Abstract
Phosphate starvation is one of the major factors limiting plant productivity globally. Soil microflora with an inherent trait of phosphate accumulation directly influences soil phosphorus level by regulating its labile form in soil solution. However, the detailed mechanism involved during their interaction with plants under phosphate deficient conditions is still unexplored. Hence, to dissect these complex gene regulatory networks, transcriptome analysis of A. thaliana roots grown under phosphate starved conditions in presence of phosphate accumulating bacteria (Pseudomonas putida; RAR) was performed. Plants grown under phosphate starved conditions showed upregulation of phosphate starvation responsive genes associated with cell biogenesis, stress, photosynthesis, senescence, and cellular transport. Inoculation of RAR upregulated genes linked to defense, cell wall remodeling, and hormone metabolism in stressed plants. Gene ontology analysis indicated the induction of S-glycoside, glucosinolate, and glycosinolate metabolic processes in RAR inoculated plants under phosphate stressed conditions. Further, protein–protein interaction analysis revealed upregulation of root development, cation transport, anion transport, sulfur compound metabolic process, secondary metabolic process, cellular amino metabolic process, and response to salicylic acid in RAR inoculated plants under phosphate starved conditions. These results indicate the potential role of phosphate accumulating bacteria in alleviating phosphate starvation in plants by involving multiple pathways.
Subject terms: Microbiology, Molecular biology, Plant sciences
Introduction
Phosphorus (P) is the second most important plant nutrient and its limitation severely affects the plant’s performance and productivity. Precipitation of P fertilizers with calcium, iron, and aluminium in soil restricts their availability to plants1. Phosphate availability immensely affects plants’ physiology, metabolism, and crop performance. The fully oxidized form of inorganic phosphate (Pi) is vital for the photosynthesis process, thereby, known to play a key role in regulating energy conservation and assimilation2. It is involved in an array of metabolic processes including energy metabolism, macro-molecular biosynthesis, photosynthesis, glycolysis, enzyme activation/inactivation, redox reactions, signaling, and carbohydrate metabolism3–5. Additionally, it also affects the chemical stability and cellular retention of biological macromolecules and metabolites.
Plants grown under limited phosphate conditions evolve multiple adaptive responses, together termed as phosphate stress responses (PSRs). It involves coordination and integration of local and long distance signaling which promotes enhanced acquisition of Pi along with remobilization within plants6. Multiple signaling molecules mediate the establishment of PSRs, among which Pi itself is a primary signal which further evokes other molecules viz. sugar, hormones, metabolites, reactive oxygen species, and peptides7,8. Plants adapt to Pi deficient conditions by modulating different morphological and metabolic adaptations such as changes in root system architecture, anthocyanin accumulation, galactolipid synthesis, the release of organic acids, phosphatases, and nucleases mediated through differential gene expression9–11. In A. thaliana, PHOSPHATE STARVATION RESPONSE 1 (PHR1) and its closely related transcription factors cumulatively referred to as PHR transcription factors are the master regulator of Pi sensing and signaling12. In addition, PHR1 also promotes the expression of NITRATE-INDUCIBLE GARP-TYPE TRANSCRIPTIONAL REPRESSOR1 (NIGT1) family genes resulting in reduced nitrate uptake. Therefore, PHR1 involve in two different transcriptional cascades forming a link between the regulation of both nitrogen and phosphorus13. In A. thaliana, Pi starvation and oxygen deficiency contain a set of overlapping genes and these responses are also under the control of PHR114. Besides PHR1, another transcription factor viz. WRKY, zinc finger, R2R3 MYB, MYB like, ERF and G2 like families also play a salient role in the regulation of PSRs15–20.
Despite being abundantly present, the unavailability of Pi in the soil is a major concern. Plant growth promoting rhizobacteria (PGPR) has the potential of mobilizing soil bound Pi through solubilization and mineralization. Soil microbes also accumulate P within their biomass which approximately accounts for 2 to 10% of total soil and can exceed up to 50%21,22. These phosphate accumulating microbes efficiently compete with plants for available orthophosphate from soil solution which represents the temporarily unavailable immobilized pool of P for plants. This form of P becomes available to plants over time, therefore, P immobilization is an important mechanism for maintaining P supply in soil solution23,24. Stress tolerant microbes along with their P solubilization abilities are known to play a pivotal role in stress mitigation in plants25. In a previous study, we demonstrated the efficacy of polyphosphate accumulating bacteria (PAB) in plant growth promotion and salinity stress alleviation in A. thaliana26.
The interaction between plant and PGPR is not an outcome of gene for gene interaction, indeed it is a multigene response27. Numerous gene based studies have been performed using phosphate solubilizing bacteria in plants under stress conditions. However, interaction mechanism of PAB with plants under phosphate starved condition is yet to be explored. Therefore, elucidation of complex gene regulatory networks regulating the interaction between plant and PAB will unravel many aspects for alleviating phosphate deficiency, a common but precarious constraint to the agricultural system. Hence, to get into the molecular insight underlying the interaction between PAB and plants, the transcriptome analysis of A. thaliana roots grown under phosphate starved conditions was performed. Furthermore, the study was validated through qRT-PCR using selected candidate genes belonging to different metabolic activities.
Results
Polyphosphate accumulation and stress tolerance potential of RAR
Phosphate accumulating potential and stress tolerance (different P sources) of Pseudomonas putida (RAR) was evaluated for 10 consecutive days. Results showed approximately similar accumulated phosphate content in RAR till the 7th day (Fig. 1A). Significantly higher phosphate accumulation was observed on the 10th day in RAR. In presence of unavailable sources i.e. HA and TCP, RAR extended its growth phase and survived up to the 10th day of incubation. However, RAR doesn’t survive under 50 µM P (KH2PO4) condition and attained the death phase on the 5th day of incubation (Fig. 1B).
Figure 1.
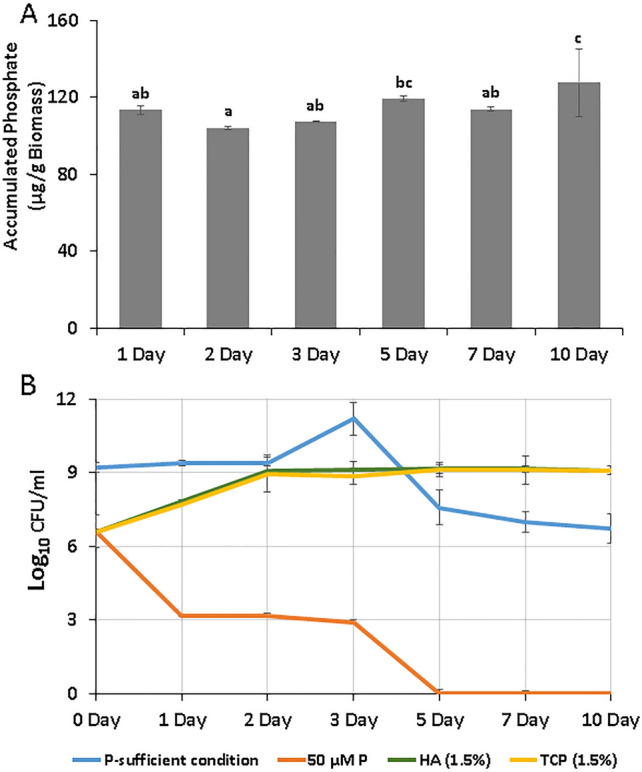
Accumulated phosphate within Pseudomonas putida biomass (A); Effect of different phosphate sources [(Normal P (0.3% KH2PO4 + 1.2%Na2HPO4), limited P (50 µM KH2PO4) and unavailable P (1.5% hydroxyapatite and tricalcium phosphate] on growth of Pseudomonas putida (B).
RAR inoculation modulates physicochemical parameters in A. thaliana under phosphate starved condition
The efficacy of P accumulating RAR on the growth of A. thaliana plants subjected to phosphate stress was evaluated (Supplementary Fig. 1). Compared to uninoculated plants, significantly higher root length, number of rosette leaves, siliques, and dry weight was observed in RAR inoculated plants (Supplementary Table 2). Phosphate stress (HA; unavailable P) resulted in declined shoot length, dry weight, and siliques of A. thaliana plants which were enhanced in RAR treated plants by 30.34%, 78.57%, and 48.55%, respectively. Additionally, lowered P content in phosphate starved plants was enhanced in presence of RAR under stress conditions. The dry weight of the plants grown under limited P (available 50 µM P) was higher compared to control conditions. Additionally, a higher reduction in P content was evident in plants grown under HA conditions as compared to 50 µM available P conditions. Therefore, based on the results HA source of P was selected for further study.
