Abstract
Acute myeloid leukemia (AML) is the most common acute leukemia in adults. While survival for younger patients over the last several decades has improved nearly sixfold with the optimization of intensive induction chemotherapy and allogeneic stem cell transplantation (alloHSCT), this effect has been largely mitigated in older and less fit patients as well as those with adverse-risk disease characteristics. However, the last 10 years has been marked by major advances in the molecular profiling of AML characterized by a deeper understanding of disease pathobiology and therapeutic vulnerabilities. In this regard, the classification of AML subtypes has recently evolved from a morphologic to a molecular and genetic basis, reflected by recent updates from the World Health Organization and the new International Consensus Classification system. After years of stagnation in new drug approvals for AML, there has been a rapid expansion of the armamentarium against this disease since 2017. Low-intensity induction therapy with hypomethylating agents and venetoclax has substantially improved outcomes, including in those previously considered to have a poor prognosis. Furthermore, targeted oral therapies against driver mutations in AML have been added to the repertoire. But with an accelerated increase in treatment options, several questions arise such as how to best sequence therapy, how to combine therapies, and if there is a role for maintenance therapy in those who achieve remission and cannot undergo alloHSCT. Moreover, certain subtypes of AML, such as those with TP53 mutations, still have dismal outcomes despite these recent advances, underscoring an ongoing unmet need and opportunity for translational advances. In this review, we will discuss recent updates in the classification and risk stratification of AML, explore the literature regarding low-intensity and novel oral combination therapies, and briefly highlight investigative agents currently in early clinical development for high-risk disease subtypes.
Keywords: Acute myeloid leukemia, Targeted therapy, Novel treatments, Combination therapy, FLT3, IDH1, IDH2, TP53
Background
Acute myeloid leukemia (AML) is the most common acute leukemia in adults. AML is thought to arise from somatically acquired mutations, which is a fairly ubiquitous process during human aging [1–11]. However, AML can arise both de novo and secondary to other processes including antecedent hematologic disorders or exposure to immunosuppressive or cytotoxic therapies. The median age of diagnosis of AML is 68 years in the USA, and the incidence continues to increase with age [12]. The median overall survival (OS) from diagnosis for patients under the age of 65 had improved from 8 months between 1975 and 1979 to 46 months between 2010 and 2014; however, survival in patients older than 65 has only marginally improved during this same interval [12, 13]. This is in part because induction with intensive cytarabine- and anthracycline-based chemotherapy (i.e., “7 + 3”) has remained the standard of care for AML for over 40 years, the tolerability of which is limited in older and less fit patients.
While optimization of intensive chemotherapy and better supportive care over the years has improved survival in AML, this benefit is largely confined to younger patients and those without adverse-risk cytogenetics. Moreover, the only potentially curative strategy for those with intermediate- or adverse-risk disease is allogeneic hematopoietic stem cell transplantation (alloHSCT) [14], which is not an option for many patients with AML due to age, frailty, and medical co-morbidities [15]. Accordingly, there has been a considerable interest in de-intensifying induction therapy, guided by an improved understanding of AML pathobiology due to advances in genomic profiling [16–18]. This has led to the approval of multiple novel agents and targeted therapies, which are now increasingly employed in the frontline, relapsed/refractory (R/R), and maintenance settings (reviewed in [19–22]). However, with 10 new Food and Drug Administration (FDA) approvals for AML in the last 5 years and increasing availability of personalized genomic data, important questions arise of how to best personalize the treatment of patients with AML and how to utilize transplantation in the context of targeted therapies (Fig. 1). In this review, we will discuss the recent updates in classification and risk stratification of AML, explore combination therapies in clinical practice and situations in which intensive chemotherapy can potentially be replaced, and highlight ongoing areas of investigation for AML therapeutics.
Fig. 1.
Approach to frontline treatment of AML with FDA-approved therapies in 2022. Treatment algorithm of AML induction and maintenance therapy is shown. AML Acute myeloid leukemia, GO Gemtuzumab ozogamicin, MIDO Midostaurin, HMA Hypomethylating agent, VEN Venetoclax, ENA Enasidenib; IVO Ivosidenib, LDAC Low-dose cytarabine, alloHSCT Allogeneic stem cell transplantation, MRD Measurable residual disease, CR Complete remission
Updates in classification and risk stratification of AML
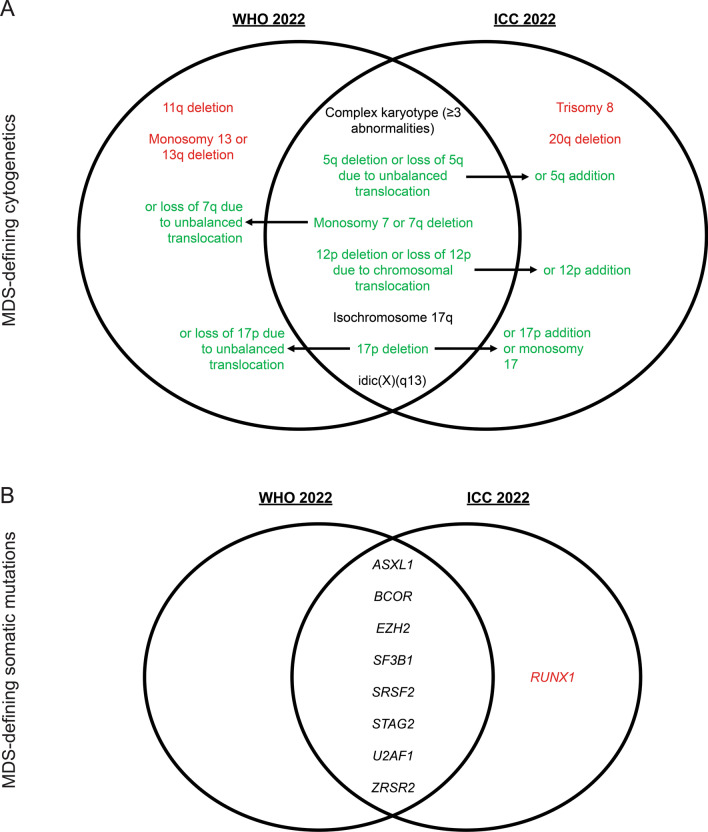
In 2022, the World Health Organization (WHO) [23] updated their classification of hematolymphoid neoplasms, and a separate International Consensus Classification (ICC) system was formed [24]. While these have led to changes in many hematologic malignancies, we will henceforth focus on changes related to AML (summarized in Table 1). Many disease entities remain the same, and most changes revolve around incorporating cytogenetic and genetic information into diagnostic classification. A significant change is that myelodysplastic syndrome with excess blasts 2 (MDS-EB2) is no longer a recognized MDS subtype, reflecting a spectrum of disease between MDS and AML rather than a discrete transition at the arbitrary cutoff of 20% blasts. The WHO allows for a diagnosis of AML with a blast count below 20% if there are defining genetic abnormalities with the exception of BCR::ABL1 fusions and CEBPA mutations. Cases of AML without a defining genetic alteration are made based on differentiation patterns and still require a blast count of at least 20%. Similarly, the ICC uses a 10% blast cutoff for most molecularly defined subtypes of AML, again with the exception of BCR::ABL1 to avoid confusion with the diagnosis of chronic myeloid leukemia (CML). Moreover, both the WHO and ICC define cytogenetic and genetic changes almost always associated with antecedent MDS (Fig. 2A–B), while the cytogenetic profile has not drastically changed with a few exceptions, mutations in ASXL1, BCOR, EZH2, SF3B1, SRSF2, STAG2, U2AF1, or ZRSR2 now define AML with myelodysplasia-related gene mutations given the high prevalence of these mutations in MDS; RUNX1 mutations are also considered MDS-defining by the ICC but not by the WHO [25–28]. By relying on molecular rather than morphologic classification of AML, this may allow for nuanced prognostication and definition of targets for measurable residual disease (MRD) monitoring during treatment, though there is still no standard of how to incorporate this into routine care. Two major differences between the updated WHO and ICC systems are how AML with CEBPA and TP53 alterations are defined. Several studies have now shown that basic leucine zipper (bZIP) domain mutations in CEBPA confer a better prognosis with a distinct gene expression profile [29–31], so this is now an AML-defining genetic alteration in both classification systems, though the WHO still also includes other bi-allelic CEBPA mutations, while the ICC does not. Furthermore, the ICC defines TP53-mutated AML and MDS/AML as a distinct genetic entity due to the characteristically poor prognosis associated with this mutation [32–34]. Any somatic TP53 mutation with a variant allelic frequency (VAF) above 10% now defines this subtype of MDS/AML or AML. The WHO system created a distinct entity for TP53-mutated AML due to the frequent co-occurrence with complex cytogenetics or therapy-related AML (tAML).
Table 1.
Updates in WHO/ICC classifications of AML
| WHO 2022 | ICC 2022* | |
|---|---|---|
| AML with defining genetic abnormalities** | APL with PML::RARA fusion | APL with t (15;17) (q24.1;q21.2)/PML::RARA§ |
| APL with other RARA rearrangements§ | ||
| AML with RUNX1::RUNX1T1 fusion | AML with t (8;21) (q22;q22.1)/RUNX1::RUNX1T1§ | |
| AML with CBFB::MYH11 fusion | AML with inv (16) (p13.1q22) or t (16;16) (p13.1;q22)/CBFB::MYH11§ | |
| AML with DEK::NUP214 fusion | AML with t (6;9) (p22.3;q34.1)/DEK::NUP214§ | |
| AML with RBM15::MRTFA fusion | Not recognized | |
| AML with BCR::ABL1 fusion | AML with t (9;22) (q34.1;q11.2)/BCR::ABL1# | |
| AML with KMT2A rearrangement | AML with t (9;11) (p21.3;q23.3)/MLLT3::KMT2A§ | |
| AML with other KMT2A rearrangements§ | ||
| AML with MECOM rearrangement | AML with inv (3) (q21.3q26.2) or t (3;3) (q21.3;q26.2)/GATA2; MECOM (EVI1)§ | |
| AML with other MECOM rearrangements§ | ||
| AML with NUP98 rearrangement | Not recognized | |
| AML with NPM1 mutation | AML with mutated NPM1§ | |
| AML with CEBPA mutation | AML with in-frame bZIP CEBPA mutations§ | |
| AML, myelodysplasia-related† | AML# and MDS/AML§ with mutated TP53 | |
| AML# and MDS/AML§ with myelodysplasia-related gene mutations | ||
| AML# and MDS/AML§ with myelodysplasia-related cytogenetic abnormalities | ||
| MDS/AML NOS§ | ||
| AML with other defined genetic alterations | AML with other rare recurring translocations# | |
| Myeloid proliferations associated with Down syndrome | ||
| AML, defined by differentiation | AML with minimal differentiation | AML NOS# |
| AML without maturation | ||
| AML with maturation | ||
| Acute basophilic leukemia | ||
| Acute myelomonocytic leukemia | ||
| Acute monocytic leukemia | ||
| Acute erythroid leukemia | ||
| Acute megakaryoblastic leukemia | ||
| Myeloid sarcoma | Myeloid sarcoma | |
| Blastic plasmacytoid dendritic cell neoplasm | Blastic plasmacytoid dendritic cell neoplasm |
AML Acute myeloid leukemia, APL Acute promyelocytic leukemia, MDS Myelodysplastic syndrome, bZIP Basic leucine zipper domain, NOS Not otherwise specified, WHO World Health Organization, ICC International Consensus Classification
*Requires mention of qualifiers (Therapy-related, Progressing from MDS, Progressing from MDS/MPN, and/or Germline predisposition)
** ≥ 20% blast cutoff is no longer required for AML with defining genetic abnormalities except for BCR::ABL fusion and CEBPA mutation
† AML, myelodysplasia-related encompasses AML transformation from MDS and MDS/MPN
§Blast cutoff ≥ 10%
#Blast cutoff ≥ 20%
Fig. 2.
Updates in WHO/ICC MDS-defining genetic alterations in AML. Venn diagrams depict overlapping and distinct MDS-defining cytogenetic alterations A and somatic mutations B determined by the WHO [23] and ICC [24] Green text with arrows denotes overlapping entities with minor differences, black text denotes completely overlapping entities, and red text denotes completely distinct entities. MDS Myelodysplastic syndrome WHO World Health Organization, ICC International Consensus Classification
These changes reflect a movement toward molecularly defining AML and related myeloid neoplasms. The natural history of these diseases suggests some overlap in pathogenesis with biological variability perhaps better attributed to distinct genetic driver events rather than morphologic differences. Thus, the European LeukemiaNet (ELN) also updated their risk stratification schema (Fig. 3) to reflect this change [35]. The favorable prognostic impact of CEBPA mutations is driven by bZIP domain mutations [29–31], so this is now specified in the favorable-risk category. Moreover, FLT3 internal tandem duplication (FLT3-ITD) mutations had previously been considered a VAF-dependent risk factor in patients who also harbor NPM1 mutations. However, there is variability in the standardization of measuring allelic ratio (AR), and the incorporation of FLT3-targeting multi-tyrosine kinase inhibitor (TKI) midostaurin (MIDO) has demonstrated benefit in patients with FLT3-ITD regardless of VAF [36]. Thus, for epidemiologic and practical reasons, FLT3-ITD is now considered intermediate-risk irrespective of AR or co-occurring NPM1 mutations. Although patients with FLT3-ITD who did not receive MIDO had worse outcomes regardless of ELN 2017 risk category [36], MRD testing could be considered for more dynamic risk stratification after induction therapy and/or prior to alloHSCT given its prognostic relevance even for patients treated with chemotherapy only [37, 38]. In concordance with the expanded list of myelodysplasia-associated genes from the WHO and ICC, mutations in ASXL1, BCOR, EZH2, RUNX1, SF3B1, SRSF2, STAG2, U2AF1, or ZRSR2 are now considered adverse-risk based on updated prognostic studies in MDS, de novo AML, and secondary AML (sAML) [25–28, 39, 40]. Lastly, new disease-defining cytogenetic changes involving MECOM [41, 42] or KAT6A::CREBBP fusion [43] have been updated in adverse-risk disease, while hyperdiploid karyotype with multiple trisomies/polysomies is no longer considered complex karyotype. The changes to the ELN risk stratification system reflect an evolving understanding of how AML biology impacts clinical phenotype. However, genetic drivers of AML do not always appear in isolation [16, 44], and, as we will discuss later in this review, co-mutation patterns often have conflicting prognostic implications. Accordingly, a more comprehensive incorporation of genetic and cytogenetic alterations along patient and disease characteristics, as has recently been implemented in MDS [40], may improve that personalization of risk stratification.
Fig. 3.
Updates in ELN risk stratification of AML. A Sankey plot depicts changes in the 2017 [65] and 2022 [35] ELN risk stratification of AML. Prognostic groups are groups by color (favorable—green, intermediate—yellow, adverse—red) and changes are tracked by dashed arrows. bZIP Basic leucine zipper domain, ELN European LeukemiaNet
Azacitidine and venetoclax: moving up the ranks
AML in those unfit for intensive chemotherapy
It should be noted that while updates from the WHO, ICC, and ELN emphasize the importance of molecular pathogenesis in AML, there are other patient characteristics that significantly impact prognosis and treatment, namely age and fitness. The National Comprehensive Cancer Network (NCCN) guidelines divide induction treatment algorithms based on age below or above 60 years [45]. While the age of 60 is not uniform in its capacity to discriminate the ability to tolerate high-intensity chemotherapy, clinical trials have traditionally been organized around this age. However, in clinical practice, these historical age cutoffs warrant reconsideration when patients are physiologically fit despite being chronologically older. Historically, non-intensive therapy included the use of single-agent azacitidine (AZA) or decitabine (DAC) with a median OS reaching a dismal 7.7 to 10.4 months [46, 47]. The combination of the BCL2-inhibitor venetoclax (VEN) with AZA or low-dose cytarabine (LDAC) has changed the standard of care for this patient population. The VIALE-A study [48] compared AZA/VEN to AZA/placebo in an elderly population (median age 76 years) and observed an improvement in the rate of complete remission (CR) plus CR with incomplete hematologic recovery (CRi) (66.4% versus 28.3%, respectively) and median OS (14.7 months versus 9.6 months, respectively). Long-term follow-up from this study confirms these findings and notably observed that patients who achieved CR/CRi and MRD negativity with AZA/VEN had a median OS of 34.2 months [49]. Similarly, the VIALE-C study [50] randomized patients with a median age of 76 years to LDAC/VEN or LDAC/placebo, noting an improvement in the rate of CR/CRi from 13 to 48% along with survival benefit at long-term follow-up [51, 52]. Recently, a study by Pollyea et al. found that the use of alloHSCT in patients over 60 years who received AZA/VEN improved median OS compared to those who deferred alloHSCT (not reached versus 17.2 months) [53]. Another retrospective study from the same group [54] compared outcomes of patients undergoing alloHSCT after AZA/VEN to intensive chemotherapy and found no significant difference in recurrence-free survival (RFS) (73.2% versus 66.1%, respectively) or OS (76.3% versus 74.7%, respectively) at 12 months. Age was not a predictive factor of death or relapse in this population, though higher hematopoietic cell transplantation-specific comorbidity index (HCT-CI) and positive pre-alloHSCT MRD were associated with worse outcomes. Theoretically, in older patients with high-risk disease, treatment with AZA/VEN may maintain or improve fitness, allowing more patients to undergo alloHSCT who may not have previously been eligible after receiving induction with 7 + 3. Currently, there is a study (NCT04801797) ongoing to address this question which randomizes patients fit for intensive chemotherapy to receive either 7 + 3 or AZA/VEN.
