Abstract
Background
Electrochemotherapy has good local effectiveness in the treatment of vulvar cancer. Most studies have reported the safety and effectiveness of electrochemotherapy for palliative treatment of gynecological cancers and mostly vulvar squamous cell carcinoma. Some tumors, however, fail to respond to electrochemotherapy. The biological features/determinants for the nonresponsiveness are not determined yet.
Patient and methods
A recurrence of vulvar squamous cell carcinoma was treated by electrochemotherapy using intravenous administration of bleomycin. The treatment was performed by hexagonal electrodes according to standard operating procedures. We analyzed the factors that could determine nonresponsiveness to electrochemotherapy.
Results
Based on the presented case of nonresponsive vulvar recurrence to electrochemotherapy, we hypothesize that the vasculature of the tumors prior to treatment may predict the response to electrochemotherapy. The histological analysis showed minimal presence of blood vessels in the tumor. Thus, low perfusion may reduce drug delivery and lead to a lower response rate because of the minor antitumor effectiveness of vascular disruption. In this case, no immune response in the tumor was elicited by electrochemotherapy.
Conclusions
In this case, of nonresponsive vulvar recurrence treated by electrochemotherapy, we analyzed possible factors that could predict treatment failure. Based on histological analysis, low vascularization of the tumor was observed, which hampered drug delivery and distribution and resulted in no vascular disrupting action of electro-chemotherapy. All these factors could contribute to ineffective treatment with electrochemotherapy.
Key words: electrochemotherapy, bleomycin, vulvar cancer, recurrence
Introduction
Vulvar cancer is the fourth most common gynecological cancer, with an incidence of 2.6 per 100,000 women per year.1 The treatment of vulvar cancer usually involves a combination of surgery and radiotherapy. Systemic treatment is rarely used. Most often, surgery includes radical vulvectomy and bilateral lymph groin node dissection or sentinel lymph node biopsy.2 Radiotherapy can be used as adjuvant therapy after initial surgery or as part of primary therapy in locally advanced disease. Most recurrences of vulvar cancer occur locally near the surgical margins or in the contralateral lymph groin region. The therapeutic modalities used depend on the location, the extent of recurrence and previously used radiotherapy or concomitant chemoradiotherapy.3 The emerging treatment modality for vulvar cancer recurrence is electrochemotherapy.
Literature review of electrochemotherapy
Electrochemotherapy is a local ablative therapy that uses the application of reversible electric pulses to the tumor to permeabilize the cell membrane, hence enabling the entry of cytotoxic drugs into the cells.4 It is most commonly used for the treatment of superficial tumors such as melanoma, sarcoma, squamous cell carcinoma, basal cell carcinoma, skin metastases from breast cancer and others.5,6 It can also be utilized for the treatment of deep-seated tumors such as primary hepatocellular carcinoma, colorectal cancer, unresectable colorectal liver metastases or pancreatic carcinoma.7-12 It is conducted following standard operating procedures, and the method is now used in nearly 180 cancer centers around the world.6
Only a small number of papers describing the use of electrochemotherapy for the palliative treatment of gynecological cancers and mostly vulvar squamous cell carcinoma have been presented.13,14 Safety and local efficacy after electrochemotherapy with bleomycin in locoregional cutaneous recurrences of vulvar carcinomas previously treated with chemotherapy, radiotherapy and surgery or unsuitable for standard treatments have been demonstrated.15-19 The effectiveness of the clinical cases and studies is presented in Table 1. The success rate of such tumors is 80%, which is lower than the response rate of other skin tumors treated with electrochemotherapy.13,16,18,20-22
The biological predictors of unsuccessful treatment have already been reviewed.5 The clinical predictive factors of the tumor response were identified to be the size of the lesions and previous treatment as well as the tumor type. However, the biology behind this process has still not been explored. Indicated were the intrinsic tumor sensitivity and tumor stroma, where the vascularization of the tumors might be the most important factor, in addition to the involvement of the immune response. The importance of the vasculature and vascular perfusion of tumors has already been shown to have an important role in the responsiveness of tumors to electrochemotherapy due to its role in drug delivery and tumor response due to the vascular disrupting action of electrochemotherapy.23 However, its importance in the response in clinical cases has not yet been discussed. With the aim of better understanding the pathophysiology of possible causes for unsuccessful treatment with electrochemotherapy in vulvar cancer recurrences, we present a case report of a 75-year-old woman with vulvar cancer recurrence in whom treatment with electrochemotherapy was ineffective and analyzed the possible causes of failure of such a treatment.
