assessing outcomes in quality of life (QoL),36,37 one was an observational study of QoL,38 two were RCTs assessing functional capacity,39,40 and one was an RCT assessing patient adherence/compliance.41 One study using WAM technology reported significantly better physical activity outcomes compared with traditional CR.42
Review of the most effective virtual healthcare intervention shows a few common features. First, most successful interventions used multi-component models, where the patients used multiple devices and apps both for monitoring their activities and for communications with the healthcare team. Devices such as home-based ECG, pedometers, connected scales, and blood-pressure monitors were connected either to a smartphone or to the internet. Data were available to both the patients and the healthcare staff. For the patient, the availability of the data provided objective feedback on their status; for the healthcare staff, the data allowed personalized patient recommendations. Second, given the novelty of the technology provided to the patients, the researchers spent a significant amount of time and effort on individualized training and education, particularly at the beginning of the study. The training included direct instruction on the correct use of the technology and on the data upload and management. Given that a significant part of the CR population is older adults, the training was adopted towards a basic level of technology understanding among the patients. Third, the successful studies placed emphasis on the continuous feedback tailored to individual patients. For example, studies targeting physical fitness created individualized exercise prescriptions based on average steps per day as measured by an accelerometer. The last two points show the importance of personalization of the intervention which was often aided by questionnaires distributed to the patients.
In searching for reasons for the relatively high efficacy of the virtual interventions targeting physical fitness, one can notice the ‘omnipresence’ of the monitoring devices. The continuous feedback received from WAMs can serve as a constant reminder that the devices are ‘watching’. Although the technology does present some ‘big brother’ privacy concerns, when handled properly, the ever-present monitoring seems to encourage a more active lifestyle. This makes intuitive sense, as the awareness of a monitoring device or app that logs exercise or physical activity data may motivate the patient simply through its presence. In various instances of clinical research, this is referred to as a trial, or ‘Hawthorne’ effect,73–75 an effect known in psychology where some individuals tend to alter their behaviour when observed or monitored. Regardless of the contribution of this effect to observed outcomes, physical activity and exercise training are core components of recommendations from the American Association of Cardiovascular and Pulmonary Rehabilitation, the American Heart Association, and the American College of Cardiology, provided in their joint Scientific Statement.76 The European Society of Cardiology also provide similar recommendations on the use of exercise training and increased physical activity to improve aerobic fitness, prognosis, and quality of life and reduce the overall risk of disease progression or recurrence.77
Patient enrolment and adherence to cardiac rehabilitation
The main challenge of the traditional CBCR concerns patient enrolment and patient adherence to the CR regimen. Lack of referral has been cited as a barrier to enrolment in CR; however, patients regularly choose not to attend CBCR sessions due to a lack of access to transport, ill health, scheduling commitments associated with returning to work, and reimbursement issues.8,10 Patient adherence was one of the primary reported outcomes in 35 studies; however, the success in boosting adherence was rather limited. Only 20% of studies targeting improvement in patient adherence reported significant results. This statistic is somehow misleading though, because many of the studies were non-comparative and, therefore, the contextual significance of the outcomes was difficult to assess. The successful interventions used some form of feedback, usually in the form of regular text messages that were targeted at specific aspects of CR, such as monitoring symptoms, medication taking, exercising, and dietary recommendations. More advanced interventions also used personalized feedback based on the data uploaded from monitoring devices (such as accelerometers or digital blood-pressure devices). In contrast to active messaging, the availability of passive information (in the form of a dedicated website) did not seem effective in engaging patients. Overall, the effect of persistent, yet unobtrusive reminders, either as targeted text messages or as direct communication from the nursing staff, was both acceptable by the patients and effective in encouraging patient adherence to the CR regimen.
In studies exploring patient acceptance, the results were presented in a qualitative form as patient feedback on the likelihood of adherence and usability of the virtual interventions. Patients expressed positive attitudes towards the technology, with the emphasis on the ease of use, user-friendliness, flexibility, and self-efficacy. The main advantage was seen in overcoming traditional participation barriers while preserving the oversight from healthcare professionals. One caveat to the applicability of the results is the fact that most studies enrolled the patients by recruitment through existing CR programmes. Therefore, there is a certain degree of selection bias towards patients positively inclined towards the virtual CR in the sampling of participants.