RAR modifies root system architecture of A. thaliana under phosphate starved condition
Effect of RAR on root system architecture was studied through in vitro interaction of A. thaliana with RAR under normal and P starved conditions. Results showed improved growth of the plant treated with RAR as compared to the control (Supplementary Fig. 2A). Treatment of RAR improved the shoot growth and root branching (Supplementary Fig. 2A,B). Furthermore, microscopy of roots also revealed root hair formation under phosphate starved conditions in both RAR inoculated and uninoculated plants as compared to the control (Supplementary Fig. 2A,B). Since the effect of phosphate starvation was more prominent on roots, therefore, in the present study root tissue was selected for transcriptome analysis.
Summary of transcriptome sequencing and mapping onto A. thaliana reference genome
For transcriptome analysis plants were grown under in-vitro condition in the hydroponic system (Fig. 2A). RNA extracted from A. thaliana roots was used for Illumina sequencing and data was processed for the generation of high quality reads and bases (Table 1). All 8 libraries of control (1,2), RAR (1,2), HA (1,2), and HA + RAR (1,2) produced an average of 15.88 million reads in the range of 11.64 to 18.36 million for each sample. The total number of reads, total bases, and data generated through transcriptome sequencing are given in Table 1.
Figure 2.
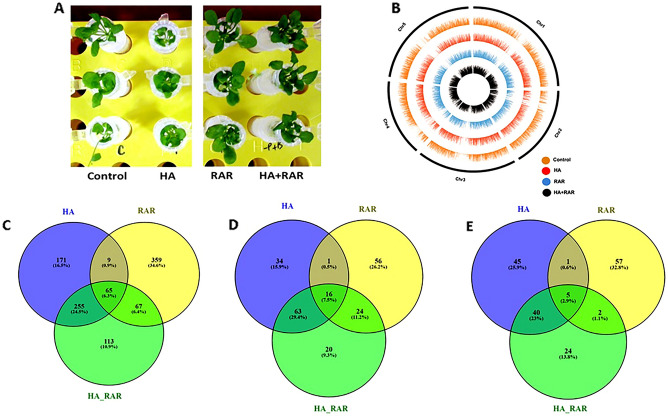
Growth of Arabidopsis thaliana in presence of RAR under phosphate starved condition in the hydroponic system (A); Circos showing the histogram of genes mapped onto 5 chromosomes of Arabidopsis thaliana (B). Venn diagram showing total number of expressed transcripts (C); Venn diagram of the differentially expressed upregulated (D) and downregulated genes (E) in RAR, HA and HA + RAR treatment as compared to control.
Table 1.
Read statistics of transcriptome of different samples.
| S. no. | Sample | No. of reads | Total bases | Data obtained (Gb) |
|---|---|---|---|---|
| 1 | Control 1 | 11,644,447 | 1,753,837,222 | ~ 1.75 |
| 2 | Control 2 | 17,513,170 | 2,637,161,861 | ~ 2.64 |
| 3 | RAR 1 | 17,459,025 | 2,628,351,214 | ~ 2.63 |
| 4 | RAR 2 | 18,365,698 | 2,764,142,720 | ~ 2.76 |
| 5 | HA 1 | 15,577,803 | 2,345,735,641 | ~ 2.35 |
| 6 | HA 2 | 16,568,894 | 2,494,141,219 | ~ 2.49 |
| 7 | HARAR 1 | 14,864,128 | 2,237,235,998 | ~ 2.24 |
| 8 | HARAR 2 | 15,110,846 | 2,274,381,789 | ~ 2.27 |
Differentially expressed genes plotted on circos plot showed gene alteration in all 5 chromosomes of A. thaliana (Fig. 2B). Venn diagram was plotted and analyzed to identify the differentially expressed genes (DEGs) between different treatments of HA, RAR, and HA + RAR (Fig. 2C–E). Overall analysis revealed that 65 DEGs were found to be common between all three treatments (Fig. 2C). Furthermore, the Venn diagram showed 9 common DEGs between HA and RAR. Results also showed overlapping of 255 and 67 DEGs between HA-HA + RAR and RAR-HA + RAR treatments, respectively. Whereas, a total of 171, 359, and 113 unique DEGs were preferentially expressed in HA, RAR, and HA + RAR treatments, respectively. Out of 1039 DEGs obtained, 114 genes were upregulated in HA, 97 in RAR, and 123 in HA + RAR treatments as compared to control (Fig. 2D). On the contrary, a lesser number of genes were found to be downregulated in HA (91), RAR (65) and HA + RAR (71) treatments (Fig. 2E).
Differential expression of genes under all growth conditions
Differential expression of genes based on P value < 0.05 was compared in plants grown under HA, RAR, and HA + RAR treatments as compared to the control (Supplementary appendix 1,2,3). Plants inoculated with RAR showed upregulation of genes involved in different metabolic processes such as hormonal metabolism, cell wall modification, defense activities, and transportation. Expression of gibberellic acid-stimulated Arabidopsis (GASA) gene i.e. GASA3 (At4g09600) and GASA5 (At3g02885) was highly induced in A. thaliana roots inoculated with RAR. The study also showed upregulation of genes associated with abscisic acid signaling viz. ABA-induced transcription repressor 5 (AITR5; At5g50360), highly ABA-induced PP2C gene 1 (HAI1; At5g59220), highly ABA-induced PP2C gene 2 (HAI2; At1g07430), HOMEOBOX 12 (HB-12; At3g61890) and ethylene signalling i.e. ethylene responsive factor54 (ERF54; At4g28140) specifically in the RAR inoculated plants. Role of RAR in cell wall remodeling through overexpression of lipid transfer protein 3 (LTP3), delta 9 desaturase 1 (ADS1; At1G06080), polygalacturonase abscission zone A. thaliana (PGAZAT; At2G41850), 3-ketoacyl-coa synthase 3 (KCS3; At1G07720), expansin A10 (EXPA10; At1g26770), xylogen protein 1 (XYP1; At5g64080) and osbp (oxysterol binding protein)-related protein 4B (ORP4B; At4g25850) was also evident in the study. Interestingly some transporters such as high affinity nitrate transporter 2.6 (NRT2.6; At3g45060), nramp metal ion transporter 6 (NRAMP6; At1g15960) were found to be upregulated in RAR inoculated plants. Expression of defense associated genes viz. myo-inositol oxygenase 4 (MIOX4; At4G26260), galactinol synthase 2 (GolS2; At1G56600), ascorbate peroxidase 5 (APX5; At4g35970) and beta glucosidase 24 (BGLU24; At5g28510) was also noted in plants treated with RAR. Results showed prominent expression of putative cytochrome P450 (At3g26200) and embryonic cell protein 63 (ECP63; At2g36640) in presence of RAR.
Plants subjected to phosphate deprived conditions showed higher induction of genes such as senescence-associated gene 12 (SAG12; At5g45890), usually multiple acids move in and out transporters 19 (UMAMIT19; At1g21890), UMAMIT17 (At4g08300), 2-oxoglutarate (2OG) and Fe(II)-dependent oxygenase superfamily protein (At2g44800), oxidoreductase 2OG-Fe(II) oxygenase family protein (At2g48080), cysteine/histidine-rich C1 domain protein (At2g37805), gibberellic acid methyltransferase 2 (GAMT2; At5g56300), fantastic four 2 (FAF2; At1g03170), dehydration response element B1A (DREB1A; At4g25480), small auxin upregulated 72 (SAUR72; At3g12830), phosphoenolpyruvate carboxylase kinase 2 (PPCK2; At3g04530) and glutathione s-transferase 14 (GSTF14; At1g49860).
Overexpression of genes associated with nitrate metabolism and transportation viz. nitrate transporter 2.2 (NRT2.2; At1g08100), NRT2.5 (At1g12940), NRT1 (At3g21670), glutamate dehydrogenase 3 (GDH3; At3g03910) and glutamine synthetase 1;4 (GLN1;4; At5g16570) was upregulated in HA and HA + RAR treatment. Phosphate stress responsive genes such as IPS1 (At3g09922) and phosphate transporter 2 (PHT1;2; At5g43370) were overexpressed in HA and HA + RAR treatment, however, the expression was more prominent in phosphate starved plants inoculated with RAR (HA + RAR). Phosphate starvation-induced gene 2 (PS2; At1g73010) was expressed in all three conditions, while, repressed expression was observed in RAR inoculated plants. Besides, sulfate [Sultr1;3 (At1g22150), Sultr2;1 (At5g10180)] and nitrate [NRT2:1 (At1g08090), NPF3.1 (At1g68570), transporters also showed overexpression in all the three treatments.