A case of unresponsive tumor
A 75-year-old woman was diagnosed with recurrence of vulvar cancer in the clitoral region. At the age of 70, simple vulvectomy of the left labium major and sentinel node biopsy (SNB) were performed because of vulvar squamous cell carcinoma. The sentinel inguinal nodes were negative, and the tumor was removed with free surgical margins. Five years after primary treatment, recurrence of vulvar squamous cell carcinoma in the clitoral region was diagnosed on regular follow up (Figure 1A). Inguinal and distal metastases were excluded after clinical assessment and imaging diagnostics. The patient was presented at the Interinstitutional Tumor Board, which decided that electrochemotherapy is a safe and viable treatment approach before eventual surgical treatment. The tumor board comprised medical oncologists, radiotherapists, and gynecological oncologists. The electrochemotherapy protocol was approved by the Institutional Medical Board and Slovenian National Ethical Committee (Number 0120-262/2021/3). Electrochemotherapy with bleomycin was performed according to the standard operating procedure.6 The patient underwent regional anesthesia. Bleomycin was administered at a dose of 15000 IU/m2 (Bleomycin medac, Medac GmbH, Germany). Eight minutes after intravenous administration of the drug, electric pulses were applied to the tumor in a way that covered all tumor nodule, including the safety margin of ~ 1 cm. Hexagonal geometry needle electrodes were used, and electric pulses were generated by Cliniporator (IGEA S.P.A., Italy). Altogether, 7 applications of electric pulses were delivered, and their delivery was verified on the screen of the generator (current > 1.5 A). The patient was discharged from the hospital the day after electrochemotherapy with no pain and no symptoms of any disturbance. At regular follow-ups one and two months after electrochemotherapy, we observed no clinical changes in the tumor (Figure 1B). Histologic analysis of repeated biopsy showed the presence of vulvar squamous cell carcinoma. Wide excision of the tumor was performed with free surgical margins. Eighteen months after vulvar cancer recurrence treatment, there were no visible signs of recurrence or progression of the disease.
Figure 1.
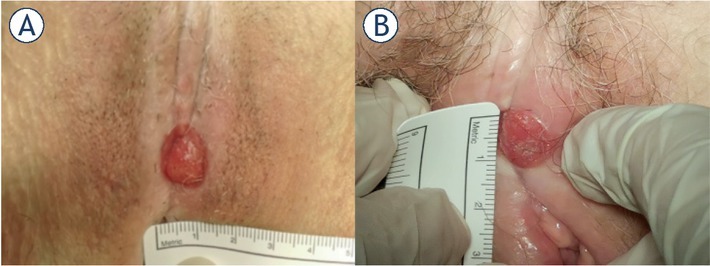
(A) Local recurrence of vulvar cancer. (B) No response to treatment two months after electrochemotherapy.
Table 1.
