Abstract
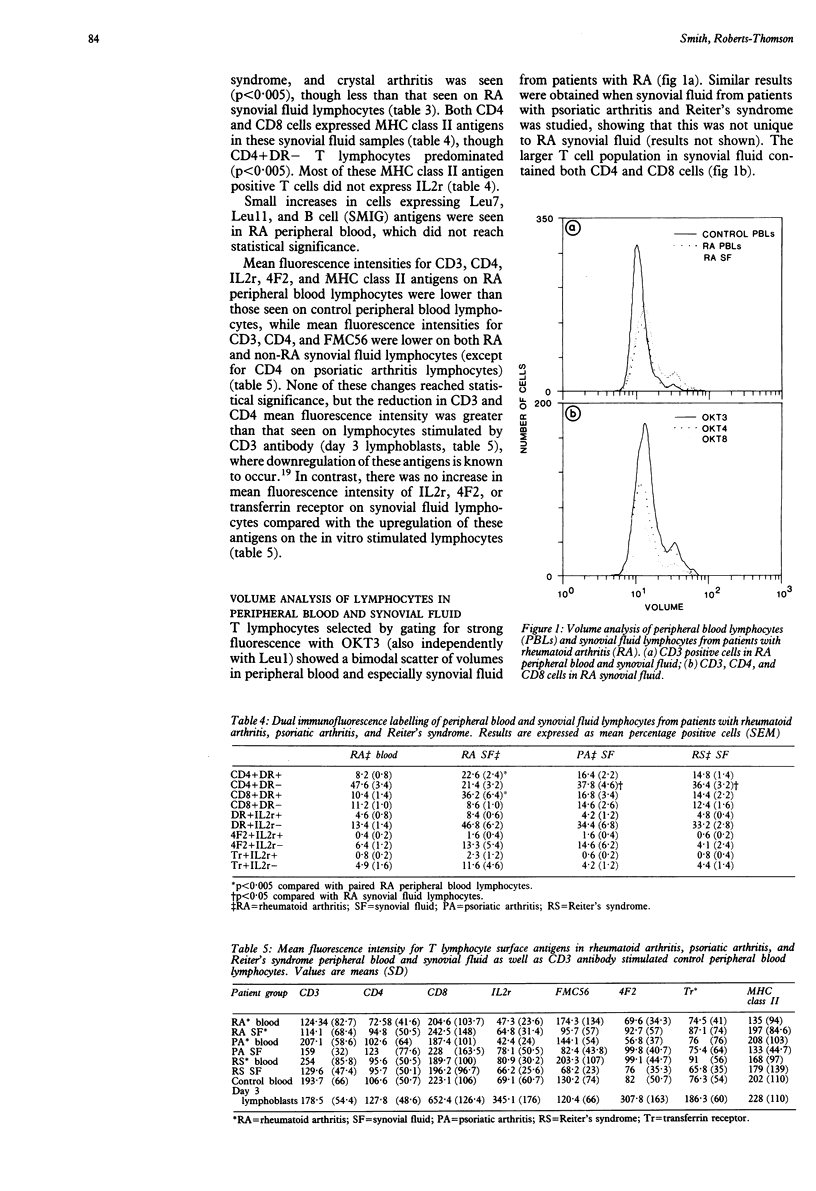
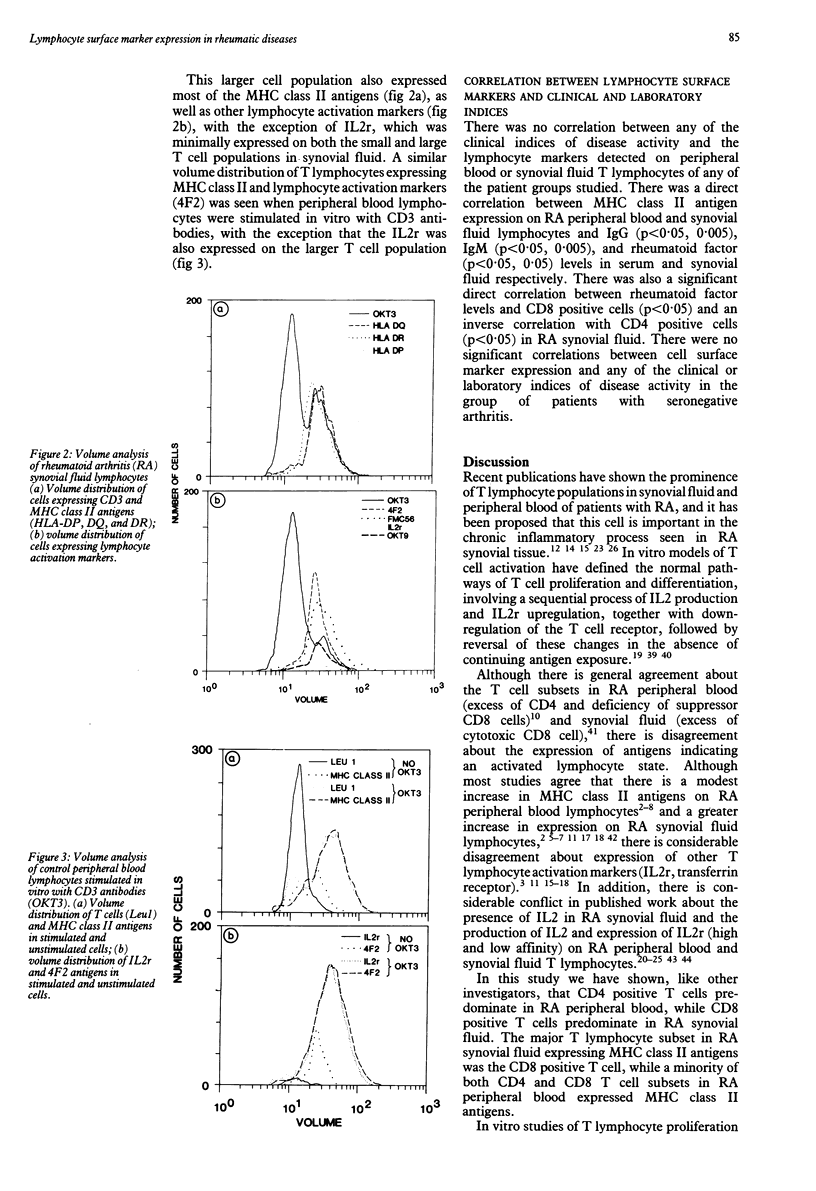
Expression of major histocompatibility complex (MHC) class II and other lymphocyte activation markers on peripheral blood and synovial fluid T lymphocytes from patients with rheumatoid arthritis (RA), psoriatic arthritis, and Reiter's syndrome were measured and the mean fluorescence intensities of these antigens were assessed. Increased expression of MHC class II antigens of synovial fluid T lymphocytes is not unique to RA, though it is quantitatively greater on RA synovial fluid T cells. There was less expression of other lymphocyte activation markers (4F2, transferin receptor) and a marked discordance between the expression of these markers and the interleukin 2 receptor (IL2r). Synovial fluid T lymphocytes contain a subpopulation of larger cells expressing MHC class II and other lymphocyte activation antigens with the exception of the IL2r. Mean fluorescence intensity of CD3 and CD4 antigens on synovial fluid T lymphocytes was decreased in all three patient groups, suggesting prior in vivo exposure of synovial fluid T lymphocytes to an unknown antigen.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- 1958 REVISION of diagnostic criteria for rheumatoid arthritis. Arthritis Rheum. 1959 Feb;2(1):16–20. doi: 10.1002/1529-0131(195902)2:1<16::aid-art1780020104>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Acuto O., Reinherz E. L. The human T-cell receptor. Structure and function. N Engl J Med. 1985 Apr 25;312(17):1100–1111. doi: 10.1056/NEJM198504253121706. [DOI] [PubMed] [Google Scholar]
- Beckman I. G., Bradley J., Brooks D., Zola H. Delineation of serologically distinct monomorphic determinants of human MHC class II antigens: evidence of heterogeneity in their topographical distribution. Mol Immunol. 1984 Mar;21(3):205–214. doi: 10.1016/0161-5890(84)90075-0. [DOI] [PubMed] [Google Scholar]
- Bertouch J. V., Roberts-Thomson P. J., Brooks P. M., Bradley J. Lymphocyte subsets and inflammatory indices in synovial fluid and blood of patients with rheumatoid arthritis. J Rheumatol. 1984 Dec;11(6):754–759. [PubMed] [Google Scholar]
- Brooks D. A., Zola H., McNamara P. J., Bradley J., Bradstock K. F., Hancock W. W., Atkins R. C. Membrane antigens of human cells of the monocyte/macrophage lineage studied with monoclonal antibodies. Pathology. 1983 Jan;15(1):45–52. doi: 10.3109/00313028309061401. [DOI] [PubMed] [Google Scholar]
