Abstract
Background
An association has been reported between celiac disease (CD) and microscopic colitis (MC). However, large, population‐based cohort studies are rare.
Objective
To systematically examine the association between CD and MC in a large, nationwide cohort.
Methods
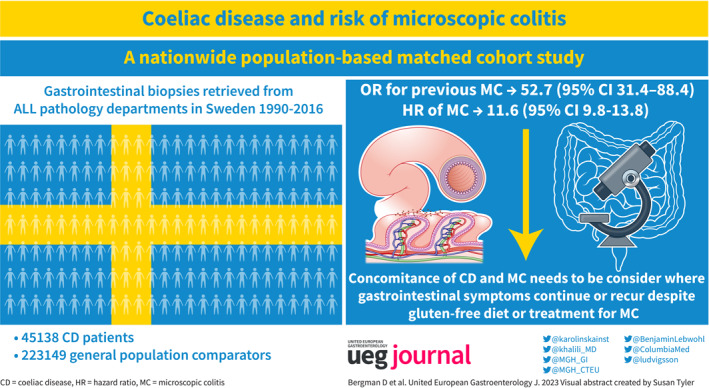
We conducted a nationwide population‐based matched cohort study in Sweden of 45,138 patients with biopsy‐verified CD (diagnosed in 1990–2016), 223,149 reference individuals, and 51,449 siblings of CD patients. Data on CD and MC were obtained from all (n = 28) pathology departments in Sweden. Adjusted hazard ratios (aHRs) were calculated using Cox regression.
Results
During follow‐up, 452 CD patients and 197 reference individuals received an MC diagnosis (86.1 vs. 7.5 per 100,000 person‐years). This difference corresponded to an aHR of 11.6 (95% confidence interval [CI] = 9.8–13.8) or eight extra MC cases in 1000 CD patients followed up for 10 years. Although the risk of MC was highest during the first year of follow‐up (aHR 35.2; 95% CI = 20.1–61.6), it remained elevated even after 10 years (aHR 8.1; 95% CI = 6.0–10.9). Examining MC subtypes lymphocytic colitis (LC) and collagenous colitis (CC) separately, the aHR was 12.4 (95% CI = 10.0–15.3) for LC and 10.2 (95% CI = 7.7–13.6) for CC. MC was also more common before CD (adjusted odds ratio [aOR] = 52.7; 95% CI = 31.4–88.4). Compared to siblings, risk estimates decreased but remained elevated (CD and later MC: HR = 6.2; CD and earlier MC: aOR = 7.9).
Conclusion
Our study demonstrated a very strong association of MC with CD with an increased risk of future and previous MC in CD patients. The magnitude of the associations underscores the need to consider the concomitance of these diagnoses in cases in which gastrointestinal symptoms persist or recur despite a gluten‐free diet or conventional MC treatment. The comparatively lower risk estimates in sibling comparisons suggest that shared genetic and early environmental factors may contribute to the association between CD and MC.
Keywords: celiac disease, collagenous colitis, epidemiology, lymphocytic colitis, microscopic colitis, non‐steroidal anti‐inflammatory drugs, NSAIDS, selective serotonin reuptake inhibitors, SSRIs, villous atrophy
Key Summary.
Summary of the established knowledge on this subject
An association between celiac disease (CD) and microscopic colitis (MC) has been reported, but large‐scale cohort studies examining the association across different strata are absent.
What are the significant and/or new findings of this study?
Using a nationwide, population‐based cohort, we found that there is a great risk of future and previous MC in patients with CD.
While the excess risk also remained in sibling analyses, lower risk estimates suggest that CD and MC share genetic risk factors, early environmental risk factors, or both.
Our results underline that the concomitance of these diagnoses should be considered in cases where gastrointestinal symptoms continue or recur despite a gluten‐free diet or conventional treatment for MC.
INTRODUCTION
Celiac disease (CD) is an autoimmune disorder where gluten intake triggers inflammation in the small intestine, resulting in villous atrophy (VA). 1 Typical symptoms of CD include fatigue, abdominal pain, weight loss, and diarrhea. A 2018 systematic review and meta‐analysis 2 found a global prevalence of CD of 1.4%. Moreover, our research group recently estimated that 1 in 49 women and 1 in 79 men in Sweden are expected to be diagnosed with CD during their lifetime. 3
Microscopic colitis (MC) is an inflammatory condition of the large intestine. 4 MC is characterized by watery, non‐bloody diarrhea, although other symptoms (e.g., fatigue, weight loss, abdominal pain) have been reported. The disorder is usually divided into collagenous colitis (CC) and lymphocytic colitis (LC) based on the histopathologic presentation of colonic biopsy. Previously, we have shown that the incidence of MC has risen in Sweden 5 over the past two decades.
While there are reports of an association between CD and MC, adequately powered cohort studies across different strata and with a high degree of generalizability are lacking. A recent case‐control study from Denmark examining 42 exposures found an odds ratio (OR) of 10.1 for earlier CD (n = 180) in patients with MC. 6 Despite this finding being consistent with the literature, the study has limitations as it was based solely on non‐validated histopathological data for MC. Also, there is a potential influence of multiple testing as associations to 42 different exposures were investigated.
The association between CD and MC has also been examined by a 2009 study from a tertiary center in New York 7 and a single‐center Canadian study from 2011. 8 The American study 7 (n = 1009) found a 70‐fold increased risk of MC in CD compared with the general population, and the Canadian study 8 reported a standardized incidence ratio (SIR) of 52.7. A 2021 systematic review and meta‐analysis 9 based on five studies including 2589 patients found that 4.5% of patients with refractory CD also had MC. This meta‐analysis also reported a pooled prevalence of 6.7% in CD in patients with refractory MC. 9
A 2019 prospective cohort study 10 reported no correlation between gluten intake and risk of MC among women without CD. However, both CD and MC are more common in women, and patients with MC are more likely to have a concomitant autoimmune disease (such as CD, diabetes, or thyroid disease). 11 Moreover, studies have found shared genetic factors 12 , 13 , 14 , 15 in CD and CC (but not for LC).
Because CD and MC are disorders with relatively high prevalence, shared symptoms, and potentially pathogenic mechanisms, it is essential to investigate the link between them. Therefore, this study systematically examines the association between CD and MC in a large population‐based cohort.
MATERIALS AND METHODS
Setting
We retrieved data on all gastrointestinal (GI) biopsies from all pathology departments in Sweden from 1990 to 2016. All Swedish citizens are assigned a unique personal identity number (PIN) that allows researchers to link data from various health care registers. 16 In addition, the healthcare system in Sweden is primarily government‐funded, decentralized, and provides equal access to health care to all its citizens.
