Abstract
Given the significant heterogeneity of alcohol use disorder (AUD) and the increasing priority to understand individual profiles of AUD, pursuing symptom-level examinations of AUD is important. Disturbances in sleep and circadian rhythms have demonstrated robust associations with alcohol consumption and AUD, yet little research has examined these associations at the symptom- or problem-levels and research to date has focused on one or two sleep/circadian characteristics. We sought to investigate the associations between (a) specific AUD symptoms and (b) domains of alcohol-related problems, and multiple sleep characteristics, collected at a daily level in the naturalistic environment. Young adult drinkers were recruited from the community (N=159, Mage=23.9, 58.5% female, 6.3% Asian, 35.9% Black or African American, 51.6% White, 5.0% multiracial) and completed a baseline visit as well as up to 18 days of naturalistic assessment. Several sleep/circadian characteristics, including eveningness, later midsleep timing, and shorter total sleep time, were consistently associated with the hazardous use symptom, above and beyond alcohol consumption. Eveningness (beta[SE]=0.21[0.00], p <.01) was a significant predictor of the alcohol-related problem domain of role interference. Exploratory analyses did not find significant associations between sleep/circadian characteristics and cannabis-related problems. The relationship between sleep/circadian characteristics and AUD and related problems may be driven by a narrower set of symptoms, such as hazardous use and role interference. This may be due to shared mechanistic dysfunction in domains such as reward processing or cognitive control. Thus, these alcohol-related symptoms and problems may be addressed through transdiagnostic treatment approaches that target these underlying mechanisms.
Keywords: alcohol use disorder, alcohol-related problems, heterogeneity, sleep and circadian characteristics, precision medicine
Approximately 14.8 million United States citizens aged 12 and older met criteria for alcohol use disorder (AUD) in 2018 (Lipari & Park-Lee, 2019). Yet, treatments demonstrate only modest efficacy (Witkiewitz et al., 2019). This is likely in part a result of the profound phenotypic heterogeneity of AUD (Litten et al., 2015). To better understand AUD heterogeneity, it is imperative to study risk factors for specific AUD symptoms and alcohol-related problems. Sleep and circadian characteristics show consistent relationships with alcohol use, AUD, and problems (Hasler & Pedersen, 2020; Miller et al., 2020) but have yet to be studied with respect to specific AUD symptoms or problems. Identifying modifiable risk factors, such as specific sleep characteristics, for specific AUD symptoms and problems may help enhance personalized medicine approaches by targeting factors specific to distinct types of problems, rather than assuming homogeneity of AUD. Moving away from studying AUD at the diagnosis, or aggregate, level may therefore be a key next step in improving treatment efficacy. Towards this aim, the current study examines sleep/circadian characteristics as risk factors for specific AUD symptoms, alcohol problem domains, and cannabis problem domains in a sample of young adults who use alcohol.
A range of individual differences in sleep and circadian rhythms have demonstrated robust associations with alcohol consumption and AUD (Chakravorty et al., 2016; Hasler et al., 2012; Hasler & Pedersen, 2020; Miller et al., 2020). For example, difficulty sleeping, shorter sleep duration, and later sleep/circadian timing are associated with alcohol consumption, AUD onset, and alcohol-related problems in cross-sectional (McKnight-Eily et al., 2011; Sivertsen et al., 2015) and longitudinal research (Hasler et al., 2017; Miller et al., 2017; Wong et al., 2004) across a range of developmental periods. There are two major limitations of this work. First, research to date has almost entirely examined AUD at the diagnosis level or general alcohol problems, rather than at the symptom-level or specific problem domains. Second, research has tended to focus on one or two sleep characteristics in isolation (e.g., difficulty sleeping), rather than multiple sleep characteristics, consistent with the multidimensional nature of sleep (Buysse, 2014).
A robust preclinical and clinical literature suggests that sleep and circadian processes, such as a tendency towards later sleep/circadian timing (“eveningness”) and/or insufficient sleep, modulate reward-related behaviors and their underlying physiological processes (Hasler & Pedersen, 2020; Logan et al., 2018). Thus, individuals with a circadian preference for eveningness might also be at a higher risk for experiencing AUD symptoms and problems that index reward dysregulation, such as incentive salience or greater delay discounting (Boness et al., 2021; Hasler et al., 2019). This could include symptoms and problems related to craving, giving up/reducing important activities to use, a failure to fulfil role obligations due to use, spending a significant amount of time obtaining, using, or recovering from drinking, continued use despite social/interpersonal harm, and recurrent use in hazardous situations (Boness et al., 2021).
In addition to reward-related processes, mechanistic pathways linking sleep/circadian disturbances and AUD may also involve negative emotionality. For example, sleep characteristics (e.g., insomnia) predict negative emotionality (e.g., anxiety/depression), which subsequently predict AUD (Baglioni et al., 2011; Hardee et al., 2018; Taylor & Hasler, 2018). In addition, alcohol is often used to reduce anxiety, suggesting a process of negative reinforcement related to the alleviation of negative emotions (e.g., Boness et al., 2021; Hasler & Pedersen, 2020; Kushner, 2000). Alcohol may be especially reinforcing for individuals with sleep/circadian disturbances due to larger reductions in anxiety (Hasler & Pedersen, 2020). For example, individuals with insomnia exhibit relatively greater reductions in tension, as well as sleep benefits, after alcohol use compared to individuals without insomnia (Roehrs, 1999). As such, we might expect individuals with insomnia-related sleep disturbances, such as longer sleep onset latency or greater wake after sleep onset, to be at higher risk for experiencing AUD symptoms and problems indexing processes such as drinking to alleviate negative emotions or withdrawal (Babson et al., 2017; Boness et al., 2021).
