Abstract
Background:
In the classic model of obstructive sleep apnea (OSA), respiratory events occur with sleep-related dilator muscle hypotonia, precipitating increased neural ventilatory “drive”. By contrast, a drive-dependent model has been proposed whereby falling drive promotes dilator muscle hypotonia to precipitate respiratory events. Here we determine the extent to which the classic v. drive-dependent models of OSA are best supported by direct physiological measurements.
Methods:
In N=50 OSA patients (5-91 events/hr), we recorded ventilation (“flow”, oronasal mask and pneumotach) and ventilatory drive (calibrated intra-esophageal diaphragm EMG) overnight. Flow and drive during events were ensemble-averaged; patients were classified as drive-dependent if flow fell/rose simultaneously with drive. Overnight effects of lower drive on flow, genioglossus muscle activity (EMGgg), and event risk were quantified (mixed models).
Results:
On average, ventilatory drive fell (rather than rose) during events (−20[−42,3]%baseline, median [IQR]) and was strongly correlated with flow (R = 0.78[0.24,0.94]). Most patients (30/50, 60%) were classified as exhibiting drive-dependent event pathophysiology. Lower drive during sleep was associated with lower flow (−17[−20,−14]%/drive) and EMGgg (−3.5[−3.8, −3.3]%max/drive), and greater event risk (OR: 2.2[1.8,2.5] per drive reduction of 100%eupnea); associations were concentrated in patients with drive-dependent OSA (i.e. flow: −37[−40,−34]%/drive, OR: 6.8[5.3,8.7]). Esophageal pressure—without tidal volume correction—falsely suggested rising drive during events (classic model).
Conclusions:
In contrast to the prevailing view, patients with OSA predominantly exhibit drive-dependent event pathophysiology whereby flow is lowest at nadir drive, and lower drive raises event risk. Preventing ventilatory drive decline is therefore considered a target for OSA intervention.
Keywords: pathophysiology, personalized medicine, endotype
Introduction
Obstructive sleep apnea (OSA) is a highly-prevalent disorder with major health consequences [1] but the pathophysiology is heterogeneous [2 ,3] and remains incompletely understood. In the prevailing “classic” model of OSA, apneas and hypopneas occur at sleep onset with a state-related decrement in pharyngeal dilator muscle tone; the attendant pharyngeal obstruction and loss of airflow then elicits a reflex rise in neural ventilatory drive that fails to reopen the airway until arousal occurs and facilitates ventilatory recovery [2–4].
In contrast, an alternative “drive-dependent” model of pharyngeal obstruction in OSA has been proposed [5–9], whereby decrements in drive—and an attendant parallel loss of dilator muscle tone—lead to obstruction occurring at nadir drive levels. This notion that flow falls and rises in parallel with drive is consistent with notable work by Onal et al. and Dempsey et al. [8 ,10 ,11] that imply a major role for ventilatory control and pharyngeal muscles in the obstruction that characterizes OSA. However, until now this drive-dependent model has been considered, by us and others, to apply to only a minority subset of patients i.e. the ~15-30% of individuals with elevated ventilatory instability [12 ,13] who respond favorably to ventilatory control interventions [13–15]. Yet a substantial body of under-appreciated evidence suggests that OSA may be predominantly drive-dependent: Mechanistically, loss of ventilatory drive is known to lower pharyngeal dilator muscle activity [16 ,17] and ventilation under experimental conditions in most patients [2 ,18–21]. Increasing ventilatory drive experimentally activates pharyngeal muscles [5 ,9], and resolves respiratory events in the majority of patients [22]. Moreover, ventilatory effort appears lower during obstructive respiratory events than during periods of spontaneous stable breathing [23]. Events are also typically resolved in slow wave sleep where ventilatory drive is elevated [24]. To date, no study has critically-evaluated the drive-dependent model of OSA.
Here we determine whether the drive-dependent model or classic model of OSA is best supported by direct physiological measurement, using gold standard recordings of neural ventilatory drive (calibrated intra-esophageal diaphragm EMG) and oronasal ventilation (flow). Support for the drive-dependent model will be inferred if 1) a prevalent reduction—rather than increase—in drive is observed during individual apneas/hypopneas, 2) a strong temporal correlation exists between drive and flow during events, 3) associations between reduced drive during sleep and lowered flow, lowered dilator muscle activity, and increased risk of respiratory events, are observed and 4) there is greater reduction in ventilatory drive during apneas v. hypopneas.
