Abstract
Background
We aimed to investigate the efficacy of postoperative adjuvant transarterial chemoembolisation (PA‐TACE) in patients with hepatocellular carcinoma (HCC) complicated by microvascular invasion (MVI).
Methods
A retrospective analysis of 1505 patients with HCC who underwent hepatectomy at four medical centers, including 782 patients who received PA‐TACE and 723 patients who did not receive adjuvant PA‐TACE, has been conducted. Propensity score matching (PSM) (1:1) was performed on the data to minimise selection bias, which resulted in a balanced clinical profile between groups.
Results
After PSM, 620 patients who received PA‐TACE and 620 patients who did not receive PA‐TACE were included. Disease‐free survival (DFS, 1‐, 2‐, and 3‐year: 88%‐68%‐61% vs. 70%‐58%‐51%, p < 0.001) and overall survival (OS, 1‐, 2‐, and 3‐year: 96%‐89%‐82% vs. 89%‐77%‐67%, p < 0.001) were significantly higher in patients who received PA‐TACE than in those who did not. Patients with MVI who received PA‐TACE had significantly higher DFS (1‐, 2‐, and 3‐year: 68%‐57%‐48% vs. 46%‐31%‐27%, p < 0.001) and OS (1‐, 2‐, and 3‐year: 96%‐84%‐77% vs. 79%‐58%‐40%, p < 0.001) than those who did not receive PA‐TACE. Among the six different liver cancer stages, MVI‐negative patients did not have significant survival outcomes from PA‐TACE (p > 0.05), whereas MVI‐positive patients achieved higher DFS and OS from it (p < 0.05). Liver dysfunction, fever, and nausea/vomiting were the most common adverse events in patients receiving PA‐TACE. There was no significant difference in grade 3 or 4 adverse events between the groups (p > 0.05).
Conclusions
Postoperative adjuvant transarterial chemoembolisation has a good safety profile and may be a potentially beneficial treatment modality for survival outcomes in patients with HCC, especially those with concomitant MVI.
Keywords: adjuvant, HCC, hepatectomy, hepatocellular carcinoma, microvascular invasion, propensity score, survival, TACE, transarterial chemoembolisation

Key summary.
Established knowledge on this subject
Postoperative adjuvant transarterial chemoembolisation (TACE) significantly improves the survival benefit of patients with hepatocellular carcinoma (HCC), especially those with microvascular invasion (MVI).
Significant new findings of this study
Postoperative adjuvant TACE improves survival outcomes for patients with different HCC stages;
Postoperative adjuvant TACE improves survival outcomes in patients with MVI but not in patients without MVI.
The adverse effects associated with postoperative adjuvant TACE are mild and manageable, and the procedure is well tolerated.
INTRODUCTION
Hepatocellular carcinoma (HCC) is one of the most common malignancies in the world and ranks third among the causes of death from malignancy with approximately 900,000 new cases and 830,000 deaths each year. 1 , 2 With the development of medical technology, the current treatment methods for liver cancer include hepatectomy, radiofrequency ablation, transarterial chemoembolisation(TACE), and immune targeted therapy, etc. 1 , 2 , 3 As we all know, liver transplantation is far superior to hepatectomy in the treatment of liver cancer, but it is often limited to the shortage of organs, difficult medical technology and harsh medical conditions. 1 , 2 , 3 , 4 However, although radiofrequency ablation is compared to hepatectomy in the treatment of liver cancer, it is often limited to the size and number of tumours in patients. 1 , 2 , 3 , 4 It can be seen that hepatectomy is still the preferred treatment for HCC, which can provide longer survival time for the patients compared with other palliative treatments. 2 , 3 , 4 Unfortunately, the majority of patients with HCC in China have reached the intermediate and advanced stages at the time of initial diagnosis, which makes the median survival rate only about 2 years. 3 , 4 , 5 Even though a carefully selected minority of patients in this category can undergo surgical resection, the efficacy of which may exceed that of non‐surgical treatment, the desired survival outcome is still not achieved. 4 , 5 , 6 Postoperative adjuvant therapy may be a good option for high‐risk patients who are susceptible to tumour recurrence.
The proposed mechanism of postoperative adjuvant transarterial chemoembolisation (PA‐TACE) is the elimination of intrahepatic micro‐metastases, residual small foci, or dissociated cancer cells due to an extrusion at the time of surgery. 7 , 8 , 9 However, the role of TACE in tumour recurrence remains somewhat controversial with some investigators suggesting that it may only be beneficial for specific subgroups of patients and has no impact on survival outcomes beyond 1 year. 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 A meta‐analysis showed that certain subgroups of patients with HCC benefited most from PA‐TACE, especially those with concomitant microvascular invasion (MVI). 9 In contrast, there appeared to be no benefit of PA‐TACE when assessing only tumour size (⩾ 5 cm) alone. 9 Microvascular invasion represents a marker of tumours with aggressive biological behavior, which has long been confirmed to be related to intrahepatic tumour micrometastasis and has been regarded as one of the important high‐risk factors for early postoperative recurrence of HCC. 18 , 19 , 20 It is well known that MVI can be detected in postoperative pathological specimens even in patients with early HCC. 20 , 21 Thus, the value of PA‐TACE for the prognosis of patients with early stage HCC accompanied by MVI deserves further investigation. The lack of standardised chemotherapy regimens and intra‐arterial treatment techniques are the main limitations of PA‐TACE. Also, the consistency and representation of patients enrolled in different studies are not optimal. This heterogeneity in patient selection may have contributed to a selection bias. In addition, not all randomised controlled trials (RCTs) are high‐quality studies, and many non‐RCTs are primarily retrospective. Therefore, some studies may have inherent selection bias, leading to differences in their views. 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 21 This study evaluates the prognostic effect of PA‐TACE in patients with HCC with or without MVI through multicenter large‐scale data, which is expected to provide rational treatment decisions for clinical work. To obtain more reliable results, propensity score matching analysis (PSM) was used to minimise the effect of patient selection bias.
METHODS
Patients
A retrospective analysis of 1505 patients with HCC from four medical centers in China between January 2018 and September 2021 was performed. Patients eligible for this study were screened according to the following inclusion Criteria: (1) All patients received surgical treatment and were confirmed as a negative margin by pathological results; (2) postoperative pathology confirmed only HCC; (3) none of the patients received any preoperative chemoradiotherapy, immunotargeted therapy, interventional therapy, and other anti‐tumour treatments; (4) no history of other malignant tumours; (5) all patients received complete follow‐ups. Exclusion criteria include (1) missing clinical data or incomplete follow‐up data; (2) patients with extrahepatic metastases were found on preoperative imaging; (3) no multiple organ failure, such as heart, lung, or kidney; and (4) patients who died in the perioperative period. Data for this study were provided by the First Affiliated Hospital of Nanchang University (FAHNU), The Second Affiliated Hospital of Nanchang University (SAHNU), Shenzhen People's Hospital (SPH), and Zhongshan People's Hospital (ZPH). Meanwhile, the study was conducted based on the Declaration of Helsinki (revised in 2013), approved by the ethics committees of the above four clinical centers, and informed consent was obtained from each patient for the data used in the study. A flow chart of patients enrolled in this study is shown in Figure S1.