Finally, cognitive control, or executive function are also relevant to both sleep/circadian disturbances and AUD. Included within the domain of cognitive control are mechanisms such as conscientiousness, impulsivity, and response inhibition (Boness et al., 2021; Kwako et al., 2016). For example, sleep/circadian factors, such as later sleep/circadian timing and less sleep are related to higher impulsivity (McGowan & Coogan, 2018) and also predict alcohol consumption and related problems (Dick et al., 2010; Stautz & Cooper, 2013). Accordingly, it might be that individuals with later sleep timing and less total sleep time would be at higher risk for experiencing AUD symptoms and problems that index impulsive behaviors such as hazardous use (e.g., drinking and driving), drinking more or longer than intended, and drinking despite physical or psychological problems (Boness et al., 2021; Logan et al., 2018; Treloar et al., 2012).
The current project serves as a preliminary step towards addressing an important gap in the literature by investigating the associations between multiple sleep indices and (a) specific AUD symptoms and (b) domains of alcohol-related problems. Sleep indices capturing variation in timing, duration, and continuity were collected both at a daily level in the naturalistic environment and through global self-report. Hypothesized associations based on shared mechanisms are reflected in Supplemental Figure 1. As an exploratory analysis, we also examined associations between sleep indices and cannabis-related problem domains given sleep and circadian characteristics, such as eveningness and later sleep timing, may be associated with cannabis involvement, including onset of use and problems (Babson et al., 2017; Hasler et al., 2017; Winiger et al., 2019, 2020; Wong et al., 2019).
1. Method
1.1. Participants
The current project combined data from two ongoing alcohol administration studies. Data was collected prior to the onset of COVID-19 (March 2020). Given overlap in methods, the samples are described as one and differences are noted when applicable. Participants were recruited via posted flyers, word of mouth, internet advertisements, and through a research registry. Potential participants underwent a phone screening to determine initial eligibility. As a function of following guidelines for ethical alcohol administration (National Institute on Alcohol Abuse and Alcoholism, 2005) and study aims, individuals currently abstaining from alcohol or in treatment for alcohol or substance use problems were excluded. Additionally, any individuals who had reported prior adverse reactions to alcohol, had a current or past diagnosis of bipolar I/II disorder or a psychotic disorder, reported past head injury with loss of consciousness >5min., were pregnant or breastfeeding, weighed over 300 pounds, or were taking medications contraindicated with alcohol or would affect alcohol response (e.g., benzodiazepines; SSRIs, SNRIs, and birth control were allowed). Participants were required to be between 21 and 30 years of age and engage in weekly or more alcohol use. There were additional study specific exclusion criteria. One study, focused on sleep and alcohol use and excluded most sleep disorders (i.e., narcolepsy, restless legs syndrome, REMS sleep disorder, current night shift work). Current insomnia disorder and delayed sleep phase disorder were not exclusionary given aims of the study to examine the role of sleep timing, duration, and efficacy in relationship to alcohol outcomes. The other study was focused on inequities in alcohol problems and excluded individuals who did not identify their race as Black or African American or White or European American.
1.2. Study Design
Both studies were approved by the University of Pittsburgh Institutional Review Board. Participants completed a baseline session (compensated $50.00) that assessed alcohol and cannabis problems, circadian preference, and psychiatric illnesses including past 12-month AUD (assessed via semi-structured interview). Following this session, participants completed the additional components of the research protocols (e.g., within-subjects alcohol administration, ecological momentary assessment protocol, sleep lab visit [one study]) as well as a 6- and 12- (one study) month follow-up.
The ecological momentary assessment (EMA) protocol was comprised of 17 consecutive days or two 9-day runs (18 days total), depending on the study, and completed on each participant’s personal smartphone or a study-provided smartphone. EMA prompts were sent four times per day via automated text messages; sleep diaries were included in the first EMA prompt sent 15 minutes after habitual wake time (and 2nd daily prompt if the morning prompt was not completed). Participants had 40 minutes to complete the assessment and received two reminder text messages 10 and 20 minutes after the first text prompt was sent. Participants could earn up to $10/day for the EMA component (≥3=$10.00; 2=$5.00; 1=$2.50: one study: one completed prompt was required to be the morning assessment for full payment). Those who completed 85% or more of the EMA prompts received a bonus compensation of $55 or $56 depending on the study. Total possible payment was $225 in one study and $256 in the other for completing the EMA protocol.
1.3. Measures
1.3.1. Sociodemographic.
Sociodemographic characteristics (e.g., self-identified race, sex assigned at birth, gender identity) were collected at baseline.
1.3.2. Sleep and Circadian Characteristics.
Eveningness, a circadian preference for later sleep/activity timing, was assessed at baseline by reverse scoring the Composite Scale of Morningness (Smith et al., 1989) such that higher scores (possible range=0-55) indicate a greater preference for eveningness. Sleep onset latency, wake after sleep onset, midsleep timing, and total sleep time were assessed daily via EMA. Outcomes were aggregated within person across days to represent an overall person average. Sleep onset latency is the time from “lights out”, or trying to fall asleep, until sleep onset. Wake after sleep onset is the total duration of time awake between sleep onset and final wake time. Midsleep timing describes the midpoint between sleep onset and wake time and reflects a measure of sleep timing. Total sleep time is the total duration of sleep through the night (duration from sleep onset to final wake time minus time awake).
