Abstract
Background.
While ADHD has been associated with differences in the structural connections formed by the brain’s white matter tracts, studies of such differences have returned inconsistent findings, likely reflecting small sample sizes. Thus, we conducted a mega-analysis on in vivo measures of white matter microstructure obtained through diffusion tensor imaging of over 6000 participants, from five cohorts.
Methods.
In a mega-analysis, linear mixed models tested for associations between the fractional anisotropy of 42 white matter tracts and ADHD traits and diagnosis. Contrasts were made against measures of mood, anxiety, and other externalizing problems.
Findings.
Overall, 6993 participants (between ages 6 to 18 years, mean 10.62 [SD 1.99]; 3,368 girls, 3,625 boys; 4146 white, non-Hispanic, 764 African American, 2083 other race/ethnicities) had either measures of ADHD and other emotional/behavioral symptoms (N=6933) and/or enough clinical data to allow a diagnosis of ADHD (N=951) or its absence (N=4884). Both the diagnosis and symptoms of ADHD were associated with lower fractional anisotropy of inferior longitudinal and left uncinate fasciculi (at FDR adjusted p<0.05). Associated effect sizes were small (the strongest association with ADHD traits had an effect size of partial-r=−0.14, while the largest case control difference was associated with an effect size of d=−0.3). Similar microstructural anomalies were not present for anxiety, mood, or externalizing problems. Findings held when ADHD cases and controls were matched on in-scanner motion.
Interpretation.
While present across cohorts, ADHD-associated microstructural differences had small effects, underscoring the limited clinical utility of this imaging modality in isolation.
Keywords: ADHD, diffusion tensor imaging, mega-analysis, white matter tracts, big data, fractional anisotropy
Introduction
ADHD is increasingly conceptualized as a disorder of connectivity, a “dysconnectome” in which microstructural differences in the white matter tracts that form structural connections in the brain underlie disruptions in large-scale brain systems that are tied to symptoms (1, 2). The evidence for such altered structural connectivity rests mainly upon cross-sectional demonstrations of ADHD-associated differences in a marker of white matter microstructure, fractional anisotropy, as measured by diffusion tensor imaging (DTI). While four meta-analyses point to alterations both in the longitudinal fasciculi that connect different cortical regions and to the corpus callosum that connects the two hemispheres, there is little consensus on the exact regions and tracts that are compromised (3–6). Furthermore, one meta-analysis posited that head motion may be driving most diagnostic differences, highlighting the need for strict control of this parameter (4). The lack of consistent findings likely reflects differences in criteria for data selection, methods of synthesis and the reliance upon published findings via coordinates-based meta-analytic methods, rather than the use of ‘raw’ diffusion tensor imaging data. These meta-analyses have typically used Activation Likelihood Estimation (ALE) or Seed-based d Mapping (SDM), or both (7, 8). These methods differ in the way that they synthesize data. For example, only SDM allows for overlapping increases and decreases from different studies to be synthesized together, so that contradictory findings cancel each other out (7). Furthermore, for inclusion in most coordinate-based meta-analyses, a cluster must meet statistical significance in the original paper, with subthreshold group differences often unavailable or not includable. This is problematic when considering the lack of statistical power in most neuroimaging studies and the problems related to publication bias, which each inflate type-I and type-II error rates, and which thus cause significant problems for retrospective quantitative summaries of the literature (9, 10).
Here, we attempt to overcome these issues by analyzing data from five datasets, with a total of 6993 participants, in an effort to identify signals that emerge when combining multiple cohorts. The use of original DTI data, processed at one site in a uniform manner, also allows us to control for critical potential confounders including in-scanner motion through procedures such as nearest neighbor matching based on motion parameters.
In this study we consider both ADHD traits as measured by a parent/caregiver completed Child Behavior Check List (CBCL), and the diagnosis of ADHD, ascertained by DSM-based interviews (11, 12). We predicted that ADHD traits and diagnoses would be associated with similar differences in white matter microstructure, as has been found for ADHD-related differences in cortical anatomy (13). We also aimed to determine the specificity of white matter differences related to ADHD symptoms through a contrast with the other empirical scales extracted from the CBCL.
Methods
Cohorts
We included data from the Healthy Brain Network (HBN), Adolescent Brain and Cognitive Development (ABCD), Neurobehavioral Clinical Research (NCR), National Consortium on Alcohol and Neurodevelopment in Adolescence (NCANDA), and Human Connectome Project – Developmental (HCP-D) datasets (14–19). The Supplemental Methods summarize each study’s recruitment methods and sampling strategies, protocols and image acquisition parameters. All studies had IRB/ethical approval and acquired informed assent and/or consent using IRB approved procedures. For the questionnaire-based analyses, the main inclusion criteria were ages ≥6 years and ≤18 with an IQ>70, availability of all covariate data, and availability of parent/caregiver completed questionnaire CBCL data. In the primary analyses we consider raw scores of the six empirical scales which are based on factor analyses of the items and include attention problems (11, 12). Raw scores were analyzed in this study because age and sex were included as covariates in the regression models. For the case-control analyses, ADHD was defined using DSM criteria (diagnostic data were available for all cohorts except HCP-D). Unaffected controls were those with no ADHD diagnosis and not on any psychotropic medications. See Supplemental Methods for further details.
Diffusion tensor imaging of white matter tracts
Choice of primary outcome measure
The primary outcome variable was fractional anisotropy calculated for the brain’s 42 major white matter tracts as defined by the IIT Human Brain Atlas version 5.0 (20). This measure was chosen as it is the most widely used index of microstructure, and it has been the focus of the four meta-analyses of DTI studies in ADHD (3–6). We also include results for other DTI indexes in the supplemental material.
We conducted analyses at two levels of data quality. In the main analyses, we excluded individuals who had any of the six-motion parameters that lay in the worst 10%. This left 6993 (79.8% of subjects with processed DTI images and necessary covariate data) individuals with scans, of whom 6933 had CBCL scores available and 5835 had ADHD diagnostic data. We next removed as an outlier any tract values that lay outside the mean +− 3 SDs interval for that tract within each cohort (at this stage the removal was of tract values not entire individual datasets). We repeated analyses at a more stringent level of quality control, removing those who had any motion parameter in the top 20%, leaving 5533 (63.14% of subjects with processed DTI images and necessary covariate data). Further discussions on excluded subjects are given in the Supplementary Methods. Comparisons between subjects included and excluded at each level of stringency are provided in Supplementary Tables 1 and 2, and Supplementary Figure 1.
Analyses
In the primary analysis, we tested if tracts differed in their associations with ADHD traits and diagnoses. Specifically, we tested for the interaction between tract identity and attention problems scores or ADHD diagnosis on fractional anisotropy, while including demographic and in-scanner motion covariates as fixed effects, and including subject as a random intercept term, nested within cohort, site, and family. A significant interaction would indicate that the association between fractional anisotropy and ADHD differs according to anatomical location, ruling out a uniform global association between fractional anisotropy and the disorder. Following this, linear mixed models tested for an association between the predictor (the empirical CBCL problem scales, or ADHD diagnosis) and the fractional anisotropy for each individual tract. Following this, linear mixed models tested for an association between the predictor (the empirical CBCL problem scales, or ADHD diagnosis) and the fractional anisotropy for each individual tract. We control for variables for which there is evidence they are a cause of ADHD, or of white matter tract variation, or of both (21). We adjusted for age, sex, socio-economic status and race/ethnicity, given evidence of effects of these demographic variables on ADHD prevalence (22, 23) and white matter tract microstructure (24, 25). We also controlled for in-scanner motion. The individual motion parameters were highly correlated and we thus conducted a principal components analysis of the six parameters and their squares, and retained the first three principal components for motion which explained 85.4% of the total variance – Supplementary Figure 2.