Methods
Participants
Participants with diagnosed or suspected OSA were eligible; exclusion criteria included use of respiratory stimulants or depressants (including opioids, benzodiazepines), heart failure or lung diseases, central sleep apnea, and pregnancy. Participants provided written informed consent and approval was granted by the Partners’ Institutional Review Board. Of the N=62 participants enrolled, N=50 patients exhibited at least mild OSA (AHI>5 events/hr) and provided data for current analyses of respiratory event physiology. Additional details are provided in the Online Supplement.
Procedure and Setup
Patients attended a single overnight polysomnographic study with additional physiological measurements of ventilation (pneumotachograph, tightly fit oronasal mask; care was taken to minimize leak) and ventilatory drive (intra-esophageal diaphragm EMG). Drive was chosen for analysis over esophageal pressure (also recorded) because of the known confounding increase in esophageal pressure swings that occur with sudden pharyngeal obstruction (even in the presence of falling drive)[16 ,25–27]. Genioglossus EMG was recorded as a representative pharyngeal dilator muscle [12]. Sleep, arousals and respiratory events were scored according to standard criteria (hypopneas: 30% reduction in flow with ≥3% desaturation or arousal; hypopneas defined as obstructive versus central based on evidence of flow limitation [28] with all invasive and non-invasive signals available to facilitate scoring).
Breath-By-Breath Ventilation and Ventilatory Drive
Breath-by-breath ventilation (“flow”: tidal volume × respiratory rate) and ventilatory drive (“drive”: swings in root-mean-squared diaphragm EMG; processing included removal of cardiogenic artifact) [19] were tabulated and presented as percentage of eupneic ventilation for each individual (calibrated to match flow during wakefulness over a moving time window to account for any position change on mechanics, see online Supplement for more details about signal processing). Peak and tonic genioglossus muscle activity were also calculated (units: %max).
Definitions
Based on establish concepts and clinical definitions, we sought to place objective numerical values on the degree of obstruction during events, and the degree to which falling drive may explain this obstruction, as follows: The degree of obstruction during events was quantified by flow:drive [29], where flow:drive=0% indicates obstructive apnea, flow:drive=100% indicates complete absence of obstruction (open airway). The magnitude of reduction in drive from a pre-event baseline was calculated and presented as a percentage of the reduction in flow: Δdrive/Δflow. The flow-drive correlation coefficient within events (R) determined whether falling flow occurred in synchrony with a reduction in drive. Based on these quantitative variables there are at least two types of events: 1) Events that are obstructive (flow:drive<100%), but the obstruction is not explained by a loss of drive since drive is unchanged or rises (Δdrive/Δflow and R are small or negative), and 2) events that are obstructive (flow:drive<100%) but have a drive-dependent (“more central”) nature, i.e. drive has fallen in parallel with flow (Δdrive/Δflow and R are positive). This latter behavior characterizes what we refer to as drive-dependent OSA. (Central events would have flow:drive=100% but are not the focus of investigation here).
Individual Event Analysis
Individual respiratory events were analyzed to determine flow and drive values at baseline (at pre-event eupneic flow) and at nadir flow (lowest 10 s average, see online Supplement), allowing calculation of flow:drive and Δdrive/Δflow for each event. We emphasize that ‘rising drive’ (per Δdrive/Δflow <0) does not preclude falling drive earlier at event onset; rather ‘rising drive’ is intended to capture obstruction that does not resolve with rising drive.
Ensemble-Average Respiratory Event Analysis
Primary analysis of respiratory events were based on the ensemble-averaging method [1 ,30 ,31]: Within a patient, flow and drive signals from each event were aligned at event termination, overlaid, and mean-averaged. Flow-drive correlation (R), flow:drive and Δdrive/Δflow were determined from the ensemble event. Since thresholds for R and Δdrive/Δflow to discriminate between drive-dependent v. classic OSA have not been established, patients were first classified visually by two scientists as exhibiting a drive-dependent phenotype of OSA if—during the ensemble-averaged event—drive fell and rose simultaneously with flow, thus, the classic phenotype was defined by any evidence of increasing drive during events without a contemporaneous increase in flow (e.g. flow rises after drive rises on a time scale of greater than one breath). Visual scoring was corroborated by use of a multivariable model with objective measures (R and Δdrive/Δflow) that successfully discriminated between groups (100% within sample accuracy).
