Abstract
Essential oils are highly concentrated natural extracts obtained from plants, rich in bioactive constituents with antimicrobial properties, but the distinctive climate of the Western Himalayan region influences the same. Aromatic and medicinal plants, viz., Origanum majorana, Origanum vulgare, Cymbopogon winterianus, Pelargonium graveolens, and Nepeta cataria were grown in the foothills of the Western Himalayan condition and evaluated for essential oil content, composition, and their effect on some of the most common pathogenic microorganisms. The essential oil content (%) was 0.77, 0.45, 1.37, 0.15 and 0.17% in O. majorana, O. vulgare, C. winterianus, P. graveolens, and N. cataria, respectively. The major essential oil constituents of the isolated oils were terpinen-4-ol, thymol, citronellal, citronellol, and nepetalactone, contributing 41.24%, 31.81%, 43.13%, 43.35% and 91.43% in O. majorana, O. vulgare, C. winterianus, P. graveolens, and N. cataria, respectively. Well-diffusion assay revealed that the essential oil of O. majorana and O. vulgare was active against both the tested Gram-positive, viz., Bacillus subtilis MTCC 121, Micrococcus luteus MTCC 2470, and Staphylococcus aureus MTCC 96; and Gram-negative, viz., Escherichia coli MTCC 43, Klebsiella pneumoniae MTCC 109, and Pseudomonas aeruginosa MTCC 2453 bacteria, while the essential oil of C. winterianus, P. graveolens, and N. cataria showed activity against only some Gram-positive bacteria. Minimum inhibitory concentration (v/v) values indicated the highest efficacy of O. majorana essential oil against B. subtilis (0.5%), M. luteus (1%), and S. aureus (1%), while O. vulgare was most efficient to E. coli (2%) and K. pneumoniae (2%). C. winterianus essential oil did not inhibit any bacterial strains. M. luteus was susceptible to the essential oil of P. graveolens (1%) and N. cataria (0.5%) at low concentrations. Present findings showed the association between the chemical constituents’ profile of isolated essential oils from the Himalayan region and their antimicrobial activity, indicating their perspective to be utilized as antibacterial means.
Subject terms: Biochemistry, Microbiology, Plant sciences
Introduction
The geographic location of the Indian Western Himalayan region is hot to sub-humid tropical in the southern tracks, warm-temperate to cool-temperate and cold alpine (2400–4800 m) mountain ranges in northern and eastern region. The region is rich in geological variability, biodiversity, and abundance of plant species. Usually, the geographical change and growing location of the plant affect the biosynthesis of chemical constituents and must be an important aspect in determining the content and chemical profile of essential oil1. The essential oils are fragrant fluids isolated from diverse portions of aromatic plants, potently producing distinctive plant taste or aroma. Essential oils play a well-known role in the food and perfume industry as flavoring and fragrance agents. More than 3000 essential oils are well-known, among which about 300 are of industrial connotation. Essential oils are a composite mixture of terpenes (monoterpenes and sesquiterpenes) and oxygenated derivatives2. The worth of a specific essential oil relies on the essential oil yield percentage, production rate, and demand. Essential oil yield varies in a broad range, i.e. 0.05–18.0% among various plant species3 and composition is a result of diverse compounds, even though in numerous plant species, only a single component may prevail over the others4. Aromatic plants have recently been reviewed for increased consideration by the research and scientific group because of their biological activities, viz., antimicrobial, and antioxidant properties, utilization in food, cosmetics, and perfumery industries5. In recent times, there has been a considerable increase in the market and utilization of many herbal plants, including Origanum majorana L. (marjoram), Origanum vulgare L. (oregano), Cymbopogon winterianus Jowitt ex Bor (Java citronella), Pelargonium graveolens L'Hér. (rose scented geranium), and Nepeta cataria L. (catnip). Since antiquity, Origanum species have been utilized in medicines and as spices primarily due to their essential oils, which contain a significant amount of two essential oil constituents, i.e. carvacrol and thymol. The other constituents in significant quantities include γ-terpinene, terpinene, cis-sabinene hydrate, and sabinene, which are being utilized in the food and medicine industry6. O. majorana has enormous industrial potential owing to its potent properties (antioxidant and antimicrobial) against various fungal and bacterial infections7–9. A few constituents, viz., cis-sabinene hydrate, terpinen-4-ol, and α-terpineol showed anti-inflammatory, antimicrobial, and anticancerous properties10. The O. vulgare plants possess thymol, p-cymene and γ-terpinene as major essential oil constituents11 while carvacrol, (Z)-α-bisabolene, caryophyllene oxide, linalyl acetate, and (E)-β-caryophyllene were recognized as the key components of O. vulgare essential oil12. Moreover, aromatic grass, i.e. java citronella is utilized for its essential oil which is rich in citronellal, citronellol, and geraniol being utilized in soap, incense, candles, perfumery, cosmetic, and flavouring industries; also has wider applications in pharmaceuticals13. Citronella essential oil chiefly contains monoterpenes with major contribution of aldehydes and alcohols14,15. The plant possesses a broad range of ethnopharmacological properties that rationalize its utilization in cosmetics, pest control, or as an anti-inflammatory agent16 and also possesses anti-inflammatory, antimicrobial, and antioxidant properties17,18. P. graveolens is an important scented aromatic plant that grows around the world for its essential oil, which is considered among the top 20 essential oils of the globe19. Furthermore, pelargonium essential oil is well-recognized for antimicrobial, anti-inflammatory, and anti-fungal properties20,21. The major constituents in P. graveolens essential oil are citronellol, geraniol, and 10-epi-γ-eudesmol22. N. cataria, a tropical aromatic plant, and its aerial parts (leaves and flowers) are utilized in the preparation of soup, sauce, and cheese. The medicinal usefulness of Nepeta species is generally ascribed towards the occurrence of flavonoids and volatile essential oils23. N. cataria essential oil is dominant with the presence of a constituent, i.e. 4a-α, 7-α, 7a-β nepetalactone (53.87%)24.
Several researchers have reported the compositional profile of essential oils from diverse regions around the globe. The composition varies depending on region, climatic conditions, grown variety, extraction/analytical methods, and vegetation period25. The utilization of man-made antioxidants, viz., butylated hydroxytoluene (BHT) and butylated hydroxyanisole (BHA), in the preservation of food has been reported to produce a few harmful effects26. Currently, the use of natural extracts has increased because of their potential to improve individual health and prevent pathologies, cancer, and atherosclerosis8,27. Several research studies have been carried out for essential oil composition determination in diverse aromatic and medicinal crops28.
The increasing interest in the replacement of man-made antimicrobial means with natural ones has fueled research activities on natural resources and essential oils, which have reported the antimicrobial activity to a varied extent29–31. Previous research has found that essential oils have antibacterial properties against animal/human pathogens, poisoning/spoilage of food, and plant bacterial pathogens32,33. Furthermore, the search for new antimicrobial agents has been necessitated because of the resistance developed to the presently available antimicrobial chemicals. There is a dearth of literature examining the production and antimicrobial potential of economically important aromatic crops grown under Western Himalayan conditions. Besides this, essential oil content and composition are also reported to be significantly affected by environmental conditions34,35, climate36, and growing conditions37. Therefore, the hypothesis of this study was that the essential oil content, profile, and bioactivity of economically important aromatic crops grown in the unique environmental conditions of the Western Himalayan region would be distinctive with varied constituent profiles and eventually lead to antimicrobial activity.
Materials and methods
Plant materials
Five plant species: O. majorana O. vulgare, C. winterianus, P. graveolens, and N. cataria were grown in the Western Himalayan region and experimental observation were recorded during 2020–2021 at an experimental farm of the Council of Scientific and Industrial Research-Institute of Himalayan Bioresource Technology, Palampur, India situated 1325 m above mean sea level (amsl) altitude (32°11′39"N latitude and 76°56′51"E longitude). The cultivated plants’ harvesting was done at the stage of their maturity. The experimental field study complies with relevant institutional guidelines and carried out in accordance with relevant regulations. The plant material identification was carried out by Dr Vikas Kumar (Plant Taxonomist) of the Environmental Technology Division of CSIR-IHBT. The specimens of O. majorana and O. vulgare have been deposited in the herbarium repository of CSIR-IHBT, Palampur, with voucher numbers 22070 and 22071, respectively. Variety Jor Lab C-5 of Java citronella (C. winterianus), and selections of Rose geranium (P. graveolens) selection IHBT/PG-1, catnip (N. cataria) selection IHBT/NC-1 were used in the present study and not deposited in herbarium repository of CSIR-IHBT as the plants were non native to Himalayan region and cultivated for commercial cultivation potential under homogeneous environmental conditions.
Essential oil isolation and GC and GC/MS analysis
The plant parts (aerial) were harvested, and the material was brought for essential oil extraction to the laboratory. The essential oil extraction was initiated by hydrodistillation in a Clevenger-type apparatus by following a method similar to an earlier study38. GC and GC–MS analysis of essential oil of aromatic plants was initiated by a Shimadzu GC-2010 gas chromatograph (Shimadzu, Tokyo, Japan) which was connected with a flame ionization detector (FID) and a capillary column. The used column, carrier gas, flow rate, injector temperature, acquisition mass range, ionization energy, oven temperature conditions, and essential oil constituents’ quantification was done by a similar method as followed by39. Essential oil constituents’ identification was carried out by calculating retention indices (RI) by using a homologous hydrocarbons (C8–C24) series and peak area percentage in the chromatogram. The constituent’s identification was completed by comparing the RIs (calculated and literature) and mass fragmentation with the library database of NIST-MS (National Institute of Standards and Technology) Version 3.0240,41. The test samples were run in triplicates, and final observations were presented as mean ± standard deviation. The identified constituents were then analyzed in multivariate principal component analysis (PCA) software i.e. PAST3 (Paleontological Statistics Software Package for Education and Data Analysis version 3.2242 to evaluate the association of grown aromatic crops and essential oil constituents.