Identification of patients with CD
The data used in this study were collected as part of the ESPRESSO study, 17 which includes data on all GI biopsies in Sweden from 1965 to 2017. In Sweden, biopsies are classified according to the Systematized Nomenclature of Medicine (SNOMED) system. This system assigns a specific code to each biopsy based on its histopathological presentation. We retrieved data on all duodenal/jejunal biopsies coded as M58 (with subgroups) or the CD diagnostic code D6218. In a previous validation study, 95% of patients with duodenal biopsy indicating VA were found to have CD according to patient charts. 18
General population reference individuals (comparators)
As part of the ESPRESSO study, 17 all patients with SNOMED codes signifying CD were matched to five general population comparators. Matching was done by age, sex, county of residence at the time of diagnosis, and index date (i.e., reference individuals started follow‐up on the date of CD diagnosis). Matched reference individuals were identified through the Total Population Register. 19
Sibling comparators
Siblings of patients with CD were identified through the Multigeneration Register. This identification process allowed us to control shared intrafamilial confounding, including genetic and early environmental factors. Using this register, we identified 51,449 siblings of 45,138 patients with CD (Figure 1).
FIGURE 1.
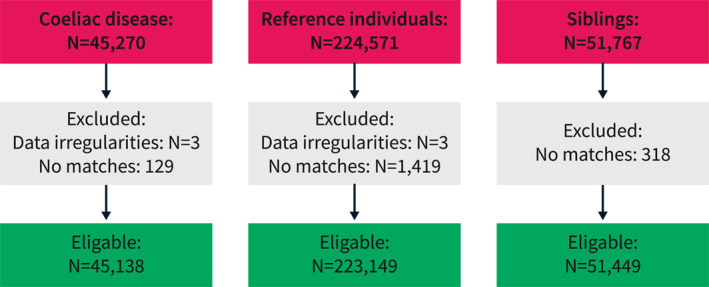
Flowchart of celiac disease patients, reference individuals, and siblings between 1990 and 2016.
Ascertainment of outcomes
Data on outcomes were identified through the ESPRESSO study. 17 We identified our outcome (CC or LC) using the SNOMED codes M40600 for CC and M47170 for LC. The validity of these codes has been ascertained in a previous study 20 showing a positive predictive value (PPV) of 95% for MC.
Follow‐up
Follow‐up started 1 day after the first biopsy date with CD (for the exposed group) and on the same date for the matched comparators. This follow‐up procedure was performed to exclude prevalent cases of MC and patients undergoing duodenoscopy and colonoscopy on the same day and focus our study on future risk. We limited our study period to 1 January 1990 to 31 December 2016, given that MC was rarely diagnosed in Sweden before 1990. 5 Follow‐up ended on the date of MC diagnosis, date of death, date of emigration, or 31 December 2016, whichever occurred first. If a reference individual developed MC, he or she was reclassified as exposed and given an own set of reference individuals.
Other covariates
Data on baseline characteristics (age, sex, county of residence, country of birth, and emigration) were collected from the Total Population Register. 19 To control for socio‐economic status, we collected data on the highest level of education from the longitudinal integrated database for health insurance and labor market studies (LISA). 21 Study participants were classified according to their years of completed education. Categories were defined as compulsory school (≤9 years), upper secondary school (10–12 years), or college (≥13 years). If information on education level was missing, the parents' highest education level was imputed. In cases where this information was also missing, the study participants were placed in a missing category.
Information on comorbidities was collected from the Swedish National Patient Register. 22 Using ICD codes, we identified all study participants with a diagnosis (prior to exposure) of diabetes, inflammatory bowel disease (IBD), thyroid disease, and rheumatoid arthritis (RA). We also used data from the Swedish Prescribed Drug Register 23 to identify patients to whom prescribed diabetes medication had been dispensed. We identified these medications by ATC codes A10AB‐AE and A10BA‐BX.
Starting, 1 July 2005, the Swedish prescribed drug register stores data on all dispensed prescribed medications. As there is a known link between MC and certain classes of drugs 24 , 25 (i.e non‐steroidal anti‐inflammatory drugs [NSAIDS], selective serotonin reuptake inhibitors [SSRIs], proton pump inhibitors [PPIs]), we conducted a sensitivity analysis (starting 1 January 2006 to rule out prevalent therapy) where all study participants with a recorded dispensation of either drug (after inclusion in the study and before end of follow up) were excluded. These drugs were also identified by ATC‐codes (NSAID: M01A, PPI: A02BC, SSRI: N06AB).
Patient and public involvement
No patient participated in the planning or design of this study.
Statistical analysis
Using a matched cohort study design, we calculated adjusted hazard ratios (aHRs) and 95% confidence intervals (CIs) based on the occurrence of MC in our exposed group (patients with CD) and their matched comparators. To minimize a potential confounding effect, we adjusted for the matching variables. 26 We also adjusted for education level and comorbidities (diabetes, IBD, thyroid disease, RA) to compute our main result. This calculation was done with a Cox proportional hazards model. Because the Schoenfeld residuals test provided strong evidence against proportionality, our main result should be interpreted as a mean aHR across the study period. To address non‐proportionality, we performed an additional analysis in which an interaction term for exposure and follow‐up time expressed in years was included. Focusing on the model at different years of follow‐up (0, 1, 5, 10, 20 years) allowed us to compute the aHR at these time points. We additionally tested whether the calendar period (1990–2000, 2001–2010, 2011–2016) and the calendar period with a maximum of 3 years of follow‐up (1990–2000, 2001–2010, 2011–2013, to allow for ≥3 years of follow‐up in all study participants) influenced the results. Further analyses were conducted to determine whether the correlation changed according to age at the start of follow‐up (<18, 18–39, 40–59, ≥60 years), years of follow‐up (<1, 1 to <5, 5 to <10, ≥10 years) (<18, 18–39, 40–59, ≥60 years), or education level (≤9, 10–12, ≥13 years). Statistical significance of interaction was tested by introducing an interaction term that included CD status and each of the above strata into the main model. We performed the same analyses using unaffected siblings of our exposed individuals as reference individuals. These analyses were stratified according to family.
In an effort to control for misclassification and surveillance bias, a sensitivity analysis was conducted where all patients diagnosed within 1 year of enrollment in the study were excluded.
To further elucidate the temporal relationship between CD and MC, a case‐control study design was used to establish the association between CD and previous MC. Using logistic regression, we computed adjusted odds ratios (aORs) to contrast the frequency of prior MC in the CD population compared with that of their matched reference individuals. In this analysis, we included the same adjustments as in our main analysis.
All statistical analyses were conducted using Stata/IC 14.2 for Mac (StataCorp).