Measures of intelligence may in part be impacted by ADHD, and not just temporally antecedent and thus we conduct analyses both with and without this variable (26–28). Random intercept terms for cohort, site, and nuclear family identity were included. To examine the specificity of findings to the attention problems subscale, we also examined associations between FA and scores on the remaining CBCL empirical scales. Adjustment was made for tests conducted for the 42 tracts and the nine predictors (diagnosis and the eight CBCL empirical scales) for a total of 378 tests, using FDR procedures with adjusted p<0.05 taken as significant (29). We used the linear.hypothesis function from the car package (30) for R (version 4.0.2; http://www.r-project.org) to test whether the strength of the associations between each tract and the attention problems scale was significantly different from the associations involving each of the remaining CBCL empirical scales (see Supplementary Methods for details).
Robustness analyses
We checked the robustness of results by using an approach based on complementary pairs stability (31). In this approach, the entire sample is randomly split into two independent halves (resamples) multiple times, such that there are no statistical differences between halves in any of the covariates within cohort (p > .1), and individuals of the same family are confined to the same half. We test repeatedly for associations between the predictor (ADHD traits or diagnosis) and outcome (white matter tract) on each half in each resample, declaring an association to be corroborated if it is present in both halves at a p<0.05. We then note the proportion of ‘corroborated’ associations (e.g., if it is present in both halves in 20 of the 100 splits, then n=20%). We interpret the distribution of n against a null distribution of n, derived through permutations that randomly shuffle the white matter tract values across subjects. This approach is similar in spirit to testing for association with independent discovery and replication samples. The approach is superior to a single split of our cohort, multiple splits can reduce the variability of statistics due to sampling variability (32, 33), and has been applied to GWAS (34).
We furthermore performed a number of sensitivity analyses to assess the robustness of the results. As noted above, we examined the results using a stricter threshold (being in the worst 20% of any motion-related parameter). We also performed analyses on samples matched on in-scanner motion, using the propensity matching without replacement algorithms available in the MatchIt package version 4.3.2 in R (35)– see Supplemental Methods.
Additionally, we include analyses controlling for IQ, as well as analyses performed after removing those with possible bipolar affective and/or psychotic symptoms. We also consider the possibility that parents under-report attention problems and ADHD symptoms as they think of their child’s symptoms while ‘on medication’. In line with previous similar studies (36), we replaced the attention problem score of a child on stimulants with the mean score for all children who were on psychostimulants or other medication for ADHD that had the same or a higher attention problems score. Finally, we contrasted the results from ‘clinical’ (cohorts with diagnosis of ADHD >50%) and ‘population’ cohorts (which had prevalence rates of ADHD at rates of 10% or lower). Further details on these robustness checks are given in the Supplement.
Exploratory axial diffusivity, radial diffusivity and voxelwise analyses
While in the primary analysis we focused on tract-level estimates of fractional anisotropy, we also report supplementary findings based on axial diffusivity, which is the coefficient of diffusion along the axon (or the long axis of the tensor ellipsoid) along with radial diffusivity, or the coefficient of diffusion perpendicular to the long axis.
We further supplemented our fractional anisotropy analyses performed at the tract level with voxelwise examinations. This allowed us to examine for any potential local, sub-tract associations between fractional anisotropy and ADHD traits and diagnosis, in addition to leveraging even further the benefits of using a single analysis pipeline across cohorts. Full details are given in the Supplemental Methods.
Results
There was a highly significant interaction between tract identity and the attention problem scale (χ(41)2=299, p<0.00001) as well as between tract identity and ADHD diagnosis (χ(41)2=632, p<0.00001) on fractional anisotropy, suggesting that tracts differed in their associations with ADHD traits and diagnosis, rather than having uniform, global associations with these clinical variables. Overall, 6933 subjects contributed to the analyses using the CBCL - Table 1. Significant associations emerged in the mega-analysis between attention problem scores and lower fractional anisotropy of the left inferior longitudinal fasciculus (B=−0.0002 [SE 0.00007], t=−3.53, p=0.0004, adjusted p=0.048), and the left uncinate fasciculus (B=−0.0003 [SE 0.00008], t=−4.13, p=0.00004, adjusted p=0.02). The effect sizes were small. For left inferior longitudinal fasciculus the effect size was partial-r=−0.12 (95%CI −0.19 to −0.05), while for the left uncinate fasciculus the effect size was partial-r=−0.14 (95%CI −0.21 to −0.07). The associations between the uncinate fasciculus and the attention problems scales were, for the most part, significantly different than their associations with all other CBCL empirical scales, except for the social problems scale. For the inferior longitudinal fasciculus, the association with the attention problems scale differed relative to those observed for the anxious-depressed, somatic problems, aggressive problems and thought problem scales. No other CBCL problems, measured by the empirical scales, were associated with fractional anisotropy of any tract at adjusted levels of significance, although nominal associations emerged particularly for social problems. See Figures 1 & 2, Table 3 and Supplementary Tables 3 and 4.
Table 1.
Demographic details on the five cohorts used in the current study for N=6933 subjects included in the primary analyses of associations between white matter microstructure and scores on the empirical CBCL scales.
| Cohort | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| ABCD | HBN | HCP-D | NCANDA | NCR | Total | |
| Total (N) | N=5420 | N=564 | N=292 | N=516 | N=141 | N=6933 |
|
| ||||||
| Female N (%) | 2639 | 219 | 166 | 264 | 63 | 3351 |
| (48.69%) | (38.83%) | (56.85%) | (51.16%) | (44.68%) | (48.33%) | |
| Male N (%) | 2781 | 345 | 126 | 252 | 78 | 3582 |
| (51.31%) | (61.17%) | (43.15%) | (48.84%) | (55.32%) | (51.67%) | |
|
| ||||||
| Age: mean (SD) years | 9.96 (0.63) | 11.36 (3.21) | 12.62 (2.6) | 15.05 (1.66) | 12.07 (2.91) | 10.6 (1.97) |
|
| ||||||
| IQ: mean (SD) | - | 101.26 (15.89) | - | 114.62 (13.53) | 110.29 (15.49) | 107.95 (16.17) |
|
| ||||||
| Scaled matrix: mean (SD) | 10.24 (2.8) | - | 11.62 (3.28) | - | - | 10.31 (2.84) |
|
| ||||||
| Race/ethnicity | ||||||
|
| ||||||
| White, non-Hispanic | 3211 (59.24%) | 271 (48.05%) | 192 (65.75%) | 333 (64.53%) | 100 (70.92%) | 4107 (59.24%) |
| African American/Black | 604 (11.14%) | 54 (9.57%) | 18 (6.16%) | 66 (12.79%) | 13 (9.22%) | 755 (10.89%) |
| Other | 1605 (29.61%) | 239 (42.38%) | 82 (28.08%) | 117 (22.67%) | 28 (19.86%) | 2071 (29.87%) |
|
| ||||||
| Household income | ||||||
|
| ||||||
| <$50k per annum | 1437 (26.51%) | 115 (20.39%) | 36 (12.33%) | 86 (16.67%) | 19 (13.48%) | 1693 (24.42%) |
| $50-$100k | 1599 (29.5%) | 127 (22.52%) | 66 (22.6%) | 134 (25.97%) | 31 (21.99%) | 1957 (28.23%) |
| $100-$200k | 1744 (32.18%) | 116 (20.57%) | 138 (47.26%) | 170 (32.95%) | 38 (26.95%) | 2206 (31.82%) |
| >200k | 640 (11.81%) | 206 (36.52%) | 52 (17.81%) | 126 (24.42%) | 53 (37.59%) | 1077 (15.53%) |
Abbreviations. ABCD, Adolescent Brain Cognitive Development; HBN, Healthy Brain Network; HCP-D, Human Connectome Project – Development; IQ, intelligence quotient; NCANDA, National Consortium on Alcohol and Neurodevelopment in Adolescence; NCR, Neurobehavioral Clinical Research.