Antibacterial assay
The essential oils of O. vulgare, O. majorana, C. winterianus, P. graveolens, and N. cataria were assessed for antibacterial activity against some bacterial opportunistic pathogens following the well-diffusion43 and broth microdilution methods44,45. The bacterial strains comprised of Gram-positive Micrococcus luteus MTCC 2470, Bacillus subtilis MTCC 121, and Staphylococcus aureus MTCC 96, and Gram-negative Klebsiella pneumoniae MTCC 109, Escherichia coli MTCC 43, and Pseudomonas aeruginosa MTCC 2453. The growing of broth temperature conditions, essential oil concentrations added into the holes punched, positive and negative control, essential oil diffusion and incubation duration and temperature were similar as used earlier31. The inhibition zones were recorded as per the earlier reported method46. The tests were run in triplicates, and final readings were presented as mean ± standard deviation. The minimum inhibitory concentration (MIC) values of the essential oil against the test strains were examined in a 96-well microtitre plate, where each essential oil was set in the concentration ranges of 8–0.125%. To the 50 µL of the essential oil, 50 µL of bacterial inoculum was added and kept at 37 °C for 12–24 h. This was followed by the addition of 30 µL resazurin (0.015%) and further incubation for 2–4 h. The MIC was considered the lowest concentration that showed no visible colour change from blue to pink.
Results and discussion
Climate and weather conditions
The climatic conditions of the experimental site were sub temperate sub humid, with a clay loam texture of the soil. Weather parameters, viz., temperature (°C) (minimum and maximum), relative humidity (RH%), and average bright sunshine (BSS) (hours) for two years of crop growth cycle were obtained from meteorological observatory of Chaudhary Sarwan Kumar Himachal Pradesh Agricultural University, Palampur, HP, through “Crop weather outlook”47 and are illustrated in Fig. 1. The mean maximum and minimum temperatures ranged from 3 to 28 and 3 to 29 °C during 2020 and 2021, respectively. Relative humidity was 54 to 90% and 50 to 89%, while BSS ranged from 5 to 10 and 4 to 9 h during 2020 and 2021, respectively. The maximum rainfall was recorded during August (673 mm) and July (1104 mm), while no rainfall was recorded during October and November in 2020 and 2021, respectively.
Figure 1.
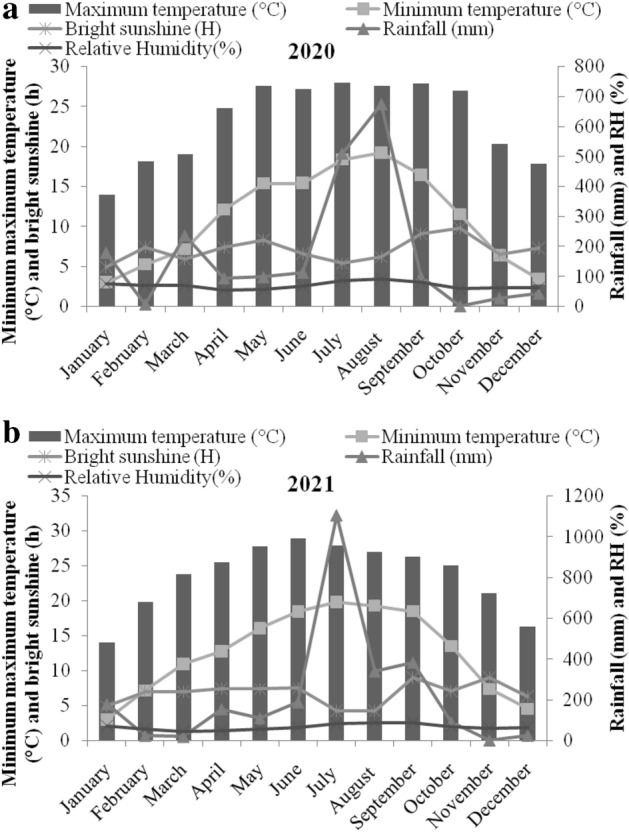
Mean weather conditions during the growth season of aromatic crops (a) 2020 and (b) 2021 at experimental site, Palampur, HP, India.
Content and composition of essential oil
The essential oil content obtained from various aromatic plants is detailed in Fig. 2. The essential oil content of O. majorana, O. vulgare, C. winterianus, P. graveolens, and N. cataria was 0.77 ± 0.01, 0.45 ± 0.01, 1.37 ± 0.01, 0.15 ± 0.01, and 0.17 ± 0.01%, respectively. The major essential oil constituents obtained through hydro distillation are detailed in Table 1. The constituents contributing to more than 5% essential oil area in O. majorana were sabinene (5.4 ± 0.01%), β-myrecene (4.92 ± 0.19%), α-terpinene (4.02 ± 0.09%), α-phellandrene (4.13 ± 0.17%), cis-sabinene hydrate (10.26 ± 0.28%), terpinolene (5.02 ± 0.10%), 2-cyclohexen-1-ol (15.54 ± 0.46%), terpinen-4-ol (31.81 ± 0.34%), and caryophyllene (4.46 ± 0.04%). In marjoram essential oil, the key component (Table 1) was terpinen-4-ol (31.81 ± 0.34) followed by 2-cyclohexen-1-ol, cis-sabinene hydrate, sabinene, and terpinolene contributing for more than 5% of essential oil area while β-myrcene, α-terpinene, α-phellandrene, and caryophyllene contributed less than 5% in essential oil area. Terpinen-4-ol with cis-sabinene hydrate is accountable for the distinguishing fragrance and flavour; additionally, terpinene (α and γ) and terpinolene were other main components while carvacrol and thymol were present in lesser amounts9. Earlier study reported terpinen-4-ol as a major essential oil component while linalool, α-terpinene, α -terpinolene, α -terpineol, β-caryophyllene, α -terpinene, and spathulenol as minor constituents in marjoram9. Similarly, terpinen-4-ol followed by cis-sabinene hydrate was detailed as the foremost essential oil constituents of marjoram essential oil48,49. The essential oil constituents in O. vulgare were α-phellandrene (2.28 ± 0.02%), cis-sabinene hydrate (27.48 ± 0.17%), terpinen-4-ol (14.62 ± 0.12%), and thymol (43.13 ± 0.97%). The major essential oil of O. vulgare was thymol contributed to 43.13 ± 0.97% in the oregano essential oil followed by cis-sabinene hydrate and terpinen-4-ol. The present findings were similar to earlier report with thymol49 as the major essential oil component, while another recorded carvacrol as the major component50. The essential oil chemical profile of oregano is not homogeneous, as it includes two main chemotypes (thymol and carvacrol rich), while intermediate types contain both thymol and carvacrol. Oregano in the present study was rich in thymol and thus considered a thymol chemotype. The types with high content of p-cymene and γ-terpinene have also been identified in oregano of different origins51. Based on essential oil components, the oregano is divided in different categories: p-cymene > 14% and/or thymol > 6% is found only in Greek oregano, while borneol > 2% content was found only in Turkish oregano52,53.
Figure 2.
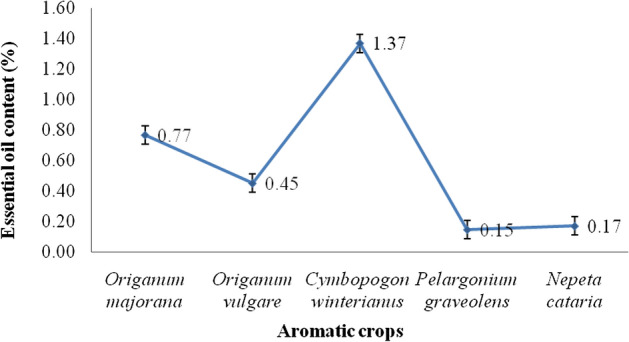
Essential oil content of aromatic medicinal plants grown in the mid hill conditions of the western Himalaya.
Table 1.
The main volatile essential oil compounds in aromatic medicinal plants grown in the mid hill conditions of the western Himalaya.
