Abstract
Alcohol‐related liver disease (ArLD) is a major cause of chronic liver disease globally. Traditionally, ArLD was mostly a concern in men rather than in women; however, such a sex gap is rapidly narrowing due to increasing chronic alcohol consumption among women. Female sex is more vulnerable to the harmful effects of alcohol with a higher risk of progression to cirrhosis and development of associated complications. The relative risk of cirrhosis and liver‐related mortality is significantly higher in women than in men. Our review endeavors to summarize the current knowledge on sex differences in alcohol metabolism, pathogenesis of ArLD, disease progression, indication for liver transplant and pharmacological treatments of ArLD, and provide evidence in support of a sex‐specific management of these patients.
Keywords: alcohol, cirrhosis, gender, portal hypertension, sex
Abbreviations
- AAH
acute alcoholic hepatitis
- ACLF
acute on chronic liver failure
- ADH
alcohol dehydrogenase
- ArLD
Alcohol‐related liver disease
- AUD
alcohol use disorders
- CBI
combined behavioral intervention
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- LPS
lipopolysaccharide
- LT
liver transplant
- MELD
model for end‐stage liver disease
- NAFLD
non‐alcoholic fatty liver disease
- NASH
non‐alcoholic steatohepatitis
- PEth
phosphatidylethanol
- TLRs
toll like receptors
INTRODUCTION
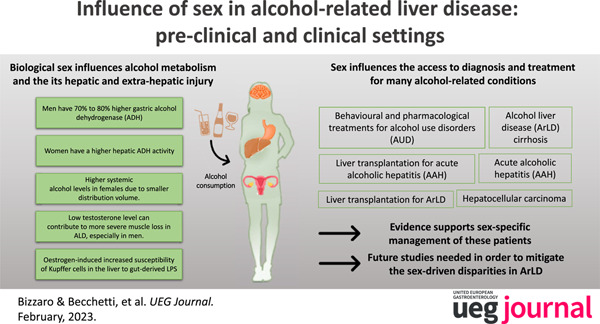
Alcohol‐related liver disease (ArLD) is a major cause of liver disease globally. 1 Traditionally, ArLD was thought to be more prevalent and severe in men than in women; however, this sex gap is now narrowing. Recent data demonstrate that alcohol intake is significantly growing among women, and that female sex is more vulnerable to the harmful effects of alcohol. Young people and female individuals from austral Asia, western Europe, and southern Latin America have the highest rates of harmful alcohol consumption. 2 This results in an overall disease burden and substantial health loss, especially in this population group. 3 In this review, we summarize the latest findings on the influence of biological sex on the metabolism of alcohol, pathogenesis of liver injury, disease progression and therapeutic options.
PRE‐CLINICAL SETTINGS
Hepatic and extrahepatic ethanol metabolism and differences among sex
Ethanol is a psychoactive compound that acts as a central nervous system depressant. It is absorbed by the gastrointestinal tract and is rapidly distributed throughout the body into peripheral tissues. 4 The main factors that influence the rate of ethanol absorption include the amount of ethanol consumed, body composition, gastric emptying, and enzymatic activity. The enzymes that are responsible for ethanol metabolization are located throughout the gastrointestinal tract and the liver, as well as in other tissues including adipose, breast, brain, and whole blood. 4 The liver metabolic pathways of ethanol are summarized in Figure 1.
FIGURE 1.
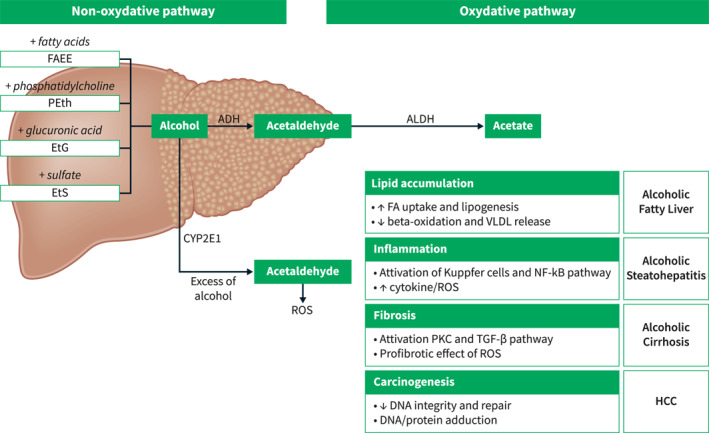
Liver pathways involved in alcohol metabolism. ADH, alcohol dehydrogenase; ALDH, aldehyde dehydrogenase; EtG, ethyl glucuronide; EtS, ethyl sulfate; FAEE, fatty acid ethyl esters; PEth, phosphatidylethanol; ROS, reactive oxygen species; VLDL, very‐low‐density lipoprotein.
The sex differences in enzymatic activities are responsible, at least in part, for the sex differences in alcohol related liver injury. 5 Men have a 70%–80% higher gastric alcohol dehydrogenase (ADH) activity compared with women. Conversely, women have a higher hepatic ADH activity. A study by Kwo et al. 6 demonstrated that the alcohol elimination rate normalized for lean body mass was higher in women than in men, suggesting a possibly higher concentration of toxic metabolites, especially acetaldehyde, in the liver.
Although the liver being the principal site of ethanol metabolism and a target for alcohol‐induced injury, important evidence suggests that ethanol could promote adipose tissue dysfunction, which in turn results in the progression of liver damage. 7 Inflammation of adipose tissue is one of the most important co‐factors in ArLD pathogenesis. 8 , 9 The expression of specific Toll‐Like Receptors (TLRs) (including TLR2, TLR3, and TLR9) was found to be higher in alcohol‐fed female mice but not in male mice 9 , confirming sexual dimorphism in alcohol‐stimulated inflammation in the adipose tissue. The clarification of the molecular mechanism of alcohol‐associated adipose tissue dysfunction could be crucial for the identification of efficient therapeutic agents for the treatment of ArLD.