1.3.3. Alcohol Use Disorder.
Past 12-month and lifetime AUD criteria from the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) (American Psychiatric Association, 2013) were assessed with the Structured Clinical Interview for DSM-5–Research Version (SCID-5-RV) (First et al., 2015) at baseline. For the current manuscript, we focus on past 12-month AUD criteria to evaluate the time period most proximal the assessment of sleep characteristics and to parallel the timeframe of the alcohol use problems. The SCID-5 assesses 11 criteria: (1) drinking more or longer than intended (larger/longer); (2) persistent desire or unsuccessful attempts to cut down or control alcohol use (quit/cut down); (3) a great deal of time spent obtaining, using, or recovering from alcohol (time spent); (4) craving or a strong desire to use alcohol (craving); (5) failure to fulfill role obligations due to recurrent alcohol use (failure to fulfill); (6) continued alcohol use despite social or interpersonal problems (social/interpersonal); (7) important activities given up or reduced because of alcohol (give up); (8) recurrent use in hazardous situations (hazardous use); (9) continued alcohol use despite physical or psychological problem that is caused or made worse by alcohol (physical/psychological); (10) alcohol tolerance (tolerance); and (11) alcohol-related withdrawal (withdrawal). Participants who scored a 2 or higher on a given criterion were coded as “1” to indicate this symptom was present in the past 12 months (otherwise coded “0” to indicate absent or subthreshold). See Supplemental Table 1 for symptom base rates.
1.3.4. Alcohol-Related Problems.
The 27-item Young Adult Alcohol Problems Screening Test (YAAPST) (Hurlbut & Sher, 1992) assessed past 12-month alcohol-related problems at baseline. Items (e.g., “Have you gotten into physical fights when drinking”) were scored a “1” if participants reported experiencing the event at least once in the past year (otherwise coded “0”). For the current study, nine items were dropped due to low endorsement (<2.5%). Two factors were indicated by exploratory factor analysis (EFA; see Supplemental Table 2) of the remaining 18 items in the full baseline sample (N=192). Items with loadings <.3 or that cross-loaded (>.3) on the other factor were removed, resulting in 12 items. Confirmatory Factor Analysis using the WLSMV estimator was then conducted, yielding domains of hazardous use/impairment and role interference (see Supplemental Table 3). Factor scores for each domain were used as dependent variables in analyses.1
1.3.5. Cannabis-Related Problems.
The 29-item Marijuana Problems Inventory (Vandrey et al., 2005) assessed past 12-month cannabis-related problems at baseline. Items (e.g., “Had trouble thinking clearly in everyday activities”) were scored a “1” if participants reported experiencing the event at least once in the past year. For the current study, two items were excluded due to irrelevance to the current sample given that not everyone was in school (i.e., “Not able to do your homework or study for a test” and “Felt that you had a problem with school”) and eight additional items were dropped due to low endorsement (<2.5%). Two factors were indicated by EFA of the remaining 19 items in the full baseline sample (N=192; see Supplemental Table 4). Items with loadings <.3 or that cross-loaded (>.3) on the other factor were removed, resulting in 14 items. Confirmatory Factor Analysis using the WLSMV estimator was then conducted, yielding problem domains of role interference/dependence and physical/psychological problems (see Supplemental Table 5). Factor scores for each domain were used as dependent variables in analyses.2
1.3.6. Consumption.
Alcohol consumption was assessed with six items indexing past 12-month frequency of use, quantity of use, frequency of drunkenness, frequency of 4+ (female)/5+ (male) drinks in a two-hour period, maximum quantity of drinks in a 24-hour period, and frequency of maximum quantity. Factor scores were estimated for each participant to index a “heaviness of consumption” composite (α=0.82, ω=0.60). Cannabis consumption was assessed with a single item indexing past 12-month frequency of use on a 12-point scale from “Not at all” to “Several times a day”.
1.4. Analytic Strategy
We estimated the associations between each of the sleep and circadian characteristics and (1) each of the 11 AUD criteria and (2) the two primary alcohol-related problem domains (hazardous use/impairment and role interference). As a secondary aim, we also estimated associations between the sleep and circadian characteristics and cannabis-related problem domains (role interference/dependence and physical/psychological). Each model included study (1 or 2), alcohol or cannabis consumption (described in Methods), age (continuous), sex assigned at birth (male or female), race (dummy codes for White, Black, or Mixed additional marginalized racial or ethnic identities), and past-year income as covariates given known sex differences in metabolism of substances, differences in patterns of consumption associated with age, and inequities in AUD, alcohol problems, cannabis problems and sleep for individuals who identify as Black or African American. Regression analyses were performed using the R’s lm() function in the stats package (R Core Team, 2020). We corrected for multiple comparisons for a given outcome by dividing by the number of models (e.g., p<.05/5[separate sleep outcomes]=pcorrected<.01) (Dunn, 1961).
2. Results
2.1. Sample
Participants were 192 young adults who reported drinking weekly or more. Of these, 159 completed the EMA protocol and were included in the current subsample. Sample characteristics are reported in Table 1.
Table 1.
Overall characteristics of full sample (N = 159)
| Characteristic | n | % | M | SD | range |
|---|---|---|---|---|---|
| Demographics | |||||
| Age | 23.94 | 2.84 | 21-30 | ||
| Sex assigned at birth (female) | 96 | 60.38 | |||
| Gender | |||||
| Female | 93 | 58.49 | |||
| Male | 63 | 39.62 | |||
| Nonbinary | 2 | 1.55 | |||
| Racial identity | |||||
| Asian | 10 | 6.29 | |||
| Black or African American (only) | 57 | 35.85 | |||
| Multiracial* | 8 | 5.03 | |||
| White (only) | 82 | 51.57 | |||
| Hispanic or Latinx | 5 | 3.14 | |||
| Household income | $ 25,974.84 | $ 22,627.82 | $10,000-$125,000 | ||
| Sleep Characteristics | |||||
| Eveningness** | 19.99 | 7.55 | 1.00-38.00 | ||
| Midsleep (24-hour clock time) | 4:31 | 1:07 | 1:59-7:08 | ||
| Sleep onset latency (hours) | 0.26 | 0.22 | 0.01-1.25 | ||
| Total sleep time (hours) | 7.56 | 0.99 | 5.38-11.98 | ||
| Wake after sleep onset (hours) | 0.12 | 0.18 | 0.00-1.67 | ||
| Substance-Related Characteristics | |||||
| Alcohol use disorder diagnosis | 30 | 18.87 | |||
| Alcohol-related problems | 3.87 | 3.13 | 0.00-23.00 | ||
| Cannabis-related problems | 1.72 | 3.12 | 0.00-15.00 | ||
| Other Psychopathology | |||||
| Major depressive disorder (past 30 days) | 3 | 1.89 | |||
| Generalized anxiety disorder (past 12 months) | 11 | 6.92 | |||
| Social anxiety disorder (past 12 months) | 4 | 2.52 | |||
| Panic disorder (past 12 months) | 3 | 1.89 | |||
| Obsessive compulsive disorder (past 12 months) | 0 | 0.00 | |||
| Posttraumatic stress disorder (past 12 months) | 4 | 2.52 |
Note.