Review of studies evaluating electrochemotherapy in vulvar cancer
| First author, year published | Included no. of patients | Average age | Histology | Response of vulvar cancer |
|
|---|---|---|---|---|---|
| OR | NR | ||||
| Perrone, 201320 | 8 | 84y | 8 SCC | 6/8 (75%) | 2/8 (25%) |
| Perrone, 201521 | 25 | 85y | 25 SCC | 20/25 (80%) | 5/20 (20%) |
| Pellegrino, 201618 | 10 | 68y | 1 9 Paget’s SCC | 6/10 (60%) | 4/10 (40%) |
| Perrone, 201913 | 55 | 79y | 57 SCC 3 Paget’s 1 melanoma | 46/55 (84%) | 9/55 (16%) |
| Corrado, 202016 | 15 | 83y | 14 1 SCC CS | 12/15 (80%) | 3/15 (20%) |
CS = carcinosarcoma; No = number; NR = no response; OR = objective response; SCC = squamous cell carcinoma; y = year(s)
Histological examination of the first excisional biopsy performed in 2016 showed well-differentiated squamous cell carcinoma arising in the background of differentiated vulvar intraepithelial neoplasm (VIN) (Figure 2A). The absence of high-risk HPV was proven by the negative immunohistochemical reaction to p16. Staining for p16 was negative in the invasive component as well as in precancerous lesions. Additionally, two sentinel lymph nodes were excised and found to be negative. The patient again underwent an excisional biopsy in 2018 showing differentiated VIN and in 2021 after electrochemotherapy. Histological examination of the post electrochemotherapy biopsy (Figure 2B) showed residual, well to moderately differentiated squamous cell carcinoma, measuring approximately 1.3 cm in the largest diameter, invading 0.4 cm in depth. There was no lymphovascular or perineural invasion present. In the surrounding parenchyma, there were some thrombosed and recanalized blood vessels. Additionally, an immunohistochemical panel consisting of anti CD3, CD20, CD68 PGM1, CD163, and ERG antibodies (markers for T and B lymphocytes, macrophages and blood vessels) was stained on the primary biopsy from 2016 and the post electrochemotherapy biopsy from 2021. Negligible number of cells on the primary as well as post-electrochemotherapy biopsy was stained positive for CD20, therefore we did not include these sections into analysis. For other markers, five different fields of immunohistochemically stained sections were captured with a DP72 CCD camera connected to a BX-51microscope (Olympus,Hamburg,Germany) and analyzed with AxioVision program (Carl Zeiss, Jena, Germany) to determine the number of positive regions per section. These were then averaged and t-test were performed to determine the statistical significance using GraphPad Prism 9 (La Jolla, CA, USA). Detailed analysis showed significant differences only in the amount of small blood vessels being more numerous in the first pretreatment biopsy in comparison to the posttreatment biopsy (Figure 3). On the other hand, no difference was found in the number of CD3-positive lymphocytes or CD68-positive (pan macrophages marker) or CD163-positive M2 macrophages between the primary biopsy and post electrochemotherapy biopsy.
Figure 2.
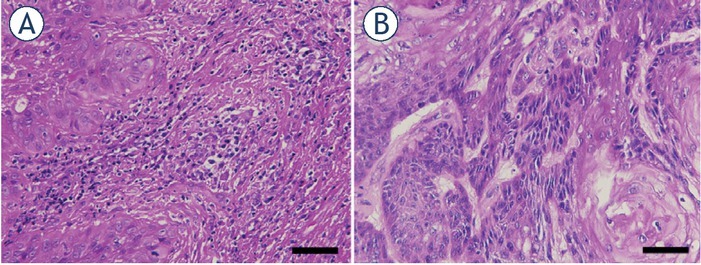
Hematoxylin and eosin stained sections of primary (A) and post-electrochemotherapy (B) biopsy. Scale bar represents 50 μm.
Figure 3.
