Abstract
Kidney, bladder, and prostate cancer are the three major tumor types of the urologic system that seriously threaten human health. Circular RNAs (CircRNAs), special non-coding RNAs with a stabile structure and a unique back-splicing loop-forming ability, have received recent scientific attention. CircRNAs are widely distributed within the body, with important biologic functions such as sponges for microRNAs, as RNA binding proteins, and as templates for regulation of transcription and protein translation. The abnormal expression of circRNAs in vivo is significantly associated with the development of urologic tumors. CircRNAs have now emerged as potential biomarkers for the diagnosis and prognosis of urologic tumors, as well as targets for the development of new therapies. Although we have gained a better understanding of circRNA, there are still many questions to be answered. In this review, we summarize the properties of circRNAs and detail their function, focusing on the effects of circRNA on proliferation, metastasis, apoptosis, metabolism, and drug resistance in kidney, bladder, and prostate cancers.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12943-023-01766-2.
Keywords: CircRNA, Kidney cancer, Bladder cancer, And prostate cancer
Introduction
Prostate, renal, and bladder cancer (BCa) are the most common tumors of the urinary system [1]. Urinary system tumors account for approximately 10% of total tumor incidence, with prostate cancer accounting for 29% of male cancer cases [2]. Surgery is currently the most effective treatment for urological tumors, but the 5-year survival rate is still unsatisfactory [3, 4]. For locally progressive and metastatic prostate cancer (PCa), androgen ablation is usually the best treatment option [5]. This treatment option effectively extends overall patient survival [6]. Targeted and immune checkpoint therapies have been shown to improve the prognosis for renal cell carcinoma (RCC) patients, with tyrosine kinase inhibitors widely used for clinical treatment [7, 8]. However, patients with BCa have a high recurrence rate and are prone to distant metastases. Patients with advanced or metastatic BCa have a poor prognosis [9]. Post-operative supplementation with bladder instillation of BCG or gemcitabine significantly improves the quality of life for patients with BCa and effectively reduce the recurrence rate [10, 11]. Although there have been many improvements in BCa treatment, early diagnosis of disease results in the best possible patient outcomes. Therefore, identification of novel biomarkers for tumor is a significant and current clinical objective, with circRNAs promising biomarkers for disease [12, 13].
Non-coding RNAs (ncRNAs) are a class of RNA that are not translated into proteins and are usually classified by size into small RNAs such as microRNA (miRNA) (about 22nt) and long-stranded non-coding RNAs (lncRNA, > 200 nt) [14]. NcRNAs are involved in tumor development and play an important role in disease [15]. CircRNAs are a family of naturally occurring long non-coding RNAs that are widely expressed in eukaryotes. Covalently closed circular RNA molecules were first discovered in 1976 within viroids [16]. Subsequently, circRNAs were found to exist in the cytoplasm of eukaryotic cells [3] and to be expressed in a cell- and organ-specific manner with important biological functions [17, 18]. With the development and popularization of sequencing technology, the importance of circRNAs has become clearer. As a special lncRNA, circRNAs have a unique covalent single-stranded closed-loop structure, which lack a 5’ cap or 3’ poly (A) tail [19]. Compared to mRNAs, circRNA have a more stable structure and are not easily degraded by RNase A. Recent studies have shown that circRNAs play important roles in many cancers by; acting as microRNA sponges [20], interacting with RNA-binding proteins [21], gene transcriptional regulation, alternative splicing [22–24], and protein translation [25].
In this review, we briefly describe the principles of circRNA formation and summarize the impact of circRNAs on the occurrence and development of RCC, PCa, and BCa. Further, the use of circRNAs as diagnostic and prognostic biomarkers is discussed. A deeper understanding of circRNAs will provide valuable clues and useful information for future urologic cancer treatment.
Overview of CircRNAs
In 1976, Sanger initiated the study of circRNAs [16]. Nigro et al. were the first to identify circular RNA transcripts in mammals - a phenomenon of “scrambled exons” in which exons were linked end-to-end to form a circular structure [26]. With improved technology and in-depth research, ncRNAs, once considered a “miscellaneous signal”, have been extensively studied. CircRNAs have a unique circular structure. Compared with linear RNAs, circRNAs are more stable and resistant to degradation by nucleic acid exonucleases [27]. The structure is highly conserved throughout development and evolution.
Biogenesis and regulation of CircRNAs
CircRNAs can be classified based on the method of splicing. Exon circRNAs (EcircRNAs), exon-intron circRNAs (EIcircRNAs), circular intron RNAs (ciRNAs), and tRNAintronic circular RNAs (tricRNAs) are formed after splicing of precursor transfer RNAs (pre-tRNAs) [28] (Fig. 1). EcircRNAs account for the majority of circRNAs and are mainly located within the cytoplasm [29]. EIcircRNAs and ciRNAs are primarily located within the nucleus [30]. The current common circRNA looping models are; direct back splicing, lasso-driven circularization, and RNA-binding protein-mediated circularization [31]. The formation of ecircRNAs is usually by direct back splicing. Direct back splicing is a common shearing method in which precursor-mRNA is looped by linking the 5′ splice donor site downstream of the exon to the 3′ splice acceptor site upstream via covalent bonding. The process forms a cord-tailed splice loop, which is followed by removal of the intron by shearing, producing a closed-loop circRNA [22]. EIcircRNAs also use the method of direct back splicing, but in the process of back splicing, some intron sequences are not removed but remain within the circRNA [32]. The formation of ciRNAs is thought to result from lasso formation from introns removed during pre-mRNA splicing. The formation of such ciRNAs can be recapitulated using expression vectors, wherein processing depends on a consensus motif containing a 7 nt GU-rich element near the 5′ splice site and an 11 nt C-rich element close to the branch point site [33].
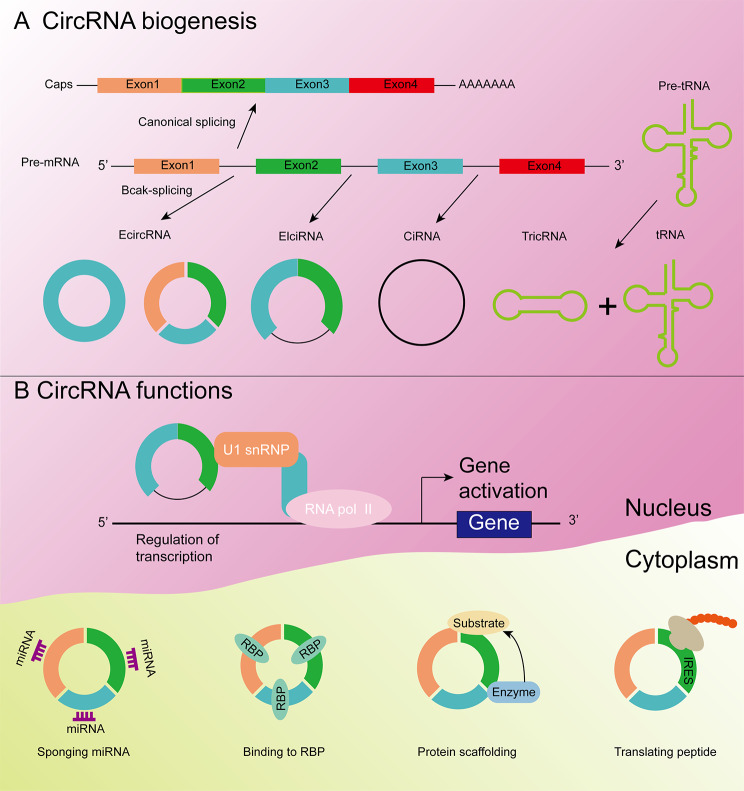
Fig. 1.
Biogenesis and function of circular RNA (circRNA)
Pre-mRNA can undergo either canonical splicing to generate a linear RNA with exon inclusion or back-splicing to produce both a circular RNA and an alternatively spliced linear RNA with exon exclusion. (A) The biogenesis of circRNAs. Biogenesis of circRNAs generates three types of circRNAs: EcircRNAs, ciRNAs, and EIciRNAs. The formation of ecircRNAs is usually by direct back splicing. Direct back splicing is a common shearing method in which precursor-mRNA is looped by linking the 5′ splice donor site downstream of the exon to the 3′ splice acceptor site upstream via covalent bonding. EIcircRNAs also use the method of direct back splicing, but in the process of back splicing, some intron sequences are not removed but remain within the circRNA. The formation of ciRNAs is thought to result from lasso formation from introns removed during pre-mRNA splicing. TricRNA is a circular RNA produced by tRNA precursor splicing. (B) The function of circRNAs. CircRNAs enhance the transcription and splicing of their parental genes by interacting with RNA pol II or U1 small nuclear ribonucleoproteins (U1 snRNPs). CircRNAs have basic functions as microRNA (miRNA) sponges, RNA-binding proteins (RBP), and templates for regulating transcription and protein translation. CircRNAs can function as microRNA sponges or decoys, protecting target mRNAs from miRNA-dependent degradation. circRNAs containing RBP binding motifs may function as sponges or decoys for these proteins and indirectly regulate their functions. circRNAs have been shown to function as protein scaffolds, facilitating the colocalization of enzymes and their substrates. CircRNA with internal ribosomal entry site elements may be translated under certain circumstances to produce unique peptides
RNA-binding proteins (RBPs) are also a means by which circRNAs are formed. RBPs include; muscle blind-like protein 1, the RNA-editing enzyme, quaking, and fusion sarcoma protein [34–37]. Exon loops can also be driven in a similar fashion by the dimerization of RBPs that bind to specific motifs within flanking introns [36]. In large-scale transcriptome analysis, 61% of human genes can generate both circular and linear transcripts [38, 39]. CircRNA biogenesis uses canonical splice sites, with back-splicing in competition with linear splicing of mRNAs. Transcript levels of circRNAs are generally at lower levels than mRNAs. Back splicing is reduced during normal physiological conditions, with decreased activity of spliceosomal components resulting in increased circRNA expression [40]. CircRNA exons and their flanking introns are much longer than linear RNAs [18]. These longer introns contain reverse complementary sequence elements (e.g. inverted-repeat Alu elements) that bring the downstream 5-donor and upstream 3-acceptor splice sites into proximity [41, 42].
Detection and identification of CircRNAs
More than 100,000 different human circRNAs have been discovered in the past decades [43, 44]. However, the unique circular structure, low abundance, and lack of 5′-caps and 3′-poly (A) tails make it difficult to efficiently detect and identify circRNAs by commonly used techniques [45].
Northern blotting and PCR techniques have played an important role in the initial detection and discovery of circRNAs (Table 1) [46]. Northern blotting is the gold standard for circRNA validation by hybridization with RNA probes and separation of circRNAs into linear counterparts by denaturing polyacrylamide gel electrophoresis [33, 41, 47]. The disadvantages of northern blotting are a poor detection rate for low abundance circRNAs and the lack of a means for high-throughput detection [48]. RT-qPCR is the most commonly used method for circRNAs analysis using different primers that flank the BSJ locus for circRNA fragment amplification and circRNA detection [47, 49]. Due to strand displacement and rolling circle replication, circRNA molecules can produce false positive signals during reverse transcription [50].
Table 1.
Methods for detecting and quantifying circRNA
| Method | Sensitivity /Accuracy |
Throughput | Advantages and Disadvantages | Ref |
|---|---|---|---|---|
| Northern blotting | Low | Low | Gold standard analysis of circRNA validation;Insensitive to low expression of circRNA | [33, [41, 46–48] |
| RT-qPCR | Medium-High | Low-Medium | Provide quantitative data; False positive signal may be generated | [47, 49, 50] |
| RCA | High | Medium | Simple operation, no need for high-precision temperature cycle and additional separation steps. | [51, 52] |
| NanoString Technologies nCounter assays | High | Medium-High | Quantify circRNA;Requires special equipment and is expensive | [54] |
| Microarrays | Medium | High | High throughput and high detection efficiency;Difficult to compare between different studies | [46, 55, 56] |
| FISH | High | Low | Forming stable DNA-RNA hybrid;Highly affected by personnel operations and instrumentation and costly | [57, 58] |
| RNA-seq | Medium-High | High | Widely used in the discovery of novel circRNAs;Overlap with linear molecular signals, data processing capabilities are required | [59, 60, 62, 64] |
Rolling circle amplification (RCA) is a recently developed technique wherein the circular structure of circRNAs is an ideal template for initiating the rolling circle amplification process, making this technique particularly suitable for circRNA analysis [51]. The reverse transcription RCA method selectively amplifies target circRNAs, with the advantages of ease of operation and low cost [52]. Enzyme-based circRNA detection reduces the need for reliance upon primers for detection of circRNAs. Duplex-specific nucleases (DSN) digest the DNA strand of a DNA/circRNA hybrid, releasing a fluorescent probe fragment and circRNA, which enhances the fluorescent signal in the presence of target circRNA [53]. NanoString Technologies nCounter assays are the latest method for accurate and sensitive detection of circRNA. NanoString uses dual probe hybridization with biotinylated capture probes and unique color-coded reporter probes to accurately quantify circRNA without enzymatic reactions or bias [54]. However, this technology requires specialized equipment that is expensive. Microarrays are also widely used as a high-throughput assay with good accuracy and sensitivity for circRNAs detection. These assays are not affected by low levels of transcript expression and are suitable for circRNA expression studies [55, 56]. However, the data generated by microarrays are difficult to compare between different studies [46]. Fluorescence in situ hybridization (FISH) is a common tool for studying the subcellular localization of RNA, using fluorescently labeled DNA probes [57, 58]. However, this technique is time-consuming and requires expensive signal detection equipment [46].
The development of RNA-seq technology has greatly advanced biomedical research as well as the detection of circRNAs [59, 60]. The low abundance of circRNAs in vivo and the lack of poly (A) tails require biochemical enrichment of circRNAs prior to short-read deep sequencing [45]. Treatment of samples with RNase R significantly enriches circRNAs [49, 61]. Performing rRNA depletion prior to sequencing and construction of circRNAs libraries, without polyA screening and transcriptome sequencing, is widely used for early circRNA analysis [62]. Ribo-RNA seq provides expression data for both coding and non-coding RNAs and has identified hundreds of circRNAs [24]. Ribo-RNA-seq has the advantage of direct quantitative comparison of the expression levels of circRNAs and their cognate linear RNAs [63]. However, this approach leads to overlapping signals of circRNAs and linear RNAs in exonic regions that cannot be separated [64]. Poly(A)-RNA-seq is suitable for circRNAs lacking poly(A) tails, and this technique has identified hundreds of human genes expressing circular RNA isoforms [65]. The limitations of this technique are that it may not detect small circRNAs and cannot accurately identify and quantify rare circular isoforms [65]. The RNase R RNA-seq method can identify more individual circRNAs than ribo- or poly (A)-RNA-seq, maximizing circRNA enrichment [41, 47]. There is a need for development of a simple, accurate, sensitive, and effective method for circRNA detection and analysis.
Functions of CircRNAs
CircRNAs act as MiRNA sponges
The most widely studied circRNA function is the capacity of circRNAs to serve as miRNA sponges. MiRNAs, an important class of small non-coding RNAs, can bind to the 3′ untranslated regions (3′ UTR) of mRNA, regulating gene expression, protein degradation, and translational repression [66]. By study of circRNA, circRNAs were found to bind miRNA and compete with mRNA for miRNA binding, serving as miRNA sponges and indirectly affecting gene expression [67, 68]. When circRNA adsorbs functional miRNA, it reduces the availability of miRNA and upregulates the expression of miRNA-targeted mRNA. The mRNA can then be recombined by the ribosome and converted into protein [24]. CiRS-7 is a canonical circRNA that acts as miRNA sponge that contains more than 70 miR-7 binding sites [69, 70]. A recent study found that ciRS-7 promotes the progression and metastasis of RCC through the PI3K/AKT signaling pathway by binding to miR-139-3p and blocking its inhibitory effect on TAGLN [71]. Many other circRNAs have also been shown to function as miRNA sponges. CircEYA3 exerts its oncogenic function by up-regulation of c-Myc expression through adsorption of miR-1294 [72]. Androgen receptor (AR) inhibits circHIAT1 expression, reducing expression of miR-195-5p/29a-3p/29c-3p, thereby increasing CDC42 expression, enhancing migration and invasion of Clear cell renal cell carcinomas (ccRCC) [73]. CircFAT1 contains miR-375 binding sites and adsorbs miR-375, up-regulating YES-associated protein 1 expression. Furthermore, inhibition of miR-375 reverses the attenuation of cell proliferation, migration, and invasion induced by circFAT1 knockdown, thereby promoting tumorigenesis [74].
CircRNAs affect splicing and regulate transcription
Alternative splicing in eukaryotes ensures the diversity of biological proteins. The biogenesis of circRNAs at the cellular level relies on typical splice sites and spliceosome mechanisms and competes with pre-mRNA splicing, which in turn affects the expression of transcribed genes [42]. Studies have found that circRNAs have the ability to transcribe and control transcription and gene expression during development and disease. EIcircRNAs and ciRNAs in the nucleus play a very important role in gene transcription. EICircRNAs interact with U1 small nuclear ribonucleoproteins (U1 snRNPs) and with the polymerase II complex, promoting transcription of their parent genes [75]. CircDONSON is expressed in the nucleus and positively related with a poor advanced TNM stage and poor prognosis for gastric cancer patients. CircDONSON recruits the NURF complex to initiate SOX4 expression, which promotes gastric cancer progression by direct interaction with the SNF2L subunit [76]. High expression of circRHOT1 in hepatocellular carcinoma (HCC) significantly promotes the growth and metastasis of HCC, with circRHOT1 recruiting TIP60 to the NR2F6 promoter, initiating NR2F6 transcription. Knockdown of circRHOT1 inhibits the transcription of NR2F6, and also inhibits the proliferation and metastasis of hepatoma cells [77].