Patient diversity
One caveat associated with the published research is the limited diversity of included patient population. Since most of the studies are run by academic hospitals, the included patient populations tend to be more urban and middle class. The included populations also skew to more males than females, which reflects the underrepresentation of female patients in CR programmes in general.78 Recruitment for the studies was mostly through CR programmes at major research universities, and the patients were broadly familiar with the use of cell phones, internet, and personal technology in general. To extend the benefits of CR outside the technologically advanced segment of the population (the ‘digital divide’), additional barriers need to be overcome. Particularly rural populations and less affluent populations face not only limited access to high-speed internet but also limited access to technology education often resulting in reluctance to engage in the advanced programmes such as virtual CR. In the future expansion of the reach of virtual CR into the real-world settings, those imbalances will provide additional challenges.
Real-world applicability of virtual cardiac rehabilitation
Duration of intervention is important as sustained physical activity is a key to improving outcomes. Relatively fewer studies have investigated the longer-term benefits of CR.79 One of the advantages of virtual CR is the potential extended duration beyond the limit of 36 weeks typically seen in centre-based CR. In our selection, 22 out of 113 (19.5%) studies extended CR beyond 36 weeks and of these, 19 studies lasted 1 year or more. Although it is reasonable to presume virtual CR interventions (in case they were effective) lead to improving clinical outcomes, further studies are needed to understand the long-term impacts of these interventions. However, promising results from individual studies suggest that virtual CR is at least as effective in maintaining multiple intervention outcomes as centre-based programmes.27,80 A cost-utility analysis based on the TELEREH-HF trial in patients with HF confirmed the cost-effectiveness of the hybrid telerehabilitation programme compared with standard care, from the perspective of the Polish National Health Fund.81 Additional studies are needed to assess the economic viability of virtual CR in other aetiologies such as post MI. Future research efforts should focus on the key health behaviour change techniques in technology-based interventions that enable full persistence of long-term behaviour change in large and diverse populations of affected patients. One of the main challenges facing the widespread implementation of virtual CR may not be necessarily a lack of efficacy but rather an acceptance of these technologies and their continuous use by CR patients. This issue was investigated in a recently published study on the CR barriers scale in the Czech Republic.82 The study highlighted the most relevant real-world barriers from patients’ point of view and can serve as a starting point for further explorations. The focus should be on the issues such as seamless integration of the mobile technologies into the daily routines, simplification of the interactions between the patient and the device, and complementary role of technology and care personnel in the CR programme. This review clearly established the superiority of the integrated approach using both the technology and human interaction in patient care. Future studies should focus on making the technology transparent to both patients and healthcare professionals, allowing both to focus on the ultimate goal of improving the lives of cardiac patients.83
Finally, COVID-19 has exacerbated the constraints on healthcare sector resources. For patients requiring CR, COVID-19 is a dual-edged sword since pre-existing CVD poses a higher risk for worse COVID-linked outcomes, including death.84,85 CR centres worldwide were closed, with notably a two-third decrease in centre-based CR from the pre-COVID period (4969 patients; May 2019—January 2020) to the COVID period (1474 patients; February 2020—August 2020).86 However, the proportion of patients receiving home-based CR increased substantially over the same time interval, from 22.2 to 72.4%. Additionally, the mobilization of virtual CR resources from research settings to more routine care to make up for the loss of CBCR has been suggested.87
Limitations
This is not a systematic review capturing all published studies within this domain. Rather, the review is focused on the recent technological advancements in monitoring programme adherence and providing guidance and means of communication for CR patients. Many technologies reviewed here are in an early stage of development. The reviewed trials are often pilot studies with small sample populations. This review does not address the potential challenges facing the technologies in larger and more diverse populations. Also, this review does not attempt to evaluate the feasibility of using these technologies in routine clinical practice across a variety of clinical settings. The nature of this review is qualitative with the intention to provide a narrative summary of the most relevant findings related to the stated objectives of the study. The quantitative information provided here is selected based on the representativeness of the data without providing additional statistical analyses.
Conclusions
Although efficacy outcomes with virtual CR sometimes, but not always, improve on the centre-based CR outcomes, superior clinical efficacy may not necessarily be the most relevant aspect of the virtual CR. This is particularly the case considering that many patients are likely to have a hybrid approach combining different doses of centre based and virtual CR. The overall results suggest that the promise of this technology is in its potential for increased user adherence, longer-term patient engagement, and broader availability. Given the relatively low risk and cost of such interventions, they should be considered as an adjunctive therapy in the management of patients in need of CR. The main challenge of the traditional CR is patient access and patient adherence to the CR regimen. If the virtual CR solutions can improve adherence/compliance of cardiac patients, that would be a significant justification for using this technology.