Evaluation of microvascular invasion and selection of postoperative adjuvant transarterial chemoembolisation
Two senior pathologists interpreted and confirmed the pathological diagnosis of surgically resected specimens by hematoxylin‐eosin staining and immunohistochemistry to determine the presence of MVI. Microvascular invasion is defined as the microscopic presence of tumour cells in the portal vein, hepatic vein, or large encapsulated vessels of liver tissue near the edge of the tumour. 20 , 21 , 22 Inclusion criteria of PA‐TACE include 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 (1) Liver function Child–Pugh grade A or B, and Eastern collaborative oncology group (ECOG) functional status score 0–2; (2) no serious coagulation dysfunction; (3) no serious infection that cannot be effectively controlled; (4) no history of iodine contrast agent allergy; and (5) no multiple organ failure such as heart, lung, and kidney. Exclusion criteria include 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 (1) severe liver dysfunction with Child – Pugh C grade, including severe jaundice, hepatic encephalopathy, intractable ascites, or hepatorenal syndrome; (2) ECOG score>2 with cachexia or multiple organ failure; (3) renal dysfunction, blood creatinine >176.8 μmol/L or creatinine clearance <30 mL/min; (4) peripheral white blood cells <3.0 × 109/L, platelet <50 × 109/L, and cannot be corrected. All patients who met these criteria were recommended to receive PA‐TACE about 4 weeks after hepatectomy. However, patients decide whether to follow the recommendation based on their medical adherence, financial status, or other social factors. Before receiving PA‐TACE, patients will be routinely tested for liver function, tumour markers, computed tomography (CT), and/or magnetic resonance imaging (MRI) to determine whether the tumour has recurred or metastasized. During the operation of PA‐TACE, we placed the hepatic arterial catheter through the femoral artery into the proper hepatic artery using the Seldinger technique and injected a mixture of appropriate chemotherapeutic (Fluorouracil, epirubicin, and platinum) and embolic agents (lipiodol and gelatin sponge) through the catheter into the residual liver based on a comprehensive assessment of the patient's body surface area, physical fitness, and residual liver volume. 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17
Follow‐up
All patients were followed up either outpatient or inpatient. Patients were followed up every 1–2 months for 6 months after discharge and every 3–6 months thereafter. During the follow‐up period, routine examinations, such as liver function test, alpha‐fetoprotein (AFP) analysis, CT, and MRI, were performed for each patient. Tumour recurrence was defined as new tumour nodules confirmed by enhanced CT and enhanced MRI. Patients who relapsed were subsequently treated with liver transplantation, rehepatectomy, local ablation, TACE, chemoradiotherapy, and immunotargeted therapy. Among them, liver transplantation, rehepatectomy, and local ablation are categorised as curative treatment, while TACE, chemoradiotherapy, and immunotargeted therapy are categorised as palliative treatment. Disease‐free survival (DFS) and Overall survival (OS) were used as study endpoints. Disease‐free survival was defined as the time from hepatectomy to the diagnosis of tumour recurrence, while OS was defined as the time from hepatectomy to death or the last follow‐up. All patients were followed up until 1 April 2022.
Statistical methods
To reduce the selection bias and confounding factors between groups, propensity score matching (PSM) analysis was used to eliminate inter‐group imbalances. A 1:1 nearest neighbor matching algorithm was applied with a caliper width of 0.01. Continuous data that fit a normal distribution were detected by an independent samples t‐test, which was expressed as mean ± standard deviation (SD). Continuous data with non‐normal distribution were detected by the Mann‐Whitney U test, which was expressed as the median (quartile distance, IQR). The chi‐square test was used to detect classified data, which were expressed as numbers (n) and proportions (%). Univariate and multivariate analyses were performed by the Cox proportional risk model before and after PSM to determine the independent prognostic factors of DFS and OS. In the univariate analysis, variables with P < 0.05 were used for multivariate analysis. Kaplan‐meier survival analysis was used to assess DFS and OS for independent prognostic factors screened after PSM, and the difference between curves was estimated by the logarithmic rank test. SPSS 26.0 statistical software (IBM Corp, Armonk, NY, USA) and R software (Version 4.2.1 http://www.r‐project.org) were used for statistical analysis of all data. All p values were obtained by the two‐tailed test, and P < 0.05 was considered statistically significant.
RESULTS
Patient and clinical characteristics
1240 patients (620 cases without PA‐TACE and 620 cases with PA‐TACE) were screened out of 1505 patients (723 cases without PA‐TACE, 782 cases with PA‐TACE) after PSM. Table 1 shows the clinical characteristics of patients with HCC who received PA‐TACE or not. Age, AFP, alanine aminotransferase (ALT), aspartate aminotransferase (AST), albumin (Alb), platelet‐to‐lymphocyte ratio (PLR), maximum tumour diameter, anatomical liver resection, MVI, and differentiation were significantly different between groups before PSM (All p < 0.05). After propensity matching analysis, there were no significant differences in clinical characteristics between the groups (all p > 0.05).
TABLE 1.
Clinical characteristics of patients with Hepatocellular carcinoma (HCC) who underwent Postoperative adjuvant transarterial chemoembolisation (PA‐TACE) or not.
| Before PSM | After PSM | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Total (n = 1505) | PA‐TACE | P | Total (n = 1240) | PA‐TACE | P | ||||
| Clinical characteristics | No (n = 723) | Yes (n = 782) | No (n = 620) | Yes (n = 620) | |||||