Different amount of alcohol metabolized among sex
Females are generally smaller than males. Furthermore, females have smaller body water content per kilogram of body weight, which leads to a smaller distribution volume. 10 In addition, alcohol distribution is influenced by the different composition of total body fat between the two sexes, that is higher in females. 11 Therefore, the same quantity of alcohol consumption results in higher systemic alcohol levels in females. The amount of alcohol intake ‘at‐risk’ for the development of hepatitis or cirrhosis is variable between individuals; however, it is estimated that the consumption of 40 g per day for 10 years is associated with an increased risk of cirrhosis. The sex‐specific definition of excessive alcohol ingestion is defined as two standard drinks (20 g ethanol) daily for men and one standard drink (10 g ethanol) daily for women.
Metabolic alterations resulting from alcohol metabolism affecting lipid, glucose, and protein metabolism
Data on alcohol metabolism and lipid, glucose and protein metabolism according to sex difference are scant.
Chronic alcohol consumption increases adipose tissue lipolysis and liver fat deposition, which leads to the development of fatty liver disease. Lipid metabolism in response to long‐term alcohol intake promotes adipose tissue loss and free fatty acid release. 12
Chronic alcohol intake impairs insulin regulation and glucose tolerance. Preclinical models suggest that alcohol inhibits insulin secretion. Therefore, a chronic, excessive alcohol intake can antagonize insulin‐stimulated glucose disposal in peripheral tissues and the suppression of hepatic glucose production might promote the development of type 2 diabetes mellitus. 13 On the other hand, a moderate alcohol consumption seems associated with a reduced risk of type 2 diabetes, especially in women. 14
Regarding protein metabolism, the alcohol effect in the skeletal muscle is associated with an increased expression of alcohol metabolizing enzymes, including ADH inhibitors. 15 Interestingly, sexual hormones are also responsible for the development of sarcopenia. 16 Low testosterone levels can contribute to more severe muscle loss in ArLD, especially in men. 15 Additionally, alcohol abuse favors malnutrition, providing futile calories and generating a vicious cycle. 16
Sex differences in metabolism and alcohol‐induced liver injury
In case of prolonged heavy alcohol consumption, the principal metabolic pathways responsible for alcohol metabolism become overwhelmed, affecting mostly the liver. This is particularly relevant in women in whom the baseline enzymatic activity is lower than in men, thus leading to a higher alcohol toxicity and liver injury. 5
Sex hormones are also involved since estrogen stimulates the lipopolysaccharide (LPS) activation of Kupffer cells, which leads to a higher inflammatory response. Indeed, females have a higher inflammatory activation in response to enteric endotoxins, the amount of which is increased by exposure to alcohol compared to males. Animal models have demonstrated that increased Kupffer cell activation causes more severe hepatic injury and necrosis. Additionally, estrogen blockade in mouse models has been shown to attenuate alcohol‐related injury in females. 17 On the other hand, chronic alcohol ingestion considerably modifies the hormonal milieu in both sexes, significantly altering both sex hormone levels and functions. 10 , 11 In men, alcohol abuse induces hormone imbalance, with a reduction of serum testosterone and elevation of estrogen levels, with consequent erectile dysfunction, infertility, loss of muscle mass, gynecomastia. In women, it was demonstrated that altered ethanol intake induces deep alterations in hormone homeostasis and reproductive potential. 18 The principal alterations seen in women are menstrual cycle disorders, including amenorrhea, anovulatory, or irregular cycles, and luteal phase dysfunction, impaired fertility, and earlier menopause. 19
In summary, women are more susceptible to the toxic effects of ethanol than men. The mechanisms responsible for this increased susceptibility to ArLD include differences in first‐pass metabolism in the stomach, different enzymatic activities in the liver, differences in ethanol distribution volumes in the body, differences in gut permeability to endotoxin and estrogen‐induced increased susceptibility of Kupffer cells in the liver to gut‐derived LPS (Figure 2).
FIGURE 2.
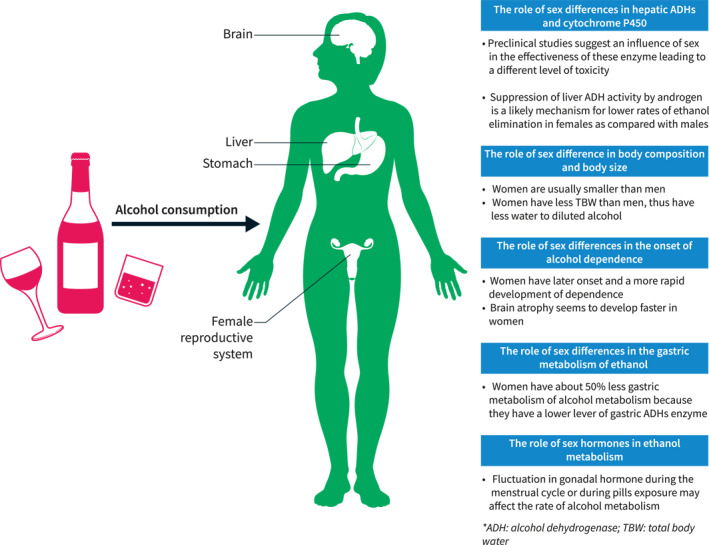
Role of sex differences in alcohol metabolism and its harmful effect. ADH, alcohol dehydrogenase; TBW, total body water.
CLINICAL SETTINGS
Alcohol‐related liver disease
Alcohol‐driven hepatic injury can be acute, chronic, or acute‐on‐chronic. Acute alcohol intake results in a predominantly steatosis‐like injury, whereas chronic intake leads to fibrosis, cirrhosis, and potentially hepatocellular carcinoma (HCC). Furthermore, alcohol may lead to significant alterations in cerebral blood flow. 20 , 21 Each of these conditions can be significantly influenced by biological sex.