CircRNAs function by interaction with proteins
In addition to serving as miRNA sponges and as a means by which to bind polymerase II complexes, circRNAs can also bind proteins. As a protein sponge, circRNA can regulate RNA indirectly, with some circRNAs containing only a few miRNA binding sites and are therefore particularly important for protein binding [42].
However, the RBP binding density of circRNA is less than that of linear mRNA [78]. CircFoxo3 is highly expressed in non-cancer cells and is associated with cell cycle progression. CircFoxo3 can bind CDK2 and P21 to inhibit cell cycle progression to form a ternary complex [79]. High expression of circAmotl1 in neonatal cardiac tissue can enhance AKT and enhance cardiomyocyte survival. CircAmotl1 binds to PDK1 and AKT1 to activate AKT phosphorylation and nuclear translocation [80].
CircRNAs undergo translation
Since circRNA acts as a non-coding RNAs, there is no free 5′ cap or 3′ poly(A) tail or easily recognizable open reading frame necessary for translation control [81]. Initially, circRNAs were thought to have no protein-coding ability, although some studies have shown circRNAs to code for protein. Due to the presence of a specific sequence, insertion of a synthetic internal ribosome entry site (IRES) upstream of the initial codon can directly recruit ribosome translation initiation [82]. The presence of methylated adenosine nucleotides in the form of N6-methyladenosine (m6A), which directly binds eIF3, has also been shown to drive the translation of circRNAs [81]. CircZNF609, which specifically controls myogenic cell proliferation, contains an open reading frame at the start codon, identical to the linear transcript, which terminates at an in-frame stop codon generated after cycling. CircZNF609 is associated with heavy multimers and is translated into protein in a splicing-dependent and cap-independent manner, providing an example of protein-encoding circRNAs in eukaryotes [83]. Zhang et al. found evidence that circSHPRH, a novel functional protein that translates SHPRH-146 A, acts as a tumor suppressor in glioblastoma [84].
Application of CircRNAs
With a unique structure, stability, and immunogenicity, there has been an increased interest in the possible biological usefulness of circRNAs. CircRNAs are stable and with their translation capacity can be used as a complement to mRNA-based therapeutics using adeno-associated vector technology [85, 86]. Delivery of nano-formulations of circRNA containing IRES showed enhanced translational duration [85, 87]. The circRNA vaccine developed against SARS-CoV-2 showed durable antigen production and the capacity to produce neutralizing antibodies [88]. For the first time, the team of Wei Wensheng from Peking University prepared a circRNA vaccine against the delta variant of COVID-19. The vaccine had broad-spectrum protection against a variety of new coronavirus variants [88]. However, the means to improve production and delivery of circRNA and the means by which to reduce the cellular immune response triggered by circRNA are issues that require further development and refinement [89, 90].
Dysregulated circRNA expression is involved in a variety of diseases, including but not limited to autoimmune diseases, cancer, liver diseases, neurological diseases, cardiovascular diseases, and diabetes [64, 91–94]. There are many DNA, RNA and protein-based biomarkers currently used clinically as disease diagnostic aids [95]. CircRNAs are not easily degraded by nucleic acid exonucleases and therefore have a much longer half-life than linear RNAs, making circRNAs ideal candidates as biomarkers [96]. Significant expression levels of circRNAs have been found in blood, gastric juice, saliva, and urine from prostate cancer patients, suggesting that circRNAs have the potential to become biomarkers for early cancer diagnosis [43, 97–100]. In clinical studies, the combination of prostate specific antigen testing and circRNAs detection, greatly improved diagnosis of prostate cancer [101].
CircRNAs are also promising clinical therapeutic targets for a number of diseases. For example, F-circRNA aids acute promyelocytic leukemia cell transformation, cell survival, and resistance to treatment. As such it is a potential therapeutic target for disease treatment [102]. CircSLC8A1 is abundant within the heart, with inhibition of circSLC8A1 in vivo decreasing myocardial ischemia-reperfusion injury and stress overload-induced cardiac hypertrophy [103, 104]. However, the development of circRNA therapies is still in its infancy and there are a number of issues that require further improvement. Common issues are design and optimization of circRNAs overexpression vectors, improved cyclization, development of efficient delivery systems, improved chemical manufacturing procedures, and control process development [62]. There is much to be learned regarding circRNAs but they will likely become a powerful weapon for clinical treatment and diagnosis in the near future.
Expression and biological function of CircRNAs in urinary system tumors
Expression and biological function of CircRNAs in kidney Cancer
CircRNAs play a critical role in the development and progression of kidney cancer. One study identified approximately 2000 differentially expressed circRNAs in kidney cancer cells using RNA-seq [105]. CircRNAs are involved in kidney cell proliferation, apoptosis, cell metastasis, invasion, epithelial-mesenchymal transition (EMT), cell metabolism, and can be used for patient prognosis (Fig. 2). In this section, we will describe the role of circRNAs in regulation of these cellular processes and will highlight the involved molecular mechanisms (Table 2).
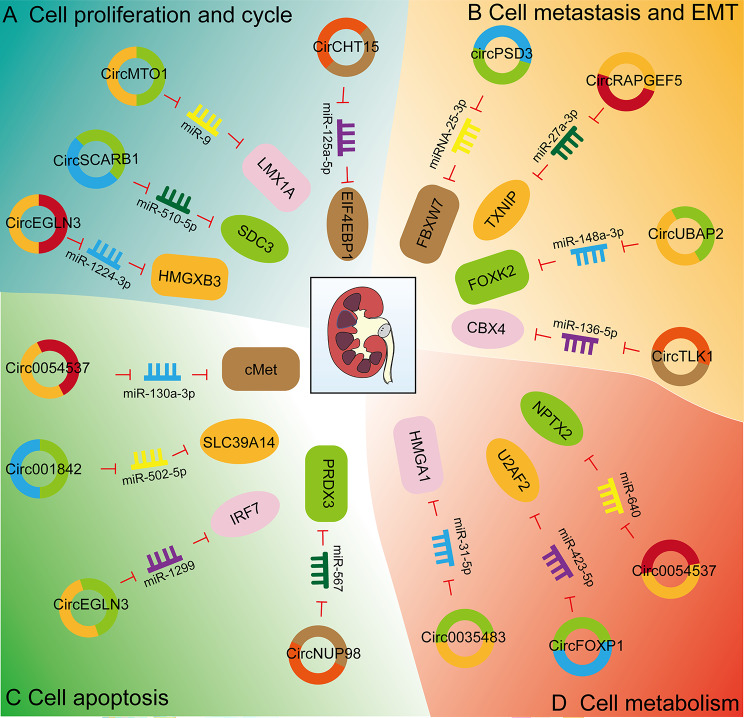
Fig. 2.
Biological functions of circRNAs in kidney cancer
(A) CircRNAs are involved in the cell proliferation and cycle of kidney cancer. CircCHT15, circSCARB1, circMTO1, and circEGLN3 affect the cell proliferation of kidney cancer cells by regulating the expression of miRNAs and related proteins. (B) CircRNAs are involved in the metastasis and EMT of kidney cancer. CircPSD3, circRAPGEF5, circUBAP2, and circTLK1 affect the cell metastasis and EMT of kidney cancer cells by regulating the expression of miRNAs and related proteins. (C) CircRNAs are involved in the apoptosis of kidney cancer. Circ0054537, circ001842, circEGLN3, and circNUP98 affect the cell apoptosis of kidney cancer cells by regulating the expression of miRNAs and related proteins. (D) CircRNAs are involved in the cell metabolism of kidney cancer. Circ0035483, circFOXP1, and circ0054537 affect the cell metabolism of kidney cancer cells by regulating the expression of miRNAs and related proteins
Table 2.
Overview of deregulated circRNAs in renal cell carcinoma
| CircRNA | Expression | Function | Target microRNA | miRNA target genes/protein | Ref |
|---|---|---|---|---|---|
| Circ000926 | Up |
Proliferation(+) Metastasis(+) |
miR-411 | CDH2 | [106] |
| CircEGLN3 | Up |
Proliferation(+) Metastasis(+) |
miR-1224-3p | HMGXB3 | [107] |
| CircRAPGEF5 | Down |
Proliferation(-) Metastasis(-) |
miR-27a-3p | TXNIP | [115] |
| Circ0001368 | Down |
Proliferation(-) Metastasis(-) |
miR-492 | LATS2 | [116] |
| Circ0054537 | Up |
Proliferation(+) Metastasis(+) Apoptosis(−) |
miR- 130a-3p | cMet | [122] |
| CircSCARB1 | Up |
Proliferation(+) Metastasis(+) Apoptosis(−) |
miR-510-5p | SDC3 | [108] |
| CircUBAP2 | Down |
Proliferation(-) Metastasis(-) |
miR-148a-3p | FOXK2 | [117] |
| CircMTO1 | Down |
Proliferation(-) Metastasis(-) |
miR-9 | LMX1A | [109] |
| CircAKT3 | Down | Metastasis(-) | miR-296-3p | E-cadherin | [118] |
| CircTLK1 | Up |
Proliferation(+) Metastasis(+) |
miR-136-5p | CBX4 | [110] |
| CircSDHC | Up |
Proliferation(+) Metastasis(+) |
miR-127-3p | CDKN3/E2F1 | [111] |
| CircCHST15 | Up |
Proliferation(+) Metastasis(+) |
miR-125a-5p | EIF4EBP1 | [112] |
| CircESRP1 | Down |
Proliferation(-) Metastasis(-) |
miR-3942 | CTCF | [119] |
| Circ0005875 | Up |
Proliferation(+) Metastasis(+) Apoptosis(−) |
miR-502-5p | ETS1 | [123] |
| Circ0035483 | Up |
Proliferation(+) Metastasis(+) |
miR-31-5p | HMGA1 | [127] |
| CircDVL1 | Down |
Proliferation(-) Metastasis(-) Apoptosis(+) |
miR-412-3p | PCDH7 | [113] |
| CircEGLN3 | Up |
Proliferation(+) Metastasis(+) Apoptosis(−) |
miR-1299 | IRF7 | [124] |
| CircMYLK | Up |
Proliferation(+) Metastasis(+) |
miR-513a-5p | VEGFC | [120] |
| Circ001842 | Up |
Proliferation(+) Metastasis(+) Apoptosis(−) |
miR-502-5p | SLC39A14 | [125] |
| CircNUP98 | Up |
Proliferation(+) Metastasis(+) Apoptosis(−) |
miR-567 | PRDX3 | [126] |
| CircFOXP1 | Up |
Proliferation(+) Metastasis(+) |
miR-423-5p | U2AF2 | [128] |
| CircPSD3 | Down |
Proliferation(-) Metastasis(-) |
miR-25-3p | FBXW7 | [114] |
| CircTXNDC11 | Up | Metastasis(+) | - | MAPK/ERK | [121] |
| Circ0054537 | UP |
Glycolysis(+) Apoptosis(−) |
miR-640 | NPTX2 | [129] |
Proliferation and cell cycle
Studies have shown that abnormal expression of circRNAs is associated with excessive renal cancer cell proliferation and disruption of cell cycle control. High expression levels of circ000926 in RCC are associated with a poor patient prognosis [106]. Silencing of circ000926 inhibits proliferation of RCC cells and inhibits the growth of xenografted tumors. Circ000926, a miRNA sponge, directly inhibits miR-411 and up-regulates CDH2. CircEGLN3 is highly expressed in kidney cancer, with inhibition of circEGLN3 reducing the proliferative ability of RCC ACHN and 769-P cell lines [107]. CircEGNL3 acts as a sponge for miR-1224-3p, which targets HMGXB3. CircEGNL3 indirectly up-regulates HMGXB3 by targeting miR-1224-3p, with overexpression of circEGLN3 reversing the inhibitory effect of miR-1224-3p on RCC. That study found circSCARB1 to be elevated in 30 pairs of RCC tissues and multiple RCC cell lines [108]. Inhibition of cell proliferation by silencing circSCARB1 is mediated by a direct interaction between circSCARB1 and miR-510-5p. SDC3 is a target gene of miR-510-5p. Transfection of miR-510-5p mimics not only suppresses SDC3 expression but also cell proliferation, with SDC3 co-transfection partially restoring cell proliferation control. Furthermore, overexpression of circMTO1 inhibits the proliferation of A498 and 786-O renal cancer cells, while circMTO1 silencing promotes the progression of SN12C and OS-RC-2 renal cancer cells [109]. CircMTO1 acts as a sponge for miR-9 and miR-223, suppressing their levels. Silencing circMTO1 downregulates LMX1A, a target of miR-9, promoting RCC cell proliferation. Transfection of a miR-9 mimic inhibited LMX1A expression, which confirmed that miR-9 exerts its function in RCC by regulation of LMX1A expression. Overexpression of a miR-9 inhibitor, with LMX1A, blocked the tumor-promoting effect of circMTO1 silencing. Similarly, high-throughput sequencing of circRNA in RCC cell lines found circTLK1 to be a novel candidate circRNA derived from the TLK1 gene [110]. CircTLK1 is highly expressed in RCC, with silencing of circTLK1 inhibiting the proliferation of RCC cells.
High expression levels of circSDHC in RCC tissues were found by GSE100186 and GSE137836 sequencing to be associated with advanced TNM stage and poor survival of RCC patients [111]. CircSDHC promotes tumor cell proliferation both in vivo and in vitro. RNA-pulldown and luciferase reporter assays demonstrated circSDHC to compete with miR-127-3p for binding, preventing inhibition of downstream CDKN3 and E2F1 pathways, resulting in malignant progression of RCC. Knockdown of circSDHC reduces CDKN3 expression, which inhibits the E2F1 pathway and can be rescued by miR-127-3p inhibitor therapy. CirCHT15 has been reported to be an independent prognostic indicator of overall survival and progression-free survival after surgical resection of ccRCC patients [112]. CirCHT15 is highly expressed in ccRCC tissues and renal cancer cell lines. In vivo and in vitro data suggest that cirCHT15 promotes the proliferation of ccRCC cells. CirCHT15 directly interacts with miR-125a-5p and regulates the expression of EIF4EBP1. CircDVL1 is poorly expressed in serum and tissues of ccRCC patients and is negatively related to malignant features of ccRCC [113]. Overexpression of circDVL1 inhibits the proliferation of various ccRCC cells and induces G1/S phase arrest. CircDVL1 acts as a molecular sponge for oncogenic miR-412-3p, preventing miR-412-3p-mediated repression of PCDH7 in ccRCC cells.
Metastasis and EMT
Cancer cell invasion and metastasis play a very important role in the occurrence and development of renal cancer. CircRNAs have been shown to regulate this process. For example, downregulation of circPSD3 in ccRCC tissues suggests that low levels of circPSD3 are associated with tumor metastasis in ccRCC patients [114]. CircPSD3 significantly inhibited cell migration, invasion, and EMT in vitro and blocked lung metastasis in vivo. CircPSD3 binds to miRNA-25-3p to regulate the expression of FBXW7. CircPSD3 inhibits tumor metastasis by suppressing the miR-25-3p/FBXW7-EMT axis and is expected to be a potential diagnostic and therapeutic target for ccRCC. The role of circRAPGEF5 in RCC is unclear, but analysis of samples from 245 RCC patients revealed that down-regulation of circRAPGEF5 was positively associated with aggressive clinical features [115]. We found that circRAPGEF5 inhibits the development and progression of RCC through the circRAPGEF5/miR-27a-3p/TXNIP pathway. A study showed that circ0001368 was downregulated in 786-O, ACHN, and A498 cell lines, as well as in RCC tissues [116]. That study found miR-492 to target miRNA of circ0001368. Inhibition of miR-492 inhibited cell metastasis, whereas overexpression of miR-492 enhanced cell metastasis. MiR-492 regulates LATS2 expression by binding to the 3′UTR region of LATS2. Previous studies have found that LATS2 inhibits HCC through the EZH2/DNMT1 axis and also inhibits glioma cell metastasis by up-regulation of tafazzin. Overexpression of LATS2 significantly inhibits the proliferation and invasion of ACHN and 786-O cells. Circ0001368 upregulates LATS2 expression by secreting miR-492, which inhibits renal cancer cell growth and invasion. Further, the circSCARB1/miR-510-5p/SDC3 signaling axis promotes invasion and migration of renal cancer cells [108]. CircUBAP2 expression is significantly downregulated in ccRCC tissues and cell lines [117]. Overexpression of circUBAP2 significantly inhibits the migration and invasion of ccRCC cells. CircUBAP2 targets miR-148a-3p, and miR-148a-3p reverses the inhibitory effect of circUBAP2 on ccRCC cell migration and invasion. Furthermore, FOXK2 was found to be a target gene of miR-148a-3p, with knockdown of FOXK2 reversing the inhibitory effect of the miR-148a-3p inhibitor on ccRCC cells. Further, circUBAP2 exerts a novel tumor-suppressive effect in ccRCC by regulating the miR-148a-3p/FOXK2 axis. Xia et al. found that circAKT3 was stably downregulated in ccRCC tissue samples by sequencing 60 ccRCC tissues and adjacent normal tissues as well as ccRCC cell lines [118]. Knockdown of circAKT3 promoted ccRCC migration and invasion, while overexpression of circAKT3 suppressed ccRCC metastasis. CircAKT3/miR-296-3p/E-cadherin is associated with the inhibition of ccRCC metastasis by circAKT3.