= participant indicated “mixed racial background” or selected more than one race (i.e., biracial or multiracial).
The Composite Scale of Morningness was reverse-scored such that higher scores now indicate greater eveningness.
2.2. Sleep and Alcohol Use Disorder Criteria (Figure 1, Supplemental Table 6)
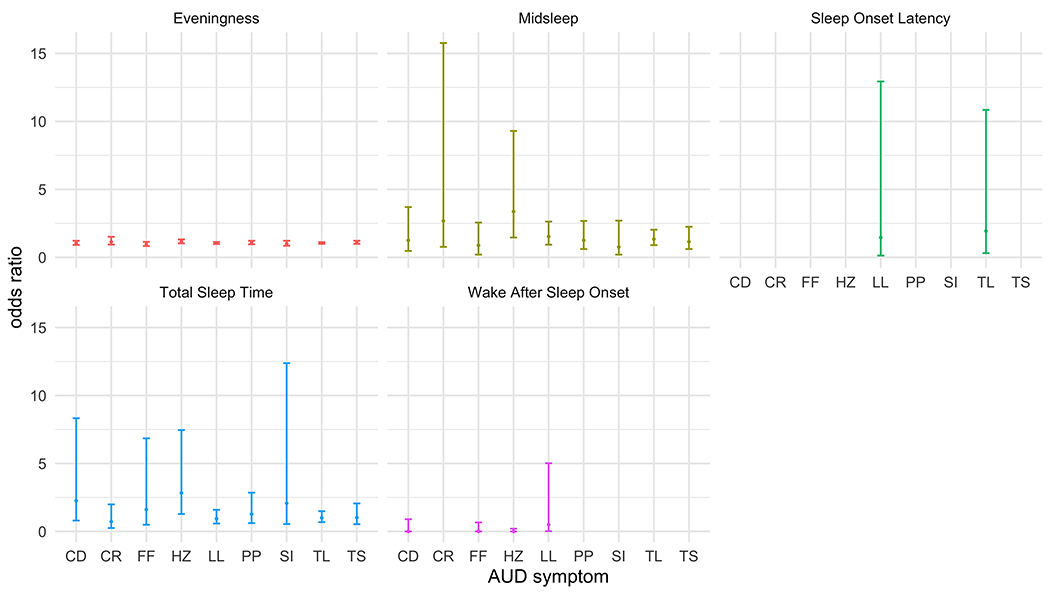
Figure 1.
Logistic regression models predicting Alcohol Use Disorder (AUD) symptom endorsement. Error bars represent standard errors. Models for withdrawal and giving up activities would not converge and are not displayed. Other symptoms missing estimates are not displayed due to imprecise estimates, likely due to low base rate and/or skew in the dependent variables. Odds ratios (OR) for Total Sleep Time are inversed such that for each unit decrease in Total Sleep Time, there is an increased odd of endorsing a given symptom (if OR > 1). AUD symptoms include: (1) alcohol-related withdrawal (withdrawal); (2) alcohol tolerance (tolerance); (3) a great deal of time spent obtaining, using, or recovering from alcohol (time spent); (4) important activities given up or reduced because of alcohol (give up); (5) craving or a strong desire to use alcohol (craving); (6) persistent desire or unsuccessful attempts to cut down or control alcohol use (quit/cut down); (7) drinking more or longer than intended (larger/longer); (8) continued alcohol use despite social or interpersonal problems (social problems); (9) recurrent use in hazardous situations (hazardous use); (10) continued alcohol use despite physical or psychological problem that is caused or made worse by alcohol (physical/psychological); (11) failure to fulfill role obligations due to recurrent alcohol use (failure to fulfill). Full estimates reported in Supplemental Table 4. * = significant at p<.05 level; † = no longer significant after Bonferroni correction (p<.05/5 sleep outcomes = .01).
Several sleep/circadian characteristics were consistently associated with the hazardous use criterion, above and beyond alcohol consumption. Eveningness (OR=1.16, 95%CI=1.03, 1.32), later midsleep timing (OR=3.37, 95%CI=1.46, 9.40), and shorter total sleep time (OR=2.83, 95%CI=1.28, 7.46) were all predictive of the likelihood of endorsing hazardous use. Later midsleep timing predicting an increased likelihood of endorsing hazardous use survived correction for multiple comparisons. Sleep onset latency and wake after sleep onset did not demonstrate any significant associations with AUD criteria.
2.3. Alcohol-Related Problems (Figure 2, Supplemental Table 7)
Figure 2.