Supplementary Material
Acknowledgements
K.C.S.L. and A.U. contributed to the study design and conceptualization; B.B. carried out data configuration; F.K. and S.S.M. provided clinical input and all authors were responsible for reviewing and editing the manuscript. We appreciate the coordination of the development of this manuscript and assistance with revisions as provided by Cecile Dessapt-Baradez, PhD, and Sai Krishnaveni Chevooru, PhD, at Sanofi. We also thank Christopher Crotty, PhD (Evidinno Outcomes Research Inc.), for medical writing support and Mir-Masoud Pourrahmat (Evidinno Outcomes Research Inc.) for project management.
Contributor Information
Keni C S Lee, Sanofi, General Medicines Global Business Unit, Paris 75008, France.
Boris Breznen, Evidinno Outcomes Research Inc., Vancouver, BC V5Y1K2, Canada.
Anastasia Ukhova, Sanofi, General Medicines Global Business Unit, Moscow 125009, Russia.
Friedrich Koehler, Division for Cardiovascular Telemedicine, Charité – Universitätsmedizin Berlin, Berlin 10117, Germany.
Seth S Martin, Ciccarone Center for the Prevention of Cardiovascular Disease, Division of Cardiology, Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, MD 21287, USA; Center for Mobile Technologies to Achieve Equity in CV Health, Baltimore (mTECH), Johns Hopkins University School of Medicine, Baltimore, MD 21287, USA.
Supplementary material
Supplementary material is available at European Heart Journal – Digital Health.
Funding
The literature review and medical writing were performed by Evidinno Outcomes Research Inc, funded by Sanofi.
Data availability
No new data were generated or analysed in support of this research.
References
- 1. Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. . Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 study. J Am Coll Cardiol 2020;76:2982–3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dalal HM, Doherty P, Taylor RS. Cardiac rehabilitation. BMJ 2015;351:h5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wenger NK. Current status of cardiac rehabilitation. J Am Coll Cardiol 2008;51:1619–1631. [DOI] [PubMed] [Google Scholar]
- 4. Kumar KR, Pina IL. Cardiac rehabilitation in older adults: new options. Clin Cardiol 2020;43:163–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Taylor RS, Dalal HM, McDonagh STJ. The role of cardiac rehabilitation in improving cardiovascular outcomes. Nat Rev Cardiol 2022;19:180–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Beatty AL, Truong M, Schopfer DW, Shen H, Bachmann JM, Whooley MA. Geographic variation in cardiac rehabilitation participation in medicare and veterans affairs populations: opportunity for improvement. Circulation 2018;137:1899–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bethell HJ, Evans JA, Turner SC, Lewin RJ. The rise and fall of cardiac rehabilitation in the United Kingdom since 1998. J Public Health 2007;29:57–61. [DOI] [PubMed] [Google Scholar]
- 8. Daly J, Sindone AP, Thompson DR, Hancock K, Chang E, Davidson P. Barriers to participation in and adherence to cardiac rehabilitation programs: a critical literature review. Prog Cardiovasc Nurs 2002;17:8–17. [DOI] [PubMed] [Google Scholar]
- 9. Lynggaard V, Nielsen CV, Zwisler AD, Taylor RS, May O. The patient education—learning and coping strategies—improves adherence in cardiac rehabilitation (LC-REHAB): a randomised controlled trial. Int J Cardiol 2017;236:65–70. [DOI] [PubMed] [Google Scholar]
- 10. Yohannes AM, Yalfani A, Doherty P, Bundy C. Predictors of drop-out from an outpatient cardiac rehabilitation programme. Clin Rehabil 2007;21:222–229. [DOI] [PubMed] [Google Scholar]
- 11. Brouwers RWM, van Exel HJ, van Hal JMC, Jorstad HT, de Kluiver EP, Kraaijenhagen RA, et al. . Cardiac telerehabilitation as an alternative to centre-based cardiac rehabilitation. Neth Heart J 2020;28:443–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Castellanos LR, Viramontes O, Bains NK, Zepeda IA. Disparities in cardiac rehabilitation among individuals from racial and ethnic groups and rural communities—a systematic review. J Racial Ethn Health Disparities 2019;6:1–11. [DOI] [PubMed] [Google Scholar]