Differential expression analysis of A. thaliana genes under phosphate starved condition
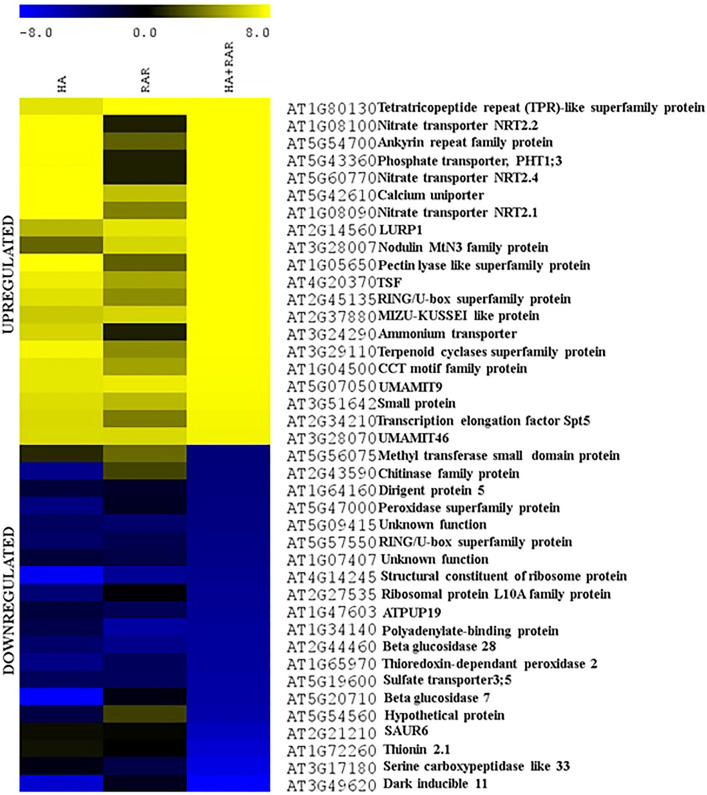
A heat map of the top 20 upregulated and downregulated genes in HA, RAR, and HA + RAR treatments as compared to non-inoculated control was generated (Fig. 3). Inorganic phosphate transporter PHT1;3 involved in transmembrane transport of phosphate was found to be upregulated in HA and HA + RAR treatment among which the expression was higher in RAR treated plants under Pi starved condition. A study showed the upregulation of nitrate transporters (NRT2.2, NRT2.4, and NRT2.1) in HA and HA + RAR treatment, however, its expression was not evident in RAR inoculated plants. In addition, results showed upregulation of ammonium and amino acid transporter (UMAMIT9 and UMAMIT46). Sulfate transporter was downregulated in all three growth conditions as compared to the control. Nodulin MtN3 family protein involved in sugar transmembrane transportation was upregulated in the RAR inoculated plants under both P-sufficient and starved conditions. However, the gene was not expressed in phosphate starved plants. Tetratricopeptide repeat (TPR)-like superfamily protein involved in oxidative stress management was highly upregulated in RAR and HA + RAR treatment, however, its repressed expression was noted in plants grown under P stress condition. SAUR6 and Thionin 21 was expressed only under phosphate starved condition, whereas, it was downregulated in RAR inoculated treatments. Results showed the downregulation of peroxidase family protein, ATPUP19, thioredoxin peroxidase 2, dark inducible 11,beta glucosidase 28 and polyadenylate binding protein genes in all three treatments.
Figure 3.
Differential expression of top 20 upregulated and downregulated genes in Arabidopsis thaliana roots grown under phosphate deprived condition in presence of Pseudomonas putida.
Comparative expression analysis of genes associated with plant growth promotion, root system architecture, defense metabolism, phytohormones, and phosphate transport
Expression analysis of genes belonging to different processes including root system architecture, defense metabolism, phytohormones, phosphate transport and plant growth promotion was studied. Most of the genes associated with plant growth promotion were found to be upregulated in presence of RAR (Table 2). Genes specifically upregulated in RAR inoculated plants included anthranilate synthase (At5g05730), IAA1 (At4g14560), WRKY60 (At2g25000), WRKY70 (At3g56400), amino acid biosynthetic pathway and nutrient uptake (At5g63890) and IAA production during plant–microbe interaction (At4g36110). In addition, genes WAK1 (At1g21250), hormone synthesis (At3g23890), and mitotic and meiotic division (At5g20850) were upregulated in both RAR and HA + RAR treatments. Genes associated with carbohydrate metabolism (At1g05030), jasmonic acid LOX2 (At3g45140), glutathione-s-transferase (At5g17220), and ethylene biosynthesis (At4g26200) showed upregulation in HA and HA + RAR treatment. However, these genes were either downregulated or not expressed in RAR alone treatment.
Table 2.
Log fold change value of genes associated with plant growth promotion, root system architecture, defense metabolism, phytohormones, and phosphate transport in HA, RAR, and HA + RAR treatments.
| Locus identifier | Gene | HA | RAR | HA + RAR | |
|---|---|---|---|---|---|
| Log fold change | |||||
| Plant growth promotion | AT5G05730 | ASA1, anthranilate synthase 1 | − 0.60502 | 0.233222 | − 1.19295 |
| AT4G14560 | IAA1 | − 0.45933 | 1.245183 | − 0.31336 | |
| AT2G25000 | WRYK60 | − 0.11835 | 0.67778 | 0.178139 | |
| AT3G56400 | WRKY70 | − 1.00746 | 0.907445 | − 1.36474 | |
| AT1G21250 | WAK1 | 0.320022 | 3.180495 | 1.194565 | |
| AT3G45140 | jasmonic acid (JA) LOX2 | 1.612366 | − 1.81294 | 1.884971 | |
| AT1G05030 | Carbohydrate metabolism | 0.174888 | − 0.33848 | 0.290582 | |
| AT1G06730 | Plastid nucleoside kinase | 0.123424 | − 0.08761 | 0.056039 | |
| AT1G53730 | STRUBBELIG-receptor family 6 | 0.025972 | 0.15064 | 0.160056 | |
| AT1G12890 | Transcription factor | − 0.27459 | 1.217637 | 0.556539 | |
| AT1G01260 | Transcription factor | 0.140424 | − 0.24861 | − 0.11045 | |
| AT5G63890 | Histidinol dehydrogenase | 0.100673 | 0.302311 | 0.037689 | |
| AT3G23890 | Topoisomerase II | 0.511329 | 1.067717 | 1.130213 | |
| AT2G46370 | Jasmonate-amido synthetase | 0.022017 | − 0.02026 | 0.01576 | |
| AT5G20850 | RAD51 | 0.563679 | 1.213952 | 1.086052 | |
| AT4G36110 | SAUR9 | − 1.23748 | 0.222742 | − 2.10603 | |
| AT5G17220 | Glutathione S-transferase | 2.265442 | 2.553179 | 2.069202 | |
| AT1G74930 | ERF/AP2 transcription factor family | 1.081301 | 0.158679 | 1.217591 | |
| AT4G26200 | Ethylene biosynthesis genes | 0.36849 | − 0.70514 | 0.486748 | |
| Root system architecture | AT1G79700 | AP2/ERF-type transcriptional activator | − 1.33638 | 0.487252 | − 0.13305 |
| AT1G23010 | Low phosphate root 1 | 0.132059 | 0.587806 | 0.432539 | |
| AT4G28610 | PHR1 | 0.161888 | 0.028235 | − 0.06149 | |
| AT3G03710 | Chloroplast polynucleotide phosphorylase | 0.03284 | − 0.19015 | 0.399371 | |
| AT3G25710 | Helix-loop-helix transcription factor | 0.074142 | − 0.61421 | − 0.39981 | |
| AT5G21040 | F box protein | 0.091412 | − 0.09987 | − 0.04077 | |
| AT5G42810 | Inositol-pentakisphosphate 2-kinase | 0.335513 | 0.328126 | 0.4272 | |
| AT3G20630 | Ubiquitin-specific protease | − 0.02404 | − 0.11438 | 0.093818 | |
| AT1G27740 | Root hair defective like | − 0.16931 | 0.158572 | − 0.16314 | |
| AT1G13620 | Root meristem growth factor− 2 | 0.383748 | 1.090409 | 0.669725 | |
| AT2G04025 | Root meristem growth factor− 3 | 0.561811 | 0.888145 | 1.084354 | |
| AT5G60810 | Root meristem growth factor− 1 | 1.036245 | 1.525388 | 1.825072 | |
| AT3G07360 | E3 ligase 9 | 0.190537 | 0.042808 | 0.027918 | |
| AT1G65800 | Receptor Kinase 2 | − 0.24397 | 0.69488 | 0.542802 | |
| AT5G21040 | F box motifs | 0.091412 | − 0.09987 | − 0.04077 | |
| AT1G66470 | Root hair defective 6 | 0.372088 | 0.977157 | 1.129053 | |
| AT1G01380 | ETC1 | 1.357817 | − 0.46166 | 0.557949 | |
| Defense | AT3G12500 | Basic chitinase | − 0.79084 | 0.580785 | − 0.61569 |
| AT2G43590 | Putative endochitinase | − 4.26441 | 2.12599 | − 3.67553 | |
| AT3G49120 | Putative peroxidase | − 1.41458 | 0.499832 | − 0.9197 | |
| AT5G64100 | Putative peroxidase | − 1.15536 | 0.835064 | − 0.52223 | |