Secondary AML
As previously discussed, most cases of AML arise de novo, but sAML presents a unique clinical challenge. While the incidence of sAML increases with age [55], sAML frequently harbors adverse-risk cytogenetics and mutational profiles that are often associated with treatment resistance [56–60]. CPX-351, a fixed 5:1 molar ratio of cytarabine/daunorubicin liposome, was developed with the intention of optimizing a synergistic molar ratio of the two chemotherapeutics [61] and was noted to have activity in sAML in early data [62]. A subsequent phase 3 study assessed CPX-351 versus 7 + 3 in patients aged 60–75 with newly diagnosed (ND) tAML, antecedent MDS or chronic myelomonocytic leukemia, or de novo AML with MDS-related cytogenetics (AML-MRC) [63] and observed improved median OS (9.56 months versus 5.95 months) and CR/CRi rates (47.7% versus 33.3%), leading to FDA approval of CPX-351 for patients with newly ND tAML or AML-MRC. Moreover, long-term survival with CPX-351 was attributed to more patients proceeding to alloHSCT, which was performed in 34% of patients [63].
In clinical practice, there is significant overlap in patients who may be eligible for AZA/VEN or CPX-351 in the frontline setting, though these two regimens have not been compared head-to-head. A recent real-world analysis of patients with ND AML who received AZA/VEN or CPX-351 found that those receiving AZA/VEN were more likely to be older (median age 75 years versus 67 years, respectively) [64]. Despite the heterogeneity between cohorts, median OS for all patients was similar between the AZA/VEN and CPX-351 cohorts with no significant difference even when controlling for multiple factors including performance status, MRC, ELN 2017 risk category [65], high-risk mutations, and HCT-CI score. Although fewer patients underwent transplant with AZA/VEN induction, median OS was not significantly affected by the choice of CPX-351 versus AZA/VEN. Thus, while a randomized control trial is lacking, this study provides some clinical equipoise between AZA/VEN and CPX-351 in patients who may be eligible for either therapy.
AML with high-risk mutations
With advances in next-generation sequencing (NGS) technology, the detection of molecular driver mutations in AML has improved. Follow-up analyses of several landmark studies have allowed for the identification of certain mutational subgroups which may derive further benefit from AZA/VEN compared to intensive chemotherapy. Perhaps the most challenging scenario is with TP53-mutant AML. Recent data suggest that patients with TP53 mutations have worse outcomes compared to other adverse-risk features with a 2-year OS of only 12.8% even with intensive treatment [33]. The presence of TP53 mutations confers similarly poor outcomes irrespective of de novo disease, sAML, or blast count [34]. However, there is heterogeneity in TP53-mutant AML based on types of mutations, involved domains, and VAF; a recent report found that patients without abnormal p53 protein expression, intact copy number, and low VAF had a more favorable prognosis in the spectrum of TP53-mutant AML [66]. Nonetheless, even in patients who undergo alloHSCT, relapse rates are very high with median OS often reported as less than 6 months after transplant [67, 68], though this may be longer based on more recent retrospective studies [69]. In the VIALE-A study, CR/CRi for patients with TP53 alterations was drastically improved with AZA/VEN (55.3%) compared to AZA/placebo (0%), though the duration of remission (DOR) was brief, and median OS was only 7.2 months [48, 70].
Notably, AZA/VEN achieved CR/CRi rates of 70% compared to 23% with AZA alone in patients with adverse-risk cytogenetics without TP53 mutations, along with durable remissions (18.4 months versus 8.51 months) and improved median OS (23.4 months versus 11.3 months). However, the benefit in DOR or OS was lost with the addition of TP53 mutations despite improvements in CR/CRi (41% with AZA/VEN versus 17% with AZA) [71]. Unfortunately, several follow-up studies with hypomethylating agents (HMA) and VEN have produced similarly discouraging results [71–73]. The lack of durable response may stem from a requirement of intact p53 protein to maintain long-term response to BH3-mimetic drugs such as VEN [74]. While HMA/VEN-based regimens have not yet demonstrably moved the needle forward for patients with TP53-mutant AML, several studies are underway combining these agents in this high-risk disease; these will be discussed later in this review.
Two other high-risk gene mutations of interest in the context of AZA/VEN are ASXL1 and RUNX1. Preclinical data from isogenic leukemic cells harboring ASXL1 mutations demonstrated that genetic correction of this variant slows leukemic cell growth and induces differentiation [75]. CD34 cells with ASXL1 mutations from patients were also shown to have higher BCL2 expression and gene-body methylation, rendering them more sensitive to VEN and AZA, respectively [75]. These findings appear clinically relevant as retrospective studies in patients with R/R AML [76] and MDS-EB2 [77] treated with HMA/VEN observed improved CR/CRi rates in patients with ASXL1 mutations. In the latter study, patients harboring ASXL1 mutations were found to have a better median OS (not reached) compared to those without (10.2 months). However, an independent study could not reproduce improved CR/CRi rates in patients with ASXL1 mutations treated with AZA/VEN compared to intensive chemotherapy [78]. Rather, they observed improved CR/CRi rates in patients with RUNX1 mutations when using AZA/VEN compared to intensive chemotherapy. A significant survival benefit was observed in older patients with RUNX1 mutations receiving AZA/VEN as well. Although retrospective in nature, these data suggest a preferential benefit of HMA/VEN in patients with RUNX1 and ASXL1 mutations and highlight the importance of mutational testing in treatment planning.
Targeted oral therapy combinations
The last 5 years has led to multiple targeted therapy approvals for patients with AML and mutations in IDH1 (ivosidenib [IVO], olutasidenib [OLU]), IDH2 (enasidenib [ENA]), and FLT3 (MIDO, gilteritinib [GILT]). We will briefly review the landmark studies regarding these agents and discuss the emerging roles for approved therapies (Table 2) as well as ongoing areas of investigation into their use.
Table 2.
Published studies of approved and investigational uses of FDA-approved and/or NCCN-recommended targeted therapies in AML
| Targeted therapy | AML study indication (Ref.) | Study arms | Median age (years) | N | CR (%) | CRc (definition, %) | mOS (months) | Final dose/schedule in study | FDA-approved use | NCCN-recommended use |
|---|---|---|---|---|---|---|---|---|---|---|
| IVO | R/R [87] | IVO, single arm | 67 | 125 | 21.6 | CR/CRh, 30.4 | 8.8 | 500 mg daily |
1. Frontline monotherapy for ND IDH1-AML if ≥ 75 years or unfit for IC 2. Frontline IVO/AZA for ND IDH1-AML if ≥ 75 years or unfit for IC 2. Monotherapy for R/R IDH1-AML |
1. Frontline monotherapy for IDH1-AML if ≥ 60 years and unfit for IC 2. Frontline IVO/AZA for IDH1-AML if ≥ 60 years old and unfit for IC 3. Maintenance monotherapy after low-intensity induction for IDH1-AML 4. Monotherapy for R/R IDH1-AML |
| ND [88] | IVO, single arm | 76.5 | 34 | 30.3 | CR/CRh, 42.4 | 12.6 | ||||
| ND [93] | IC/IVO, single arm | 62.5 | 60 | 68.3 | CR/CRi/CRp, 76.7 |
NR Estimated 12-month OS 78% |
500 mg daily starting day 1 of IC ± consolidation ± maintenance | |||
| ND [97] | AZA/IVO | 76 | 23 | 60.9 | CR/CRi/CRp, 69.6 |
NR Estimated 12-month OS 82.0% |
IVO 500 mg daily (day 1–28) AZA 75 mg/m2 daily (day 1–7) |
|||
| ND [98] | AZA/IVO vs. AZA/PBO | 76 vs. 75.5 | 72 vs. 74 | 47.2 vs. 14.9 | CR/CRi/CRp, 54.2 vs. 16.2 | 24.0 vs. 7.9 | ||||
| OLU | R/R [91] | OLU vs. AZA/OLU | 72 vs. 67 | 22 vs. 26 | 18 vs. 12 |
CR/CRp, 32 vs. 15 |
8.7 vs. 12.1 |
OLU 150 mg twice daily AZA 75 mg/m2 daily (day 1–7) |
1. Monotherapy for IDH1-AML | None |
| ND [91] | OLU vs. AZA/OLU | 72 vs. 67 | 4 vs. 13 | 0 vs. 54 |
CR/CRp, 0 vs. 54 |
8.8 vs. NR | ||||
| ENA | R/R [82, 83] | ENA, single arm | 68 | 214 | 19.6 | CR/CRi/CRp, 29.0 | 8.8 | 100 mg daily | 1. Monotherapy for R/R IDH2-AML |
1. Frontline monotherapy for IDH2-AML if ≥ 60 years and unfit for IC 2. Maintenance monotherapy after low-intensity induction for IDH2-AML 3. Monotherapy for R/R IDH2-AML |
| R/R [85] | ENA vs. Conventional care | 72 vs. 71 | 158 vs. 161 | 23.4 vs. 3.7 | CR/CRi/CRp, 29.7 vs. 6.2 | 6.5 vs. 6.2 | ||||
| R/R [100] | AZA/ENA, single arm | 64 | 19 | 27.7 | CR/CRi, 61.1 |
Not reported Estimated 12-month OS if treated in 1st relapse 75% vs. ≥ 2nd relapse 10% |
ENA 100 mg daily (day 1–28) AZA 75 mg/m2 daily (day 1–7) |
|||
| ND [86] | ENA, single arm | 77 | 39 | 17.9 | CR/CRi/CRp, 20.5 | 11.3 | 100 mg daily | |||
| ND [93] | IC/ENA, single arm | 63 | 93 | 54.9 | CR/CRi/CRp, 73.6 | 25.6 | 100 mg daily starting day 1 or day 8 of IC | |||
| ND [101] | AZA/ENA vs. AZA/PBO | 75 vs. 75 | 68 vs. 33 | 54.4 vs. 12.1 | CR/CRi/CRp, 63.2 vs. 30.3 | 22.0/22.3 |
ENA 100 mg daily (day 1–28) AZA 75 mg/m2 daily (day 1–7) |
|||
| ND [100] | AZA/ENA, single arm | 77 | 7 | 71.4 | CR/CRi, 100 |
NR Estimated 12-month OS for ND group was 83% |
||||
| MIDO | ND [114] | IC/MIDO, single arm |
Total (48.5) FLT3 mutant [46] |
Total [40] FLT3 mutant [13] |
Total [80] FLT3 mutant [92] |
Not reported |
Not reported Estimated 12-month OS for FLT3-mutant 85% |
50 mg BID on days 1–7 and days 14–21 (concomitant) or days 8–21 (sequential) of IC ± consolidation ± maintenance | 1. MIDO + IC, re-induction, and consolidation for FLT3-TKD or ITD AML if eligible for IC | 1. MIDO + IC, re-induction, and consolidation for FLT3-TKD or ITD AML if eligible for IC and with intermediate/poor-risk cytogenetics |
| ND [118] | IC/MIDO vs. IC | 47.1 vs. 48.6 | 360 vs. 357 | 58.9 vs. 53.5 | Not reported | 74.7 vs. 25.6 | 50 mg BID on days 8–21 of IC ± consolidation ± maintenance | |||
| ND [121, 122] | IC/MIDO, single arm | 54.1 | 440 | 37 | CR/CRi, 74.9 | 36.2 | 50 mg BID on days 8 of IC continuously until 48 h prior to next cycle ± consolidation ± maintenance | |||
| GILT | R/R [117] | GILT vs. Conventional care | 62.0 vs. 61.5 | 247 vs. 124 | 21.1 vs. 10.5 | CR/CRi/CRp, 54.3 vs. 24.8 | 9.3 vs. 5.6 | 120 mg daily | 1. GILT monotherapy for R/R FLT3-TKD or ITD AML | 1. GILT monotherapy for R/R FLT3-TKD or ITD AML |
| R/R [135] | VEN/GILT, single arm | 63 | 56 | 18 | CR/CRi/CRp, 39 | 10.0 |
GILT 120 mg daily VEN 400 mg daily |
|||
| SORA | R/R [130] | AZA/SORA, single arm | 64 | 43 | 16 | CR/CRi, 43 | 6.2 |
SORA 400 mg BID (days 1–28) AZA 75 mg/m2 daily (day 1–7) |
None |
1. SORA + AZA or DAC + for R/R FLT3-ITD AML 2. Frontline SORA + AZA or DAC for FLT3-ITD AML if unfit for IC 3. SORA + AZA or DAC + SORA maintenance after low-intensity induction for FLT3-ITD AML 4. SORA maintenance after alloHSCT in FLT3-ITD AML |
| ND [136] | AZA/SORA, single arm | 74 | 27 | 26 | CR/CRi/CRp, 70 | 8.3 | ||||
| ND [144, 145] | IC/SORA vs. IC | 50 vs. 50 | 134 vs. 133 | 60 vs. 59 | Not reported |
NR vs. NR 5-year OS 61% vs. 53% |
400 mg BID on days 10–19 of IC ± consolidation ± maintenance | |||
| MAIN [146] | SORA vs PBO | 54.2 vs. 53.6 | 43 vs. 40 | N/a | N/a |
NR vs. NR Estimated 2-year OS 90.5% vs. 66.2% |
400 mg BID | |||
| MAIN [148] | SORA vs PBO | 35 vs. 35 | 100 vs. 102 | N/a | N/a |
NR vs. NR Estimated 2-year OS 82.1 vs. 68.0 |
CR Complete remission, CRc Composite complete remission, CRi Complete remission with incomplete hematologic recovery, CRp Complete remission with incomplete platelet recovery, CRh Complete response with partial hematologic recovery, mOS Median overall survival, FDA Food and Drug Administration, NCCN National Comprehensive Cancer Network, R/R Relapsed/refractory, ND Newly diagnosed, MAIN Maintenance, alloHSCT Allogeneic stem cell transplant, IVO Ivosidenib, OLU Olutasidenib, ENA Enasidenib, MIDO Midostaurin, GILT Gilteritinib, SORA Sorafenib, AZA Azacitidine, VEN Venetoclax, IC Intensive induction chemotherapy, PBO Placebo, NR Not reached, BID Twice daily
IDH1 and IDH2-mutant AML
IDH1 and IDH2 mutations are reported at a frequency of 7–14% and 8–19%, respectively, in AML [16, 79]. Mutations in these genes typically occur in the conserved arginine residues (IDH1R132, IDH2R140, and IDH2R172) of the catalytic domain of isocitrate dehydrogenase. The prognostic implications of IDH1/IDH2 mutations are not entirely clear. Some reports have suggested that IDH2 mutations are associated with better outcomes while IDH1 mutations confer worse outcomes [80, 81], though there is significant heterogeneity in the prognostic impact of co-mutations such as NPM1 [16, 81].
In a phase 1/2 study, patients with R/R AML harboring IDH2R140 or IDH2R172 mutations (mean age 67 years) were treated with ENA [82, 83]. Patients with IDH2R140 mutations had 2-HG reductions greater than 90% regardless of response, while 2-HG levels in those with IDH2R172 mutations correlated with response (82.0% reduction from baseline if CR, 44.3% reduction from baseline if non-CR response, and 38.4% reduction from baseline if no response). CR/CRi/CR with incomplete platelet recovery (CRp) was observed in 29% of patients. Median OS for all patients was 8.8 months but extended to 22.9 months in patients achieving CR. While reduction in mutant IDH2 VAF was not required for response, follow-up data demonstrated that clearance of IDH2-mutated clones was associated with 100% CR [83]. Furthermore, co-occurring mutations in NRAS or MAPK pathway were suggested to contribute to treatment resistance [84]. In a phase 3 randomized study from the BEAT AML Master trial, older patients with IDH2-mutant R/R AML were randomized to ENA or conventional care [85]; patients in the ENA arm had a doubling of event-free survival (EFS) and significant improvements in CR/CRi/CRp rates and hematologic response. The presence of DNMT3A co-mutations has been shown to be associated with CR, and no deleterious effects of RAS signaling pathway co-mutations were observed; however, the presence of ≥ 4 co-mutations decreased the overall response rates (ORR) significantly (27.3% compared to 47.1% with < 4 co-mutations) [86]. Thus, although ENA only has an FDA-approval label for R/R AML with IDH2 mutations, the NCCN guidelines provide a recommendation to consider frontline ENA use for patients older than 60 years who are not candidates for intensive remission induction [45].
In a phase 1 study, DiNardo et al. assessed the use of IVO in IDH1-mutant R/R AML [87]. The median age of patients was 67 years, and CR/CRp rates were 30.4% with a median DOR of 8.2 months. Median OS was 8.8 months with an 18-month OS rate of 50.1% in patients with CR/CRp. It was noted that patients with a lower co-mutational burden had improved CR/CRp rates, but no specific predictive co-mutations were identified. As a follow-up to this study, 34 patients with ND AML and IDH1 mutations (median age 76.5 years) received IVO in the frontline setting [88]. The composite CR (CRc) rate was 42.4% with over 60% of patients maintaining CRc at 1 year. Patients who received prior HMA therapy for antecedent hematologic disorder achieved CRc approximately half as frequently as those without prior HMA. Receptor tyrosine kinase (RTK) pathway mutations were observed in 36.8% of patients who did not achieve CRc compared to no patients who achieved CRc. IDH1 mutant clone clearance was reported in 64.3% of patients who achieved CRc and was not observed in any patients without CRc. These two studies ultimately led to the approval of IVO in both R/R AML with IDH1 mutations and ND AML with IDH1 mutations in patients who are ineligible for standard chemotherapy.
On December 1, 2022, the FDA approved another IDH1 inhibitor, OLU [89], for use in R/R AML based on a phase 1/2 trial of patients with IDH1-mutant R/R AML who were naïve to IDH1 inhibitors [90]. Patients with a median age of 71 years were treated with OLU until progression. Notably, CR/CRh rates were 35% and were achieved at a median of 1.9 months. Responses appeared durable with a median DOR of 25.9 months in patients who achieved CR/CRh. Prior VEN exposure did not appear to decrease response efficacy. Survival data will require further maturation. Another phase 1/2 study examining OLU with or without AZA in ND and R/R AML demonstrated similar CR/CRh rates in R/R AML patients treated with monotherapy while responses were surprisingly worse when combined with AZA [91]. While the role of OLU is not clear considering experience with using IVO, one compelling scenario could be in the setting of IVO resistance through IDH1 mutations, though this is solely based on preclinical data [92] with no current data on response rates after prior IVO exposure.