- Burmester G. R., Jahn B., Gramatzki M., Zacher J., Kalden J. R. Activated T cells in vivo and in vitro: divergence in expression of Tac and Ia antigens in the nonblastoid small T cells of inflammation and normal T cells activated in vitro. J Immunol. 1984 Sep;133(3):1230–1234. [PubMed] [Google Scholar]
- Burmester G. R., Yu D. T., Irani A. M., Kunkel H. G., Winchester R. J. Ia+ T cells in synovial fluid and tissues of patients with rheumatoid arthritis. Arthritis Rheum. 1981 Nov;24(11):1370–1376. doi: 10.1002/art.1780241106. [DOI] [PubMed] [Google Scholar]
- Combe B., Andary M., Klein B., Clot J., Sany J. Regulation of interleukin-2 production in rheumatoid arthritis. J Rheumatol. 1987 Apr;14(2):226–229. [PubMed] [Google Scholar]
- Combe B., Pope R. M., Fischbach M., Darnell B., Baron S., Talal N. Interleukin-2 in rheumatoid arthritis: production of and response to interleukin-2 in rheumatoid synovial fluid, synovial tissue and peripheral blood. Clin Exp Immunol. 1985 Mar;59(3):520–528. [PMC free article] [PubMed] [Google Scholar]
- Cush J. J., Lipsky P. E. Phenotypic analysis of synovial tissue and peripheral blood lymphocytes isolated from patients with rheumatoid arthritis. Arthritis Rheum. 1988 Oct;31(10):1230–1238. doi: 10.1002/art.1780311003. [DOI] [PubMed] [Google Scholar]
- Duke O., Panayi G. S., Janossy G., Poulter L. W. An immunohistological analysis of lymphocyte subpopulations and their microenvironment in the synovial membranes of patients with rheumatoid arthritis using monoclonal antibodies. Clin Exp Immunol. 1982 Jul;49(1):22–30. [PMC free article] [PubMed] [Google Scholar]
- Duke O., Panayi G. S., Janossy G., Poulter L. W., Tidman N. Analysis of T cell subsets in the peripheral blood and synovial fluid of patients with rheumatoid arthritis by means of monoclonal antibodies. Ann Rheum Dis. 1983 Aug;42(4):357–361. doi: 10.1136/ard.42.4.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery P., Wood N., Gentry K., Stockman A., Mackay I. R., Bernard O. High-affinity interleukin-2 receptors on blood lymphocytes are decreased during active rheumatoid arthritis. Arthritis Rheum. 1988 Sep;31(9):1176–1181. doi: 10.1002/art.1780310914. [DOI] [PubMed] [Google Scholar]
- Fox R. I., Fong S., Sabharwal N., Carstens S. A., Kung P. C., Vaughan J. H. Synovial fluid lymphocytes differ from peripheral blood lymphocytes in patients with rheumatoid arthritis. J Immunol. 1982 Jan;128(1):351–354. [PubMed] [Google Scholar]