| Essential oil compounds | RI | Aromatic medicinal plants | Identification methods | |||||
|---|---|---|---|---|---|---|---|---|
| Experimental | Literature | Origanum majorana | Origanum vulgare | Cymbopogon winterianus | Pelargonium graveolens | Nepeta cataria | ||
| Area (%) | ||||||||
| α-Thujene | 925 | 924 | 0.76 ± 0.03 | 1.77 ± 0.01 | – | – | – | RI, GC, GC/MS |
| α-Pinene | 934 | 932 | 0.64 ± 0.01 | 0.83 ± 0.01 | – | – | – | RI, GC, GC/MS |
| Sabinene | 973 | 969 | 5.4 ± 0.01 | – | – | – | – | RI, GC, GC/MS |
| β-Pinene | 979 | 974 | 1.70 ± 0.01 | – | – | – | – | RI, GC, GC/MS |
| β-Myrcene | 986 | 988 | 4.92 ± 0.19 | 1.69 ± 0.01 | – | – | – | RI, GC, GC/MS |
| α-Terpinene | 1017 | 1014 | 4.02 ± 0.03 | 0.59 ± 0.01 | – | – | – | RI, GC, GC/MS |
| Limonene | 1030 | 1024 | – | 2.76 ± 0.01 | – | – | RI, GC, GC/MS | |
| α-Phellandrene | 1026 | 1025 | 4.13 ± 0.17 | 2.28 ± 0.02 | – | 0.53 ± 0.11 | – | RI, GC, GC/MS |
| cis-Sabinene hydrate | 1030 | 1065 | 10.26 ± 0.28 | 27.48 ± 0.17 | – | – | – | RI, GC, GC/MS |
| Terpinolene | 1074 | 1086 | 5.02 ± 0.10 | – | – | – | – | RI, GC, GC/MS |
| Linalool | 1102 | 1095 | – | – | 0.72 ± 0.01 | 1.18 ± 0.01 | – | RI, GC, GC/MS |
| trans-Sabinene hydrate | 1086 | 1098 | 1.87 ± 0.76 | – | – | – | RI, GC, GC/MS | |
| Rose oxide B | 1113 | 1106 | – | – | – | 2.54 ± 0.05 | – | RI, GC, GC/MS |
| 2-Cyclohexen-1-ol | 1104 | 1118 | 15.54 ± 0.46 | – | – | 0.89 ± 0.11 | – | RI, GC, GC/MS |
| Menthone | 1132 | 1148 | – | – | – | – | – | RI, GC, GC/MS |
| Citronellal | 1155 | 1148 | – | – | 41.24 ± 0.37 | – | – | RI, GC, GC/MS |
| Menthone < iso- > | 1149 | 1158 | – | – | – | – | – | RI, GC, GC/MS |
| Terpinen-4-ol | 1185 | 1174 | 31.81 ± 0.34 | 14.62 ± 0.12 | – | – | – | RI, GC, GC/MS |
| Menthan-2-one < cis-ρ- > | 1178 | 1194 | – | – | – | 12.67 ± 0.06 | – | RI, GC, GC/MS |
| Citronellol | 1230 | 1223 | – | – | 9.91 ± 0.02 | 43.35 ± 0.48 | – | RI, GC, GC/MS |
| Geraniol | 1256 | 1249 | – | – | 16.8 ± 2.65 | – | – | RI, GC, GC/MS |
| Phenol | 1273 | 1264 | – | – | 0.51 ± 0.12 | – | – | RI, GC, GC/MS |
| Citronellyl formate | 1277 | 1271 | – | – | – | 20.99 ± 1.73 | – | RI, GC, GC/MS |
| Citral < dimethoxy-(Z)- > | 1305 | 1316 | – | – | – | 0.54 ± 0.21 | – | RI, GC, GC/MS |
| Thymol | 1296 | 1289 | – | 43.13 ± 0.97 | – | – | – | RI, GC, GC/MS |
| Citronellyl acetate | 1348 | 1350 | – | – | 2.90 ± 0.05 | – | – | RI, GC, GC/MS |
| Neryl acetate | 1378 | 1359 | – | – | 2.66 ± 0.57 | – | – | RI, GC, GC/MS |
| Nepetalactone < 4aα,7α,7aβ- > | 1380 | 1386 | – | – | – | 91.43 ± 0.30 | RI, GC, GC/MS | |
| β-Elemene | 1388 | 1389 | – | – | 2.07 ± 0.40 | – | – | RI, GC, GC/MS |
| Caryophyllene | 1417 | 1408 | 4.46 ± 0.04 | – | – | – | – | RI, GC, GC/MS |
| Citronellyl propanoate | 1440 | 1444 | – | – | – | 2.26 ± 0.10 | 2.25 ± 0.02 | RI, GC, GC/MS |
| Germacrene D | 1482 | 1484 | – | – | 1.18 ± 0.03 | – | – | RI, GC, GC/MS |
| β-Selinene | 1498 | 1489 | – | – | 0.67 ± 0.45 | – | – | RI, GC, GC/MS |
| γ-Cadinene | 1519 | 1513 | – | – | 1.58 ± 0.02 | – | – | RI, GC, GC/MS |
| Citronellyl butanoate | 1526 | 1530 | – | – | 1.58 ± 0.10 | – | RI, GC, GC/MS | |
| Elemol | 1554 | 1548 | – | – | 5.34 ± 0.20 | – | RI, GC, GC/MS | |
| Phenyl ethyl tiglate < 2- > | 1597 | 1584 | – | – | – | 0.71 ± 0.48 | – | RI, GC, GC/MS |
| Eudesmol < 10-epi-γ- > | 1624 | 1622 | – | – | – | 2.71 ± 0.27 | - | RI, GC, GC/MS |
| γ-Eudesmol | 1642 | 1630 | – | – | 0.58 ± 0.04 | – | – | RI, GC, GC/MS |
| α-Cadinol | 1653 | 1652 | – | – | 1.37 ± 0.19 | – | – | RI, GC, GC/MS |
| Eudesmol | 1668 | 1661 | – | – | 4.66 ± 0.03 | – | – | RI, GC, GC/MS |
| Citronellyl tiglate | 1668 | 1666 | – | – | – | 1.33 ± 0.09 | – | RI, GC, GC/MS |
RI retention index, GC gas chromatography, GC/MS gas chromatography/mass spectrometry.
The values are mean ± standard deviation.
Similarly, the essential oil constituents identified in C. winterianus were limonene (2.76 ± 0.01%), citronellal (41.24 ± 0.37%), citronellol (9.91 ± 0.02%), geraniol (16.8 ± 2.65%), citronellyl acetate (2.90 ± 0.05%), neryl acetate (2.66 ± 0.57%), elemol (5.34 ± 0.20%), and eudesmol (4.66 ± 0.03%). The earlier studies depicted citronellal, citronellol, and geraniol as major constituents of C. winterianus15. However, the key constituent in C. winterianus essential oil is citronellal which is reported in current findings and gives a distinctive lemongrass aroma to the plant15,54. The composition of essential oil might be varied according to genotype and cultivars; lower percentage of citronellal and higher geraniol content in Medini cultivar of java citronella was recorded in earlier findings13. The major constituents identified in P. graveolens were rose oxide (2.54 ± 0.57%), menthan-2-one < cis-ρ- > (12.67 ± 0.06%), citronellol (43.35 ± 0.48%), citronellyl formate (20.99 ± 1.73%), citronellyl propanoate (2.26 ± 0.10%), and eudesmol < 10-epi-γ- > (2.71 ± 0.27%). The essential oil components of P. graveolens in the present study showed the highest amount of components such as citronellol (43.35 ± 0.48%) followed by menthan-2-one < cis-ρ- > (12.67 ± 0.06%), citronellyl formate (20.99 ± 1.73%), eudesmol < 10-epi-γ- > (2.71 ± 0.27%), rose oxide B (2.54 ± 0.05%), citronellyl propanoate (2.26 ± 0.10%), and citronellyl butanoate (1.58 ± 0.10%) while geraniol content was relatively low (< 0.50% not shown in data). Earlier studies also reported citronellol, geraniol, citronellyl formate, linalool, geranyl formate, menthone, isomenthone, and cis-rose oxide as major essential oil constituents55. However, N. cataria recorded nepetalactone < 4aα,7α,7aβ- > as a major essential oil constituent (91.43 ± 0.30%) followed by citronellyl propanoate (2.25 ± 0.02%) in Western Himalayan growing conditions. It can be noticed that few of the chief constituents were observed in more than one plant, viz., β-myrcene, α-terpinene, α-phellandrene, linalool, 2-cyclohexen-1-ol, terpinen-4-ol, citronellol, nepetalactone < 4aα,7α,7aβ- > , citronellyl propanoate, however, others were particular to specific plant species (Table 1). The essential oil components of N. cataria in the current finding showed the highest amount of nepetalactone < 4aα,7α,7aβ- > (91.43 ± 0.30%) followed by citronellyl propanoate (2.25 ± 0.02%); while the earlier findings reported 4a-α,7-α,7a-β-nepetalactone, 4a-α,7-β,7a-α-nepetalactone, and α-pinene as key essential oil constituents at 1810 m amsl altitude in sandy-loam slightly alkaline soil56.
The climatic and geographical variations produce significant discrepancy in the essential oil profile of aromatic plants grown under different soil and environmental conditions. In present study, terpinen-4-ol was recorded as major constituent of marjoram while in Mediterranean warm climatic condition of Turkey and temperate Argentinean conditions, thymol was the leading contributor to the essential oil57,58. The variations in the essential oil composition in the current study with Argentinean and Turkish condition might be ascribed to unique climatic situations and assorted agro-climatic stipulations of the growing region and acclimatized plants’ metabolism8. Similarly, the two different regions, viz., Nagarjun, Kathmandu (1537 m amsl) and Sanothimi, Bhaktapur (1336 m amsl) at different altitudinal variation in Nepal recorded slight variability in the content of terpinen-4-ol contributing 32.10 and 33.35% in the essential oil of marjoram, thus corroborating the present proposition that there might be a disparity in composition with geographical and atmospheric variability59.
Additionally, the geographical regions variability in Northern and Southern parts of Greece produced lower and higher thymol content, respectively, in essential oil of oregano53. However, carvacrol (70.0–77.4%) was the most dominant compound in neutral (pH 6.7) soils of Germany in a greenhouse setting followed by γ-terpinene and p-cymene60. Similarly, altitude is also an important environmental factor which plays a major role in manipulating the essential oil composition with high thymol at lower elevations and influencing the phenol pathway by thermal variability, thus increasing the total concentration of constituents with increased heat61. The different altitudinal ranges, viz., Auli (2744 m), Pithoragarh (1524 m), and Haldwani (412 m) in Western Himalayan region under natural field conditions specified thymol as the foremost component with higher concentration (52.83%) in intermediate altitude, i.e. 1524 m amsl and lowest in lower altitude62 thus corroborates the present findings with 43.13 ± 0.97% of thymol at an almost similar range of altitude at 1325 m amsl in the Western Himalayan region. The constituents 3'-terpinene and p-cymene (biosynthetic precursors) of carvacrol and thymol in oregano were influenced by environmental factors (thermal) thus influencing the qualitative composition of oregano63. Similarly, the genetic expression also influences the accumulation of key constituents; the dominant allele contributes to carvacrol, while the recessive one accumulates thymol64. Thus, in the present findings, environmental factors such as high bright sunshine hours (Fig. 1a,b) during the complete growth cycle may be responsible for the establishment of thymol-rich oregano. Carvacrol is not recorded in the present finding, which corroborates the earlier findings where thymol is produced at the expense of carvacrol and both varied inversely61. The different bioclimatic and geographical zones reported variation in constituents of oregano with a high concentration of carvacrol, linalyl acetate, (Z)-α-bisabolene, (E)-β-caryophyllene, and caryophyllene oxide in Iranian oregano12.