Recent studies have evaluated the use of IVO or ENA in combination with other frontline therapies in ND AML. A phase 1 study assessed the use of IVO or ENA in combination with 7 + 3 (or bioequivalent dose of idarubicin) in patients with IDH1/IDH2 mutations [93]. Patients received IVO or ENA throughout induction, consolidation, and maintenance, though IVO or ENA were discontinued in patients who underwent alloHSCT. CR/CRi/CRp rates were 72% for IVO and 63% for ENA at the end of induction, which is slightly better compared to historical controls with IDH1/2 mutations [94]. In an updated analysis [95], the authors reported CR/CRi/CRp rates of 78.3% in the IVO subgroup and 73.6% in the ENA subgroup. Responses for the sAML subgroup were improved if there was no prior HMA exposure, consistent with previously reported data [87]. Co-mutations did not impact response rates in the IVO cohort, but in the ENA cohort, co-mutations with ASXL1, NRAS, U2AF1, and TP53 were associated with worse response rates, while DNMT3A co-mutations were associated with marginally improved CR/CRi/CRp. When assayed by digital polymerase chain reaction (dPCR), 39% of patients treated with IVO cleared IDH1-mutant clones and 23% of patients treated with ENA cleared IDH2-mutant clones. Approximately half of the patients receiving IVO or ENA ultimately proceeded to alloHSCT. The use of IVO or ENA in combination with induction therapy was tolerable and ultimately did not significantly impact the time to recovery of the absolute neutrophil count or platelet count. An unanswered question that will require further investigation is the role of maintenance IVO or ENA after alloHSCT, particularly in patients who are unable to clear their mutant clone prior to transplantation. In patients eligible for intensive chemotherapy, the improved response rates compared to historical controls with the addition of IVO or ENA [93, 95] provides a compelling argument for this practice. However, in the absence of randomized head-to-head comparison, this combination is neither FDA-approved nor recommended by the NCCN guidelines [45].
The majority of IDH1/2 mutations have been shown to be exquisitely sensitive to the combination of AZA/VEN with response rates similar to or higher than those achieved with standard induction. Therefore, it is reasonable to consider low-intensity therapy in this patient population without compromising outcomes. In a pooled analysis of from VIALE-A [48] and a phase 1b HMA/VEN study [70], the patients with IDH1/2 mutations achieved CR/CRi rates of 79% with AZA/VEN compared with 11% with AZA alone [96]. Median DOR was 29.5 months and 9.5 months, respectively, and median OS was 24.5 months and 6.2 months, respectively. CR/CRi and OS were relatively better with IDH2 mutations compared to IDH1.
The combination of AZA with IVO has recently emerged as an alternative non-intensive treatment option [97]. The AGILE study was a phase 3 trial randomizing patients with ND IDH1-mutant AML ineligible for intensive chemotherapy to receive AZA/IVO or AZA/placebo [98]. At a median follow-up of 12.4 months, EFS was significantly longer in the AZA/IVO group compared to AZA/placebo with an estimated 12-month EFS of 37% and 12%, respectively. As a secondary endpoint, the median OS was 24 months for AZA/IVO and 7.9 months for AZA/placebo. CR/CRp rates were 53% with AZA/IVO compared to 18% with AZA/placebo, and DOR was longer for AZA/IVO compared to AZA/placebo (22.1 months versus 9.2 months). Patients with RTK pathway mutations (FLT3, KIT, NRAS, KRAS, PTPN11) and TP53 mutations were more likely to respond to AZA/IVO, and follow-up data suggest that relapse appears to preferentially occur with the acquisition of secondary high-risk mutations, independent of IDH1 [99]. The findings of this study led to the recent FDA approval of AZA/IVO for the frontline treatment of patients with ND IDH1-mutant AML. While the rate of differentiation syndrome in these patients approaches 20%, the rate of cytopenias compared to AZA/VEN is significantly less. Therefore, when thinking about the various options for patients with IDH1 mutations, toxicity, quality of life, and sequencing of treatment should be considered.
The combination of AZA/ENA has also been studied in patients with R/R [100] and ND AML with IDH2 mutations ineligible for intensive chemotherapy [100, 101]. In the phase 2 analysis of patients with ND AML, patients were randomized to AZA/ENA or AZA monotherapy [101]. The median age of patients was 75 years with CR/CRi/CRp rates of 63% with the AZA/ENA group compared to 30% with AZA alone; similar ORR were seen regardless of R140 or R172 mutations. Both ORR and CR were more durable with AZA/ENA compared to AZA (24.1 months versus 9.9 months and not reached versus 12.7 months, respectively). In a 2-year post hoc analysis, median EFS with AZA/ENA was 15.7 months compared to 11.9 months with AZA alone and OS was 22 months with AZA/ENA compared to 18.6 months with AZA alone; while the survival differences were not statistically significant, this study was not powered to detect significant differences in survival outcomes.
A major question remains as to whether therapies such as AZA/IVO or AZA/ENA would outperform AZA/VEN for ND IDH1/2-mutant AML. The lack of survival advantage with AZA/ENA compared to AZA monotherapy [101] would suggest that AZA/VEN is superior in IDH2 mutations with the caveat that the median OS of patients with AZA in this study was significantly longer than reported for patients with IDH2 mutations in the AZA/VEN studies [96]. For patients with IDH1 mutations, CR rates appear to be better with AZA/VEN [96] than with AZA/IVO [97], though median OS was essentially the same. At present, the widespread availability of AZA/VEN and its ability to bridge to alloHSCT favors its use in the frontline setting for patients with IDH1/2 mutations who are ineligible for intensive chemotherapy, thereby preserving IVO, OLU, or ENA in the case of R/R disease. However, AZA/IVO could be considered in patients who are at high risk of complications with the myelosuppression of AZA/VEN. While ENA and IVO monotherapy are both NCCN-recommended options for frontline therapy [45], only IVO is approved in this setting, and these should only be considered in patients with a very poor performance status. An area for future study will be triplet therapies. In an exploratory study of patients with treatment-naïve and R/R AML with IDH1 mutations [102], patients who received AZA/VEN/IVO had a CRc rate of 85–100% depending on dose intensity; however, this compares similarly to CRc rates of 67–100% with IVO/VEN alone. Nevertheless, the use of a triplet regimen improved MRD to 86% from 25% with doublet therapy. Studies combining different permutations of ENA, OLU, or IVO with HMA and/or VEN are currently underway (NCT04092179, NCT03471260, NCT04774393, NCT02719574) as are studies of these agents in the maintenance setting (NCT05010772, NCT03728335, NCT03564821, NCT03515512, NCT04522895).
FLT3-mutant AML
FLT3 encodes a type 3 RTK (FMS-like tyrosine kinase 3) and is widely expressed on AML blasts [103]. Mutations in FLT3 are seen in about 25–32% of cases of ND AML with 25% harboring ITDs and 7–10% harboring TKD mutations [104, 105]. Previous reports prior to the era of FLT3-targeting TKIs (henceforth referred to as FLT3i) have suggested that AR of FLT3-ITD and the presence of NPM1 co-mutations variably affect outcomes [106–108]. Nevertheless, recent data suggest that relapse risk is higher in patients with FLT3-ITD AML irrespective of AR or presence of NPM1 mutation, and these patients should be considered for alloHSCT in first remission (CR1) if eligible [109, 110]; this is reflected in the updated ELN recommendations [24]. FLT3i can be divided into Type I and Type II inhibitors [111], which are active against both ITD and TKD mutations or ITD only, respectively. The first approved FLT3i in AML was the Type I staurosporine-derived inhibitor MIDO [112, 113] with early reports of its synergy with chemotherapy in patients with ND FLT3-mutant AML [114]. Newer and more selective FLT3i, GILT (Type I) and quizartinib (QUIZ) (Type II), have demonstrated promising responses in patients with R/R AML [115–117].
The RATIFY trial [118] was a randomized, placebo-controlled phase 3 trial investigating the addition of MIDO to standard induction chemotherapy and high-dose cytarabine (HiDAC) consolidation in adult patients under the age of 60 with ND AML and FLT3 mutations (TKD or ITD). CR rates were similar between both groups, though median EFS and OS were significantly improved with MIDO (8.2 months versus 3 months and 74.7 months versus 25.6 months, respectively). OS was durable with 51.4% of patients in the MIDO group surviving at 4 years. More patients underwent alloHSCT in CR1 with MIDO compared to placebo (28% versus 23%, respectively); notably, follow-up studies after the publication of the RATIFY trial demonstrated deeper molecular remission with the addition of FLT3i to induction therapy [119, 120], perhaps explaining in part the durable differences in OS despite similar CR and EFS rates.
Recent findings from a phase 2 study have also established the efficacy of adding to MIDO to induction, HiDAC consolidation, and maintenance in patients up to the age of 70 with ND FLT3-mutant AML [121, 122]. It should be noted that at present, MIDO is not currently approved as monotherapy and therefore is not recommended for post-consolidation maintenance given minimal benefit demonstrated after alloHSCT [36, 118, 121–123]. Preliminary data from a phase 1 study (NCT02236013) evaluating GILT in combination with 7 + 3, consolidation, and maintenance in ND AML [124] noted a median OS of 35.8 months with CRc achieved by 81.8% of all patients. AlloHSCT was performed in 30.4% of all patients. These data have led to ongoing clinical trials of GILT versus MIDO in addition to induction chemotherapy and consolidation (NCT04027309, NCT03836209).
GILT is the only FDA-approved FLT3i for use in R/R AML with FLT3 mutations. The ADMIRAL study was a phase 3 randomized control trial of patients with R/R AML and FLT3-mutations who received GILT or salvage chemotherapy [117]. Similar rates of prior FLT3i exposure were noted in both arms, and approximately 20% of patients in either group had previously undergone alloHSCT. Median OS for patients receiving GILT was 9.3 months versus 5.6 months for those receiving salvage chemotherapy. [125]. CRc rates were 54.3% with GILT and 24.8% with chemotherapy, and the median DOR was 11 months in the GILT group. Median OS for the FLT3-ITD and FLT3-TKD groups that received GILT were 9.3 months and 8 months, respectively. An important aspect of this study was the efficacy in both FLT3-ITD and FLT3-TKD populations, as the latter has been demonstrated to confer secondary resistance to type II FLT3i [126].
Given the increasing use of low-intensity regimens in AML, pooled data from VIALE-A [48] and a phase 1b HMA/VEN study [70] showed that patients with FLT3-ITD had a CR/CRi rate of 63% with AZA/VEN and a median OS of 9.9 months, while those with FLT3-TKD had a CR/CRi rate of 77% and a median OS of 19.2 months [127]. Of patients with FLT3 mutations, approximately 36% had NPM1 mutations in each of the AZA/VEN and AZA groups. Of those with concurrent FLT3-ITD and NPM1 mutation, AZA/VEN conferred a CR/CRi rate of 70% and a median OS of 9.1 months; patients with FLT3-ITD and wild-type NPM1 had a median OS of 10.6 months. This study has two important takeaways for older patients with mutated FLT3. First, the rate of FLT3 mutations in this population was lower, and the patients were older than typical FLT3-driven AML seen in younger patients, possibly suggesting different disease kinetics and biology. Second, while CR/CRi rates were worse for patients with FLT3-ITD mutations with wild-type NPM1 compared to those with mutated NPM1, the overall survival did not differ significantly, suggesting that NPM1 status has an unclear prognostic value for patients treated with HMA/VEN.
Trials combining GILT [128] or MIDO [129] with HMA have not yielded encouraging results to date, though early data suggest that AZA/sorafenib (SORA) may be effective in patients with R/R AML and FLT3-ITD [130]. Data demonstrating the efficacy and tolerability of DAC/SORA in patients with R/R AML with FLT3-ITD [131] have led to NCCN recommendations for the use of AZA/SORA or DAC/SORA as low-intensity therapy in elderly patients with FLT3-ITD AML or in R/R AML with FLT3-ITD [45], though it does not carry FDA approval for these indications. Despite the modest benefit with HMA, there seems to be synergy between FLT3i and VEN [132–134]. A phase 1b study for VEN/GILT enrolled patients with FLT3-wild-type or FLT3-mutant (dose escalation) and FLT3-mutant (dose expansion) R/R AML [135]. The median age of patients was 63 years, 31% of whom had received prior alloHSCT and 16% of whom received prior VEN. No patients had previously received GILT, though 64% of patients with FLT3 mutations had received other prior FLT3i. Patients with FLT3-ITD had CR/CRi/CRp rates of 43%, while those with FLT3-TKD has rates of 33%, and response rates were slightly better in those who were FLT3i-naïve. Median OS was 10 months for all FLT3-mutated patients, though there was a significant improvement in those who had undergone alloHSCT after VEN/GILT (not reached) compared to those who did not receive alloHSCT (6.3 months).
Furthermore, a phase 2 trial evaluated the use of triplet therapy (DAC/VEN/FLT3i) in older patients with ND FLT3-mutant AML and all adult patients with R/R FLT3-mutant AML. In ND AML, the CRc rate was 92% with high rates of 91% MRD negativity in responders by PCR. In patients with R/R AML, CRc rates were 63% with MRD negativity by PCR in all patients who responded. At a median follow-up of 14.5 months, the median OS was not reached in ND patients (2-year OS estimated at 80%); the median OS in R/R patients was 6.8 months. Approximately one-third of patients underwent alloHSCT in either group. These results compare favorably to other reports of FLT3i/HMA in the ND setting [128, 131, 136], though CRc rates appear to be higher with VEN/GILT in patients with R/R AML [135].
Although not approved, two other FLT3i deserve mention given recent reports of their efficacy in AML. QUIZ is a second-generation type I FLT3i that can achieve significant marrow remissions in R/R FLT3-mutant AML [116, 137–139], though survival advantage was minimal compared to salvage chemotherapy in the phase 3 QuANTUM-R study [138]. Due to these underwhelming results and concerns about cardiotoxicity and increased myelosuppression compared to other FLT3i, QUIZ has not been approved in the USA or Europe, though it is approved for use in Japan. In the frontline setting, the phase 3 QuANTUM-FIRST (NCT02668653) trial [140] enrolled patients up to age 75 with ND AML and FLT3-ITD and randomized them to QUIZ or placebo in addition to induction therapy with 7 + 3. Patients who achieved CR/CRi received up to 4 cycles of HiDAC with QUIZ or placebo and/or alloHSCT followed by up to 3 years of maintenance therapy with QUIZ or placebo. CR/CRi rates were 71.6% and 64.9% in the QUIZ and placebo arms, respectively, with DOR of 38.6 months and 12.4 months, respectively. Median OS and RFS were 31.9 months versus 15.1 months and 39.3 months versus 13.6 months in the QUIZ and placebo arms, respectively. AlloHSCT was performed in CR1 at similar rates between both arms; when censored for alloHSCT, OS trended toward a benefit with QUIZ over placebo. Moreover, an updated report from the study found that QUIZ conferred a deeper molecular remission compared to the placebo arm, perhaps underscoring the durability of benefit [141]. Although RATIFY [118] had already demonstrated a benefit to the addition of MIDO to induction chemotherapy, the QuANTUM-FIRST study is unique in that it evaluates the addition of an FLT3i for the higher-risk FLT3-ITD mutation.
Lastly, a type I FLT3i emerging in clinical discussion is crenolanib (CREN). Long-term data were recently reported regarding the use of CREN in combination with 7 + 3 in adult patients with FLT-mutant ND AML [142]. CREN maintenance was offered up to 1 year after HiDAC or alloHSCT. The median age of patients enrolled was 57 years, 34% of which were over the age of 60 years. FLT3 mutations were 75% ITD, 18% TKD, and 7% both ITD and TKD. CR/CRi rates above 80% were reported across several subgroups including those with FLT3-ITD mutations or concomitant FLT3/DNMT3A/NPM1 mutations. MRD-negative CR/CRi was achieved in 94% of evaluable patients, and 50% of patients underwent alloHSCT. Median OS has not been reached at a median follow-up of 45 months. Furthermore, translational studies found that no FLT3 mutant clones were found at relapse in patients who completed protocol therapy.
In considering the role of FLT3i in ND FLT3-mutant AML, intensive induction chemotherapy plus MIDO remains a standard of care for eligible patients. However, the formal release of data from QuANTUM-FIRST is awaiting, and ongoing trials will assess other frontline combinations with QUIZ (NCT04209725, NCT04047641), CREN (NCT03258931), and GILT (NCT04027309, NCT03836209), including head-to-head comparisons against MIDO. If QUIZ is approved for ND AML, it should be emphasized that its use would be limited to patients with FLT3-ITD, while those with TKD mutations should still receive MIDO. For patients who are ineligible for intensive chemotherapy, AZA/VEN is effective for those with FLT3-TKD mutations; unfortunately, better frontline options for those with FLT3-ITD are currently limited. Nonetheless, GILT remains a very active FLT3i in the relapsed setting, and early data from doublet and triplet FLT3i combinations are encouraging in ND and R/R AML [143]. Several trials exploring triplet combinations with DAC/VEN/QUIZ (NCT03661307) and AZA/VEN/GILT (NCT04140487) are currently enrolling with results highly anticipated.
Maintenance therapy and consideration of treatment-free remissions
While the historical focus of induction therapy in AML is to achieve remission and proceed with transplantation in eligible patients, the emergence of tolerable oral therapies posits the role of maintenance therapy, particularly in patients with a higher risk of relapse, such as those with pre-transplantation MRD positivity. There are limited data to support the use of targeted therapies as maintenance after transplant except for SORA. Although SORA has limited efficacy in the frontline setting [144, 145], the SORMAIN trial evaluated SORA maintenance in patients with FLT3-ITD after alloHSCT [146]. The investigators noted a 25–30% absolute improvement in 2-year RFS and OS compared to the placebo. Patients treated with SORA had higher rates of GVHD and skin toxicity, consistent with previous reports about its immunogenicity [147]. These results have since been corroborated by another phase 3 study of SORA maintenance post-alloHSCT in CR1 for patients with FLT3-ITD [148]; thus, while SORA does not have an FDA label indication for use in the treatment of ND or R/R FLT3-mutant AML, it is recommended for use in patients with FLT3-ITD who achieve remission after alloHSCT [45]. While no other agents are currently recommended for post-alloHSCT maintenance, the MORPHO trial (NCT02997202) is evaluating the efficacy of GILT versus placebo in this setting for patients with AML and FLT3 mutations and has completed enrollment.