One study found circTLK1 to be mainly distributed within the cytoplasm, with high levels of expression positively related to distant metastasis and poor patient prognosis. Expression of CBX4 is positively regulated by miR-136-5p sponging [110]. Enhanced CBX4 expression reverses the phenotypic suppression of RCC cells induced by circTLK1 inhibition. Additionally, in RCC tissues CBX4 expression is positively related to VEGFA expression. CBX4 knockout significantly inhibits the expression of VEGFA in RCC cells. Another study found that circESRP1 was poorly expressed in renal cancer tissues and renal cancer cells, and its expression level was negatively related to advanced tumor size, TNM stage, and distant metastasis of ccRCC [119]. CircESRP1 competitively binds to miR-394. A downstream target of miR-394 is CTCF, which specifically promotes circESRP1 transcription, regulating the circESRP1/miR-3942 pathway and forming a positive feedback loop. This process regulates ccRCC cell function through a c-Myc-mediated EMT mechanism. CircMYLK is significantly up-regulated in RCC, with circMYLK binding miR-513a-5p and promoting the expression of VEGFC, which promotes the tumorigenesis of RCC cells [120]. Silencing circMYLK inhibits RCC metastasis in vitro and in vivo. Overexpression of circTXNDC11 is associated with advanced TNM staging and lymph node metastasis in renal cancer [121]. Knockdown of circTXNDC11 inhibits cell invasion and metastasis in vitro. CircTXNDC11 promotes RCC migration and invasion by activating the MAPK/ERK pathway.
Apoptosis
CircRNAs have been reported to regulate apoptosis in renal cancer cells. MiR-130a-3p is expressed at abnormally low levels in RCC cells, with up-regulation of miR-130a-3p promoting apoptosis of RCC cells [122]. A direct interaction between circ0054537 and miR-130a-3p was found by RNA-pulldown and luciferase reporter assay. The oncogene c-Met can be targeted by miR-130a-3p and is jointly controlled by circ0054537 and miR-130a-3p, affecting the tumorigenesis and development of RCC. Similarly, high expression levels of circSCARB1 are found in renal cancer cells with knockdown of circSCARB1 promoting apoptosis of 786-O and A498 cell lines [108]. Circ0005875 is up-regulated in RCC tissues and cells, with knockdown of the circ0005875 gene increasing the expression of miR-502-5p, thus promoting the apoptosis of renal cancer cells [123]. Sequencing results identified elevated circEGLN3 in RCC tissues and cell lines and predicted a poor prognosis for RCC patients [124]. Silencing of circEGLN3 promotes apoptosis in RCC cells. CircEGLN3 is primarily located in the cytoplasm where it inhibits RCC progression by targeting miR-1299, which regulates the expression of IRF7. In addition, circ001842 is highly expressed in RCC. Circ001842 is involved in RCC pathogenesis through a miR-502-5p-dependent SLC39A14 mechanism [125]. Knockdown of circ001842 promotes apoptosis of RCC cells. Another study found that circNUP98 was selectively up-regulated in 78 pairs RCC tissues, when compared to adjacent normal tissues. Silencing of circNUP98 down-regulated PRDX3 by up-regulation of miR-567, thereby promoting the apoptosis of RCC and inhibiting the progression of RCC [126]. Further, STAT3 has been identified as an inducer of circNUP98 in RCC cells. CircNUP98 functions as an oncogene through a novel STAT3/circNUP98/miR-567/PRDX3 axis, providing a potential biomarker and therapeutic target for RCC therapy.
Metabolism
CirCRNAs have been shown to control kidney cancer cell metabolism. For example, circ0035483 is upregulated in RCC tissues and cells. Downregulation of circ0035483 suppresses glucose consumption and lactate production in RCC cells, suggesting that overexpression of circ0035483 promotes glycolytic metabolism in RCC cells [127]. MiR-31-5p up-regulation is associated with circ0035483 knockout in RCC cells. MiR-31-5p targets HMGA1 and suppresses the malignant behavior of RCC cells by negative regulation of HMGA1. Moreover, circFOXP1 is highly expressed in RCC tissues and cells, with down-regulation of circFOXP1 inhibiting the glycolysis of renal cancer cells [128]. ZNF263, upstream of circFOXP1, promotes RCC progression through the miR-423-5p/U2AF2 axis. That study found circ0054537 to be distributed within the cytoplasm and to be up-regulated in RCC tissues and cells [129]. Circ0054537 gene knockout inhibits glycolysis in RCC cells but promotes apoptosis. Luciferase reporter assay and RNA-pulldown confirmed that circ0054537 binds miR-640 and targets NPTX2.
Expression and biological function of CircRNAs in bladder Cancer
Aberrant expression of circRNAs is associated with the development of BCa. Several high-throughput experiments have shown that circRNAs expression profiles are dysregulated in BCa [130]. CircRNAs are associated with patient prognosis, cell proliferation, apoptosis, cell metastasis, invasion, EMT, and drug resistance (Fig. 3). In this section, we will describe the role of circRNAs in regulating these cellular processes and will highlight circRNA molecular mechanisms (Table 3).
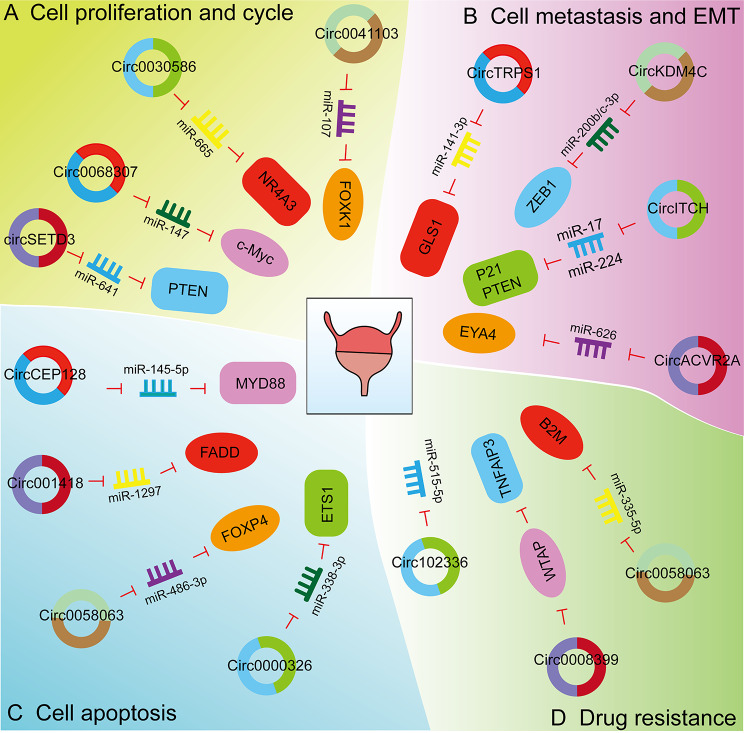
Fig. 3.
Biological functions of circRNAs in bladder cancer (BCa)
(A) CircRNAs are involved in the cell proliferation and cycle of BCa. CircSETD3, circ0068307, circ0030586, and circ0041103 affect the cell proliferation of BCa cells by regulating the expression of miRNAs and related proteins. (B) CircRNAs are involved in the metastasis and EMT of BCa. CircTRPS1, circKDM4C, circITCH, and circACVR2A affect the cell metastasis and EMT of BCa cells by regulating the expression of miRNAs and related proteins. (C) CircRNAs are involved in the apoptosis of BCa. CircCEP128, circ001418, circ0058063, and circ0000326 affect the cell apoptosis of BCa cells by regulating the expression of miRNAs and related proteins. (D) CircRNAs are involved in the drug resistance of BCa. Circ102336, circ0008399, and circ0058063 affect the drug resistance of BCa cells by regulating the expression of miRNAs and related proteins
Table 3.
Overview of deregulated circRNAs in bladder cancer
| CircRNA | Expression | Function | Target microRNA | miRNA target genes/protein | Ref |
|---|---|---|---|---|---|
| Circ0030586 | Down | Proliferation(-) | miR-665 | NR4A3 | [131] |
| Circ102336 | Up |
Proliferation(+) Resistance(-) |
miR-515-5p | - | [153] |
| CircTRPS1 | Up |
Proliferation(+) Metastasis(+) |
miR-141-3p | GLS1 | [130] |
| Circ001418 | Up |
Proliferation(+) Metastasis(+) Apoptosis(−) |
miR-1297 | EphA2 | [148] |
| Circ0091017 | Up |
Proliferation(+) Metastasis(+) |
miR-589-5p | - | [132] |
| Circ0041103 | Up |
Proliferation(+) Metastasis(+) |
miR-107 | FOXK1 | [133] |
| Circ0058063 | Up |
Proliferation(+) Apoptosis(−) Resistance(−) |
miR-335-5p | B2M | [155] |
| Circ0068307 | Up |
Proliferation(+) Metastasis(+) |
miR-147 | c-Myc | [134] |
| CircITCH | Down |
Proliferation(-) Metastasis(-) Apoptosis(+) |
miR-17/ miR-224 | p21/ PTEN | [140] |
| CircACVR2A | Down |
Proliferation(-) Metastasis(-) |
miR-626 | EYA4 | [141] |
| Circ0068871 | Up |
Proliferation(+) Metastasis(+) Apoptosis(−) |
miR-181a-5p | STAT3 | [142] |
| Circ0008532 | Up | Metastasis(+) | miR-155-5p /miR-330-5p | MTGR1 | [143] |
| Circ0001944 | Up |
Proliferation(+) Metastasis(+) |
miR-548 | PROK2 | [135] |
| Circ0000658 | Up | Metastasis(+) | miR-498 | HMGA2 | [144] |
| Circ0023642 | Up | Metastasis(+) | miR-490-5p | EGFR | [145] |
| CircST6GALNAC6 | Down |
Proliferation(-) Metastasis(-) |
miR-200a-3p | STMN1 | [146] |
| CircUBE2K | Up |
Proliferation(+) Metastasis(+) |
miR-516b-5p | ARHGAP5 | [136] |
| CircKDM4C | Up | Metastasis(+) | miR-200bc-3p | ZEB1 | [147] |
| Circ0058063 | Up |
Metastasis(+) Apoptosis(−) |
miR-486-3p | FOXP4 | [149] |
| Circ100984 | Up |
Proliferation(+) Metastasis(+) |
miR-432-3p | c-Jun/YBX-1/β-catenin | [137] |
| CircSETD3 | Down |
Proliferation(-) Metastasis(-) |
miR-641 | PTEN | [138] |
| Circ0006948 | Up |
Proliferation(+) Metastasis(+) |
- | N-cadherin | [139] |
| Circ0000629 | Down |
Proliferation(-) Metastasis(-) Apoptosis(+) |
miR-1290 | CDC73 | [150] |
| CircCEP128 | Up |
Proliferation(+) Metastasis(+) Apoptosis(−) |
miR-145-5p | MYD88 | [151] |
| Circ0000326 | Up |
Proliferation(+) Metastasis(+) Apoptosis(−) |
miR-338-3p | ETS1 | [152] |
| Circ0008399 | Up | Resistance(+) | - | WTAP | [154] |
Proliferation and the cell cycle
CircRNAs play an important regulatory role in the malignant behavior of BCa cells. Circ0030586 is localized to the cytoplasm where it is poorly expressed in BCa tissues and cells [131]. Overexpression of circ0030586 inhibits BCa cell proliferation and stemness in vitro and also inhibits tumor growth in vivo. Circ0030586 binds miR-665, which is highly expressed in BCa tissues and cells, while NR4A3 is down-regulated in BCa. MiR-665 overexpression or NR4A3 silencing reverses the inhibitory effects of circ0030586 overexpression on BCa cell proliferation and stemness. The level of circ0091017 expression is significantly down-regulated in BCa tissues and cell lines, while the expression of miR-589-5p is up-regulated [132]. In vitro studies found that overexpression of circ0091017 inhibits the proliferation of BCa cells and the expression of miRNA-589-5p. However, overexpression of miR-589-5p reverses the inhibitory effect of circ0091017 on the malignant phenotype of BCa cells. Circ0041103 is aberrantly upregulated in BCa tissues and cell lines [133]. Knockdown of circ0041103 inhibits the proliferation and metastasis of T24 and UM-UC-3 cells. MiR-107 is the binding target of circ0041103, with FOXK1 a downstream gene target of miR-107. Overexpression of circ0041103 reverses the suppressed proliferation and metastatic capacity of T24 and UM-UC-3 cells that overexpress miR-107. Circ0068307 is highly expressed in BCa cell lines, with silencing of circ0068307 resulting in reduced cancer stem cell differentiation by up-regulation of miR-147 expression [134]. The transcription of c-Myc is inhibited after miR-147 activation. MiR-147 down-regulation of the CSC-related proteins (OCT-4, NANOG, and Sox-2) results in decreased cell proliferation and migration. One study found that the expression of circ0001944 was increased in BCa tissues and related to a poor prognosis for BCa patients. Downregulation of circ0001944 reduced BCa invasion and metastasis in vivo and in vitro [135]. Circ0001944 expression promotes BCa progression by adsorbing miR-548 and enhancing PROK2 expression. Another study found that circUBE2K was located on chromosome 4, with high expression of circUBE2K associated with a poor prognosis in BCa patients [136]. In vitro studies found that down-regulation of circUBE2K reduced the proliferative capacity of BCa cells. By subcutaneous xenograft, circUBE2K was found to significantly increase the proliferation of BCa cells in vivo. Qi et al. found that circ100984 was highly expressed in BCa tissues, with knockdown of circ100984 inhibiting the growth of BCa tumors [137]. Circ100984 adsorbs miR-432-3p and indirectly regulates YBX-1 and EMT-related molecules. YBX-1 and c-Jun act as transcriptional regulators of β-catenin and YBX-1, respectively, in BCa cells. Knockdown of YBX-1 suppresses the expression of β-catenin and c-Jun, whereas down-regulation of c-Jun conversely inhibits the expression of YBX-1 and β-catenin. Mei et al. found that circSETD3, a tumor suppressor, inhibits the proliferation of BCa cells [138]. CircSETD3 is significantly down-regulated in cancerous clinical tissues and cell lines, and is often deficient in BCa patients with larger tumors, advanced clinical stage, positive lymph node metastasis, and poor prognosis. We found that the circSETD3/miR-641/PTEN axis regulates the malignant phenotype of BCa cells in vitro.
Metastasis and EMT
A number of circRNAs contribute to metastasis and EMT. CircTRPS1 is significantly increased in exosomes derived from BCa tissue as well as from urine and serum of patients [130]. Metabolomics and RNA-seq have demonstrated circTRPS1 to enhance the function of GLS1, a key enzyme for glutamine metabolism. Overexpression of GLS1 enhances both proliferation and invasiveness of BCa cells and impairs anti-tumor immunity within the BCa microenvironment. Therefore, circTRPS1 may be a novel biomarker and therapeutic target for BCa patients. Circ0006948 is upregulated in BCa tissues and cell lines [139]. Increased expression of cir0006948 is related to advanced tumor stage, lymph node metastasis, high distance transfer rate, and poor prognosis. Knockdown of circ0006948 reduces the proliferation and metastatic capacity of BCa cells, resulting in upregulation of E-cadherin and downregulation of N-cadherin, Vimentin, β-catenin, and MMP-9. CircITCH was found to be poorly expressed in BCa tissues and cell lines, with circITCH expression inhibiting cell proliferation, migration, invasion, and metastasis [140]. CircITCH up-regulates the expression of miR-17 and miR-224 that target p21 and PTEN, respectively, which reduce the invasiveness of BCa. CircACVR2A is poorly expressed in BCa tissues and cell lines [141]. Reductions in circACVR2A are positively related to an aggressive clinical pathology. CircACVR2A is an independent risk factor for overall survival rate in patients with BCa. CircACVR2A directly interacts with miR-626, functioning as a miRNA sponge, which regulates the expression of EYA4. One study found that circ0068871 was overexpressed in BCa tissues and cell lines, whereas miR-181a-5p expression was inhibited [142]. Deletion of circ0068871 or up-regulation of miR-181a-5p inhibited BCa cell proliferation and migration in vitro and in vivo. Circ0068871 up-regulates FGFR3 expression and activates STAT3 to promote BCa progression by targeting miR-181a-5p. Hou et al. found that circ0008532 expression was significantly up-regulated in BCa tissues and cell lines and positively related to BCa progression by secreting miR-155-5p/miR-330-5p, which affects MTGR1 expression and Notch signaling activity [143]. Circ0008532 knockdown suppresses the invasion of BCa cells. Zhao et al. found that circ0000658 was highly expressed in BCa tissue samples and cell lines and related to a poor prognosis for BCa patients [144]. Circ0000658 binds competitively to miR-498, thereby limiting miR-498 expression. Further, circ0000658 weakens binding of miR-498 to the target gene, HMGA2, which up-regulates the expression of HMGA2. Overexpression of circ0000658 results in decreased expression of E-cadherin and increased expression of N-cadherin, snail, slug, ZEB1 and Twist in BCa cells. Estrogen receptor α (ERα) plays a protective role in BCa by reducing BCa invasion through reduced expression of circ0023642 [145]. ERα decreases circ0023642 levels and subsequently increases miR-490-5p expression, resulting in decreased EGFR expression, thereby inhibiting BCa cell invasion. Another study found that circST6GALNAC6 expression was down-regulated in BCa tissues and cells [146]. Overexpression of circST6GALNAC6 effectively inhibited cell proliferation, migration, invasion, and EMT in vitro and inhibited BCa metastasis in vivo. Binding of the transcription factor, SP1, to circST6GALNAC6 mRNA transcripts activates circST6GALNAC6 transcription. CircKDM4C is derived from the KDM4C gene and is highly expressed in BCa cell lines and tissues. Overexpression of circKDM4C significantly enhances the migration and invasion of BCa cells, while silencing of circKDM4C inhibits migration and invasion of BCa cells [147]. CircKDM4C can interact with miR-200b-3p and miR-200c-3p, thereby enhancing ZEB1 expression and promoting EMT.