Acute alcoholic hepatitis (AAH)
Acute alcoholic hepatitis (AAH) is a clinical syndrome characterized by jaundice, ascites, hypoalbuminemia, hyperbilirubinemia, prothrombin time prolongation, and mild‐to‐moderate increase in transaminases. Severe cases not responding to corticosteroid therapy have a 6‐month mortality rate of 75%. 22
The epidemiology of AAH is poorly understood. However, since alcohol abuse is increasing sharply, it is expected that the incidence of AAH will also increase worldwide. A large Spanish cohort of AAH demonstrated that male sex, older age, model for end‐stage liver disease (MELD) score and failure to withdraw alcohol were independently associated with mortality. Interestingly, patients with recurrent AAH were more frequently men (93% vs. 72%) and there was a trend towards a lower survival (83% vs. 92%). 23
In a study of the NHANES database on 15,981 subjects, male sex and Hispanic race were associated with harmful alcohol use. However, when considering liver disease, female sex was associated with 8% higher risk of acute on chronic liver failure (ACLF). The risk of ACLF grade ≥2 was 1.7‐fold higher in people <35 years old and 1.5‐fold higher in females. 24
Alcohol liver disease (ArLD) cirrhosis
A systematic analysis regarding cirrhosis‐related mortality and morbidity due to hepatitis C virus (HCV), hepatitis B virus (HBV), ArLD and non‐alcoholic steatohepatitis (NASH) conducted across 195 countries demonstrated that the global burden of alcohol‐related cirrhosis is 27.3% in males and 20.6% in females, respectively. The highest age‐standardized death rate due to alcohol‐related cirrhosis for both males and females is shown in central Asia (male 38.7%; female 33.3%), central and eastern Europe (male 44.8%; female 43.6% and male 40.3%; female 32.8%, respectively), and Latin America (male 42.8% and female 30.7%). These patterns closely follow the distribution of alcohol consumption in these regions. Furthermore, the risk for the development of cirrhosis was consistently higher in women than in men at all levels of alcohol consumption and cirrhosis‐driven mortality is increasing mostly in women than in men. 25
In the US, hospitalizations due to alcoholic cirrhosis increased by 19.8% from 2007 to 2014; notably, the increase was more important in women than in men (33.5% vs. 14.7%). 26
Genetic polymorphisms are often advocated among the factors influencing ArLD progression. A large cohort of studies including twins has shown heritability for alcohol dependence ranging from −16% to 72%, with higher rates in men. However, a selection bias on including few women‐twins can explain these results. 27 Three loci have been identified which are associated with an increased risk of developing ArLD, namely, PNPLA3, TM6SF2, and MBOAT7. 28 Interestingly, some of these genetic polymorphisms are shared with the pathogenesis of non‐alcoholic fatty liver disease (NAFLD), suggesting a mutual influence among alcohol consumption and metabolic disease. A recent study evaluating alcohol consumption through phosphatidylethanol (PEth) in blood demonstrated that PEth ≥48 ng/mL and binge drinking have the highest risk of significant fibrosis progression in patients with NAFLD. 29 On the other hand, in a study on biopsy‐proven ArLD, insulin resistance was found to be the strongest predictor of liver fibrosis stage and hepatic inflammation among various metabolic markers. 30
Additionally, also different patterns of alcohol consumption can influence ArLD progression. A meta‐analysis including data on two million participants with 5505 cases of cirrhosis showed that there was no increased risk of ArLD for occasional drinkers. However, consumption of 1 drink per day in comparison to long‐term abstainers showed an increased risk of liver cirrhosis in women but not in men. 25
Hepatocellular carcinoma (HCC)
HCC is the most common type of primary liver cancer. In 90% of cases, HCC develops in the context of chronic liver disease. HCC is the fifth most common cancer in men and the ninth in women. 31
In Europe, 33% and 18% of the total number of HCCs in men and women, respectively, is caused by past and/or current alcohol intake. 31
Alcohol promotes hepatocarcinogenesis indirectly through the development of cirrhosis, and directly through the formation of acetaldehyde, reactive oxygen species, DNA damage, up regulation of the production of cytokines, and alteration of immune surveillance. 11
The risk of HCC is 5 times higher in women than in men per the same amount of alcohol consumption (>80 g/day). 32
Besides epidemiological differences, the role of sex in the carcinogenic cascade driven by alcohol is currently under investigation. Estrogen may play a protective role. In a study by Hassan et al., 33 the use of estrogenic therapy during menopause was associated with a reduced risk of HCC and increased overall survival.
In conclusion, biological factors make women more sensitive to alcohol‐mediated liver damage and therefore more at risk of developing HCC than men. On the other hand, socio‐cultural factors make the prevalence of HCC higher in males.
It should also be highlighted that socio‐cultural differences are evolving, especially in developing countries. Thus, it is likely that the epidemiological scenario will change in the future.
Therapeutic approach in ArLD
Behavioral and pharmacological treatments for alcohol use disorders (AUD)
Alcohol use disorder (AUD) is a chronic disease defined by the loss of control, craving, and failure to fulfill major role obligations related to alcohol misuse.
Considering the drinking pattern, compared to men, more women are lifetime abstainers, drink less, and are less likely to engage in problem‐drinking and develop AUD or alcohol withdrawal symptoms. 34 However, evidence shows that racial/ethnic minority women, sexual minority women, and women belonging to low socioeconomic status (based on education, income, or residence in disadvantaged neighborhoods) are more likely to experience AUD. Additionally, when considering these characteristics, harmful drinking patterns, including binge drinking and intoxication, are present. 35 Unfortunately, these psycho‐socio‐cultural factors have an impact on morbidity and mortality outcomes. 36
In general, most patients with ArLD do not receive AUD therapy, which becomes even more evident when considering alcohol‐related disparities among sexes. In a study by Mellinger et al. women were less likely to receive a face‐to‐face visit (HR 0.84, p < 0.001) or an approved relapse prevention medication (0.89, p = 0.05) than men. 37
Indeed, relatively few studies have examined sex differences in the effectiveness of specific behavioral or pharmacologic treatment for AUD. While women may be less likely to undergo alcohol treatment, sex itself is not necessarily a predictor of outcome among patients receiving alcohol treatment. 38
Several factors might contribute to gender disparities in AUD access to treatments, and these can be even more pronounced when ethnical minorities are considered. One factor is the stigma of AUD, which can be particularly rooted in some cultures. Other deterrents could be related to the fear of losing child care. Lastly, logistic barriers and inadequate insurance might play a role.