Ethics
This study was approved by the Stockholm Ethics Review Board. Because the study is strictly register‐based, informed consent was not required. 27
RESULTS
Some 45,138 patients with CD, 223,149 reference individuals, and 51,449 siblings were included in the study (Table 1). As expected, most patients (62.6%) with CD were female. Mean age at diagnosis was 32.4 years (standard deviation [SD] = 25.1) and mean follow‐up was 11.6 years (SD = 7.1). Some 95% of the exposed were born in a Nordic country and 34.2% had an education level corresponding to college or university. Diabetes was more prevalent in exposed individuals (5.1%) compared with reference individuals (1.6%). Other immune‐mediated disorders (IBD, thyroid disease, RA) were also more prevalent in individuals with CD (Table 1).
TABLE 1.
Summary statistics for celiac disease patients, reference individuals and siblings.
| Celiac disease | Reference | Siblings | |
|---|---|---|---|
| n [%] | n [%] | n [%] | |
| Total | 45,138 [100.0] | 223,149 [100.0] | 51,449 [100.0] |
| Male | 16,886 [37.4] | 83,177 [37.3] | 26,416 [51.3] |
| Female | 28,252 [62.6] | 139,972 [62.7] | 25,033 [48.7] |
| Age at start follow up | |||
| Mean [SD] years | 32.4 [25.1] | 32.0 [24.9] | 30.8 [21.0] |
| Median [IQR] years | 28.3 [9.1–53.7] | 27.8 [8.9–53.1] | 28.3 [11.7–48.8] |
| <18 years | 17,670 [39.0] | 88,286 [39.6] | 18,480 [35.9] |
| 18 < 40 years | 10,114 [22.4] | 50,235 [22.5] | 14,285 [27.8] |
| 40 < 60 years | 8850 [19.6] | 43,834 [19.6] | 13,260 [25.8] |
| ≥60 years | 8504 [18.9] | 40,794 [18.3] | 5424 [10.5] |
| Years of follow up | |||
| Mean [SD] years | 11.6 [7.1] | 11.8 [7.1] | 11.7 [7.1] |
| Median [IQR] years | 10.9 [5.7–16.9] | 11.0 [5.8–17.1] | 11.0 [5.8–16.9] |
| <1 year | 1568 [3.5] | 5964 [2.7] | 1591 [3.1] |
| 1 < 5 years | 8157 [18.0] | 40,561 [18.3] | 9417 [18.3] |
| 5 < 10 years | 10,893 [24.1] | 53,850 [24.2] | 12,088 [23.5] |
| ≥10 years | 24,520 [54.4] | 122,774 [54.8] | 28,353 [55.1] |
| Year of start follow up | |||
| 1990–2000 | 15,275 [33.8] | 75,656 [33.8] | 16,126 [31.3] |
| 2001–2010 | 20,149 [44.7] | 99,419 [44.6] | 23,595 [45.9] |
| 2011–2016 | 9723 [21.5] | 48,074 [21.6] | 11,728 [22.8] |
| Reason for end of follow‐up | |||
| Emigration | 476 [1.1] | 4292 [1.9] | 588 [1.1] |
| 31 December 2016 | 39,249 [86.9] | 196,612 [88.0] | 47,169 [91.6] |
| Diagnosed with microscopic colitis | 452 [1.0] | 197 [0.09] | 88 [0.2] |
| Diagnosed with lymphocytic colitis | 305 [0.7] | 126 [0.06] | 61 [0.1] |
| Diagnosed with collagenous colitis | 147 [0.3] | 71 [0.03] | 27 [0.05] |
| Death | 4961 [11.0] | 21,444 [9.7] | 2088 [4.0] |
| Diagnosed with celiac disease | 0 [0] | 604 [0.27] | 1516 [2.9] |
| Country of birth | |||
| Nordic | 43,165 [95.4] | 203,881 [91.3] | 50,104 [97.4] |
| Other | 1973 [4.5] | 19,264 [8.7] | 1343 [2.6] |
| Missing | 0 [0.00] | 4 [0.00] | 2 [0.00] |
| Education | |||
| Compulsory school (≤9 years) | 9140 [20.2] | 47,218 [21.2] | 9707 [18.9] |
| Upper secondary school (10–12 years) | 18,121 [40.1] | 92,051 [41.2] | 17,917 [34.9] |
| College or university (≥13 years) | 15,460 [34.2] | 78,699 [35.2] | 11,259 [21.9] |
| Missing | 2417 [5.4] | 5181 [2.3] | 12,566 [24.3] |
| Comorbidity at start of follow up | |||
| Diabetes | 2287 [5.1] | 3515 [1.6] | 965 [1.8] |
| Inflammatory bowel disease | 1107 [2.5] | 356 [0.2] | 418 [0.8] |
| Thyroid disease | 963 [2.2] | 1598 [0.7] | 477 [0.9] |
| Rheumatoid arthritis | 333 [0.7] | 944 [0.4] | 262 [0.5] |
CD and later MC
During the study period, 452 patients with CD and 197 reference individuals were diagnosed with MC, which translates to incidence rates (IRs) of 86.6 per 100,000 person‐years (95% CI = 78.6–94.5) (Table 2) in the exposed population (patients with CD) and 7.5 per 100,000 person‐years (95% CI = 6.5–8.6) in reference individuals. Figure 2 outlines the difference in cumulative events between CD patients and reference individuals during the study period. Notably, when diagnosed with MC, patients with CD were almost 10 years younger (mean age at onset of MC = 53.7, SD = 18.9) than reference individuals who on average developed MC at age 62.1 (SD = 16.5).
TABLE 2.
MC incidence rates for CD patients, reference individuals and siblings.