Pathophysiological Mechanisms of Obstruction in Drive-Dependent and Classic OSA
To determine physiological differences between drive-dependent and classic OSA phenotypes, endotypic traits were quantified using flow and drive during sleep (non-REM only): Pharyngeal collapsibility was determined by the ventilation at eupneic level of ventilatory drive (“Vpassive”, lower values represents greater collapsibility). Pharyngeal muscle effectiveness was taken as the flow-drive slope during sleep [2 ,19]. Loop gain (drive response to lowered flow) was determined from a chemoreflex model fit to flow data [32]. Arousal threshold was taken as the median drive immediately prior to arousal [33]. Plots of flow v. drive were constructed by sorting breath data in deciles based on drive; median values of flow and drive characterized each decile. Genioglossus EMG and respiratory event probability for each decile was also recorded.
Statistical Analysis
Several tests were used to support or refute the drive-dependent model of OSA: 1) The proportion of individual events observed with a fall rather than rise in drive was compared (against equivalent proportions) using the Fisher-exact test. 2) Primary analysis examined the flow-drive correlation R during events (ensemble-average analysis using Pearson correlation: see Supplemental Figure 1): A significant average correlation above zero (Wilcoxon sign-rank) was taken to support the drive-dependent model of OSA as predominant. 3) Linear mixed model analysis formally assessed the association between flow (dependent variable) and drive (fixed effect) during sleep, with subject included as a random effect: Models were built using the flow and drive deciles for each patient (above). Analysis was repeated using genioglossus activity (dependent variable), and again using respiratory event probability (dependent variable) in logistic mixed model regression. 4) Additional reduction in drive during apneas v. hypopneas was examined using mixed model analysis, with event type (apnea v. hypopnea) as a fixed effect.
We also report the prevalence of drive-dependent v. classic OSA; a simple majority of patients with drive-dependent OSA was taken to support the drive-dependent model. Students t-test (unpaired) compared endotypic traits between the classic and drive-dependent groups. Variables were tested for normality and presented as mean±SD or median[IQR]. Significance was accepted at P<0.05.
Results
50 subjects were available for analysis. Baseline characteristics are detailed in Table 1. Patients exhibited a broad range of OSA severities (5-92 events/hr) on the study night. The vast majority (96%) of respiratory events were classified as obstructive by clinical criteria.
Table 1.
Patient Characteristics
| Characteristic | All Subjects (N=50) | Classic (N=20) | Drive-Dependent (N=30) |
|---|---|---|---|
| Demographics | |||
| Age (years) | 55±11 | 54±14 | 57±9 |
| Sex (M:F) | 35:15 | 10:10 | 25:5 |
| Race (Black:White:Asian:Other) | 13:34:1:2 | 6:11:1:2 | 7:23:0:0 |
| Body mass index (kg/m2) | 32.8±6.4 | 32.2±6.3 | 33.2±6.4 |
| Neck circumference (cm) | 41.7±4.6 | 39.4±4.1 | 43.3±4.3 |
| Polysomnography # | |||
| OSA severity (mild:moderate:severe) | 14:9:27 | 4:4:12 | 10:5:15 |
| Apnea-hypopnea index, total (events/h) | 40[14,54] | 42[16,59] | 34[13,50] |
| Central apneas (% respiratory events) | 0[0,0] | 0[0,0] | 0[0,2] |
| Central apnea or hypopnea (% respiratory events) | 0[0,1] | 0[0,0] | 0[0,2] |
| Central or mixed events (% respiratory events) | 0[0,2] | 0[0,1] | 0[0,2] |
| Hypopneas (% respiratory events) | 52[29,85] | 58[25,86] | 49[33,85] |
| Arousal Index, total (events/h) | 49[37,72] | 51[39,84] | 49[37,62] |
| REM-NREM balance (%)* | −29[−100,11] | −38[−100,−10] | −21[−100,18] |
| Apnea-hypopnea index, NREM (events/h) | 41[12,52] | 43[14,59] | 33[12,51] |
| Mean event duration (seconds) | 22±6 | 24±5 | 21±5 |
| Total sleep time (min) | 243[181,284] | 258[192,285] | 240[175,276] |
| Sleep time, spontaneous breathing off CPAP (min) | 154[78,242] | 177[75,242] | 149[88,219] |
| NREM 1 (% sleep off CPAP) | 40[25,64] | 47[28,65] | 32[25,64] |
| NREM 2 (% sleep off CPAP) | 40[30,54] | 35[26,51] | 41[31,61] |
| NREM 3 (% sleep off CPAP) | 1[0,11] | 0[0,9] | 1[0,12] |
| REM (% sleep off CPAP) | 6[0,12] | 6[0,13] | 4[0,11] |
Values are mean±S.D or median[IQR] as determined by normality. REM = Rapid eye movement sleep, NREM = non-REM sleep.