Figure 1.
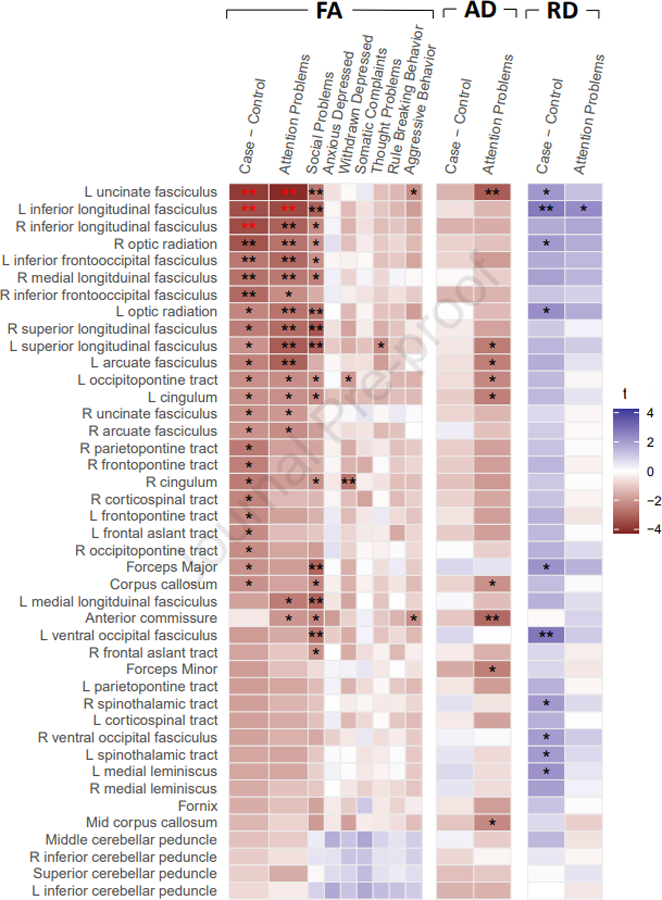
Association between fractional anisotropy of white matter tracts and ADHD diagnosis, and scores on the empirical subscales from the Child Behavior Checklist. Colors indicate t-statistic, single black stars are nominally significant p < .05, double black stars at p < .01, and double red stars mark associations significant at FDR adjusted p < .05. Results for AD and RD metrics are exploratory only, and displayed for comparison to the main FA results.
Figure 2.
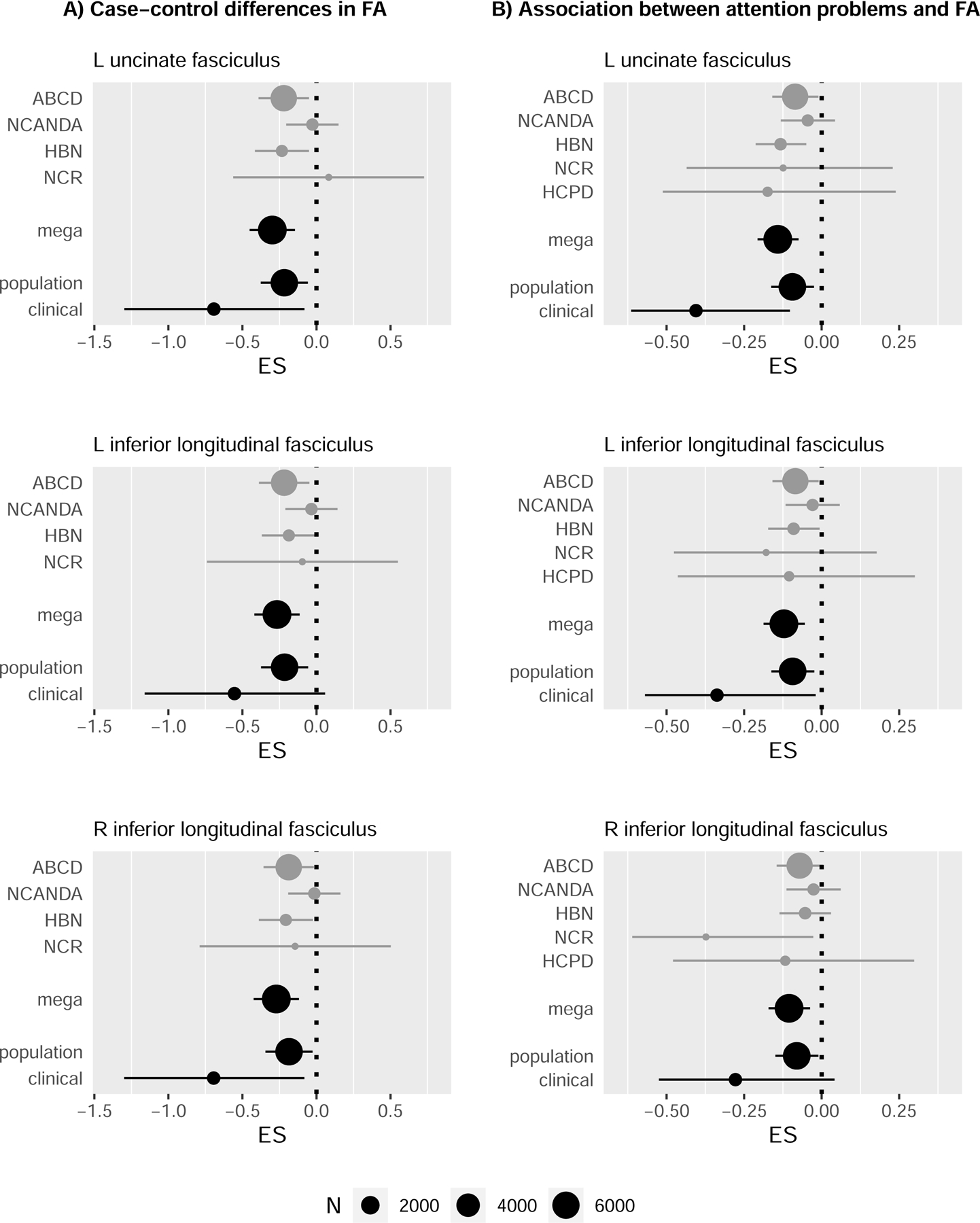
Forest plots showing the effect sizes for associations between fractional anisotropy and (A) the diagnosis of ADHD; and (B) CBCL attention problems scale. The individual cohorts are given, followed by the mega-analytic effect size, then results for the population and clinic-based cohorts. Effect sizes are estimated using Cohen’s d in (A) and partial-r in (B). The association between the FA in the right inferior longitudinal fasciculus and attention problems (lower, right plot) only reached nominal significance p < 0.01 in the main analysis, but it is displayed here for completeness.
Table 3.