| Celiac disease | Reference | Siblings | |
|---|---|---|---|
| N total | 45,138 | 223,149 | 51,449 |
| N events | 452 | 197 | 88 |
| Incidence proportion [%] | 1.0 | 0.09 | 0.2 |
| Person years | 524,695 | 2,602,493 | 584,392 |
| Incidence rate/100,000 person‐years [95% CI] | |||
| Overall | 86.1 [78.6–94.5] | 7.5 [6.5–8.6] | 14.9 [12.1–18.4] |
| Sex | |||
| Males | 66.4 [55.8–78.9] | 5.2 [3.9–6.8] | 10.0 [7.0–14.3] |
| Females | 97.6 [87.6–108.9] | 8.8 [7.5–10.4] | 20.0 [15.4–26.0] |
| Age at start follow up | |||
| <18 years | 16.7 [12.1–22.9] | 0.7 [0.4–1.4] | 3.2 [1.5–6.8] |
| 18 < 40 years | 97.2 [80.6–117.1] | 6.1 [4.3–8.5] | 9.4 [5.6–15.5] |
| 40 < 60 years | 155.1 [133.6–180.2] | 13.8 [11.0–17.2] | 30.7 [23.3–40.4] |
| ≥60 years | 185.4 [156.5–219.8] | 20.7 [16.5–25.9] | 32.8 [19.5–55.5] |
| Years of follow up | |||
| <1 | 227.8 [187.4–276.8] | 6.5 [3.9–11.0] | 10.5 [4.4–25.3] |
| 1 < 5 | 82.0 [69.0–97.3] | 5.6 [4.1–7.5] | 15.2 [10.4–22.4] |
| 5 < 10 | 66.9 [55.0–81.4] | 8.5 [6.6–10.9] | 13.5 [8.9–20.5] |
| ≥10 | 70.2 [58.8–83.9] | 9.0 [7.2–11.2] | 18.0 [13.0–25.1] |
| Start of follow up | |||
| 1990–2000 | 61.5 [53.1–71.4] | 7.1 [5.8–8.6] | 12.1 [8.8–16.7] |
| 2001–2010 | 105.4 [92.3–120.3] | 8.2 [6.6–10.2] | 16.7 [12.2–22.7] |
| 2011–2016 | 178.2 [137.7–230.5] | 6.8 [3.8–12.3] | 25.8 [13.9–48.0] |
| Start of follow up (with max 3 years of follow up) | |||
| 1990–2000 | 64.9 [45.1–93.4] | 1.8 [0.7–4.8] | 4.4 [1.1–17.4] |
| 2001–2010 | 177.4 [146.6–214.9] | 6.9 [4.5–10.7] | 16.6 [9.2–30.0] |
| 2011–2013 | 236.4 [173.4–322.2] | 9.5 [4.8–19.0] | 30.4 [13.7–67.8] |
| Country of birth | |||
| Nordic | 87.91 [79.4.1–95.7] | 8.0 [6.9–9.2] | 14.9 [12.3–18.8] |
| Other | 59.1 [32.7–106.7] | 1.1 [0.3–4.5] | NA [NA‐NA] |
| Education | |||
| Compulsory school (≤9 years) | 119.7 [100.3–142.9] | 10.6 [8.2–13.8] | 15.5 [9.8–24.6] |
| Upper secondary school (10–12 years) | 81.1 [70.1–93.8] | 7.3 [5.9–9.0] | 20.3 [15.0–27.5] |
| College or university (≥13 years) | 84.9 [72.2–100.0] | 5.9 [4.5–7.7] | 20.0 [13.4–29.9] |
| Missing | 10.7 [3.4–33.1] | 7.3 [2.3–22.5] | 2.1 [0.7–6.6] |
| Comorbidity at start of follow up | |||
| Diabetes | 90.8 [58.6–140.7] | 29.1 [13.9–61.1] | 12.0 [1.7–84.9] |
| No diabetes | 85.9 [78.2–94.4] | 7.3 [6.3–8.4] | 14.9 [12.1–18.4] |
| Inflammatory bowel disease | 91.7 [52.1–161.4] | 24.2 [3.4–171.7] | 51.4 [12.9–205.6] |
| No inflammatory bowel disease | 86.0 [78.3–94.4] | 7.5 [6.5–8.6] | 14.6 [11.8–18.1] |
| Thyroid disease | 211.7 [129.7–345.5] | 43.1 [18.0–103.6] | NA [NA‐NA] |
| No thyroid disease | 84.3 [76.8–92.6] | 7.3 [6.4–8.5] | 15.0 [12.1–18.5] |
| Rheumatoid arthritis | 147.8 [55.5–393.7] | 40.3 [13.0–124.9] | 96.3 [24.1–385.1] |
| No rheumatoid arthritis | 85.8 [78.2–94.2] | 7.4 [6.4–8.5] | 14.6 [11.8–18.1] |
Abbreviations: CD, celiac disease; MC, microscopic colitis.
FIGURE 2.
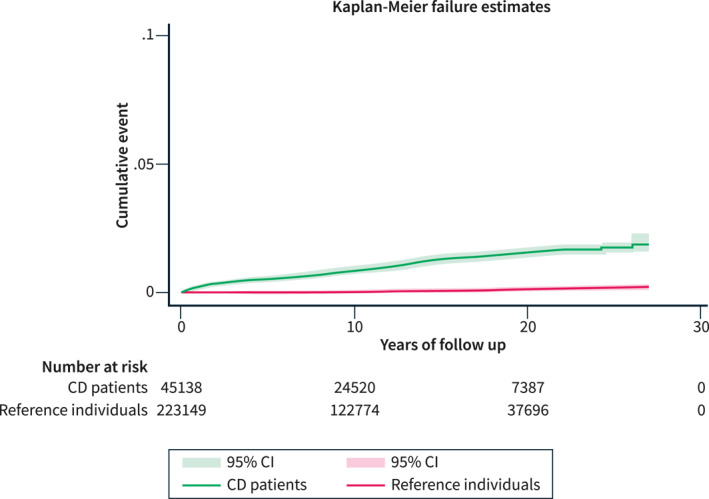
Absolute risk differences (and 95% confidence intervals) in MC diagnosis between CD patients and reference individuals during follow‐up. CD, celiac disease; MC, microscopic colitis.
Siblings had an IR of 14.9 (95% CI = 12.1–18.4) per 100,000 person‐years.
Adjustment for age, sex, county, calendar period, education level, and comorbidities (diabetes, IBD, thyroid disease, RA) yielded an aHR of 11.6 (95% CI = 9.8–13.8) compared to reference individuals (Table 3). This aHR corresponded to eight extra MC cases in 1000 CD patients followed up for 10 years. Stratifying by sex, the aHR was 12.7 (95% CI = 9.2–17.7) for males and 11.2 (95% CI = 9.2–13.7) for females (p for interaction = 0.49). Examining LC and CC separately revealed LC to be the more common subtype, constituting 66% (n = 431) of MC diagnoses (Table 1). The aHR was 12.4 (95% CI = 10.0–15.3) for LC and 10.2 (95% CI = 7.7–13.6) for CC (Table 4).
TABLE 3.
Adjusted hazard ratios (MC) for CD patients versus reference individuals and siblings.