- 13. Grace SL, Turk-Adawi K, Santiago de Araujo Pio C, Alter DA. Ensuring cardiac rehabilitation access for the majority of those in need: a call to action for Canada. Can J Cardiol 2016;32:S358–SS64. [DOI] [PubMed] [Google Scholar]
- 14. Valencia HE, Savage PD, Ades PA. Cardiac rehabilitation participation in underserved populations. Minorities, low socioeconomic, and rural residents. J Cardiopulm Rehabil Prev 2011;31:203–210. [DOI] [PubMed] [Google Scholar]
- 15. Frederix I, Vanhees L, Dendale P, Goetschalckx K. A review of telerehabilitation for cardiac patients. J Telemed Telecare 2015;21:45–53. [DOI] [PubMed] [Google Scholar]
- 16. Anderson L, Thompson DR, Oldridge N, Zwisler AD, Rees K, Martin N, et al. . Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst Rev 2016;2016:CD001800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stefanakis M, Batalik L, Papathanasiou J, Dipla L, Antoniou V, Pepera G. Exercise-based cardiac rehabilitation programs in the era of COVID-19: a critical review. Rev Cardiovasc Med 2021;22:1143–1155. [DOI] [PubMed] [Google Scholar]
- 18. Antoniou V, Davos CH, Kapreli E, Batalik L, Panagiotakos DB, Pepera G. Effectiveness of home-based cardiac rehabilitation, using wearable sensors, as a multicomponent, cutting-edge intervention: a systematic review and meta-analysis. J Clin Med 2022;11:3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ruano-Ravina A, Pena-Gil C, Abu-Assi E, Raposeiras S, van′t Hof A, Meindersma E, et al. . Participation and adherence to cardiac rehabilitation programs. A systematic review. Int J Cardiol 2016;223:436–443. [DOI] [PubMed] [Google Scholar]
- 20. Pepera G, Tribali MS, Batalik L, Petrov I, Papathanasiou J. Epidemiology, risk factors and prognosis of cardiovascular disease in the Coronavirus Disease 2019 (COVID-19) pandemic era: a systematic review. Rev Cardiovasc Med 2022;23:28. [DOI] [PubMed] [Google Scholar]
- 21. Besnier F, Gayda M, Nigam A, Juneau M, Bherer L. Cardiac rehabilitation during quarantine in COVID-19 pandemic: challenges for center-based programs. Arch Phys Med Rehabil 2020;101:1835–1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al.. Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane; 2021. www.training.cochrane.org/handbook. [Google Scholar]
- 23. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. . The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. PLoS Med 2021;18:e1003583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Duan YP, Liang W, Guo L, Wienert J, Si GY, Lippke S. Evaluation of a web-based intervention for multiple health behavior changes in patients with coronary heart disease in home-based rehabilitation: pilot randomized controlled trial. J Med Internet Res 2018;20:e12052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Martin S, Anderson B, Vincenzo JL, Zai SY. A retrospective comparison of home telehealth and nursing care with or without rehabilitation therapy on rehospitalization rates of individuals with heart failure. J Cardiopulm Rehabil Prev 2017;37:207–213. [DOI] [PubMed] [Google Scholar]
- 26. Avila A, Claes J, Goetschalckx K, Buys R, Azzawi M, Vanhees L, et al. . Home-based rehabilitation with telemonitoring guidance for patients with coronary artery disease (short-term results of the TRiCH study): randomized controlled trial. J Med Internet Res 2018;20:e225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Batalik L, Dosbaba F, Hartman M, Batalikova K, Spinar J. Benefits and effectiveness of using a wrist heart rate monitor as a telerehabilitation device in cardiac patients: a randomized controlled trial. Medicine (Baltimore) 2020;99:e19556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rosario MBD, Lovell NH, Fildes J, Holgate K, Yu J, Ferry C, et al. . Evaluation of an mHealth-based adjunct to outpatient cardiac rehabilitation. IEEE J Biomed Health Inform 2018;22:1938–1948. [DOI] [PubMed] [Google Scholar]