Significant associations (FDR p<0.05) from the primary analysis between fractional anisotropy and scores on the attention problems scale and/or ADHD diagnosis. Also shown are the results of the sensitivity analyses examining the robustness of the association for these tracts. Results are summarized for all tracts and models in the Supplementary Results.
| Independent variable | Model | N | Β | SE | t | Uncorrected p | FDR-p | Effect size: partial-r or cohen’s d (95% confidence interval). |
|---|---|---|---|---|---|---|---|---|
| Left inferior longitudinal fasciculus | ||||||||
|
| ||||||||
| Primary analysis | Attention problems scale | 6904 | −0.0002 | 0.00007 | −3.53 | 0.0004 | 0.02 | −0.12 (−0.19 to −0.05) |
| Stringent QC threshold (worst 20% of subjects on any motion parameter excluded) | Attention problems scale | 5453 | −0.0002 | 0.00008 | −2.94 | 0.003 | - | −0.12 (−0.2 to −0.04) |
| Matched on covariates including QC variables | Attention problems scale | 6096 | −0.0002 | 0.00007 | −3.13 | 0.002 | - | −0.12 (−0.19 to −0.04) |
| Controlling for IQ | Attention problems scale | 6904 | −0.0002 | 0.00007 | −3.28 | 0.001 | - | −0.11 (−0.18 to −0.05) |
| Excluding subjects with possible bipolar and/or psychosis symptoms | Attention problems scale | 6473 | −0.0003 | 0.00008 | −3.91 | 0.0001 | - | −0.14 (−0.21 to −0.07) |
| CBCL scores adjusted for medication status | Attention problems scale | 6904 | −0.0002 | 0.00006 | −3.58 | 0.0004 | - | −0.12 (−0.19 to −0.06) |
| Primary analysis | ADHD diagnosis | 5811 | −0.003 | 0.0008 | −3.43 | 0.0006 | 0.048 | −0.27 (−0.42 to −0.11) |
| Stringent QC threshold (worst 20% of subjects on any motion parameter excluded) | ADHD diagnosis | 4665 | −0.003 | 0.0009 | −2.95 | 0.003 | - | −0.26 (−0.44 to −0.09) |
| Matched on covariates including QC variables | ADHD diagnosis | 2084 | −0.003 | 0.001 | −2.61 | 0.01 | - | −0.52 (−0.92 to −0.12) |
| Controlling for IQ | ADHD diagnosis | 5811 | −0.003 | 0.0008 | −3.2 | 0.001 | - | −0.25 (−0.4 to −0.1) |
| Excluding subjects with possible bipolar and/or psychosis symptoms | ADHD diagnosis | 5504 | −0.003 | 0.0009 | −3.51 | 0.0005 | - | −0.28 (−0.44 to −0.12) |
|
| ||||||||
| Left uncinate fasciculus | ||||||||
|
| ||||||||
| Primary analysis | Attention problems scale | 6911 | −0.0003 | 0.00008 | −4.13 | 0.00004 | 0.02 | −0.14 (−0.21 to −0.07) |
| Stringent QC threshold (worst 20% of subjects on any motion parameter excluded) | Attention problems scale | 5454 | −0.0003 | 0.00009 | −3.41 | 0.0007 | - | −0.14 (−0.21 to −0.06) |
| Matched on covariates including QC variables | Attention problems scale | 6104 | −0.0003 | 0.00008 | −3.39 | 0.0007 | - | −0.13 (−0.2 to −0.05) |
| Controlling for IQ | Attention problems scale | 6911 | −0.0003 | 0.00008 | −3.88 | 0.0001 | - | −0.13 (−0.2 to −0.07) |
| Excluding subjects with possible bipolar and/or psychosis symptoms | Attention problems scale | 6478 | −0.0004 | 0.00009 | −4.26 | 0.00002 | - | −0.15 (−0.22 to −0.08) |
| CBCL scores adjusted for medication status | Attention problems scale | 6911 | −0.0003 | 0.00007 | −4.18 | 0.00003 | - | −0.14 (−0.21 to −0.08) |
| Primary analysis | ADHD diagnosis | 5816 | −0.003 | 0.0009 | −3.85 | 0.0001 | 0.02 | −0.3 (−0.45 to −0.15) |
| Stringent QC threshold (worst 20% of subjects on any motion parameter excluded) | ADHD diagnosis | 4665 | −0.003 | 0.001 | −3.09 | 0.002 | - | −0.28 (−0.45 to −0.1) |
| Matched on covariates including QC variables | ADHD diagnosis | 2091 | −0.003 | 0.001 | −2.40 | 0.02 | - | −0.48 (−0.88 to −0.08) |
| Controlling for IQ | ADHD diagnosis | 5816 | −0.003 | 0.0009 | −3.65 | 0.0003 | - | −0.28 (−0.44 to −0.13) |
| Excluding subjects with possible bipolar and/or psychosis symptoms | ADHD diagnosis | 5509 | −0.004 | 0.001 | −3.96 | 0.00008 | - | −0.32 (−0.48 to −0.16) |
|
| ||||||||
| Right inferior longitudinal fasciculus | ||||||||
|
| ||||||||
| Primary analysis | ADHD diagnosis | 5814 | −0.003 | 0.0009 | −3.49 | 0.0005 | 0.048 | −0.27 (−0.42 to −0.12) |
| Stringent QC threshold (worst 20% of subjects on any motion parameter excluded) | ADHD diagnosis | 4664 | −0.003 | 0.001 | −3.25 | 0.001 | - | −0.29 (−0.47 to −0.11) |
| Matched on covariates including QC variables | ADHD diagnosis | 2087 | −0.003 | 0.001 | −2.49 | 0.01 | - | −0.5 (−0.9 to −0.1) |
| Controlling for IQ | ADHD diagnosis | 5814 | −0.003 | 0.0009 | −3.28 | 0.001 | −0.26 (−0.41 to −0.1) | |
| Excluding subjects with possible bipolar and/or psychosis symptoms | ADHD diagnosis | 5506 | −0.003 | 0.001 | −3.42 | 0.0007 | - | −0.28 (−0.43 to −0.12) |
Abbreviations: ADHD, attention deficit hyperactivity disorder; CBCL, Child Behavior Checklist; IQ, intelligence quotient; QC, quality control.
p<.05
p<.01
p<.001.
These associations between ADHD and lower fractional anisotropy held in the same tracts in diagnostic contrasts of 951 ADHD cases against 4884 unaffected controls: the left inferior longitudinal fasciculus (B=−0.003 [SE 0.0008], t=−3.43, p=0.0006, adjusted p=0.048) the left uncinate fasciculus (B=−0.003 [SE 0.0009], t=−3.84, p=0.0001, adjusted p=.02) and, in addition the right inferior longitudinal fasciculus (B=−0.003 [SE 0.0009], t=−3.49, p=0.0005, adjusted p=.048) met FDR adjusted significance- Table 2, Figures 1 & 2, Supplementary Table 5. Effect sizes were small. Similar effect sizes were observed for both the left and right inferior longitudinal fasciculus (left: −0.27 (95%CI −0.42 to −0.11); right: −0.27 (95%CI −0.42 to −0.12), while for the left uncinate fasciculus, an effect size of −0.3 (95%CI −0.45 to −0.15) was observed. Nominally significant associations between ADHD and reduced fractional anisotropy of other association tracts, some projection tracts (fronto-, parieto and occipito-pontine and corticospinal tracts) and callosal tracts are noted in Supplementary Table 5.
Table 2.