| AT4G19810 | Chitinase | − 0.05348 | 0.337477 | 0.176733 | |
| AT1G72260 | Thionin | 0.627182 | − 0.00836 | − 6.34483 | |
| AT3G50970 | Dehydrin Xero2 | − 0.08879 | 0.755893 | − 0.48026 | |
| AT4G25780 | Putative pathogenesis-related protein | 1.644367 | 0.672576 | 1.285413 | |
| AT5G47910 | Respiratory oxidase oxidase protein | 0.468684 | − 1.20605 | 0.162101 | |
| AT5G39580 | Peroxidase | − 0.77574 | 1.265149 | − 0.70767 | |
| AT5G39720 | Avirulence responsive protein | 1.358477 | 1.700944 | 2.177848 | |
| AT5G17220 | Glutathione S-transferase, putative | 2.265442 | 2.553179 | 2.069202 | |
| AT3G47540 | Chitinase | − 0.29663 | 0.75535 | − 0.44857 | |
| AT1G19200 | Senescence-associated protein | 1.467621 | 1.319232 | 1.296234 | |
| AT3G09940 | Monodehydroascorbate reductase | 0.813197 | − 0.67246 | 0.325767 | |
| AT1G69080 | Universal stress protein | 0.879286 | − 0.41984 | 0.797882 | |
| AT1G08830 | superoxide dismutase | 0.26508 | − 0.26706 | 0.026401 | |
| Phytohormones | AT1G68320 | MYB62 | − 0.91856 | 0.254571 | 0.016459 |
| AT5G13080 | WRKY75 | − 1.41862 | 0.111279 | − 1.72453 | |
| AT1G68320 | MYB62 | − 0.91856 | 0.254571 | 0.016459 | |
| AT2G47190 | MYB2 | 0.430055 | − 1.44589 | 0.386696 | |
| AT3G62980 | AtTIR1, Transport inhibitor response | 0.111033 | 0.27616 | 0.469989 | |
| AT1G19220 | Auxin response factor 19 | 0.731804 | − 0.84521 | 0.568221 | |
| AT2G27050 | Ethylene-insensitive3-like1 | 0.087121 | 0.276828 | 0.233629 | |
| AT2G01830 | Cytokinin response 1 | − 0.0748 | 0.270271 | 0.243102 | |
| AT5G67030 | Zeaxanthin epoxidase | 0.1306 | − 0.02059 | 0.188658 | |
| AT4G26080 | ABA insensitive 1 | − 0.34884 | 0.677509 | − 0.07961 | |
| AT5G57050 | ABA signal transduction | − 0.10187 | 1.476148 | 0.468082 | |
| Phosphate transporters | AT2G38940 | PHT1;4; Inorganic phosphate transporter | 1.574022 | 0.534836 | 1.95555 |
| AT5G20150 | SPX domaincontaining proteins | 3.249601 | 1.643516 | 3.192507 | |
| AT2G26660 | SPX domaincontaining proteins | 0.907458 | 0.416665 | 0.911567 | |
| AT1G68740 | PHO1 | 1.962392 | 0.154712 | 1.761031 | |
| AT5G43360 | PHT1;3 | 7.743493 | 0 | 9.22805 | |
| AT3G47420 | Glycerol-3-P transporter | 2.725013 | 0.968898 | 2.280317 | |
| AT5G43370 | PHT1;2 | 4.763233 | 0.20171 | 5.615784 | |
| AT3G52190 | PHF1 | 1.362951 | 0.529231 | 1.240638 | |
| AT2G32830 | PHT1;5 | 1.930059 | 1.704908 | 1.366535 | |
| AT5G54800 | Glucose-6-phosphate/phosphate translocator | 0.135257 | 0.188966 | − 0.0262 | |
| AT5G43370 | PHT2 | 4.763233 | 0.20171 | 5.615784 | |
| AT5G43350 | PHT1;1 | 1.397895 | − 0.60161 | 1.491866 | |
| AT1G76430 | PHT1;9 | 0.196505 | − 0.78779 | 0.289093 | |
| AT1G20860 | PHT1;8 | 2.288292 | 1.475084 | 2.466618 | |
| AT1G61800 | PT2 | 0.768295 | 5.129416 | 4.814035 | |
| AT2G32830 | PHT1;5 | 1.930059 | 1.704908 | 1.366535 | |
Plants grown under phosphate starved conditions showed upregulation of disease resistance response protein-related (At1g22900), thionin (At1g72260) and respiratory burst oxidase protein (At5g47910) linked to defense activities in plants (Table 2). However, inoculation of RAR resulted in downregulation of these genes in plants under phosphate starved condition (HA + RAR). RAR inoculation upregulated the expression of defense associated genes including [basic chitinase (At3g12500), putative endochitinase (At2g43590), putative peroxidase (At3g49120, At5g64100), dehydrin xero2 (At3g50970), peroxidase ATP24a (At5g39580) and chitinase (At3g47540)], while, these genes were either downregulated or not expressed in other two treatments.
Genes associated with modification in root system architecture were differentially expressed in HA, RAR, and HA + RAR treatments (Table 2). Plants grown under phosphate starved conditions showed upregulation of PHR1 (At4g28610) and U box/armadillo repeat-containing E3 ligase9 (AtPUB9; At3g07360). Root meristem growth factor (RGF2; At1g13620), RGF3 (At2g04025), RGF1 (At5g60810), root hair defective 6 (At1g66470) and, ipk (At5g42810) were upregulated under all growth conditions. Among all, the gene involved in root hair patterning (ETC1; At1g01380) was upregulated in HA and HA + RAR treatment. RAR induced the expression of ethylene insensitive-3 like 1 (At2g27050), auxin receptor (At3g62980) and ABA signal transduction (At5g57050) encoding genes directly involved in induction and development of root hair under P starved condition.
High affinity phosphate transporters belonging to PHT1 family viz. PHT1;1 (At5g43350), PHT1;2 (At5g43370), PHT1;3 (At5g43360), PHT1;4 (At2g38940), PHT1;5 (At2g32830), and PHT1;8 (At1g20860) were upregulated in HA and HA + RAR treatments (Table 2). These transporters are responsible for Pi acquisition and mobilization in plants under starved conditions. Among these, PHT1;5 (Pi homeostasis) and PHT1;8 (root to shoot translocation of orthophosphate) were also expressed in RAR inoculated plants. Phosphate transporter (PT2; At1g61800) identical to AtPT2 was specifically upregulated in RAR and HA + RAR treatment depicting role of inoculum in mediating expression of particular transporter for P uptake.
Gene ontology and Kyoto encyclopedia of genes and genomes pathways analysis of differentially expressed genes
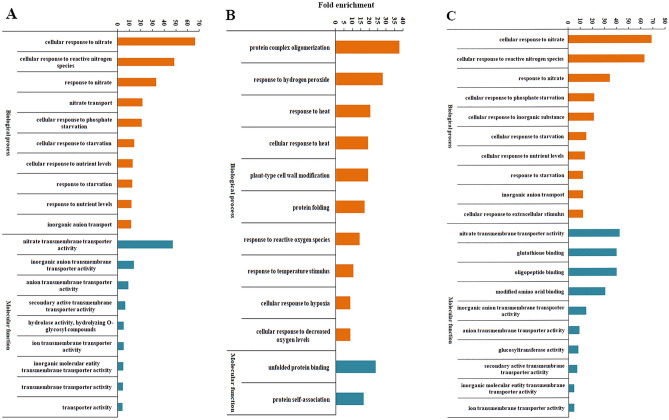
We identified different biological processes (BP) categories enriched in treatments HA, RAR and HA + RAR (Fig. 4A–C; Supplementary Table 3). Top overrepresented categories were most common in HA and HA + RAR treatments. These processes were associated with response to nitrate stress, reactive nitrogen species, phosphate starvation, nutrient levels, detoxification, toxic substance and secondary metabolic processes. BP categories uniquely enriched in HA treatment included nitrate transport, aging and ion transport. RAR inoculated plants exhibited BP associated with protein complex oligomerization, hydrogen peroxide, heat, plant cell wall modification, reactive oxygen species, temperature, oxidative stress, salt stress, osmotic stress, water deprivation, abscisic acid and abiotic stimulus. BP categories such as S-glycoside, glucosinolate, glycosinolate metabolic process were specifically enriched in phosphate starved plants inoculated with RAR (HA + RAR).
Figure 4.
Gene ontology analysis of genes differentially expressed in HA (A) RAR (B) and HA + RAR (C) treatment. Gene ontologies were categorized by their significance.