- Goto M., Miyamoto T., Nishioka K. 2 dimensional flow cytometric analysis of activation antigens expressed on the synovial fluid T cells in rheumatoid arthritis. J Rheumatol. 1987 Apr;14(2):230–233. [PubMed] [Google Scholar]
- Goto M., Miyamoto T., Nishioka K., Okumura K. Selective loss of suppressor T cells in rheumatoid arthritis patients: analysis of peripheral blood lymphocytes by 2-dimensional flow cytometry. J Rheumatol. 1986 Oct;13(5):853–857. [PubMed] [Google Scholar]
- Goto M., Miyamoto T., Nishioka K., Uchida S. T cytotoxic and helper cells are markedly increased, and T suppressor and inducer cells are markedly decreased, in rheumatoid synovial fluids. Arthritis Rheum. 1987 Jul;30(7):737–743. doi: 10.1002/art.1780300703. [DOI] [PubMed] [Google Scholar]
- Haynes B. F., Hemler M. E., Mann D. L., Eisenbarth G. S., Shelhamer J., Mostowski H. S., Thomas C. A., Strominger J. L., Fauci A. S. Characterization of a monoclonal antibody (4F2) that binds to human monocytes and to a subset of activated lymphocytes. J Immunol. 1981 Apr;126(4):1409–1414. [PubMed] [Google Scholar]
- Hemler M. E., Glass D., Coblyn J. S., Jacobson J. G. Very late activation antigens on rheumatoid synovial fluid T lymphocytes. Association with stages of T cell activation. J Clin Invest. 1986 Sep;78(3):696–702. doi: 10.1172/JCI112629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn B., Burmester G. R., Gramatzki M., Weseloh G., Stock P., Kalden J. R. Intraarticular T lymphocytes in monoarticular and oligoarticular inflammatory joint diseases. Normal subset distribution and less numbers of activated T cells indicate major differences as compared to rheumatoid arthritis. J Rheumatol. 1986 Apr;13(2):254–258. [PubMed] [Google Scholar]
- Jahn B., Burmester G. R., Stock P., Rohwer P., Kalden J. R. Functional and phenotypical characterization of activated T cells from intra-articular sites in inflammatory joint diseases. Possible modulation of the CD3 antigen. Scand J Immunol. 1987 Dec;26(6):745–754. doi: 10.1111/j.1365-3083.1987.tb02312.x. [DOI] [PubMed] [Google Scholar]
- Karlsson-Parra A., Svenson K., Hällgren R., Klareskog L., Forsum U. Peripheral blood T lymphocyte subsets in active rheumatoid arthritis--effects of different therapies on previously untreated patients. J Rheumatol. 1986 Apr;13(2):263–268. [PubMed] [Google Scholar]
- Kitas G. D., Salmon M., Farr M., Gaston J. S., Bacon P. A. Deficient interleukin 2 production in rheumatoid arthritis: association with active disease and systemic complications. Clin Exp Immunol. 1988 Aug;73(2):242–249. [PMC free article] [PubMed] [Google Scholar]