| Age (years) | 56.00 (47.00, 64.00) | 57.00 (48.00, 66.00) | 55.00 (47.00, 63.00) | 0.004 | 56.00 (47.00, 64.00) | 56.00 (47.00, 65.00) | 56.00 (48.00, 64.00) | 0.987 | |
| AFP (ng/mL) | 53.45 (6.30, 894.70) | 39.07 (5.05, 604.10) | 77.200 (7.68, 1000.00) | 0.005 | 47.80 (6.00, 795.05) | 43.00 (5.45, 871.85) | 65.57 (6.68, 750.88) | 0.552 | |
| ALT (U/L) | 30.84 (22.00, 46.00) | 29.00 (21.00, 44.00) | 32.86 (23.00, 48.00) | 0.004 | 30.00 (21.42, 45.00) | 29.00 (21.18, 44.96) | 31.00 (21.60, 45.00) | 0.281 | |
| AST (U/L) | 35.00 (27.00, 50.50) | 33.87 (26.00, 47.34) | 37.00 (28.00, 52.97) | 0.002 | 34.48 (26.24, 49.00) | 33.96 (26.00, 47.04) | 35.41 (26.73, 51.04) | 0.149 | |
| GGT (U/L) | 54.00 (30.28, 104.60) | 54.55 (30.24, 105.35) | 52.59 (30.53, 104.00) | 0.834 | 50.72 (30.00, 99.00) | 53.00 (30.00, 101.25) | 48.36 (29.95, 97.50) | 0.523 | |
| ALP (U/L) | 96.20 (76.00, 123.29) | 94.00 (73.00, 123.00) | 98.13 (77.29, 124.80) | 0.083 | 96.07 (76.00, 123.00) | 94.25 (75.00, 122.84) | 98.00 (77.11, 123.00) | 0.295 | |
| Alb (g/L) | 41.08 (37.98, 43.80) | 40.50 (37.60, 43.40) | 41.59 (38.59, 44.39) | <0.001 | 41.00 (37.80, 43.80) | 40.70 (37.80, 43.52) | 41.38 (37.92, 44.28) | 0.058 | |
| TB (mol/L) | 14.60 (10.80, 19.78) | 14.20 (10.40, 19.80) | 14.80 (11.19, 19.68) | 0.128 | 14.60 (10.74, 19.73) | 13.99 (10.20, 19.73) | 14.90 (11.20, 19.74) | 0.062 | |
| WBC (109/L) | 5.30 (4.28, 6.55) | 5.25 (4.23, 6.66) | 5.34 (4.33, 6.50) | 0.914 | 5.26 (4.23, 6.48) | 5.24 (4.20, 6.63) | 5.29 (4.26, 6.37) | 0.764 | |
| CR (μmol/L) | 72.70 (62.40, 82.79) | 72.70 (62.35, 82.75) | 72.86 (62.45, 82.86) | 0.704 | 73.00 (62.39, 82.90) | 73.00 (63.34, 82.50) | 72.70 (61.98, 83.14) | 0.957 | |
| PT (s) | 11.90 (11.30, 12.60) | 11.90 (11.30, 12.60) | 11.90 (11.30, 12.60) | 0.968 | 11.90 (11.30, 12.60) | 11.90 (11.30, 12.50) | 11.90 (11.30, 12.70) | 0.179 | |
| NLR | 2.21 (1.61, 3.23) | 2.21 (1.60, 3.17) | 2.22 (1.62, 3.28) | 0.293 | 2.20 (1.60, 3.18) | 2.20 (1.60, 3.08) | 2.19 (1.61, 3.22) | 0.684 | |
| LMR | 3.41 (2.56, 4.76) | 3.44 (2.55, 4.89) | 3.40 (2.59, 4.72) | 0.933 | 3.41 (2.56, 4.80) | 3.44 (2.53, 4.90) | 3.40 (2.57, 4.75) | 0.891 | |
| PLR | 110.38 (82.28, 153.52) | 104.72 (78.28, 149.79) | 113.51 (86.96, 158.14) | 0.001 | 108.92 (82.16, 151.91) | 107.13 (79.00, 151.82) | 110.46 (85.06, 151.91) | 0.166 | |
| Operation time (mins) | 220.00 (165.00, 280.00) | 215.00 (160.00, 273.75) | 220.00 (176.25, 280.00) | 0.058 | 220.00 (168.75, 280.00) | 220.00 (163.75, 271.25) | 220.00 (170.00, 280.00) | 0.518 | |
| Maximum tumour diameter (mm) | 44.00 (27.00, 71.00) | 40.00 (25.00, 67.00) | 49.00 (29.25, 76.00) | <0.001 | 43.00 (27.00, 71.00) | 43.00 (27.00, 70.00) | 43.00 (27.75, 72.00) | 0.548 | |
| Gender (n(%)) | Male | 1274 (84.65) | 611 (84.51) | 663 (84.78) | 0.940 | 1051 (84.76) | 529 (85.32) | 522 (84.19) | 0.636 |
| Female | 231 (15.35) | 112 (15.49) | 119 (15.22) | 189 (15.24) | 91 (14.68) | 98 (15.81) | |||
| HBV (n(%)) | Negative | 199 (13.22) | 107 (14.80) | 92 (11.76) | 0.097 | 161 (12.98) | 82 (13.23) | 79 (12.74) | 0.866 |
| Positive | 1306 (86.78) | 616 (85.20) | 690 (88.24) | 1079 (87.02) | 538 (86.77) | 541 (87.26) | |||
| Child–Pugh classification (n(%)) | A | 1434 (95.28) | 683 (94.47) | 751 (96.04) | 0.190 | 1181 (95.24) | 585 (94.35) | 596 (96.13) | 0.182 |
| B | 71 (4.72) | 40 (5.53) | 31 (3.96) | 59 (4.76) | 35 (5.65) | 24 (3.87) | |||
| Liver cirrhosis (n(%)) | No | 374 (24.85) | 179 (24.76) | 195 (24.94) | 0.984 | 304 (24.52) | 153 (24.68) | 151 (24.35) | 0.948 |
| Yes | 1131 (75.15) | 544 (75.24) | 587 (75.06) | 936 (75.48) | 467 (75.32) | 469 (75.65) | |||
| Number of tumours (n(%)) | Single | 1315 (87.38) | 638 (88.24) | 677 (86.57) | 0.370 | 1095 (88.31) | 547 (88.23) | 548 (88.39) | 1.000 |
| Multiple | 190 (12.62) | 85 (11.76) | 105 (13.43) | 145 (11.69) | 73 (11.77) | 72 (11.61) | |||
| Tumour location (n(%)) | Left | 474 (31.50) | 234 (32.37) | 240 (30.69) | 0.759 | 385 (31.05) | 197 (31.77) | 188 (30.32) | 0.838 |
| Right | 955 (63.46) | 454 (62.79) | 501 (64.07) | 802 (64.68) | 396 (63.87) | 406 (65.48) | |||
| Double | 76 (5.05) | 35 (4.84) | 41 (5.24) | 53 (4.27) | 27 (4.35) | 26 (4.19) | |||
| Tumour margin (n(%)) | Non‐smooth | 383 (25.45) | 188 (26.00) | 195 (24.94) | 0.678 | 297 (23.95) | 149 (24.03) | 148 (23.87) | 1.000 |
| Smooth | 1122 (74.55) | 535 (74.00) | 587 (75.06) | 943 (76.05) | 471 (75.97) | 472 (76.13) | |||
| Vascular invasion (imaging) (n(%)) | Negative | 1372 (91.16) | 663 (91.70) | 709 (90.66) | 0.537 | 1134 (91.45) | 562 (90.65) | 572 (92.26) | 0.361 |
| Positive | 133 (8.84) | 60 (8.30) | 73 (9.34) | 106 (8.55) | 58 (9.35) | 48 (7.74) | |||
| Anatomical liver resection (n(%)) | No | 462 (30.70) | 250 (34.58) | 212 (27.11) | 0.002 | 370 (29.84) | 200 (32.26) | 170 (27.42) | 0.072 |
| Yes | 1043 (69.30) | 473 (65.42) | 570 (72.89) | 870 (70.16) | 420 (67.74) | 450 (72.58) | |||
| Laparoscopic surgery (n(%)) | No | 911 (60.53) | 435 (60.17) | 476 (60.87) | 0.821 | 761 (61.37) | 388 (62.58) | 373 (60.16) | 0.414 |
| Yes | 594 (39.47) | 288 (39.83) | 306 (39.13) | 479 (38.63) | 232 (37.42) | 247 (39.84) | |||
| MVI (n(%)) | Negative | 842 (55.95) | 453 (62.66) | 389 (49.74) | <0.001 | 725 (58.47) | 371 (59.84) | 354 (57.10) | 0.357 |
| Positive | 663 (44.05) | 270 (37.34) | 393 (50.26) | 515 (41.53) | 249 (40.16) | 266 (42.90) | |||
| Satellite nodules (n(%)) | Negative | 1277 (84.85) | 616 (85.20) | 661 (84.53) | 0.770 | 1053 (84.92) | 520 (83.87) | 533 (85.97) | 0.341 |
| Positive | 228 (15.15) | 107 (14.80) | 121 (15.47) | 187 (15.08) | 100 (16.13) | 87 (14.03) | |||
| Differentiation (n(%)) | High‐medium | 1243 (82.59) | 616 (85.20) | 627 (80.18) | 0.013 | 1040 (83.87) | 522 (84.19) | 518 (83.55) | 0.817 |
| Low | 262 (17.41) | 107 (14.80) | 155 (19.82) | 200 (16.13) | 98 (15.81) | 102 (16.45) | |||
Note: Bold values indicate the comparison of clinical characteristics of patients who received PA‐TACE and those who did not receive PA‐TACE before PSM and after PSM.