REM-NREM balance is given by (AHIrem – AHInrem)/(AHIrem + AHInrem)*100, where −100% indicates events exclusively in NREM, +100% indicates events exclusively in REM, and 0 indicates equal AHI in NREM and REM states.
Polysomnographic respiratory event data refers to the period off CPAP. OSA severity categories: mild (5-15 events/h), moderate (15-30 events/h), severe (≥30 events/hr).
Falling Ventilatory Drive as the Predominant Behavior in OSA
Individual Events.
Illustrative examples of a patient who exhibits rising drive during events (classic phenotype) and a patient who exhibits drive-dependent events are shown in Figure 1 (A–D). In the 4747 individual events analyzed, ventilatory drive fell from pre-event baseline during the majority of events (74%, P<1×10−9 v. equal proportions). On average, drive fell by 20%baseline while flow fell by 68%baseline (Table 2, Figure 2A); drive fell by about a third of the fall in flow (Δdrive/Δflow=34%). Drive falling in events remained predominant in separate sensitivity analysis isolated to obstructive hypopneas and to obstructive apneas, and during non-REM and REM (see Supplemental Table 1).
Figure 1. Illustrative Examples of Classic and Drive-Dependent OSA.
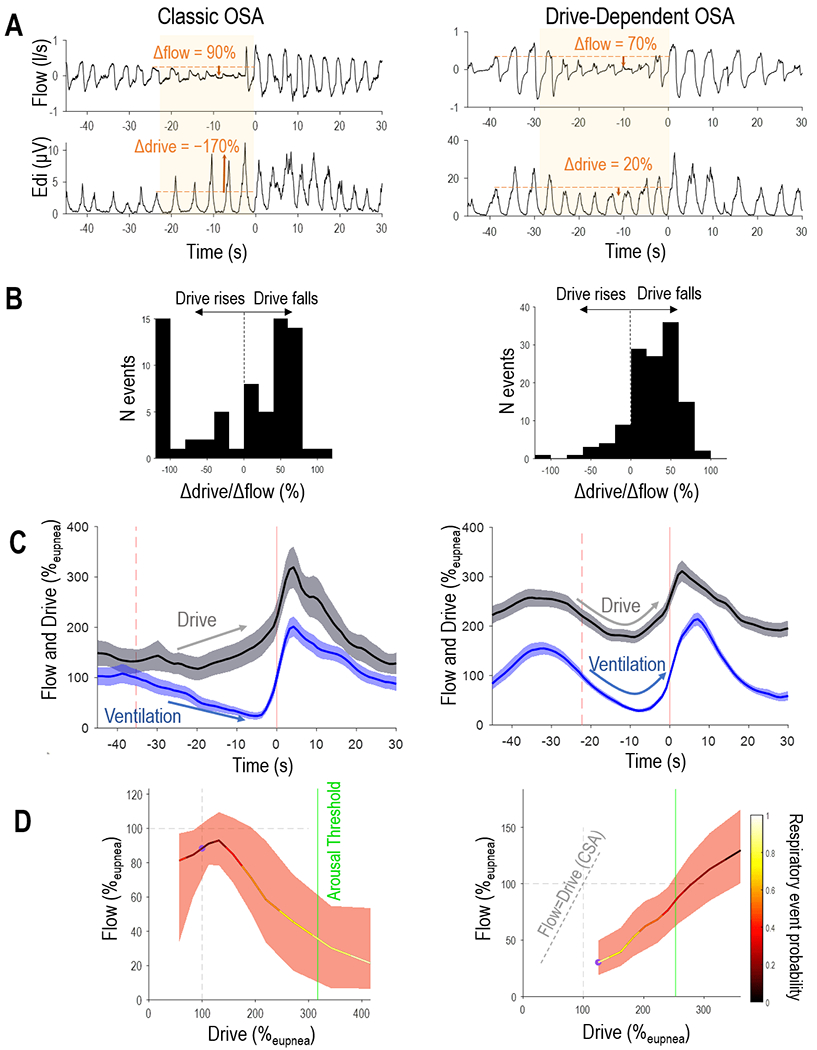
Compared to the patient exhibiting classic OSA (left), the individual with drive-dependent OSA (right) exhibits a characteristic fall in drive during individual events, seen in raw signals data (A) and histogram of drive changes (Δdrive/Δflow in B). Note that the drive-dependent patient shows a fall in drive that is in synchrony with the event-related loss of flow (ensemble average in C; shading describes 95%CI) and raises ventilation when ventilatory drive is spontaneously elevated during sleep (“endogram” plot of flow v. drive in D; black line show median flow for 10 drive deciles; shading describes IQR). In contrast, the example individual with classic OSA exhibits falling flow despite rising drive. Note also that the flow-drive profile in drive-dependent OSA is to the right of the line of unity (dashed line), indicating pharyngeal obstruction (in contrast with central sleep apnea [CSA] where flow=drive).