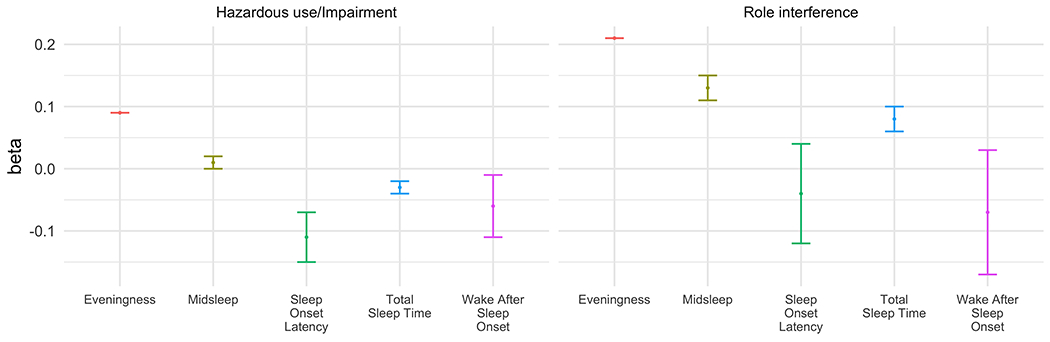
Sleep and circadian characteristics predicting alcohol-related problem domains. Models control for data set, alcohol consumption, age, sex, race, and income (N = 159). Bars represent standard errors. * = significant at p<.05 level and after Bonferroni correction (p<.05/5 sleep outcomes = .01).
Eveningness (beta[SE]=0.21[0.00], p<.01) was a significant predictor of alcohol-related role interference (see Figure 2, Supplemental Table 7). The association remained after correcting for multiple comparisons. There were no other significant relationships between sleep and circadian characteristics and hazardous use/impairment or role interference.
2.4. Cannabis-Related Problems
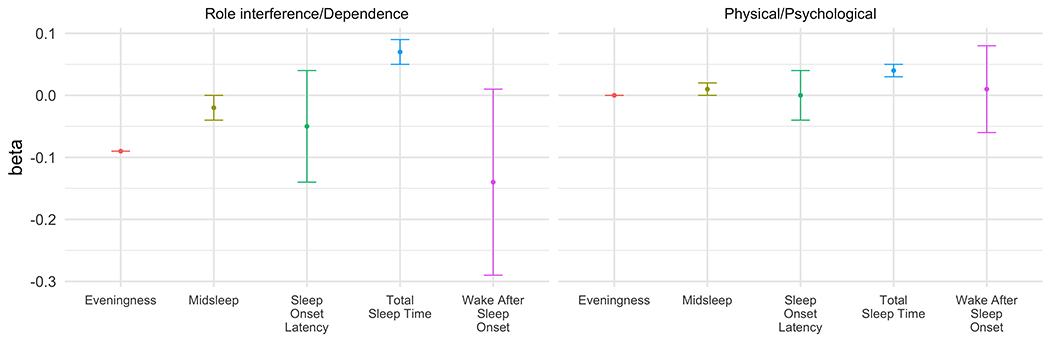
There were no significant relationships between sleep and circadian characteristics and the role interference/dependence or physical/psychological problem domains (see Figure 3, Supplemental Table 8).
Figure 3.
Sleep and circadian characteristics predicting cannabis-related problem domains. Models control for data set, cannabis consumption, age, sex, race, and income (N = 159). Bars represent standard errors.
3. Discussion
Given that significant heterogeneity among SUDs may limit the ability to effectively treat them, there is an increasing need to better understand risk for specific symptoms or aspects of addiction. Towards this aim, we sought to examine associations between sleep/circadian characteristics and specific AUD symptoms and alcohol-/cannabis-related problems. To our knowledge, this is the first examination of AUD and alcohol/cannabis problem-level associations with a range of sleep/circadian characteristics measured at both daily and global levels. We found that certain sleep/circadian characteristics may be uniquely related to indicators of hazardous use (e.g., risky behavior while drinking) and role interference over and above quantity and frequency of alcohol consumption. Our findings demonstrate the importance of examining specific alcohol-related problems and AUD criteria to identify underlying mechanisms between sleep and alcohol-risk and can further tailor treatments.
Sleep/circadian characteristics, particularly later midsleep timing, relating to hazardous use, while accounting for level of alcohol consumption, highlights the possibility that behaviors people engage in while drinking may be particularly affected by sleep. This could be partially driven by sleep and circadian factors altering reward sensitivity (Logan et al., 2018) and/or decreasing cognitive control (Kusztor et al., 2019; Logan et al., 2018). Alcohol has been shown to increase disinhibition (see Boness et al., 2021) and eveningness is associated with greater state-level impulsivity, including urgency (Hasler et al., 2022). Providing further support for the role of reward-related dysfunction in sleep and AUD is work demonstrating associations between (a) greater eveningness and altered neural activity to reward as well as greater alcohol use (Hasler, Casement, et al., 2017; Hasler et al., 2013) and (b) later midsleep timing and increased sensitivity to the stimulating effects of alcohol (Hasler et al., 2019). Additional research examining sleep characteristics as predictors of individual differences in the sensitivity to the disinhibiting effects of alcohol is needed.
Cognitive control’s involvement in these associations is also consistent with previous research suggesting an association between sleep/circadian characteristics, such as later midsleep timing and impulsivity (McGowan & Coogan, 2018). Impulsivity is also a well-established correlate of risky or hazardous alcohol use (Hamilton et al., 2012; Stautz & Cooper, 2013), and may interact with negative emotionality (negative urgency) or reward mechanisms (positive urgency) in sleep-AUD pathways (Hasler & Pedersen, 2020; Le et al., 2022).
Eveningness was also associated with alcohol-related role interference. Greater eveningness may confer risk for role interference, such as showing up late to work, and neglecting obligations because of altered reward response (Hasler et al., 2013) or lack of planning (Hasler et al., 2022), which may result in people choosing to continue drinking rather than considering future responsibilities that they might be required to complete (e.g., work the next day). Incorporating measures of delay discounting could further test this possibility.
While the current results are unable to examine the directionality of the associations between sleep/circadian characteristics and AUD/problems, they suggest that it may be useful to intervene on such shared mechanisms with transdiagnostic interventions. For example, mindfulness-based interventions are thought to modify core cognitive, emotional, neural, physiological, and behavioral processes implicated in psychopathology, including reward and cognitive control processes (Garland, 2016; Garland & Howard, 2018; Ie et al., 2014). Thus, transdiagnostic interventions, such as mindfulness-based interventions, may be effective in reducing both sleep/circadian disturbances and hazardous use by acting on their shared reward and cognitive control processes.