The growing conditions produced significant variation in essential oil composition; recorded citronellal, geraniol, and citronellol rich java citronella essential oil in Brazilian conditions65. However, java citronella recorded citronellal as major essential oil constituent followed by geraniol and citronellol14; the sequence of occurrence of constituents relative to their quantity is slightly different from current findings; the variability of essential oil might be because of diverse weather conditions in growing region. However, the key constituents were citronellal while citronellol and geraniol in C. winterianus under tropical monsoon climate with an average maximum temperature of 25 °C with hot and cold climate during summer and winter, respectively in Kumaon region, Uttarakhand under Indian Western Himalayan region13 which is comparable to present findings with similar range (30–45%) of major essential oil constituent i.e. citronellal which might be due to related environmental conditions viz., average temperature, sunshine, and rainfall settings (Fig. 1a,b). In contrast, the essential oil produced in much warmer conditions in Andhra Pradesh, India also recorded higher citronellal (50.93%) and comparatively lower citronellol and geraniol possibly be due differences in geographical location, climatic conditions, pedogenetic factors, season, and harvesting time66. Furthermore, the chemical constituents of the plant species differ depending on geographical origin, cultivars, cultivation method, photoperiod, harvest period and plant age67.
However, P. graveolens essential oil recorded in present study is at variance from that of Pauri Garhwal region of North India when citronellol, geraniol, linalool, citronellyl formate, and p-menthone were reported as major components while α-selinene and α-humulene as minor components68. Citronellol (51.0–63.4%) and isomenthone (9.8–17.8%) were reported as major constituents in cultivar “Kelkar”, while geraniol content (0.9% to 2.1%) was comparatively low22, which is corroborating the present findings with negligible geraniol content. The worth of rose scented geranium essential oil in the perfumery industry is primarily determined by C/G (citronellol/geraniol), which differed significantly according to cultivar, region, and location. The essential oil composition is affected by environmental conditions and reported citronellol and geraniol as key constituents of P. graveolens in North of Tunisia at 17 m amsl altitude69 while Egyptian P. graveolens, reported citronellol, trans-geraniol, and 10-epi-γ-eudesmol. In addition, the geraniol content is temperature dependent and generally decreases with falling night temperatures, whereas citronellol increases with a decrease in night temperatures70–72. In contrast, the cv. Kelkar recorded too high C/G ratio (30.19–70.44) in the essential oil; that too during summer, which is because of the presence of a lower geraniol area percentage as both the constituents are inversely related. Usually, rose scented geranium essential oil, which possesses an approximately equal amount of citronellol and geraniol (C/G between 0.5 and 2.0) is regarded as the greatest quality for commercial purposes73. The chemical profile of essential oil for geraniol content with previous findings74,75 showed sharp variability. The external factors, viz., varied developmental stages, harvest/collection times, soil and climatic conditions, cultivation region, geographic origin, or chemotype, may cause variability in essential oil (quantity and quality of constituents) of the identical species at diverse locations76–78. In sandy-loam neutral soils of Iranian conditions, the constituents such as α-pinene, β-pinene, and 4aα,7α,7aβ-nepetalactone were recorded at the full flowering stage of N. cataria25. The findings from Kashan, Iran, at 1550 m amsl, reported similar content of components of N. cataria79 as detected in the present study, which might be due to similar altitudinal and climatic conditions of the current study site. The earlier findings reported 4a-α,7-α,7a-β-nepetalactone, 1,8-cineole, and 4a-α, 7-β, 7a-α-nepetalactone as key constituents in the wild-growing N. cataria in Northern region of Iran80 while nepetalacones, β-caryophyllene and caryophyllene oxide were identified as chief constituents of the essential oil of N. cataria collected from Cordoba province of Argentina81. The quantitative and qualitative differences in essential oil composition may be because of chemotypes, mode of distillation, geographical and climatic factors80. The constituent profile of essential oil might be influenced by the phenological stages and geographical conditions of the growing region32,82, environmental conditions, plant nutritional status, season, and others83. Conversely, a few other studies reported deficiencies in nepetalactones but reported thymol84 and 1,8-cineole85 as the most abundant constituent of the N. cataria essential oil. The chief constituent reported was pinene (α and β) in the essential oil, which showed a gradual increase following the maturation of the plant25. Some of the key essential oil constituents were comparable to a few of the previously reported essential oils isolated from plants but differed from some of the other studies around the world. The geographical and growing location variation affected the biosynthesis of chemical constituents of N. cataria, reported 4aα,7α,7aβ-nepetalactone as a major constituent which has industrial utilization and chemotaxonomic markers in the essential oil.
The most important constituents of essential oils primarily belong to seven chemical groups: monoterpenes (α-thujene, sabinene, α-pinene, β-myrcene, β-pinene, α-terpinene, limonene, α-phellandrene, terpinolene), bicyclic monoterpenoids (cis & trans -sabinene hydrate), acyclic monoterpenoids (linalool), cyclic monoterpenoids (rose oxide b, 2-cyclohexen-1-ol, menthone, menthone, citronellal, isomenthone, menthan-2-one < cis-ρ- > , phenol/2-methoxy-3-(2-propenyl)-, citronellyl formate, citral < dimethoxy-(z)- > , thymol, citronellyl acetate, neryl acetate, nepetalactone < 4aα,7α,7aβ- >), alcoholic monoterpenoids (terpinen-4-ol, citronellol, geraniol), sesquiterpenes (β-elemene, caryophyllene, germacrene D, γ-cadinene, α-cadinol) and sesquiterpenoids (citronellyl propanoate, citronellyl butanoate, elemol, phenyl ethyl tiglate < 2- > , eudesmol < 10-epi-γ- > , γ-eudesmol, citronellyl tiglate) (Table 2). The chemical constituent profile of O. majorana and O. vulgare isolated essential oils remained in harmony with earlier reported studies49,86. The components of C. winterianus, P. graveolens, and N. cataria are similar to the earlier reported studies15,25,51,53,68,87.
Table 2.
The grouped components (area %) of essential oil in aromatic medicinal plants grown in the mid hill conditions of the western Himalaya.
| Grouped components | Aromatic crops | ||||
|---|---|---|---|---|---|
| O. majorana | O. vulgare | C. winterianus | P. graveolens | N. cataria | |
| Monoterpenes | 26.59 ± 0.12 | 7.16 ± 0.04 | 2.76 ± 0.02 | 0.53 ± 0.03 | ‒ |
| Bicyclic monoterpenoids | 12.13 ± 0.05 | 27.48 ± 0.17 | ‒ | ‒ | ‒ |
| Acyclic monoterpenoids | ‒ | ‒ | 0.72 ± 0.03 | 1.18 ± 0.02 | ‒ |
| Cyclic monoterpenoids | 15.54 ± 0.06 | 43.13 ± 0.24 | 49.38 ± 0.36 | 37.63 ± 0.67 | 91.43 ± 0.45 |
| Alcoholic monoterpenoids | 31.81 ± 0.16 | 14.62 ± 0.21 | 26.71 ± 0.27 | 43.35 ± 0.80 | ‒ |
| Sesquiterpene | 4.46 ± 0.02 | ‒ | 3.43 ± 0.03 | ‒ | ‒ |
| Sesquiterpenoids | ‒ | ‒ | 11.95 ± 0.25 | 7.26 ± 0.04 | 2.25 ± 0.02 |
| Total | 90.53 | 92.39 | 94.95 | 89.95 | 93.68 |
The values are mean ± standard deviation.
Antibacterial activity
The well-diffusion assay revealed the strong antibacterial activities of O. majorana and O. vulgare against all the tested Gram-positive strains viz., B. subtilis MTCC 121 (6–14.33 mm), M. luteus MTCC 2470 (8.66–14.66 mm) and S. aureus MTCC 96 (7.5–8.66 mm), and the Gram-negative strains E. coli MTCC 43 (6–10 mm) and K. pneumoniae MTCC 109 (6–11 mm) (Table 3). These essential oils exhibited lesser activity against the Gram-negative P. aeruginosa MTCC 2453 (3.33–5.66 mm). However, only M. luteus and S. aureus fell under the sensitive category towards O. majorana, according to46. Earlier findings reported the efficacy of O. majorana essential oil in opposition to both the Gram-positive and Gram-negative bacteria, with good activities against S. aureus and Bacillus sp59,88. Likewise, the strains M. luteus, B. subtilis, E. coli, and K. pneumoniae may be marked to fall under the sensitive category towards O. vulgare. Similar to our findings, in an earlier report, the O. vulgare essential oil showed higher inhibitory activity against the Gram-positive bacteria than the Gram-negative ones89. On the other hand, C. winterianus and N. cataria showed lower inhibition zones against B. subtilis MTCC 121(2–4 mm) and M. luteus MTCC 2470 (3–3.33 mm), while no activities were observed against any other strains (Table 3). The essential oil of P. graveolens also exhibited very low activity against M. luteus MTCC 2470 (3 mm) and S. aureus MTCC 96 (4 mm) (Table 3). In case of C. winterianus, P. graveolens and N. cataria, the Gram-positive bacterial strains fell under the ‘not sensitive’ category. Also, these essential oils showed no antibacterial activities against the Gram-negative bacteria in the qualitative plate assay (Table 3). The results of well-diffusion assay demonstrate the essential oils of O. majorana and O. vulgare as potential antibacterial agents, among others. The MIC (% v/v) of each of these essential oils for antagonistic activity against the test bacterial strains was determined that show the lowest MIC values of O. majorana against the Gram-positive B. subtilis (0.5%), M. luteus (1%) and S. aureus (1%) (Fig. 3). According to the broth microdilution method, a lower MIC value corresponds to better activity of the test compound90. Among Gram-negative bacteria, E. coli was more sensitive (2% MIC) to O. majorana than K. pneumoniae (4%) and P. aeruginosa (4%) (Fig. 3). In O. vulgare, the Gram-negative Strains E. coli and K. pneumoniae showed higher sensitivity (2% MIC) than P. aeruginosa (4% MIC) and all the tested Gram-positive strains (4% MIC) (Fig. 3). The results signify that the essential oils of O. majorana and O. vulgare showed inhibitory activities against both the Gram-positive and Gram-negative strains. C. winterianus did not show any inhibitory activity in the microdilution assay. Contrary to this, the essential oil of citronella has been reported with bactericidal activity against human pathogenic strains91. Interestingly, M. luteus was found to be more susceptible to P. graveolens (1%) and N. cataria (0.5%) in the microdilution assay. Earlier, P. graveolens essential oil came out to be ineffective against bacterial pathogens at lower concentrations92. B. subtilis also showed susceptibility to N. cataria (1%). N. cataria extracts have been reported to show high inhibitions of B. subtilis and M. luteus as indicated by the MIC values93. It might be inferred from the results that P. graveolens and N. cataria show potent inhibitory activity against some selective Gram-positive pathogenic strains.