Abbreviations: AFP, Alpha‐fetoprotein; Alb, Albumin; ALP, Alkaline phosphatase; ALT, Alanine aminotransferase; AST, Aspartate aminotransferase; CR, Creatinine; GGT, Gamma‐glutamyltransferase; HBV, Hepatitis B virus; HCC, Hepatocellular carcinoma; LMR, Lymphocyte‐to‐monocyte ratio; MVI, Microvascular invasion; NLR, Neutrophil‐to‐lymphocyte ratio; PA‐TACE, Postoperative adjuvant transarterial chemoembolisation; PLR, Platelet‐to‐lymphocyte ratio; PSM, Propensity score matching; PT, Prothrombin time; TB, Total bilirubin; WBC, White blood cell.
Adverse events and follow‐up antitumour therapy
Liver dysfunction, fever, and nausea/vomiting were the most common adverse events in patients receiving PA‐TACE (Table 2). There was no significant difference in grade 3 or 4 adverse events between groups (all p > 0.05). Both groups received follow‐up antitumour therapy after tumour recurrence, including liver transplantation, rehepatectomy, local ablation, TACE, chemoradiotherapy, and immunotargeted therapy. Patients with tumour recurrence in the PA‐TACE group received more curative treatment, while patients with tumour recurrence in the Non‐PA‐TACE group received more palliative treatment (Table 3, before PSM, p < 0.001; after PSM, p < 0.001).
TABLE 2.
Comparison of adverse events occurring in patients with Hepatocellular carcinoma (HCC) who received Postoperative adjuvant transarterial chemoembolisation (PA‐TACE) or not.
| Adverse events | Before PSM | After PSM | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Non‐PA‐TACE (n = 732) | PA‐TACE (n = 782) | P | P* | Non‐PA‐TACE (n = 620) | PA‐TACE (n = 620) | P | P* | |||||
| Grade 1–2 | Grade 3–4 | Grade 1–2 | Grade 3–4 | Grade 1–2 | Grade 3–4 | Grade 1–2 | Grade 3–4 | |||||
| Liver dysfunction | 102 | 31 | 273 | 34 | < 0.001 | 0.954 | 84 | 22 | 251 | 25 | < 0.001 | 0.656 |
| Neutropenia | 68 | 23 | 119 | 29 | 0.001 | 0.576 | 54 | 17 | 99 | 22 | < 0.001 | 0.416 |
| Thrombocytopenia | 57 | 16 | 78 | 21 | 0.118 | 0.554 | 46 | 11 | 61 | 13 | 0.116 | 0.680 |
| Anemia | 52 | 9 | 54 | 13 | 0.928 | 0.500 | 39 | 7 | 45 | 8 | 0.463 | 0.759 |
| Pain | 79 | 11 | 121 | 14 | 0.007 | 0.683 | 67 | 6 | 104 | 9 | 0.001 | 0.436 |
| Fever | 60 | 6 | 176 | 11 | < 0.001 | 0.290 | 57 | 4 | 153 | 6 | < 0.001 | 0.525 |
| Nausea/vomiting | 83 | 14 | 135 | 17 | 0.002 | 0.746 | 75 | 12 | 123 | 12 | < 0.001 | 1.000 |
| Fatigue | 78 | 7 | 81 | 6 | 0.701 | 0.674 | 75 | 3 | 77 | 4 | 0.799 | 0.705 |
Note: P: Comparison of adverse event grades 1–4. P*: Comparison of adverse event grades 3–4.
Abbreviations: HCC, Hepatocellular carcinoma; PA‐TACE, Postoperative adjuvant transarterial chemoembolisation; PSM, Propensity score matching.
TABLE 3.
Comparison of follow‐up antitumour therapy after tumour recurrence in patients with Hepatocellular carcinoma (HCC) who received Postoperative adjuvant transarterial chemoembolisation (PA‐TACE) or not.
| Anti‐tumour therapy | Before PSM | After PSM | ||||
|---|---|---|---|---|---|---|
| Non‐PA‐TACE (n = 282) | PA‐TACE (n = 274) | P | Non‐PA‐TACE (n = 252) | PA‐TACE (n = 193) | P | |
| Curative treatment | 129 | 177 | < 0.001 | 111 | 139 | < 0.001 |
| Liver transplantation | 4 | 12 | 0.037 | 4 | 11 | 0.017 |
| Rehepatectomy | 10 | 18 | 0.103 | 9 | 16 | 0.032 |
| Local ablation | 115 | 147 | 0.002 | 98 | 112 | < 0.001 |
| Palliative care | 153 | 97 | < 0.001 | 141 | 54 | < 0.001 |
| TACE | 89 | 42 | < 0.001 | 84 | 19 | < 0.001 |
| Chemoradiotherapy | 15 | 8 | 0.155 | 14 | 5 | 0.125 |
| Immunotargeted therapy | 49 | 47 | 0.954 | 43 | 30 | 0.668 |
Abbreviations: HCC, Hepatocellular carcinoma; PA‐TACE, Postoperative adjuvant transarterial chemoembolisation; PSM, Propensity score matching; TACE, Transarterial chemoembolisation.