- 29. Bernocchi P, Vitacca M, La Rovere MT, Volterrani M, Galli T, Baratti D, et al. . Home-based telerehabilitation in older patients with chronic obstructive pulmonary disease and heart failure: a randomised controlled trial. Age Ageing 2018;47:82–88. [DOI] [PubMed] [Google Scholar]
- 30. Frederix I, Driessche NV, Hansen D, Berger J, Bonne K, Alders T, et al. . Increasing the medium-term clinical benefits of hospital-based cardiac rehabilitation by physical activity telemonitoring in coronary artery disease patients. Eur J Prev Cardiol 2015;22:150–158. [DOI] [PubMed] [Google Scholar]
- 31. Frederix I, Solmi F, Piepoli MF, Dendale P. Cardiac telerehabilitation: a novel cost-efficient care delivery strategy that can induce long-term health benefits. Eur J Prev Cardiol 2017;24:1708–1717. [DOI] [PubMed] [Google Scholar]
- 32. Skobel E, Knackstedt C, Martinez-Romero A, Salvi D, Vera-Munoz C, Napp A, et al. . Internet-based training of coronary artery patients: the Heart Cycle Trial. Heart Vessels 2017;32:408–418. [DOI] [PubMed] [Google Scholar]
- 33. Pfaeffli Dale L, Whittaker R, Jiang Y, Stewart R, Rolleston A, Maddison R. Text message and internet support for coronary heart disease self-management: results from the Text4Heart Randomized Controlled Trial. J Med Internet Res 2015;17:e237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Varnfield M, Karunanithi M, Lee CK, Honeyman E, Arnold D, Ding H, et al. . Smartphone-based home care model improved use of cardiac rehabilitation in postmyocardial infarction patients: results from a randomised controlled trial. Heart 2014;100:1770–1779. [DOI] [PubMed] [Google Scholar]
- 35. Claes J, Cornelissen V, McDermott C, Moyna N, Pattyn N, Cornelis N, et al. . Feasibility, acceptability, and clinical effectiveness of a technology-enabled cardiac rehabilitation platform (Physical Activity Toward Health-I): randomized controlled trial. J Med Internet Res 2020;22:e14221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Eyles H, Neal B, Jiang Y, Doughty RN, McLean R, Ni Mhurchu C. A salt-reduction smartphone app supports lower-salt food purchases for people with cardiovascular disease: findings from the SaltSwitch randomised controlled trial. Eur J Prev Cardiol 2017;24:1435–1444. [DOI] [PubMed] [Google Scholar]
- 37. Sjolin I, Ogmundsdottir Michelsen H, Back M, Tanha T, Gonzalez M, Sandberg C, et al. . A lifestyle and self-care focused smartphone application can improve risk factor outcomes in cardiac rehabilitation for patients after myocardial infarction. Eur J Prev Cardiol 2019;26:S33–SS4. [Google Scholar]
- 38. Widmer RJ, Allison T, Lerman L, Lerman A. The augmentation of usual cardiac rehabilitation with an online and smartphone-based program improves cardiovascular risk factors and reduces rehospitalizations. J Am Coll Cardiol 2014;63:A1296. [Google Scholar]
- 39. Cai C, Yang G, Bao Z, Zhang F, Ju W, Chen H, et al. . Home-based cardiac rehabilitation versus conventional care for patients with atrial fibrillation treated with catheter ablation: a randomized controlled trial. J Arrhythm 2019;35:95–96. [Google Scholar]
- 40. Yudi MB, Clark DJ, Tsang D, Jelinek M, Kalten K, Joshi S, et al. . SMARTphone-based, early cardiac REHABilitation in patients with acute coronary syndromes: a randomized controlled trial. Coron Artery Dis 2021;32:432–440. [DOI] [PubMed] [Google Scholar]
- 41. Ding H, Varnfield M, Lee CK, VanVeen J, Arnold D, Keightley A, et al. . An innovative home care delivery model reduces the rate of dropouts found with traditional cardiac rehabilitation. Circulation 2012;125:A031. [Google Scholar]
- 42. Izawa KP, Watanabe S, Hiraki K, Morio Y, Kasahara Y, Takeichi N, et al. . Determination of the effectiveness of accelerometer use in the promotion of physical activity in cardiac patients: a randomized controlled trial. Arch Phys Med Rehabil 2012;93:1896–1902. [DOI] [PubMed] [Google Scholar]
- 43. Alharbi M, Bauman A, Neubeck L, Gallagher R. Validation of Fitbit-Flex as a measure of free-living physical activity in a community-based phase III cardiac rehabilitation population. Eur J Prev Cardiol 2016;23:1476–1485. [DOI] [PubMed] [Google Scholar]