| CD compared to reference individuals | CD compared to siblings | |
|---|---|---|
| Adjusted hazard ratio | Adjusted hazard ratio | |
| Total number of events/1000 person‐years | 11.6 [9.8–13.8] | 6.2 [4.7–8.2] |
| Sex | ||
| Males | 12.7 [9.2–17.7] | 9.6 [4.3–21.2] |
| Females | 11.2 [9.2–13.7] | 4.9 [3.3–7.3] |
| Excluding MC diagnosed within 1 year of CD | 9.8 [8.2–11.8] | 4.9 [3.7–6.6] |
| Age at start follow up (years) | ||
| <18 | 24.3 [11.2–52.6] | 12.4 [1.4–108.9] |
| 18 < 40 | 15.9 [10.8–23.5] | 18.9 [7.3–48.9] |
| 40 < 60 | 11.0 [8.4–14.4] | 5.5 [3.5–8.6] |
| ≥60 | 8.9 [6.7–11.8] | 17.5 [5.4–56.0] |
| Follow up (years) | ||
| <1 | 35.2 [20.1–61.6] | 5.7 [4.4–7.5] |
| 1 < 5 | 15.1 [10.6–21.5] | 7.0 [4.1–12.2] |
| 5 < 10 | 7.9 [5.6–10.9] | 5.3 [2.8–10.0] |
| ≥10 | 8.1 [6.0–10.9] | 4.4 [2.7–7.0] |
| Year of start follow up | ||
| 1990–2000 | 8.7 [6.8–11.1] | 5.4 [3.5–8.3] |
| 2001–2010 | 12.9 [10.1–16.9] | 7.4 [4.8–11.3] |
| 2011–2016 | 26.7 [14.0–50.9] | 15.9 [4.5–55.8] |
| Year of start follow up (with max 3 years of follow up) | ||
| 1990–2000 | 35.4 [ 12.4–101.2] | 59.5 [1.7–2096.2] |
| 2001–2010 | 25.9 [16.0–41.9] | 14.3 [5.9–34.7] |
| 2011–2013 | 25.4 [11.9–54.4] | 39.2 [4.6–333.7] |
| Country of birth | ||
| Nordic | 11.1 [9.3–13.2] | 6.1 [4.7–8.1] |
| Other | NA a | NA a |
| Education | ||
| Compulsory school (<9 years) | 10.6 [6.9–16.3] | 12.6 [4.0–39.4] |
| Upper secondary school (10–12 years) | 9.2 [6.6–12.8] | 8.5 [4.2–17.3] |
| College or university (≥13 years) | 15.0 [8.9–25.3] | 11.1 [3.9–31.2] |
| Missing | NA b | NA c |
| Comorbidity | ||
| Diabetes | 0.44 [0.04–5.4] | NA a |
| No diabetes | 11.0 [10.0–14.3] | 6.3 [4.8–8.4] |
| Inflammatory bowel disease | NA d | NA d |
| No inflammatory bowel disease | 11.7 [9.9–14.0] | 6.5 [4.9–8.7] |
| Thyroid disease | NA d | NA a |
| No thyroid disease | 11.7 [9.9–13.9] | 6.3 [4.8–8.4] |
| Rheumatoid arthritis | NA d | NA d |
| No rheumatoid arthritis | 11.9 [10.0–14.1] | 6.4 [4.8–8.4] |
Note: Adjusted for sex, age, calendar period, county, education, RA, diabetes, thyroid disease, IBD.
Abbreviations: CD, celiac disease; IBD, inflammatory bowel disease; MC, microscopic colitis; RA, rheumatoid arthritis.
MC only occurred among the exposed.
MC only occurred among the reference population.
No events of MC within the same family specific stratum.
No events of MC recorded.
TABLE 4.
Adjusted hazard ratios (LC and CC) for CD patients versus reference individuals.
| Compared to reference individuals | Compared to reference individuals | |
|---|---|---|
| Adjusted hazard ratio (LC) | Adjusted hazard ratio (CC) | |
| Overall | 12.4 [10.0–15.3] | 10.2 [7.7–13.6] |
| Sex | ||
| Males | 12.7 [8.6–18.8] | 12.5 [6.7–23.4] |
| Females | 12.2 [9.5–15.7] | 9.7 [7.0–13.4] |
| Excluding LC/CC diagnosed within 1 year of CD | 10.3 [8.2–12.9] | 8.9 [6.6–12.1] |
| Age at start of follow up (years) | ||
| <18 | 21.5 [9.3–49.5] | 43.1 [5.3–347.3] |
| 18 < 40 | 13.4 [8.6–20.7] | 27.6 [11.5–66.1] |
| 40 < 60 | 11.9 [8.4–16.8] | 9.4 [6.1–14.6] |
| ≥60 | 10.4 [7.2–15.0] | 6.8 [4.3–10.7] |
| Years of follow up | ||
| <1 | 30.4 [16.5–56.0] | 64.2 [15.2–270.7] |
| 1 < 5 | 13.6 [9.0–20.7] | 19.0 [9.8–36.9] |
| 5 < 10 | 10.1 [6.6–15.5] | 5.0 [2.9–8.5] |
| ≥10 | 7.9 [5.4–11.5] | 8.0 [4.9–13.1] |
| Calendar period | ||
| 1990–2000 | 8.3 [6.0–11.4] | 9.3 [6.2–13.8] |
| 2001–2010 | 15.0 [11.0–20.5] | 9.5 [6.1–14.7] |
| 2011–2016 | 23.4 [11.4–48.2] | 41.2 [9.5–179.7] |
| Calendar period with max 3 years of follow up | ||
| 1990–2000 | 21.8 [7.3–64.9] | NA [NA–NA] |
| 2001–2010 | 23.4 [13.6–40.3] | 35.6 [12.5–101.5] |
| 2011–2013 | 20.1 [8.7–46.1] | 62.5 [8.1–481.0] |
| Country of birth | ||
| Nordic | 12.0 [9.6–14.8] | 9.5 [7.1–12.7] |
| Other | NA [NA–NA] | NA [NA–NA] |
| Level of education | ||
| Compulsory school (<9 years) | 12.8 [7.2–22.9] | 8.1 [4.2–15.5] |
| Upper secondary school (10–12 years) | 9.6 [6.4–14.4] | 8.3 [4.7–14.7] |
| College or university (≥13 years) | 14.9 [7.9–28.1] | 15.0 [5.9–38.1] |
| Missing | NA [NA–NA] | NA [NA–NA] |
| Comorbidity at baseline | ||
| Diabetes | NA [NA–NA] | NA [NA–NA] |
| No diabetes | 12.5 [10.0–15.5] | 10.9 [8.1–14.7] |
| Inflammatory bowel disease | NA [NA–NA] | NA [NA–NA] |
| No inflammatory bowel disease | 12.5 [10.1–15.5] | 10.4 [7.8–13.8] |
| Thyroid disease | NA [NA–NA] | NA [NA–NA] |
| No thyroid disease | 12.7 [10.2–15.7] | 10.0 [7.5–13.4] |
| Rheumatoid arthritis | NA [NA–NA] | NA [NA–NA] |
| No rheumatoid arthritis | 12.7 [10.3–15.7] | 10.3 [7.7–13.8] |
Note: Adjusted for sex, age, calendar period, county, education, RA, diabetes, thyroid disease, IBD.
Abbreviations: CC, collagenous colitis; CD, celiac disease; IBD, inflammatory bowel disease; LC, lymphocytic colitis; RA, rheumatoid arthritis.
Compared to siblings, individuals with CD were at a six‐fold increased risk of later MC (95% CI = 4.7–8.2). The aHRs were similar for both subtypes: LC (aHR = 6.9 [95% CI = 4.9–9.8]) and CC (aHR = 7.0 [95% CI = 4.1–11.8]).