- Kluin-Nelemans H. C., van der Linden J. A., Gmelig Meyling F. H., Schuurman H. J. HLA-DR positive T lymphocytes in blood and synovial fluid in rheumatoid arthritis. J Rheumatol. 1984 Jun;11(3):272–276. [PubMed] [Google Scholar]
- Luyten F., Suykens S., Veys E. M., Van Lerbeirghe J., Ackerman C., Mielants H., Verbruggen G. Peripheral blood T lymphocyte subpopulations determined by monoclonal antibodies in active rheumatoid arthritis. J Rheumatol. 1986 Oct;13(5):864–869. [PubMed] [Google Scholar]
- Meijer C. J., de Graaff-Reitsma C. B., Lafeber G. J., Cats A. In situ localization of lymphocyte subsets in synovial membranes of patients with rheumatoid arthritis with monoclonal antibodies. J Rheumatol. 1982 May-Jun;9(3):359–365. [PubMed] [Google Scholar]
- Nordström D., Konttinen Y. T., Bergroth V., Leirisalo-Repo M. Synovial fluid cells in Reiter's syndrome. Ann Rheum Dis. 1985 Dec;44(12):852–856. doi: 10.1136/ard.44.12.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pincus S. H., Clegg D. O., Ward J. R. Characterization of T cells bearing HLA-DR antigens in rheumatoid arthritis. Arthritis Rheum. 1985 Jan;28(1):8–15. doi: 10.1002/art.1780280103. [DOI] [PubMed] [Google Scholar]
- Pitzalis C., Kingsley G., Lanchbury J. S., Murphy J., Panayi G. S. Expression of HLA-DR, DQ and DP antigens and interleukin-2 receptor on synovial fluid T lymphocyte subsets in rheumatoid arthritis: evidence for "frustrated" activation. J Rheumatol. 1987 Aug;14(4):662–666. [PubMed] [Google Scholar]
- Plater-Zyberk C., Rockett K. A., Maini R. N. Spontaneous recovery of the decreased expression in vitro of interleukin 2 receptors in rheumatoid arthritis. Clin Exp Immunol. 1988 Jul;73(1):93–97. [PMC free article] [PubMed] [Google Scholar]
- Ruschen S., Lemm G., Warnatz H. Interleukin-2 secretion by synovial fluid lymphocytes in rheumatoid arthritis. Br J Rheumatol. 1988 Oct;27(5):350–356. doi: 10.1093/rheumatology/27.5.350. [DOI] [PubMed] [Google Scholar]
- Salmon M., Bacon P. A., Symmons D. P., Blann A. D. Transferrin receptor bearing cells in the peripheral blood of patients with rheumatoid arthritis. Clin Exp Immunol. 1985 Nov;62(2):346–352. [PMC free article] [PubMed] [Google Scholar]