Considering behavioral therapy, despite the small number of sex‐oriented studies, the results are conflicting on adherence to programs by women. In a study evaluating the effectiveness of the Network Support group, women fared less well than men, suggesting that a careful evaluation of the pre‐existing social support networks is necessary prior to propose this approach in female. 39 Certainly, some differences emerge between the risk factors for the failure of behavioral therapy for AUD in males and females. Failure to initiate treatment was predicted in women by mental health diagnoses and in men by less education, whereas treatment completion was predicted in women by higher income and legal/agency referral, and in men by older age. 40 Sex‐related differences emerge in considering pharmacological treatment for AUD (Table 1).
TABLE 1.
Pharmacotherapy agents and behavioral treatment for alcohol use disorders specifically considering gender differences.
| Treatment | Recommendation | Use in advanced liver disease | Hepatotoxicity | Special consideration/barriers based on gender |
|---|---|---|---|---|
| Behavioral therapy (i.e., cognitive; motivational enhancement) | First line | Yes | NA | Consider for women:
|
| Naltrexone | First line | Avoid in Child‐Pugh class C | Possible |
|
| Acamprosate | Second line | Yes | Not reported |
|
| Baclofen | NA | Yes | Not reported |
|
| Gabapentin | Second line | Yes | Not reported |
|
| Topiramate | Second line | Avoid in patients with hepatic encephalopathy | Not reported |
|
A study among alcohol‐ and cocaine‐dependent patients treated with oral naltrexone demonstrated that, compared with placebo, men had reductions in cocaine and alcohol use and drug severity; however, this effect was not observed in women. The addition of psychosocial treatment did not affect the outcome for both sexes. 41 Suh et al. showed that in the absence of clear predictors of treatment discontinuation in men, women were more likely to discontinue naltrexone treatment because of nausea. 42 In contrast, a separate study of sex differences in naltrexone treatment outcomes among alcohol‐addicted individuals demonstrated that the effect sizes for naltrexone over placebo were the same for women and men. 43
The COMBINE study (e.g., Combined Pharmacotherapies and Behavioral Interventions for Alcohol Dependence) investigated alcohol treatment among 8 groups of patients (378 women, 848 men) who received medical management with 16 weeks of placebo, naltrexone (100 mg daily), acamprosate (3 g daily), or their combination with or without a specialist delivered combined behavioral intervention (CBI). 44 A ninth group received CBI alone with no pills. Greenfield and co‐authors reported a sex‐focused analysis of the COMBINE study and confirmed that women with AUD responded to naltrexone similar to the men on a wide range of outcome measures. We speculate that clinicians can feel reasonably comfortable prescribing naltrexone for alcohol dependence in both men and women. In a recent trial confirming the efficacy of baclofen in reducing heavy drinking and increasing abstinent days in individuals with AUD, an interesting difference among sexes emerged. 45 Indeed, men benefit from 90 mg of baclofen/day but not from 30 mg/day, women show benefit from 30 mg/day of baclofen, marginal benefit from 90 mg/day with increased side effects at this dose, suggesting that dose adjustment should be considered when baclofen is prescribed.
To conclude, AUD treatment is a cornerstone in preventing ArLD progression. Sex unbalance in treatment access exists and has to be counteracted. Acknowledging sex‐differences in both behavioral and pharmacological treatments is paramount to achieve the best results and address the most suitable therapeutic choice.
Liver transplantation for ArLD
In recent years, the survival rate of liver transplant (LT) patients with ArLD has significantly improved, making this etiology one of the most suitable indications for LT. 46 Indeed, graft and patient survival at 5 years after LT for ArLD are 74% and 79%, respectively. 47
The relapse rates of alcohol use after LT for ArLD are comparable to those seen in other indications. 48 Male sex was one of the risk factors for relapsing. 49
A UNOS study showed that, among 51,329 adults registered on LT waiting lists from January 2014 to March 2019, ArLD was the leading etiology among men without HCC in 2019. 50 In Italy, where the indication to LT is more important in men than in women (76% vs. 24%), a recent study on 18,362 adults listed for LT from January 2004 to December 2020 showed that ArLD was a major cause of waitlisting for cirrhosis without HCC and that this was more relevant in men than in women (82% vs. 18%). 51 Furthermore, donor‐recipient matching has been recently suggested to influence the long‐term outcome of liver‐transplanted patients, independent of indication. 52
Recent data indicate that, among LT candidates with ArLD, women were less likely to be listed and, once listed, were less likely to be transplanted. McElroy et al. examined 949 patients with ArLD. They showed that the listing rate of women with ArLD was significantly lower (10% vs. 19%; p < 0.05) and also the LT rate (40% vs. 44%; p < 0.05). Despite women represented only 30% of LT recipients, they were more likely to die on the waiting list than men. There are different potential factors responsible for these differences. Firstly, women are disadvantaged by the MELD score. Secondly, there is a higher prevalence of psychiatric comorbidities and history of psychiatric pharmacotherapy in women. 53 Cullaro et al. recently found women to be 10% more likely to be delisted than men. 54 In a sub‐analysis including only ArLD etiology, delisting was significantly more common in women than in men (5.4% vs. 2.6% respectively). 54
Regarding the post‐LT follow‐up, no difference in short‐ and long‐term survival between women and men recipients has been reported. Legaz et al. showed similar short‐term survival and death rates between men and women (15.8% vs. 18.7%). Liver graft failure was one of the main causes of death in male recipients (19.5%), followed by bacterial sepsis (16.8%) and multiple organ failure (15.9%), whereas, in female recipients, the main cause of death was not related to LT (77.7%). However, a more recent study found a substantial mortality risk in women with ArLD aged <40 years who underwent transplantation for ArLD. In this cohort, recurrent disease was the most important driver for post‐transplant mortality 55 (Table 2A).