For patients in remission after induction therapy but unfit for transplant, maintenance options have been limited in the absence of FLT3-ITD. The QUAZAR AML-001 study [149] randomized patients who had achieved CR/CRi after intensive chemotherapy but were not fit for alloHSCT to receive oral azacitidine (CC-486) maintenance or placebo. Median OS after randomization was longer in patients receiving CC-486 compared to placebo (24.7 months versus 14.8 months), though it should be noted that only 14% of patients had adverse-risk cytogenetics. Indeed, studies have demonstrated disease-free survival (DFS) but no OS benefit with subcutaneous AZA maintenance in older patients in CR/CRi after induction [150] or with CC-486 in patients with adverse-risk cytogenetics after alloHSCT [151]. Combining VEN with AZA may improve these outcomes based on preliminary data from a phase 2 study (NCT04062266), particularly in those who received VEN-based induction [152]. Furthermore, the VIALE-M study (NCT04102020) is also investigating the role of CC-486 in combination with VEN for patients with CR1 after induction, and the VIALE-T study (NCT04161885) is assessing AZA/VEN maintenance after alloHSCT.
With several options for low-intensity maintenance therapies, the question remains as to whether treatment needs to be indefinite. A retrospective study reported their experience with discontinuing HMA/VEN or LDAC/VEN in transplant-ineligible patients older than 65 years who achieved CRc after receiving either combination for at least 12 months in the frontline setting [153]. Patients who stopped therapy experienced a median treatment-free remission (TFR) of 45.8 months with over half still in remission at end of data collection. No significant differences in RFS or OS were noted between cohorts. One caveat is that patients were highly selected; the vast majority of patients achieved true CR with MRD negativity at the time of discontinuation. Moreover, of patients who sustained treatment-free remission, 86% had a prior NPM1 or IDH2 mutation with MRD negativity at cessation. Consequently, in this specific population, MRD negativity may be a reasonable impetus to interrupt treatment, though prospective studies formally evaluating this question are warranted.
Investigational agents and future directions
The aforementioned studies emphasize an increasingly nuanced approach to treatment decision-making in AML as molecular data such as types of mutations (i.e., FLT3-ITD versus FLT3-TKD) and co-mutational patterns (i.e., IDH1/2 with DNMT3A or RTK pathway mutations) have important prognostic and treatment implications. Although TP53-mutant myeloid neoplasms remain one of the largest unmet needs in care, it is encouraging that detailed mechanistic studies may open the door to the development of further targeted therapies. Moreover, with the adoption of genomic data into routine care for patients with AML [18, 154], larger population-based studies will hopefully improve the personalization of treatments. Below we briefly highlight investigational agents (Fig. 4, Table 3), with a focus on those in later clinical development.
Fig. 4.
Mechanisms of novel targeted therapies in AML in later-stage clinical development. A Mutations in TP53 lead to altered conformation of p53 leading to a subtype of AML characterized by treatment resistance, high relapse rates, and poor overall survival. Novel agents such as eprenetapopt are metabolized into MQ which covalently modifies the mutant p53 protein leading to a wild-type-like conformational change and restoration of normal p53 activity [155]. Recent reports have demonstrated that MQ can drive p53-independent cell death through ROS accumulation [156, 157] and ferroptosis [158]. B Leukemic cells can evade immune surveillance by upregulation of CD47, which binds SIRPa; this emits a “don’t eat me signal” to macrophages [163–165]. Antibodies targeting CD47 can block this inhibitory signal and allow for phagocytosis of leukemic cells [165–167]. C Certain types of AML are characterized by mutated NPM1c or oncogenic fusion partners associated with MLL [173, 174, 179]. These lead to complex formation with menin and LEDGF, ultimately resulting in transcriptional activation of leukemia stem cell promoting genes [175–177]. Blocking this pathway with menin inhibitors such as KO-539 or SNDX-5613 can repress this transcriptional program allowing for differentiation of granulocytes [178, 180, 181]. AML Acute myeloid leukemia, MQ Methylene quinuclidinone, ROS Reactive oxidative species, SIRPa Signal regulatory protein alpha, NPM1c Cytoplasmic NPM1 (mutant NPM1), MLL Histone lysine N-methyltransferase 2A (KMT2A), LEDGF Lens epithelium-derived growth factor, MI Menin inhibitor
Table 3.
Investigational agents in clinical development for AML and/or MDS
| Class/Pathway | Investigational agent | Alternative names | Proposed MOA/Target | AML/MDS trials |
|---|---|---|---|---|
| FLT3 inhibitor | Quizartinib | Type I FLT3 inhibitor | NCT04107727, NCT03135054, NCT04493138, NCT04128748, NCT03735875, NCT02668653, NCT01892371, NCT03661307, NCT03793478, NCT04047641, NCT04687761, NCT04112589, NCT02984995, NCT03723681, NCT02039726, NCT02834390, NCT01565668, NCT02675478, NCT00462761, NCT01468467, NCT01390337, NCT00989261, NCT01411267 | |
| Crenolanib | Type I FLT3 inhibitor | NCT03258931, NCT02400255, NCT03250338, NCT01657682, NCT01522469, NCT02400281, NCT02626338, NCT02283177, NCT02270788 | ||
| Ponatinib | Type I FLT3 inhibitor | NCT02428543, NCT03690115 | ||
| Luxeptinib | CG-806 | Type I FLT3 inhibitor | NCT04477291 | |
| HM43239 | Type I FLT3 inhibitor | NCT03850574 | ||
| FF-10101 | Type I FLT3 inhibitor | NCT03194685, NCT02193958 | ||
| IDH inhibitor | LY3410738 | IDH1/2 inhibitor | NCT04603001 | |
| BCL2/MCL1 | S65487 | VOB560 | BCL2 inhibitor | NCT04742101, NCT03755154, NCT04702425 |
| S55746 | BCL201 | BCL2 inhibitor | NCT02920541 | |
| AMG 176 | MCL1 inhibitor | NCT05209152, NCT02675452 | ||
| AZD5991 | MCL1 inhibitor | NCT03013998 | ||
| S64315 | MIK665 | MCL1 inhibitor | NCT04629443, NCT03672695, NCT02979366, NCT02992483, NCT04702425 | |
| Mutant P53 | Eprenetapopt | APR-246 | Mutant p53 reactivation, cellular redox modification, ferroptosis | NCT03931291, NCT04214860, NCT03745716, NCT03072043, |
| Epigenetic pathways | Ziftomenib | KO-539 | Menin-MLL inhibitor | NCT04067336 |
| Revumenib | SNDX-5613, VTP50469 | Menin inhibitor | NCT05406817, NCT05326516, NCT04065399, NCT05360160, NCT03013998 | |
| Pinometostat | EPZ5676 | DOT1L inhibitor | NCT03701295 | |
| Iadademstat | ORY-1001 | KDM1A inhibitor | NCT05546580 | |
| Entinostat | SYNDX-275, MS-275 | HDAC1/3 inhibitor | NCT00101179, NCT00313586, NCT00462605, NCT00015925, NCT02936752 | |
| Other oncogenic pathways | Pevonedistat | TAK-924, MLN4924 | NEDD8 activator | NCT03459859, NCT03772925, NCT04266795, NCT03813147, NCT03268954 |
| Entospletinib | GS-9973 | Syk inhibitor | NCT05020665, NCT03013998 | |
| Olaparib | PARP inhibitor | NCT03953898 | ||
| Milademetan | DS-3032, RAIN-32 | MDM2/HDM2 inhibitor | NCT03671564, NCT03634228 | |
| Siremadlin | HDM201 | MDM2/HDM2 inhibitor | NCT05447663, NCT05155709 | |
| Uproleselan | GMI-1271 | E-selectin antagonist | NCT04964505, NCT05569512, NCT05054543, NCT04839341, NCT04848974, NCT03616470, NCT02306291 | |
| Tamibarotene | SY-1425 | Retinoic acid receptor alpha agonist | NCT04797780, NCT04905407, NCT02807558 | |
| JNJ-74856665 | Dihydroorotate dehydrogenase inhibitor | NCT04609826 | ||
| PRT543 | PRMT5 inhibitor | NCT03886831 | ||
| H3B-8800 | RVT-2001 | Splicing modulator | NCT0281540 | |
| Emavusertib | CA-4948 | IRAK4 inhibitor | NCT04278768, NCT05178342 | |
| Targeted Immune inhibitors | Magrolimab | Hu5F9-G4, ONO-7913 | Anti-CD47 | NCT04313881, NCT05367401, NCT05079230, NCT04435691, NCT03248479, NCT04778397, NCT04778410, NCT02678338 |
| Lemzoparlimab | TJ011133, TJC4 | Anti-CD47 | NCT04202003, NCT04912063 | |
| Ipilimumab | Anti-CTLA4 | NCT01757639, NCT02890329, NCT03600155, NCT02397720, NCT03912064, NCT02846376, NCT00060372, NCT01822509, NCT02530463 | ||
| Nivolumab | Anti-PD-1 | NCT03417154, NCT02530463, NCT02464657, NCT03600155, NCT03358719, NCT03092674, NCT02846376, NCT02532231, NCT02397720, NCT01822509, NCT04913922, NCT03825367, NCT02275533, NCT04277442 | ||
| Pembrolizumab | Anti-PD-1 | NCT02996474, NCT02845297, NCT02708641, NCT03769532, NCT02768792, NCT02771197, NCT04284787, NCT04214249, NCT03969446, NCT04372706, NCT03761914, NCT03144245, NCT02981914, NCT03094637, NCT02936752, NCT01953692 | ||
| Spartalizumab | PDR001 | Anti-PD-1 | NCT03066648 | |
| Relatlimab | Anti-LAG3 | NCT04913922 | ||
| Sabatolimab | MBG453 | Anti-TIM3 | NCT04812548, NCT04623216, NCT04878432, NCT05367401, NCT05201066, NCT04150029, NCT03946670, NCT04266301 | |
| Decoy receptor | Evorpacept | ALX148 | Anti-CD47 | NCT04755244, NCT04417517 |
| ADC | IMGN632 | Anti-CD123 | NCT03386513, NCT04086264, NCT05320380 | |
| Cusatuzumab | ARGX-110 | Anti-CD70 | NCT04023526, NCT04241549, NCT04150887, NCT03030612 | |
| Vadastuximab | SGN-CD33A | Anti-CD33 | NCT02326584, NCT01902329 | |
| BITE/DART | AMV564 | CD33 x CD3 | NCT03144245, NCT03516591 | |
| AMG 427 | FLT3 x CD3 | NCT03541369 | ||
| Flotetuzumab | MGD006 | CD123 x CD3 | NCT05506956, NCT04158739, NCT04582864, NCT04681105 | |
| Vibecotamab | XmAB14045 | CD123 x CD3 | NCT02730312, NCT05285813 | |
| APVO436 | CD123 x CD3 | NCT03647800 |
A summary of agents without FDA approval or NCCN recommendation for the treatment of AML/MDS but under investigation for the treatment of AML and/or MDS is shown along with proposed mechanisms of action and clinical trials that are not yet recruiting, recruiting, enrolling, active but not recruiting, or completed. Terminated or suspended studies have been omitted. Cellular therapies (i.e., CAR T cells) are not shown. MOA Mechanism of action, AML Acute myeloid leukemia, MDS Myelodysplastic syndrome, ADC Antibody–drug conjugate, BITE Bispecific T-cell engager, DART Dual affinity retargeting protein
P53 reactivation
Eprenetapopt (APR-246) is a small-molecular inhibitor originally thought to work through covalent modification of mutated p53, which restores wild-type-like p53 conformation, thus functionally reactivating it [155]. However, its mechanism may also target other synergistic pathways which can drive p53-independent cell death including modulation of cellular redox [156, 157] and increasing glutathione turnover, leading to ferroptosis [158]. Two phase 2 studies are evaluating the use of this agent in combination with AZA in TP53-mutant MDS and AML [159, 160]. In these studies, CR rates of patients with MDS were 47–50% with durable responses. In patients with AML, however, CR was only 17%. Notably, responding patients in both studies had significant reductions in TP53 VAF. Median OS was 10.8–12.1 months for MDS patients and 13.9 months for AML patients. Notable toxicities were febrile neutropenia and neurologic toxicity. Recent data from a phase 2 study (NCT03931291) was presented, which assessed eprenetapopt/AZA as post-alloHSCT maintenance in patients with TP53-mutant MDS and AML [161]. Patients received a median of 7 cycles of treatment with a median RFS of 12.5 months and a median OS of 20.6 months. No 30-day mortalities were noted from the first dose. These results, while modest, are encouraging in this very high-risk population. Preclinical studies suggest that XPO1 upregulation may contribute to eprenetapopt resistance in AML and can be overcome with agents like selinexor, though this will require in vivo validation [162]. An additional trial combining eprenetapopt with AZA/VEN has completed enrollment and awaiting data release (NCT04214860). A phase 1 study of APR-548, which is a next-generation molecule, in combination with AZA (NCT04638309) had opened but was terminated by the sponsor.
CD47—targeting the “don’t eat me” signal
CD47 is a heavily glycosylated cell surface protein and is expressed by virtually all cells in the body, including those that do not express integrin, such as red blood cells [163]. It provides an anti-phagocytic signal in healthy cells but was discovered as an adverse prognostic factor in AML as it is overexpressed on leukemic stem cells compared to non-leukemic stem cells; preclinical murine models demonstrated that blockade of CD47 with monoclonal antibodies could enable phagocytosis of leukemic stem cells and prevent in vivo engraftment [164, 165]. These findings led to the development of a humanized anti-CD47 antibody known as Hu5F9-G4 or magrolimab [166]. A phase 1b study explored the tolerability of magrolimab in combination with AZA (NCT03248479) for patients with untreated intermediate- to very high-risk MDS and with untreated AML unfit for intensive chemotherapy [167]. TP53 mutations were noted in 27% of patients. Common adverse events included anemia, neutropenia, thrombocytopenia, and infusion reactions. In transfusion-dependent MDS and AML patients, 58% and 64% were able to achieve transfusion independence; moreover, CR/CRi rate was 56% in AML patients. The median duration of response was not reached in MDS, AML, or TP53-mutant AML subpopulations. Preliminary data from a phase 1b/2 study evaluating the combination of magrolimab/AZA/VEN (NCT04435691) noted CR/CRi rates of 63% and 86% in patients with ND AML with or without TP53 mutations, respectively, conferring 1-year OS rates of 53% and 83%, respectively [168]. In patients with R/R AML, median OS was only 7.4 months with responses especially limited in those with prior VEN exposure. Given these findings, particularly in the very high-risk TP53-mutant group, three phase 3 trials opened which are comparing magrolimab/AZA versus AZA in patients with untreated intermediate- to very high-risk MDS (NCT04313881), magrolimab/AZA versus AZA/VEN or intensive chemotherapy in patients with untreated TP53-mutant AML (NCT04778397), and magrolimab/AZA/VEN versus AZA/VEN in patients with untreated AML who are ineligible for standard intensive chemotherapy (NCT05079230). A phase 2 study of magrolimab with various anti-leukemic therapies in patients with untreated AML is enrolling as well (NCT04778410). An additional combination study of another anti-CD47 antibody, lemzoparlimab, had opened for patients with a higher-risk MDS or AML ineligible for intensive chemotherapy (NCT04912063) but was recently stopped.
Menin inhibition
Several types of AML have overexpression of HOXA/B cluster genes and MEIS1, which are critical regulators of hematopoietic stem cell self-renewal and differentiation [169–171]. These can be dysregulated with alterations of histone lysine N-methyltransferase 2A (KMT2A or MLL) and/or NPM1 mutations [172–174]. KMT2A binds menin as part of a histone methyltransferase complex, and when it is involved in an oncogenic fusion, it may lead to aberrant transactivation of leukemia-promoting genes [175–177]. While mechanistically unclear, cytoplasmic localization of the mutant NPM1 is associated with a similar phenotype and genetic signature as KMT2A-driven AML [173, 178, 179]. Consequently, several preclinical models examined the role of menin inhibition in these subtypes of AML and observed efficacy [178, 180, 181]. This has led to the opening of multiple phase 1/2 studies in patients with R/R AML with KMT2A-rearrangement or NPM1-mutation using the menin-MLL inhibitor KO-539 (NCT04067336) or menin inhibitor SNDX-5613 (NCT04065399, NCT05326516, NCT05406817, NCT05360160, NCT03013998) which are currently enrolling patients or opening soon. Early data from NCT04067336 and NCT04065399 suggest similar CR/CRh rates (25–30%) and MRD negativity rates in responding patients (75–78%) with either KO-539 or SNDX-5613, respectively [182–184]. Toxicities include QTc prolongation with SNDX-5613 and differentiation syndrome, particularly with KO-539. Of note, however, the activity of KO-539 appears largely restricted to patients with NPM1-mutated AML, in whom differentiation syndrome was not observed.
Other therapies in clinical development
In addition to the aforementioned therapies, several other pathways have been identified as possible therapeutic vulnerabilities in AML and are emerging in early clinical development [185, 186]. Some of these agents target epigenetic and oncogenic signaling pathways and may allow for the augmentation of available therapies. The ALICE study is evaluating the use of iadademstat, a lysine-specific demethylase 1 inhibitor, in combination with AZA for the frontline treatment of AML in patients unfit for intensive chemotherapy. Preliminary data suggest CR/CRi rates of 64% with median OS extending to 14.3 months in responding patients. Strikingly, 75% of patients with TP53 alterations responded [187]. There has also been a considerable interest in exploiting advances in immunotherapy and cellular therapy for the treatment of AML. Unfortunately, findings from early studies on these agents have been underwhelming thus far. Flotetuzumab is a dual affinity retargeting antibody that targets CD123 and CD3. A phase 1/2 study reported flotetuzumab could achieve CR/CRh/CRi rates of 30% with 12-month OS of 75% in patients with R/R AML [188] with recent data suggesting similar outcomes in pediatric and adolescent/young adult patients [189]. The majority of patients who benefit from flotetuzumab are primary refractory and TP53-mutated, which is related to a distinct immune microenvironment compared to non-TP53-mutated AML. An ongoing phase 1b/2 study is also evaluating the combination of pivekimab sunirine (IMGN632), a CD123-targeting antibody–drug conjugate, with AZA/VEN [190]. Patients with R/R AML treated with this were reported to have CRc rates of 31%, including 26% in those with ELN adverse disease and 64% of those with FLT3-ITD, with minimal additive myelosuppression beyond that of AZA/VEN. In regard to checkpoint inhibition, findings from the unpublished REMAIN trial did not demonstrate a PFS or OS benefit with the use of nivolumab maintenance in patients with CR/CRi who are ineligible for alloHSCT [191]. Nevertheless, immune dysregulation is important in AML, though it is not yet clear how to effectively target the tumor microenvironment or which factors can be used to predict response to immune-based therapies.