Risk factors for Disease‐free survival and Overall survival
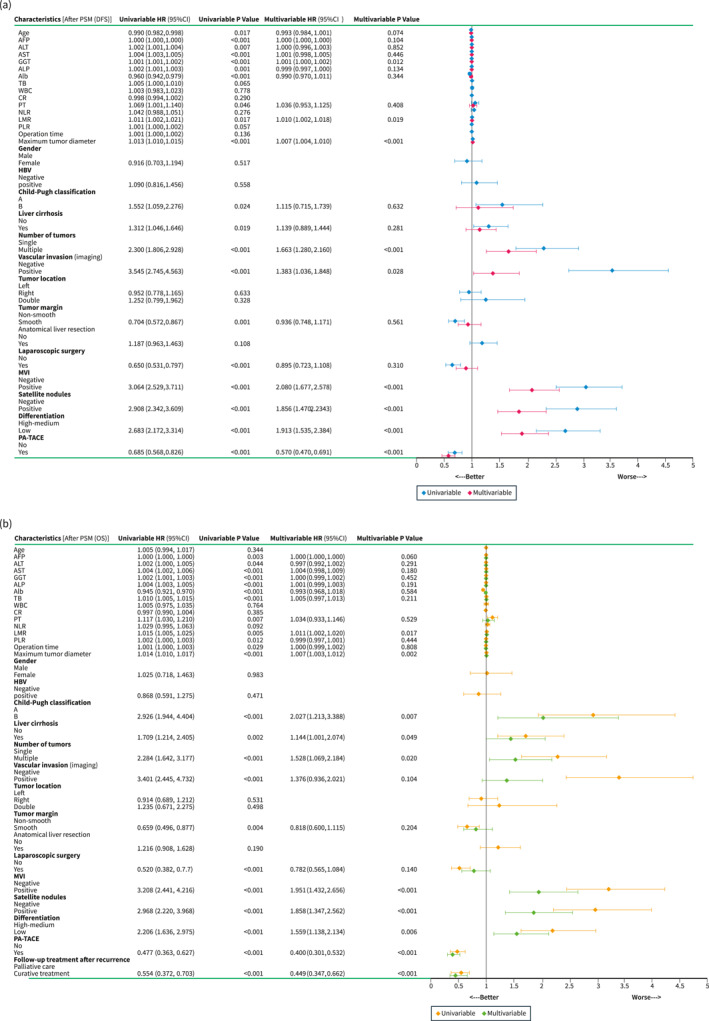
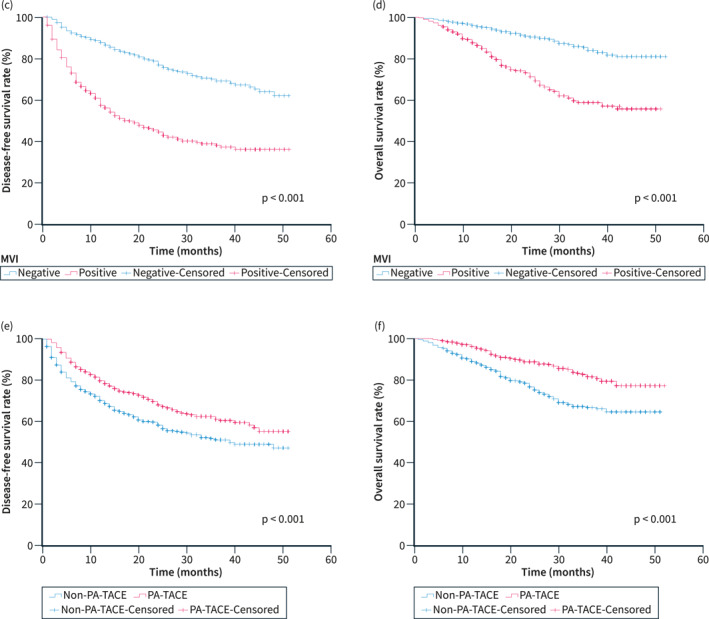
During the follow‐up period, there were 556 tumour recurrences and 271 deaths after hepatectomy in all patients with HCC before PSM (after PSM: 445 tumour recurrences and 227 deaths). Independent risk factors for DFS and OS were assessed by univariate and multivariate analyses after PSM as shown in Figure 1. MVI [DFS: Hazard ratio (HR), 2.080, p < 0.001; OS: HR, 1.951, p < 0.001)] and Non‐PA‐TACE (DFS: HR, 0.570, p < 0.001; OS: HR, 0.449, p < 0.001) were found to be independent risk factors affecting DFS and OS after hepatectomy in patients with HCC. Patients who received PA‐TACE had significantly higher DFS (1‐, 2‐, and 3‐year: 88%‐68%‐61% vs. 70%‐58%‐51%, p < 0.001) and OS (1‐, 2‐, and 3‐year: 96%‐89%‐82% vs. 89%‐77%‐67%, p < 0.001) than those who did not receive PA‐TACE. Results similar to the above were seen before PSM (Figure S2).
FIGURE 1.
Forest plots of univariate and multivariate analysis of Cox regression models for DFS (a) and OS (b) in patients with HCC after PSM. Curves of DFS (c) and OS (d) for all patients with or without MVI after PSM. Curves of DFS (e) and OS (f) for all patients with or without PA‐TACE after PSM. AFP, Alpha‐fetoprotein; Alb, Albumin; ALP, Alkaline phosphatase; ALT, Alanine aminotransferase; AST, Aspartate aminotransferase; Cl, Confidence interval; CR, Creatinine; DFS, Disease‐free survival; GGT, Gamma‐glutamyltransferase; HBV, Hepatitis B virus; HCC, Hepatocellular carcinoma; HR, Hazard ratio; LMR, Lymphocyte‐to‐monocyte ratio; MVI, Microvascular invasion; NLR, Neutrophil‐to‐lymphocyte ratio; OS, Overall survival; PA‐TACE, Postoperative adjuvant transarterial chemoembolisation; PLR, Platelet‐to‐lymphocyte ratio; PSM, Propensity score matching; PT, Prothrombin time; TB, Total bilirubin; WBC, White blood cell.
Subgroup analysis of Disease‐free survival and Overall survival
DFS and OS were assessed for different subgroups of the population after PSM. MVI‐negative patients did not achieve significant survival outcomes from PA‐TACE (Figure 2a, DFS, p = 0.324; Figure 2b, OS, p = 0.213), whereas MVI‐positive patients achieved higher DFS (Figure 2c, 1‐, 2‐, and 3‐year: 68%‐57%‐48% vs. 46%‐31%‐27%, p < 0.001) and OS (Figure 2d, 1‐, 2‐, and 3‐year: 96%‐84%‐77% vs. 79%‐58%‐40%, p < 0.001) from it. Among the six different liver cancer stages, MVI‐negative patients did not have significant survival outcomes from PA‐TACE (all p > 0.05), while MVI‐positive patients achieved higher DFS [Figure 3: Within Milan criteria, p < 0.001; Beyond Milan criteria, p < 0.001; Barcelona Clinic Liver Cancer (BCLC) stage O‐A, p < 0.001; BCLC stage B‐C, p = 0.001; China liver cancer (CNLC) stage I, p < 0.001; CNLC stage Il‐Illa, p = 0.001; American Joint Committee on Cancer (AJCC) Tumour Node Metastasis (TNM) (8th) stage I, p < 0.001; AJCC TNM (8th) stage Il‐IlI, p < 0.001; Japan Integrated Staging (JIS) score 0–1, p < 0.001; JIS score 2–3, p < 0.001; Hong Kong Liver Cancer (HKLC) stage I, p < 0.001; HKLC stage Il‐Ill, p < 0.001] and OS (Figure 4: Within Milan criteria, p < 0.001; Beyond Milan criteria, p < 0.001; BCLC stage O‐A, p < 0.001; BCLC stage B‐C, p < 0.001; CNLC stage I, p < 0.001; CNLC stage Il‐Illa, p < 0.001; AJCC TNM (8th) stage I, p < 0.001; AJCC TNM (8th) stage Il‐IlI, p < 0.001; JIS score 0–1, p < 0.001; JIS score 2–3, p < 0.001; HKLC stage I, p < 0.001; HKLC stage Il‐Ill, p < 0.001) from it. Results similar to the above were seen before PSM (Figures S3–S5).
FIGURE 2.
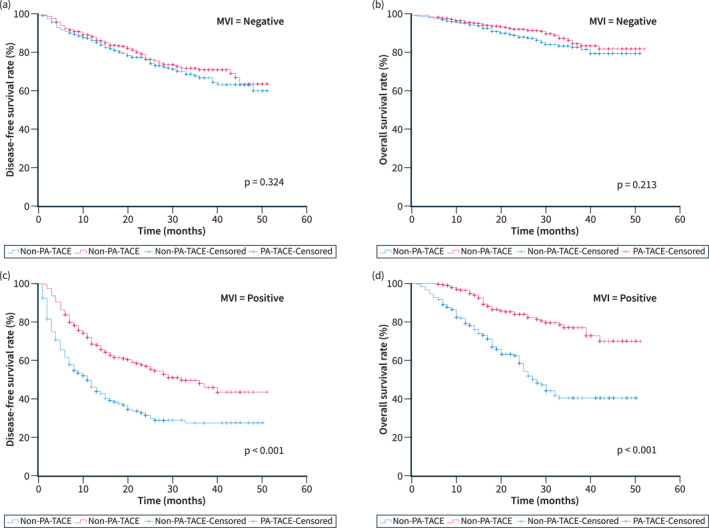
Subgroup curves of DFS (a, c) and OS (b, d) in patients with negative and positive MVI who received PA‐TACE after PSM. DFS, Disease‐free survival; MVI, Microvascular invasion; OS, Overall survival; PA‐TACE, Postoperative adjuvant transarterial chemoembolisation; PSM, Propensity score matching.