Apoptosis
Apoptosis is a normal physiological process that is usually inhibited in tumor tissues. Some circRNAs can promote apoptosis. Circ001418 expression is increased in BCa patients [148]. In vitro studies found that knockdown of circ001418 inhibited cell proliferation and induced apoptosis. By inhibiting miR-1297, circ001418 overexpression increases the expression of EphA2 and cytochrome c proteins while suppressing the expression of FADD protein. CircST6GALNAC6 binds miR-200a-3p to regulate STMN1 [146]. STMN1 is involved in BCa EMT, with metastasis regulated by the circST6GALNAC6/miR-200a-3p axis. Further, circRNA 0058063 was found to be aberrantly expressed in different BCa cell lines and BCa cancer tissues [149]. Knockdown of circ0058063 significantly reduced cell proliferation and invasion and promoted apoptosis. Circ0058063 and FOXP4 directly bind miR-486-3p, indicating that circ0058063 regulates FOXP4 expression by competitively binding miR-486-3p. That study found circ0000629 to be expressed in the cytoplasm and to inhibit the development and metastasis of BCa cells [150]. By enhancing the expression of BAX and PUMA, BCL-2 expression was inhibited, promoting cellular apoptosis. Circ0000629 binds miR-1290 to regulate the expression of CDC73. Overexpression of circ0000629 reduces BCa tumor growth in vivo. The circCEP128/miR-145-5p/MYD88 axis plays an important role in BCa [151]. Knockdown of circCEP128 reduces cell viability and migration and induces cell cycle arrest, promoting apoptosis of BCa cells. Overexpression of miR-145-5p or knockdown of circCEP128 promotes the expression of the MAPK signaling pathway and related proteins. Furthermore, knockdown of circCEP128 inhibits the growth of BCa tissue in vivo. CircCEP128 overexpression promotes BCa progression by regulation of miR-145-5p and MYD88 through MAPK signaling. Circ0000326 expression is significantly elevated in BCa cell lines and tissues, with circ0000326 promoting BCa cell growth and migration as well as inhibiting apoptosis [152]. Circ0000326 and ETS1 bind directly to miR-338-3p. In addition, circ0000326 binds miR-338-3p and up-regulates ETS1 expression. Circ0000326 also activates the PI3K/AKT pathway through the miR-338-3p/ETS1 axis.
Drug resistance
Acquired drug resistance is a significant impediment to tumor therapy. Some cirRNAs are capable of reversing tumor drug resistance. In BCa tissue samples and cell lines, circ102336 is highly expressed and relates to poor overall survival of BCa patients [153]. Circ102336 knockdown increases sensitivity of T24 and 5637 cell lines for CDDP. Circ102336 directly binds and negatively regulates miR-515-5p. EIF4A3 up-regulates circ0008399 in BCa tissues and cells [154]. Circ0008399 binds WTAP to promote the formation of the WTAP/METTL3/METTL14 m6A methyltransferase complex. Circ0008399 increases the expression of TNFAIP3 by increasing mRNA stability in an m6A-dependent manner. Activation of the circ0008399/WTAP/TNFAIP3 pathway reduces the sensitivity of BCa to cisplatin, with targeting of the circ0008399/WTAP/TNFAIP3 axis enhancing the efficacy of cisplatin. Circ0058063 is highly expressed in CDDP-resistant BCa tissues and cells [155]. Knockdown of circ0058063 inhibits cell proliferation and tumor growth, while inducing apoptosis in CDDP-resistant BCa cells in vitro and in vivo. Circ0058063 promotes CDDP resistance in BCa cells by secreting miR-335-5p, which up-regulates B2M levels by promoting the expression of genes associated with cancer stem cell properties. In addition, miR-335-5p inhibition reverses inhibition of cell proliferation and promotes apoptosis by silencing circ0058063.
Expression and biological functions of CircRNAs in prostate Cancer
CircRNAs are involved in PCa cell proliferation, apoptosis, cell metastasis, invasion, EMT, and drug resistance. CircRNAs are also useful for patient prognosis (Fig. 4). In this section, we will describe the role of circRNAs in the regulation of PCa cellular processes and will highlight the involved circRNA molecular mechanisms (Table 4).
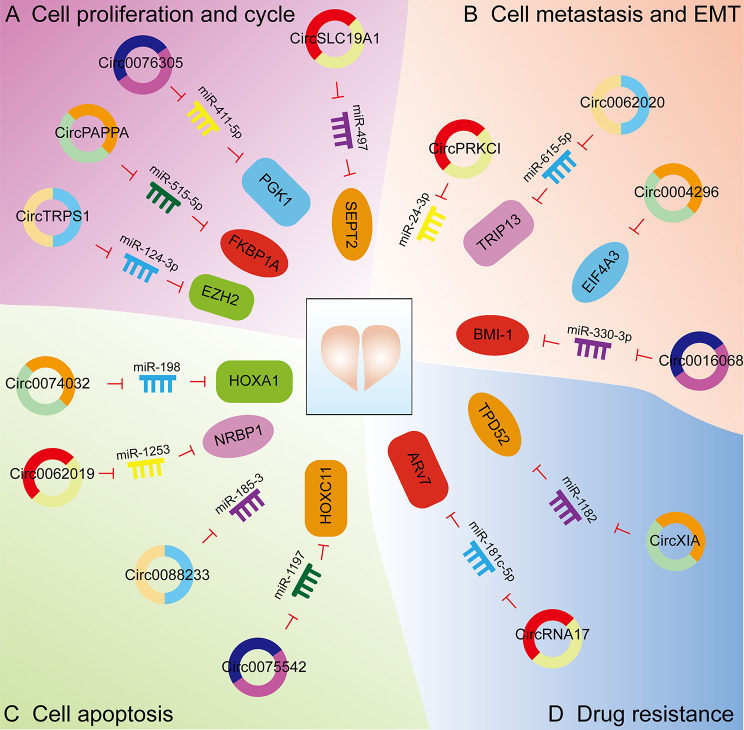
Fig. 4.
Biological functions of circRNAs in prostate cancer (PCa)
(A) CircRNAs are involved in the cell proliferation and cycle of PCa. CircTRPS1, circPAPPA, circ0076305, and circSLC19A1 affect the cell proliferation of PCa cells by regulating the expression of miRNAs and related proteins. (B) CircRNAs are involved in the metastasis and EMT of PCa. CircPRKCI, circ0062020, circ0004296, and circ0016068 affect the cell metastasis and EMT of PCa cells by regulating the expression of miRNAs and related proteins. (C) CircRNAs are involved in the apoptosis of PCa. Circ0074032, circ0062019, circ0088233, and circ0075542 affect the cell apoptosis of PCa cells by regulating the expression of miRNAs and related proteins. (D) CircRNAs are involved in the drug resistance of PCa. CircRNA17 and circXIA affect the drug resistance of PCa cells by regulating the expression of miRNAs and related proteins
Table 4.
Overview of deregulated circRNAs in prostate cancer
| CircRNA | Expression | Function | Target microRNA | miRNA target genes/protein | Ref |
|---|---|---|---|---|---|
| CircPRKCI | Up |
Proliferation(+) Metastasis(+) |
miR-24-3p | - | [165] |
| Circ102004 | Up |
Proliferation(+) Metastasis(+) apoptosis(−) |
- | - | [164] |
| Circ0003258 | Up | Metastasis(+) | miR-653-5p | ARHGAP5 | [166] |
| Circ0004296 | Down |
Proliferation(-) Metastasis(-) |
- | EIF4A3/ETS1 | [167] |
| Circ17 | Down | Resistance(-) | miRNA-181c-5p | ARv7 | [179] |
| CircTRPS1 | Up |
Proliferation(+) Metastasis(+) |
miR-124-3p | EZH2 | [157] |
| Circ0074032 | Up |
Proliferation(+) Metastasis(+) Apoptosis(−) |
miR-198 | HOXA1 | [172] |
| Circ0062019 | Up |
Proliferation(+) Metastasis(+) Apoptosis(−) |
miR-1253 | NRBP1 | [173] |
| CircPAPPA | Up |
Proliferation(+) Metastasis(+) Apoptosis(−) |
miR-515-5p | FKBP1A | [158] |
| Circ0076305 | Up |
Metastasis(+) Apoptosis(−) |
miR-411-5p | PGK1 | [159] |
| CircSLC19A1 | Up |
Metastasis(+) Apoptosis(−) |
miR-497 | SEPT2 | [160] |
| Circ0062020 | Down |
Metastasis(-) Resistance(-) |
miR-615-5p | TRIP13 | [168] |
| CircXIAP | Up |
Proliferation(+) Metastasis(+) Apoptosis(−) Resistance(-) |
miR-1182 | TPD52 | [181] |
| Circ0016068 | Up | Metastasis(+) | miR-330-3p | BMI-1 | [169] |
| Circ0088233 | Up |
Proliferation(+) Metastasis(+) Apoptosis(−) |
miR-185-3p | E2F1/WNT2B | [174] |
| Circ0057558 | Up | Proliferation(+) | miR-206 | USP33/c-Myc | [161] |
| Circ0062019 | Up |
Proliferation(+) Metastasis(+) |
miR-195-5p | HMGA2 | [162] |
| Circ0075542 | Down |
Proliferation(-) Metastasis(-) |
miR-1197 | HOXC11 | [175] |
| Circ0030586 | Up |
Proliferation(+) Metastasis(+) |
miR-145-3p | - | [170] |
| CircFMN2 | Up |
Proliferation(+) Metastasis(+) |
miR-1238 | LHX2 | [163] |
| Circ0057553 | Up |
Proliferation(+) Metastasis(+) Apoptosis(−) |
miR-515-5p | YES1 | [176] |
| Circ0006404 | Up |
Proliferation(+) Metastasis(+) Apoptosis(−) |
miR-1299 | CFL2 | [177] |
| CircDPP4 | Up |
Proliferation(+) Metastasis(+) Apoptosis(−) |
miR-564 | ZIC2 | [178] |
| Circ0001686 | Up |
Proliferation(+) Metastasis(+) |
miR-411-5p |
SMAD3 /TGFBR2 |
[171] |
| Circ0001275 | Up | Resistance(+) | - | - | [180] |
Proliferation and the cell cycle
PCa cells can maintain an active proliferative state by activation of cell proliferation signaling pathways. CirRNAs enhance this process. Circ0001206 is downregulated in PCa and significantly related to the clinical features of PCa patients [156]. ROC curves show circ0001206 to have good diagnostic value as a biomarker for PCa. Overexpression of circ0001206 inhibits PCa cell proliferation in vitro and prevents tumor growth in vivo. Dual-luciferase reporter gene assays demonstrated circ0001206 to bind to miR-1285-5p. Smad4 expression is increased by overexpression of circ0001206, and this effect is partially reversed by co-transfection with miR-1285-5p mimics. Similarly, the expression of circTRPS1 is increased in PCa patient samples and cell lines, with circTRPS1 acting as an oncogene with miR-124-3p, resulting in enhanced EZH2 expression and promotion of cancer stem-like cell-mediated PCa progression [157]. In vitro and in vivo experiments demonstrated circTRPS1 silencing to inhibit proliferation of PCa cells. CircPAPPA was found to be highly expressed in PCa tissues. Knockdown of circPAPPA effectively inhibited the proliferative capacity of PCa cell lines DU145 and LNCap, and also inhibited tumor metastasis by promoting apoptosis [158]. CircPAPPA binds and negatively relates to miR-515-5p. MiR-515-5p silencing greatly reduces circPAPPA knockdown-mediated effects in PCa cells. MiR-515-5p directly binds to FKBP1A, with the effects of miR-515-5p overexpression partially offset by FKBP1A overexpression. CircPAPPA silencing partially reduces FKBP1A protein levels by increasing miR-515-5p expression. CircPAPPA knockout also significantly inhibits tumor growth in vivo. In addition, circ0076305 is highly expressed in PCa [159]. Circ0076305 silencing inhibits cell growth and glycolysis while triggering apoptosis in PCa cells. Circ0076305 targets miR-411-5p, with miR-411-5p inhibition counteracting the effects of circ0076305 silencing in PCa cells. Furthermore, miR-411-5p directly targets PGK1, with miR-411-5p up-regulation suppressing PCa cell malignant behavior by decreasing PGK1. Lin et al. found increased expression of circSLC19A1 in PCa cells and PCa secreted extracellular vesicles (EVs) [160]. EVs with high expression of circSLC19A1 can be taken up by PCa cells and in that manner promote cell proliferation and invasion. CircSLC19A1 can directly bind to miR-497. In PCa cells, the expression of miR-497 is down-regulated, while the expression of its target, septin 2 (SEPT2), is significantly up-regulated. This up-regulation activates the ERK1/2 pathway and regulates the growth and invasion of PCa cells. These results suggest that the miR-497/SEPT2/ERK1/2 pathway, regulated by EV-derived circSLC19A1, is important in PCa growth and invasion.
A study found that circ0057558 was highly expressed in PCa tissues and cell lines [161]. Circ0057558 knockout in PCa cells significantly inhibited cell proliferation and colony formation but promoted cell arrest and enhanced sensitivity to doxorubicin. Circ0057558 can bind miR-206, which is negatively related to PCa tissue expression. Further, miR-206 targets USP33, with USP33 overexpression partially blocking the effects of miR-206 mimics on prostate cell proliferation. USP33 can bind to c-Myc. Increased c-Myc protein induced by circ0057558 overexpression partially reverses a miR-206 mimic. The proliferation-inhibitory activity of the c-Myc inhibitor 361 was pronounced in primary PCa cells and in patient-derived xenograft models with higher circ0057558 levels. In another study, circ0062019 was found to be highly expressed in PCa cell lines [162]. Silencing circ0062019 can effectively inhibit cell proliferation and metastasis. Circ0062019 binds miR-195-5p and miR-195-5p binds HMGA2. The circ0062019-induced malignant phenotype of PCa cells is reversed by the down-regulation of HMGA2. CircFMN2 expression is significantly elevated in PCa tissues [163]. Knockdown of circFMN2 suppresses cell proliferation and inhibits tumor growth in vitro and in vivo. CircFMN2 can act as a sponge for miR-1238, which up-regulates the expression of LHX2.
Metastasis and EMT
Blocking metastasis of PCa cells is critical to the inhibition of tumor progression. Circ102004 is expressed at greater levels in PCa samples than in paracancerous tissues [164]. Circ102004 exerts an oncogenic role in PCa by stimulating cancer cell migration and invasion. Overexpression of Circ102004 promotes PCa cell metastasis and overgrowth of xenografted tumors, as well as affects signaling pathways such as ERK, JNK, and Hedgehog. CircPRKCI was found to be up-regulated in PCa tissues and related to patient metastatic rate in an analysis of 40 PCa tissues and normal paracancerous tissues [165]. The metastatic capacity of DU-145 and PC-3 cell lines was inhibited after knockdown of circPRKCI, which binds miR-24-3p and exhibits a negative relationship with circPRKC. Likewise, circ0003258 was significantly up-regulated in PCa tissues and cells and related to PCa cell metastais [166]. High expression of circ0003258 promotes PCa cell migration by inducing EMT in vitro and tumor metastasis in vivo. Circ0003258 increases the expression of Rho GTPase activating protein 5 by binding to miR-653-5p. Moreover, circ0003258 binds to IGF2BP3 within the cytoplasm, enhancing stability of HDAC4 mRNA, which activates the ERK signaling pathway and triggers EMT, ultimately accelerating PCa metastasis.