GO molecular function (MF) categories enriched in HA, RAR and HA + RAR treatments is represented in Fig. 4A–C and Supplementary Table 3. Common MF categories in HA and HA + RAR treatment included nitrate, inorganic anion, anion, ion and inorganic molecular transmembrane transporter activity. MF specifically enriched in HA + RAR treatments constituted glutathione binding, oligopeptide binding, oligopeptide binding and glucosyltransferase activity. However, RAR treated plants showed enrichment of MF associated with unfolded protein binding and protein self-association.
Functional enrichment analysis of clusters
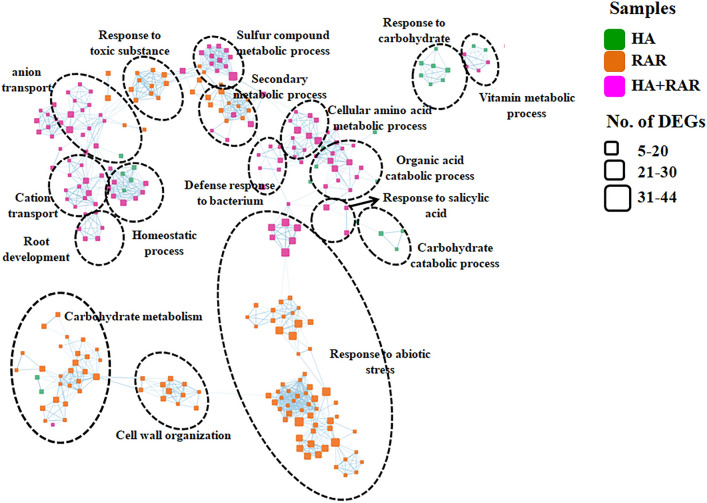
Protein–protein interaction (PPI) analysis showed presence of eleven significant clusters in the plants grown under different growth conditions viz. RAR, HA and HA + RAR (Fig. 5). Among all three treatments, the majority of DEGs were evident in HA + RAR treatments. These DEGs were associated with various processes such as root development, cation and anion transport, sulfur compound metabolic process, secondary metabolic process, cellular amino metabolic process and response to salicylic acid. RAR inoculated plants showed involvement of DEGs belonging to carbohydrate metabolism, cell wall organization, response to toxic substance and abiotic stress. Secondary metabolic process and response to abiotic stress was common in both RAR and HA + RAR treatments. Plants grown under phosphate starved condition showed enrichment of DEGs associated with response to carbohydrate.
Figure 5.
Functional enrichment analysis of the HA, RAR and HA + RAR treatments and its interactome analyses uncovers functional significance in these three samples. Node colour represent the different sample such as green, orange and pink colour showing the HA, RAR and HA + RAR samples. The size of the nodes shows the number (setSize) of differentially expressed genes identified in enrichment.
Validation of differentially expressed genes through real time qPCR
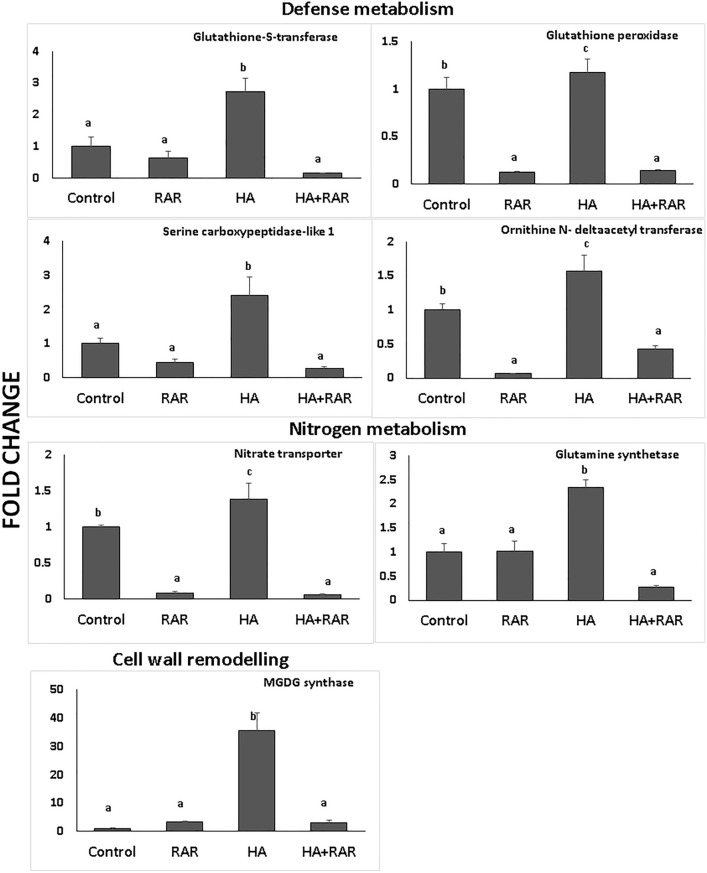
Overall transcriptome analysis revealed that phosphate starvation affected the defense metabolism, nitrogen metabolism, and cell wall remodelling in plants. Henceforth, differential expression of genes associated with these metabolic processes was assessed. Expression of genes associated with defense metabolism viz. glutathione S-transferase [GST; At1g78340.1], glutathione peroxidase [GPX; At4g31870.1], serine carboxypeptidase-like 1 [At5g36180.1] and ornithine N-delta-acetyltransferase [At2g39030.1] were significantly upregulated in A. thaliana under P starved condition as compared to control. On the contrary, their reduced expression was evident in the presence of RAR (Fig. 6). Elevated expression of genes associated with nitrogen metabolism i.e. nitrate transporter (At1g08090.1) glutamine synthetase gene (At5g16570.2) was found in the A. thaliana grown under P starved condition, however, inoculation of RAR significantly reduced the expression of both genes under stress condition. Similarly, genes involved in glycolipid biosynthesis [monogalactosyldiacylglycerol synthase (At5g20410.1)] was upregulated by ~ 35 under stress condition, while, significantly reduced expression was evident in RAR treated plants (RAR and HA+RAR). The results obtained were consistent with the transcriptome data indicating the reliability of high throughput NGS technologies for gene expression studies (Supplementary Fig. 3).
Figure 6.
Effect of phosphate accumulating Pseudomonas putida inoculation on expression of genes associated with defense metabolism, nitrogen metabolism and cell wall remodelling in Arabidopsis thaliana roots grown under phosphate starved condition.
Discussion
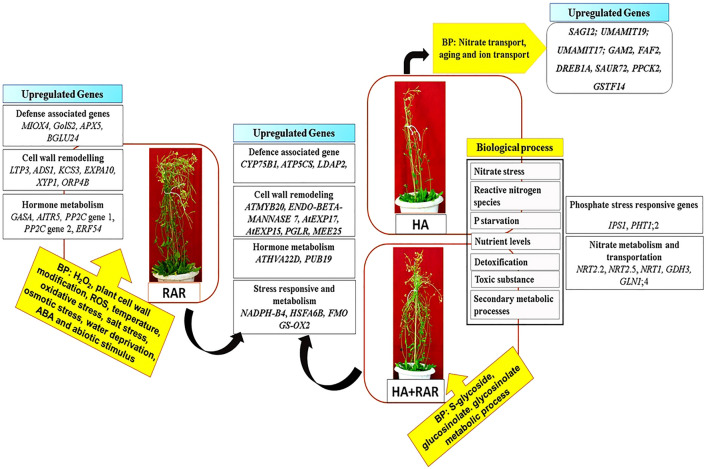
The study involves the elucidation of detailed molecular mechanisms occurring during the interaction of polyphosphate accumulating bacteria P. putida (RAR) with A. thaliana ecotype Col-0 grown under phosphate deprived conditions. Transcriptome analysis using RNA-Seq has been used to analyze genes that are differentially regulated by Pi deprivation and inoculation of RAR. Environmental factors substantially regulate the developmental program of root. Phosphate deprivation resulted in induced root hair formation under in vitro conditions in the present study. Increased density of root hairs enable the plants to meet the nutrient demand of the plant under inadequate supply28,29. RAR mediated enhanced root hair formation under both normal and stressed conditions is as per the earlier report30,31. Phosphorus acquisition by plants depends on a concerted action of a range of physiological and morphological adaptations which involve numerous signaling pathways32. Transcriptome analysis revealed differential expression of genes associated with the different processes in A. thaliana grown under different growth conditions viz. HA, RAR and HA + RAR. Modulations in different processes in plants grown in presence of RAR under phosphate starved condition is summarized in Fig. 7.
Figure 7.
A figure demonstrating the summary of overall modulations in different processes in Arabidopsis thaliana grown under phosphate starved condition in presence of phosphate accumulating Pseudomonas putida.
P. putida mediated altered metabolism, growth, and development of A. thaliana under phosphate starved condition
Phosphate starvation responsive genes are linked with multiple processes such as cell biogenesis, cellular transport mechanism, amino acid metabolism, response to stresses, photosynthesis, senescence, and others33. Present study also showed upregulation of different genes associated with these functions in plants. Upregulation of senescence associated gene (SAG12) under phosphate starved condition is in accordance to earlier report34. Pi starved condition in plants represses the photosynthesis resulting in carbon starvation which activate proteins and amino acid catabolism for release of free ammonium33. Present study showed the upregulation of usually multiple acids, in and out transporter genes (UMAMIT17 and UMAMIT19) responsible for amino acid transportation in plants.