FIGURE 3.
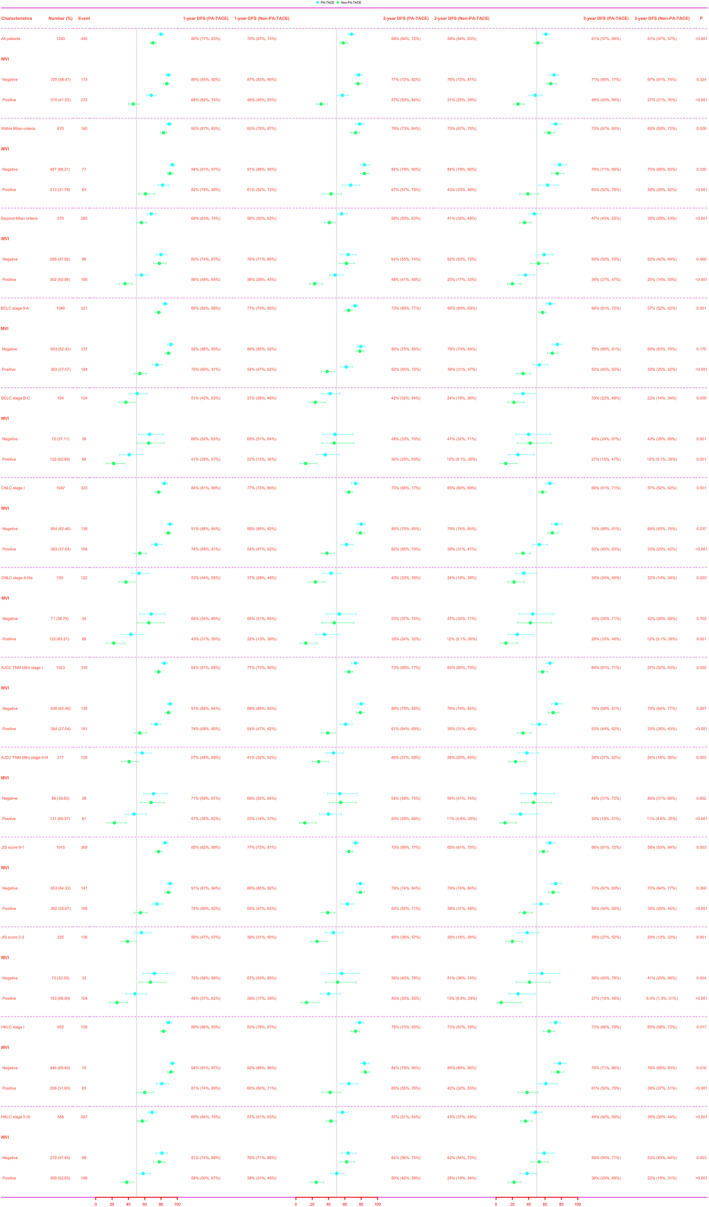
Subgroup forest plots of DFS at 1, 2, and 3 years in patients with or without MVl who received PA‐TACE in different liver cancer stages after PSM. AJCC, American Joint Committee on Cancer; BCLC, Barcelona Clinic Liver Cancer; CNLC, China liver cancer; DFS, Disease‐free survival; HKLC, Hong Kong Liver Cancer; JIS, Japan Integrated Staging; MVI, Microvascular invasion; PA‐TACE, Postoperative adjuvant transarterial chemoembolization; PSM, Propensity score matching; TNM, Tumor Node Metastasis.
FIGURE 4.

Subgroup forest plots of OS at 1, 2, and 3 years in patients with or without MVI who received PA‐TACE in different liver cancer stages after PSM. AJCC, American Joint Committee on Cancer; BCLC, Barcelona Clinic Liver Cancer; CNLC, China liver cancer; HKLC, Hong Kong Liver Cancer; JIS, Japan Integrated Staging; MVI, Microvascular invasion; OS, Overall survival; PA‐TACE, Postoperative adjuvant transarterial chemoembolisation; PSM, Propensity score matching; TNM, Tumour Node Metastasis.
DISCUSSION
Microvascular invasion generally reflects the high invasive and metastatic capacity of the tumour, and its presence significantly worsens the surgical outcome of HCC. 18 , 19 , 20 , 21 , 22 Even in small HCCs (<3 cm), the incidence of MVI remained higher than 20%. 23 , 24 Internationally, scholars have emphasised that MVI is an important basis for assessing the risk of recurrence of HCC and selecting treatment options, and it should be used as an indicator for routine pathological examination. 18 , 19 , 20 , 21 , 22 , 23 , 24 In the present study, approximately 44% of patients with HCC harboured MVI, which was an independent risk factor for DFS and OS. In this study, MVI‐positive patients who received PA‐TACE had significantly higher survival rates. Many studies have shown that PA‐TACE can significantly prolong survival in MVI‐positive patients but not in MVI‐negative patients. 11 , 12 , 13 , 14 , 15 , 16 , 17 A meta‐analysis showed that PA‐TACE not only failed to improve outcomes in MVI‐negative patients but may potentially promote postoperative recurrences in certain patients. 25 This suggests that PA‐TACE is not a necessary treatment option for MVI‐negative patients. In addition, analysis of six different liver cancer stages revealed no significant survival benefit from PA‐TACE in MVI‐negative patients, whereas MVI‐positive patients had higher survival outcomes from it. It is worth noting that the above results were the same in patients with early and intermediate liver cancer stages. Thus, it is evident that the detection of MVI may help to guide the selection of PA‐TACE.
Some scholars have found through prospective studies that the postoperative tumour recurrence is mostly seen within 6 months after surgery, especially the highest risk of recurrence in the third to fourth months after surgery. 26 All patients in this study were followed up every 1–2 months for the first 6 months after surgery to ensure the earlier detection of tumour recurrence and to enable patients with tumour recurrence to receive timely follow‐up anti‐tumour therapy. Interestingly, we analysed the important reasons for the significantly longer OS in the PA‐TACE group by the different subsequent antitumour regimens for patients with tumour recurrence. After the diagnosis of tumour recurrence, significantly more patients in the PA‐TACE group received curative treatment (liver transplantation, rehepatectomy, or local ablation) than in the Non‐PA‐TACE group, which may have led to longer OS in the PA‐TACE group. This may reflect the fact that the tumour recurrence in patients in the PA‐TACE group was usually localised and manageable. In contrast, more patients with tumour recurrence in the Non‐PA‐TACE group received relatively palliative treatment, which may be associated with more extensive tumour recurrence and unfavorable factors, such as large vessel cancer thrombosis and extrahepatic metastases.