Table 2.
Drive-Related Event Pathophysiology
| Characteristic | All Subjects | Classic | Drive-Dependent |
|---|---|---|---|
| Individual Events | |||
| N events | 4747 | 2094 | 2653 |
| Reduction in drive (%baseline) | 20[3,42] | 12[12,32] | 27[4,53] |
| Reduction in flow (%baseline) | 68[38,100] | 70[39,100] | 66[38,96] |
| Δdrive/Δflow (%) | 34[−2,70] | 20[−16,50] | 47[10,83] |
| Proportion of events with falling drive (%) | 74 | 66 | 80 |
| Ensemble-Averaged Events | |||
| N patients | 50 | 20 | 30 |
| Reduction in drive (%baseline) | 16[9,28] | 5[12,−5] | 23[16,37] |
| Reduction in flow (%baseline) | 59[48,73] | 65[46,79] | 59[49,68] |
| Δdrive/Δflow (%) | 26[16,46] | 10[−10,18] | 44[27,59] |
| Flow-drive correlation, R | 0.78[0.24,0.94] | 0.18[−0.38,0.30] | 0.92[0.81,0.96] |
| Flow-drive nadir time difference (s) | 4[2,8] | 8[6.5,9.5] | 2[1,5] |
Continuous data shown are median[IQR]. Drive denotes diaphragm EMG (calibrated to units of ventilation, L/min). Flow denotes ventilation (tidal volume × respiratory rate). Reduction in drive denotes change in drive from baseline (below) to that measured during events (at nadir flow, see Methods). Reduction in flow denotes the change in flow during events (i.e. a 30% reduction is the theoretical minimum for a hypopnea; a value of 100% represents apnea). Baseline (pre-event) drive is measured at the time when flow is closest to eupneic levels. The fall in drive represented by Δdrive/Δflow seeks to quantify an aspect of the central nature of events: a value of 100% indicates that the fall in drive (Δdrive) is of equal magnitude to the fall in Δflow (and no exacerbation of obstruction). Flow-drive correlation (R) indicates the synchrony between flow and drive during events; a high value indicates that falling drive potentially explains (directly or indirectly) the reduction in flow.
Figure 2. Predominance of Drive-Dependent Pathophysiology in OSA.