- Smith K. A., Cantrell D. A. Interleukin 2 regulates its own receptors. Proc Natl Acad Sci U S A. 1985 Feb;82(3):864–868. doi: 10.1073/pnas.82.3.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. D., Roberts-Thomson P. J. Interleukin 2 and interleukin 2 inhibitors in human serum and synovial fluid. II. Mitogenic stimulation, interleukin 2 production and interleukin 2 receptor expression in rheumatoid arthritis, psoriatic arthritis and Reiter's syndrome. J Rheumatol. 1989 Jul;16(7):897–903. [PubMed] [Google Scholar]
- Sowden J. A., Roberts-Thomson P. J., Zola H. Evaluation of CD5-positive B cells in blood and synovial fluid of patients with rheumatic diseases. Rheumatol Int. 1987;7(6):255–259. doi: 10.1007/BF00270525. [DOI] [PubMed] [Google Scholar]
- Veys E. M., Hermanns P., Goldstein G., Kung P., Schindler J., Van Wauwe J. Determination of T lymphocyte subpopulations by monoclonal antibodies in rheumatoid arthritis. Influence of immunomodulating agents. Int J Immunopharmacol. 1981;3(3):313–319. doi: 10.1016/0192-0561(81)90025-4. [DOI] [PubMed] [Google Scholar]
- Veys E. M., Hermanns P., Schindler J., Kung P. C., Goldstein G., Symoens J., Van Wauwe J. Evaluation of T cell subsets with monoclonal antibodies in patients with rheumatoid arthritis. J Rheumatol. 1982 Jan-Feb;9(1):25–29. [PubMed] [Google Scholar]
- Watson A. J., DeMars R., Trowbridge I. S., Bach F. H. Detection of a novel human class II HLA antigen. 1983 Jul 28-Aug 3Nature. 304(5924):358–361. doi: 10.1038/304358a0. [DOI] [PubMed] [Google Scholar]
- Welte K., Andreeff M., Platzer E., Holloway K., Rubin B. Y., Moore M. A., Mertelsmann R. Interleukin 2 regulates the expression of Tac antigen on peripheral blood T lymphocytes. J Exp Med. 1984 Nov 1;160(5):1390–1403. doi: 10.1084/jem.160.5.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willkens R. F., Arnett F. C., Bitter T., Calin A., Fisher L., Ford D. K., Good A. E., Masi A. T. Reiter's syndrome. Evaluation of preliminary criteria for definite disease. Arthritis Rheum. 1981 Jun;24(6):844–849. doi: 10.1002/art.1780240612. [DOI] [PubMed] [Google Scholar]
- Young C. L., Adamson T. C., 3rd, Vaughan J. H., Fox R. I. Immunohistologic characterization of synovial membrane lymphocytes in rheumatoid arthritis. Arthritis Rheum. 1984 Jan;27(1):32–39. doi: 10.1002/art.1780270106. [DOI] [PubMed] [Google Scholar]
- Zola H., Furness V., Barclay S., Zowtyj H., Smith M., Melo J. V., Neoh S. H., Bradley J. The p24 leucocyte membrane antigen: modulation associated with lymphocyte activation and differentiation. Immunol Cell Biol. 1989 Feb;67(Pt 1):63–70. doi: 10.1038/icb.1989.8. [DOI] [PubMed] [Google Scholar]