Conclusion
AML is a very complex and heterogeneous disease as evidenced by the expansion of genetic and cytogenetic qualifiers in the updated WHO [23] and ICC [24] classification systems. While outcomes for AML continually improve by decade, a refined understanding of patient and tumor characteristics is needed to continue this upward trend. This is especially true when selecting patients for intensive chemotherapy and/or alloHSCT, as a standardized and validated metric of physiologic age or fitness would greatly improve our ability to personalize treatments and design clinical trials more representative of the actual patient population. However, we also now have several active, low-intensity therapies approved or in the developmental pipeline. Perhaps, the most transformative has been the combination of AZA/VEN, which has supplanted conventional cytotoxic chemotherapy in many cases. Unfortunately, outcomes in patients who progress on HMA/VEN are poor, particularly in patients who harbor TP53 or RAS-pathway mutations [192], and clinical development of additional therapeutic options is critical. Moreover, several groups will soon be reporting data from trials investigating the addition of VEN to induction chemotherapy in both the ND and R/R AML setting to answer the question if there is any benefit to further intensification of therapy [193–196].
We are entering a unique era of precision oncology whereby molecularly informed data can be exploited to tailor treatments based on disease pathobiology. But while options have rapidly increased for patients IDH1/2 and FLT3 mutations, progress has unfortunately been slow for those with the highest risk forms of AML, such as TP53-mutant disease. Nevertheless, the incorporation of data such as co-mutational burden and MRD analysis will hopefully allow us to better define patients at the highest risk of relapse [197–199] and who may benefit from early relapse intervention [200–202]. Pertinent questions in this regard will be whether induction of deeper molecular remissions with combination therapies would improve outcomes compared to sequencing therapies, how we can minimize toxicity associated with combinations of novel agents, and if we can use MRD negativity to interrupt treatment.
The multitude of active studies addressing these questions will lend nuance to clinical practice and contribute to improved outcomes for patients.
Acknowledgements
Figure 4 is made using www.BioRender.com.
Abbreviations
- 2-HG
2-Hydroxyglutarate
- AlloHSCT
Allogeneic stem cell transplantation
- AML
Acute myeloid leukemia
- AR
Allelic ratio
- AZA
Azacitidine
- bZIP
Basic leucine zipper
- CR
Complete remission
- CR1
First remission
- CRc
Composite complete remission
- CREN
Crenolanib
- CRh
Complete response with partial hematologic recovery
- CRi
Complete remission with incomplete hematologic recovery
- CRp
Complete remission with incomplete platelet recovery
- DAC
Decitabine
- DOR
Duration of remission
- dPCR
Digital polymerase chain reaction
- EB2
Excess blasts-2
- EFS
Event-free survival
- ELN
European LeukemiaNet
- ENA
Enasidenib
- FDA
Food and drug administration
- FLT3i
FLT3 inhibitor
- GILT
Gilteritinib
- HCT-CI
Hematopoietic cell transplantation-specific comorbidity index
- HiDAC
High-dose cytarabine
- HMA
Hypomethylating agent
- ICC
International consensus classification
- ITD
Internal tandem duplication
- IVO
Ivosidenib
- KMT2A/MLL
Histone lysine N-methyltransferase 2A
- LDAC
Low-dose cytarabine
- MDS
Myelodysplastic syndrome
- MIDO
Midostaurin
- MRD
Measurable residual disease
- NCCN
National comprehensive cancer network
- ND
Newly diagnosed
- NGS
Next-generation sequencing
- OLU
Olutasidenib
- OS
Overall survival
- QUIZ
Quizartinib
- R/R
Relapsed/refractory
- RFS
Recurrence-free survival
- RTK
Receptor tyrosine kinase
- sAML
Secondary acute myeloid leukemia
- SORA
Sorafenib
- tAML
Therapy-related AML
- TFR
Treatment-free remission
- TKD
Tyrosine kinase domain
- TKI
Tyrosine kinase inhibitor
- VAF
Variant allelic frequency
- VEN
Venetoclax
- WHO
World health organization
Author contributions
RSB and CL conceived the organization and scope of the review and participated in writing the manuscript. KWP provided key insights into manuscript preparation and organization. RSB, KWP, and CL read and agreed to the published version of the manuscript. All authors read and approved the final manuscript.
Funding
RSB is supported by the Hematopoiesis Training Program grant at the University of Pennsylvania (T32DK07780) from the National Institutes of Health (NIH).
Availability of data and materials
The material supporting the information of this review has been included within this article.
Declarations
Ethics approval and consent to participate
This is not applicable to this review.
Consent for publication
This is not applicable to this review.
Competing interests
RSB receives consultancy fees from Alva10. CL serves on advisory boards for BMS, Jazz Pharma, Genentech, Novartis, Abbvie, Daiichi, Astellas, Macrogenics, Servier, and Taiho. Previous but not current speakers bureau: Astellas, Jazz KP has received research funding from AbbVie, Agios, Daiichi Sankyo, Millennium; served as an advisory board member for AbbVie, Astellas, Astra Zeneca, Boston BioMedical, Bristol-Myers Squibb, Celgene, Novartis, Jazz Pharmaceuticals, and Servier.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bejar R, Stevenson K, Abdel-Wahab O, Galili N, Nilsson B, Garcia-Manero G, et al. Clinical effect of point mutations in myelodysplastic syndromes. N Engl J Med. 2011;364(26):2496–2506. doi: 10.1056/NEJMoa1013343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Busque L, Patel JP, Figueroa ME, Vasanthakumar A, Provost S, Hamilou Z, et al. Recurrent somatic TET2 mutations in normal elderly individuals with clonal hematopoiesis. Nat Genet. 2012;44(11):1179–1181. doi: 10.1038/ng.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jan M, Snyder TM, Corces-Zimmerman MR, Vyas P, Weissman IL, Quake SR, et al. Clonal evolution of preleukemic hematopoietic stem cells precedes human acute myeloid leukemia. Sci Transl Med- 2012;4(149):14918. doi: 10.1126/scitranslmed.3004315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Welch JS, Ley TJ, Link DC, Miller CA, Larson DE, Koboldt DC, et al. The origin and evolution of mutations in acute myeloid leukemia. Cell. 2012;150(2):264–278. doi: 10.1016/j.cell.2012.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Genovese G, Kahler AK, Handsaker RE, Lindberg J, Rose SA, Bakhoum SF, et al. Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N Engl J Med. 2014;371(26):2477–2487. doi: 10.1056/NEJMoa1409405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jaiswal S, Fontanillas P, Flannick J, Manning A, Grauman PV, Mar BG, et al. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. 2014;371(26):2488–2498. doi: 10.1056/NEJMoa1408617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Young AL, Challen GA, Birmann BM, Druley TE. Clonal haematopoiesis harbouring AML-associated mutations is ubiquitous in healthy adults. Nat Commun. 2016;7:12484. doi: 10.1038/ncomms12484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Desai P, Mencia-Trinchant N, Savenkov O, Simon MS, Cheang G, Lee S, et al. Somatic mutations precede acute myeloid leukemia years before diagnosis. Nat Med. 2018;24(7):1015–1023. doi: 10.1038/s41591-018-0081-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spencer Chapman M, Ranzoni AM, Myers B, Williams N, Coorens THH, Mitchell E, et al. Lineage tracing of human development through somatic mutations. Nature. 2021;595(7865):85–90. doi: 10.1038/s41586-021-03548-6. [DOI] [PubMed] [Google Scholar]
- 10.Fabre MA, de Almeida JG, Fiorillo E, Mitchell E, Damaskou A, Rak J, et al. The longitudinal dynamics and natural history of clonal haematopoiesis. Nature. 2022;606(7913):335–342. doi: 10.1038/s41586-022-04785-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitchell E, Spencer Chapman M, Williams N, Dawson KJ, Mende N, Calderbank EF, et al. Clonal dynamics of haematopoiesis across the human lifespan. Nature. 2022;606(7913):343–350. doi: 10.1038/s41586-022-04786-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shallis RM, Wang R, Davidoff A, Ma X, Zeidan AM. Epidemiology of acute myeloid leukemia: recent progress and enduring challenges. Blood Rev. 2019;36:70–87. doi: 10.1016/j.blre.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 13.Kantarjian H, Kadia T, DiNardo C, Daver N, Borthakur G, Jabbour E, et al. Acute myeloid leukemia: current progress and future directions. Blood Cancer J. 2021;11(2):41. doi: 10.1038/s41408-021-00425-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vasu S, Kohlschmidt J, Mrozek K, Eisfeld AK, Nicolet D, Sterling LJ, et al. Ten-year outcome of patients with acute myeloid leukemia not treated with allogeneic transplantation in first complete remission. Blood Adv. 2018;2(13):1645–1650. doi: 10.1182/bloodadvances.2017015222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koreth J, Schlenk R, Kopecky KJ, Honda S, Sierra J, Djulbegovic BJ, et al. Allogeneic stem cell transplantation for acute myeloid leukemia in first complete remission: systematic review and meta-analysis of prospective clinical trials. JAMA. 2009;301(22):2349–2361. doi: 10.1001/jama.2009.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patel JP, Gonen M, Figueroa ME, Fernandez H, Sun Z, Racevskis J, et al. Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. N Engl J Med. 2012;366(12):1079–1089. doi: 10.1056/NEJMoa1112304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cancer Genome Atlas Research Network Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. New England J Med. 2013;368(22):2059–2074. doi: 10.1056/NEJMoa1301689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duncavage EJ, Schroeder MC, O'Laughlin M, Wilson R, MacMillan S, Bohannon A, et al. Genome sequencing as an alternative to cytogenetic analysis in myeloid cancers. N Engl J Med. 2021;384(10):924–935. doi: 10.1056/NEJMoa2024534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lai C, Doucette K, Norsworthy K. Recent drug approvals for acute myeloid leukemia. J Hematol Oncol. 2019;12(1):100. doi: 10.1186/s13045-019-0774-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bazinet A, Assouline S. A review of FDA-approved acute myeloid leukemia therapies beyond '7 + 3'. Expert Rev Hematol. 2021;14(2):185–197. doi: 10.1080/17474086.2021.1875814. [DOI] [PubMed] [Google Scholar]
- 21.Daver N, Wei AH, Pollyea DA, Fathi AT, Vyas P, DiNardo CD. New directions for emerging therapies in acute myeloid leukemia: the next chapter. Blood Cancer J. 2020;10(10):107. doi: 10.1038/s41408-020-00376-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roloff GW, Odenike O, Bajel A, Wei AH, Foley N, Uy GL. Contemporary approach to acute myeloid leukemia therapy in 2022. Am Soc Clin Oncol Educ Book. 2022;42:1–16. doi: 10.1200/EDBK_349605. [DOI] [PubMed] [Google Scholar]
- 23.Khoury JD, Solary E, Abla O, Akkari Y, Alaggio R, Apperley JF, Bejar R, Berti E, Busque L, Chan JK, Chen W. The 5 th of the World Health Organization classification of haematolymphoid tumours: myeloid and histiocytic/dendritic neoplasms. Leukemia. 2022;36(7):1703–1719. doi: 10.1038/s41375-022-01613-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arber DA, Orazi A, Hasserjian RP, Borowitz MJ, Calvo KR, Kvasnicka HM, et al. International consensus classification of myeloid neoplasms and acute leukemias: integrating morphologic, clinical, and genomic data. Blood. 2022;140(11):1200–1228. doi: 10.1182/blood.2022015850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Papaemmanuil E, Gerstung M, Malcovati L, Tauro S, Gundem G, Van Loo P, et al. Clinical and biological implications of driver mutations in myelodysplastic syndromes. Blood. 2013;122(22):3616–3627. doi: 10.1182/blood-2013-08-518886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haferlach T, Nagata Y, Grossmann V, Okuno Y, Bacher U, Nagae G, et al. Landscape of genetic lesions in 944 patients with myelodysplastic syndromes. Leukemia. 2014;28(2):241–247. doi: 10.1038/leu.2013.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Makishima H, Yoshizato T, Yoshida K, Sekeres MA, Radivoyevitch T, Suzuki H, et al. Dynamics of clonal evolution in myelodysplastic syndromes. Nat Genet. 2017;49(2):204–212. doi: 10.1038/ng.3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walter MJ, Shen D, Ding L, Shao J, Koboldt DC, Chen K, et al. Clonal architecture of secondary acute myeloid leukemia. N Engl J Med. 2012;366(12):1090–1098. doi: 10.1056/NEJMoa1106968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tarlock K, Lamble AJ, Wang YC, Gerbing RB, Ries RE, Loken MR, et al. CEBPA-bZip mutations are associated with favorable prognosis in de novo AML: a report from the children's oncology group. Blood. 2021;138(13):1137–1147. doi: 10.1182/blood.2020009652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taube F, Georgi JA, Kramer M, Stasik S, Middeke JM, Rollig C, et al. CEBPA mutations in 4708 patients with acute myeloid leukemia: differential impact of bZIP and TAD mutations on outcome. Blood. 2022;139(1):87–103. doi: 10.1182/blood.2020009680. [DOI] [PubMed] [Google Scholar]
- 31.Wakita S, Sakaguchi M, Oh I, Kako S, Toya T, Najima Y, et al. Prognostic impact of CEBPA bZIP domain mutation in acute myeloid leukemia. Blood Adv. 2022;6(1):238–247. doi: 10.1182/bloodadvances.2021004292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haase D, Stevenson KE, Neuberg D, Maciejewski JP, Nazha A, Sekeres MA, et al. TP53 mutation status divides myelodysplastic syndromes with complex karyotypes into distinct prognostic subgroups. Leukemia. 2019;33(7):1747–1758. doi: 10.1038/s41375-018-0351-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grob T, Al Hinai ASA, Sanders MA, Kavelaars FG, Rijken M, Gradowska PL, et al. Molecular characterization of mutant TP53 acute myeloid leukemia and high-risk myelodysplastic syndrome. Blood. 2022;139(15):2347–2354. doi: 10.1182/blood.2021014472. [DOI] [PubMed] [Google Scholar]
- 34.Weinberg OK, Siddon A, Madanat YF, Gagan J, Arber DA, Dal Cin P, et al. TP53 mutation defines a unique subgroup within complex karyotype de novo and therapy-related MDS/AML. Blood Adv. 2022;6(9):2847–2853. doi: 10.1182/bloodadvances.2021006239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Döhner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Büchner T, Dombret H, Ebert BL, Fenaux P, Larson RA, Levine RL. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel Blood. J Am Soc Hematol. 2017;129(4):424–447. doi: 10.1182/blood-2016-08-733196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dohner K, Thiede C, Jahn N, Panina E, Gambietz A, Larson RA, et al. Impact of NPM1/FLT3-ITD genotypes defined by the 2017 European LeukemiaNet in patients with acute myeloid leukemia. Blood. 2020;135(5):371–380. doi: 10.1182/blood.2019002697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grob T, Sanders MA, Vonk CM, Kavelaars FG, Rijken M, Hanekamp DW, et al. Prognostic value of FLT3-internal tandem duplication residual disease in acute myeloid leukemia. J Clin Oncol. 2023;41(4):756–765. doi: 10.1200/JCO.22.00715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Loo S, Dillon R, Ivey A, Anstee NS, Othman J, Tiong IS, et al. Pretransplant FLT3-ITD MRD assessed by high-sensitivity PCR-NGS determines posttransplant clinical outcome. Blood. 2022;140(22):2407–2411. doi: 10.1182/blood.2022016567. [DOI] [PubMed] [Google Scholar]
- 39.Awada H, Durmaz A, Gurnari C, Kishtagari A, Meggendorfer M, Kerr CM, et al. Machine learning integrates genomic signatures for subclassification beyond primary and secondary acute myeloid leukemia. Blood. 2021;138(19):1885–1895. doi: 10.1182/blood.2020010603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bernard E, Tuechler H, Greenberg PL, Hasserjian RP, Ossa JEA, Nannya Y, et al. Molecular international prognostic scoring system for myelodysplastic syndromes. NEJM Evidence. 2022;1(7):2200008. doi: 10.1056/EVIDoa2200008. [DOI] [PubMed] [Google Scholar]
- 41.Lugthart S, Groschel S, Beverloo HB, Kayser S, Valk PJ, van Zelderen-Bhola SL, et al. Clinical, molecular, and prognostic significance of WHO type inv (3) (q21q26.2)/t (3;3) (q21;q26.2) and various other 3q abnormalities in acute myeloid leukemia. J Clin Oncol. 2010; 28(24):3890–8. [DOI] [PubMed]
- 42.Ottema S, Mulet-Lazaro R, Beverloo HB, Erpelinck C, van Herk S, van der Helm R, et al. Atypical 3q26/MECOM rearrangements genocopy inv (3)/t (3;3) in acute myeloid leukemia. Blood. 2020;136(2):224–234. doi: 10.1182/blood.2019003701. [DOI] [PubMed] [Google Scholar]
- 43.Kayser S, Hills RK, Langova R, Kramer M, Guijarro F, Sustkova Z, et al. Characteristics and outcome of patients with acute myeloid leukaemia and t (8;16) (p11;p13): results from an international collaborative study. Br J Haematol. 2021;192(5):832–842. doi: 10.1111/bjh.17336. [DOI] [PubMed] [Google Scholar]
- 44.Papaemmanuil E, Dohner H, Campbell PJ. Genomic classification in acute myeloid leukemia. N Engl J Med. 2016;375(9):900–901. doi: 10.1056/NEJMc1608739. [DOI] [PubMed] [Google Scholar]
- 45.Network NCC. Acute myeloid leukemia (Version 2.2022) 2022 [Available from: https://www.nccn.org/login?ReturnURL=https://www.nccn.org/professionals/physician_gls/pdf/aml.pdf.