- 44. Alter DA, O’Sullivan M, Oh PI, Redelmeier DA, Marzolini S, Liu R, et al. . Synchronized personalized music audio-playlists to improve adherence to physical activity among patients participating in a structured exercise program: a proof-of-principle feasibility study. Sports Med Open 2015;1:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bahler C, Bjarnason-Wehrens B, Schmid JP, Saner H. SWISSPAQ: validation of a new physical activity questionnaire in cardiac rehabilitation patients. Swiss Med Wkly 2013;143:w13752. [DOI] [PubMed] [Google Scholar]
- 46. Etiwy M, Akhrass Z, Gillinov L, Alashi A, Wang R, Blackburn G, et al. . Accuracy of wearable heart rate monitors in cardiac rehabilitation. Cardiovasc Diagn Ther 2019;9:262–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Freene N, Borg S, McManus M, Mair T, Tan R, Davey R, et al. . Comparison of device-based physical activity and sedentary behaviour following percutaneous coronary intervention in a cohort from Sweden and Australia: a harmonised, exploratory study. BMC Sports Sci Med Rehabil 2020;12:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Freene N, McManus M, Mair T, Tan R, Davey R. High sedentary behaviour and low physical activity levels at 12 months after cardiac rehabilitation: a prospective cohort study. Ann Phys Rehabil Med 2020;63:53–58. [DOI] [PubMed] [Google Scholar]
- 49. Lee YH, Hur SH, Sohn J, Lee HM, Park NH, Cho YK, et al. . Impact of home-based exercise training with wireless monitoring on patients with acute coronary syndrome undergoing percutaneous coronary intervention. J Korean Med Sci 2013;28:564–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Orme M, Clague-Baker N, Robinson T, Drewry S, Singh S. Does cardiac rehabilitation change physical activity and sedentary behaviour for people with mild-to-moderate stroke in the sub-acute phase of recovery? Eur Stroke J 2018;3:123–124. [Google Scholar]
- 51. Shen H, Zhao J, Zhou X, Li J, Wan Q, Huang J, et al. . Impaired chronotropic response to physical activities in heart failure patients. BMC Cardiovasc Disord 2017;17:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bravo-Escobar R, Gonzalez-Represas A, Gomez-Gonzalez AM, Montiel-Trujillo A, Aguilar-Jimenez R, Carrasco-Ruiz R, et al. . Effectiveness and safety of a home-based cardiac rehabilitation programme of mixed surveillance in patients with ischemic heart disease at moderate cardiovascular risk: a randomised, controlled clinical trial. BMC Cardiovasc Disord 2017;17:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chokshi N, Adusumalli S, Small D, Morris A, Feingold J, Ha Y, et al. . Effect of loss-framed financial incentives and personalized goal-setting on physical activity among ischemic heart disease patients using wearable devices: the active reward randomized clinical trial. J Gen Intern Med 2018;33:176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Dale LP, Whittaker R, Eyles H, Mhurchu CN, Ball K, Smith N, et al. . Cardiovascular disease self-management: pilot testing of an mHealth healthy eating program. J Pers Med 2014;4:88–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. De Kluiver EP, Van Der Velde AE, Meindersma EP, Prins LF, Wilhelm M, Iliou MC, et al. . A European randomised controlled trial for m-Health guided cardiac rehabilitation in the elderly; results of the EU-CaRE RCT study. Eur Heart J 2019;40:1217. [Google Scholar]
- 56. Devi R, Powell J, Singh S. A web-based program improves physical activity outcomes in a primary care angina population: randomized controlled trial. J Med Internet Res 2014;16:e186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Duscha BD, Piner LW, Patel MP, Craig KP, Brady M, McGarrah RW, et al. . Effects of a 12-week mHealth program on peak VO2 and physical activity patterns after completing cardiac rehabilitation: a randomized controlled trial. Am Heart J 2018;199:105–114. [DOI] [PubMed] [Google Scholar]