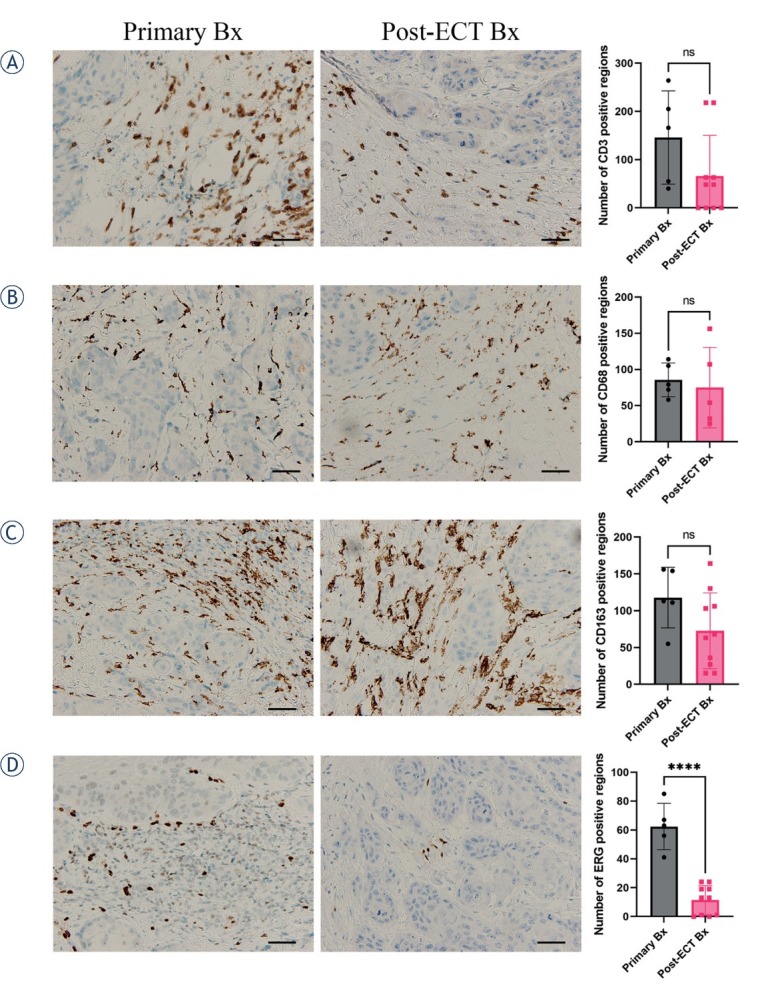
Immunohistologically stained sections for lymphocytes CD3 (A), macrophages CD68 PMG1 (B) and CD163 (C), and blood vessels ERG (D), from primary biopsy (Primary Bx) and post-electrochemotherapy biopsy (post-ECT Bx) samples. Scale bar represents 50 μm. The number of positively stained regions ± standard error of the mean (SEM) is presented.
Discussion
Response to electrochemotherapy is evaluated in accordance with the modified Response Evaluation Criteria in Solid Tumors (RECIST).24 Complete response for squamous cell carcinoma is observed in 63% of cases, and objective response is observed in 80%.22 For vulvar cancer, stable disease and progression of disease are observed in 16 to 40% of cases.14
There are many clinical factors contributing to predicting the response to treatment with electrochemotherapy. The possibility for unsuccessful treatment increases with tumor size, and a marked drop in response rate occurs after chemotherapy or in previously irradiated tissue compared with nonirradiated tissue.22
There are, although, differences in the response rate of different tumor histologies; i.e., melanoma was the most resistant, and basal cell carcinoma was the most sensitive to electrochemotherapy.22 The underlying biological factors have not yet been fully explored. As indicated in the review5, stromal factors may play a significant role. Vasculature has already been shown in preclinical studies to play a crucial role in the perfusion of tumors and consequently in drug delivery to tumors.23 To overcome this obstacle in less perfused tumors, intratumoral drug delivery could be an approach to overcome insufficient drug delivery. The vascular component and its destruction by electrochemotherapy can be a significant factor in the tumor response. The vascular disrupting effect of electrochemotherapy is based on the apoptosis of endothelial cells in small vessels, where the abrogation of the blood flow induces hypoxia in tumors and consequently indirect tumor cell death. The bigger vessels, as demonstrated in a study on electrochemotherapy of normal liver in pigs, are not affected by electrochemotherapy. The histological analysis was performed 2 and 7 days after electrochemotherapy and no thrombosis or other clinically significant damage to large blood vessels and bile ducts in the liver was observed.25 Another biological factor may contribute to non-responsiveness, tumor cells in low oxygenated parts of tumors are more aggressive and are more resistant to therapy, leading also to higher recurrence rate of the treated tumors.26,27 Therefore, in less responsive tumors, lower vascularity and slow perfusion could be predictors of a lower response rate. In two clinical studies on electrochemotherapy of liver tumors, one on colorectal liver metastases and the other on hepatocellular carcinoma, the effect of vascularity was evident.7,8 A clear difference in the response rate of these two tumor types was observed, although the treatment was performed in the same way, even by the same team of experts. Based on the known tumor histology features, colorectal metastases are less perfused tumors than hepatocellular carcinoma. Based on this, it can be deduced that the vascular component is important factor contributing to the response of the tumors. Namely, the vasculature is important for drug delivery and distribution and the vascular disruptive component of electrochemotherapy.