Postoperative adjuvant transarterial chemoembolisation can accelerate the deterioration of liver function, suppress host immunity to tumour progression, and affect hepatocyte regeneration. 27 , 28 These may adversely affect the long‐term survival of patients after radical resection of HCC. In our study, patients had relatively mild adverse reactions to PA‐TACE with abnormal liver dysfunction, fever, and nausea/vomiting as the most common adverse events. Most adverse events were minor and manageable, and no toxicity‐related deaths occurred. In particular, there was no increase in grade 3–4 adverse events in the PA‐TACE group compared with the non‐PA‐TACE group. In addition, other studies have found that postoperative adjuvant TACE is accompanied by mild adverse effects, and most recover quickly and well after symptomatic management. 29 , 30 No patients have been identified with serious adverse events or toxicity‐related deaths in PA‐TACE, but its safety still needs to be investigated in more prospective large clinical experiences.
Although the data in this study were screened by strict inclusion and exclusion criteria, there were still inevitable limitations: (1) As a retrospective study, even though we minimised patient selection bias through PSM, it was difficult to completely avoid retrospective bias and confounders between groups; (2) due to the lack of formal clinical guidelines for PA‐TACE, it is difficult to have the same drug type, drug dose, and operation cycle for TACE in different medical centers. It is hoped that larger, multi‐center, prospective trials will be conducted in the future to verify the findings of this study.
CONCLUSIONS
Among the six different conventional liver cancer stages in this study, PA‐TACE showed a survival benefit not only for patients in the early stages but also for those in the intermediate stages. However, it had limited efficacy in patients with HCC without MVI. Overall, PA‐TACE has a good safety profile and may be a potentially beneficial treatment modality for survival outcomes in patients with HCC, especially those with concomitant MVI.
AUTHOR CONTRIBUTIONS
Laihui Luo, Renfeng Shan, Lifeng Cui, and Zhao Wu: Concepts, designs, data collection, analysis, manuscript preparation, and editing. Junlin Qian, Shuju Tu, WenJian Zhang, Yuanpeng Xiong, Wei Lin, and Hongtao Tang: Data collection, analysis, manuscript preparation, and editing. Yang Zhang, Jisheng Zhu, Yong Li, Zeyu Huang, Zhigang Li, Shengping Mao, Hui Li, Zemin Hu, Peng Peng, and Kun He: Analysis, manuscript preparation, and editing. Yong Li, Yongzhu He, Liping Liu, and Wei Shen: Guarantee the integrity of the entire study and manuscript review. All authors have read and approved the final version to be submitted.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no competing interests.
ETHICS STATEMENT
The study was approved by the ethics committees of the First Affiliated Hospital of Nanchang University, the Second Affiliated Hospital of Nanchang University, Shenzhen People's Hospital and Zhongshan People's Hospital, and followed the guidelines of the Declaration of Helsinki (Ethics number: 2022‐CDYFYYLK‐08‐015).
CONSENT FOR PUBLICATION
Written informed consent was obtained from patients or their immediate family.
GUARANTORS
Yongzhu He, MD, PhD Division of Hepatobiliary and Pancreas Surgery, Department of General Surgery, The First Affiliated Hospital of Nanchang University(The First Clinical Medical College of Nanchang University), No. 17 Yongwaizheng Street, Donghu District, Nanchang City, Jiangxi Province, China 330006, Tel: +86 0791‐88694131; Department of Hepatobiliary Surgery, Zhongshan People's Hospital (Zhongshan Hospital Affiliated to Sun Yat‐sen University), No. 2, Sunwen East Road, Shiqi District, Zhongshan City, Guangdong Province, China 528400, Tel:+86 0760‐89880551, E‐mail: yongzhuhe@email.ncu.edu.cn.
Supporting information
Supplementary Material
Figure S1
Figure S2a
Figure S2b
Figure S3
Figure S4
Figure S5
ACKNOWLEDGEMENTS
This work was funded by Zhongshan Science and Technology Plan Project of Guangdong Province (Project Number: 2021B1040), Key research and development projects of Jiangxi Provincial Department of Science and Technology (Project Number: 20202BBGL73092), Natural Science Foundation of Jiangxi Provincial (Project Number: 20171BAB205064), and National Natural Science Foundation of China (Project Number: 81860432) that play no role in the collection, analysis, interpretation of results, or writing of the manuscripts.
Luo L, Shan R, Cui L, Wu Z, Qian J, Tu S, et al. Postoperative adjuvant transarterial chemoembolization improves survival of hepatocellular carcinoma patients with microvascular invasion: a multicenter retrospective cohort. United European Gastroenterol J. 2023;11(2):228–41. 10.1002/ueg2.12365
Laihui Luo, Renfeng Shan, Lifeng Cui and Zhao Wu contributed equally to this work.
Contributor Information
Yong Li, Email: dryongli@163.com.
Liping Liu, Email: liuliping@mail.sustech.edu.cn.
Wei Shen, Email: shenweiniu@163.com.
Yongzhu He, yongzhuhe@email.ncu.edu.cn.
DATA AVAILABILITY STATEMENT
The datasets generated and analysed during the current study are not publicly available due to privacy and ethical concerns, but are available from the corresponding author on reasonable request.
REFERENCES
- 1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a Cancer J clinicians. 2021;71(3):209–49. 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2. Erratum. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a Cancer J clinicians. 2020;70(4):313. [DOI] [PubMed] [Google Scholar]
- 3. Kloeckner R, Galle PR, Bruix J. Local and regional therapies for hepatocellular carcinoma. Baltimore: Hepatology; 2021. p. 137–49. [DOI] [PubMed] [Google Scholar]
- 4. Park JW, Chen M, Colombo M, Roberts LR, Schwartz M, Chen P, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE Study. Liver Int. 2015;35(9):2155–66. 10.1111/liv.12818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Xie DY, Ren ZG, Zhou J, Fan J, Gao Q. 2019 Chinese clinical guidelines for the management of hepatocellular carcinoma: updates and insights. Hepatobiliary Surg Nutr. 2020;9(4):452–63. 10.21037/hbsn-20-480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Prince D, Liu K, Xu W, Chen M, Sun JY, Lu XJ, et al. Management of patients with intermediate stage hepatocellular carcinoma. Ther Adv Med Oncol. 2020;12:1758835920970840. 10.1177/1758835920970840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Reig M, Cabibbo G. Antiviral therapy in the palliative setting of HCC (BCLC‐B and ‐C). J Hepatol. 2021;74(5):1225–33. 10.1016/j.jhep.2021.01.046 [DOI] [PubMed] [Google Scholar]