TABLE 2.
Summary of the principal studies considering liver transplant (LT) as an option for alcohol‐related liver disease (ArLD) cirrhosis (A) and acute alcoholic hepatitis (AAH) (B) according to sex.
| Author (year) | Country | N of women versus men on wait list (%) | N of women versus men underwent LT (%) | Survival of women versus men | Graft loss women versus men | Relapse women versus men |
|---|---|---|---|---|---|---|
| (A) | ||||||
| Askgaard et al. (2016) | Denmark | No data reported | 28 (18%) versus 128 (82%) | No data reported according to sex | No data reported according to sex | 10 (35%) versus 25 (19%) |
| Relative risk: Women 1.2 (0.6; 2.5) versus men 1 (0.7; 0.9); p = 0.6 | ||||||
| Wigg et al. (2017) | Australia | No data reported | 14 (16%) versus 73 (84%) | No data reported according to sex | No data reported according to sex | Harmful relapse: 2 (14%) versus 12 (16%) |
| Weeks et al. (2018) | USA | No data reported | 12 (35%) versus 22 (65%) | No data reported according to sex | No data reported according to sex | 4 (33%) versus 4 (22%) |
| Legaz et al. (2019) | Spain | No data reported | 32 (9%) versus 368 (81%) | 23 (72%) versus 255 (69%) at 10 years follow up (p = 0.89) | 1 (11%) versus 22 (19%) | No data reported |
| Matsuoka et al. (2020) | USA | No data reported | 2857 (21%) versus 10,924 (79%) | No difference in patient survival according to sex (p = 0.197) | No difference in graft survival according to sex (p = 0.066) | No data reported |
| McElroy et al. (2020) | USA | 33 (21%) versus 121 (79%) | 14 (19%) versus 57 (81%) | No data reported according to sex | No data reported according to sex | No data reported according to sex |
| Lee et al. (2021) | USA | No data reported | 2782 (22%) versus 9502 (88%) | Women aged <40 years had lower adjusted 5 years mortality versus women aged >40 years and versus men | No data reported | Age <40 years: Women 32% died for alcohol relapse versus male 25% |
| Louvet et al. (2022) | France and Belgium | 128 patients | 25 (27%) versus 68 (73%) | 81 (63% of total); No data reported according to sex | No data reported according to sex | 23 (25% of total) No data reported according to sex |
| (B) | ||||||
| Im et al. (2016) | USA | 9 (45%) versus 11 (65%) | 4 (44%) versus 5 (66%) | 4/4 (100%) versus 4/5 (80%) | No graft loss | 1 (25%) versus 0 (0%) |
| Lee et al. (2018) | USA | No data reported according to sex | 42 (27%) versus 105 (73%) | Sex female not associated with increased risk of death 1.16 (0.43–3.08) p = 0.77 | No data reported according to sex | Sex female not associated with increased risk of relapse |
| 1.32 (0.60–2.92) 0.49 | ||||||
| Week et al. (2018) | USA | No data reported according to sex | 13 (28%) versus 33 (72%) | No data reported according to sex | No data reported according to sex | 4 (30%) versus 9 (27%) |
| Germani et al. (2022) | Italy | 20 patients; No data reported according to sex | 5 (31%) versus 11 (69%) | 15 survived. No data reported according to sex | No data reported according to sex | 2 alcohol relapse (32%); No data reported according to sex |
| Louvet et al. (2022) | France and Belgium | 102 patients; No data reported according to sex | 22 (32%) versus 46 (68%) | 61 patients survived. No data reported according to sex | No data reported according to sex | 23 alcohol relapses (34% of total). No data reported according to sex |
In conclusion, among LT candidates with ArLD, women are less likely to be listed and, once listed, are less likely to be transplanted and more likely to be delisted. Post‐transplant survival seems comparable between sexes. Further efforts to mitigate the sex‐related differences in the field of LT for ArLD are eagerly expected.
Liver transplantation for acute alcoholic hepatitis (AAH)
Severe AAH is associated with a mortality rate of 75% at 6 months. 22 Despite these high mortality rates and the lack of effective therapies, AAH was traditionally considered an absolute contraindication to LT worldwide due to the lack of pre‐LT abstinence and the postulated high risk of post‐LT relapse. 56 However, if performed under stringent selection criteria, LT can significantly increase survival rates in patients with AAH not responding to steroid therapy. 57 There is an ongoing debate as whether to allocate a limited resource, such as liver grafts, to individuals who have not demonstrated a period of alcohol abstinence. However, the existing data on LT in these patients indicate that outcomes, namely survival and relapse, are acceptable in this subset of patients. 58
No study has yet evaluated the outcome of LT for AAH according to recipient sex, perhaps reflecting the relatively recent introduction of AAH as a potential indication for LT (Table 2B). Current data indicate that LT in AAH is performed mostly in males (58% in Mathurin et al. 57 and 66% in Germani et al. 59 ). In an independent cohort from the US 60 in early LT for AAH, 49/111 women were evaluated in the study. In detail, 4/9 (44%) receive early LT. Among the 45 women who received psychosocial assessment, 36 were declined for LT for poor psychosocial profile.
CONCLUSION
In patients with ArLD, sex influences not only the different metabolism of alcohol but also the level of hepatic and extra‐hepatic injury and phenotypic expression of diseases. Sex also influences the access to diagnosis and treatment for many alcohol‐related conditions, including liver transplantation. A better understanding of the impact of sex in these patients is paramount in order to mitigate the sex‐driven disparities in ArLD.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ACKNOWLEDGMENTS
The Authors wish to thank the AISF Secretary General Prof. Alessio Aghemo and the Coordinating Committee for their critical revision of this manuscript. The article publication charge (APC) for this review article was funded by the Gastroenterology Fellowship of Padova University.