- 58. Ensom E, Albuquerque D, Erskine N, Peterson A, Dickson E, Ding EY, et al. . Feasibility of, and adherence to, a novel, home-based cardiac tele-rehabilitation program for heart attack survivors: the MI-PACE study. Circulation 2019;140:A11873. [Google Scholar]
- 59. Ewa Piotrowicz E, Wolszakiewicz J, Korzeniowska-Kubacka I, Chrapowicka A, Dobraszkiewicz-Wasilewska B, Jasionowska A, et al. . Home-based cardiac telerehabilitation—TeleInterMed study findings and results. Eur J Prev Cardiol 2013;1:S64. [Google Scholar]
- 60. Fang J, Huang B, Xu D, Li J, Au WW. Innovative application of a home-based and remote sensing cardiac rehabilitation protocol in Chinese patients after percutaneous coronary intervention. Telemed J E Health 2019;25:288–293. [DOI] [PubMed] [Google Scholar]
- 61. Knudsen MV, Petersen AK, Angel S, Hjortdal VE, Maindal HT, Laustsen S. Tele-rehabilitation and hospital-based cardiac rehabilitation are comparable in increasing patient activation and health literacy: a pilot study. Eur J Cardiovasc Nurs 2020;19:376–385. [DOI] [PubMed] [Google Scholar]
- 62. Laustsen S, Oestergaard LG, van Tulder M, Hjortdal VE, Petersen AK. Telemonitored exercise-based cardiac rehabilitation improves physical capacity and health-related quality of life. J Telemed Telecare 2020;26:36–44. [DOI] [PubMed] [Google Scholar]
- 63. Lear SA, Singer J, Banner-Lukaris D, Horvat D, Park JE, Bates J, et al. . Improving access to cardiac rehabilitation using the internet: a randomized trial. Stud Health Technol Inform 2015;209:58–66. [PubMed] [Google Scholar]
- 64. McDermott C, McCormack C, Claes J, McDermott L, O'Shea O, Buys R, et al. . Efficacy of a technology-enabled, home-based cardiac rehabilitation program. J Am Coll Cardiol 2019;73:1895. [Google Scholar]
- 65. Pfaeffli L, Maddison R, Jiang Y, Dalleck L, Lof M. Measuring physical activity in a cardiac rehabilitation population using a smartphone-based questionnaire. J Med Internet Res 2013;15:e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Piotrowicz E, Jasionowska A, Banaszak-Bednarczyk M, Gwilkowska J, Piotrowicz R. ECG telemonitoring during home-based cardiac rehabilitation in heart failure patients. J Telemed Telecare 2012;18:193–197. [DOI] [PubMed] [Google Scholar]
- 67. Piotrowicz E, Korzeniowska-Kubacka I, Chrapowicka A, Wolszakiewicz J, Dobraszkiewicz-Wasilewska B, Batogowski M, et al. . Feasibility of home-based cardiac telerehabilitation: results of TeleInterMed study. Cardiol J 2014;21:539–546. [DOI] [PubMed] [Google Scholar]
- 68. Song Y, Ren C, Liu P, Tao L, Zhao W, Gao W. Effect of smartphone-based telemonitored exercise rehabilitation among patients with coronary heart disease. J Cardiovasc Transl Res 2020;13:659–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Skobel E, Martinez-Romero A, Scheibe B, Schauerte P, Marx N, Luprano J, et al. . Evaluation of a newly designed shirt-based ECG and breathing sensor for home-based training as part of cardiac rehabilitation for coronary artery disease. Eur J Prev Cardiol 2014;21:1332–1340. [DOI] [PubMed] [Google Scholar]
- 70. Byun W, Ozemek C, Riggin K, Strath S, Kaminsky L. Correlates of objectively measured physical activity in cardiac patients. Cardiovasc Diagn Ther 2014;4:406–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Gallagher R, Roach K, Sadler L, Glinatsis H, Belshaw J, Kirkness A, et al. . Mobile technology use across age groups in patients eligible for cardiac rehabilitation: survey study. JMIR Mhealth Uhealth 2017;5:e161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Nabutovsky I, Nachshon A, Klempfner R, Shapiro Y, Tesler R. Digital cardiac rehabilitation programs: the future of patient-centered medicine. Telemed J E Health 2020;26:34–41. [DOI] [PubMed] [Google Scholar]
- 73. Braunholtz DA, Edwards SJ, Lilford RJ. Are randomized clinical trials good for us (in the short term)? Evidence for a “trial effect”. J Clin Epidemiol 2001;54:217–224. [DOI] [PubMed] [Google Scholar]