Transcriptome analysis revealed enrichment of GO functions associated with transportation viz. phloem transport, nucleocytoplasmic transport, nuclear transport and vascular transport in RAR inoculated plants. These functions are involved in transportation of water, metabolites, sugar, constitutive nuclear proteins, import/export of key signalling molecules which is essential for growth, development, hormone signalling and responses to environmental stimuli35,36. In addition, RAR inoculated plants showed enrichment of indole glucosinolate biosynthetic process. As per earlier reports, indole glucosinolates are metabolically associated with auxin homeostasis in plants and its disruption negatively affect the plant growth and development6,37. Enrichment of pathways associated with stress regulation in the plants grown under P deprived condition in presence of RAR shows its involvement in stress management in plants under P deficient condition. Gene ontology study showed abundance of biological functions associated with the hypoxic conditions in the plants grown under phosphate deprived condition. The overlapping of transcriptional response to phosphate and oxygen deficiency leads to the induction of set of commonly induced genes which is under control of transcription factor phosphate starvation response1 (PHR1)14. Involvement of C2H2-type zinc finger like protein (At2g28710) in present study demonstrates its role in regulation of Pi starvation as reported earlier17.
P. putida mediated modulation in phytohormones in A. thaliana under phosphate starved condition
Phytohormones are known to regulate various biological processes linked to plant growth and stress response cascades38. Plants inoculated with RAR showed upregulation of numerous genes associated with hormone regulation such as gibberellic acid (GASA3 and GASA5), abscisic acid (AITR5, HAI1 and HAI2) and ethylene signalling (ERF54). Abscisic acid and gibberellins are well associated with plants developmental processes involving seed dormancy, root growth, flowering time and seed maturation38. Additionally, interaction of ethylene with other hormones regulates both growth and senescence in plants39. Auxin responsible for altering root system architecture under phosphate starvation condition40 was majorly upregulated in the plants treated with HA. SMALL AUXIN UP RNAs (SAURs) are early auxin response genes which regulate the growth and development of plants41. Present study showed upregulation of SAUR genes viz. SAUR6, SAUR41 and SAUR72 in plants subjected to phosphate starvation condition indicating the involvement of these genes under nutrient deprived condition.
P. putida mediated modulation in transporters under phosphate starved condition
Uptake of nutrient from soil is pivotal for plants’ growth and development which is achieved by a set of specialized transporters. These transporters are involved in sensing and radial transportation of water and nutrients to vascular tissues42. Present study showed modulations in transporters linked with different nutrient uptake in plants. Upregulation of nitrate transporters in HA and HA + RAR treatment observed in present study has also been demonstrated earlier that the role of nitrate transporters in regulating phosphate starvation response in plants43. High-affinity phosphate transporter 2 (PHT1;2; At5g43370) responsible for external inorganic phosphate uptake was overexpressed under both HA and HA + RAR conditions. Higher expression in HA + RAR as compared to HA treatment suggest the involvement of RAR in enhanced uptake of Pi under starved condition. CC-type glutaredoxins are expressed in plants to mediate signalling under nitrate deprived condition44. However, present study reports the involvement of CC-type glutaredoxin (ROXY) family (At5g11930) in plants grown under phosphate starved condition.
P. putida mediated management of reactive oxygen species in A. thaliana under phosphate starved condition
Significant accumulation of reactive oxygen species is being reported under phosphate deprived condition30. Genes associated with quenching of reactive oxygen species i.e. glutathione S-transferase was upregulated in phosphate starved plants as per earlier reports45. DREB1 gene activates the AtTPPF transcription which regulates the level of reactive oxygen species, trehalose and sucrose in plants during drought stress46. Present study also showed the upregulation of DREB1 gene under phosphate starved condition.
Gene expression study through qPCR revealed the activation of defense metabolism in A. thaliana under phosphate starved condition evident through higher expression of glutathione peroxidase, glutathione S-transferase, serine carboxypeptidase-like 1 and ornithine N-delta acetyl transferase. Lower expression of these genes in the presence of RAR reveals the availability of P to the plants under starved condition along with important role of PAB in regulating P homeostasis. Henceforth, the present study provides a detailed molecular mechanism of phosphate accumulating RAR mediated phosphate stress alleviation in A. thaliana. Numerous modification in metabolic pathways in RAR inoculated plants under phosphate starved condition indicates the substantial role of PAB in regulating P homeostasis in plants. Present study is the first report encompassing a regulatory network involve during the interaction of phosphate accumulating bacteria with A. thaliana under phosphate starved conditions. This progress will be beneficial in consideration of phosphate accumulation as an important trait of microorganisms for mediating P availability and regulating stress response in plants under P deficient conditions.
Material and methods
Characterization of phosphate accumulating P. putida
P. putida MTCC 5279, (RAR) already characterized as abiotic stress tolerant plant growth promoting bacterial strain47, with an ability to promote the growth of model plant A. thaliana under P starved salinity stressed conditions30. In present study, RAR was further characterized for phosphate accumulating potential and its growth in presence of different P sources. For phosphate accumulation, RAR was inoculated in NBRI-PA medium comprised of available form of P source i.e. disodium hydrogen orthophosphate (Na2HPO4) and potassium dihydrogen phosphate (KH2PO4) and accumulated Pi was determined upto 10 days. Inoculated NBRI-PA media was incubated at 28 °C for 48 h and bacterial cells were harvested by centrifugation at 10,000 rpm for 10 min at 4 °C. Extraction of Pi from bacterial cells was performed as described earlier by48. Extracted Pi was estimated by molybdenum blue method as described earlier49. Further, growth of RAR under varied P conditions viz. sufficient and deficient (50 µM KH2PO4, 1.5% HA and TCP) was evaluated by monitoring their growth followed by their viable cell count determination (CFU/ml) at different time intervals upto 10 days.
In-vitro interaction of P. putida with A. thaliana under phosphate starved condition
To evaluate the plant growth promoting potential of P. putida, its interaction with A. thaliana was performed on solid MS medium (Murashige and Skoog medium; 0.5x) and pot (soil rite) condition. Further, to elucidate the mechanism of interaction, a plant growth experiment was set up in a closed hydroponic system.
A. thaliana plants were grown on solid MS media plate supplemented with 1% sucrose (pH adjusted to 5.6) and 0.8% agar (solidifying agent). Surface sterilized pre-germinated one week old seedlings of A. thaliana was transferred in MS media plates in a single row containing available and unavailable source of P. P-starved condition was created by adding 500 µM of hydroxyapatite (HA) as unavailable phosphate source in MS media50. RAR was streaked at the bottom of the plates.
For pot experiments, surface sterilized seeds of A. thaliana was sown in soil rite as described earlier30. Log phase grown culture of RAR was inoculated around the roots and after 20 days phosphate starvation condition was created by supplying unavailable P (HA; 500 µM) and available limited P (50 µM P) to the plants. Treatments included control, RAR, HA, HA + RAR, 50 µM P and 50 µM P + RAR. Plants were harvested after 2 weeks of stress and root length, shoot length and dry weight of plants were recorded. Phosphate content was estimated in A. thaliana plants grown under pot conditions according to Tsvetkova and Georgiev (2003).
Further, for hydroponic experiment, surface sterilized pre-germinated seedlings of A. thaliana (Col-0) were transferred in liquid ½ MS media supplemented with 1% sucrose (pH adjusted to 5.6) in micro-centrifuge tubes. After 4 days, plants were inoculated with log phase grown culture of P. putida RAR (@1%, spun and resuspended in 20 mM magnesium sulphate, O.D. ~ 0.6). After 5 days, plants were transferred to the new micro-centrifuge tubes containing MS media supplemented with either available P source (KH2PO4) or unavailable source (500 µM of HA) and again RAR was inoculated in the plants. Treatments in the experiment were control (CONT), PAB (RAR), 500 µM of HA and HA + RAR. After 8 days of phosphate stress, root tissue of the plant was excised, washed and stored at − 80 °C for transcriptome study. This study involve the experiment based on Arabidopsis thaliana which comply with institutional, national, and international guidelines and legislation.