Bizzaro D, Becchetti C, Trapani S, Lavezzo B, Zanetto A, D'Arcangelo F, et al. Influence of sex in alcohol‐related liver disease: pre‐clinical and clinical settings. United European Gastroenterol J. 2023;11(2):218–27. 10.1002/ueg2.12370
Debora Bizzaro and Chiara Becchetti shared co‐authorship.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Karlsen TH, Sheron N, Zelber‐Sagi S, Carrieri P, Dusheiko G, Bugianesi E, et al. The EASL‐lancet liver commission: protecting the next generation of Europeans against liver disease complications and premature mortality. Lancet. 2022;399(10319):61–116. 10.1016/s0140-6736(21)01701-3 [DOI] [PubMed] [Google Scholar]
- 2. GBD 2020 Alcohol Collaborators . Population‐level risks of alcohol consumption by amount, geography, age, sex, and year: a systematic analysis for the global burden of disease study 2020. Lancet. 2022;400(10347):185–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. GBD 2020 Alcohol Collaborators . Alcohol use and burden for 195 countries and territories, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet. 2018;392(10152):1015–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Edenberg HJ, McClintick JN. Alcohol dehydrogenases, aldehyde dehydrogenases, and alcohol use disorders: a critical review. Alcohol Clin Exp Res. 2018;42(12):2281–97. 10.1111/acer.13904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li TK, Beard JD, Orr WE, Kwo PY, Ramchandani VA, Thomasson HR. Variation in ethanol pharmacokinetics and perceived gender and ethnic differences in alcohol elimination. Alcohol Clin Exp Res. 2000;24(4):415–6. 10.1111/j.1530-0277.2000.tb02002.x [DOI] [PubMed] [Google Scholar]
- 6. Kwo PY, Ramchandani VA, O'Connor S, Amann D, Carr LG, Sandrasegaran K, et al. Gender differences in alcohol metabolism: relationship to liver volume and effect of adjusting for body mass. Gastroenterology. 1998;115(6):1552–7. 10.1016/s0016-5085(98)70035-6 [DOI] [PubMed] [Google Scholar]
- 7. Gopal T, Ai W, Casey CA, Donohue TM, Jr. , Saraswathi V. A review of the role of ethanol‐induced adipose tissue dysfunction in alcohol‐associated liver disease. Alcohol Clin Exp Res. 2021;45(10):1927–39. 10.1111/acer.14698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Parker R, Kim SJ, Im GY, Nahas J, Dhesi B, Vergis N, et al. Obesity in acute alcoholic hepatitis increases morbidity and mortality. EBioMedicine. 2019;45:511–8. 10.1016/j.ebiom.2019.03.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fulham MA, Mandrekar P. Sexual dimorphism in alcohol induced adipose inflammation relates to liver injury. PLoS One. 2016;11(10):e0164225. 10.1371/journal.pone.0164225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Baraona E, Abittan CS, Dohmen K, Moretti M, Pozzato G, Chayes ZW, et al. Gender differences in pharmacokinetics of alcohol. Alcohol Clin Exp Res. 2001;25(4):502–7. 10.1111/j.1530-0277.2001.tb02242.x [DOI] [PubMed] [Google Scholar]
- 11. Dey A, Cederbaum AI. Alcohol and oxidative liver injury. Hepatology. 2006;43(2 Suppl 1):S63–74. 10.1002/hep.20957 [DOI] [PubMed] [Google Scholar]
- 12. Steiner JL, Lang CH. Alcohol, adipose tissue and lipid dysregulation. Biomolecules. 2017;7(1):16. 10.3390/biom7010016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Avogaro A, Fontana P, Valerio A, Trevisan R, Riccio A, Del Prato S, et al. Alcohol impairs insulin sensitivity in normal subjects. Diabetes Res. 1987;5(1):23–7. [PubMed] [Google Scholar]
- 14. Hodge AM, English DR, O'Dea K, Giles GG. Alcohol intake, consumption pattern and beverage type, and the risk of type 2 diabetes. Diabet Med. 2006;23(6):690–7. 10.1111/j.1464-5491.2006.01864.x [DOI] [PubMed] [Google Scholar]
- 15. Thapaliya S, Runkana A, McMullen MR, Nagy LE, McDonald C, Naga Prasad SV, et al. Alcohol‐induced autophagy contributes to loss in skeletal muscle mass. Autophagy. 2014;10(4):677–90. 10.4161/auto.27918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dasarathy S, Merli M. Sarcopenia from mechanism to diagnosis and treatment in liver disease. J Hepatol. 2016;65(6):1232–44. 10.1016/j.jhep.2016.07.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Järveläinen HA, Lukkari TA, Heinaro S, Sippel H, Lindros KO. The antiestrogen toremifene protects against alcoholic liver injury in female rats. J Hepatol. 2001;35(1):46–52. 10.1016/s0168-8278(01)00050-2 [DOI] [PubMed] [Google Scholar]
- 18. Jensen SB, Gluud C. Sexual dysfunction in men with alcoholic liver cirrhosis. A comparative study. Liver. 1985;5(2):94–100. 10.1111/j.1600-0676.1985.tb00221.x [DOI] [PubMed] [Google Scholar]
- 19. Rachdaoui N, Sarkar DK. Pathophysiology of the effects of alcohol abuse on the endocrine system. Alcohol Res. 2017;38(2):255–76. [PMC free article] [PubMed] [Google Scholar]
- 20. Burra P, Senzolo M, Pizzolato G, Ermani M, Chierichetti F, Bassanello M, et al. Does liver‐disease aetiology have a role in cerebral blood‐flow alterations in liver cirrhosis? Eur J Gastroenterol Hepatol. 2004;16(9):885–90. 10.1097/00042737-200409000-00012 [DOI] [PubMed] [Google Scholar]