Circ0004296 expression is reduced in PCa tissues, patient blood, as well as urine, with a negative relationship to metastasis [167]. In vitro and in vivo experiments demonstrated circ0004296 to inhibit PCa cell proliferation, migration, invasion, and EMT. Circ0004296 is expressed in the nucleus and interacts with the RNA-binding protein, EIF4A3. The expression of EIF4A3 is significantly up-regulated in PCa tissues and relates to PCa cell metastasis. Silencing of EIF4A3 inhibits PCa cell proliferation, migration, invasion, and EMT. Cai et al. found that circ0062020 was up-regulated in PCa tissues and cells, especially in radiation-hardened PCa tissues [168]. Further, circ0062020 knockdown enhanced radio-sensitivity by modulation of the miR-615-5p/thyroid hormone receptor interactor 13 (TRIP13) axis in PCa cells. Furthermore, circ0062020 knockout inhibited PCa tumor growth in vivo. These findings may contribute to improvements in PCa therapy. Circ0016068 is highly expressed in PCa tissues and cell lines [169]. Circ0016068 promotes PCa cell EMT, growth, migration, and invasion in vitro, as well as tumor growth and metastasis in a mouse model of PCa. Circ0016068 competes with B lymphoma Moloney mouse leukemia virus insertion region-1 (BMI-1) for binding to miR-330-3p. Circ0016068 sequesters miR-330-3p and releases BMI-1 to enhance PCa cell proliferation, migration, and invasion, as well as xenograft tumor metastasis. Circ0030586 was found to be highly expressed in PCa cells using the GEO database [170]. Knockdown of circ0030586 in PC3 cells inhibited PCa cell proliferation, migration and invasion, and resulted in significant up-regulation of E-cadherin and significant down-regulation of p-AKT/AKT, IKKα, PIK3CB, and Twist. Circ0001686 expression was up-regulated in PCa cells, while miR-411-5p expression was down-regulated [171]. Furthermore, circ0001686 promoted cell proliferation, migration and invasion. Circ0001686 decreased miR-411-5p levels, affecting the downstream target genes, SMAD3 and TGFBR2.
Apoptosis
Promotion of PCa apoptosis may contribute to better outcomes for PCa patients. Circ0074032 is overexpressed in PCa tissues, with high expression levels of circ0074032 associated with a poor prognosis for PCa patients [172]. Circ0074032 silencing reduces xenograft tumor growth in vivo and induces apoptosis and inhibition of PCa cell proliferation. Circ0074032 binds miR-198, with miR-198 inhibition abrogating the effects of circ0074032 on silencing-mediated PCa cell proliferation and apoptosis. Further, miR-198 directly targets homeobox A1, which attenuates miR-198 mimetic-mediated effects on the malignant phenotype of PCa cells.
A study found that circ0062019 is highly expressed in PCa tissue and multiple PCa cell lines [173]. Knockdown of circ0062019 inhibits the proliferation and the cell cycle of PCa cells, promoting cellular apoptosis. Inhibition of PCNA and BCL-2 enhances the expression of BAX. Circ0062019 promotes PCa cell progression by binding miR-1253. MiR-1253 impedes PCa cell progression by regulation of NRBP1. By analysis of 46 pairs of PCa and adjacent normal tissues, circ0088233 was found to be up-regulated and its expression level related to TNM stage [174]. Knockdown of circ0088233 reduced cell proliferation, migration, and invasion, as well as induced G1 phase arrest and apoptosis. Circ0088233 binds miR-185-3, with expression levels negatively related. Circ0088233 overexpression blocked the effects of miR-185-3p on cell proliferation, migration, invasion, cell cycle progression, and apoptosis. Huang et al. found that circ0075542 expression was down-regulated in prostate tumor tissues [175]. Overexpression of circ0075542 inhibited cell proliferation, decreased migration, decreased invasion, and promoted apoptosis. Circ0075542 targeted HOXC11 via adsorption of miR-1197. Circ0057553 is significantly up-regulated in PCa tissues and cells [176]. Knockdown of circ0057553 inhibits PCa cell viability, migration, invasion, and glycolysis, while promoting apoptosis. Circ0057553 binds to miR-515-5p and miR-515-5p, targeting YES1. MiR-515-5p inhibition partially rescues the function of circ0057553 knockdown, while YES1 restores the effect of miR-515-5p overexpression. Further, circ0006404 is highly expressed in PCa patients [177]. Circ0006404 promotes PCa cell survival, metastasis, and proliferation, as well as inhibits PCa cellular apoptosis. Circ0006404 directly targets miR-1299, with silencing of miR-1299 reversing the effects of circ0006404 interference on PCa cells. CFL2 binds directly to miR-1299, with effects induced by miR-1299 in PCa cells largely attenuated by CFL2 overexpression. CFL2 is regulated by the circ0006404/miR-1299 axis in PCa cells. Circ0006404 promotes PCa progression through the miR-1299/CFL2 axis in vivo. In an assessment of 60 patients and PC cell lines, circDPP4 expression was found to be up-regulated in PC tumors and to predict a lower overall patient survival rate [178]. Reduction of circDPP4 inhibited PCa cell proliferation and metastasis, while promoting apoptosis rate. Overexpression of miR-564 and inhibition of ZIC2 inhibited PCa progression. CircDPP4 functions as a miR-564 sponge and regulates the expression of the miR-564 target gene, ZIC2. Tumor growth is inhibited by silencing of circDPP4, which is accompanied by elevated miR-564 and attenuation of Ki-67 and ZIC2.
Drug resistance
Androgen deprivation therapy (ADT) induced by drugs such as enzalutamide (Enz), can lead to longer survival in castration-resistant prostate cancer (CRPC). However, most patients develop drug resistance with prolonged dosing. Increased expression of the AR splice variant ARv7 may play a key role in the development of Enz resistance in CRPC. CircRNA17 expression is lower in PCa patients with higher Gleason scores. In vitro cell line studies demonstrated CRPC C4–2 anti-Enz cells (EnzR-C4–2) to express less of the molecule than parental Enz-sensitive (EnzS-C4-2) cells [179]. Inhibition of circRNA17 in EnzS-C4–2 cells increased ARv7 expression, which lead to increased Enz resistance and cell invasiveness. Mechanistic analysis showed that Enz suppressed the expression of circRNA17 at the transcriptional level by inhibition of host gene, PDLIM5, transcription. CircRNA17 also regulates the expression of ARv7 by altering the expression of miR-181c-5p, which involves miR-181c-5p and ARv7 direct binding to the 3’UTR. Circ0001275 levels are highly up-regulated in enzalutamide-resistant cell lines, with overexpression shown to increase resistance to enzalutamide [180]. CircXIAP expression was upregulated in DTX-resistant PCa tissue specimens and cell lines [181]. CircXIAP is also overexpressed in exosomes of DTX-resistant cells. CircXIAP knockout enhanced DTX sensitivity by inhibiting the proliferation, migration, and invasion of DTX-resistant cells, as well as inducing cell cycle arrest and apoptosis. CircXIAP directly targets miR-1182, down-regulating miR-1182 in DTX-resistant cells and reversing the effects of circXIAP knockout. TPD52 is a target of miR-1182, with up-regulation attenuating the effect of miR-1182 on DTX sensitivity. Importantly, circXIAP deletion suppresses tumor growth and increases DTX sensitivity in vivo.
CircRNAs are potential diagnostic and prognostic biomarkers of urinary system tumors
CircRNAs are potential diagnostic and prognostic biomarkers for kidney Cancer
Aberrant expression of circRNAs is always associated with poor prognosis in urinary system cancer (Table 5). CircPPP6R3 is highly expressed in clear cell renal cell carcinoma tissues and cell lines and may serve as a diagnostic marker for ccRCC [182]. High expression levels of circPPP6R3 are positively related to higher histological grade, T stage, M stage, and advanced clinical stage in ccRCC patients. Furthermore, KM analysis showed that patients with RCC and low levels of circCYP24A1 had shorter overall survival, higher tumor burden, and higher tumor grade [183]. Deficiency of circCYP24A1 in RCC is associated with worse clinical outcomes. High expression levels of circ0085576 are significantly associated with tumor size, clinical stage, metastatic status, and poor survival [184]. Circ0085576 is found to be upregulated in ccRCC tissues and cell lines, thus circ0085576 may serve as a predictor of clinical prognosis in ccRCC patients. A study showed that circ0065217 is abundantly expressed in RCC tissues and cell lines, and its expression is related to advanced TNM stage, large tumor size, and lymph node metastasis [185]. CircAMOTL1L expression is down-regulated in RCC tissues and cell lines [186]. Decreased expression of circAMOTL1L in RCC patients is associated with tumor stage, metastasis, and poor prognosis. Another study found that increased expression of circPRRC2A is positively associated with advanced clinical stage and poor survival in RCC patients [187]. High levels of circPTCH1 were significantly associated with worse patient survival, higher Fuhrman grade, and higher risk of metastasis [188].
Table 5.
CircRNAs are Potential Diagnostic and Prognostic Biomarkers in Urinary System Tumors
| CircRNAs | Tumor Type | Expression in Tumor | Clinical relevance | Ref |
|---|---|---|---|---|
| CircPPP6R3 | RCC | Upregulated | Diagnostic | [182] |
| CircCYP24A1 | RCC | Downregulated | Prognosis | [183] |
| Circ0085576 | RCC | Upregulated | Prognosis | [184] |
| Circ0065217 | RCC | Upregulated | Prognosis | [185] |
| CircAMOTL1L | RCC | Downregulated | Prognosis | [186] |
| CircPRRC2A | RCC | Upregulated | Prognosis | [187] |
| CircPTCH1 | RCC | Upregulated | Prognosis | [188] |
| CircSMARCA5 | BCa | Upregulated | Prognosis | [189] |
| Circ0077837 | BCa | Downregulated | Prognosis | [190] |
| Circ0004826 | BCa | Downregulated | Prognosis | [190] |
| Circ0139402 | BCa | Upregulated | Prognosis | [191] |
| Circ0014130 | BCa | Upregulated | Prognosis | [192] |
| Circ0001361 | BCa | Upregulated | Prognosis | [193] |
| Circ0137439 | BCa | Upregulated | Diagnostic/Prognosis | [194] |
| Circ0086722 | PCa | Upregulated | Prognosis | [195] |
| CircPDHX | PCa | Upregulated | Prognosis | [196] |
| CircSOBP | PCa | Downregulated | Diagnostic | [197] |
| CircITCH | PCa | Downregulated | Diagnostic/Prognosis | [198] |
CircRNAs are potential diagnostic and prognostic biomarkers of bladder Cancer
Dysregulated circRNA expression is closely related to the clinic-pathological features of BCa patients (Table 5). Accurate BCa prognosis for patients guides treatment decisions, thereby improving outcomes for patients.
CircSMARCA5 is up-regulated in BCa tissues compared to adjacent tissues and is associated with larger tumor size, higher tumor stage, and lymph node metastasis [189]. High expression of circSMARCA5 is associated with shorter disease-free survival (DFS) and overall survival (OS), and may serve as a potential prognostic marker for BCa patients. Sequencing of bladder cancer samples found that circ0077837 and circ0004826 are significantly downregulated and significantly associated with worse clinico-pathological features and a poor prognosis in BCa patients [190]. Furthermore, circ0139402 and circ0014130 are highly expressed in bladder cancer samples and correlated with aggressive features and poor survival [191, 192]. A study found that circ0001361 is expressed at high levels in BCa tissues and cell lines, with a positive relationship found for pathology grade and muscle infiltration [193]. Kaplan-Meier survival analysis showed that BCa patients with high levels of circ0001361 expression had lower overall survival rates. Screening and validation in 30 normal and 116 BCa samples revealed circ0137439 to be significantly up-regulated in BCa samples [194]. ROC curves are used to assess the diagnostic value of circ0137439. The Kaplan-Meier method is used to evaluate the prognostic significance of circ0137439 in BCa. Increased expression of circ0137439 is associated with higher tumor stage, higher tumor grade, higher lymph node status, and history of muscle-invasive bladder cancer (MIBC). Circ0137439 can be used to not only differentiate BCa from control, but also to differentiate MIBC from non-muscle invasive bladder cancer. Circ0137439 urine levels can be used as an independent prognostic predictor of recurrence-free survival and overall survival of BCa patients.
CircRNAs are potential diagnostic and prognostic biomarkers of prostate Cancer
Screening and early diagnosis of PCa are critical to improved treatment outcomes and reducing patient morbidity (Table 5). Each has been identified as a research priority. Circ0086722 is highly expressed in PCa tissues and cells, with highly levels of circ0086722 is positively correlated with pT3 stage, higher sample Gleason score (> 7) and worse biochemical recurrence-free survival in PCa patients [195]. CircPDHX is highly expressed in PCa tissues and cells, with increased expression of circPDHX related to Gleason score and pathogenic T-stage and serves as an independent prognostic factor for poor survival in PCa patients [196]. Among 56 paired tissues, circSOBP expression is lower in PCa tissues compared to paraneoplastic tissues [197]. The ROC curve showed that the U1 small nuclear ribonucleoproteins of circSOBP was 0.763, indicating that circSOBP is a promising biomarker for differentiating PCa tissues. Low expression of circITCH in tumor tissue is associated with a high risk of advanced pathological T-stage and lymph node metastasis in patients with prostate cancer [198]. CircITCH is able to effectively distinguish tumor tissue from paired adjacent tissue with an Area under the curve(AUC) of 0.812 (95% CI: 0.780–0.845). Patients with high circITCH expression had longer DFS and OS, suggesting that it is a good diagnostic and prognostic biomarker.
CircRNAs Show Promise as a clinical therapeutic target
As circRNAs have shown an important role in the pathogenesis and progression of urological tumors, there has been an increasing number of studies using circRNAs as clinical therapeutic targets [199]. ciRS-7 is highly expressed in RCC tissues and is associated with high Fuhrman grading and poor survival [71]. PBAE/si-ciRS-7 nanocomplexes are effective for RCC progression and metastasis, therefore targeting ciRS-7 of PBAE/si-ciRS-7 nanocomplexes for drug development may be a promising strategy for RCC gene therapy [71]. CircSNX6 is highly expressed in sunitinib-resistant renal cancer cells [200]. CircSNX6 is found to promote sunitinib resistance in RCC in a cohort sample analysis. CircSNX6 is found to regulate intracellular lysophosphatidic acid levels and promote sunitinib resistance. It is promising to be a new prognostic indicator and a promising therapeutic target. CircME1 interacts with U1 snRNP to upregulate ME1 expression and thereby promote resistance to sunitinib in renal cancer cells [201]. The study suggests that circME1 may be a promising biomarker for predicting sunitinib resistance and a therapeutic target for ccRCC. High circ0008399 expression is associated with poor prognosis in patients with bladder cancer [154]. Targeting the circ0008399/WTAP/TNFAIP3 axis enhances the efficacy of Cisplatin. Regulation of target RNA expression by m6A modification reduces the sensitivity of cisplatin in bladder cancer and has potential therapeutic value in bladder cancer. Circ0001361 is expressed at high levels in BCa tissues and cell lines and exerts oncogenic effects in BCa invasion and metastasis by targeting the miR-491-5p/MMP9 axis and is expected to be a potential new target for BCa therapy [193]. HnRNP-L can regulate circCSPP1 to induce autophagy in tumor cells, leading to prostate tumor progression. CircCSPP1 has future promise for the clinical prognosis of PCa and for the development of new therapeutic options [202]. CircFoxo3 inhibits PCa progression and alleviates the sensitivity of PCa cells to doxorubicin by enhancing Foxo3 expression [203]. Targeting circFoxo3/Foxo3/EMT may provide an applicable strategy for exploring potential prostate cancer prognosis and therapeutic approaches.
However, the clinical application of circRNAs in urologic tumors remains largely unexplored, and further research is needed before circRNAs can truly enter clinical practice. On the one hand, a large number of samples, multiple centers, and independent external cohort validation are required for circRNA to become a widely accepted biomarker. On the other hand, the number of cirRNAs reported to affect tumors is still very small, and only a few circRNAs have clarified their biological functions and molecular mechanisms in urological tumors, and more cirRNAs need to be explored.
Conclusions
This paper reviews the biogenesis and regulation of circRNAs and describes the detection methods and the main functions of circRNAs in vivo. The effects of circRNAs on tumor proliferation, metastasis, apoptosis, metabolism and drug resistance in kidney, bladder, and prostate cancers are summarized. Some potential clinical applications of circRNAs are identified.
In recent years, the development of new detection technologies has facilitated our understanding of previously unknown circRNAs. More and more circRNAs have been found to play an important role in the development and progression of urologic tumors. CircRNA-based tumor diagnosis and treatment options have received much attention, with results suggesting their useful clinical application. However, the current academic understanding of circRNAs is lacking, with a complete appreciation of the importance of circRNA still in its infancy. Our understanding of the role of circRNAs in tumorigenesis and development is not comprehensive. Most current studies of the role and mechanism of action of circRNAs in cancer are limited to single or a few circRNAs, which limits a systematic, comprehensive, and integrated understanding of their biological importance.
Looking ahead, circRNAs require continuous validation and development, if they are to be truly useful for clinical diagnosis and treatment. Current studies do not include clinical data for large cohorts and as such requires further validation. The recent development of single-cell RNA-seq and CRISPR-Cas-based editing technologies will provide for a deeper understanding of circRNAs.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Not applicable.