Illumina sequencing and mapping
Total RNA was extracted from the roots of hydroponically grown A. thaliana under different growth conditions viz. CONT., HA, RAR and HA + RAR. RNA was extracted using the ZR plant RNA miniprep (ZYMO Research) kit using the manufacturer’s protocol. The RNA sequencing library was prepared using TruSeq stranded mRNA library prep kit as per the manufacturer's protocol. The sequencing was done on the Illumina NextSeq500 platform to generate 2 × 75 bp reads. The sequencing data was generated using the fastaq format. The quality of the raw data was checked using FastQC52. Further, to eliminate the low quality reads Trimmomatic 0.36 tool was used53. These filtered and high quality reads were used for further analysis. The high quality reads were mapped onto the A. thaliana reference genome using the HISAT2 software54 using default parameters. After mapping, the resulting files were sorted and converted to BAM files using standard SAMtools55,56 followed by annotation and abundance estimation using the Stringtie program57.
Differential gene expression and GO analysis
The differential gene expression analysis was carried out using the edgeR package of the R studio58. The differential expression of the treated samples were carried out in comparison to the untreated control (RAR vs Cont, HA vs Cont, HA + RAR vs Cont). The differential genes were filtered based on P-value < 0.05 and a log2 fold change ≤ − 1 or ≥ 1. The differentially expressed genes in all three treatments were used for the plotting of heat maps using the MeV tool59. Based on the annotation from the TAIR database the differentially expressed genes were functionally categorized. Further, the differentially expressed genes selected from previous reports belonging to different processes viz. plant growth promotion, root system architecture, phytohormones, defense, and phosphate transportation in plants were visualized using the MapMan 3.5.1 software60. To know the function of genes, the GO annotations of the differentially expressed genes were fetched from the Arabidopsis information resource (TAIR) database using the bulk retrieval tool. The GO annotations were used for plotting the functional categories viz. biological processes, molecular functions, and cellular components. The GO enrichment analysis was done using the String database61. Further, the WebGestalt toolkit62 was used to identify the top ranking genes in each of the treatments.
Protein–protein interaction (PPI) network analysis
The sets of genes with log2FC ≥ 2 for up-regulated and ≤ -2 for down-regulated genes with Pvalue ≤ 0.05 were selected for functional enrichment study (FES). These putative enriched functions were analyzed and visualized in Cytoscape version 3.9.063 using the enrichment map module.
Availability of supporting data and accession number
The raw reads data is available at the NCBI Sequencing Read Archive (SRA; SUB11641967) database (Bioproject: PRJNA851914; https://www.ncbi.nlm.nih.gov/bioproject/?term=PRJNA851914).
Quantitative real time PCR analysis
Real time qPCR analysis of randomly selected genes from the study was performed for validation of results. Total RNA was extracted from hydroponically grown A. thaliana root tissue using RNA easy mini kit (Qiagen) according to the manufacturer’s instructions. DNase enzyme treated RNA was used for cDNA preparation through revertaid H minus cDNA synthesis kit (Thermo). Real time PCR was carried out with Quanti-Tect TM SYBR® Green PCR kit (Qiagen) on Stratagene Mx3000P systems with a 10 μl reaction system. The reaction mixture comprised of forward and reverse primer (0.5 μl each of 10 μM concentration), 5 μl SYBR green master, and cDNA. Cycle conditions included a preliminary step at 95 °C for 10 min, 40 cycles of denaturation and amplification at 94 °C for 30 s, 55 °C for the 30 s, and 72 °C for 30 s. Fold change was calculated from ct value by the delta-delta ct method. Each sample was analyzed in triplicate. The primer pairs used in the study are provided in Supplementary Table 1.
Statistical analysis
One-way analysis of variance (ANOVA) was performed to identify the significantly different treatments using SPSS 16.0.
Acknowledgements
Study is supported by network in-house project (OLP0109) of CSIR. So.S is thankful to Department of Science and Technology, India for research fellowship. So.S also thank AcSIR for academic support.
Author contributions
S.S. conceived the idea. So.S. performed the experiments. M.R., N.B. and M.H.A. analyzed the data and prepared figures. So.S. wrote the main manuscript. All authors reviewed the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mehar Hasan Asif, Email: mh.asif@nbri.res.in.
Suchi Srivastava, Email: ssnbri@gmail.com.
References
- 1.Benjamin P, Mathilde C, Laurent N, Thierry D. Root developmental adaptation to phosphate starvation: Better safe than sorry. Trends Plant Sci. 2011;16:442–450. doi: 10.1016/j.tplants.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 2.Carstensen A, et al. The impacts of phosphorus deficiency on the photosynthetic electron transport chain1[OPEN] Plant Physiol. 2018;177:271–284. doi: 10.1104/pp.17.01624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uhde-Stone C, et al. Acclimation of white lupin to phosphorus deficiency involves enhanced expression of genes related to organic acid metabolism. Plant Soil. 2003;248:99–116. doi: 10.1023/A:1022335519879. [DOI] [Google Scholar]
- 4.Vance CP, Uhde-Stone C, Allan DL. Phosphorus acquisition and use: Critical adaptations by plants for securing a nonrenewable resource. New Phytol. 2003;157:423–447. doi: 10.1046/j.1469-8137.2003.00695.x. [DOI] [PubMed] [Google Scholar]
- 5.Shenoy VV, Kalagudi GM. Enhancing plant phosphorus use efficiency for sustainable cropping. Biotechnol. Adv. 2005;23:501–513. doi: 10.1016/j.biotechadv.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 6.Chien PS, Chiang CP, Leong SJ, Chiou TJ. Sensing and signaling of phosphate starvation: From local to long distance. Plant Cell Physiol. 2018;59:1714–1722. doi: 10.1093/pcp/pcy148. [DOI] [PubMed] [Google Scholar]
- 7.Karthikeyan AS, et al. Phosphate starvation responses are mediated by sugar signaling in Arabidopsis. Planta. 2007;225:907–918. doi: 10.1007/s00425-006-0408-8. [DOI] [PubMed] [Google Scholar]
- 8.Pant BD, et al. Identification of primary and secondary metabolites with phosphorus status-dependent abundance in Arabidopsis, and of the transcription factor PHR1 as a major regulator of metabolic changes during phosphorus limitation. Plant Cell Environ. 2015;38:172–187. doi: 10.1111/pce.12378. [DOI] [PubMed] [Google Scholar]
- 9.Liu TY, et al. Identification of plant vacuolar transporters mediating phosphate storage. Nat. Commun. 2016;7:11095. doi: 10.1038/ncomms11095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song L, et al. The molecular mechanism of ethylene-mediated root hair development induced by phosphate starvation. PLoS Genet. 2016;12:e1006194. doi: 10.1371/journal.pgen.1006194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Plaxton WC, Tran HT. Metabolic adaptations of phosphate-starved plants. Plant Physiol. 2011;156:1006–1015. doi: 10.1104/pp.111.175281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ueda Y, Kiba T, Yanagisawa S. Nitrate-inducible NIGT1 proteins modulate phosphate uptake and starvation signalling via transcriptional regulation of SPX genes. Plant J. 2020;102:448–466. doi: 10.1111/tpj.14637. [DOI] [PubMed] [Google Scholar]
- 13.Maeda Y, et al. A NIGT1-centred transcriptional cascade regulates nitrate signalling and incorporates phosphorus starvation signals in Arabidopsis. Nat. Commun. 2018;9:1376. doi: 10.1038/s41467-018-03832-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klecker M, et al. A shoot-specific hypoxic response of arabidopsis sheds light on the role of the phosphate-responsive transcription factor PHOSPHATE STARVATION RESPONSE1. Plant Physiol. 2014;165:774–790. doi: 10.1104/pp.114.237990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang H, et al. Arabidopsis WRKY45 transcription factor activates Phosphate transporter1;1 expression in response to phosphate starvation. Plant Physiol. 2014;164:2020–2029. doi: 10.1104/pp.113.235077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dai X, Wang Y, Zhang WH. OsWRKY74, a WRKY transcription factor, modulates tolerance to phosphate starvation in rice. J. Exp. Bot. 2016;67:947–960. doi: 10.1093/jxb/erv515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Devaiah BN, Nagarajan VK, Raghothama KG. Phosphate homeostasis and root development in arabidopsis are synchronized by the zinc finger transcription factor ZAT6. Plant Physiol. 2007;145:147–159. doi: 10.1104/pp.107.101691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu H, et al. Overexpressing HRS1 confers hypersensitivity to low phosphate-elicited inhibition of primary root growth in Arabidopsis thaliana. J. Integr. Plant Biol. 2009;51:382–392. doi: 10.1111/j.1744-7909.2009.00819.x. [DOI] [PubMed] [Google Scholar]