- 74. McCarney R, Warner J, Iliffe S, van Haselen R, Griffin M, Fisher P. The Hawthorne effect: a randomised, controlled trial. BMC Med Res Methodol 2007;7:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Menezes P, Miller WC, Wohl DA, Adimora AA, Leone PA, Miller WC, et al. . Does HAART efficacy translate to effectiveness? Evidence for a trial effect. PLoS One 2011;6:e21824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Thomas RJ, Beatty AL, Beckie TM, Brewer LC, Brown TM, Forman DE, et al. . Home-based cardiac rehabilitation: a scientific statement from the American Association of Cardiovascular and Pulmonary Rehabilitation, the American Heart Association, and the American College of Cardiology. J Cardiopulm Rehabil Prev 2019;39:208–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ambrosetti M, Abreu A, Corra U, Davos CH, Hansen D, Frederix I, et al. . Secondary prevention through comprehensive cardiovascular rehabilitation: from knowledge to implementation. 2020 update. A position paper from the Secondary Prevention and Rehabilitation Section of the European Association of Preventive Cardiology. Eur J Prev Cardiol 2020;28:460–495. [DOI] [PubMed] [Google Scholar]
- 78. Balady GJ, Ades PA, Bittner VA, Franklin BA, Gordon NF, Thomas RJ, et al. . Referral, enrollment, and delivery of cardiac rehabilitation/secondary prevention programs at clinical centers and beyond: a presidential advisory from the American Heart Association. Circulation 2011;124:2951–2960. [DOI] [PubMed] [Google Scholar]
- 79. Hannan AL, Harders MP, Hing W, Climstein M, Coombes JS, Furness J. Impact of wearable physical activity monitoring devices with exercise prescription or advice in the maintenance phase of cardiac rehabilitation: systematic review and meta-analysis. BMC Sports Sci Med Rehabil 2019;11:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Piotrowicz E, Pencina MJ, Opolski G, Zareba W, Banach M, Kowalik I, et al. . Effects of a 9-Week Hybrid Comprehensive Telerehabilitation Program on long-term outcomes in patients with heart failure: the Telerehabilitation in Heart Failure Patients (TELEREH-HF) randomized clinical trial. JAMA Cardiol 2020;5:300–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Niewada M, Tabor B, Piotrowicz E, Piotrowicz R, Opolski G, Banach M, et al. . Cost-effectiveness of telerehabilitation in patients with heart failure in Poland: an analysis based on the results of Telerehabilitation in the Heart Failure Patients (TELEREH-HF) randomized clinical trial. Kardiol Pol 2021;79:510–516. [DOI] [PubMed] [Google Scholar]
- 82. Winnige P, Filakova K, Hnatiak J, Dosbaba F, Bocek O, Pepera G, et al. . Validity and Reliability of the Cardiac Rehabilitation Barriers Scale in the Czech Republic (CRBS-CZE): determination of key barriers in East-Central Europe. Int J Environ Res Public Health 2021;18:13113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Johnson T, Isakazde N, Mathews L, Gao Y, MacFarlane Z, Spaulding EM, Martin SS, Marvel FA. Building a hybrid virtual cardiac rehabilitation program to promote health equity: lessons learned. Cardiovasc Digit Health J 2022;3:158–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, et al. . Cardiovascular implications of fatal outcomes of patients with Coronavirus Disease 2019 (COVID-19). JAMA Cardiol 2020;5:811–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Nishiga M, Wang DW, Han Y, Lewis DB, Wu JC. COVID-19 and cardiovascular disease: from basic mechanisms to clinical perspectives. Nat Rev Cardiol 2020;17:543–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Dalal HM, Doherty P, McDonagh ST, Paul K, Taylor RS. Virtual and in-person cardiac rehabilitation. BMJ 2021;373:n1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Scherrenberg M, Wilhelm M, Hansen D, Voller H, Cornelissen V, Frederix I, et al. . The future is now: a call for action for cardiac telerehabilitation in the COVID-19 pandemic from the secondary prevention and rehabilitation section of the European Association of Preventive Cardiology. Eur J Prev Cardiol 2020;28:524–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No new data were generated or analysed in support of this research.