Abbreviations
- CircRNA
Circular RNA
- ccRCC
Clear cell renal cell carcinoma
- RCC
Renal cell carcinoma
- BCa
Bladder cancer
- PCa
Prostate cancer
- NcRNA
Non-coding RNA
- LncRNA
Long-stranded non-coding RNA
- EcircRNA
Exon circRNA
- EIcircRNA
Exon-intron circRNA
- CiRNA
Circular intronic RNA
- TricRNA
tRNA intronic circular RNA
- Pre-tRNAs
Precursor transfer RNAs
- RBP
RNA-binding proteins
- MiRNA
microRNA
- RCA
Rolling circle amplification
- FISH
Fluorescence in situ hybridization
- DSN
Duplex-specific nucleases
- AR
Androgen receptor
- U1 snRNPs
U1 small nuclear ribonucleoproteins
- HCC
Hepatocellular carcinoma
- IRES
Internal ribosome entry site
- m6A
N6-methyladenosine
- EMT
Epithelial-mesenchymal transition
- Erα
Estrogen receptorα
- EVs
Extracellular vesicles
- SEPT2
Septin 2
- TRIP13
Thyroid hormone receptor interactor 13
- BMI-1
B lymphoma Moloney mouse leukemia virus insertion region-1
- ADT
Androgen deprivation therapy
- Enz
Enzalutamide
- CRPC
Castration-resistant prostate cancer
- DFS
Disease-free survival
- OS
Overall survival
- MIBC
Muscle-invasive bladder cancer
- AUC
Area under the curve
Author Contribution
The work presented here was carried out in collaboration among all authors. YDW, CDL and WZL identified the topic of the review and directed the completion of the paper. ZSF, TX, Mierxiati and WZD conceived the structure of the manuscript; ZZH, WY and ZY drafted the original draft; ZWK, ZHL and SGH revised the manuscript. FT was involved in the revision back of the manuscript. All of the authors had participated in the preparation of this manuscript.
Funding
This work is supported by grants from the National Natural Science Foundation of China (No.81872099).
Data Availability
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare no competing interests.
Consent for publication
Not applicable.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zi-hao Zhang, Yue Wang, Ya Zhang contributed equally to this work.
Contributor Information
Zi-Liang Wang, Email: huf_zlwang@126.com.
Da-Long Cao, Email: fudan_kenly@163.com.
Ding-Wei Ye, Email: dwyelie@163.com.
References
- 1.Hashemi M, et al. Curcumin in the treatment of urological cancers: therapeutic targets, challenges and prospects. Life Sci. 2022;309:120984. doi: 10.1016/j.lfs.2022.120984. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17–48. doi: 10.3322/caac.21763. [DOI] [PubMed] [Google Scholar]
- 3.Massari F, et al. Adjuvant therapy in renal cell carcinoma. Cancer Treat Rev. 2017;60:152–7. doi: 10.1016/j.ctrv.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 4.Yang L, Zou X, Zou J, Zhang G. Functions of circular RNAs in bladder, prostate and renal cell cancer (review). Mol Med Rep. 2021;23. 10.3892/mmr.2021.11946. [DOI] [PMC free article] [PubMed]
- 5.Rebello RJ, et al. Prostate cancer. Nat Rev Dis Primers. 2021;7:9. doi: 10.1038/s41572-020-0024.3-0. [DOI] [PubMed] [Google Scholar]
- 6.Damodaran S, Lang JM, Jarrard DF. Targeting metastatic hormone sensitive prostate Cancer: Chemohormonal Therapy and New Combinatorial Approaches. J Urol. 2019;201:876–85. doi: 10.1097/ju.0000000000000117. [DOI] [PubMed] [Google Scholar]
- 7.Regan MM, et al. Treatment-free survival over extended follow-up of patients with advanced melanoma treated with immune checkpoint inhibitors in CheckMate 067. J Immunother Cancer. 2021;9. 10.1136/jitc-2021-003743. [DOI] [PMC free article] [PubMed]
- 8.Wei G et al. The thermogenic activity of adjacent adipocytes fuels the progression of ccRCC and compromises anti-tumor therapeutic efficacy. Cell Metab 33, 2021–2039.e2028, 10.1016/j.cmet.2021.08.012 (2021). [DOI] [PubMed]
- 9.Nadal R, Bellmunt J. Management of metastatic bladder cancer. Cancer Treat Rev. 2019;76:10–21. doi: 10.1016/j.ctrv.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Lenis AT, Lec PM, Chamie K, Mshs MD. Bladder Cancer: A Review Jama. 2020;324:1980–91. doi: 10.1001/jama.2020.17598. [DOI] [PubMed] [Google Scholar]
- 11.Coen JJ, et al. Bladder preservation with twice-a-Day Radiation Plus Fluorouracil/Cisplatin or once Daily Radiation Plus Gemcitabine for muscle-invasive bladder Cancer: NRG/RTOG 0712-A randomized phase II trial. J Clin Oncol. 2019;37:44–51. doi: 10.1200/jco.18.00537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou WY, et al. Circular RNA: metabolism, functions and interactions with proteins. Mol Cancer. 2020;19:172. doi: 10.1186/s12943-020-01286-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kristensen LS, Jakobsen T, Hager H, Kjems J. The emerging roles of circRNAs in cancer and oncology. Nat Rev Clin Oncol. 2022;19:188–206. doi: 10.1038/s41571-021-00585-y. [DOI] [PubMed] [Google Scholar]
- 14.Mohapatra S, Pioppini C, Ozpolat B, Calin GA. Non-coding RNAs regulation of macrophage polarization in cancer. Mol Cancer. 2021;20. 10.1186/s12943-021-01313-x. [DOI] [PMC free article] [PubMed]
- 15.Sheng R, Li X, Wang Z, Wang X. Circular RNAs and their emerging roles as diagnostic and prognostic biomarkers in ovarian cancer. Cancer Lett. 2020;473:139–47. doi: 10.1016/j.canlet.2019.12.043. [DOI] [PubMed] [Google Scholar]
- 16.Sanger HL, Klotz G, Riesner D, Gross HJ, Kleinschmidt AK. Viroids are single-stranded covalently closed circular RNA molecules existing as highly base-paired rod-like structures. Proc Natl Acad Sci U S A. 1976;73:3852–6. doi: 10.1073/pnas.73.11.3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hsu MT, Coca-Prados M. Electron microscopic evidence for the circular form of RNA in the cytoplasm of eukaryotic cells. Nature. 1979;280:339–40. doi: 10.1038/280339a0. [DOI] [PubMed] [Google Scholar]
- 18.van Zonneveld AJ, Kölling M, Bijkerk R, Lorenzen JM. Circular RNAs in kidney disease and cancer. Nat Rev Nephrol. 2021;17:814–26. doi: 10.1038/s41581-021-00465-9. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y, Zhang Y, Wang P, Fu X, Lin W. Circular RNAs in renal cell carcinoma: implications for tumorigenesis, diagnosis, and therapy. Mol Cancer. 2020;19:149. doi: 10.1186/s12943-020-01266-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deng G, et al. Targeting androgen receptor (AR) with antiandrogen Enzalutamide increases prostate cancer cell invasion yet decreases bladder cancer cell invasion via differentially altering the AR/circRNA-ARC1/miR-125b-2-3p or miR-4736/PPARgamma/MMP-9 signals. Cell Death Differ. 2021;28:2145–59. doi: 10.1038/s41418-021-00743-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen Q, et al. Circular RNA ACTN4 promotes intrahepatic cholangiocarcinoma progression by recruiting YBX1 to initiate FZD7 transcription. J Hepatol. 2022;76:135–47. doi: 10.1016/j.jhep.2021.08.027. [DOI] [PubMed] [Google Scholar]
- 22.Yu J, Xie D, Huang N, Zhou Q. Circular RNAs as novel diagnostic biomarkers and therapeutic targets in kidney disease. Front Med (Lausanne) 2021;8:714958. doi: 10.3389/fmed.2021.714958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Papatsirou M, Artemaki PI, Karousi P, Scorilas A, Kontos CK. Circular RNAs: emerging regulators of the Major Signaling Pathways involved in Cancer Progression. Cancers (Basel). 2021;13. 10.3390/cancers13112744. [DOI] [PMC free article] [PubMed]
- 24.Kristensen LS, et al. The biogenesis, biology and characterization of circular RNAs. Nat Rev Genet. 2019;20:675–91. doi: 10.1038/s41576-019-0158-7. [DOI] [PubMed] [Google Scholar]
- 25.Wen SY, Qadir J, Yang BB. Circular RNA translation: novel protein isoforms and clinical significance. Trends Mol Med. 2022;28:405–20. doi: 10.1016/j.molmed.2022.03.003. [DOI] [PubMed] [Google Scholar]
- 26.Nigro JM, et al. Scrambled exons. Cell. 1991;64:607–13. doi: 10.1016/0092-8674(91)90244-s. [DOI] [PubMed] [Google Scholar]
- 27.Wang S, et al. Circular RNAs in body fluids as cancer biomarkers: the new frontier of liquid biopsies. Mol Cancer. 2021;20:13. doi: 10.1186/s12943-020-01298-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ghetti M, Vannini I, Storlazzi CT, Martinelli G, Simonetti G. Linear and circular PVT1 in hematological malignancies and immune response: two faces of the same coin. Mol Cancer. 2020;19:69. doi: 10.1186/s12943-020-01187-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patop IL, Wust S, Kadener S. Past, present, and future of circRNAs. EMBO J. 2019;38:e100836. doi: 10.15252/embj.2018100836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li X, Yang L, Chen LL. The Biogenesis, Functions, and Challenges of Circular RNAs. Mol Cell. 2018;71:428–42. doi: 10.1016/j.molcel.2018.06.034. [DOI] [PubMed] [Google Scholar]
- 31.Chen LL, Yang L. Regulation of circRNA biogenesis. RNA Biol. 2015;12:381–8. doi: 10.1080/15476286.2015.1020271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh V, Uddin MH, Zonder JA, Azmi AS, Balasubramanian SK. Circular RNAs in acute myeloid leukemia. Mol Cancer. 2021;20:149. doi: 10.1186/s12943-021-01446-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Y, et al. Circular intronic long noncoding RNAs. Mol Cell. 2013;51:792–806. doi: 10.1016/j.molcel.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 34.Itskovich SS, et al. MBNL1 regulates essential alternative RNA splicing patterns in MLL-rearranged leukemia. Nat Commun. 2020;11:2369. doi: 10.1038/s41467-020-15733-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ivanov A, et al. Analysis of intron sequences reveals hallmarks of circular RNA biogenesis in animals. Cell Rep. 2015;10:170–7. doi: 10.1016/j.celrep.2014.12.019. [DOI] [PubMed] [Google Scholar]
- 36.Conn SJ, et al. The RNA binding protein quaking regulates formation of circRNAs. Cell. 2015;160:1125–34. doi: 10.1016/j.cell.2015.02.014. [DOI] [PubMed] [Google Scholar]
- 37.Errichelli L, et al. FUS affects circular RNA expression in murine embryonic stem cell-derived motor neurons. Nat Commun. 2017;8:14741. doi: 10.1038/ncomms14741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salzman J, Chen RE, Olsen MN, Wang PL, Brown P. O. Cell-type specific features of circular RNA expression. PLoS Genet. 2013;9:e1003777. doi: 10.1371/journal.pgen.1003777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guo JU, Agarwal V, Guo H, Bartel DP. Expanded identification and characterization of mammalian circular RNAs. Genome Biol. 2014;15:409. doi: 10.1186/s13059-014-0409-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.He AT, Liu J, Li F, Yang BB. Targeting circular RNAs as a therapeutic approach: current strategies and challenges. Signal Transduct Target Ther. 2021;6:185. doi: 10.1038/s41392-021-00569-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang XO, et al. Complementary sequence-mediated exon circularization. Cell. 2014;159:134–47. doi: 10.1016/j.cell.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 42.Ashwal-Fluss R, et al. circRNA biogenesis competes with pre-mRNA splicing. Mol Cell. 2014;56:55–66. doi: 10.1016/j.molcel.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 43.Vo JN, et al. The Landscape of circular RNA in Cancer. Cell. 2019;176:869–881e813. doi: 10.1016/j.cell.2018.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Glažar P, Papavasileiou P, Rajewsky N. circBase: a database for circular RNAs. RNA. 2014;20:1666–70. doi: 10.1261/rna.043687.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ma XK, Xue W, Chen LL, Yang L. CIRCexplorer pipelines for circRNA annotation and quantification from non-polyadenylated RNA-seq datasets. Methods. 2021;196:3–10. doi: 10.1016/j.ymeth.2021.02.008. [DOI] [PubMed] [Google Scholar]
- 46.Mi Z, et al. Circular RNA detection methods: a minireview. Talanta. 2022;238:123066. doi: 10.1016/j.talanta.2021.123066. [DOI] [PubMed] [Google Scholar]
- 47.Memczak S, et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. 2013;495:333–8. doi: 10.1038/nature11928. [DOI] [PubMed] [Google Scholar]
- 48.D’Ambra E, Morlando M. Study of circular RNA expression by Nonradioactive Northern Blot Procedure. Methods Mol Biol. 2021;2348:371–83. doi: 10.1007/978-1-0716-1581-2_23. [DOI] [PubMed] [Google Scholar]
- 49.Jeck WR, et al. Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA. 2013;19:141–57. doi: 10.1261/rna.035667.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cocquet J, Chong A, Zhang G, Veitia RA. Reverse transcriptase template switching and false alternative transcripts. Genomics. 2006;88:127–31. doi: 10.1016/j.ygeno.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 51.Goo NI, Kim DE. Rolling circle amplification as isothermal gene amplification in molecular diagnostics. Biochip J. 2016;10:262–71. doi: 10.1007/s13206-016-0402-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu Y, et al. Direct detection of circRNA in real samples using reverse transcription-rolling circle amplification. Anal Chim Acta. 2020;1101:169–75. doi: 10.1016/j.aca.2019.12.027. [DOI] [PubMed] [Google Scholar]
- 53.Jiao J, et al. A method to directly assay circRNA in real samples. Chem Commun (Camb) 2018;54:13451–4. doi: 10.1039/c8cc08319c. [DOI] [PubMed] [Google Scholar]
- 54.Dahl M, et al. Enzyme-free digital counting of endogenous circular RNA molecules in B-cell malignancies. Lab Invest. 2018;98:1657–69. doi: 10.1038/s41374-018-0108-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tang W, et al. CircRNA microarray profiling identifies a novel circulating biomarker for detection of gastric cancer. Mol Cancer. 2018;17:137. doi: 10.1186/s12943-018-0888-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li S, et al. Microarray is an efficient tool for circRNA profiling. Brief Bioinform. 2019;20:1420–33. doi: 10.1093/bib/bby006. [DOI] [PubMed] [Google Scholar]
- 57.Chrzanowska NM, Kowalewski J, Lewandowska MA. Use of fluorescence in situ hybridization (FISH) in diagnosis and tailored therapies in solid tumors. Molecules. 2020;25. 10.3390/molecules25081864. [DOI] [PMC free article] [PubMed]
- 58.Lim AS, Lim TH. Fluorescence in situ hybridization on tissue sections. Methods Mol Biol. 2017;1541:119–25. doi: 10.1007/978-1-4939-6703-2_11. [DOI] [PubMed] [Google Scholar]
- 59.Wang Z, Gerstein M, Snyder M. RNA-Seq: a revolutionary tool for transcriptomics. Nat Rev Genet. 2009;10:57–63. doi: 10.1038/nrg2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zoabi Y, Shomron N. Processing and Analysis of RNA-seq data from Public Resources. Methods Mol Biol. 2021;2243:81–94. doi: 10.1007/978-1-0716-1103-6_4. [DOI] [PubMed] [Google Scholar]
- 61.Lu T et al. Transcriptome-wide investigation of circular RNAs in rice. Rna 21, 2076–2087, doi:10.1261/rna.052282.115 (2015). [DOI] [PMC free article] [PubMed]
- 62.Zhao X, Zhong Y, Wang X, Shen J, An W. Advances in circular RNA and its applications. Int J Med Sci. 2022;19:975–85. doi: 10.7150/ijms.71840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ma XK, et al. CIRCexplorer3: a CLEAR Pipeline for Direct Comparison of Circular and Linear RNA expression. Genomics Proteom Bioinf. 2019;17:511–21. doi: 10.1016/j.gpb.2019.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen LL. The expanding regulatory mechanisms and cellular functions of circular RNAs. Nat Rev Mol Cell Biol. 2020;21:475–90. doi: 10.1038/s41580-020-0243-y. [DOI] [PubMed] [Google Scholar]
- 65.Salzman J, Gawad C, Wang PL, Lacayo N, Brown PO. Circular RNAs are the predominant transcript isoform from hundreds of human genes in diverse cell types. PLoS ONE. 2012;7:e30733. doi: 10.1371/journal.pone.0030733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu B, Li J, Cairns MJ. Identifying miRNAs, targets and functions. Brief Bioinform. 2014;15:1–19. doi: 10.1093/bib/bbs075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Krek A, et al. Combinatorial microRNA target predictions. Nat Genet. 2005;37:495–500. doi: 10.1038/ng1536. [DOI] [PubMed] [Google Scholar]
- 68.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 69.Hansen TB, et al. Natural RNA circles function as efficient microRNA sponges. Nature. 2013;495:384–8. doi: 10.1038/nature11993. [DOI] [PubMed] [Google Scholar]