A major limitation of this research is that it cannot shed light on causality. We are unable to determine whether sleep/circadian characteristics increased the risk of hazardous use, whether hazardous use impacted sleep/circadian characteristics, or whether these occurred jointly due to underlying mechanisms (e.g., disrupted reward processes, impulsivity). In addition, due to relatively low base rates of AUD symptoms and alcohol-/cannabis-related problems in the current sample, we were unable to examine differential associations by individual characteristics such as sex and race. Future examinations should prioritize this especially in light of research suggesting that there are sex differences in alcohol-related sensitivity (Miller et al., 2009) and sleep characteristics (Knutson et al., 2010; Roenneberg et al., 2007) as well as racial differences in associations between midsleep timing and sensitivity to the stimulating effects of alcohol (Hasler et al., 2019). Low base rates also resulted in imprecise estimates, leading to large standard errors and confidence intervals in some instances. While we expect these estimates to stabilize in a clinical sample focused on individuals with an AUD, future research on this topic is needed. With a larger sample, there may also be sufficient power to examine models with all sleep/circadian characteristics in the same model to examine the predominant predictors of AUD symptoms and related problems that emerge. Last, because this study relied on self-reported sleep and circadian preference, future research should use objective measures of sleep (e.g., wrist actigraphy) and circadian timing (e.g., dim light melatonin onset) to better parse the role of sleep versus circadian factors in observed associations with AUD and related problems. In addition, it will be important for future work to include measures of sleep quality and daytime alertness to ensure full coverage of sleep as a multi-dimensional construct (Buysse, 2014).
Taken together, these results suggest that the relationship between sleep/circadian characteristics and AUD and related problems may be driven by hazardous use. This provides further justification for the need to investigate symptom- and problem-level associations in addition to syndrome-level associations, which may obscure these more nuanced relationships and potentially important treatment targets. Greater consideration of sleep/circadian timing and duration may enhance treatment benefits for cognitive-behavioral sleep therapies in individuals with AUD exhibiting hazardous use; previous studies have consistently achieved benefits for sleep without clearly impacting alcohol use (Arnedt et al., 2011; Miller, Donahue, et al., 2017). The current research therefore serves as an important step in understanding profiles of risk among those with AUD, and CUD, towards the future refinement and application of precision medicine approaches.
Supplementary Material
Funding:
This study was funded by the National Institute on Alcohol Abuse and Alcoholism (AA-026249; AA-025617). The funding agency had no role in the analysis, interpretation of data, or writing of this report.
Footnotes
Factor scores were based on 11 of the 12 items included in the confirmatory factor analysis because, although model fit was acceptable with the 12 items, one item loaded <.3 (see Supplemental Table 3).
One of the 14 items was excluded from the confirmatory factor analysis due to negative variance. Additionally, although model fit was acceptable, a second item included in the confirmatory factor analysis was removed from the estimation of factor scores because it loaded <.3 in the confirmatory model (see Supplemental Table 5). Factor scores were therefore estimated from 12 items.
References
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). American Psychiatric Association. [Google Scholar]
- Arnedt JT, Conroy DA, Armitage R, & Brower KJ (2011). Cognitive-behavioral therapy for insomnia in alcohol dependent patients: A randomized controlled pilot trial. Behaviour Research and Therapy, 49(4), 227–233. 10.1016/j.brat.2011.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babson KA, Sottile J, & Morabito D (2017). Cannabis, Cannabinoids, and Sleep: A Review of the Literature. Current Psychiatry Reports, 19(4), 23. 10.1007/s11920-017-0775-9 [DOI] [PubMed] [Google Scholar]
- Baglioni C, Battagliese G, Feige B, Spiegelhalder K, Nissen C, Voderholzer U, Lombardo C, & Riemann D (2011). Insomnia as a predictor of depression: A meta-analytic evaluation of longitudinal epidemiological studies. Journal of Affective Disorders, 135(1–3), 10–19. 10.1016/j.jad.2011.01.011 [DOI] [PubMed] [Google Scholar]
- Boness CL, Watts AL, Moeller KN, & Sher KJ (2021). The Etiologic, Theory-Based, Ontogenetic Hierarchical Framework of Alcohol Use Disorder: A Translational Systematic Review of Reviews. Psychological Bulletin. 10.31219/osf.io/bscuh [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ (2014). Sleep Health: Can We Define It? Does It Matter? Sleep, 37(1), 9–17. 10.5665/sleep.3298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravorty S, Chaudhary NS, & Brower KJ (2016). Alcohol Dependence and Its Relationship With Insomnia and Other Sleep Disorders. Alcoholism: Clinical and Experimental Research, 40(11), 2271–2282. 10.1111/acer.13217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick DM, Smith G, Olausson P, Mitchell SH, Leeman RF, O’Malley SS, & Sher K (2010). Understanding the construct of impulsivity and its relationship to alcohol use disorders. Addiction Biology, 15(2), 217–226. 10.1111/j.1369-1600.2009.00190.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn OJ (1961). Multiple Comparisons among Means. Journal of the American Statistical Association, 56(293), 52–64. 10.1080/01621459.1961.10482090 [DOI] [Google Scholar]