Examining aHRs across age categories (<18, 18–39, 40–59, ≥60 years), we found the highest increase in risk in the youngest age category with an aHR of 24.3 (95% CI = 11.2–52.6) (Table 3). aHRs decreased with increasing age, albeit with overlapping CIs (p = 0.005 for interaction between CD and age category).
Similar analyses were performed according to strata defined by calendar period and education level. We found no evidence for a significant effect modification by education level (p = 0.37). Moreover, analyses of the calendar period revealed increasing aHRs over time with the highest aHR (26.7 [95% CI = 14.0–50.9] in 2011–2016, p for interaction = 0.001). Restricting the follow‐up to only include the first 3 years after CD diagnosis, aHRs were generally higher, although with wider CIs (Table 3). Stratifying on disease duration, we found an aHR of 35.2 (95% CI = 20.1–61.6) for the first follow‐up year. In subsequent time intervals, HRs were lower (Table 3). Examining LC and CC separately, aHRs were highest during the first year of follow‐up but remained elevated even after >10 years of follow‐up for both subtypes, with an aHR of 7.9 (95% CI = 5.4–11.5) for LC and 8.0 (95% CI = 4.9–13.1) for CC (Table 4).
Adding an interaction term for exposure and follow‐up time, the aHR at time 0 was 19.3 (95% CI = 14.7–25.4), with an annual change of 0.93 (95% CI = 0.91–0.96). Table S1 outlines the corresponding figures for 1, 5, 10, and 20 years of follow‐up.
CD and earlier MC
We also performed a case‐control analysis to assess the association between CD and previous MC (n = 159) (Table 5). The aOR for earlier CD in MC patients was 52.7 (95% CI = 31.4–88.4). The aOR was 94.3 (95% CI = 29.4–302.7) in males and 43.2 (95% CI = 24.2–77.1) in females. The aOR was 114.4 (95% CI = 27.7–472.7) in the 40 to <60 age group and 36.6 (95% CI = 20.5–65.6) in the ≥60 age group. No estimate was calculated for patients aged 18 to <40 years because of lack of events. In the youngest age group, we found only one previous case of MC in the exposed group and none in the controls. No significant effect modification by age group was identified (p = 0.35).
TABLE 5.
Adjusted odds ratios (prior MC) for CD patients versus reference individuals and siblings.
| Reference individuals | Siblings | |
|---|---|---|
| Adjusted odds ratio | Adjusted odds ratio | |
| Overall | 52.7 [31.4–88.4] | 7.9 [4.8–13.2] |
| Sex | ||
| Males | 94.3 [29.4–302.7] | 21.3 [4.6–98.9] |
| Females | 43.2 [24.2–77.1] | 6.9 [3.1–15.2] |
| Excluding MC diagnosed within 7 days of CD | 50.7 [30.2.4–85.1] | 7.9 [4.8–13.2] |
| Age at start follow up (years) | ||
| <18 years | 0.28 [0.008–9.8] | NA |
| 18 < 40 years | NA | 4.7 [1.4–16.0] |
| 40 < 60 years | 114.4 [27.7–472.7] | 6.7 [2.9–15.8] |
| ≥60 years | 36.6 [20.5–65.6] | 7.7 [3.2–18.4] |
Note: Adjusted for sex, age, calendar period, county, education, RA, diabetes, thyroid disease, IBD.
Abbreviations: CD, celiac disease; IBD, inflammatory bowel disease; MC, microscopic colitis; RA, rheumatoid arthritis.
Compared to siblings, the aOR for earlier MC was 7.9 (95% CI = 4.8–13.2) (males 21.3 [95% CI = −4.6 to 98.9], females 6.9 [3.1–15.2]).
Sensitivity analysis
Due to the known link between certain drugs (i.e., NSAIDS, SSRIs, PPIs) and MC, we conducted additional analyses to assess the potential influence of these drugs in the causal pathway. First, we computed the aHR for the complete study population from 2006 to 2016, aHR = 13.3 (95% CI = 9.6–18.4). In a second analysis, we examined the association between CD and MC in a population in which all study participants with a recorded dispensation of at least one of the aforementioned drugs had been excluded. This analysis yielded a similar aHR of 13.4 (95% CI = 9.6–18.7).
A patient with newly diagnosed CD may have lymphocytic infiltration (which resolves upon adoption of a gluten‐free diet) in the colonic mucosa as a manifestation of CD. Therefore, biopsies from CD patients can be misclassified as LC. To control for this possibility, we performed analyses excluding all cases of MC diagnosed within 1 year of the diagnostic duodenal/jejunal biopsy. These analyses resulted in a somewhat lower aHR of 9.8 (95% CI = 8.2–11.8): 10.3 (95% CI = 8.2–12.9) for LC and 8.9 (95% CI = 6.6–12.1) for CC.
A similar restriction was applied in the case‐control analysis to control for surveillance bias. Excluding all MC cases diagnosed 1–7 days before CD diagnosis, the risk estimate (50.7 [95% CI = 30.2–85.1]) was similar to that of the unrestricted analysis.
DISCUSSION
In this nationwide, matched cohort study of 45,138 patients with CD, we found an 11.6‐fold increased risk of MC (95% CI = 9.8–13.8). Of note, patients with CD were diagnosed with MC at an earlier age than the reference population. Examining MC risk according to the follow‐up, we found the highest aHR in the first year after CD diagnosis, indicating that surveillance bias may have contributed to our findings. Still, after >10 years of follow‐up, aHRs remained elevated for all outcomes (MC, LC, CC), suggesting an association independent of surveillance bias and lymphocytic infiltration related to an active, untreated CD between CD and MC.
To assess the temporal association with MC, we also performed a case‐control analysis. Previous MC was more than 50 times more common in individuals with CD than in the general population.
Comparison with the literature
Our study confirms findings that MC is overrepresented in patients with CD. One of the largest studies to date, 7 based on 1000 patients from a tertiary center in New York, observed a 70‐fold increase in MC occurrence in patients with CD compared with the general population. A similar finding reported from a Canadian team in 2019 found a SIR of 52.7. Finally, a Danish study recently found an OR of 10.1 for earlier CD in patients with MC. 6 The concomitance of these conditions was supported by a 2021 systematic review and meta‐analysis. 9 Because of their respective overrepresentation, the authors concluded that MC and CD should be considered if either of these diagnoses is refractory. 10 This reasoning is consistent with the American Gastroenterological Association 2016 guideline 28 on the medical management of MC, which states that CD should be excluded as a cause of ongoing symptoms in patients on medical treatment for MC.