- 70.Hansen TB, Kjems J, Damgaard CK. Circular RNA and miR-7 in cancer. Cancer Res. 2013;73:5609–12. doi: 10.1158/0008-5472.CAN-13-1568. [DOI] [PubMed] [Google Scholar]
- 71.Mao W, et al. ciRS-7 is a prognostic biomarker and potential gene therapy target for renal cell carcinoma. Mol Cancer. 2021;20:142. doi: 10.1186/s12943-021-01443-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rong Z, et al. Circular RNA CircEYA3 induces energy production to promote pancreatic ductal adenocarcinoma progression through the miR-1294/c-Myc axis. Mol Cancer. 2021;20:106. doi: 10.1186/s12943-021-01400-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang K, Sun Y, Tao W, Fei X, Chang C. Androgen receptor (AR) promotes clear cell renal cell carcinoma (ccRCC) migration and invasion via altering the circHIAT1/miR-195-5p/29a-3p/29c-3p/CDC42 signals. Cancer Lett. 2017;394:1–12. doi: 10.1016/j.canlet.2016.12.036. [DOI] [PubMed] [Google Scholar]
- 74.Liu G, et al. CircFAT1 sponges miR-375 to promote the expression of yes-associated protein 1 in osteosarcoma cells. Mol Cancer. 2018;17:170. doi: 10.1186/s12943-018-0917-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li Z, et al. Exon-intron circular RNAs regulate transcription in the nucleus. Nat Struct Mol Biol. 2015;22:256–64. doi: 10.1038/nsmb.2959. [DOI] [PubMed] [Google Scholar]
- 76.Ding L, et al. Circular RNA circ-DONSON facilitates gastric cancer growth and invasion via NURF complex dependent activation of transcription factor SOX4. Mol Cancer. 2019;18. 10.1186/s12943-019-1006-2. [DOI] [PMC free article] [PubMed]
- 77.Wang L, et al. Circular RNA circRHOT1 promotes hepatocellular carcinoma progression by initiation of NR2F6 expression. Mol Cancer. 2019;18:119. doi: 10.1186/s12943-019-1046-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.You X, et al. Neural circular RNAs are derived from synaptic genes and regulated by development and plasticity. Nat Neurosci. 2015;18:603–10. doi: 10.1038/nn.3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Du WW, et al. Foxo3 circular RNA retards cell cycle progression via forming ternary complexes with p21 and CDK2. Nucleic Acids Res. 2016;44:2846–58. doi: 10.1093/nar/gkw027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zeng Y, et al. A circular RNA binds to and activates AKT phosphorylation and nuclear localization reducing apoptosis and enhancing Cardiac Repair. Theranostics. 2017;7:3842–55. doi: 10.7150/thno.19764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fontemaggi G, Turco C, Esposito G, Di Agostino S. New Molecular Mechanisms and Clinical Impact of circRNAs in Human Cancer. Cancers (Basel). 2021;13. 10.3390/cancers13133154. [DOI] [PMC free article] [PubMed]
- 82.Wang Y, Wang Z. Efficient backsplicing produces translatable circular mRNAs. RNA 21, 172–179, doi:10.1261/rna.048272.114 (2015). [DOI] [PMC free article] [PubMed]
- 83.Legnini I, et al. Circ-ZNF609 is a circular RNA that can be translated and functions in myogenesis. Mol Cell. 2017;66:22–37e29. doi: 10.1016/j.molcel.2017.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang M, et al. A novel protein encoded by the circular form of the SHPRH gene suppresses glioma tumorigenesis. Oncogene. 2018;37:1805–14. doi: 10.1038/s41388-017-0019-9. [DOI] [PubMed] [Google Scholar]
- 85.Wesselhoeft RA, et al. RNA circularization diminishes immunogenicity and can extend translation duration in vivo. Mol Cell. 2019;74:508–520e504. doi: 10.1016/j.molcel.2019.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Meganck RM, et al. Tissue-dependent expression and translation of circular RNAs with recombinant AAV vectors in vivo. Mol Ther Nucleic Acids. 2018;13:89–98. doi: 10.1016/j.omtn.2018.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wesselhoeft RA, Kowalski PS, Anderson DG. Engineering circular RNA for potent and stable translation in eukaryotic cells. Nat Commun. 2018;9:2629. doi: 10.1038/s41467-018-05096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Qu L, et al. Circular RNA vaccines against SARS-CoV-2 and emerging variants. Cell. 2022;185:1728–44. doi: 10.1016/j.cell.2022.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chen CK, et al. Structured elements drive extensive circular RNA translation. Mol Cell. 2021;81:4300–18. doi: 10.1016/j.molcel.2021.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liu CX, et al. RNA circles with minimized immunogenicity as potent PKR inhibitors. Mol Cell. 2022;82:420–434e426. doi: 10.1016/j.molcel.2021.11.019. [DOI] [PubMed] [Google Scholar]
- 91.Liu CX, et al. Structure and degradation of circular RNAs regulate PKR activation in Innate Immunity. Cell. 2019;177:865–880e821. doi: 10.1016/j.cell.2019.03.046. [DOI] [PubMed] [Google Scholar]
- 92.Yu J, et al. Circular RNA cSMARCA5 inhibits growth and metastasis in hepatocellular carcinoma. J Hepatol. 2018;68:1214–27. doi: 10.1016/j.jhep.2018.01.012. [DOI] [PubMed] [Google Scholar]
- 93.Zhao Q, et al. Targeting Mitochondria-Located circRNA SCAR alleviates NASH via reducing mROS output. Cell. 2020;183:76–93e22. doi: 10.1016/j.cell.2020.08.009. [DOI] [PubMed] [Google Scholar]
- 94.Dube U, et al. An atlas of cortical circular RNA expression in Alzheimer disease brains demonstrates clinical and pathological associations. Nat Neurosci. 2019;22:1903–12. doi: 10.1038/s41593-019-0501-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang H, Review, et al. RNA-based diagnostic markers discovery and therapeutic targets development in cancer. Pharmacol Ther. 2022;234:108123. doi: 10.1016/j.pharmthera.2022.108123. [DOI] [PubMed] [Google Scholar]
- 96.Enuka Y, et al. Circular RNAs are long-lived and display only minimal early alterations in response to a growth factor. Nucleic Acids Res. 2016;44:1370–83. doi: 10.1093/nar/gkv1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang L, Liao Y, Tang L. MicroRNA-34 family: a potential tumor suppressor and therapeutic candidate in cancer. J Exp Clin Cancer Res. 2019;38:53. doi: 10.1186/s13046-019-1059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Preußer C, et al. Selective release of circRNAs in platelet-derived extracellular vesicles. J Extracell Vesicles. 2018;7:1424473. doi: 10.1080/20013078.2018.1424473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bahn JH, et al. The landscape of microRNA, Piwi-interacting RNA, and circular RNA in human saliva. Clin Chem. 2015;61:221–30. doi: 10.1373/clinchem.2014.230433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shao Y, et al. Global circular RNA expression profile of human gastric cancer and its clinical significance. Cancer Med. 2017;6:1173–80. doi: 10.1002/cam4.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Xia Q, et al. Circular RNA expression profiling identifies prostate Cancer- specific circRNAs in prostate Cancer. Cell Physiol Biochem. 2018;50:1903–15. doi: 10.1159/000494870. [DOI] [PubMed] [Google Scholar]
- 102.Wu K, et al. Circular RNA F-circSR derived from SLC34A2-ROS1 fusion gene promotes cell migration in non-small cell lung cancer. Mol Cancer. 2019;18:98. doi: 10.1186/s12943-019-1028-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li M, et al. A circular transcript of ncx1 gene mediates ischemic myocardial injury by targeting miR-133a-3p. Theranostics. 2018;8:5855–69. doi: 10.7150/thno.27285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lim TB, et al. Targeting the highly abundant circular RNA circSlc8a1 in cardiomyocytes attenuates pressure overload induced hypertrophy. Cardiovasc Res. 2019;115:1998–2007. doi: 10.1093/cvr/cvz130. [DOI] [PubMed] [Google Scholar]
- 105.Huang Z, et al. Upregulated circPDK1 promotes RCC Cell Migration and Invasion by regulating the miR-377-3P-NOTCH1 Axis in Renal Cell Carcinoma. Onco Targets Ther. 2020;13:11237–52. doi: 10.2147/ott.S280434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang D, et al. Down-regulation of circular RNA_000926 attenuates renal cell carcinoma progression through miRNA-411-Dependent CDH2 inhibition. Am J Pathol. 2019;189:2469–86. doi: 10.1016/j.ajpath.2019.06.016. [DOI] [PubMed] [Google Scholar]
- 107.Zhang G, et al. Circular RNA EGLN3 silencing represses renal cell carcinoma progression through the miR-1224-3p/HMGXB3 axis. Acta Histochem. 2021;123:151752. doi: 10.1016/j.acthis.2021.151752. [DOI] [PubMed] [Google Scholar]
- 108.Sun J, Pan S, Cui H, Li H. CircRNA SCARB1 promotes renal cell Carcinoma Progression Via Mir- 510-5p/SDC3 Axis. Curr Cancer Drug Targets. 2020;20:461–70. doi: 10.2174/1568009620666200409130032. [DOI] [PubMed] [Google Scholar]
- 109.Li K, Wan CL, Guo Y, Circular RNA circMTO1 suppresses RCC Cancer Cell Progression via miR9/LMX1A Axis. Technol Cancer Res Treat. 2020;19:1533033820914286. doi: 10.1177/1533033820914286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li J, et al. CircTLK1 promotes the proliferation and metastasis of renal cell carcinoma by sponging miR-136-5p. Mol Cancer. 2020;19:103. doi: 10.1186/s12943-020-01225-2. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 111.Cen J, et al. Circular RNA circSDHC serves as a sponge for mir-127-3p to promote the proliferation and metastasis of renal cell carcinoma via the CDKN3/E2F1 axis. Mol Cancer. 2021;20:19. doi: 10.1186/s12943-021-01314-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gui CP, et al. circCHST15 is a novel prognostic biomarker that promotes clear cell renal cell carcinoma cell proliferation and metastasis through the miR-125a-5p/EIF4EBP1 axis. Mol Cancer. 2021;20:169. doi: 10.1186/s12943-021-01449-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wang Y, et al. Circular RNA circDVL1 inhibits clear cell renal cell carcinoma progression through the miR-412-3p/PCDH7 axis. Int J Biol Sci. 2022;18:1491–507. doi: 10.7150/ijbs.69351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Xie X et al. Downregulation of Circular RNA circPSD3 Promotes Metastasis by Modulating FBXW7 Expression in Clear Cell Renal Cell Carcinoma. J Oncol 2022, 5084631, 10.1155/2022/5084631 (2022). [DOI] [PMC free article] [PubMed]
- 115.Chen Q, et al. CircRNA cRAPGEF5 inhibits the growth and metastasis of renal cell carcinoma via the miR-27a-3p/TXNIP pathway. Cancer Lett. 2020;469:68–77. doi: 10.1016/j.canlet.2019.10.017. [DOI] [PubMed] [Google Scholar]
- 116.Chen L, Wu D, Ding T. Circular RNA circ_0001368 inhibited growth and invasion in renal cell carcinoma by sponging miR-492 and targeting LATS2. Gene 753, 144781, 10.1016/j.gene.2020.144781 (2020). [DOI] [PubMed]
- 117.Sun J, et al. CircUBAP2 inhibits proliferation and metastasis of Clear Cell Renal Cell Carcinoma via Targeting miR-148a-3p/FOXK2 pathway. Cell Transpl. 2020;29:963689720925751. doi: 10.1177/0963689720925751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Xue D, et al. Circ-AKT3 inhibits clear cell renal cell carcinoma metastasis via altering miR-296-3p/E-cadherin signals. Mol Cancer. 2019;18:151. doi: 10.1186/s12943-019-1072-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gong LJ, Wang XY, Yao XD, Wu X, Gu WY. CircESRP1 inhibits clear cell renal cell carcinoma progression through the CTCF-mediated positive feedback loop. Cell Death Dis. 2021;12:1081. doi: 10.1038/s41419-021-04366-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Li J, Huang C, Zou Y, Yu J, Gui Y. Circular RNA MYLK promotes tumour growth and metastasis via modulating miR-513a-5p/VEGFC signalling in renal cell carcinoma. J Cell Mol Med. 2020;24:6609–21. doi: 10.1111/jcmm.15308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yang CY, Wang J, Zhang JQ, Cai HM. Human circular RNA hsa_circRNA_101705 (circTXNDC11) regulates renal cancer progression by regulating MAPK/ERK pathway. Bioengineered. 2021;12:4432–41. doi: 10.1080/21655979.2021.1955579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Li R, Luo S, Zhang D, Circular RNA hsa_circ_0054537 sponges miR-130a-3p to promote the progression of renal cell carcinoma through regulating cMet pathway. Gene. 2020;754:144811. doi: 10.1016/j.gene.2020.144811. [DOI] [PubMed] [Google Scholar]
- 123.Luo S, Deng F, Yao N, Zheng F. Circ_0005875 sponges mir-502-5p to promote renal cell carcinoma progression through upregulating E26 transformation specific-1. Anticancer Drugs. 2022;33:e286–98. doi: 10.1097/CAD.0000000000001205. [DOI] [PubMed] [Google Scholar]
- 124.Lin L, Cai J, Circular RNA circ-EGLN3 promotes renal cell carcinoma proliferation and aggressiveness via miR-1299-mediated IRF7 activation. J Cell Biochem. 2020;121:4377–85. doi: 10.1002/jcb.29620. [DOI] [PubMed] [Google Scholar]
- 125.Zeng J, et al. Circular RNA circ_001842 plays an oncogenic role in renal cell carcinoma by disrupting microRNA-502-5p-mediated inhibition of SLC39A14. J Cell Mol Med. 2020;24:9712–25. doi: 10.1111/jcmm.15529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yu R, Yao J, Ren Y, A novel circRNA circNUP98, a potential biomarker, acted as an oncogene via the miR-567/ PRDX3 axis in renal cell carcinoma. J Cell Mol Med. 2020;24:10177–88. doi: 10.1111/jcmm.15629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Liu Z, Wang R, Zhu G. Circ_0035483 functions as a tumor promoter in renal cell Carcinoma via the miR-31-5p-Mediated HMGA1 Upregulation. Cancer Manag Res. 2021;13:693–706. doi: 10.2147/cmar.S282806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Fang L, Ye T, An Y, Circular. RNA FOXP1 Induced by ZNF263 Upregulates U2AF2 Expression to Accelerate Renal Cell Carcinoma Tumorigenesis and Warburg Effect through Sponging miR-423-5p. J Immunol Res 2021, 8050993, 10.1155/2021/8050993 (2021). [DOI] [PMC free article] [PubMed]
- 129.Pei L et al. Silencing circular RNA circ_0054537 and upregulating microRNA-640 suppress malignant progression of renal cell carcinoma via regulating neuronal pentraxin-2 (NPTX2). Bioengineered 12, 8279–8295, 10.1080/21655979.2021.1984002 (2021). [DOI] [PMC free article] [PubMed]
- 130.Yang C, et al. Exosome-derived circTRPS1 promotes malignant phenotype and CD8 + T cell exhaustion in bladder cancer microenvironments. Mol Ther. 2022;30:1054–70. doi: 10.1016/j.ymthe.2022.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Fan L, Yang J, Shen C, Wu Z, Hu H. Circ_0030586 inhibits cell proliferation and stemness in bladder cancer by inactivating the ERK signaling via miR-665/NR4A3 axis. Acta Histochem. 2021;123:151745. doi: 10.1016/j.acthis.2021.151745. [DOI] [PubMed] [Google Scholar]
- 132.Zhang L, Xia HB, Zhao CY, Shi L, Ren XL. Cyclic RNA hsa_circ_0091017 inhibits proliferation, migration and invasiveness of bladder cancer cells by binding to microRNA-589-5p. Eur Rev Med Pharmacol Sci. 2020;24:86–96. doi: 10.26355/eurrev_202001_19898. [DOI] [PubMed] [Google Scholar]
- 133.Chen LQ, et al. Hsa_circ_0041103 induces proliferation, migration and invasion in bladder cancer via the miR-107/FOXK1 axis. Eur Rev Med Pharmacol Sci. 2021;25:1282–90. doi: 10.26355/eurrev_202102_24832. [DOI] [PubMed] [Google Scholar]
- 134.Chen Q, et al. Hsa_circ_0068307 mediates bladder cancer stem cell-like properties via miR-147/c-Myc axis regulation. Cancer Cell Int. 2020;20:151. doi: 10.1186/s12935-020-01235-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Jin M, et al. Hsa_circ_0001944 promotes the growth and metastasis in bladder cancer cells by acting as a competitive endogenous RNA for miR-548. J Exp Clin Cancer Res. 2020;39:186. doi: 10.1186/s13046-020-01697-6. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 136.Yang C, et al. High-throughput sequencing identified circular RNA circUBE2K mediating RhoA associated bladder cancer phenotype via regulation of miR-516b-5p/ARHGAP5 axis. Cell Death Dis. 2021;12:719. doi: 10.1038/s41419-021-03977-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tong L, et al. circ_100984-miR-432-3p axis regulated c-Jun/YBX-1/beta-catenin feedback loop promotes bladder cancer progression. Cancer Sci. 2021;112:1429–42. doi: 10.1111/cas.14774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tian Y, et al. Circular RNA circSETD3 hampers cell growth, migration, and stem cell properties in bladder cancer through sponging miR-641 to upregulate PTEN. Cell Cycle. 2021;20:1589–602. doi: 10.1080/15384101.2021.1954758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Liu F, et al. Circ_0006948 drives the malignant development of bladder cancer via activating the epithelial-mesenchymal transition. J BUON. 2021;26:1491–7. [PubMed] [Google Scholar]