- 46.Kantarjian HM, Thomas XG, Dmoszynska A, Wierzbowska A, Mazur G, Mayer J, et al. Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J Clin Oncol. 2012;30(21):2670–2677. doi: 10.1200/JCO.2011.38.9429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dombret H, Seymour JF, Butrym A, Wierzbowska A, Selleslag D, Jang JH, et al. International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood. 2015;126(3):291–299. doi: 10.1182/blood-2015-01-621664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.DiNardo CD, Jonas BA, Pullarkat V, Thirman MJ, Garcia JS, Wei AH, et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med. 2020;383(7):617–629. doi: 10.1056/NEJMoa2012971. [DOI] [PubMed] [Google Scholar]
- 49.Pratz KW, Jonas BA, Pullarkat VA, Thirman MJ, Garcia JS, Fiedler W, et al. Long-term follow-up of the phase 3 viale-a clinical trial of venetoclax plus azacitidine for patients with untreated acute myeloid leukemia ineligible for intensive chemotherapy. Blood. 2022;140(Supplement 1):529–531. doi: 10.1182/blood-2022-158518. [DOI] [Google Scholar]
- 50.Wei AH, Montesinos P, Ivanov V, DiNardo CD, Novak J, Laribi K, et al. Venetoclax plus LDAC for newly diagnosed AML ineligible for intensive chemotherapy: a phase 3 randomized placebo-controlled trial. Blood. 2020;135(24):2137–2145. doi: 10.1182/blood.2020004856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wei AH, Panayiotidis P, Montesinos P, Laribi K, Ivanov V, Kim I, Novak J, Champion R, Fiedler W, Pagoni M, Bergeron J. Long-term follow-up of VIALE-C in patients with untreated AML ineligible for intensive chemotherapy. Blood, J Am Soc Hematol. 2022;140(25):2754–2756. doi: 10.1182/blood.2022016963. [DOI] [PubMed] [Google Scholar]
- 52.Wei AH, Panayiotidis P, Montesinos P, Laribi K, Ivanov V, Kim I, et al. 6-month follow-up of VIALE-C demonstrates improved and durable efficacy in patients with untreated AML ineligible for intensive chemotherapy (141/150) Blood Cancer J. 2021;11(10):163. doi: 10.1038/s41408-021-00555-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pollyea DA, Winters A, McMahon C, Schwartz M, Jordan CT, Rabinovitch R, et al. Venetoclax and azacitidine followed by allogeneic transplant results in excellent outcomes and may improve outcomes versus maintenance therapy among newly diagnosed AML patients older than 60. Bone Marrow Transplant. 2022;57(2):160–166. doi: 10.1038/s41409-021-01476-7. [DOI] [PubMed] [Google Scholar]
- 54.Winters AC, Bosma G, Abbott D, Minhajuddin M, Jordan C, Pollyea DA, Gutman JA. outcomes are similar after allogeneic hematopoietic stem cell transplant for newly diagnosed acute myeloid leukemia patients who received venetoclax+ azacitidine versus intensive chemotherapy. Transp Cel Ther. 2022;28(10):694–e1. doi: 10.1016/j.jtct.2022.07.022. [DOI] [PubMed] [Google Scholar]
- 55.Granfeldt Ostgard LS, Medeiros BC, Sengelov H, Norgaard M, Andersen MK, Dufva IH, et al. Epidemiology and clinical significance of secondary and therapy-related acute myeloid leukemia: a national population-based cohort study. J Clin Oncol. 2015;33(31):3641–3649. doi: 10.1200/JCO.2014.60.0890. [DOI] [PubMed] [Google Scholar]
- 56.Godley LA, Larson RA. Therapy-related myeloid leukemia. Semin Oncol. 2008;35(4):418–429. doi: 10.1053/j.seminoncol.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kern W, Haferlach T, Schnittger S, Hiddemann W, Schoch C. Prognosis in therapy-related acute myeloid leukemia and impact of karyotype. J Clin Oncol. 2004;22(12):2510–2511. doi: 10.1200/JCO.2004.99.301. [DOI] [PubMed] [Google Scholar]
- 58.Schoch C, Kern W, Schnittger S, Hiddemann W, Haferlach T. Karyotype is an independent prognostic parameter in therapy-related acute myeloid leukemia (t-AML): an analysis of 93 patients with t-AML in comparison to 1091 patients with de novo AML. Leukemia. 2004;18(1):120–125. doi: 10.1038/sj.leu.2403187. [DOI] [PubMed] [Google Scholar]
- 59.Kayser S, Dohner K, Krauter J, Kohne CH, Horst HA, Held G, et al. The impact of therapy-related acute myeloid leukemia (AML) on outcome in 2853 adult patients with newly diagnosed AML. Blood. 2011;117(7):2137–2145. doi: 10.1182/blood-2010-08-301713. [DOI] [PubMed] [Google Scholar]
- 60.Leith CP, Kopecky KJ, Godwin J, McConnell T, Slovak ML, Chen IM, et al. Acute myeloid leukemia in the elderly: assessment of multidrug resistance (MDR1) and cytogenetics distinguishes biologic subgroups with remarkably distinct responses to standard chemotherapy. Southwest Oncol Group Stud Blood. 1997;89(9):3323–3329. [PubMed] [Google Scholar]
- 61.Mayer LD, Harasym TO, Tardi PG, Harasym NL, Shew CR, Johnstone SA, et al. Ratiometric dosing of anticancer drug combinations: controlling drug ratios after systemic administration regulates therapeutic activity in tumor-bearing mice. Mol Cancer Ther. 2006;5(7):1854–1863. doi: 10.1158/1535-7163.MCT-06-0118. [DOI] [PubMed] [Google Scholar]
- 62.Lancet JE, Cortes JE, Hogge DE, Tallman MS, Kovacsovics TJ, Damon LE, et al. Phase 2 trial of CPX-351, a fixed 5:1 molar ratio of cytarabine/daunorubicin, vs cytarabine/daunorubicin in older adults with untreated AML. Blood. 2014;123(21):3239–3246. doi: 10.1182/blood-2013-12-540971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lancet JE, Uy GL, Cortes JE, Newell LF, Lin TL, Ritchie EK, et al. CPX-351 (cytarabine and daunorubicin) liposome for injection versus conventional cytarabine plus daunorubicin in older patients with newly diagnosed secondary acute myeloid leukemia. J Clin Oncol. 2018;36(26):2684–2692. doi: 10.1200/JCO.2017.77.6112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Matthews AH, Perl AE, Luger SM, Loren AW, Gill SI, Porter DL, et al. Real-world effectiveness of CPX-351 vs venetoclax and azacitidine in acute myeloid leukemia. Blood Adv. 2022;6(13):3997–4005. doi: 10.1182/bloodadvances.2022007265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dohner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Buchner T, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129(4):424–447. doi: 10.1182/blood-2016-08-733196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tashakori M, Kadia T, Loghavi S, Daver N, Kanagal-Shamanna R, Pierce S, et al. TP53 copy number and protein expression inform mutation status across risk categories in acute myeloid leukemia. Blood. 2022;140(1):58–72. doi: 10.1182/blood.2021013983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bejar R, Stevenson KE, Caughey B, Lindsley RC, Mar BG, Stojanov P, et al. Somatic mutations predict poor outcome in patients with myelodysplastic syndrome after hematopoietic stem-cell transplantation. J Clin Oncol. 2014;32(25):2691–2698. doi: 10.1200/JCO.2013.52.3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Britt A, Mohyuddin GR, McClune B, Singh A, Lin T, Ganguly S, et al. Acute myeloid leukemia or myelodysplastic syndrome with chromosome 17 abnormalities and long-term outcomes with or without hematopoietic stem cell transplantation. Leuk Res. 2020;95:106402. doi: 10.1016/j.leukres.2020.106402. [DOI] [PubMed] [Google Scholar]
- 69.Badar T, Atallah EL, Shallis RM, Saliba AN, Stahl MF, Bewersdorf JP, et al. Predictors of long-term outcome in TP53-mutated acute myeloid leukemia patients receiving allogeneic stem cell transplant after first- or second-line therapy: results from the consortium on myeloid malignancies and neoplastic diseases (COMMAND) Blood. 2022;140(Supplement 1):1435–1437. doi: 10.1182/blood-2022-167731. [DOI] [Google Scholar]
- 70.DiNardo CD, Pratz K, Pullarkat V, Jonas BA, Arellano M, Becker PS, et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood. 2019;133(1):7–17. doi: 10.1182/blood-2018-08-868752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pollyea DA, Pratz KW, Wei AH, Pullarkat VA, Jonas BA, Recher C, Babu S, Schuh AC, Dail M, Sun Y, Potluri J. Outcomes in patients with poor-risk cytogenetics with or without TP53 mutations treated with venetoclax combined with hypomethylating agents. Blood. 2021;23(138):224. doi: 10.1182/blood-2021-145639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Morsia E, McCullough K, Joshi M, Cook J, Alkhateeb HB, Al-Kali A, et al. Venetoclax and hypomethylating agents in acute myeloid leukemia: mayo clinic series on 86 patients. Am J Hematol. 2020;95(12):1511–1521. doi: 10.1002/ajh.25978. [DOI] [PubMed] [Google Scholar]
- 73.Venugopal S, Shoukier M, Konopleva M, Dinardo CD, Ravandi F, Short NJ, et al. Outcomes in patients with newly diagnosed TP53-mutated acute myeloid leukemia with or without venetoclax-based therapy. Cancer. 2021;127(19):3541–3551. doi: 10.1002/cncr.33675. [DOI] [PubMed] [Google Scholar]
- 74.Thijssen R, Diepstraten ST, Moujalled D, Chew E, Flensburg C, Shi MX, et al. Intact TP-53 function is essential for sustaining durable responses to BH3-mimetic drugs in leukemias. Blood. 2021;137(20):2721–2735. doi: 10.1182/blood.2020010167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rahmani NE, Ramachandra N, Sahu S, Gitego N, Lopez A, Pradhan K, et al. ASXL1 mutations are associated with distinct epigenomic alterations that lead to sensitivity to venetoclax and azacytidine. Blood Cancer J. 2021;11(9):157. doi: 10.1038/s41408-021-00541-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Aldoss I, Yang D, Pillai R, Sanchez JF, Mei M, Aribi A, et al. Association of leukemia genetics with response to venetoclax and hypomethylating agents in relapsed/refractory acute myeloid leukemia. Am J Hematol. 2019;94(10):E253–E255. doi: 10.1002/ajh.25567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gangat N, McCullough K, Johnson I, Al-Kali A, Begna KH, Patnaik MM, et al. Real-world experience with venetoclax and hypomethylating agents in myelodysplastic syndromes with excess blasts. Am J Hematol. 2022;97(6):E214–E216. doi: 10.1002/ajh.26539. [DOI] [PubMed] [Google Scholar]
- 78.Cherry EM, Abbott D, Amaya M, McMahon C, Schwartz M, Rosser J, et al. Venetoclax and azacitidine compared with induction chemotherapy for newly diagnosed patients with acute myeloid leukemia. Blood Adv. 2021;5(24):5565–5573. doi: 10.1182/bloodadvances.2021005538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dohner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. N Engl J Med. 2015;373(12):1136–1152. doi: 10.1056/NEJMra1406184. [DOI] [PubMed] [Google Scholar]
- 80.Chou WC, Lei WC, Ko BS, Hou HA, Chen CY, Tang JL, et al. The prognostic impact and stability of Isocitrate dehydrogenase 2 mutation in adult patients with acute myeloid leukemia. Leukemia. 2011;25(2):246–253. doi: 10.1038/leu.2010.267. [DOI] [PubMed] [Google Scholar]
- 81.Paschka P, Schlenk RF, Gaidzik VI, Habdank M, Kronke J, Bullinger L, et al. IDH1 and IDH2 mutations are frequent genetic alterations in acute myeloid leukemia and confer adverse prognosis in cytogenetically normal acute myeloid leukemia with NPM1 mutation without FLT3 internal tandem duplication. J Clin Oncol. 2010;28(22):3636–3643. doi: 10.1200/JCO.2010.28.3762. [DOI] [PubMed] [Google Scholar]
- 82.Stein EM, DiNardo CD, Pollyea DA, Fathi AT, Roboz GJ, Altman JK, et al. Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood. 2017;130(6):722–731. doi: 10.1182/blood-2017-04-779405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stein EM, DiNardo CD, Fathi AT, Pollyea DA, Stone RM, Altman JK, et al. Molecular remission and response patterns in patients with mutant-IDH2 acute myeloid leukemia treated with enasidenib. Blood. 2019;133(7):676–687. doi: 10.1182/blood-2018-08-869008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Amatangelo MD, Quek L, Shih A, Stein EM, Roshal M, David MD, et al. Enasidenib induces acute myeloid leukemia cell differentiation to promote clinical response. Blood. 2017;130(6):732–741. doi: 10.1182/blood-2017-04-779447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.de Botton S, Montesinos P, Schuh AC, Papayannidis C, Vyas P, Wei AH, et al. Enasidenib vs conventional care in mutant-IDH2 relapsed/refractory acute myeloidleukemia: a randomized, phase 3 trial. Blood. 2022;141(2):156. doi: 10.1182/blood.2021014901. [DOI] [PubMed] [Google Scholar]
- 86.Pollyea DA, Tallman MS, de Botton S, Kantarjian HM, Collins R, Stein AS, et al. Enasidenib, an inhibitor of mutant IDH2 proteins, induces durable remissions in older patients with newly diagnosed acute myeloid leukemia. Leukemia. 2019;33(11):2575–2584. doi: 10.1038/s41375-019-0472-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.DiNardo CD, Stein EM, de Botton S, Roboz GJ, Altman JK, Mims AS, et al. Durable remissions with ivosidenib in IDH1-mutated relapsed or refractory AML. N Engl J Med. 2018;378(25):2386–2398. doi: 10.1056/NEJMoa1716984. [DOI] [PubMed] [Google Scholar]
- 88.Roboz GJ, DiNardo CD, Stein EM, de Botton S, Mims AS, Prince GT, et al. Ivosidenib induces deep durable remissions in patients with newly diagnosed IDH1-mutant acute myeloid leukemia. Blood. 2020;135(7):463–471. doi: 10.1182/blood.2019002140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Caravella JA, Lin J, Diebold RB, Campbell AM, Ericsson A, Gustafson G, et al. Structure-based design and identification of FT-2102 (Olutasidenib), a potent mutant-selective IDH1 inhibitor. J Med Chem. 2020;63(4):1612–1623. doi: 10.1021/acs.jmedchem.9b01423. [DOI] [PubMed] [Google Scholar]
- 90.Cortes JE, Fenaux P, Yee K, Recher C, Wei AH, Montesinos P, et al. Olutasidenib (FT-2102) induces durable complete remissions in patients with relapsed/refractory mIDH1 acute myeloid leukemia. Results from a planned interim analysis of a phase 2 pivotal clinical trial. Blood. 2022;140(Supplement 1):6193–6. doi: 10.1182/blood-2022-167330. [DOI] [Google Scholar]
- 91.Watts JM, Baer MR, Yang J, Prebet T, Lee S, Schiller GJ, et al. Olutasidenib alone or with azacitidine in IDH1-mutated acute myeloid leukaemia and myelodysplastic syndrome: phase 1 results of a phase 1/2 trial. Lancet Haematol. 2022;10(1):46. doi: 10.1016/S2352-3026(22)00292-7. [DOI] [PubMed] [Google Scholar]
- 92.Reinbold R, Hvinden IC, Rabe P, Herold RA, Finch A, Wood J, et al. Resistance to the isocitrate dehydrogenase 1 mutant inhibitor ivosidenib can be overcome by alternative dimer-interface binding inhibitors. Nat Commun. 2022;13(1):4785. doi: 10.1038/s41467-022-32436-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Stein EM, DiNardo CD, Fathi AT, Mims AS, Pratz KW, Savona MR, et al. Ivosidenib or enasidenib combined with intensive chemotherapy in patients with newly diagnosed AML: a phase 1 study. Blood. 2021;137(13):1792–1803. doi: 10.1182/blood.2020007233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.DiNardo CD, Ravandi F, Agresta S, Konopleva M, Takahashi K, Kadia T, et al. Characteristics, clinical outcome, and prognostic significance of IDH mutations in AML. Am J Hematol. 2015;90(8):732–736. doi: 10.1002/ajh.24072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Stein EM, DiNardo CD, Fathi AT, Mims AS, Savona MR, Stein AS, et al. Updated survival and response analyses from a phase 1 study of ivosidenib or enasidenib combined with induction and consolidation chemotherapy in patients with newly diagnosed AML with an IDH1 or IDH2 mutation. Blood. 2021;138(Supplement 1):1276. doi: 10.1182/blood-2021-146507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pollyea DA, DiNardo CD, Arellano ML, Pigneux A, Fiedler W, Konopleva M, et al. Impact of venetoclax and azacitidine in treatment-naive patients with acute myeloid leukemia and IDH1/2 mutations. Clin Cancer Res. 2022;28(13):2753–2761. doi: 10.1158/1078-0432.CCR-21-3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.DiNardo CD, Stein AS, Stein EM, Fathi AT, Frankfurt O, Schuh AC, et al. Mutant isocitrate dehydrogenase 1 inhibitor ivosidenib in combination with azacitidine for newly diagnosed acute myeloid leukemia. J Clin Oncol. 2021;39(1):57–65. doi: 10.1200/JCO.20.01632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Montesinos P, Recher C, Vives S, Zarzycka E, Wang J, Bertani G, et al. Ivosidenib and azacitidine in IDH1-mutated acute myeloid leukemia. N Engl J Med. 2022;386(16):1519–1531. doi: 10.1056/NEJMoa2117344. [DOI] [PubMed] [Google Scholar]
- 99.Döhner H, Marchione DM, Choe S, Montesinos P, Recher C, Vives S, et al. Molecular characterization of clinical response and relapse in patients with IDH1m ND-AML treated with Ivo+AZA in the AGILE study. Blood. 2022;140(Supplement 1):539–542. doi: 10.1182/blood-2022-159473. [DOI] [Google Scholar]
- 100.Venugopal S, Takahashi K, Daver N, Maiti A, Borthakur G, Loghavi S, et al. Efficacy and safety of enasidenib and azacitidine combination in patients with IDH2 mutated acute myeloid leukemia and not eligible for intensive chemotherapy. Blood Cancer J. 2022;12(1):10. doi: 10.1038/s41408-021-00604-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.DiNardo CD, Schuh AC, Stein EM, Montesinos P, Wei AH, de Botton S, et al. Enasidenib plus azacitidine versus azacitidine alone in patients with newly diagnosed, mutant-IDH2 acute myeloid leukaemia (AG221-AML-005): a single-arm, phase 1b and randomised, phase 2 trial. Lancet Oncol. 2021;22(11):1597–1608. doi: 10.1016/S1470-2045(21)00494-0. [DOI] [PubMed] [Google Scholar]
- 102.Lachowiez CA, Garcia JS, Borthakur G, Loghavi S, Zeng Z, Tippett GD, et al. A phase Ib/II study of ivosidenib with venetoclax +/- azacitidine in IDH1-mutated hematologic malignancies. J Clinic Oncol 2022; 40 (16_suppl):7018.