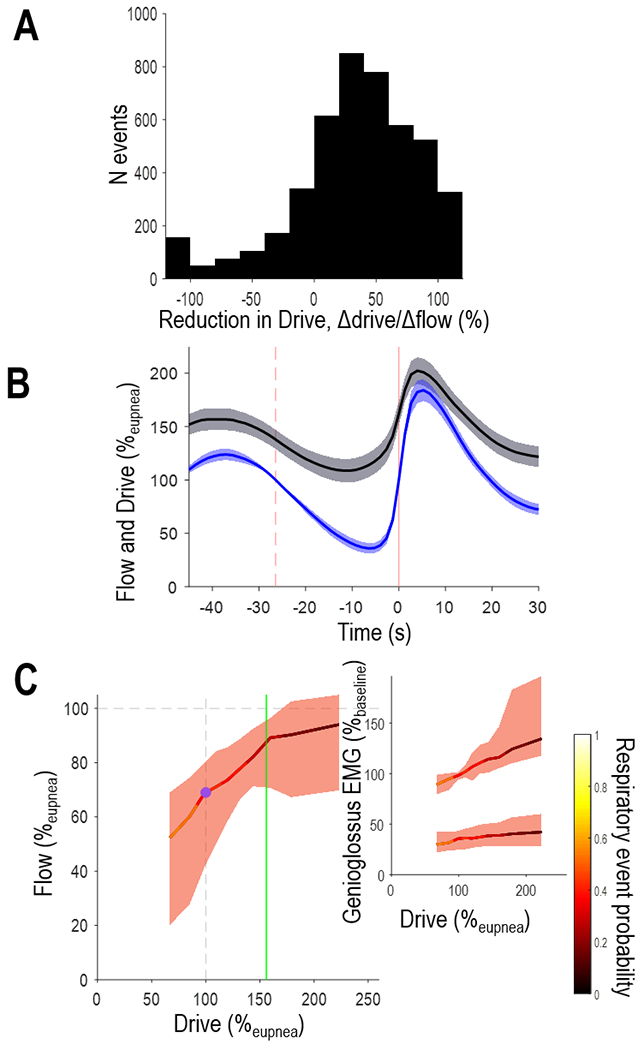
Pooled data from all N=50 patients are shown. A) Histogram of individual events (N=4747) illustrates that drive typically falls rather than rises during events in OSA. B) Multi-patient ensemble-average traces exhibit ventilation and ventilatory drive synchrony; vertical dashed line illustrates the event start and solid vertical line illustrates event end. For illustrative purposes, multi-patient plots were generated by stretching/compressing individual patient ensemble averages (to align start and end times) before averaging (shading denotes 95%CI). Event duration was normalised to the group mean. C) During breaths within sleep, lowered drive is associated with a reduction in flow and genioglossus EMG, as well as an increased likelihood of respiratory events (color-bar).
Ensemble-Averaged Events.
The multi patient ensemble-average flow and drive signals (Figure 2B) illustrate that drive fell and rose in synchrony with the event-related loss and recovery of flow. Drive and flow were strongly and positively correlated (R=0.78[0.24,0.94], P<1×10−9, Table 2); lowered drive (16%baseline; Table 2) was accompanied by a reduction in genioglossus activity (17%baseline; Table 3) and an attendant loss of neuromechanical conductance (flow:drive ratio, 48%baseline; Table 3).
Table 3.
Pharyngeal Obstruction and Dilator Muscle Hypotonia During Events
| Characteristic | All Subjects (N=50) | Classic (N=20) | Drive-Dependent (N=30) |
|---|---|---|---|
| Flow:drive baseline (%) | 71[58,79] | 68[57,72] | 75[60,84] |
| Flow:drive during events (%) | 32[20,52] | 22[16,32] | 41[26,62] |
| Reduction in flow:drive (%baseline) | 48[33,69] | 67[48,79] | 44[26,55] |
| EMGgg baseline (%max) | 9.0[4.7,13.0] | 6.7[4.4,15.0] | 9.9[4.7,11.8] |
| EMGgg during events (%max) | 6.4[3.4,11.6] | 5.9[3.8,15.1] | 7.7[3.4,9.6] |
| Reduction in EMGgg (%baseline) | 17.2[−1.3,31.0] | 0.7[−6.4,31.3] | 19.4[13.4,31.0] |
Data (median[IQR]) were calculated from ensemble-averaged events. See Table 2 footnote for definitions (flow, drive, baseline, during events). Flow:drive represents the magnitude of pharyngeal obstruction (neuromechanical conductance) calibrated to wakefulness levels (i.e. a value of 100% is considered an “open” airway); note that baseline pre-event levels are reduced from wakefulness levels. Reduction in flow:drive describes event-related exacerbation of obstruction: e.g. a fall in flow:drive by 50%baseline accompanying a fall in flow by 50%baseline would indicate no change in drive. EMGgg denotes genioglossus EMG peak.
Drive-Dependent v. Classic OSA—Prevalence and Mechanisms
Events.
The majority of patients (N=30/50, prevalence=60[46,74]%, estimate[95%CI]) were characterized as exhibiting a drive-dependent phenotype v. classic based on visual review of ensemble-averaged events, verified by objective measures (R and Δdrive/Δflow, Figure 3). Quantitatively, drive-dependent patients exhibited a reduction in drive (by 23[16,37]%baseline, Δdrive/Δflow=44[27,59]%, median[IQR]) during events that was tightly correlated with the reduction in flow (R=0.92[0.81,0.95]). By contrast, those with classic OSA exhibited a negligible fall in drive (Δdrive/Δflow=10[−10,18]%, P=6×10−9 v. drive-dependent) that was uncorrelated with flow (R=0.18[−0.38,0.30], P=1×10−9 v. drive-dependent; see Table 2 and Figure 3). Notably only 7/50 individuals exhibited an increase in ventilatory drive (also R<0; Figure 3A–C). Disease severity (per apnea-hypopnea index) was similar between groups (Figure 3D).