- 8. Zhang YF, Shang H, Zeng XL, Ji H, Li YM, Lu HW. Postoperative adjuvant chemo (embolization) therapy for hepatocellular carcinoma with portal vein tumor thrombosis. OncoTargets Ther. 2018;11:5407–17. 10.2147/ott.s171612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liang L, Li C, Diao YK, Jia HD, Xing H, Pawlik TM, et al. Survival benefits from adjuvant transarterial chemoembolization in patients undergoing liver resection for hepatocellular carcinoma: a systematic review and meta‐analysis. Ther Adv Gastroenterol. 2020;13:1756284820977693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu F, Guo X, Dong W, Zhang W, Wei S, Zhang S, et al. Postoperative adjuvant TACE‐associated nomogram for predicting the prognosis of resectable Hepatocellular Carcinoma with portal vein Tumor Thrombus after Liver Resection. Int J Biol Sci. 2020;16(16):3210–20. 10.7150/ijbs.46896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang H, Du PC, Wu MC, Cong WM. Postoperative adjuvant transarterial chemoembolization for multinodular hepatocellular carcinoma within the Barcelona Clinic Liver Cancer early stage and microvascular invasion. Hepatobiliary Surg Nutr. 2018;7(6):418–28. 10.21037/hbsn.2018.09.05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang K, Xiang YJ, Yu HM, Cheng YQ, Qin YY, Wang WJ, et al. A novel classification in predicting prognosis and guiding postoperative management after R0 liver resection for patients with hepatocellular carcinoma and microvascular invasion. Eur J Surg Oncol : J Eur Soc Surg Oncol Br Assoc Surg Oncol. 2022;48(6):1348–55. 10.1016/j.ejso.2021.12.466 [DOI] [PubMed] [Google Scholar]
- 13. Qi YP, Zhong JH, Liang ZY, Zhang J, Chen B, Chen CZ, et al. Adjuvant transarterial chemoembolization for patients with hepatocellular carcinoma involving microvascular invasion. Am J Surg. 2019;217(4):739–44. 10.1016/j.amjsurg.2018.07.054 [DOI] [PubMed] [Google Scholar]
- 14. Liu S, Guo L, Li H, Zhang B, Sun J, Zhou C, et al. Postoperative adjuvant trans‐arterial chemoembolization for patients with hepatocellular carcinoma and portal vein tumor thrombus. Ann Surg Oncol. 2018;25(7):2098–104. 10.1245/s10434-018-6438-1 [DOI] [PubMed] [Google Scholar]
- 15. Ye JZ, Chen JZ, Li ZH, Bai T, Zhu SL, Li LQ, et al. Efficacy of postoperative adjuvant transarterial chemoembolization in hepatocellular carcinoma patients with microvascular invasion. World J Gastroenterol. 2018;23(41):7415–24. 10.3748/wjg.v23.i41.7415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hu S, Gan W, Qiao L, Ye C, Wu D, Liao B, et al. A new prognostic algorithm predicting HCC recurrence in patients with Barcelona clinic liver cancer stage B who received PA‐TACE. Front Oncol. 2018;11:742630. 10.3389/fonc.2021.742630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang YY, Wang LJ, Xu D, Liu M, Wang HW, Wang K, et al. Postoperative adjuvant transarterial chemoembolization should be considered selectively in patients who have hepatocellular carcinoma with microvascular invasion. HPB : official J Int Hepato Pancreato Biliary Assoc. 2019;21(4):425–33. 10.1016/j.hpb.2018.08.001 [DOI] [PubMed] [Google Scholar]
- 18. Lee S, Kang TW, Song KD, Lee MW, Rhim H, Lim HK, et al. Effect of microvascular invasion risk on early recurrence of hepatocellular carcinoma after surgery and radiofrequency ablation. Ann Surg. 2021;273(3):564–71. 10.1097/sla.0000000000003268 [DOI] [PubMed] [Google Scholar]
- 19. Costentin CE, Ferrone CR, Arellano RS, Ganguli S, Hong TS, Zhu AX. Hepatocellular carcinoma with macrovascular invasion: defining the optimal treatment strategy. Liver cancer. 2017;6(4):360–74. 10.1159/000481315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yang L, Gu D, Wei J, Yang C, Rao S, Wang W, et al. A radiomics nomogram for preoperative prediction of microvascular invasion in hepatocellular carcinoma. Liver cancer. 2019;8(5):373–86. 10.1159/000494099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li Y, Zhang Y, Fang Q, Zhang X, Hou P, Wu H, et al. Radiomics analysis of [F]FDG PET/CT for microvascular invasion and prognosis prediction in very‐early‐ and early‐stage hepatocellular carcinoma. Eur J Nucl Med Mol Imag. 2021;48(8):2599–614. 10.1007/s00259-020-05119-9 [DOI] [PubMed] [Google Scholar]
- 22. Liao B, Liu L, Wei L, Wang Y, Chen L, Cao Q, et al. Innovative synoptic reporting with seven‐point sampling protocol to improve detection rate of microvascular invasion in hepatocellular carcinoma. Front Oncol. 2021;11:726239. 10.3389/fonc.2021.726239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pawlik TM, Delman KA, Vauthey JN, Nagorney DM, Ng IOL, Ikai I, et al. Tumor size predicts vascular invasion and histologic grade: implications forselection of surgical treatment for hepatocellular carcinoma. Liver Transpl. 2005;11(9):1086–92. 10.1002/lt.20472 [DOI] [PubMed] [Google Scholar]
- 24. Onaca N, Davis GL, Jennings LW, Goldstein RM, Klintmalm GB. Improved results of transplantation forhepatocellular carcinoma: a report from the international registry of hepatic tumors in LiverTransplantation. Liver Transpl. 2009;15(6):574–80. 10.1002/lt.21738 [DOI] [PubMed] [Google Scholar]
- 25. Chen W, Ma T, Zhang J, Zhang X, Chen W, Shen Y, et al. A systematic review and meta‐analysis of adjuvant transarterial chemoembolization after curative resection for patients with hepatocellular carcinoma. HPB Oxf. 2020;22(6):795–808. 10.1016/j.hpb.2019.12.013 [DOI] [PubMed] [Google Scholar]
- 26. Lu X, Zhao H, Yang H, Mao Y, Sang X, Miao R, et al. A prospective clinical study on early recurrence of hepatocellular carcinoma after hepatectomy. J Surg Oncol. 2009;100(6):488–93. 10.1002/jso.21354 [DOI] [PubMed] [Google Scholar]
- 27. Raoul JL, Forner A, Bolondi L, Cheung TT, Kloeckner R, de Baere T. Updated use of TACE for hepatocellular carcinoma treatment: how and when to use it based on clinical evidence. Cancer Treat Rev. 2019;72:28–36. 10.1016/j.ctrv.2018.11.002 [DOI] [PubMed] [Google Scholar]
- 28. Lencioni R, de Baere T, Soulen MC, Rilling WS, Geschwind JF. Lipiodol transarterial chemoembolization for hepatocellular carcinoma: a systematic review of efficacy and safety data. Hepatology. 2016;64(1):106–16. 10.1002/hep.28453 [DOI] [PubMed] [Google Scholar]
- 29. Wang Z, Ren Z, Chen Y, Hu J, Yang G, Yu L, et al. Adjuvant transarterial chemoembolization for HBV‐related hepatocellular carcinoma after resection: a randomized controlled study. Clin Cancer Res. 2018;24(9):2074–81. 10.1158/1078-0432.ccr-17-2899 [DOI] [PubMed] [Google Scholar]
- 30. Wei W, Jian PE, Li SH, Guo ZX, Zhang YF, Ling YH, et al. Adjuvant transcatheter arterial chemoembolization after curative resection for hepatocellular carcinoma patients with solitary tumor and microvascular invasion: a randomized clinical trial of efficacy and safety. Cancer Commun (Lond). 2018;38(1):61. 10.1186/s40880-018-0331-y [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Figure S1
Figure S2a
Figure S2b
Figure S3
Figure S4
Figure S5
Data Availability Statement
The datasets generated and analysed during the current study are not publicly available due to privacy and ethical concerns, but are available from the corresponding author on reasonable request.