- First MB, Williams JB, Karg RS, & Spitzer RL (2015). Structured clinical interview for DSM-5—Research version (SCID-5 for DSM-5, research version; SCID-5-RV) (pp. 1–94). American Psychiatric Association. [Google Scholar]
- Garland EL (2016). Restructuring reward processing with Mindfulness-Oriented Recovery Enhancement: Novel therapeutic mechanisms to remediate hedonic dysregulation in addiction, stress, and pain: Mindfulness and hedonic regulation. Annals of the New York Academy of Sciences, 1373(1), 25–37. 10.1111/nyas.13034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, & Howard MO (2018). Mindfulness-based treatment of addiction: Current state of the field and envisioning the next wave of research. Addiction Science & Clinical Practice, 13(1), 14. 10.1186/s13722-018-0115-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton KR, Sinha R, & Potenza MN (2012). Hazardous Drinking and Dimensions of Impulsivity, Behavioral Approach, and Inhibition in Adult Men and Women. Alcoholism: Clinical and Experimental Research, 36(6), 434–449. 10.1111/j.1530-0277.2011.01708.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardee JE, Cope LM, Martz ME, & Heitzeg MM (2018). Review of Neurobiological Influences on Externalizing and Internalizing Pathways to Alcohol Use Disorder. Current Behavioral Neuroscience Reports, 5(4), 249–262. 10.1007/s40473-018-0166-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler BP, Casement MD, Sitnick SL, Shaw DS, & Forbes EE (2017). Eveningness among late adolescent males predicts neural reactivity to reward and alcohol dependence 2 years later. Behavioural Brain Research, 327, 112–120. 10.1016/j.bbr.2017.02.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler BP, Franzen PL, de Zambotti M, Prouty D, Brown SA, Tapert SF, Pfefferbaum A, Pohl KM, Sullivan EV, De Bellis MD, Nagel BJ, Baker FC, Colrain IM, & Clark DB (2017). Eveningness and Later Sleep Timing Are Associated with Greater Risk for Alcohol and Marijuana Use in Adolescence: Initial Findings from the National Consortium on Alcohol and Neurodevelopment in Adolescence Study. Alcoholism: Clinical and Experimental Research, 41(6), 1154–1165. 10.1111/acer.13401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler BP, & Pedersen SL (2020). Sleep and circadian risk factors for alcohol problems: A brief overview and proposed mechanisms. Current Opinion in Psychology, 34, 57–62. 10.1016/j.copsyc.2019.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler BP, Sitnick SL, Shaw DS, & Forbes EE (2013). An altered neural response to reward may contribute to alcohol problems among late adolescents with an evening chronotype. Psychiatry Research: Neuroimaging, 214(3), 357–364. 10.1016/j.pscychresns.2013.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler BP, Smith LJ, Cousins JC, & Bootzin RR (2012). Circadian rhythms, sleep, and substance abuse. Sleep Medicine Reviews, 16(1), 67–81. 10.1016/j.smrv.2011.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler BP, Soehner AM, & Clark DB (2015). Sleep and circadian contributions to adolescent alcohol use disorder. Alcohol, 49(4), 377–387. 10.1016/j.alcohol.2014.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler BP, Wallace ML, Graves JL, Molina BSG, & Pedersen SL (2022). Circadian preference is associated with multiple domains of trait and state level impulsivity. Chronobiology International, 1–13. 10.1080/07420528.2022.2035392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler BP, Wallace ML, White SJ, Molina BSG, & Pedersen SL (2019). Preliminary Evidence That Real World Sleep Timing and Duration are Associated With Laboratory-Assessed Alcohol Response. Alcoholism: Clinical and Experimental Research, 43(7), 1575–1584. 10.1111/acer.14076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurlbut SC, & Sher KJ (1992). Assessing Alcohol Problems in College Students. Journal of American College Health, 41(2), 49–58. 10.1080/07448481.1992.10392818 [DOI] [PubMed] [Google Scholar]
- Ie A, Ngnoumen CT, & Langer EJ (Eds.). (2014). Mindfulness: A transtherapeutic approach for transdiagnostic mental processes. In The Wiley Blackwell Handbook of Mindfulness (1st ed.). Wiley. 10.1002/9781118294895 [DOI] [Google Scholar]
- Knutson KL, Van Cauter E, Rathouz PJ, DeLeire T, & Lauderdale DS (2010). Trends in the Prevalence of Short Sleepers in the USA: 1975–2006. Sleep, 33(1), 37–45. 10.1093/sleep/33.1.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner M (2000). The relationship between anxiety disorders and alcohol use disorders A review of major perspectives and findings. Clinical Psychology Review, 20(2), 149–171. 10.1016/S0272-7358(99)00027-6 [DOI] [PubMed] [Google Scholar]
- Kusztor A, Raud L, Juel BE, Nilsen AS, Storm JF, & Huster RJ (2019). Sleep deprivation differentially affects subcomponents of cognitive control. Sleep, 42(4). 10.1093/sleep/zsz016 [DOI] [PubMed] [Google Scholar]
- Kwako LE, Momenan R, Litten RZ, Koob GF, & Goldman D (2016). Addictions Neuroclinical Assessment: A Neuroscience-Based Framework for Addictive Disorders. Biological Psychiatry, 80(3), 179–189. 10.1016/j.biopsych.2015.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le TM, Chen Y, Chaudhary S, & Li C-SR (2022). Problem drinking and the interaction of reward, negative emotion, and cognitive control circuits during cue-elicited craving. Addiction Neuroscience, 1, 100004. 10.1016/j.addicn.2021.100004 [DOI] [Google Scholar]