Clinical cases of nonresponsive tumors to electrochemotherapy are lacking. This is the first detailed analyzed case of recurrent vulvar cancer that has not responded to electrochemotherapy. The analysis of the immune component of the tumor stroma showed no significant changes after electrochemotherapy, with lymphocytes present in the margin of the tumor, while macrophages were also distributed in the tumors; however, we can presume that their phenotype was M2, as there were no antitumor effects. In responsive tumors, at least some increase in immune cell infiltration would be expected since electrochemotherapy induces immunogenic cell death.28,29 On the other hand, the vascularization of the treated lesion was minimal, which may indicate a poor response to electrochemotherapy due to the minimal delivery of bleomycin after intravenous administration to the tumor site, lack of distribution around the tumor and absence of vascular disrupting action of electrochemotherapy.
This case clearly indicates that we have to search for biological determinants of failure of electrochemotherapy. As already suggested, the explanations for the heterogeneity in tumor response may reside in the altered vasculature that occurs with tumor growth and the difference in cell susceptibility or aggressiveness in the hypoxic environment.5
In conclusion, we propose to analyze the tumor vasculature with pathohistological biopsy or ultrasound prior to electrochemotherapy. The investigation of tumor vasculature may allow us to predict the treatment response of vulvar cancer with electrochemotherapy, which will help to determine the best individual treatment option and may ultimately improve patient outcomes.
Acknowledgement
The authors acknowledge the financial support from the state budget by the Slovenian Research Agency, program no. P3-0003.
Disclosure
No potential conflicts of interest were disclosed.
References
- 1.United States Cancer Statistics (USCS) U.S. Cancer Statistics Data Visualizations Tool. CDC, Centers for Disease Control and prevention. 2022. https://www.cdc.gov/cancer/uscs/dataviz/index.htm May 24. [cited 2022 Aug 19]. Available at.