Demographic details of N=5835 subjects included in the primary ADHD case versus unaffected control comparisons.
| Cohort | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| ABCD | HBN | NCANDA | NCR | Total | ||||||
|
| ||||||||||
| Case | Controls | Case | Controls | Case | Controls | Case | Controls | Cases | Controls | |
| Total (N) | N=481 | N=4187 | N=334 | N=146 | N=12 | N=499 | N=124 | N=52 | N=951 | N=4884 |
|
| ||||||||||
| Female N (%) | 157 (32.64%) | 2197 (52.47%) | 102 (30.54%) | 75 (51.37%) | 1 (8.33%) | 260 (52.1%) | 44 (35.48%) | 27 (51.92%) | 304 (31.97%) | 2559 (52.4%) |
| Male N (%) | 324 (67.36%) | 1990 (47.53%) | 232 (69.46%) | 71 (48.63%) | 11 (91.67%) | 239 (47.9%) | 80 (64.52%) | 25 (48.08%) | 647 (68.03%) | 2325 (47.6%) |
|
| ||||||||||
| Age: mean (SD) years | 9.91 (0.63) | 9.96 (0.63) | 11.28 (3.17) | 11.23 (3.14) | 15.02 (1.66) | 15.05 (1.66) | 12.02 (3.09) | 12.45 (2.76) | 10.73 (2.43) | 10.54 (1.85) |
|
| ||||||||||
| IQ: mean (SD) | - | - | 99.64 (16.03) | 104.01 (15.42) | 105.17 (14.48) | 114.88 (13.45) | 106.44 (13.76) | 113.35 (14.48) | 101.57 (15.69) | 112.49 (14.61) |
|
| ||||||||||
| Scaled matrix: mean (SD) | 9.78 (2.9) | 10.37 (2.75) | - | - | - | - | - | - | 9.78 (2.9) | 10.37 (2.75) |
|
| ||||||||||
| Race/ethnicity | ||||||||||
|
| ||||||||||
| White, non-Hispanic | 287 (59.67%) | 2519 (60.16%) | 155 (46.41%) | 70 (47.95%) | 8 (66.67%) | 323 (64.73%) | 84 (67.74%) | 36 (69.23%) | 534 (56.15%) | 2948 (60.36%) |
| African American | 66 (13.72%) | 434 (10.37%) | 29 (8.68%) | 16 (10.96%) | 1 (8.33%) | 64 (12.83%) | 14 (11.29%) | 5 (9.62%) | 110 (11.57%) | 519 (10.63%) |
| Other | 128 (26.61%) | 1234 (29.47%) | 150 (44.91%) | 60 (41.1%) | 3 (25%) | 112 (22.44%) | 26 (20.97%) | 11 (21.15%) | 307 (32.28%) | 1417 (29.01%) |
|
| ||||||||||
| Household income | ||||||||||
|
| ||||||||||
| <$50k per annum | 147 (30.56%) | 1015 (24.24%) | 70 (20.96%) | 26 (17.81%) | 2 (16.67%) | 83 (16.63%) | 19 (15.32%) | 7 (13.46%) | 238 (25.03%) | 1131 (23.16%) |
| $50-$100k | 152 (31.6%) | 1247 (29.78%) | 74 (22.16%) | 37 (25.34%) | 3 (25%) | 129 (25.85%) | 38 (30.65%) | 10 (19.23%) | 267 (28.08%) | 1423 (29.14%) |
| $100-$200k | 140 (29.11%) | 1398 (33.39%) | 71 (21.26%) | 30 (20.55%) | 4 (33.33%) | 164 (32.87%) | 34 (27.42%) | 13 (25%) | 249 (26.18%) | 1605 (32.86%) |
| >200k | 42 (8.73%) | 527 (12.59%) | 119 (35.63%) | 53 (36.3%) | 3 (25%) | 123 (24.65%) | 33 (26.61%) | 22 (42.31%) | 197 (20.72%) | 725 (14.84%) |
Robustness checks
Results of the complementary pairs stability analysis are shown in Supplementary Table 6. In brief, we find that the association between FA of the left uncinate fasciculus and CBCL attention scale had n=77%, p < .001 (i.e. it was nominally significant p < .05 in both halves of the data for 77 out of the 100 random splits, and such a high n was never observed in any of the 1000 permutations of the data). Similarly, the association between that index and ADHD diagnosis was n=64% (p < .001). The relationship between FA in the left inferior longitudinal fasciculus and CBCL attention problems was n=50% (p < 0.001), and ADHD diagnosis was 47% (p < 0.001). That same value for n was also observed for the relationship between FA in the right inferior longitudinal fasciculus.
Although motion parameters were considered as covariates in the main analyses, we conducted further analyses to ensure that findings were not driven by motion. The pattern of findings held at the more stringent level of quality control (that retained 62.4% of the individuals in the original cohort- Table 2 and Supplemental Tables 7 & 8). Moreover, those with ADHD problems were propensity matched on motion parameters and demographic variables with those who had no ADHD problems (2560 and 3561 participants in each group). These groups did not differ in any motion parameters thus ensuring that motion was not contributing to findings. Effects of the matching procedure in the relationship between ADHD variables and motion parameters are given in Supplementary Table 9. The findings of associations between the fractional anisotropy of long association tracts and attention problems scores all held– Supplementary Tables 10 & 11. The pattern of association between attention problems and tract fractional anisotropy held when covarying for estimates of general intelligence – Supplementary Tables 12 & 13. Following adjustment for a possible under-reporting of attention problems (due to ADHD medication), we found that associations with white matter tract microstructure were more pronounced - Supplementary Table 14. This was also the case after excluding subjects with possible bipolar disorder and/or psychosis symptoms - Supplementary Tables 15 & 16.
We further examined whether associations between white matter microstructure and ADHD traits and diagnoses changed or remained stable with age. To limit confounds between age range and cohort, we explored this question in the four datasets with suitably wide age ranges, excluding the ABCD cohort as subjects were aged 9–10 years old at time of scanning. We first fit models examining linear, quadratic, and cubic trends across all included subjects, which were compared using Akaike’s Information Criterion (AIC). The best fitting terms for age were then examined in interactions with (1) attention problems and (2) ADHD diagnosis. The results of these analyses are reported in Supplementary Table 17 and, importantly, do not overlap with the tracts implicated in ADHD in the primary analysis. For completion we examined interactions in the ABCD cohort, focusing on linear trends given the small age range covered. No tracts met FDR-corrected significance for either the interaction with attention problems or ADHD diagnosis for the ABCD cohort.
There were consistently lower mean effect sizes for the clinical cohort combined (mean −0.34 for attention problems, −0.65 for ADHD Diagnosis) than the population cohorts combined (−0.09 for attention problems, −0.21 for ADHD Diagnosis) within the white fiber tracts shown in Figure 2. Significant interactions between diagnosis and the nature of the cohorts (clinic-based vs population) were observed in the right inferior longitudinal fasciculus (t=1.98, p=.048), and between attention problems in the left uncinate fasciculus (t=3.41, p=.0007). Trends were also observed between the nature of the cohorts and ADHD diagnosis in the left uncinate fasciculus (t=1.87, p=.06), and attention problems in the left inferior longitudinal fasciculus (t=1.86, p=.06).
Exploratory axial diffusivity, radial diffusivity and voxelwise analyses
To supplement our examination of the associations between white matter microstructure assessed using fractional anisotropy and ADHD traits and diagnosis, we examined associations involving alternative measures of white matter microstructure. We found that the axial diffusivity and radial diffusivity of the inferior longitudinal fasciculi and the left uncinate fasciculus were also associated with ADHD at nominal levels of significance. See Figure 2 and Supplementary Tables 18–21.
Two clusters in the supplementary voxelwise analysis for the association between attention problems and FA were significant at p=.02 and p=.04, with one cluster intercepting with the left frontopontine tract, and a second located around the middle cerebellar peduncle. No clusters reached statistical significance for ADHD diagnosis. See Supplementary Table 22 and Supplementary Figure 3.
Discussion
Both ADHD traits and diagnosis showed significant associations with altered microstructure of the inferior longitudinal fasciculi and the left uncinate fasciculus. The overall effect sizes were small, with the strongest association involving ADHD traits as assessed using the CBCL associated with an effect size of partial-r=−0.14, and the largest group difference when comparing cases and controls associated with an effect size of d=−0.3. White matter tract microstructural anomalies were not as prominently associated with problems related to mood, anxiety or other externalizing problems. The findings held in multiple sensitivity analyses and robustness checks, including after matching ADHD cases and unaffected controls on the basis of motion parameters.