Common genetic risk factors, specifically shared alleles (primarily DQ2.5) in the HLA region, have been found to help explain the association between CD and CC. 12 However, a study investigating the same associations in a cohort of LC patients reported no significant associations. 13 Because almost all patients with CD are carriers of the HLA DQ2 allele, 29 these findings suggest a fundamental difference in the etiology of the two subtypes. Hence, we consider the almost identical aHRs (compared to siblings) for LC (aHR = 6.9 [95% CI = 4.9–9.8]) and CC (aHR = 7.0 [95% CI = 4.1–11.8]) as a compelling result as this may suggest the involvement of a yet unknown genetic link between CD and LC.
Strengths and weaknesses
This study has several strong points. First, nationwide coverage minimizes the risk of selection bias. Second, the Swedish PIN allows us to monitor all study participants and outcomes of interest. A third strength is the size of our study population, which permits precise calculations of relative risks across several strata. Fourth, CD and MC are primarily diagnosed by the histological presentation of biopsy taken during endoscopy and the PPV of having a diagnosis of CD or MC in Swedish pathology registers is nearly 100%. 16
We also had data on the siblings of our exposed individuals. Using CD‐free siblings as comparators yielded lower aHRs, indicating that shared genetics or early environmental factors may play a role in the pathogenesis of CD and MC.
A potential limitation is our lack of data on smoking. Smoking is more prevalent among patients with MC. 30 However, large‐scale data from Sweden show no association between CD and smoking. 31 , 32 In addition, a large proportion of CD patients were diagnosed before the typical age of smoking initiation. 3 Hence, even though smoking has been linked to MC, the lack of an association with CD suggests that it is doubtful to have any meaningful impact. Furthermore, patients with CD may be under increased medical surveillance compared with the general population. This could introduce a bias in that our exposed population may be more likely to undergo colonoscopy due to GI symptoms than our reference population. The significant interaction between exposure and length of follow‐up (meaning that the relative risk decreases as a function of time) may support the notion that surveillance bias partially explains our findings.
In conclusion, our study confirms an association between CD and MC before and after CD diagnosis. The magnitude and long‐term persistence of the associations strongly suggest that the concurrence of CD and MC should be considered in special cases (e.g., persistence or recurrence of GI symptoms despite a gluten‐free diet in patients with CD or adequate treatment for those diagnosed with MC). The risk was most pronounced during the first year of follow‐up and towards the end of the study period, possibly reflecting surveillance bias and raising awareness of MC. Nevertheless, the risk was markedly increased after >10 years of follow‐up. Also, our findings indicate that patients with CD are being diagnosed with MC at an earlier age than the general population.
AUTHOR CONTRIBUTIONS
Guarantor: Bergman and Ludvigsson had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: all authors. Acquisition of data: Ludvigsson. Analysis: Bergman and Roelstrate. Interpretation of data: All authors. Writing the first draft of the manuscript: Bergman. Critical revision of the manuscript for important intellectual content and approval of the final version: All authors.
CONFLICT OF INTEREST STATEMENT
Dr. Khalili receives grant funding from Takeda and Pfizer. He has received consulting fees from Takeda. Dr. Ludvigsson coordinates a study on behalf of the Swedish IBD quality register (SWIBREG). That study has received funding from the Janssen corporation.
ETHICS APPROVAL
This study was approved by the Regional Ethics Committee of Stockholm, Sweden (Protocol nos. 2014/1287‐31/4 and 2018/972‐32).
Supporting information
Table S1
ACKNOWLEDGMENTS
Associate professor Mark Clements advised on survival analysis. This work was supported by the Karolinska Institutet (Ludvigsson), Stockholm County Council (Ludvigsson), Mag‐TarmFonden, Swedish Gastroenterology Society and the NIH (National Institutes of Health NIA R01 [AG068390; Ludvigsson and Khalili]). None of the funding organizations played any role in the design and conduct of the study; in the collection, management, and analysis of the data; or in the preparation, review, and approval of the manuscript.
Bergman D, Khalili H, Lebwohl B, Roelstraete B, Green PHR, Ludvigsson JF. Celiac disease and risk of microscopic colitis: a nationwide population‐based matched cohort study. United European Gastroenterol J. 2023;11(2):189–201. 10.1002/ueg2.12374
DATA AVAILABILITY STATEMENT
In accordance with the Swedish regulations, the data from this study are not publicly available.
REFERENCES
- 1. Lebwohl B, Ludvigsson JF, Green PH. Celiac disease and non‐celiac gluten sensitivity. BMJ. 2015;351:h4347. 10.1136/bmj.h4347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Singh P, Arora A, Strand TA, Leffler DA, Catassi C, Green PH, et al. Global prevalence of celiac disease: systematic review and meta‐analysis. Clin Gastroenterol Hepatol. 2018;16(6):823–36.e2. 10.1016/j.cgh.2017.06.037 [DOI] [PubMed] [Google Scholar]
- 3. Bergman D, King J, Lebwohl B, Clements MS, Roelstraete B, Kaplan GG, et al. Two waves of coeliac disease incidence in Sweden: a nationwide population‐based cohort study from 1990 to 2015. Gut. 2021;71(6):1088–94. 10.1136/gutjnl-2021-324209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Burke KE, D'Amato M, Ng SC, Pardi DS, Ludvigsson JF, Khalili H. Microscopic colitis. Nat Rev Dis Primers. 2021;7(1):39. 10.1038/s41572-021-00273-2 [DOI] [PubMed] [Google Scholar]
- 5. Bergman D, Clements MS, Khalili H, Agreus L, Hultcrantz R, Ludvigsson JF. A nationwide cohort study of the incidence of microscopic colitis in Sweden. Aliment Pharmacol Ther. 2019;49(11):1395–400. 10.1111/apt.15246 [DOI] [PubMed] [Google Scholar]
- 6. Wildt S, Munck LK, Winther‐Jensen M, Jess T, Nyboe Andersen N. Autoimmune diseases in microscopic colitis: a Danish nationwide case‐control study. Aliment Pharmacol Ther. 2021;54(11‐12):1454–62. 10.1111/apt.16614 [DOI] [PubMed] [Google Scholar]
- 7. Green PH, Yang J, Cheng J, Lee AR, Harper JW, Bhagat G. An association between microscopic colitis and celiac disease. Clin Gastroenterol Hepatol. 2009;7(11):1210–6. 10.1016/j.cgh.2009.07.011 [DOI] [PubMed] [Google Scholar]