- 2.Merlo S. Modern treatment of vulvar cancer. Radiol Oncol. 2020;54:371–6. doi: 10.2478/raon-2020-0053. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salom EM, Penalver M. Recurrent vulvar cancer. Curr Treat Options Oncol. 2002;3:143–53. doi: 10.1007/s11864-002-0060-x. doi. [DOI] [PubMed] [Google Scholar]
- 4.Cemazar M, Sersa G. Recent advances in electrochemotherapy. Bioelectricity. 2019;1:204–13. doi: 10.1089/bioe.2019.0028. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sersa G, Ursic K, Cemazar M, Heller R, Bosnjak M, Campana LG. Biological factors of the tumour response to electrochemotherapy: review of the evidence and a research roadmap. Eur J Surg Oncol. 2021;47:1836–46. doi: 10.1016/j.ejso.2021.03.229. doi. [DOI] [PubMed] [Google Scholar]
- 6.Gehl J, Sersa G, Matthiessen LW, Tobian M, Soden D, Occhini A. Updated standard operating procedures for electrochemotherapy of cutaneous tumours and skin metastases. Acta Oncol. 2018;57:874–82. doi: 10.1080/0284186X.2018.1454602. et al. doi. [DOI] [PubMed] [Google Scholar]
- 7.Djokic M, Cemazar M, Bosnjak M, Dezman R, Badovinac D, Miklavcic D. A prospective phase II study evaluating intraoperative electrochemotherapy of hepatocellular carcinoma. Cancers. 2020;12:E3778. doi: 10.3390/cancers12123778. et al. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edhemovic I, Brecelj E, Cemazar M, Boc N, Trotovsek B, Djokic M. Intraoperative electrochemotherapy of colorectal liver metastases: a prospective phase II study. Eur J Surg Oncol. 2020;46:1628–33. doi: 10.1016/j.ejso.2020.04.037. et al. doi. [DOI] [PubMed] [Google Scholar]
- 9.Casadei R, Ricci C, Ingaldi C, Alberici L, Di Marco M, Guido A. Intraoperative electrochemotherapy in locally advanced pancreatic cancer: indications, techniques and results-a single-center experience. Updat Surg. 2020;72:1089–96. doi: 10.1007/s13304-020-00782-x. et al. doi. [DOI] [PubMed] [Google Scholar]
- 10.Spallek H, Bischoff P, Zhou W, de Terlizzi F, Jakob F, Kovàcs A. Percutaneous electrochemotherapy in primary and secondary liver malignancies - local tumor control and impact on overall survival. Radiol Oncol. 2022;56:102–10. doi: 10.2478/raon-2022-0003. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schipilliti FM, Onorato M, Arrivi G, Panebianco M, Lerinò D, Milano A. Electrochemotherapy for solid tumors: literature review and presentation of a novel endoscopic approach. Radiol Oncol. 2022;56:285–91. doi: 10.2478/raon-2022-0022. et al. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bosnjak M, Jesenko T, Markelc B, Cerovsek A, Sersa G, Cemazar M. Sunitinib potentiates the cytotoxic effect of electrochemotherapy in pancreatic carcinoma cells. Radiol Oncol. 2022;56:164–72. doi: 10.2478/raon-2022-0009. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perrone AM, Galuppi A, Pirovano C, Borghese G, Covarelli P, De Terlizi F. Palliative electrochemotherapy in vulvar carcinoma: preliminary results of the ELECHTRA (Electrochemotherapy Vulvar Cancer) multicenter study. Cancers. 2019;11:E657. doi: 10.3390/cancers11050657. et al. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tranoulis A, Georgiou D, Founta C, Mehra G, Sayasneh A, Nath R. Use of electrochemotherapy in women with vulvar cancer to improve quality-of-life in the palliative setting: a meta-analysis. Int J Gynecol Cancer. 2020;30:107–14. doi: 10.1136/ijgc-2019-000868. doi. [DOI] [PubMed] [Google Scholar]
- 15.Merlo S, Vivod G, Bebar S, Bosnjak M, Cemazar M, Srsa G. Literature review and our experience with bleomycin-based electrochemotherapy for cutaneous vulvar metastases from endometrial cancer. Technol Cancer Res Treat. 2021;20:15330338211010134. doi: 10.1177/15330338211010134. et al. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corrado G, Cutillo G, Fragomeni SM, Bruno V, Tagliaferri L, Mancini E. Palliative eletrochemotherapy in primary or recurrent vulvar cancer. Int J Gynecol Cancer. 2020;30:927–31. doi: 10.1136/ijgc-2019-001178. et al. doi. [DOI] [PubMed] [Google Scholar]