ADHD problems were associated with lower fractional anisotropy of the long association tracts, specifically the inferior longitudinal and uncinate fasciculi. This is consistent with prior meta-analyses that found lower fractional anisotropy in ADHD in the sagittal stratum (which contain fibers that form the inferior longitudinal fasciculus (4, 5)). Our finding is also consistent with models of ADHD as the result of disruptions to several large-scale brain networks mediating key cognitive functions in the disorder. For example, the inferior longitudinal fasciculus has been implicated previously in ADHD, mainly through its role in visuospatial attention (37). Finally, the uncinate fasciculus has been implicated in ADHD as a core component of both emotion and reward processing systems (38, 39).
The findings were similar in analyses that treated ADHD as a trait and as a diagnostic category, complementing demonstrations that ADHD traits and diagnosis have similar cortical anatomic changes and share a common genetic basis (13, 40, 41). In both cases, small effect sizes were reported. Small effects are in line with other recent large-scale investigations of associations between clinical and brain phenotypes in psychiatry, most saliently the recent work of Marek and colleagues, as well as the emerging conclusion that real, reproducible associations between individual differences in complex biological systems and multifaceted, heterogenous disorders such as ADHD will almost certainly involve small univariate effect sizes (10, 13, 42).
The study represents six advances. First, we analyze ‘raw’ data, rather than meta-analyzing published summary results, as the latter is biased by signals that attained thresholds for declaring significance within each study. In light of the small effect sizes reported here, it is likely that such results would have been missed using retrospective voxelwise meta-analytic methods, as they are unlikely to have survived the corrections for multiple comparisons at the level of single studies. Second, data were analyzed at one site using a uniform processing pipeline, thus circumventing the introduction of confounds tied to software (43). Third, the use of ‘raw’ DTI data allows us to control for critical confounders, particularly motion, at the individual level, and to perform follow-up analyses on nearest neighbor matched samples. Such procedures essentially exclude the possibility that the diagnostic and trait differences are artifacts of in-scanner motion. Fourth, we were able to show that white matter differences tied to ADHD were largely ADHD-specific relative to anxiety, mood or non-ADHD externalizing problems assessed using the CBCL. Fifth, we were able to begin to explore whether clinically enriched samples may be able to detect differences of larger effects than those seen in more population-based, representative cohorts, reporting that effect sizes tended to be larger in clinically ascertained cohorts (HBN, NCR) than cohorts of the general population (ABCD, HCP-D and NCANDA). Finally, we employed a stability computational framework that can serve as a viable alternative to foster reproducibility of results when replication samples are unavailable.
Limitations of the study include the fact that subjects with ADHD diagnoses, lower IQ, lower socioeconomic status and who were male were disproportionately excluded due to excessive in-scanner motion, at both levels of stringency. This likely made the sample unrepresentative of ADHD subjects as a whole, which is an issue known to impact neuroimaging studies of neurodevelopmental disorders, and we cannot consider our effect size estimates to be population level estimates for the population of U.S. children (44, 45). Second, as we considered cross-sectional studies, we were limited in our ability to examine developmental questions, including whether the associations between brain structure and ADHD traits and diagnoses changed with age(46). While we examined this question cross-sectionally, such cross-sectional approaches are potentially confounded by cohort effects, and are unable to capture changes in brain structure and ADHD traits at the individual level. Furthermore, our use of cross-sectional data meant that we were unable to make statements about the direction of effects. While it is possible that anomalies in white matter tract development leads to ADHD symptom formation, it is also feasible that the experience of ADHD symptoms per se has an impact on white matter tract development. Indeed, using longitudinal data from a large Dutch population cohort, it was found that higher externalizing and internalizing symptoms at baseline predicted smaller increases in global fractional anisotropy over time, but baseline white matter properties were not associated over time with later symptoms (47). Third, given its prominence in the field, we opted to analyze the fractional anisotropy of white matter tracts derived using DTI methods. However, it is possible that recent advances in diffusion weighted imaging could return diagnostic signals which have larger effect sizes, including the acquisition of multi-shell diffusion weight sequences, analyzed using methods that more accurately reconstruct fiber orientation within voxels (such as the ‘fixel’ based approach) (48–50).
In summary, we conducted a large, multi-cohort study of white matter differences associated with ADHD diagnosis and traits, in which we processed multiple large datasets according to a harmonized imaging pipeline, thereby overcoming issues known to inflate effect sizes and false positive rates of both smaller individual neuroimaging studies and retrospective meta-analyses of neuroimaging studies based on the published literature (9, 10). The results showed ADHD-associated differences in white matter microstructure within long association tracts that were not found for anxiety, mood and non-ADHD externalizing problems, supporting the hypothesized role for white matter alterations in the pathophysiology of ADHD. Nonetheless, while our findings do not preclude the possibility that DTI data could add value to other data sources in building tools for diagnosis, prognosis and treatment guidance, particularly when machine learning rather than traditional statistical approaches are employed, the small effect sizes observed limit the clinical utility of this imaging modality in isolation, as such differences cannot reliably distinguish individuals with and without ADHD.
Supplementary Material
KEY RESOURCES TABLE
| Resource Type | Specific Reagent or Resource | Source or Reference | Identifiers | Additional Information |
|---|---|---|---|---|
| Add additional rows as needed for each resource type | Include species and sex when applicable. | Include name of manufacturer, company, repository, individual, or research lab. Include PMID or DOI for references; use “this paper” if new. | Include catalog numbers, stock numbers, database IDs or accession numbers, and/or RRIDs. RRIDs are highly encouraged; search for RRIDs at https://scicrunch.org/resources. | Include any additional information or notes if necessary. |
| Deposited Data; Public Database | #2573, #2846 and #3165 | NIMH Data Archive | RRID:SCR_004434 | |
| Deposited Data; Public Database | NCANDA_PUBLIC_4Y_REDCAP_V01 | https://dx.doi.org/10.7303/syn22216455 | N/A | |
| Deposited Data; Public Database | NCANDA_PUBLIC_BASE_DIFFUSION_V01 | https://dx.doi.org/10.7303/syn11565329 | N/A | |
| Deposited Data; Public Database | NCANDA_PUBLIC_BASE_STRUCTURAL_V01 | https://dx.doi.org/10.7303/syn11541569 | N/A | |
| Deposited Data; Public Database | LORIS - Longitudinal Online Research and Imaging System | https://doi.org/10.3389/fninf.2011.00037 | RRID:SCR_000590 | |
| Deposited Data; Public Database | Healthy Brain Network | https://doi.org/10.1038/sdata.2017.181 | N/A | |
| Deposited Data; Public Database | ABCD study | http://dx.doi.org/10.15154/1519007 | RRID:SCR_015769 | |
| Software; Algorithm | QSIprep | https://doi.org/10.1038/s41592-021-01185-5 | N/A | |
| Software; Algorithm | SynthSR | https://doi.org/10.1016/j.neuroimage.2021.118206 | N/A | |
| Software; Algorithm | R | https://www.r-project.org/ | RRID:SCR_001905 | |