- 21. Dam M, Burra P, Tedeschi U, Cagnin A, Chierichetti F, Ermani M, et al. Regional cerebral blood flow changes in patients with cirrhosis assessed with 99mTc‐HM‐PAO single‐photon emission computed tomography: effect of liver transplantation. J Hepatol. 1998;29(1):78–84. 10.1016/s0168-8278(98)80181-5 [DOI] [PubMed] [Google Scholar]
- 22. Zanetto A, Shalaby S, Gambato M, Germani G, Senzolo M, Bizzaro D, et al. New indications for liver transplantation. J Clin Med. 2021;10(17):3867. 10.3390/jcm10173867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gratacos‐Gines J, Rodriguez M, Giraldez‐Gallego A, Cabezas J, Vazquez IF, Cots MV, et al. Impact of sex and recurrence in the prognosis of alcoholic hepatitis. J Hepatol. 2022;77:S127. 10.1016/s0168-8278(22)00638-9 [DOI] [Google Scholar]
- 24. Singal AK, Arsalan A, Dunn W, Arab JP, Wong RJ, Kuo YF, et al. Alcohol‐associated liver disease in the United States is associated with severe forms of disease among young, females and Hispanics. Aliment Pharmacol Ther. 2021;54(4):451–61. 10.1111/apt.16461 [DOI] [PubMed] [Google Scholar]
- 25. Roerecke M, Vafaei A, Hasan OSM, Chrystoja BR, Cruz M, Lee R, et al. Alcohol consumption and risk of liver cirrhosis: a systematic review and meta‐analysis. Am J Gastroenterol. 2019;114(10):1574–86. 10.14309/ajg.0000000000000340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dang K, Hirode G, Singal AK, Sundaram V, Wong RJ. Alcoholic liver disease epidemiology in the United States: a retrospective analysis of 3 US databases. Am J Gastroenterol. 2020;115(1):96–104. 10.14309/ajg.0000000000000380 [DOI] [PubMed] [Google Scholar]
- 27. Heath AC. Genetic influences on alcoholism risk: a review of adoption and twin studies. Alcohol Health Res World. 1995;19(3):166–71. [PMC free article] [PubMed] [Google Scholar]
- 28. Stickel F, Moreno C, Hampe J, Morgan MY. The genetics of alcohol dependence and alcohol‐related liver disease. J Hepatol. 2017;66(1):195–211. 10.1016/j.jhep.2016.08.011 [DOI] [PubMed] [Google Scholar]
- 29. Blomdahl J, Nasr P, Ekstedt M, Kechagias S. Moderate alcohol consumption is associated with significant fibrosis progression in NAFLD. Hepatol Commun. 2023;7(1):e0003. 10.1097/hc9.0000000000000003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Israelsen M, Juel HB, Detlefsen S, Madsen BS, Rasmussen DN, Larsen TR, et al. Metabolic and genetic risk factors are the strongest predictors of severity of alcohol‐related liver fibrosis. Clin Gastroenterol Hepatol. 2022;20(8):1784–94e9. 10.1016/j.cgh.2020.11.038 [DOI] [PubMed] [Google Scholar]
- 31. Burra P, Zanetto A, Germani G. Liver transplantation for alcoholic liver disease and hepatocellular carcinoma. Cancers (Basel). 2018;10(2):46. 10.3390/cancers10020046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Donato F, Tagger A, Gelatti U, Parrinello G, Boffetta P, Albertini A, et al. Alcohol and hepatocellular carcinoma: the effect of lifetime intake and hepatitis virus infections in men and women. Am J Epidemiol. 2002;155(4):323–31. 10.1093/aje/155.4.323 [DOI] [PubMed] [Google Scholar]
- 33. Hassan MM, Botrus G, Abdel‐Wahab R, Wolff RA, Li D, Tweardy D, et al. Estrogen replacement reduces risk and increases survival times of women with hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2017;15(11):1791–9. 10.1016/j.cgh.2017.05.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Erol A, Karpyak VM. Sex and gender‐related differences in alcohol use and its consequences: contemporary knowledge and future research considerations. Drug Alcohol Depend. 2015;156:1–13. 10.1016/j.drugalcdep.2015.08.023 [DOI] [PubMed] [Google Scholar]
- 35. Mulia N, Bensley KM. Alcohol‐related disparities among women: evidence and potential explanations. Alcohol Res. 2020;40(2):09. 10.35946/arcr.v40.2.09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Probst C, Roerecke M, Behrendt S, Rehm J. Socioeconomic differences in alcohol‐attributable mortality compared with all‐cause mortality: a systematic review and meta‐analysis. Int J Epidemiol. 2014;43(4):1314–27. 10.1093/ije/dyu043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mellinger JL, Fernandez A, Shedden K, Winder GS, Fontana RJ, Volk ML, et al. Gender disparities in alcohol use disorder treatment among privately insured patients with alcohol‐associated cirrhosis. Alcohol Clin Exp Res. 2019;43(2):334–41. 10.1111/acer.13944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Greenfield SF, Brooks AJ, Gordon SM, Green CA, Kropp F, McHugh RK, et al. Substance abuse treatment entry, retention, and outcome in women: a review of the literature. Drug Alcohol Depend. 2007;86(1):1–21. 10.1016/j.drugalcdep.2006.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Litt MD, Kadden RM, Tennen H. Network support treatment for alcohol dependence: gender differences in treatment mechanisms and outcomes. Addict Behav. 2015;45:87–92. 10.1016/j.addbeh.2015.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Green CA, Polen MR, Dickinson DM, Lynch FL, Bennett MD. Gender differences in predictors of initiation, retention, and completion in an HMO‐based substance abuse treatment program. J Subst Abuse Treat. 2002;23(4):285–95. 10.1016/s0740-5472(02)00278-7 [DOI] [PubMed] [Google Scholar]