- 140.Yang C, et al. Circular RNA circ-ITCH inhibits bladder cancer progression by sponging miR-17/miR-224 and regulating p21, PTEN expression. Mol Cancer. 2018;17:19. doi: 10.1186/s12943-018-0771-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Dong W, et al. Circular RNA ACVR2A suppresses bladder cancer cells proliferation and metastasis through miR-626/EYA4 axis. Mol Cancer. 2019;18:95. doi: 10.1186/s12943-019-1025-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mao W, et al. Circular RNA hsa_circ_0068871 regulates FGFR3 expression and activates STAT3 by targeting miR-181a-5p to promote bladder cancer progression. J Exp Clin Cancer Res. 2019;38:169. doi: 10.1186/s13046-019-1136-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chen L, et al. Circ_0008532 promotes bladder cancer progression by regulation of the miR-155-5p/miR-330-5p/MTGR1 axis. J Exp Clin Cancer Res. 2020;39:94. doi: 10.1186/s13046-020-01592-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Qiu F, et al. Circ_0000658 knockdown inhibits epithelial-mesenchymal transition in bladder cancer via miR-498-induced HMGA2 downregulation. J Exp Clin Cancer Res. 2022;41. 10.1186/s13046-021-02175-3. [DOI] [PMC free article] [PubMed]
- 145.Wu L, et al. ERalpha-mediated alterations in circ_0023642 and mir-490-5p signaling suppress bladder cancer invasion. Cell Death Dis. 2019;10:635. doi: 10.1038/s41419-019-1827-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tan S, et al. circST6GALNAC6 suppresses bladder cancer metastasis by sponging miR-200a-3p to modulate the STMN1/EMT axis. Cell Death Dis. 2021;12:168. doi: 10.1038/s41419-021-03459-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ma X, et al. circKDM4C enhances bladder cancer invasion and metastasis through miR-200bc-3p/ZEB1 axis. Cell Death Discov. 2021;7:365. doi: 10.1038/s41420-021-00712-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Peng G, Meng H, Pan H, Wang W. CircRNA 001418 promoted cell growth and metastasis of bladder carcinoma via EphA2 by miR-1297. Curr Mol Pharmacol. 2021;14:68–78. doi: 10.2174/1874467213666200505093815. [DOI] [PubMed] [Google Scholar]
- 149.Liang H, Huang H, Li Y, Lu Y, Ye T. CircRNA_0058063 functions as a ceRNA in bladder cancer progression via targeting miR-486-3p/FOXP4 axis. Biosci Rep. 2020;40. 10.1042/bsr20193484. [DOI] [PMC free article] [PubMed]
- 150.Wang J, Luo J, Wu X, Gao Z. Circular RNA_0000629 suppresses bladder Cancer progression mediating MicroRNA-1290/CDC73. Cancer Manag Res. 2021;13:2701–15. doi: 10.2147/CMAR.S292863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sun M, et al. Circular RNA CEP128 promotes bladder cancer progression by regulating Mir-145-5p/Myd88 via MAPK signaling pathway. Int J Cancer. 2019;145:2170–81. doi: 10.1002/ijc.32311. [DOI] [PubMed] [Google Scholar]
- 152.Chen Y, et al. Circular RNA_0000326 promotes bladder cancer progression via microRNA-338-3p/ETS Proto-Oncogene 1/phosphoinositide-3 kinase/Akt pathway. Bioengineered. 2021;12:11410–22. doi: 10.1080/21655979.2021.2008738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Gong P, Xu R, Zhuang Q, He X. A novel circular RNA (hsa_circRNA_102336), a plausible biomarker, promotes the tumorigenesis by sponging mir-515-5p in human bladder cancer. Biomed Pharmacother. 2020;126:110059. doi: 10.1016/j.biopha.2020.110059. [DOI] [PubMed] [Google Scholar]
- 154.Wei W, et al. Circ0008399 Interaction with WTAP promotes Assembly and Activity of the m(6)a methyltransferase complex and promotes cisplatin resistance in bladder Cancer. Cancer Res. 2021;81:6142–56. doi: 10.1158/0008-5472.Can-21-1518. [DOI] [PubMed] [Google Scholar]
- 155.Sun M, Liu X, Zhao W, Zhang B, Deng P. Circ_0058063 contributes to cisplatin-resistance of bladder cancer cells by upregulating B2M through acting as RNA sponges for miR-335-5p. BMC Cancer. 2022;22:313. doi: 10.1186/s12885-022-09419-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Song Z, et al. Hsa_Circ_0001206 is downregulated and inhibits cell proliferation, migration and invasion in prostate cancer. Artif Cells Nanomed Biotechnol. 2019;47:2449–64. doi: 10.1080/21691401.2019.1626866. [DOI] [PubMed] [Google Scholar]
- 157.Sha J, et al. Downregulation of circ-TRPS1 suppressed prostatic cancer prognoses by regulating miR-124-3p/EZH2 axis-mediated stemness. Am J Cancer Res. 2020;10:4372–85. [PMC free article] [PubMed] [Google Scholar]
- 158.Wang G, Zhao H, Duan X, Ren Z. CircRNA pappalysin 1 facilitates prostate cancer development through miR-515-5p/FKBP1A axis. Andrologia. 2021;53:e14227. doi: 10.1111/and.14227. [DOI] [PubMed] [Google Scholar]
- 159.Ding X, Sun J, Zhang X. Circ_0076305 facilitates prostate cancer development via sponging miR-411-5p and regulating PGK1. Andrologia 54, e14406, 10.1111/and.14406 (2022). [DOI] [PubMed]
- 160.Zheng Y, et al. Extracellular vesicle-derived circ_SLC19A1 promotes prostate cancer cell growth and invasion through the miR-497/septin 2 pathway. Cell Biol Int. 2020;44:1037–45. doi: 10.1002/cbin.11303. [DOI] [PubMed] [Google Scholar]
- 161.Ding T, et al. Circular RNA circ_0057558 controls prostate Cancer Cell Proliferation through regulating miR-206/USP33/c-Myc Axis. Front Cell Dev Biol. 2021;9:644397. doi: 10.3389/fcell.2021.644397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wang P, et al. hsa_circ_0062019 promotes the proliferation, migration, and invasion of prostate cancer cells via the miR-195-5p/HMGA2 axis. Acta Biochim Biophys Sin (Shanghai) 2021;53:815–22. doi: 10.1093/abbs/gmab058. [DOI] [PubMed] [Google Scholar]
- 163.Shan G, et al. circFMN2 sponges miR-1238 to promote the expression of LIM-Homeobox gene 2 in prostate Cancer cells. Mol Ther Nucleic Acids. 2020;21:133–46. doi: 10.1016/j.omtn.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 164.Si-Tu J, et al. Upregulated circular RNA circ-102004 that promotes cell proliferation in prostate cancer. Int J Biol Macromol. 2019;122:1235–43. doi: 10.1016/j.ijbiomac.2018.09.076. [DOI] [PubMed] [Google Scholar]
- 165.Kong M, Li H, Yuan W, Mao L, Chen J. The role of Circ_PRKCI/miR-24-3p in the metastasis of prostate cancer. J BUON. 2021;26:949–55. [PubMed] [Google Scholar]
- 166.Yu YZ, et al. Hsa_circ_0003258 promotes prostate cancer metastasis by complexing with IGF2BP3 and sponging miR-653-5p. Mol Cancer. 2022;21. 10.1186/s12943-021-01480-x. [DOI] [PMC free article] [PubMed]
- 167.Mao S, et al. Hsa_circ_0004296 inhibits metastasis of prostate cancer by interacting with EIF4A3 to prevent nuclear export of ETS1 mRNA. J Exp Clin Cancer Res. 2021;40:336. doi: 10.1186/s13046-021-02138-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Li H, et al. Circ_0062020 Knockdown strengthens the radiosensitivity of prostate Cancer cells. Cancer Manag Res. 2020;12:11701–12. doi: 10.2147/CMAR.S273826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Li Q, et al. Circular RNA circ-0016068 promotes the growth, Migration, and Invasion of prostate Cancer cells by regulating the miR-330-3p/BMI-1 Axis as a competing endogenous RNA. Front Cell Dev Biol. 2020;8:827. doi: 10.3389/fcell.2020.00827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Luo GC, Chen L, Fang J, Yan ZJ. Hsa_circ_0030586 promotes epithelial-mesenchymal transition in prostate cancer via PI3K-AKT signaling. Bioengineered. 2021;12:11089–107. doi: 10.1080/21655979.2021.2008217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Pan J, et al. Circ_0001686 promotes prostate Cancer progression by Up-Regulating SMAD3/TGFBR2 via miR-411-5p. World J Mens Health. 2022;40:149–61. doi: 10.5534/wjmh.200204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Feng C, et al. Hsa_circ_0074032 promotes prostate cancer progression through elevating homeobox A1 expression by serving as a microRNA-198 decoy. Andrologia. 2022;54:e14312. doi: 10.1111/and.14312. [DOI] [PubMed] [Google Scholar]
- 173.Ren X, et al. Circular RNA circ_0062019 exerts oncogenic properties in prostate cancer via mediating miR-1253/NRBP1 axis. Andrologia. 2022;54:e14343. doi: 10.1111/and.14343. [DOI] [PubMed] [Google Scholar]
- 174.Deng ZH, et al. Hsa_circ_0088233 alleviates proliferation, Migration, and Invasion of prostate Cancer by Targeting hsa-miR-185-3p. Front Cell Dev Biol. 2020;8:528155. doi: 10.3389/fcell.2020.528155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Han Y, et al. Circular RNA hsa_circ_0075542 acts as a sponge for microRNA-1197 to suppress malignant characteristics and promote apoptosis in prostate cancer cells. Bioengineered. 2021;12:5620–31. doi: 10.1080/21655979.2021.1967064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Zhang Y, et al. Circ_0057553/miR-515-5p regulates prostate Cancer Cell Proliferation, apoptosis, Migration, Invasion and Aerobic glycolysis by targeting YES1. Onco Targets Ther. 2020;13:11289–99. doi: 10.2147/OTT.S272294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Li P, Wang Z, Li S, Wang L. Circ_0006404 accelerates prostate Cancer Progression through regulating miR-1299/CFL2 signaling. Onco Targets Ther. 2021;14:83–95. doi: 10.2147/OTT.S277831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Gu H, Duan Z. Silencing of circDPP4 suppresses cell progression of human prostate cancer and enhances docetaxel cytotoxicity through regulating the miR-564/ZIC2 axis. J Gene Med. 2022;24:e3403. doi: 10.1002/jgm.3403. [DOI] [PubMed] [Google Scholar]
- 179.Wu G, et al. Preclinical study using circular RNA 17 and micro RNA 181c-5p to suppress the enzalutamide-resistant prostate cancer progression. Cell Death Dis. 2019;10:37. doi: 10.1038/s41419-018-1048-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Lim MCJ, et al. hsa_circ_0001275 is one of a number of circRNAs Dysregulated in Enzalutamide resistant prostate Cancer and confers Enzalutamide Resistance in Vitro. Cancers (Basel). 2021;13. 10.3390/cancers13246383. [DOI] [PMC free article] [PubMed]
- 181.Zhang H, Li M, Zhang J, Shen Y, Gui Q. Exosomal Circ-XIAP promotes Docetaxel Resistance in prostate Cancer by regulating miR-1182/TPD52 Axis. Drug Des Devel Ther. 2021;15:1835–49. doi: 10.2147/DDDT.S300376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Chen Z, et al. Circular RNA circPPP6R3 upregulates CD44 to promote the progression of clear cell renal cell carcinoma via sponging miR-1238-3p. Cell Death Dis. 2021;13:22. doi: 10.1038/s41419-021-04462-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Wu X, et al. CircCYP24A1 hampered malignant phenotype of renal cancer carcinoma through modulating CMTM-4 expression via sponging miR-421. Cell Death Dis. 2022;13:190. doi: 10.1038/s41419-022-04623-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Liu G, et al. Hsa_circ_0085576 promotes clear cell renal cell carcinoma tumorigenesis and metastasis through the miR-498/YAP1 axis. Aging. 2020;12:11530–49. doi: 10.18632/aging.103300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Yan JS, Chen Q, Li YL, Gao YQ. Hsa_circ_0065217 promotes growth and metastasis of renal cancer through regulating the mir-214-3p-ALPK2 axis. Cell Cycle. 2021;20:2519–30. doi: 10.1080/15384101.2021.1991123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Gao L et al. circAMOTL1L Suppresses Renal Cell Carcinoma Growth by Modulating the miR-92a-2-5p/KLLN Pathway. Oxid Med Cell Longev 2021, 9970272, 10.1155/2021/9970272 (2021). [DOI] [PMC free article] [PubMed]
- 187.Li W, et al. circPRRC2A promotes angiogenesis and metastasis through epithelial-mesenchymal transition and upregulates TRPM3 in renal cell carcinoma. Theranostics. 2020;10:4395–409. doi: 10.7150/thno.43239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Liu H, et al. circPTCH1 promotes invasion and metastasis in renal cell carcinoma via regulating miR-485-5p/MMP14 axis. Theranostics. 2020;10:10791–807. doi: 10.7150/thno.47239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Zhang Z, Sang Y, Liu Z, Shao J. Negative correlation between circular RNA SMARC5 and MicroRNA 432, and their clinical implications in bladder Cancer patients. Technol Cancer Res Treat. 2021;20:15330338211039110. doi: 10.1177/15330338211039110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Shen C, et al. Downregulated hsa_circ_0077837 and hsa_circ_0004826, facilitate bladder cancer progression and predict poor prognosis for bladder cancer patients. Cancer Med. 2020;9:3885–903. doi: 10.1002/cam4.3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Wei B, Wang Z, Lian Q, Chi B, Ma S. hsa_circ_0139402 Promotes Bladder Cancer Progression by Regulating hsa-miR-326/PAX8 Signaling. Dis Markers 2022, 9899548, 10.1155/2022/9899548 (2022). [DOI] [PMC free article] [PubMed]
- 192.Li G, et al. CircRNA hsa_circ_0014130 function as a mir-132-3p sponge for playing oncogenic roles in bladder cancer via upregulating KCNJ12 expression. Cell Biol Toxicol. 2021 doi: 10.1007/s10565-021-09668-z. [DOI] [PubMed] [Google Scholar]
- 193.Liu F, et al. Hsa_circ_0001361 promotes bladder cancer invasion and metastasis through miR-491-5p/MMP9 axis. Oncogene. 2020;39:1696–709. doi: 10.1038/s41388-019-1092-z. [DOI] [PubMed] [Google Scholar]
- 194.Song Z, et al. Identification of urinary hsa_circ _0137439 as potential biomarker and tumor regulator of bladder cancer. Neoplasma. 2020;67:137–46. doi: 10.4149/neo_2018_181214N970. [DOI] [PubMed] [Google Scholar]
- 195.Deng W, et al. Novel circular RNA circ_0086722 drives tumor progression by regulating the miR-339-5p/STAT5A axis in prostate cancer. Cancer Lett. 2022;533:215606. doi: 10.1016/j.canlet.2022.215606. [DOI] [PubMed] [Google Scholar]
- 196.Mao Y, et al. Circular RNA_PDHX promotes the Proliferation and Invasion of prostate Cancer by sponging MiR-378a-3p. Front Cell Dev Biol. 2020;8:602707. doi: 10.3389/fcell.2020.602707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Chao F, et al. Novel circular RNA circSOBP governs amoeboid migration through the regulation of the miR-141-3p/MYPT1/p-MLC2 axis in prostate cancer. Clin Transl Med. 2021;11:e360. doi: 10.1002/ctm2.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Huang E, Chen X, Yuan Y. Downregulated circular RNA itchy E3 ubiquitin protein ligase correlates with advanced pathologic T stage, high lymph node metastasis risk and poor survivals in prostate cancer patients. Cancer Biomark. 2019;26:41–50. doi: 10.3233/cbm-182111. [DOI] [PubMed] [Google Scholar]
- 199.Zhang Q, et al. Roles of circRNAs in the tumour microenvironment. Mol Cancer. 2020;19:14. doi: 10.1186/s12943-019-1125-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Huang KB, et al. Circular RNA circSNX6 promotes sunitinib resistance in renal cell carcinoma through the miR-1184/GPCPD1/ lysophosphatidic acid axis. Cancer Lett. 2021;523:121–34. doi: 10.1016/j.canlet.2021.10.003. [DOI] [PubMed] [Google Scholar]
- 201.Zhang MX, et al. CircME1 promotes aerobic glycolysis and sunitinib resistance of clear cell renal cell carcinoma through cis-regulation of ME1. Oncogene. 2022;41:3979–90. doi: 10.1038/s41388-022-02386-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Lu J, et al. HnRNP-L-regulated circCSPP1/miR-520 h/EGR1 axis modulates autophagy and promotes progression in prostate cancer. Mol Ther Nucleic Acids. 2021;26:927–44. doi: 10.1016/j.omtn.2021.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Shen Z, Zhou L, Zhang C, Xu J. Reduction of circular RNA Foxo3 promotes prostate cancer progression and chemoresistance to docetaxel. Cancer Lett. 2020;468:88–101. doi: 10.1016/j.canlet.2019.10.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.