- 19.Ramaiah M, Jain A, Raghothama KG. ETHYLENE RESPONSE FACTOR070 regulates root development and phosphate starvation-mediated responses. Plant Physiol. 2014;164:1484–1498. doi: 10.1104/pp.113.231183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Y, et al. Overexpression of a phosphate starvation response ap2/erf gene from physic nut in arabidopsis alters root morphological traits and phosphate starvation-induced anthocyanin accumulation. Front. Plant Sci. 2018;9:1186. doi: 10.3389/fpls.2018.01186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oberson A, Joner EJ. Microbial turnover of phosphorus in soil. Org. Phosphorus Environ. 2004 doi: 10.1079/9780851998220.0133. [DOI] [Google Scholar]
- 22.Achat DL, et al. Assessing turnover of microbial biomass phosphorus: Combination of an isotopic dilution method with a mass balance model. Soil Biol. Biochem. 2010;42:2231–2240. doi: 10.1016/j.soilbio.2010.08.023. [DOI] [Google Scholar]
- 23.Richardson A, Simpson R. Soil microorganisms mediating phosphorus availability update on microbial phosphorus. Plant Physiol. 2011;156:989–996. doi: 10.1104/pp.111.175448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Esberg C, du Toit B, Olsson R, Ilstedt U, Giesler R. Microbial responses to P addition in six South African forest soils. Plant Soil. 2010;329:209–225. doi: 10.1007/s11104-009-0146-3. [DOI] [Google Scholar]
- 25.Sandhya V, Ali SZ, Grover M, Reddy G, Venkateswarlu B. Effect of plant growth promoting Pseudomonas spp. on compatible solutes, antioxidant status and plant growth of maize under drought stress. Plant Growth Regul. 2010;62:21–30. doi: 10.1007/s10725-010-9479-4. [DOI] [Google Scholar]
- 26.Srivastava S, et al. Functional Genetic diversity and plant growth promoting potential of polyphosphate accumulating bacteria in soil. Microbiol. Spectr. 2022;10:e00345-21. doi: 10.1128/spectrum.00345-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chauhan PS, et al. Transcriptional alterations reveal Bacillus amyloliquefaciens-rice cooperation under salt stress. Sci. Rep. 2019;9:11912. doi: 10.1038/s41598-019-48309-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma Z, Bielenberg DG, Brown KM, Lynch JP. Regulation of root hair density by phosphorus availability in Arabidopsis thaliana. Plant Cell Environ. 2001;24:459–467. doi: 10.1046/j.1365-3040.2001.00695.x. [DOI] [Google Scholar]
- 29.Müller M, Schmidt W. Environmentally induced plasticity of root hair development in Arabidopsis. Plant Physiol. 2004;134:409–419. doi: 10.1104/pp.103.029066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Srivastava S, Srivastava S. Prescience of endogenous regulation in Arabidopsis thaliana by Pseudomonas putida MTCC 5279 under phosphate starved salinity stress condition. Sci. Rep. 2020;10:5855. doi: 10.1038/s41598-020-62725-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jatan R, Chauhan PS, Lata C. High-throughput sequencing and expression analysis suggest the involvement of Pseudomonas putida RA-responsive micrornas in growth and development of arabidopsis. Int. J. Mol. Sci. 2020;21:1–18. doi: 10.3390/ijms21155468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hell R, Stephan UW. Iron uptake, trafficking and homeostasis in plants. Planta. 2003;216:541–551. doi: 10.1007/s00425-002-0920-4. [DOI] [PubMed] [Google Scholar]
- 33.Wu P, et al. Phosphate starvation triggers distinct alterations of genome expression in arabidopsis roots and leaves. Plant Physiol. 2003;132:1260–1271. doi: 10.1104/pp.103.021022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aleksza D, Horváth GV, Sándor G, Szabados L. Proline accumulation is regulated by transcription factors associated with phosphate starvation. Plant Physiol. 2017;175:555–567. doi: 10.1104/pp.17.00791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lucas WJ, et al. The plant vascular system: Evolution, development and functions. J. Integr. Plant Biol. 2013;55:294–388. doi: 10.1111/jipb.12041. [DOI] [PubMed] [Google Scholar]
- 36.Tamura K, Hara-Nishimura I. Functional insights of nucleocytoplasmic transport in plants. Front. Plant Sci. 2014;5:118. doi: 10.3389/fpls.2014.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Malka SK, Cheng Y. Possible interactions between the biosynthetic pathways of indole glucosinolate and Auxin. Front. Plant Sci. 2017;8:2131. doi: 10.3389/fpls.2017.02131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shu K, Zhou W, Chen F, Luo X, Yang W. Abscisic acid and gibberellins antagonistically mediate plant development and abiotic stress responses. Front. Plant Sci. 2018;9:416. doi: 10.3389/fpls.2018.00416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iqbal N, et al. Ethylene role in plant growth, development and senescence: interaction with other phytohormones. Front. Plant Sci. 2017;8:475. doi: 10.3389/fpls.2017.00475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nacry P, et al. A role for auxin redistribution in the responses of the root system architecture to phosphate starvation in Arabidopsis. Plant Physiol. 2005;138:2061–2074. doi: 10.1104/pp.105.060061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ren H, Gray WM. SAUR proteins as effectors of hormonal and environmental signals in plant growth. Mol. Plant. 2015;8:1153–1164. doi: 10.1016/j.molp.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zelazny E, Vert G. Plant nutrition: Root transporters on the move. Plant Physiol. 2014;166:500–508. doi: 10.1104/pp.114.244475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bao S, An L, Su S, Zhou Z, Gan Y. Expression patterns of nitrate, phosphate, and sulfate transporters in Arabidopsis roots exposed to different nutritional regimes. Botany. 2011;89:647–653. doi: 10.1139/b11-053. [DOI] [Google Scholar]
- 44.Jung JY, Ahn JH, Schachtman DP. CC-type glutaredoxins mediate plant response and signaling under nitrate starvation in Arabidopsis. BMC Plant Biol. 2018;18:1–13. doi: 10.1186/s12870-018-1512-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fukushima A, et al. Effects of combined low glutathione with mild oxidative and low phosphorus stress on the metabolism of arabidopsis thaliana. Front. Plant Sci. 2017;8:1464. doi: 10.3389/fpls.2017.01464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lin Q, et al. Overexpression of the trehalose-6-phosphate phosphatase family gene AtTPPF improves the drought tolerance of Arabidopsis thaliana. BMC Plant Biol. 2019;19:1–15. doi: 10.1186/s12870-019-1986-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Srivastava S, et al. Gene expression profiling through microarray analysis in Arabidopsis thaliana colonized by pseudomonas putida MTCC5279, a plant growth promoting rhizobacterium. Plant Signal. Behav. 2012;7:235–245. doi: 10.4161/psb.18957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chaudhry V, Nautiyal CS. A high throughput method and culture medium for rapid screening of phosphate accumulating microorganisms. Bioresour. Technol. 2011;102:8057–8062. doi: 10.1016/j.biortech.2011.05.045. [DOI] [PubMed] [Google Scholar]
- 49.Muyima NYO, Cloete TE. Phosphate uptake by immobilized Acinetobacter calcoaceticus cells in a full scale activated sludge plant. J. Ind. Microbiol. 1995;15:19–24. doi: 10.1007/BF01570008. [DOI] [PubMed] [Google Scholar]
- 50.Hiruma K, et al. Root endophyte colletotrichum tofieldiae confers plant fitness benefits that are phosphate status dependent. Cell. 2016;165:464–474. doi: 10.1016/j.cell.2016.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tsvetkova GE. Effect of phosphorus nutrition on the nodulation, nitrogen fixation and nutrient use efficiency of Bradyrhizobiumjaponicum-soybean (Glycine max L. Merr.) symbiosis. Bulg. J. Plant. Physiol. 2003;3:331–335. [Google Scholar]
- 52.Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data (2010).
- 53.Bolger AM, Lohse M, Usadel B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim D, Langmead B, Salzberg SL. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods. 2015;12:357–360. doi: 10.1038/nmeth.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pertea M, Kim D, Pertea GM, Leek JT, Salzberg SL. Transcript-level expression analysis of RNA-seq experiments with HISAT. StringTie Ballgown. Nat. Protoc. 2016;11:1650–1667. doi: 10.1038/nprot.2016.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li H, et al. The sequence alignment/map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pertea M, et al. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015;33:290–295. doi: 10.1038/nbt.3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Robinson MD, McCarthy DJ, Smyth GK. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2009;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Howe EA, Sinha R, Schlauch D, Quackenbush J. RNA-Seq analysis in MeV. Bioinformatics. 2011;27:3209–3210. doi: 10.1093/bioinformatics/btr490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thimm O, et al. MAPMAN: A user-driven tool to display genomics data sets onto diagrams of metabolic pathways and other biological processes. Plant J. 2004;37:914–939. doi: 10.1111/j.1365-313X.2004.02016.x. [DOI] [PubMed] [Google Scholar]
- 61.Franceschini A, et al. STRING v9.1: Protein-protein interaction networks, with increased coverage and integration. Nucleic Acids Res. 2013;41:808–809. doi: 10.1093/nar/gks1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang B, Kirov S, Snoddy J. WebGestalt: An integrated system for exploring gene sets in various biological contexts. Nucleic Acids Res. 2005;33:W741–W748. doi: 10.1093/nar/gki475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shannon P, et al. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]