Table 3.
Antibacterial activity of the essential oils against Gram-positive and Gram-negative bacterial strains.
| Essential oil | Gram-positive | Gram-negative | ||||
|---|---|---|---|---|---|---|
| Bacillus subtilis MTCC 121 | Micrococcus luteus MTCC2470 | Staphylococcus aureus MTCC96 | Escherichia coli MTCC 43 | Klebsiella pneumoniae MTCC109 | Pseudomonas aeruginosa MTCC 2453 | |
| O. majorana | 6 ± 0 | 8.66 ± 0.5 | 8.66 ± 0.5 | 6 ± 0 | 6 ± 0 | 3.33 ± 0.5 |
| O. vulgare | 14.33 ± 0.5 | 14.66 ± 0.5 | 7.5 ± 0.5 | 10 ± 0 | 11 ± 0 | 5.66 ± 0.5 |
| C. winterianus | 4 ± 0 | 3.33 ± 0.5 | – | – | – | – |
| P. graveolens | – | 3 ± 0 | 4 ± 0 | – | – | – |
| N. cataria | 2 ± 0 | 3 ± 0 | – | – | – | – |
| Streptomycin (1 mg/mL) | 16.33 ± 0.5 | 6 ± 0 | 16.33 ± 0.5 | 16.33 ± 0.5 | 14 ± 0 | 7.66 ± 0.5 |
The values are mean ± standard deviation.
Values are means ± Standard Deviation (SD) of triplicate readings expressed in mm including 6 mm of well diameter.
Figure 3.
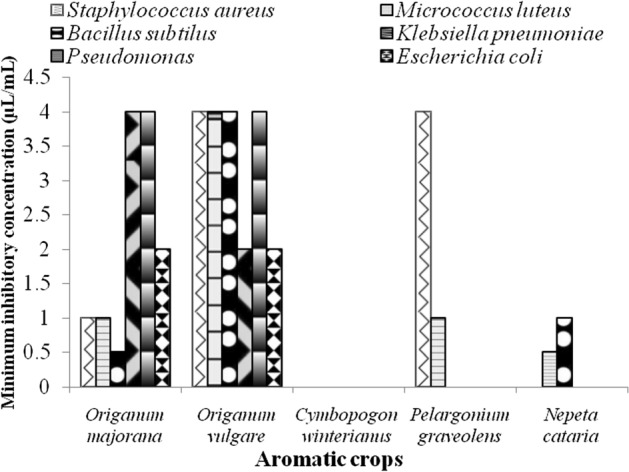
Minimum Inhibitory Concentration (MIC) (% v/v) values of essential oils against the bacterial strains. The experiment was repeated in triplicate on three separate occasions.
Principal component analysis (PCA)
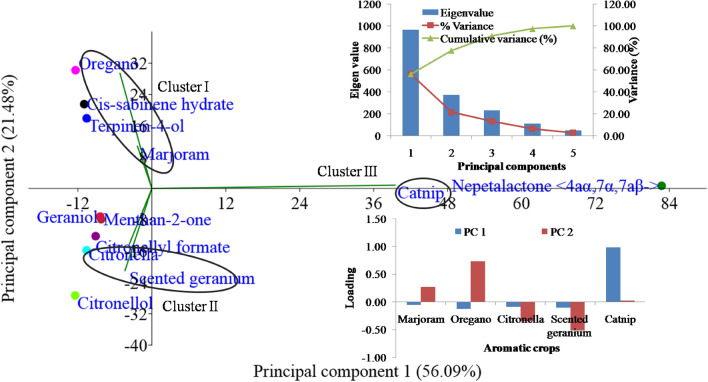
Principal component analysis (PCA) was executed to assess the relation among aromatic crops and their essential oil constituents (Fig. 4). The PC analysis revealed 77.57% of variations elucidated by PC1 and PC2. Among all essential oil constituents, cis-sabinene hydrate, terpinen-4-ol, thymol, and nepetalactone < 4aα,7α,7aβ- > showed a positive association, while citronellal, menthan-2-one, citronellol, geraniol, and citronellyl formate showed negative association in PC2. However, in PC1 only nepetalactone < 4aα,7α,7aβ- > showed a positive association, while the rest of the constituents evidenced a negative association.
Figure 4.
The multivariate analyses of mean value of major compounds of essential oil were conducted through principal component analysis. Principal component 1 and Principal component 2 jointly explained 77.57% of the total variation for aromatic plants grown in the western Himalaya; the eigenvalues and loading scores of the variables with PC1 and PC2 are presented at top right and bottom right corner of the figure, respectively.
PC analysis separated oregano and marjoram crops, making them to be positioned on the same plane, while rose scented geranium and citronella were found to get positioned on the another plane. However, catnip was the only crop that appeared to be distinctive from other studied crops and lies alone in the separate plane (Fig. 4). Nepetalactone < 4aα,7α,7aβ- > was the only constituent that showed positive contribution and strong relationships with both PCs and a major essential oil constituent of catnip. The current study showed that the five PCs were highly illuminating and showed eigen values and thus contributed to about 97.29% of the whole variance of essential oil constituents. The experiential score plot of aromatic crops could be illustrated into three distinct clusters (Fig. 4 and Table 4). Cluster I include 10.26 and 27.48% of cis-sabinene hydrate, 31.81 and 14.62% of terpinen-4-ol in marjoram and oregano, respectively, while 43.13% of thymol only in oregano. Cluster I explained a higher concentration of terpinen-4-ol and thymol in marjoram and oregano, respectively. Cluster II includes crops viz., citronella and rose scented geranium which constitutes about 9.91 and 43.35% citronellol, while 41.24% and 16.80% citronellal and geraniol, respectively, and 20.99% of citronellyl formate only in rose scented geranium. Cluster II included distinctive constituents such as citronellal, menthan-2-one, citronellol, and citronellyl formate which were not observed in other clusters and thus grouped in cluster II. Furthermore, cluster III comprised 91.43% nepetalactone < 4aα,7α,7aβ- > which contributed to the majority of area percentage in essential oil and constituted an independent cluster of the single aromatic crop, i.e. catnip. The PCA separated treatments into three distinct clusters (Fig. 4), Cluster I exhibited cis-sabinene hydrate, terpinen-4-ol, thymol as major constituents, while Cluster II exhibited citronellal, menthan-2-one, citronellol, geraniol, and citronellyl formate. Cluster III was an independent cluster with the highest nepetalactone < 4aα,7α,7aβ- > content. The PC2 separated cis-sabinene hydrate, terpinen-4-ol, and thymol from other constituents and were placed within the positive end of PC2 with 21.54, 17.92, and 30.21 loadings, respectively. However, citronellal, menthan-2-one, citronellol, geraniol, and citronellyl formate were placed in the negative end of PC1 and PC2 with − 10.60 to − 15.81, − 8.23 to − 7.85, 12.45 to 27.33, − 8.38 to − 7.18, − 9.13 to − 12.19 and − 12.39 to 30.21 loading, respectively. However, nepetalactone < 4aα,7α,7aβ- > were placed in the positive end of both PCs with 82.74 and 0.69 loadings, respectively (Fig. 4). A noteworthy disparity in major essential oil profiles was recorded in different aromatic crops grown in the Western Indian Himalayan region, while some similarity was found in some minor constituents.
Table 4.
Clusters variability in essential oil constituents (%) of the grown aromatic crops in the mid hills conditions of western Himalaya.
| Essential oil constituents | Cluster I | Cluster II | Cluster III |
|---|---|---|---|
| Cis-sabinene hydrate | 10.26‒27.48 | 0.00 | 0.00 |
| Citronellal | 0.00 | 0.00‒41.24 | 0.00 |
| Terpinen-4-ol | 14.62‒31.81 | 0.00 | 0.00 |
| Menthan-2-one | 0.00 | 0.00 | 0.00 |
| Citronellol | 0.00 | 9.91‒43.35 | 0.00 |
| Geraniol | 0.00 | 0.00‒16.80 | 0.00 |
| Citronellyl formate | 0.00 | 0.00 | 0.00 |
| Thymol | 0.00 | 0.00 | 0.00 |
| Nepetalactone < 4aα,7α,7aβ- > | 0.00 | 0.00 | 0.00‒91.43 |
Conclusion
The present study suggests that isolated essential oils from five aromatic plants grown in the Western Himalayan region have essential oil quality according to the ISO standards and previously reported studies. Some of the extracted essential oils demonstrated remarkable antibacterial activities against selected opportunistic pathogenic strains. Consequently, these oils may perhaps become alternatives to synthesized antimicrobial compounds in controlling diseases causing agents. Though, more research on the security, safety, and toxicity of the studied essential oils ought to be carried out before further commercial usage (Supplementary Figure A).