| Software; Algorithm | R package: nlme | Pinheiro J, Bates D, R Core Team (2022). nlme: Linear and Nonlinear Mixed Effects Models. R package version 3.1–159, https://CRAN.R-project.org/package=nlme. | RRID:SCR_015655 | |
| Software; Algorithm | R package: matchit | https://doi.org/10.18637/jss.v042.i08 | N/A | |
| Software; Algorithm | Biowulf at the NIH | NIH; https://hpc.nih.gov/systems/ | RRID:SCR_007169 | |
| Software; Algorithm | Analysis of Functional NeuroImages | https://doi.org/10.1006/cbmr.1996.0014 | RRID:SCR_005927 |
Acknowledgements:
Data used in the preparation of this article were obtained from the Adolescent Brain Cognitive Development (ABCD) Study (https://abcdstudy.org), held in the NIMH Data Archive (NDA). The ABCD Study is supported by the National Institutes of Health and additional federal partners under award numbers U01DA041048, U01DA050989, U01DA051016, U01DA041022, U01DA051018, U01DA051037, U01DA050987, U01DA041174, U01DA041106, U01DA041117, U01DA041028, U01DA041134, U01DA050988, U01DA051039, U01DA041156, U01DA041025, U01DA041120, U01DA051038, U01DA041148, U01DA041093, U01DA041089, U24DA041123, and U24DA041147. A full list of supporters is available at https://abcdstudy.org/federal-partners.html. A listing of participating sites and a complete listing of the study investigators can be found at https://abcdstudy.org/consortium_members/. ABCD consortium investigators designed and implemented the study and/or provided data but did not participate in analysis or writing of this report. The ABCD data repository grows and changes over time. The ABCD data used in this report came from DOI: 10.15154/1519007. The DOIs can be found at http://dx.doi.org/10.15154/1519007. The ABCD data used in this report came from the fast-track data release (raw neuroimaging data). The raw data are available at https://nda.nih.gov/edit_collection.html?id=2573. Instructions on how to create an NDA study are available at https://nda.nih.gov/training/modules/study.html). Data and/or research tools used in the preparation of this manuscript were obtained from the National Institute of Mental Health (NIMH) Data Archive (NDA). NDA is a collaborative informatics system created by the National Institutes of Health to provide a national resource to support and accelerate research in mental health. Dataset identifier(s): #2573, #2846 and #3165. This manuscript reflects the views of the authors and may not reflect the opinions or views of the NIH or of the Submitters submitting original data to NDA. DOI: 10.15154/1527794. This manuscript included data from a limited access dataset obtained from the Child Mind Institute Biobank, the HBN study (http://www.healthybrainnetwork.org). Data collection and sharing for this project was also provided by the National Consortium on Alcohol and Neurodevelopment in Adolescence (NCANDA), which is funded by the U.S. National Institute on Alcohol Abuse and Alcoholism with co-funding from the National Institute on Drug Abuse, the National Institute of Mental Health, the National Institute of Child Health and Human Development, and the National Institute of Health Office of the Director (AA021695 (MPI: SA Brown, SF Tapert), AA021697 (MPI: A Pfefferbaum, KM Pohl), AA021692 (PI: SF Tapert), AA021681 (PI: MD DeBellis), AA021690 (PI: DB Clark), AA021691 (PI: B Nagel), AA021696 (MPI: IM Colrain, FC Baker), AA021697–04S1 (PI: KM Pohl) ). NCANDA data are disseminated by the Center for Health Sciences, SRI International. We used data from NCANDA_PUBLIC_4Y_REDCAP_V01 (https://dx.doi.org/10.7303/syn22216455), NCANDA_PUBLIC_BASE_DIFFUSION_V01 (https://dx.doi.org/10.7303/syn11565329) and NCANDA_PUBLIC_BASE_STRUCTURAL_V01 (https://dx.doi.org/10.7303/syn11541569). The Neurobehavioral Clinical Research cohort is supported by the intramural research programs of the of Mental Health and the National Human Genome Research Institute (ZIAHG200378 to PS; ClinicalTrials.gov identifier: NCT01721720).
The Human Connectome Project- Development was supported by the NIMH under Award Number U01MH109589. This study reflects the views of the authors and may not reflect the opinions or views of other individuals or institutions including the NIH, the ABCD, NCANDA, HCP-D consortium investigators or other funding agencies. Image processing was conducted using the high-performance computing capabilities of the NIH Biowulf cluster. The authors thank the NIMH Data Science and Sharing Team for help with accessing and processing the ABCD-BIDS dataset.
Funding:
Intramural program of the National Institute of Mental Health and the National Human Genome Research Institute (ZIAHG200378 to Philip Shaw). The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation or review of the manuscript; and decision to submit the manuscript for publication but had a role in the approval of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial disclosure: All authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Sudre G, Bouyssi-Kobar M, Norman L, Sharp W, Choudhury S, Shaw P (2021): Estimating the Heritability of Developmental Change in Neural Connectivity, and Its Association With Changing Symptoms of Attention-Deficit/Hyperactivity Disorder. Biological Psychiatry 89:443–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Konrad K, Eickhoff SB (2010): Is the ADHD brain wired differently? A review on structural and functional connectivity in attention deficit hyperactivity disorder. Human Brain Mapping 31:904–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Ewijk H, Heslenfeld DJ, Zwiers MP, Buitelaar JK, Oosterlaan J (2012): Diffusion tensor imaging in attention deficit/hyperactivity disorder: A systematic review and meta-analysis. Neuroscience & Biobehavioral Reviews 36:1093–1106. [DOI] [PubMed] [Google Scholar]
- 4.Aoki Y, Cortese S, Castellanos FX (2018): Research Review: Diffusion tensor imaging studies of attention‐deficit/hyperactivity disorder: meta‐analyses and reflections on head motion. Journal of Child Psychology and Psychiatry 59:193–202. [DOI] [PubMed] [Google Scholar]
- 5.Chen L, Hu X, Ouyang L, He N, Liao Y, Liu Q, et al. (2016): A systematic review and meta-analysis of tract-based spatial statistics studies regarding attention-deficit/hyperactivity disorder. Neurosci Biobehav Rev 68:838–847. [DOI] [PubMed] [Google Scholar]
- 6.Zhao Y, Yang L, Gong G, Cao Q, Liu J (2022): Identify aberrant white matter microstructure in ASD, ADHD and other neurodevelopmental disorders: A meta-analysis of diffusion tensor imaging studies. Progress in Neuro-Psychopharmacology and Biological Psychiatry 113:110477. [DOI] [PubMed] [Google Scholar]
- 7.Radua J, Mataix-Cols D (2009): Voxel-wise meta-analysis of grey matter changes in obsessive–compulsive disorder. The British Journal of Psychiatry 195:393–402. [DOI] [PubMed] [Google Scholar]
- 8.Eickhoff SB, Bzdok D, Laird AR, Kurth F, Fox PT (2012): Activation likelihood estimation meta-analysis revisited. Neuroimage 59:2349–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Button KS, Ioannidis JP, Mokrysz C, Nosek BA, Flint J, Robinson ES, et al. (2013): Power failure: why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci 14:365–376. [DOI] [PubMed] [Google Scholar]
- 10.Marek S, Tervo-Clemmens B, Calabro FJ, Montez DF, Kay BP, Hatoum AS, et al. (2022): Reproducible brain-wide association studies require thousands of individuals. Nature 603:654–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Achenbach TM, Rescorla LA (2001): ASEBA School Age Forms and Profiles. Burlington, Vt: ASEBA
- 12.Achenbach TM, Ruffle TM (2000): The Child Behavior Checklist and related forms for assessing behavioral/emotional problems and competencies.[see comment]. Pediatr Rev 21:265–271. [DOI] [PubMed] [Google Scholar]
- 13.Hoogman M, Muetzel R, Guimaraes JP, Shumskaya E, Mennes M, Zwiers MP, et al. (2019): Brain imaging of the cortex in ADHD: a coordinated analysis of large-scale clinical and population-based samples. American Journal of Psychiatry 176:531–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alexander LM, Escalera J, Ai L, Andreotti C, Febre K, Mangone A, et al. (2017): An open resource for transdiagnostic research in pediatric mental health and learning disorders. Scientific data 4:1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sudre G, Sharp W, Kundzicz P, Bouyssi-Kobar M, Norman L, Choudhury S, et al. (2020): Predicting the course of ADHD symptoms through the integration of childhood genomic, neural, and cognitive features. Molecular Psychiatry 1–9. [DOI] [PMC free article] [PubMed]