- Lipari RN, & Park-Lee E (2019). Key substance use and mental health indicators in the United States: Results from the 2018 National Survey on Drug Use and Health. [Google Scholar]
- Litten RZ, Ryan ML, Falk DE, Reilly M, Fertig JB, & Koob GF (2015). Heterogeneity of Alcohol Use Disorder: Understanding Mechanisms to Advance Personalized Treatment. Alcoholism: Clinical and Experimental Research, 39(4), 579–584. 10.1111/acer.12669 [DOI] [PubMed] [Google Scholar]
- Logan RW, Hasler BP, Forbes EE, Franzen PL, Torregrossa MM, Huang YH, Buysse DJ, Clark DB, & McClung CA (2018). Impact of Sleep and Circadian Rhythms on Addiction Vulnerability in Adolescents. Biological Psychiatry, 83(12), 987–996. 10.1016/j.biopsych.2017.11.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan NM, & Coogan AN (2018). Sleep and circadian rhythm function and trait impulsivity: An actigraphy study. Psychiatry Research, 268, 251–256. 10.1016/j.psychres.2018.07.030 [DOI] [PubMed] [Google Scholar]
- McKnight-Eily LR, Eaton DK, Lowry R, Croft JB, Presley-Cantrell L, & Perry GS (2011). Relationships between hours of sleep and health-risk behaviors in US adolescent students. Preventive Medicine, 53(4–5), 271–273. 10.1016/j.ypmed.2011.06.020 [DOI] [PubMed] [Google Scholar]
- Miller MA, Weafer J, & Fillmore MT (2009). Gender Differences in Alcohol Impairment of Simulated Driving Performance and Driving-Related Skills. Alcohol and Alcoholism, 44(6), 586–593. 10.1093/alcalc/agp051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MB, Donahue ML, Carey KB, & Scott-Sheldon LAJ (2017). Insomnia treatment in the context of alcohol use disorder: A systematic review and meta-analysis. Drug and Alcohol Dependence, 181, 200–207. 10.1016/j.drugalcdep.2017.09.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MB, Freeman L, Curtis AF, Boissoneault J, & McCrae CS (2020). Sleep Health and Alcohol Use. In Neurological Modulation of Sleep (pp. 255–264). Elsevier. 10.1016/B978-0-12-816658-1.00027-2 [DOI] [Google Scholar]
- Miller MB, Janssen T, & Jackson KM (2017). The Prospective Association Between Sleep and Initiation of Substance Use in Young Adolescents. Journal of Adolescent Health, 60(2), 154–160. 10.1016/j.jadohealth.2016.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. (2020). R: A language and environment for statistical computing. Foundation for Statistical Computing. https://www.R-project.org/. [Google Scholar]
- Roehrs T (1999). Ethanol as a Hypnotic in Insomniacs Self Administration and Effects on Sleep and Mood. Neuropsychopharmacology, 20(3), 279–286. 10.1016/S0893-133X(98)00068-2 [DOI] [PubMed] [Google Scholar]
- Roenneberg T, Kuehnle T, Juda M, Kantermann T, Allebrandt K, Gordijn M, & Merrow M (2007). Epidemiology of the human circadian clock. Sleep Medicine Reviews, 11(6), 429–438. 10.1016/j.smrv.2007.07.005 [DOI] [PubMed] [Google Scholar]
- Sivertsen B, Skogen JC, Jakobsen R, & Hysing M (2015). Sleep and use of alcohol and drug in adolescence. A large population-based study of Norwegian adolescents aged 16 to 19 years. Drug and Alcohol Dependence, 149, 180–186. 10.1016/j.drugalcdep.2015.01.045 [DOI] [PubMed] [Google Scholar]
- Smith CS, Reilly C, & Midkiff K (1989). Evaluation of Three Circadian Rhythm Questionnaires With Suggestions for an Improved Measure of Morningness. Journal of Applied Psychology, 74(5), 728–738. [DOI] [PubMed] [Google Scholar]
- Stautz K, & Cooper A (2013). Impulsivity-related personality traits and adolescent alcohol use: A meta-analytic review. Clinical Psychology Review, 33(4), 574–592. 10.1016/j.cpr.2013.03.003 [DOI] [PubMed] [Google Scholar]
- Taylor BJ, & Hasler BP (2018). Chronotype and Mental Health: Recent Advances. Current Psychiatry Reports, 20(8), 59. 10.1007/s11920-018-0925-8 [DOI] [PubMed] [Google Scholar]
- Treloar HR, Morris DH, Pedersen SL, & Mccarthy DM (2012). Direct and Indirect Effects of Impulsivity Traits on Drinking and Driving in Young Adults. Journal of Studies on Alcohol and Drugs, 73(5), 794–803. 10.15288/jsad.2012.73.794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandrey R, Budney AJ, Kamon JL, & Stanger C (2005). Cannabis withdrawal in adolescent treatment seekers. Drug and Alcohol Dependence, 78(2), 205–210. 10.1016/j.drugalcdep.2004.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winiger EA, Huggett SB, Hatoum AS, Friedman NP, Drake CL, Wright KP, & Hewitt JK (2020). Onset of regular cannabis use and young adult insomnia: An analysis of shared genetic liability. Sleep, 43(5), zsz293. 10.1093/sleep/zsz293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winiger EA, Huggett SB, Hatoum AS, Stallings MC, & Hewitt JK (2019). Onset of regular cannabis use and adult sleep duration: Genetic variation and the implications of a predictive relationship. Drug and Alcohol Dependence, 204, 107517. 10.1016/j.drugalcdep.2019.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkiewitz K, Litten RZ, & Leggio L (2019). Advances in the science and treatment of alcohol use disorder. Science Advances, 5(9), eaax4043. 10.1126/sciadv.aax4043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong MM, Brower KJ, Fitzgerald HE, & Zucker RA (2004). Sleep Problems in Early Childhood and Early Onset of Alcohol and Other Drug Use in Adolescence: Alcoholism: Clinical & Experimental Research, 28(4), 578–587. 10.1097/01.ALC.0000121651.75952.39 [DOI] [PubMed] [Google Scholar]
- Wong MM, Craun EA, Bravo AJ, Pearson MR, & The Protective Strategies Study Team. (2019). Insomnia symptoms, cannabis protective behavioral strategies, and hazardous cannabis use among U.S. college students. Experimental and Clinical Psychopharmacology, 27(4), 309–317. 10.1037/pha0000273 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