Supplementary Information
Acknowledgements
The authors are grateful to the Director, CSIR-IHBT, Palampur, India, for providing necessary facilities during the study period. The authors are also thankful to Mr Kuldip Singh and Mr Shiv Kumar for providing technical support in carrying out the research work. Financial grants from the Council of Scientific and Industrial Research, New Delhi, India, under the CSIR Aroma mission project (HCP-0007 phase- II) are too acknowledged.
Author contributions
S.R.: Data curation, Methodology, Investigation, Formal analysis, Software, Writing- original draft, Visualization, Validation, paper writing, literature review & paper editing. S.M.: Experiment of antimicrobial activity and writing. R.K.: Supervised and finalized antimicrobial work. R.K.: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing—review & editing. The authors have read and agreed to the manuscript publication.
Funding
This research received funding from CSIR Aroma Mission (HCP 0007).
Data availability
The data analyzed during the current study available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-31875-3.
References
- 1.Sanli A, Karadogan T. Geographical impact on essential oil composition of endemic (Kundmannia anatolica) Hub.-Mor. (Apiaceae) Afr. J. Tradit. Complement. Altern. Med. 2016;14:131–137. doi: 10.2110/ajtcam.v14i1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Badawy MEI, Abdelgaleil SAM. Composition and antimicrobial activity of essential oils isolated from Egyptian plants against plant pathogenic bacteria and fungi. Ind. Crops Prod. 2014;52:776–782. doi: 10.1016/j.indcrop.2013.12.003. [DOI] [Google Scholar]
- 3.Sankarikutty B, Narayanan CS. Essential oils/Isolation and production. In: Trugo L, Finglas PM, editors. Encyclopedia of Food Sciences and Nutrition. 2. Academic Press; 2003. pp. 2185–2189. [Google Scholar]
- 4.Cowan MM. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999;12:564–582. doi: 10.1128/CMR.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peter KV, Babu KN. Introduction to herbs and spices: Medicinal uses and sustainable production. In: Peter KV, editor. Handbook of Herbs and Spices. Woodhead Publishing Series in Food Science; 2012. pp. 1–16. [Google Scholar]
- 6.Kimera F, Sewilam H, Fouad WM, Suloma A. Efficient utilization of aquaculture effluents to maximize plant growth, yield, and essential oils composition of Origanum majorana cultivation. Ann. Agric. Sci. 2021;66:1–7. doi: 10.1016/j.aoas.2020.11.002. [DOI] [Google Scholar]
- 7.Ezzeddine NB, Abdelkefi MM, Aissa RB, Chaabouni MM. Antibacterial screening of Origanum majorana L. oil from Tunisia. J. Essent. Oil Res. 2001;13:295–297. doi: 10.1080/10412905.2001.9699698. [DOI] [Google Scholar]
- 8.Sellami IH, Maamouri E, Chahed T, Wannes WA, Kchouk ME, Marzouk B. Effect of growth stage on the content and composition of the essential oil and phenolic fraction of sweet marjoram (Origanum majorana L.) Ind. Crops Prod. 2009;30:395–402. doi: 10.1016/j.indcrop.2009.07.010. [DOI] [Google Scholar]
- 9.Vagi E, Simandi B, Suhajda A, Hethelyi E. Essential oil composition and antimicrobial activity of Origanum majorana L. extracts obtained with ethyl alcohol and supercritical carbon dioxide. Food Res. Int. 2005;38:51–57. doi: 10.1016/j.foodres.2004.07.006. [DOI] [Google Scholar]
- 10.Nakayama K, Murata S, Ito H, Iwasaki K, Villareal MO, Zheng YW, Matsui H, Isoda H, Ohkohchi N. Terpinen-4-ol inhibits colorectal cancer growth via reactive oxygen species. Oncol. Lett. 2017;14:2015–2024. doi: 10.3892/ol.2017.6370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Napoli E, Giovino A, Carrubba A, Siong VHY, Rinoldo C, Nina O, Ruberto G. Variations of essential oil constituents in oregano (Origanum vulgare subsp. Viridulum (= O. heracleoticum) over cultivation cycles. Plants. 2020;9(1174):1–23. doi: 10.3390/plants9091174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morshedloo MR, Salami SA, Nazeri V, Maggi F, Craker L. Essential oil profile of oregano (Origanum vulgare L.) populations grown under similar soil and climate conditions. Ind. Crops Prod. 2018;119:183–190. doi: 10.1016/j.indcrop.2018.03.049. [DOI] [Google Scholar]
- 13.Verma RS, Rahman LU, Verma RK, Chauhan A, Singh A, Kukreja AK, Khanuja SPS. Qualitative performance of Java citronella (Cymbopogon winterianus Jowitt) cultivars in Kumaon Himalaya. J. Med. Aromat. Plant Sci. 2008;31:321–325. [Google Scholar]
- 14.Kakaraparthi PS, Srinivasa KVNS, Kumar JK, Kumar AN, Rajput DK, Sarma VUM. Variation in the essential oil content and composition of Citronella (Cymbopogon winterianus Jowitt.) in relation to time of harvest and weather conditions. Ind. Crops Prod. 2014;61:240–248. doi: 10.1016/j.indcrop.2014.06.044. [DOI] [Google Scholar]
- 15.Simic A, Rancic A, Sokovic MD, Ristic M, Grujic-Jovanovic S, Vukojevic J, Marin PD. Essential oil composition of Cymbopogonwinterianus. and Carumcarvi. and their antimicrobial activities. Pharm. Biol. 2008;46:437–441. doi: 10.1080/13880200802055917. [DOI] [Google Scholar]
- 16.Avoseh O, Oyedeji O, Rungqu P, Nkeh-Chungag B, Oyedeji A. Cymbopogon species; ethnopharmacology, phytochemistry and the pharmacological importance. Molecules. 2015;20:7438–7453. doi: 10.3390/molecules20057438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Staudt A, Duarte PF, Amaral BPD, Andrade BCDOP, Simas NK, Leal ICR, Sangenito LS, Santos ALSD, Oliveira DD, Junges A, Cansian RL, Paroul N. Biological properties of functional flavoring produced by enzymatic esterification of citronellol and geraniol present in Cymbopogon winterianus essential oil. Nat. Prod. Res. 2021;35:5981–5987. doi: 10.1080/14786419.2020.1810032. [DOI] [PubMed] [Google Scholar]
- 18.Tavares LA, Rezende AA, Santos JL, Estevam CS, Silva AMO, Schneider JK, Cunha JLS, Droppa-Almeida D, Correia-Neto IJ, Cardoso JC, Severino P, Souto EB, de Albuquerque-Junior RLC. Cymbopogon winterianus essential oil attenuates bleomycin-induced pulmonary fibrosis in a Murine Model. Pharmaceutics. 2021;13:679. doi: 10.3390/pharmaceutics13050679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cavar S, Maksimovic M. Antioxidant activity of essential oil and aqueous extract of Pelargonium graveolens L’Her. Food Control. 2012;23:263–267. doi: 10.1016/j.foodcont.2011.07.031. [DOI] [Google Scholar]
- 20.Boukhatem MN, Kameli A, Saidi F. Essential oil of Algerian rose scented geranium (Pelargonium graveolens): Chemical composition and antimicrobial activity against food spoilage pathogens. Food Control. 2013;34:208–213. doi: 10.1016/j.foodcont.2013.03.045. [DOI] [Google Scholar]
- 21.Abers M, Schroeder S, Goelz L, Sulser A, Rose TS, Puchalski K, Langland J. Antimicrobial activity of the volatile substances from essential oils. BMC Complement. Med. Ther. 2021;21:1–14. doi: 10.1186/s12906-021-03285-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moyo M, Van Staden J. Medicinal properties and conservation of Pelargonium sidoides DC. J. Ethonopharmacol. 2014;152:243–255. doi: 10.1016/j.jep.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verma RS, Rahman L, Verma RK, Chauhan A, Singh A. Essential oil composition of Pelargonium graveolens L’Her ex Ait. cultivars harvested in different seasons. J. Essent. Oil Res. 2013;25:372–379. doi: 10.1080/10412905.2013.782476. [DOI] [Google Scholar]
- 24.Jamzad Z, Chase MW, Ingrouille M, Simmonds MS, Jalili A. Phylogenetic relationships in Nepeta L. (Lamiaceae) and related genera based on ITS sequence data. Taxon. 2003;52:21–32. doi: 10.2307/3647299. [DOI] [Google Scholar]
- 25.Ashrafi B, Ramak P, Ezatpour B, Talei GR. Biological activity and chemical composition of the essential oil of Nepeta cataria L. J. Res. Pharm. 2019;23:336–343. doi: 10.12991/jrp.2019.141. [DOI] [Google Scholar]
- 26.Zomorodian K, Saharkhiz MJ, Shariati S, Pakshir K, Rahimi MJ, Khashei R. Chemical composition and antimicrobial activities of essential oils from Nepeta cataria L. against common causes of food-borne infections. ISRN Pharm. 2012;591953:1–6. doi: 10.5402/2012/591953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Namiki M. Antioxidants/antimutagens in food. Crit. Rev. Food Sci. Nutr. 1990;29:273–300. doi: 10.1080/10408399009527528. [DOI] [PubMed] [Google Scholar]
- 28.Tapiero H, Tew K, Nguyen Ba G, Mathe G. Polyphenols: do they play a role in the prevention of human pathologies? Biomed. Pharmacother. 2002;56:200–207. doi: 10.1016/s0753-3322(02)00178-6. [DOI] [PubMed] [Google Scholar]
- 29.Edris AE, Shalaby A, Fadel HM. Effect of organic agriculture practices on the volatile aroma components of some essential oil plants growing in Egypt II: Sweet Marjoram (Origanum marjorana L.) essential oil. Flavour Fragr. J. 2003;18:345–351. doi: 10.1002/ffj.123. [DOI] [Google Scholar]
- 30.Man A, Santacroce L, Jacob R, Mare A, Man L. Antimicrobial activity of six essential oils against a group of human pathogens: A comparative Study. Pathogens. 2019;8:1–11. doi: 10.3390/pathogens8010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rathore S, Mukhia S, Kapoor S, Bhatt V, Kumar R, Kumar R. Seasonal variability in essential oil composition and biological activity of Rosmarinus officinalis L. accessions in the western Himalaya. Sci. Rep. 2022;12:1–13. doi: 10.1038/s41598-022-07298-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burt S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004;94:223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 33.Delamare APL, Moschen-Pistorello IT, Artico L, Atti-Serafini L, Echeverrigaray S. Antibacterial activity of the essential oils of Salvia officinalis L. and Salvia triloba L. cultivated in South Brazil. Food Chem. 2007;100:603–608. doi: 10.1016/j.foodchem.2005.09.078. [DOI] [Google Scholar]
- 34.Karalija E, Dahija S, Tarkowski P, Cavar Zeljkovic S. Influence of climate-related environmental stresses on economically important essential oils of Mediterranean Salvia sp. Front. Plant Sci. 2022;13:864807. doi: 10.3389/fpls.2022.864807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yeddes W, Wannes WA, Hammami M, Smida M, Chebbi A, Marzouk B, Tounsi MS. Effect of environmental conditions on the chemical composition and antioxidant activity of essential oils from Rosmarinus officinalis L. growing wild in Tunisia. J. Essent. Oil Bear. Plants. 2018;21:972–986. doi: 10.1080/0972060X.2018.1533433. [DOI] [Google Scholar]
- 36.Mehalaine, S., Chenchouni, H. Effect of climatic factors on essential oil accumulation in two Lamiaceae species from Algerian semiarid lands. in: Exploring the Nexus of Geoecology, Geography, Geoarcheology and Geotourism: Advances and Applications for Sustainable Development in Environmental Sciences and Agroforestry Research, Advances in Science, Technology & Innovation, Chenchouni, H., (ed). pp 1‒4. 10.1007/978-3-030-01683-812 (2019).