- 103.Carow CE, Levenstein M, Kaufmann SH, Chen J, Amin S, Rockwell P, et al. Expression of the hematopoietic growth factor receptor FLT3 (STK-1/Flk2) in human leukemias. Blood. 1996;87(3):1089–1096. doi: 10.1182/blood.V87.3.1089.bloodjournal8731089. [DOI] [PubMed] [Google Scholar]
- 104.Nagel G, Weber D, Fromm E, Erhardt S, Lubbert M, Fiedler W, et al. Epidemiological, genetic, and clinical characterization by age of newly diagnosed acute myeloid leukemia based on an academic population-based registry study (AMLSG BiO) Ann Hematol. 2017;96(12):1993–2003. doi: 10.1007/s00277-017-3150-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Levis M. FLT3 mutations in acute myeloid leukemia: what is the best approach in 2013? Hematology / Edu Program Am Soc Hematol Am Soc Hematol Edu Program. 2013;2013:220–226. doi: 10.1182/asheducation-2013.1.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Schlenk RF, Kayser S, Bullinger L, Kobbe G, Casper J, Ringhoffer M, et al. Differential impact of allelic ratio and insertion site in FLT3-ITD-positive AML with respect to allogeneic transplantation. Blood. 2014;124(23):3441–3449. doi: 10.1182/blood-2014-05-578070. [DOI] [PubMed] [Google Scholar]
- 107.Gale RE, Green C, Allen C, Mead AJ, Burnett AK, Hills RK, et al. The impact of FLT3 internal tandem duplication mutant level, number, size, and interaction with NPM1 mutations in a large cohort of young adult patients with acute myeloid leukemia. Blood. 2008;111(5):2776–2784. doi: 10.1182/blood-2007-08-109090. [DOI] [PubMed] [Google Scholar]
- 108.Pratcorona M, Brunet S, Nomdedeu J, Ribera JM, Tormo M, Duarte R, et al. Favorable outcome of patients with acute myeloid leukemia harboring a low-allelic burden FLT3-ITD mutation and concomitant NPM1 mutation: relevance to post-remission therapy. Blood. 2013;121(14):2734–2738. doi: 10.1182/blood-2012-06-431122. [DOI] [PubMed] [Google Scholar]
- 109.Linch DC, Hills RK, Burnett AK, Khwaja A, Gale RE. Impact of FLT3 (ITD) mutant allele level on relapse risk in intermediate-risk acute myeloid leukemia. Blood. 2014;124(2):273–276. doi: 10.1182/blood-2014-02-554667. [DOI] [PubMed] [Google Scholar]
- 110.Oran B, Cortes J, Beitinjaneh A, Chen HC, de Lima M, Patel K, et al. Allogeneic transplantation in first remission improves outcomes irrespective of FLT3-ITD allelic ratio in FLT3-ITD-positive acute myelogenous leukemia. Biol Blood Marrow Transplant. 2016;22(7):1218–1226. doi: 10.1016/j.bbmt.2016.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Novatcheva ED, Anouty Y, Saunders I, Mangan JK, Goodman AM. FMS-like tyrosine kinase 3 inhibitors for the treatment of acute myeloid leukemia. Clin Lymphoma Myeloma Leuk. 2022;22(3):e161–e184. doi: 10.1016/j.clml.2021.09.002. [DOI] [PubMed] [Google Scholar]
- 112.Tamaoki T, Nomoto H, Takahashi I, Kato Y, Morimoto M, Tomita F. Staurosporine, a potent inhibitor of phospholipid/Ca++dependent protein kinase. Biochem Biophys Res Commun. 1986;135(2):397–402. doi: 10.1016/0006-291X(86)90008-2. [DOI] [PubMed] [Google Scholar]
- 113.Weisberg E, Boulton C, Kelly LM, Manley P, Fabbro D, Meyer T, et al. Inhibition of mutant FLT3 receptors in leukemia cells by the small molecule tyrosine kinase inhibitor PKC412. Cancer Cell. 2002;1(5):433–443. doi: 10.1016/S1535-6108(02)00069-7. [DOI] [PubMed] [Google Scholar]
- 114.Stone RM, Fischer T, Paquette R, Schiller G, Schiffer CA, Ehninger G, et al. Phase IB study of the FLT3 kinase inhibitor midostaurin with chemotherapy in younger newly diagnosed adult patients with acute myeloid leukemia. Leukemia. 2012;26(9):2061–2068. doi: 10.1038/leu.2012.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Perl AE, Altman JK, Cortes J, Smith C, Litzow M, Baer MR, et al. Selective inhibition of FLT3 by gilteritinib in relapsed or refractory acute myeloid leukaemia: a multicentre, first-in-human, open-label, phase 1–2 study. Lancet Oncol. 2017;18(8):1061–1075. doi: 10.1016/S1470-2045(17)30416-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cortes JE, Kantarjian H, Foran JM, Ghirdaladze D, Zodelava M, Borthakur G, et al. Phase I study of quizartinib administered daily to patients with relapsed or refractory acute myeloid leukemia irrespective of FMS-like tyrosine kinase 3-internal tandem duplication status. J Clin Oncol. 2013;31(29):3681–3687. doi: 10.1200/JCO.2013.48.8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Perl AE, Martinelli G, Cortes JE, Neubauer A, Berman E, Paolini S, et al. Gilteritinib or chemotherapy for relapsed or refractory FLT3-mutated AML. N Engl J Med. 2019;381(18):1728–1740. doi: 10.1056/NEJMoa1902688. [DOI] [PubMed] [Google Scholar]
- 118.Stone RM, Mandrekar SJ, Sanford BL, Laumann K, Geyer S, Bloomfield CD, et al. Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N Engl J Med. 2017;377(5):454–464. doi: 10.1056/NEJMoa1614359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Levis M, Perl AE. Gilteritinib: potent targeting of FLT3 mutations in AML. Blood Adv. 2020;4(6):1178–1191. doi: 10.1182/bloodadvances.2019000174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Levis M, Shi W, Chang K, Laing C, Pollner R, Gocke C, et al. FLT3 inhibitors added to induction therapy induce deeper remissions. Blood. 2020;135(1):75–78. doi: 10.1182/blood.2019002180. [DOI] [PubMed] [Google Scholar]
- 121.Schlenk RF, Weber D, Fiedler W, Salih HR, Wulf G, Salwender H, et al. Midostaurin added to chemotherapy and continued single-agent maintenance therapy in acute myeloid leukemia with FLT3-ITD. Blood. 2019;133(8):840–851. doi: 10.1182/blood-2018-08-869453. [DOI] [PubMed] [Google Scholar]
- 122.Dohner H, Weber D, Krzykalla J, Fiedler W, Wulf G, Salih H, et al. Midostaurin plus intensive chemotherapy for younger and older patients with AML and FLT3 internal tandem duplications. Blood Adv. 2022;6(18):5345–5355. doi: 10.1182/bloodadvances.2022007223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Maziarz RT, Levis M, Patnaik MM, Scott BL, Mohan SR, Deol A, et al. Midostaurin after allogeneic stem cell transplant in patients with FLT3-internal tandem duplication-positive acute myeloid leukemia. Bone Marrow Transplant. 2021;56(5):1180–1189. doi: 10.1038/s41409-020-01153-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pratz KW, Cherry M, Altman JK, Cooper BW, Cruz JC, Jurcic JG, et al. A Phase 1 study of gilteritinib in combination with induction and consolidation chemotherapy in patients with newly diagnosed AML: final results. Blood. 2020;136(Supplement 1):16–17. doi: 10.1182/blood-2020-137685. [DOI] [Google Scholar]
- 125.Fischer T, Stone RM, Deangelo DJ, Galinsky I, Estey E, Lanza C, et al. Phase IIB trial of oral Midostaurin (PKC412), the FMS-like tyrosine kinase 3 receptor (FLT3) and multi-targeted kinase inhibitor, in patients with acute myeloid leukemia and high-risk myelodysplastic syndrome with either wild-type or mutated FLT3. J Clin Oncol. 2010;28(28):4339–4345. doi: 10.1200/JCO.2010.28.9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Smith CC, Lin K, Stecula A, Sali A, Shah NP. FLT3 D835 mutations confer differential resistance to type II FLT3 inhibitors. Leukemia. 2015;29(12):2390–2392. doi: 10.1038/leu.2015.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Konopleva M, Thirman MJ, Pratz KW, Garcia JS, Recher C, Pullarkat V, et al. Impact of FLT3 mutation on outcomes after venetoclax and azacitidine for patients with treatment-naive acute myeloid leukemia. Clin Cancer Res. 2022;28(13):2744–2752. doi: 10.1158/1078-0432.CCR-21-3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wang ES, Montesinos P, Minden MD, Lee JH, Heuser M, Naoe T, et al. Phase 3 trial of gilteritinib plus azacitidine vs azacitidine for newly diagnosed FLT3mut+ AML ineligible for intensive chemotherapy. Blood. 2022;140(7):1845. doi: 10.1182/blood.2021014586. [DOI] [PubMed] [Google Scholar]
- 129.Strati P, Kantarjian H, Ravandi F, Nazha A, Borthakur G, Daver N, et al. Phase I/II trial of the combination of midostaurin (PKC412) and 5-azacytidine for patients with acute myeloid leukemia and myelodysplastic syndrome. Am J Hematol. 2015;90(4):276–281. doi: 10.1002/ajh.23924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ravandi F, Alattar ML, Grunwald MR, Rudek MA, Rajkhowa T, Richie MA, et al. Phase 2 study of azacytidine plus sorafenib in patients with acute myeloid leukemia and FLT-3 internal tandem duplication mutation. Blood. 2013;121(23):4655–4662. doi: 10.1182/blood-2013-01-480228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Muppidi MR, Portwood S, Griffiths EA, Thompson JE, Ford LA, Freyer CW, et al. Decitabine and sorafenib therapy in FLT-3 ITD-mutant acute myeloid leukemia. Clin Lymphoma Myeloma Leuk. 2015;15(Suppl):S73–S79. doi: 10.1016/j.clml.2015.02.033. [DOI] [PubMed] [Google Scholar]
- 132.Yamatani K, Ai T, Saito K, Suzuki K, Hori A, Kinjo S, et al. Inhibition of BCL2A1 by STAT5 inactivation overcomes resistance to targeted therapies of FLT3-ITD/D835 mutant AML. Transl Oncol. 2022;18:101354. doi: 10.1016/j.tranon.2022.101354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhu R, Li L, Nguyen B, Seo J, Wu M, Seale T, et al. FLT3 tyrosine kinase inhibitors synergize with BCL-2 inhibition to eliminate FLT3/ITD acute leukemia cells through BIM activation. Signal Transduct Target Ther. 2021;6(1):186. doi: 10.1038/s41392-021-00578-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Brinton LT, Zhang P, Williams K, Canfield D, Orwick S, Sher S, et al. Synergistic effect of BCL2 and FLT3 co-inhibition in acute myeloid leukemia. J Hematol Oncol. 2020;13(1):139. doi: 10.1186/s13045-020-00973-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Daver N, Perl AE, Maly J, Levis M, Ritchie E, Litzow M, McCloskey J, Smith CC, Schiller G, Bradley T, Tiu RV. Venetoclax plus gilteritinib for FLT3-mutated relapsed/refractory acute myeloid leukemia. J Clinic Oncol. 2022;40(35):4048–59. doi: 10.1200/JCO.22.00602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ohanian M, Garcia-Manero G, Levis M, Jabbour E, Daver N, Borthakur G, et al. Sorafenib combined with 5-azacytidine in older patients with untreated FLT3-ITD mutated acute myeloid leukemia. Am J Hematol. 2018;93(9):1136–1141. doi: 10.1002/ajh.25198. [DOI] [PubMed] [Google Scholar]
- 137.Cortes JE, Tallman MS, Schiller GJ, Trone D, Gammon G, Goldberg SL, et al. Phase 2b study of 2 dosing regimens of quizartinib monotherapy in FLT3-ITD-mutated, relapsed or refractory AML. Blood. 2018;132(6):598–607. doi: 10.1182/blood-2018-01-821629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cortes JE, Khaled S, Martinelli G, Perl AE, Ganguly S, Russell N, et al. Quizartinib versus salvage chemotherapy in relapsed or refractory FLT3-ITD acute myeloid leukaemia (QuANTUM-R): a multicentre, randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2019;20(7):984–997. doi: 10.1016/S1470-2045(19)30150-0. [DOI] [PubMed] [Google Scholar]
- 139.Cortes J, Perl AE, Dohner H, Kantarjian H, Martinelli G, Kovacsovics T, et al. Quizartinib, an FLT3 inhibitor, as monotherapy in patients with relapsed or refractory acute myeloid leukaemia: an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol. 2018;19(7):889–903. doi: 10.1016/S1470-2045(18)30240-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Erba H, Montesinos P, Vrhovac R, Patkowska E, Kim H-J, Zak P, et al. AML-029 quizartinib prolonged overall survival (OS) vs placebo plus intensive induction and consolidation therapy followed by single-agent continuation in patients aged 18–75 years with newly diagnosed FLT3–internal tandem duplication positive (FLT3-ITD+) acute myeloid leukemia (AML) Clin Lymphoma Myeloma Leuk. 2022;22:S208–S209. doi: 10.1016/S2152-2650(22)01212-5. [DOI] [Google Scholar]
- 141.Levis MJ, Erba HP, Montesinos P, Vrhovac R, Patkowska E, Kim H, et al. Quantum-first trial: FLT3-ITD-specific MRD clearance is associated with improved overall survival. Blood. 2022;140(Supplement 1):546–548. doi: 10.1182/blood-2022-162739. [DOI] [Google Scholar]
- 142.Wang ES, Goldberg AD, Walter RB, Collins R, Stone RM. Long-term results of a phase 2 trial of crenolanib combined with 7+3 chemotherapy in adults with newly diagnosed FLT3 mutant AML. J Clinic Oncol 2022; 40 (16_suppl):7007.
- 143.Short N, DiNardo CD, Daver N, Macaron W, Yilmaz M, Borthakur G, et al. Updated results from a phase i/ii study of the triplet combination of azacitidine, venetoclax and gilteritinib for patients with FLT3-mutated acute myeloid leukemia. Blood. 2022;140(Supplement 1):2007–2009. doi: 10.1182/blood-2022-157210. [DOI] [Google Scholar]
- 144.Rollig C, Serve H, Huttmann A, Noppeney R, Muller-Tidow C, Krug U, et al. Addition of sorafenib versus placebo to standard therapy in patients aged 60 years or younger with newly diagnosed acute myeloid leukaemia (SORAML): a multicentre, phase 2, randomised controlled trial. Lancet Oncol. 2015;16(16):1691–1699. doi: 10.1016/S1470-2045(15)00362-9. [DOI] [PubMed] [Google Scholar]
- 145.Rollig C, Serve H, Noppeney R, Hanoun M, Krug U, Baldus CD, et al. Sorafenib or placebo in patients with newly diagnosed acute myeloid leukaemia: long-term follow-up of the randomized controlled SORAML trial. Leukemia. 2021;35(9):2517–2525. doi: 10.1038/s41375-021-01148-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Burchert A, Bug G, Fritz LV, Finke J, Stelljes M, Rollig C, et al. Sorafenib maintenance after allogeneic hematopoietic stem cell transplantation for acute myeloid leukemia with FLT3-internal tandem duplication mutation (SORMAIN) J Clin Oncol. 2020;38(26):2993–3002. doi: 10.1200/JCO.19.03345. [DOI] [PubMed] [Google Scholar]
- 147.Metzelder SK, Schroeder T, Finck A, Scholl S, Fey M, Gotze K, et al. High activity of sorafenib in FLT3-ITD-positive acute myeloid leukemia synergizes with allo-immune effects to induce sustained responses. Leukemia. 2012;26(11):2353–2359. doi: 10.1038/leu.2012.105. [DOI] [PubMed] [Google Scholar]
- 148.Xuan L, Wang Y, Huang F, Fan Z, Xu Y, Sun J, et al. Sorafenib maintenance in patients with FLT3-ITD acute myeloid leukaemia undergoing allogeneic haematopoietic stem-cell transplantation: an open-label, multicentre, randomised phase 3 trial. Lancet Oncol. 2020;21(9):1201–1212. doi: 10.1016/S1470-2045(20)30455-1. [DOI] [PubMed] [Google Scholar]
- 149.Wei AH, Dohner H, Pocock C, Montesinos P, Afanasyev B, Dombret H, et al. Oral Azacitidine maintenance therapy for acute myeloid leukemia in first remission. N Engl J Med. 2020;383(26):2526–2537. doi: 10.1056/NEJMoa2004444. [DOI] [PubMed] [Google Scholar]
- 150.Huls G, Chitu DA, Havelange V, Jongen-Lavrencic M, van de Loosdrecht AA, Biemond BJ, et al. Azacitidine maintenance after intensive chemotherapy improves DFS in older AML patients. Blood. 2019;133(13):1457–1464. doi: 10.1182/blood-2018-10-879866. [DOI] [PubMed] [Google Scholar]
- 151.Oran B, de Lima M, Garcia-Manero G, Thall PF, Lin R, Popat U, et al. A phase 3 randomized study of 5-azacitidine maintenance vs observation after transplant in high-risk AML and MDS patients. Blood Adv. 2020;4(21):5580–5588. doi: 10.1182/bloodadvances.2020002544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Bazinet A, Kantarjian HM, Borthakur G, Yilmaz M, Bose P, Jabbour E, et al. Phase 2 study of azacitidine (AZA) and venetoclax (VEN) as maintenance therapy for acute myeloid leukemia (AML) patients in remission. J Clinic Oncol 2022; 40 (16suppl): e19018.