- 41. Pettinati HM, Kampman KM, Lynch KG, Suh JJ, Dackis CA, Oslin DW, et al. Gender differences with high‐dose naltrexone in patients with co‐occurring cocaine and alcohol dependence. J Subst Abuse Treat. 2008;34(4):378–90. 10.1016/j.jsat.2007.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Suh JJ, Pettinati HM, Kampman KM, O'Brien CP. Gender differences in predictors of treatment attrition with high dose naltrexone in cocaine and alcohol dependence. Am J Addict. 2008;17(6):463–8. 10.1080/10550490802409074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Baros AM, Latham PK, Anton RF. Naltrexone and cognitive behavioral therapy for the treatment of alcohol dependence: do sex differences exist? Alcohol Clin Exp Res. 2008;32(5):771–6. 10.1111/j.1530-0277.2008.00633.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Anton RF, O'Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295(17):2003–17. 10.1001/jama.295.17.2003 [DOI] [PubMed] [Google Scholar]
- 45. Garbutt JC, Kampov‐Polevoy AB, Pedersen C, Stansbury M, Jordan R, Willing L, et al. Efficacy and tolerability of baclofen in a U.S. community population with alcohol use disorder: a dose‐response, randomized, controlled trial. Neuropsychopharmacology. 2021;46(13):2250–6. 10.1038/s41386-021-01055-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Burra P, Senzolo M, Adam R, Delvart V, Karam V, Germani G, et al. Liver transplantation for alcoholic liver disease in Europe: a study from the ELTR (European Liver Transplant Registry). Am J Transplant. 2010;10(1):138–48. 10.1111/j.1600-6143.2009.02869.x [DOI] [PubMed] [Google Scholar]
- 47. Burra P, Samuel D, Sundaram V, Duvoux C, Petrowsky H, Terrault N, et al. Limitations of current liver donor allocation systems and the impact of newer indications for liver transplantation. J Hepatol. 2021;75(Suppl 1):S178–90. 10.1016/j.jhep.2021.01.007 [DOI] [PubMed] [Google Scholar]
- 48. Burra P, Mioni D, Cecchetto A, Cillo U, Zanus G, Fagiuoli S, et al. Histological features after liver transplantation in alcoholic cirrhotics. J Hepatol. 2001;34(5):716–22. 10.1016/s0168-8278(01)00002-2 [DOI] [PubMed] [Google Scholar]
- 49. Neuberger J. Liver transplantation for alcoholic liver disease: what is the risk and consequence of relapse? Dig Dis Sci. 2020;65(6):1600–7. 10.1007/s10620-020-06127-3 [DOI] [PubMed] [Google Scholar]
- 50. Wong RJ, Singal AK. Trends in liver disease etiology among adults awaiting liver transplantation in the United States, 2014‐2019. JAMA Netw Open. 2020;3(2):e1920294. 10.1001/jamanetworkopen.2019.20294 [DOI] [PubMed] [Google Scholar]
- 51. Manzia TM, Trapani S, Nardi A, Ricci A, Lenci I, Milana M, et al. Temporal trends of waitlistings for liver transplantation in Italy: the ECALITA (Evolution of IndiCAtion in LIver transplantation in ITAly) registry study. Dig Liver Dis. 2022;54(12):1664–71. 10.1016/j.dld.2022.08.033 [DOI] [PubMed] [Google Scholar]
- 52. Germani G, Zeni N, Zanetto A, Adam R, Karam V, Belli LS, et al. Influence of donor and recipient gender on liver transplantation outcomes in Europe. Liver Int. 2020;40(8):1961–71. 10.1111/liv.14510 [DOI] [PubMed] [Google Scholar]
- 53. McElroy LM, Likhitsup A, Scott Winder G, Saeed N, Hassan A, Sonnenday CJ, et al. Gender disparities in patients with alcoholic liver disease evaluated for liver transplantation. Transplantation. 2020;104(2):293–8. 10.1097/tp.0000000000002843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cullaro G, Sarkar M, Lai JC. Sex‐based disparities in delisting for being “too sick” for liver transplantation. Am J Transplant. 2018;18(5):1214–9. 10.1111/ajt.14608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Legaz I, Navarro Noguera E, Bolarin JM, Campillo JA, Moya R, Luna A, et al. Patient sex in the setting of liver transplant in alcoholic liver disease. Exp Clin Transpl. 2019;17(3):355–62. 10.6002/ect.2017.0302 [DOI] [PubMed] [Google Scholar]
- 56. Mathurin P, O'Grady J, Carithers RL, Phillips M, Louvet A, Mendenhall CL, et al. Corticosteroids improve short‐term survival in patients with severe alcoholic hepatitis: meta‐analysis of individual patient data. Gut. 2011;60(2):255–60. 10.1136/gut.2010.224097 [DOI] [PubMed] [Google Scholar]
- 57. Mathurin P, Moreno C, Samuel D, Dumortier J, Salleron J, Durand F, et al. Early liver transplantation for severe alcoholic hepatitis. N Engl J Med. 2011;365(19):1790–800. 10.1056/nejmoa1105703 [DOI] [PubMed] [Google Scholar]
- 58. Louvet A, Labreuche J, Moreno C, Vanlemmens C, Moirand R, Feray C, et al. Early liver transplantation for severe alcohol‐related hepatitis not responding to medical treatment: a prospective controlled study. Lancet Gastroenterol Hepatol. 2022;7(5):416–25. 10.1016/s2468-1253(21)00430-1 [DOI] [PubMed] [Google Scholar]
- 59. Germani G, Angrisani D, Addolorato G, Merli M, Mazzarelli C, Tarli C, et al. Liver transplantation for severe alcoholic hepatitis: a multicenter Italian study. Am J Transplant. 2022;22(4):1191–200. 10.1111/ajt.16936 [DOI] [PubMed] [Google Scholar]
- 60. Im GY, Kim‐Schluger L, Shenoy A, Schubert E, Goel A, Friedman SL, et al. Early liver transplantation for severe alcoholic hepatitis in the United States‐‐a single‐center experience. Am J Transplant. 2016;16(3):841–9. 10.1111/ajt.13586 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