- 37.Zheljazkov VD, Kacaniova M, Dincheva I, Radoukova T, Semerdjieva IB, Astatkie T, Schlegel V. Essential oil composition, antioxidant and antimicrobial activity of the galbuli of six juniper species. Ind. Crops Prod. 2018;124:449–458. doi: 10.1016/j.indcrop.2018.08.013. [DOI] [Google Scholar]
- 38.Rathore S, Kumar R. Dynamics of phosphorus and biostimulants on agro-morphology, yield, and essential oil profile of German chamomile (Matricaria chamomilla L.) under acidic soil conditions of the Western Himalaya. Front. Plant Sci. 2022;13:1–16. doi: 10.3389/fpls.2022.917388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rathore S, Kumar R. Essential oil content and compositional variability of Lavandula species cultivated in the mid hill conditions of the Western Himalaya. Molecules. 2022;27(3391):1–14. doi: 10.3390/molecules27113391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adams PR. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy. 4.1. Allured Publishing; 2017. [Google Scholar]
- 41.Stein SE. Mass Spectral Database and Software, Version 3.02. National Institute of Standards and Technology (NIST); 2005. [Google Scholar]
- 42.Hammer O, Harper DAT, Ryan PD. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001;4:1–9. [Google Scholar]
- 43.Balouiri M, Sadiki M, Ibnsouda SK. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016;6:71–79. doi: 10.1016/j.jpha.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Elshikh M, Ahmed S, Funston S, Dunlop P, McGaw M, Marchant R, Banat IM. Resazurin-based 96-well plate microdilution method for the determination of minimum inhibitory concentration of biosurfactants. Biotechnol. Lett. 2016;38:1015–1019. doi: 10.1007/s10529-016-2079-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Adukwu EC, Allen SC, Phillips CA. The anti-biofilm activity of lemongrass (Cymbopogon flexuosus) and grapefruit (Citrus paradisi) essential oils against five strains of Staphylococcus aureus. J. Appl. Microbiol. 2012;113:1217–1227. doi: 10.1111/j.1365-2672.2012.05418.x. [DOI] [PubMed] [Google Scholar]
- 46.Ponce AG, Fritz R, Del Valle C, Roura SI. Antimicrobial activity of essential oils on the native microflora of organic Swiss chard. LWT- Food Sci. Technol. 2003;36:679–684. doi: 10.1016/S0023-6438(03)00088-4. [DOI] [Google Scholar]
- 47.Anonymous. 2022. Crop weather outlook. All India coordinated research project on agro meteorology. http://www.cropweatheroutlook.in/crida/amis/bramis.jsp.
- 48.Komaitis ME. Composition of the essential oil of marjoram (Origanum majorana L.) Food Chem. 1992;42:117–118. doi: 10.1016/0308-8146(92)90020-3. [DOI] [Google Scholar]
- 49.Raina AP, Negi KS. Essential oil composition of Origanum majorana and Origanum vulgare ssp. hirtum growing in India. Chem. Nat. Compd. 2011;6:882–883. doi: 10.1007/s10600-012-0133-4. [DOI] [Google Scholar]
- 50.Bejaoui A, Chaabane H, Jemli M, Boulila A, Boussaid M. Essential oil composition and antibacterial activity of Origanum vulgare subsp. glandulosum Desf. at different phenological stages. J. Med. Food. 2013;16:1115–1120. doi: 10.1089/jmf.2013.0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.D’Antuono LF, Galletti GC, Bocchin I. Variability of essential oil content and composition of Origanum vulgare L. populations from a north mediterranean area (Liguria Region, Northern Italy) L. Ann. Bot. 2000;86:471–478. doi: 10.1006/anbo.2000.1205. [DOI] [Google Scholar]
- 52.Kokkini S, Karousou R, Hanlidou E, Lanaras T. Essential oil composition of Greek (Origanum vulgare ssp. hirtum) and Turkish (O. onites) Oregano: A tool for their distinction. J. Essent. Oil Res. 2004;16:334–338. doi: 10.1080/10412905.2004.9698735. [DOI] [Google Scholar]
- 53.Kokkini S, Karousou R, Dardioti A, Krigas N, Lanaras T. Autumn essential oils of Greek oregano. Phytochem. 1997;44:883–886. doi: 10.1016/s0031-9422(96)00576-6. [DOI] [Google Scholar]
- 54.Bayala B, Coulibaly AY, Djigma FW, Nagalo BM, Baron S, Figueredo G, Lobaccaro JMA, Simpore J. Chemical composition, antioxidant, anti-inflammatory and antiproliferative activities of the essential oil of Cymbopogon nardus, a plant used in traditional medicine. Biomol. Concepts. 2020;11:86–96. doi: 10.1515/bmc-2020-0007. [DOI] [PubMed] [Google Scholar]
- 55.Dzamic AM, Sokovic MD, Ristic MS, Grujic SM, Mileski KS, Marin PD. Chemical composition, antifungal and antioxidant activity of Pelargonium graveolens essential oil. J. Appl. Pharm. Sci. 2014;4:001–005. doi: 10.7324/JAPS.2014.40301. [DOI] [Google Scholar]
- 56.Mohammadi S, Saharkhiz MJ. Changes in essential oil content and composition of catnip (Nepeta cataria L.) during different developmental stages. J. Essent. Oil Bear. Plants. 2011;14:396–400. doi: 10.1080/0972060X.2011.10643592. [DOI] [Google Scholar]
- 57.Baser KHC, Kirimer N, Tumen G. Composition of the essential oil of Origanum majorana L. from Turkey. J. Essent. Oil Res. 1993;5:577–579. doi: 10.1080/10412905.1993.9698283. [DOI] [Google Scholar]
- 58.Banchio E, Bogino PC, Zygadlo J, Giordano W. Plant growth promoting rhizobacteria improve growth and essential oil yield in Origanum majorana L. Biochem. Syst. Ecol. 2008;36:766–771. doi: 10.1016/j.bse.2008.08.006. [DOI] [Google Scholar]
- 59.Paudel PN, Satyal P, Satyal R, Setzer WN, Gyawali R. Chemical Composition, enantiomeric distribution, antimicrobial and antioxidant activities of Origanum majorana L. essential oil from Nepal. Molecules. 2022;27:1–19. doi: 10.3390/molecules27186136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Azizi A, Yan F, Honermeier B. Herbage yield, essential oil content and composition of three oregano (Origanum vulgare L.) populations as affected by soil moisture regimes and nitrogen supply. Ind. Crops Prod. 2009;29:554–561. doi: 10.1016/j.indcrop.2008.11.001. [DOI] [Google Scholar]
- 61.Vokou D, Kokkini S, Bessiere JM. Geographic variation of Greek oregano (Origanum vulgare ssp. hirtum) essential oils. Biochem. Syst. Ecol. 1993;21:287–295. doi: 10.1016/0305-1978(93)90047-u. [DOI] [Google Scholar]
- 62.Goyal S, Tewari G, Pandey HK, Kumari A. Exploration of productivity, chemical composition, and antioxidant potential of Origanum vulgare L. grown at different geographical locations of Western Himalaya, India. J. Chem. 2021;2021:6683300. doi: 10.1155/2021/6683300. [DOI] [Google Scholar]
- 63.Poulose AJ, Croteau R. Biosynthesis of aromatic monoterpenes. Conversion of 3'-terpinene to pcymene and thymol in Thymus vulgaris L. Arch. Biochem. Biophys. 1978;187:307–314. doi: 10.1016/0003-9861(78)90039-5. [DOI] [PubMed] [Google Scholar]
- 64.Vernet, Ph. Le polymorphisme chimique du Thymus vulgaris L. (Labile); mode de transmission hereditaire de 3 terpenes (le thymol, le carvacrol et le linalol). C. R. Acad. Sc Paris (serie D), 284, 1289–1292. (1977).