- 16.Casey B, Cannonier T, Conley MI, Cohen AO, Barch DM, Heitzeg MM, et al. (2018): The adolescent brain cognitive development (ABCD) study: imaging acquisition across 21 sites. Developmental cognitive neuroscience 32:43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pfefferbaum A, Rohlfing T, Pohl KM, Lane B, Chu W, Kwon D, et al. (2016): Adolescent development of cortical and white matter structure in the NCANDA sample: role of sex, ethnicity, puberty, and alcohol drinking. Cerebral cortex 26:4101–4121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Somerville LH, Bookheimer SY, Buckner RL, Burgess GC, Curtiss SW, Dapretto M, et al. (2018): The Lifespan Human Connectome Project in Development: A large-scale study of brain connectivity development in 5–21 year olds. Neuroimage 183:456–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown SA, Brumback T, Tomlinson K, Cummins K, Thompson WK, Nagel BJ, et al. (2015): The National Consortium on Alcohol and NeuroDevelopment in Adolescence (NCANDA): a multisite study of adolescent development and substance use. Journal of studies on alcohol and drugs 76:895–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang S, Arfanakis K (2018): Evaluation of standardized and study-specific diffusion tensor imaging templates of the adult human brain: Template characteristics, spatial normalization accuracy, and detection of small inter-group FA differences. Neuroimage 172:40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.VanderWeele TJ (2019): Principles of confounder selection. Eur J Epidemiol 34:211–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Russell AE, Ford T, Williams R, Russell G (2016): The association between socioeconomic disadvantage and attention deficit/hyperactivity disorder (ADHD): a systematic review. Child Psychiatry & Human Development 47:440–458. [DOI] [PubMed] [Google Scholar]
- 23.Bax AC, Bard DE, Cuffe SP, McKeown RE, Wolraich ML (2019): The association between race/ethnicity and socioeconomic factors and the diagnosis and treatment of children with attention-deficit hyperactivity disorder. J Dev Behav Pediatr 40:81–91. [DOI] [PubMed] [Google Scholar]
- 24.Ozernov‐Palchik O, Norton ES, Wang Y, Beach SD, Zuk J, Wolf M, et al. (2019): The relationship between socioeconomic status and white matter microstructure in pre‐reading children: a longitudinal investigation. Human brain mapping 40:741–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ursache A, Noble KG, Pediatric Imaging N, Study G (2016): Socioeconomic status, white matter, and executive function in children. Brain and behavior 6:e00531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller GA, Chapman JP (2001): Misunderstanding analysis of covariance. J Abnorm Psychol 110:40–48. [DOI] [PubMed] [Google Scholar]
- 27.Dennis M, Francis DJ, Cirino PT, Schachar R, Barnes MA, Fletcher JM (2009): Why IQ is not a covariate in cognitive studies of neurodevelopmental disorders. J Int Neuropsychol Soc 15:331–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bridgett DJ, Walker ME (2006): Intellectual functioning in adults with ADHD: a meta-analytic examination of full scale IQ differences between adults with and without ADHD. Psychol Assess 18:1–14. [DOI] [PubMed] [Google Scholar]
- 29.Benjamini Y, Hochberg Y (1995): Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society, Series B 57:289–300. [Google Scholar]
- 30.Fox J, Weisberg S (2019): An R Companion to Applied Regression. Third ed. Thousand Oaks CA: Sage. [Google Scholar]
- 31.Meinshausen N, Bühlmann P (2010): Stability selection. Journal of the Royal Statistical Society: Series B (Statistical Methodology) 72:417–473. [Google Scholar]
- 32.Bühlmann P, Yu B (2002): Analyzing bagging. The annals of Statistics 30:927–961. [Google Scholar]
- 33.Breiman L (1996): Bagging predictors. Machine learning 24:123–140. [Google Scholar]
- 34.Sabourin JA, Cropp CD, Sung H, Brody LC, Bailey‐Wilson JE, Wilson AF (2019): ComPaSS‐ GWAS: A method to reduce type I error in genome‐wide association studies when replication data are not available. Genetic epidemiology 43:102–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ho DE, Imai K, King G, Stuart EA (2011): MatchIt: Nonparametric Preprocessing for Parametric Causal Inference. Journal of Statistical Software 42:1–28. [Google Scholar]
- 36.Hek K, Demirkan A, Lahti J, Terracciano A, Teumer A, Cornelis MC, et al. (2013): A genome-wide association study of depressive symptoms. Biological psychiatry 73:667–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Versace A, Jones N, Joseph H, Lindstrom R, Wilson T, Lima Santos J, et al. (2021): White matter abnormalities associated with ADHD outcomes in adulthood. Molecular psychiatry 1–11. [DOI] [PMC free article] [PubMed]
- 38.Nagel BJ, Bathula D, Herting M, Schmitt C, Kroenke CD, Fair D, et al. (2011): Altered white matter microstructure in children with attention-deficit/hyperactivity disorder. Journal of the American Academy of Child & Adolescent Psychiatry 50:283–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sudre G, Shaw P, Wharton A, Weingart D, Sharp W, Sarlls J (2015): White matter microstructure and the variable adult outcome of childhood Attention Deficit Hyperactivity Disorder. Neuropsychopharmacology 40:746–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thapar A (2018): Discoveries on the genetics of ADHD in the 21st century: new findings and their implications. American Journal of Psychiatry 175:943–950. [DOI] [PubMed] [Google Scholar]
- 41.Faraone SV, Larsson H (2018): Genetics of attention deficit hyperactivity disorder. Molecular psychiatry1. [DOI] [PMC free article] [PubMed]
- 42.Bernanke J, Luna A, Chang L, Bruno E, Dworkin J, Posner J (2022): Structural brain measures among children with and without ADHD in the Adolescent Brain and Cognitive Development Study cohort: a cross-sectional US population-based study. The Lancet Psychiatry 9:222–231. [DOI] [PubMed] [Google Scholar]
- 43.Ressel V, Van Hedel HJ, Scheer I, Tuura ROG (2018): Comparison of DTI analysis methods for clinical research: influence of pre-processing and tract selection methods. European Radiology Experimental 2:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.LeWinn KZ, Sheridan MA, Keyes KM, Hamilton A, McLaughlin KA (2017): Sample composition alters associations between age and brain structure. Nature Communications 8:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heeringa SG, Berglund PA (2020): A guide for population-based analysis of the Adolescent Brain Cognitive Development (ABCD) Study baseline data. BioRxiv
- 46.Kraemer HC, Yesavage JA, Taylor JL, Kupfer D (2000): How can we learn about developmental processes from cross-sectional studies, or can we? American Journal of Psychiatry 157:163–171. [DOI] [PubMed] [Google Scholar]
- 47.Muetzel RL, Blanken LM, van der Ende J, El Marroun H, Shaw P, Sudre G, et al. (2018): Tracking brain development and dimensional psychiatric symptoms in children: a longitudinal population-based neuroimaging study. American Journal of psychiatry 175:54–62. [DOI] [PubMed] [Google Scholar]
- 48.Raffelt DA, Tournier J-D, Smith RE, Vaughan DN, Jackson G, Ridgway GR, et al. (2017): Investigating white matter fibre density and morphology using fixel-based analysis. Neuroimage 144:58–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schilling KG, Janve V, Gao Y, Stepniewska I, Landman BA, Anderson AW (2018): Histological validation of diffusion MRI fiber orientation distributions and dispersion. Neuroimage 165:200–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tournier JD, Calamante F, Connelly A (2013): Determination of the appropriate b value and number of gradient directions for high‐angular‐resolution diffusion‐weighted imaging. NMR in Biomedicine 26:1775–1786. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