- 8. Stewart M, Andrews CN, Urbanski S, Beck PL, Storr M. The association of coeliac disease and microscopic colitis: a large population‐based study. Aliment Pharmacol Ther. 2011;33(12):1340–9. 10.1111/j.1365-2036.2011.04666.x [DOI] [PubMed] [Google Scholar]
- 9. Aziz M, Haghbin H, Khan RS, Khan Z, Weissman S, Kamal F, et al. Celiac disease is associated with microscopic colitis in refractory cases in adults: a systematic review and meta‐analysis of observational studies. Dig Dis Sci. 2021;67(8):3529–42. 10.1007/s10620-021-07232-7 [DOI] [PubMed] [Google Scholar]
- 10. Liu PH, Lebwohl B, Burke KE, Ivey KL, Ananthakrishnan AN, Lochhead P, et al. Dietary gluten intake and risk of microscopic colitis among US women without celiac disease: a prospective cohort study. Am J Gastroenterol. 2019;114(1):127–34. 10.1038/s41395-018-0267-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Olesen M, Eriksson S, Bohr J, Jarnerot G, Tysk C. Lymphocytic colitis: a retrospective clinical study of 199 Swedish patients. Gut. 2004;53(4):536–41. 10.1136/gut.2003.023440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Westerlind H, Mellander MR, Bresso F, Munch A, Bonfiglio F, Assadi G, et al. Dense genotyping of immune‐related loci identifies HLA variants associated with increased risk of collagenous colitis. Gut. 2017;66(3):421–8. 10.1136/gutjnl-2015-309934 [DOI] [PubMed] [Google Scholar]
- 13. Westerlind H, Bonfiglio F, Mellander MR, Hübenthal M, Brynedal B, Björk J, et al. HLA associations distinguish collagenous from lymphocytic colitis. Am J Gastroenterol. 2016;111(8):1211–3. 10.1038/ajg.2016.215 [DOI] [PubMed] [Google Scholar]
- 14. Koning F. Celiac disease: caught between a rock and a hard place. Gastroenterology. 2005;129(4):1294–301. 10.1053/j.gastro.2005.07.030 [DOI] [PubMed] [Google Scholar]
- 15. Stahl E, Roda G, Dobbyn A, Hu J, Zhang Z, Westerlind H, et al. Collagenous colitis is associated with HLA signature and shares genetic risks with other immune‐mediated diseases. Gastroenterology. 2020;159(2):549–61.e8. 10.1053/j.gastro.2020.04.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ludvigsson JF, Otterblad‐Olausson P, Pettersson BU, Ekbom A. The Swedish personal identity number: possibilities and pitfalls in healthcare and medical research. Eur J Epidemiol. 2009;24(11):659–67. 10.1007/s10654-009-9350-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ludvigsson JF, Lashkariani M. Cohort profile: ESPRESSO (Epidemiology Strengthened by histoPathology Reports in Sweden). Clin Epidemiol. 2019;11:101–14. 10.2147/clep.s191914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ludvigsson JF, Brandt L, Montgomery SM, Granath F, Ekbom A. Validation study of villous atrophy and small intestinal inflammation in Swedish biopsy registers. BMC Gastroenterol. 2009;9(1):19. 10.1186/1471-230x-9-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ludvigsson JF, Almqvist C, Bonamy AK, Ljung R, Michaelsson K, Neovius M, et al. Registers of the Swedish total population and their use in medical research. Eur J Epidemiol. 2016;31(2):125–36. 10.1007/s10654-016-0117-y [DOI] [PubMed] [Google Scholar]
- 20. Svensson M, Bergman D, Olen O, Myrelid P, Bohr J, Wickbom A, et al. Validating microscopic colitis (MC) in Swedish pathology registers. Scand J Gastroenterol. 2018. ;53(12):1469–75. 10.1080/00365521.2018.1543446 [DOI] [PubMed] [Google Scholar]
- 21. Ludvigsson JF, Svedberg P, Olen O, Bruze G, Neovius M. The longitudinal integrated database for health insurance and labour market studies (LISA) and its use in medical research. Eur J Epidemiol. 2019;34(4):423–37. 10.1007/s10654-019-00511-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim JL, Reuterwall C, et al. External review and validation of the Swedish national inpatient register. BMC Publ Health. 2011;11(1):450. 10.1186/1471-2458-11-450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wettermark B, Hammar N, Fored CM, Leimanis A, Otterblad Olausson P, Bergman U, et al. The new Swedish Prescribed Drug Register–opportunities for pharmacoepidemiological research and experience from the first six months. Pharmacoepidemiol Drug Saf. 2007;16(7):726–35. 10.1002/pds.1294 [DOI] [PubMed] [Google Scholar]
- 24. Verhaegh BP, de Vries F, Masclee AA, Keshavarzian A, de Boer A, Souverein PC, et al. High risk of drug‐induced microscopic colitis with concomitant use of NSAIDs and proton pump inhibitors. Aliment Pharmacol Ther. 2016;43(9):1004–13. 10.1111/apt.13583 [DOI] [PubMed] [Google Scholar]
- 25. Tong J, Zheng Q, Zhang C, Lo R, Shen J, Ran Z. Incidence, prevalence, and temporal trends of microscopic colitis: a systematic review and meta‐analysis. Am J Gastroenterol. 2015;110(2):265–76; quiz 77. 10.1038/ajg.2014.431 [DOI] [PubMed] [Google Scholar]
- 26. Sjolander A, Greenland S. Ignoring the matching variables in cohort studies ‐ when is it valid and why? Stat Med. 2013;32(27):4696–708. 10.1002/sim.5879 [DOI] [PubMed] [Google Scholar]
- 27. Ludvigsson JF, Haberg SE, Knudsen GP, Lafolie P, Zoega H, Sarkkola C, et al. Ethical aspects of registry‐based research in the Nordic countries. Clin Epidemiol. 2015;7:491–508. 10.2147/clep.s90589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nguyen GC, Smalley WE, Vege SS, Carrasco‐Labra A, Flamm SL, Gerson L, et al. American Gastroenterological Association Institute guideline on the medical management of microscopic colitis. Gastroenterology. 2016;150(1):242–6; quiz e17‐8. 10.1053/j.gastro.2015.11.008 [DOI] [PubMed] [Google Scholar]
- 29. Lundin KE, Scott H, Hansen T, Paulsen G, Halstensen TS, Fausa O, et al. Gliadin‐specific, HLA‐DQ (alpha 1*0501,beta 1*0201) restricted T cells isolated from the small intestinal mucosa of celiac disease patients. J Exp Med. 1993;178(1):187–96. 10.1084/jem.178.1.187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jaruvongvanich V, Poonsombudlert K, Ungprasert P. Smoking and risk of microscopic colitis: a systematic review and meta‐analysis. Inflamm Bowel Dis. 2019;25(4):672–8. 10.1093/ibd/izy296 [DOI] [PubMed] [Google Scholar]
- 31. Ludvigsson JF, Nordenvall C, Järvholm B. Smoking, use of moist snuff and risk of celiac disease: a prospective study. BMC Gastroenterol. 2014;14(1):120. 10.1186/1471-230x-14-120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ludvigsson JF, Montgomery SM, Ekbom A. Smoking and celiac disease: a population‐based cohort study. Clin Gastroenterol Hepatol. 2005;3(9):869–74. 10.1016/s1542-3565(05)00414-3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Data Availability Statement
In accordance with the Swedish regulations, the data from this study are not publicly available.