- 65.Oliveira WA, Pereira FDO, Luna GCDG, Lima IO, Wanderley PA, Lima RBD, Lima EO. Antifungal activity of Cymbopogon winterianus jowitt ex bor against Candida albicans. Braz. J. Microbiol. 2011;42:433–441. doi: 10.1590/S1517-83822011000200004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kaul PN, Bhattacharya AK, Singh K, Rajeswara Rao BR. Chemical composition of the essential oil of Java citronella (C. winterianus Jowitt.) grown in Andhra Pradesh. Pafai J. 1997;19:29–33. [Google Scholar]
- 67.Kumoro AC, Wardhani DH, Retnowati DS, Haryani K. A brief review on the characteristics, extraction and potential industrial applications of citronella grass (Cymbopogon nardus) and lemongrass (Cymbopogon citratus) essential oils. Int. Conf. Chem. Mater. Eng. 2021;1053:1–10. doi: 10.1088/1757-899X/1053/1/012118. [DOI] [Google Scholar]
- 68.Rana VS, Juyal JP, Blazquez MA. Chemical constituents of essential oil of Pelargonium graveolens leaves. Internat J. Aromather. 2002;12:216–218. doi: 10.1016/S0962-4562(03)00003-1. [DOI] [Google Scholar]
- 69.Mnif W, Dhifi W, Jelali N, Baaziz H, Hadded A, Hamdi N. Characterization of leaves essential oil of Pelargonium graveolens originating from tunisia: Chemical composition, antioxidant and biological activities. J. Essent. Oil Bear. Plant. 2011;14:761–769. doi: 10.1080/0972060X.2011.10644001. [DOI] [Google Scholar]
- 70.Motsa NM, Soundy P, Steyn JM, Mojela RA, Learmonth N, Teubes C. Plant shoot age and temperature effects on essential oil yield and oil composition of rose-scented geranium (Pelargonium sp.) growth in South Africa. J. Essent. Oil Res. 2006;18:106–110. doi: 10.1080/10412905.2006.12067129. [DOI] [Google Scholar]
- 71.Prakasa Rao EVS, Ganesha Rao RS. Seasonal variation in oil content and its composition in two chemotypes of scented geranium (Pelargonium sp) J. Essent. Oil Res. 1995;7:159–163. doi: 10.1080/10412905.1995.9698491. [DOI] [Google Scholar]
- 72.Rajeshwara Rao BR, Bhatacharya AK. History and botanical nomenclature of rose scented geranium cultivars grown in India. Indian Perfum. 1992;36:155–161. [Google Scholar]
- 73.Saxena G, Banerjee S, Rahman L, Sharma S, Kumar S. An efficient in vitro procedure for micropropagation and generation of somaclones of rose-scented Pelargonium. Plant Sci. 2000;155(2):133–140. doi: 10.1016/s0168-9452(00)00213-2. [DOI] [PubMed] [Google Scholar]
- 74.Fekri N, Amir DE, Owis A, AbouZid S. Studies on essential oil from rose-scented geranium, Pelargoniumgraveolens L'Hérit. (Geraniaceae) Nat. Prod. Res. 2021;35:2593–2597. doi: 10.1080/14786419.2019.1682581. [DOI] [PubMed] [Google Scholar]
- 75.Filippova AA, Szhenova TM, Golovina NV, Garnova NYu, Bokov DO. Standardization of geranium essential oil. Mosc. Univ. Chem. Bull. 2020;75:200–206. doi: 10.3103/S0027131420030037. [DOI] [Google Scholar]
- 76.Chalchat JC, Garry RP, Muhayimana A. Essential oil of Tagetes minuta from Rwanda and France: Chemical composition according to harvesting location, growth stage and part of plant extracted. J. Essent. Oil Res. 1995;7:375–386. doi: 10.1080/10412905.1995.9698544. [DOI] [Google Scholar]
- 77.Graven EH, Webber L, Benians G, Venter M, Gardner JB. Effect of soil type and nutrient status on the yield and composition of Tagetes oil (Tagetes minuta L.) J. Essent. Oil Res. 1991;3:303–307. doi: 10.1080/10412905.1991.9697948. [DOI] [Google Scholar]
- 78.Gill A, Ghersa CM, Leicach S. Essential oil yield and composition of Tagetes minuta accessions from Argentina. Biochem. Syst. Ecol. 2000;28:261–274. doi: 10.1016/S0305-1978(99)00062-9. [DOI] [Google Scholar]
- 79.Safaei-Ghomi J, Jafari-Bidgoli Z, Batooli H. Volatile constituents analysis of Nepeta cataria from central Iran. Chem. Nat. Compd. 2009;45:913–915. doi: 10.1007/s10600-010-9470-3. [DOI] [Google Scholar]
- 80.Morteza-Semnani K, Saeedi M. Essential oils composition of Nepeta cataria L. and Nepeta crassifolia Boiss. and Buhse from Iran. J. Essent. Oil Bear. Plant. 2004;7:120–124. doi: 10.1080/0972-060X.2004.10643376. [DOI] [Google Scholar]
- 81.Malizia RA, Molli JS, Cardell DA, Retamar JA. Volatile constituents of the essential oil of Nepeta cataria L. grown in Cordoba province (Argentina) J. Essent. Oil Res. 1996;8:565–567. doi: 10.1080/10412905.1996.9700691. [DOI] [Google Scholar]
- 82.Zomorodian K, Moein M, Lori ZG, Ghasemi Y, Rahimi MJ, Bandegani A, Pakshir K, Bazargani A, Mirzamohammadi S, Abbasi N. Chemical composition and antimicrobial activities of the essential oil from Myrtus communis leaves. J. Essent. Oil Bear. Plants. 2013;16:76–84. doi: 10.1080/0972060X.2013.764183. [DOI] [Google Scholar]
- 83.Perry RJ, Hodges JR. Attention and executive deficits in Alzheimer's disease: A critical review. Brain. 1999;122:383–404. doi: 10.1093/brain/122.3.383. [DOI] [PubMed] [Google Scholar]
- 84.Yang S, Bai M, Yang J, Yuan Y, Zhang Y, Qin J, Kuang Y, Sampietro DA. Chemical composition and larvicidal activity of essential oils from Peganum harmala, Nepeta cataria and Phellodendron amurense against Aedes aegypti (Diptera: Culicidae) Saudi Pharm. J. 2020;28:560–564. doi: 10.1016/j.jsps.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gilani AH, Shah AJ, Zubair A. Chemical composition and mechanisms underlying the spasmolytic and bronchodilatory properties of the essential oil of Nepeta cataria L. J. Ethnopharmacol. 2009;121:405–411. doi: 10.1016/j.jep.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 86.Nurzynska-Wierdak R, Dzida K. Influence of plant density and term of harvest on yield and chemical composition of sweet marjoram (Origanum majorana L.) Sci. Pol. Hortorum Cultus. 2009;8:51–61. [Google Scholar]
- 87.Aggarwal KK, Ahmad A, Kumar TRS, Jain N, Gupta VK, Kumar S, Khanuja SPS. Antimicrobial activity spectra of Pelargonium graveolens L. and Cymbopogon winterianus Jowitt oil constituents and acyl derivatives. J. Med. Aromat. Plant Sci. 2000;22:544–548. [Google Scholar]
- 88.Amor G, Caputo L, La Storia A, De Feo V, Mauriello G, Fechtali T. Chemical composition and antimicrobial activity of Artemisia herba-alba and Origanum majorana essential oils from Morocco. Molecules. 2019;24:4021. doi: 10.3390/molecules24224021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tsitlakidou P, Papachristoforou A, Tasopoulos N, Matzara A, Hatzikamari M, Karamanoli K, Mourtzinos I. Sensory analysis, volatile profiles and antimicrobial properties of Origanum vulgare L. essential oils. Flavour Fragr. J. 2022;37:43–51. doi: 10.1002/ffj.3680. [DOI] [Google Scholar]
- 90.Famuyide IM, Aro AO, Fasina FO, Eloff JN, McGaw LJ. Antibacterial activity and mode of action of acetone crude leaf extracts of under-investigated Syzygium and Eugenia (Myrtaceae) species on multidrug resistant porcine diarrhoeagenic Escherichia coli. BMC Vet. Res. 2019;15:1–14. doi: 10.1186/s12917-019-1914-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kaur H, Bhardwaj U, Kaur R, Kaur H. Chemical composition and antifungal potential of citronella (Cymbopogon nardus) leaves essential oil and its major compounds. J. Essent. Oil Bear. Plants. 2021;24:571–581. doi: 10.1080/0972060X.2021.1942231. [DOI] [Google Scholar]
- 92.Androutsopoulou C, Christopoulou SD, Hahalis P, Kotsalou C, Lamari FN, Vantarakis A. Evaluation of essential oils and extracts of rose geranium and rose petals as natural preservatives in terms of toxicity, antimicrobial, and antiviral activity. Pathogens. 2021;10:494. doi: 10.3390/pathogens10040494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nadeem A, Shahzad H, Ahmed B, Muntean T, Waseem M, Tabassum A. Phytochemical profiling of antimicrobial and potential antioxidant plant: Nepeta cataria. Front. Plant Sci. 2022;13:1–18. doi: 10.3389/fpls.2022.969316. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data analyzed during the current study available from the corresponding author on reasonable request.