- 153.Chua CC, Hammond D, Kent A, Tiong IS, Konopleva MY, Pollyea DA, et al. Treatment-free remission after ceasing venetoclax-based therapy in patients with acute myeloid leukemia. Blood Adv. 2022;6(13):3879–3883. doi: 10.1182/bloodadvances.2022007083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Duncavage EJ, Bagg A, Hasserjian RP, DiNardo CD, Godley LA, Iacobucci I, et al. Genomic profiling for clinical decision making in myeloid neoplasms and acute leukemia. Blood. 2022;140(21):2228. doi: 10.1182/blood.2022015853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lambert JM, Gorzov P, Veprintsev DB, Soderqvist M, Segerback D, Bergman J, et al. PRIMA-1 reactivates mutant p53 by covalent binding to the core domain. Cancer Cell. 2009;15(5):376–388. doi: 10.1016/j.ccr.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 156.Peng X, Zhang MQ, Conserva F, Hosny G, Selivanova G, Bykov VJ, et al. APR-246/PRIMA-1MET inhibits thioredoxin reductase 1 and converts the enzyme to a dedicated NADPH oxidase. Cell Death Dis. 2013;4:e881. doi: 10.1038/cddis.2013.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Haffo L, Lu J, Bykov VJN, Martin SS, Ren X, Coppo L, et al. Inhibition of the glutaredoxin and thioredoxin systems and ribonucleotide reductase by mutant p53-targeting compound APR-246. Sci Rep. 2018;8(1):12671. doi: 10.1038/s41598-018-31048-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Fujihara KM, Zhang BZ, Jackson TD, Ogunkola MO, Nijagal B, Milne JV, et al. Eprenetapopt triggers ferroptosis, inhibits NFS1 cysteine desulfurase, and synergizes with serine and glycine dietary restriction. Sci Adv. 2022;8(37):eabm9427. doi: 10.1126/sciadv.abm9427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sallman DA, DeZern AE, Garcia-Manero G, Steensma DP, Roboz GJ, Sekeres MA, et al. Eprenetapopt (APR-246) and azacitidine in TP53-mutant myelodysplastic syndromes. J Clin Oncol. 2021;39(14):1584–1594. doi: 10.1200/JCO.20.02341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Cluzeau T, Sebert M, Rahme R, Cuzzubbo S, Lehmann-Che J, Madelaine I, et al. Eprenetapopt plus azacitidine in TP53-mutated myelodysplastic syndromes and acute myeloid leukemia: a phase II study by the groupe francophone des myelodysplasies (GFM) J Clin Oncol. 2021;39(14):1575–1583. doi: 10.1200/JCO.20.02342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Mishra A, Tamari R, DeZern AE, Byrne MT, Gooptu M, Chen YB, Deeg HJ, Sallman D, Gallacher P, Wennborg A, Hickman DK. Eprenetapopt plus azacitidine after allogeneic hematopoietic stem-cell transplantation for TP53-mutant acute myeloid leukemia and myelodysplastic syndromes. J Clinic Oncol. 2022;40(34):3985–93. doi: 10.1200/JCO.22.00181. [DOI] [PubMed] [Google Scholar]
- 162.Kruer TL, Quintana A, Ferrall-Fairbanks M, Zhang L, Newman H, McLemore AF, et al. XPO1 overexpression is a mechanism of resistance to eprenetapopt and 5-azacitidine therapy that can be therapeutically exploited for the treatment of TP53 mutated myeloid malignancies. Blood. 2022;140(Supplement 1):99–100. doi: 10.1182/blood-2022-169765. [DOI] [Google Scholar]
- 163.Oldenborg PA, Zheleznyak A, Fang YF, Lagenaur CF, Gresham HD, Lindberg FP. Role of CD47 as a marker of self on red blood cells. Science. 2000;288(5473):2051–2054. doi: 10.1126/science.288.5473.2051. [DOI] [PubMed] [Google Scholar]
- 164.Jaiswal S, Jamieson CH, Pang WW, Park CY, Chao MP, Majeti R, et al. CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell. 2009;138(2):271–285. doi: 10.1016/j.cell.2009.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Majeti R, Chao MP, Alizadeh AA, Pang WW, Jaiswal S, Gibbs KD, Jr, et al. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell. 2009;138(2):286–299. doi: 10.1016/j.cell.2009.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Liu J, Wang L, Zhao F, Tseng S, Narayanan C, Shura L, et al. Pre-clinical development of a humanized anti-CD47 antibody with anti-cancer therapeutic potential. PLoS ONE. 2015;10(9):e0137345. doi: 10.1371/journal.pone.0137345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Sallman DA, Malki MA, Asch AS, Lee DJ, Kambhampati S, Donnellan WB, et al. Tolerability and efficacy of the first-in-class anti-CD47 antibody magrolimab combined with azacitidine in MDS and AML patients: phase Ib results. J Clinic Oncol 2020; 38 (15_suppl):7507.
- 168.Daver N, Senapati J, Maiti A, Loghavi S, Kadia TM, DiNardo CD, et al. Phase I/II study of azacitidine (AZA) with venetoclax (VEN) and magrolimab (Magro) IN Patients (pts) with newly diagnosed (ND) older/unfit or high-risk acute myeloid leukemia (AML) and relapsed/refractory (R/R) AML. Blood. 2022;140(Supplement 1):141–144. doi: 10.1182/blood-2022-170188. [DOI] [Google Scholar]
- 169.Bhatlekar S, Fields JZ, Boman BM. Role of HOX genes in stem cell differentiation and cancer. Stem Cells Int. 2018;2018:3569493. doi: 10.1155/2018/3569493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Alharbi RA, Pettengell R, Pandha HS, Morgan R. The role of HOX genes in normal hematopoiesis and acute leukemia. Leukemia. 2013;27(5):1000–1008. doi: 10.1038/leu.2012.356. [DOI] [PubMed] [Google Scholar]
- 171.Collins CT, Hess JL. Deregulation of the HOXA9/MEIS1 axis in acute leukemia. Curr Opin Hematol. 2016;23(4):354–361. doi: 10.1097/MOH.0000000000000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Spencer DH, Young MA, Lamprecht TL, Helton NM, Fulton R, O'Laughlin M, et al. Epigenomic analysis of the HOX gene loci reveals mechanisms that may control canonical expression patterns in AML and normal hematopoietic cells. Leukemia. 2015;29(6):1279–1289. doi: 10.1038/leu.2015.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Alcalay M, Tiacci E, Bergomas R, Bigerna B, Venturini E, Minardi SP, et al. Acute myeloid leukemia bearing cytoplasmic nucleophosmin (NPMc+ AML) shows a distinct gene expression profile characterized by up-regulation of genes involved in stem-cell maintenance. Blood. 2005;106(3):899–902. doi: 10.1182/blood-2005-02-0560. [DOI] [PubMed] [Google Scholar]
- 174.Armstrong SA, Staunton JE, Silverman LB, Pieters R, den Boer ML, Minden MD, et al. MLL translocations specify a distinct gene expression profile that distinguishes a unique leukemia. Nat Genet. 2002;30(1):41–47. doi: 10.1038/ng765. [DOI] [PubMed] [Google Scholar]
- 175.Yokoyama A, Wang Z, Wysocka J, Sanyal M, Aufiero DJ, Kitabayashi I, et al. Leukemia proto-oncoprotein MLL forms a SET1-like histone methyltransferase complex with menin to regulate Hox gene expression. Mol Cell Biol. 2004;24(13):5639–5649. doi: 10.1128/MCB.24.13.5639-5649.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Yokoyama A, Somervaille TC, Smith KS, Rozenblatt-Rosen O, Meyerson M, Cleary ML. The menin tumor suppressor protein is an essential oncogenic cofactor for MLL-associated leukemogenesis. Cell. 2005;123(2):207–218. doi: 10.1016/j.cell.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 177.Yokoyama A, Cleary ML. Menin critically links MLL proteins with LEDGF on cancer-associated target genes. Cancer Cell. 2008;14(1):36–46. doi: 10.1016/j.ccr.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Uckelmann HJ, Kim SM, Wong EM, Hatton C, Giovinazzo H, Gadrey JY, et al. Therapeutic targeting of preleukemia cells in a mouse model of NPM1 mutant acute myeloid leukemia. Science. 2020;367(6477):586–590. doi: 10.1126/science.aax5863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Vassiliou GS, Cooper JL, Rad R, Li J, Rice S, Uren A, et al. Mutant nucleophosmin and cooperating pathways drive leukemia initiation and progression in mice. Nat Genet. 2011;43(5):470–475. doi: 10.1038/ng.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Krivtsov AV, Evans K, Gadrey JY, Eschle BK, Hatton C, Uckelmann HJ, et al. A Menin-mll inhibitor induces specific chromatin changes and eradicates disease in models of MLL-rearranged leukemia. Cancer Cell. 2019;36(6):660–73. doi: 10.1016/j.ccell.2019.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Klossowski S, Miao H, Kempinska K, Wu T, Purohit T, Kim E, et al. Menin inhibitor MI-3454 induces remission in MLL1-rearranged and NPM1-mutated models of leukemia. J Clin Investig. 2020;130(2):981–997. doi: 10.1172/JCI129126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Issa GC, Aldoss I, DiPersio JF, Cuglievan B, Stone RM, Arellano ML, et al. The menin inhibitor SNDX-5613 (revumenib) leads to durable responses in patients (Pts) with KMT2A-rearranged or NPM1 mutant AML: updated results of a phase (Ph) 1 study. Blood. 2022;140(Supplement 1):150–152. doi: 10.1182/blood-2022-164849. [DOI] [Google Scholar]
- 183.Erba HP, Fathi AT, Issa GC, Altman JK, Montesinos P, Patnaik MM, et al. Update on a phase 1/2 first-in-human study of the menin-KMT2A (MLL) inhibitor ziftomenib (KO-539) in patients with relapsed or refractory acute myeloid leukemia. Blood. 2022;140(Supplement 1):153–156. doi: 10.1182/blood-2022-167412. [DOI] [Google Scholar]
- 184.Issa GC, Cuglievan B, Stein E, Arellano ML, Žucenka A, Khera N, et al. Outcomes after transplant in relapsed/refractory KMT2Ar (MLLr) and mNPM1 (NPM1c) leukemia patients achieving remissions after menin inhibition: SNDX-5613 (revumenib) Ph1 experience. Blood. 2022;140(Supplement 1):914–916. doi: 10.1182/blood-2022-164600. [DOI] [Google Scholar]
- 185.Himmelstein G, Mascarenhas J, Marcellino BK. Alternatives to intensive treatment in patients with AML. Clin Adv Hematol Oncol. 2021;19(8):526–535. [PubMed] [Google Scholar]
- 186.Dohner H, Wei AH, Lowenberg B. Towards precision medicine for AML. Nat Rev Clin Oncol. 2021;18(9):577–590. doi: 10.1038/s41571-021-00509-w. [DOI] [PubMed] [Google Scholar]
- 187.Salamero O, Somervaille TCP, Molero A, Acuña-Cruz E, Perez-Simon JA, Coll R, et al. Iadademstat combination with azacitidine is a safe and effective treatment in first line acute myeloid leukemia. Final results of the alice trial. Blood. 2022;140(Supplement 1):1711–3. doi: 10.1182/blood-2022-168945. [DOI] [Google Scholar]
- 188.Uy GL, Aldoss I, Foster MC, Sayre PH, Wieduwilt MJ, Advani AS, et al. Flotetuzumab as salvage immunotherapy for refractory acute myeloid leukemia. Blood. 2021;137(6):751–762. doi: 10.1182/blood.2020007732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Lamble AJ, Liu X, Minard C, Militano O, Bernhardt MB, Cooper TM, et al. Safety and activity of flotetuzumab in pediatric and young adult patients with relapsed/refractory acute myeloid leukemia: results from the COG PEPN1812 phase 1 trial. Blood. 2022;140(Supplement 1):6209–6210. doi: 10.1182/blood-2022-158029. [DOI] [Google Scholar]
- 190.Daver N, Montesinos P, Aribi A, Marconi G, Altman JK, Wang ES, et al. Broad activity for the pivekimab sunirine (PVEK, IMGN632), azacitidine, and venetoclax triplet in high-risk patients with relapsed/refractory acute myeloid leukemia (AML) Blood. 2022;140(Supplement 1):145–149. doi: 10.1182/blood-2022-158030. [DOI] [Google Scholar]
- 191.Liu H, Sharon E, Karrison TG, Zha Y, Fulton N, Streicher H, et al. Randomized phase ii study to assess the role of nivolumab as single agent to eliminate minimal residual disease and maintain remission in acute myelogenous leukemia (AML) patients after chemotherapy (NCI9706 protocol; REMAIN trial) Blood. 2022;140(Supplement 1):1716–1719. doi: 10.1182/blood-2022-157326. [DOI] [Google Scholar]
- 192.Ilyas R, Johnson IM, McCullough K, Al-Kali A, Alkhateeb HB, Begna K, et al. Outcome of patients with acute myeloid leukemia following failure of front-line venetoclax plus hypomethylating agent therapy. Blood. 2022;140(Supplement 1):1286–1287. doi: 10.1182/blood-2022-165491. [DOI] [PubMed] [Google Scholar]
- 193.Piciocchi A, Messina M, Cipriani M, Paoloni F, Simonetti G, Palmieri R, et al. The addition of venetoclax to induction chemotherapy in no low-risk AML patients: a propensity score-matched analysis of the gimema AML1718 and AML1310 trials. Blood. 2022;140(Supplement 1):137–138. doi: 10.1182/blood-2022-158890. [DOI] [Google Scholar]
- 194.Marconi G, Piciocchi A, Audisio E, Priolo G, Papayannidis C, Martelli M, et al. Gimema AML1718 part 1: planned interim analysis of a safety run-in and phase 2 open-label study of venetoclax, fludarabine, idarubicin and cytarabine (V-FLAI) in the induction therapy of non low-risk acute myeloid leukemia. Blood. 2022;140(Supplement 1):1705–1707. doi: 10.1182/blood-2022-157393. [DOI] [Google Scholar]
- 195.Chua CC, Loo S, Reynolds J, Tiong IS, Fong CY, Ting SB, et al. High response and prolonged treatment-free remission after a short-course of modified intensive chemotherapy and venetoclax in elderly AML: an updated analysis of the caveat trial. Blood. 2022;140(Supplement 1):1708–1710. doi: 10.1182/blood-2022-165021. [DOI] [Google Scholar]
- 196.Abaza Y, Khan T, Dinner S, Frankfurt O, Altman JK. Venetoclax (VEN) plus intensive chemotherapy (IC) with FLAG-IDA in patients (Pts) with newly diagnosed (ND) and relapsed/refractory (R/R) acute myeloid leukemia (AML) Blood. 2022;140(Supplement 1):9036–9038. doi: 10.1182/blood-2022-168437. [DOI] [Google Scholar]
- 197.Bazarbachi A, Galimard J-E, Labopin M, Abou Dalle I, Lioure B, Maertens JA, et al. Frequency and impact of pre-transplant somatic Co-occurring mutations on clinical outcomes of acute myeloid leukemia patients receiving allogeneic hematopoietic stem cell transplantation: On behalf of the EBMT acute leukemia working party. Blood. 2022;140(Supplement 1):735–736. doi: 10.1182/blood-2022-162162. [DOI] [Google Scholar]
- 198.Hernández Sánchez A, Villaverde Ramiro A, Sträng E, Gastone C, Heckman CA, Versluis J, et al. Machine learning allows the identification of new Co-mutational patterns with prognostic implications in NPM1 mutated AML - results of the European harmony alliance. Blood. 2022;140(Supplement 1):739–742. doi: 10.1182/blood-2022-167138. [DOI] [Google Scholar]
- 199.Torabi A, Alonzo TA, Othus M, Gerbing RB, Wang Y-C, Ries RE, et al. Characteristics and prognostic effects of DNMT3A Co-mutations. Blood. 2022;140(Supplement 1):745–747. doi: 10.1182/blood-2022-162526. [DOI] [Google Scholar]
- 200.Macaron W, Ravandi F, Kadia TM, DiNardo CD, Issa GC, Daver N, et al. Clinical outcomes and impact of therapeutic intervention in patients (pts) with acute myeloid leukemia (AML) with recurrence of measurable residual disease (MRD) after achievement of MRD-negative remission. Blood. 2022;140(Supplement 1):543–545. doi: 10.1182/blood-2022-156955. [DOI] [Google Scholar]
- 201.Othman J, Potter N, Mokretar K, Taussig D, Khan A, Krishnamurthy P, et al. High molecular response rate and overall survival with FLT3 inhibitors as MRD-guided salvage treatment for molecular failure in AML. Blood. 2022;140(Supplement 1):2002–2004. doi: 10.1182/blood-2022-159503. [DOI] [Google Scholar]
- 202.Bazinet A, Kadia TM, Short N, Borthakur G, Wang SA, Loghavi S, et al. Achievement of measurable residual disease (MRD) negativity supersedes treatment intensity in predicting the outcome of patients with acute myeloid leukemia. Blood. 2022;140(Supplement 1):2267–2269. doi: 10.1182/blood-2022-162892. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The material supporting the information of this review has been included within this article.