- 17.Perrone AM, Galuppi A, Borghese G, Corti B, Ferioli M, Della Gatta AN. Electrochemotherapy pre-treatment in primary squamous vulvar cancer. Our preliminary experience. J Surg Oncol. 2018;117:1813–7. doi: 10.1002/jso.25072. et al. doi. [DOI] [PubMed] [Google Scholar]
- 18.Pellegrino A, Damiani GR, Mangioni C, Stripoli D, Loverro G, Cappello A. Outcomes of bleomycin-based electrochemotherapy in patients with repeated loco-regional recurrences of vulvar cancer. Acta Oncol. 2016;55:619–24. doi: 10.3109/0284186X.2015.1117134. et al. doi. [DOI] [PubMed] [Google Scholar]
- 19.Vivod G, Kovacevic N, Čemažar M. Electrochemotherapy as an alternative treatment option to pelvic exenteration for recurrent vulvar cancer of the perineum region. Technol Cancer Res Treat. 2022;21:15330338221116488. doi: 10.1177/15330338221116489. et al. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perrone AM, Galuppi A, Cima S, Pozatti F, Arcelli A, Cortesi A. Electrochemotherapy can be used as palliative treatment in patients with repeated loco-regional recurrence of squamous vulvar cancer: a preliminary study. Gynecol Oncol. 2013;130:550–3. doi: 10.1016/j.ygyno.2013.06.028. et al. doi. [DOI] [PubMed] [Google Scholar]
- 21.Perrone AM, Cima S, Pozzati F, Fraculli R, Cammelli S, Tesei M. Palliative electro-chemotherapy in elderly patients with vulvar cancer: a phase II trial: Electro-chemotherapy in vulvar cancer. J Surg Oncol. 2015;112:529–32. doi: 10.1002/jso.24036. et al. doi. [DOI] [PubMed] [Google Scholar]
- 22.Clover AJP, de Terlizzi F, Bertino G, Curatolo O, Odili J, Campana LG. Electrochemotherapy in the treatment of cutaneous malignancy: Outcomes and subgroup analysis from the cumulative results from the pan-European International Network for Sharing Practice in Electrochemotherapy database for 2482 lesions in 987 patients (2008-2019) Eur J Cancer. 2020;138:30–40. doi: 10.1016/j.ejca.2020.06.020. et al. doi. [DOI] [PubMed] [Google Scholar]
- 23.Groselj A, Kranjc S, Bosnjak M, Krzan M, Kosjek T, Prevc A. Vascularization of the tumours affects the pharmacokinetics of bleomycin and the effectiveness of electrochemotherapy. Basic Clin Pharmacol Toxicol. 2018;123:247–56. doi: 10.1111/bcpt.13012. et al. doi. [DOI] [PubMed] [Google Scholar]
- 24.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sergent D, Ford R. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. et al. doi. [DOI] [PubMed] [Google Scholar]
- 25.Zmuc J, Gasljevic G, Sersa G, Edhemovic I, Boc N, Seliskar A. Large liver blood vessels and bile ducts are not damaged by electrochemotherapy with bleomycin in pigs. Sci Rep. 2019;9:1–11. doi: 10.1038/s41598-019-40395-y. et al. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wei J, Hu M, Du H. Improving cancer immunotherapy: exploring and targeting metabolism in hypoxia microenvironment. Front Immunol. 2022;13:845923. doi: 10.3389/fimmu.2022.845923. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tannock IF, Gordon Steel G. Cell proliferation, drug distribution and therapeutic effects in relation to the vascular system of solid tumours. Br J Cancer. 2022 doi: 10.1038/s41416-022-02109-6. Dec 23. Ahead of print. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Calvet CY, Mir LM. The promising alliance of anti-cancer electrochemotherapy with immunotherapy. Cancer Metastasis Rev. 2016;35:165–77. doi: 10.1007/s10555-016-9615-3. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ursic K, Kos S, Kamensek U, Cemazar M, Miceska S, Markelc B. Potentiation of electrochemotherapy effectiveness by immunostimulation with IL-12 gene electrotransfer in mice is dependent on tumor immune status. J Control Release. 2021;332:623–35. doi: 10.1016/j.jconrel.2021.03.009. et al. doi. [DOI] [PubMed] [Google Scholar]