Figure 3. Defining Characteristics of Drive-Dependent v. Classic OSA During Events.

Patients visually classified as drive-dependent (N=30) exhibited a higher flow-drive correlation during events (A) and have greater reduction in drive (B) than those classified as classic (N=20), supporting the visual scoring. Variables were quantified from ensemble-average events. The flow-drive correlation (R) and reduction in drive (Δdrive/Δflow) discriminated between groups (see dashed line drawn from a regression model). (D) OSA severity was similar between groups.
Traits.
Compared to the classic phenotype, the drive-dependent phenotype exhibited greater pharyngeal dilator muscle effectiveness (33.1 v. 0.4 %flow/drive, P=5.5×10−5, Wilcoxon rank-sum, Table 4, Figure 4) and a lower arousal threshold (139 v. 171 %eupnea, P=1×10−4). There was no difference in passive collapsibility (e.g. Figure 1D and 4C, purple dots indicate Vpassive) or loop gain between phenotypes.
Table 4.
Drive-Related Sleep Apnea Pathophysiology: Endotypes
| Endotype | All Subjects (N=50) | Classic (N=20) | Drive-Dependent (N=30) |
|---|---|---|---|
| Collapsibility per Vpassive (%eupnea) | 68[48,84] | 65[35,80] | 71[48,86] |
| Muscle Effectiveness (%flow/drive) | 24.7[1.0,38.5] | 0.4[−0.2,23.7] | 33.1[22.2,52.3]* |
| Loop Gain | 0.63[0.55,0.78] | 0.62[0.54,0.72] | 0.63[0.55,0.81] |
| Arousal Threshold (%eupnea) | 156[135,173] | 171[155,198] | 139[124,159]* |
Endotypes were calculated from flow and drive data during non-REM sleep (arousal data removed, data are shown median[IQR]). Vpassive denotes the value of flow at eupneic drive. Muscle effectiveness is the slope of the plot between flow and drive. Loop gain represents the drive response to a reduction in flow (1 cycle per minute, i.e. LG1). The arousal threshold represents the level of drive preceding arousal from sleep. The greater muscle effectiveness in drive-dependent v. classic OSA provided for a greater level of ventilation without arousal (“Vactive”): 87[74,97] v. 69[36,85] %eupnea.
Denotes a significant difference (P<0.05) between drive-dependent v. classic groups.
Figure 4. Flow, Drive, and Dilator Muscles in Drive-Dependent v. Classic OSA.

A) Note that drive falls in tight concordance with falling flow during events in drive-dependent OSA (right) in contrast to classic OSA where flow fails to rise when drive increases (left). B) Drive falling provides a reduced stimulus for genioglossus activity (also falls then rises during events). Note the temporal association between drive and genioglossus activity particularly in drive-dependent OSA (right). C) Multi-patient “endogram” shows flow-drive profiles for both groups. Drive-dependent patients exhibited greater loss of flow and increase in event risk (color bar) with falling drive v. classic patients. Endotypic differences can also be seen: drive-dependent patients had a greater muscle effectiveness (flow-drive slope), lower arousal threshold (green vertical line), but similar collapsibility (flow at drive=100%, “Vpassive”) compared with classic OSA. D) However, average genioglossus-drive profiles were similar between groups. EMG data presented as %eupnea (i.e. data normalized by peak EMG at drive=100%).
Reduced Drive is Associated with Lower Flow and Genioglossus Activity, and Greater Event Risk
Mixed model analysis (same deciles data used for traits above) revealed that—across all patients during sleep—flow and genioglossus activity were significantly, positively associated with drive (Table 5). Lower drive was also associated with greater risk of respiratory events: event odds ratio=2.2[1.8,2.5] per reduction in drive by 100%eupnea (e.g. from 200 to 100%eupnea, Table 6). A physiologically-meaningful relationship was seen between flow and drive exclusively in the drive-dependent patient group (difference from classic: P<1×10−9) in whom there was a profound association between drive and event risk (odds ratio: 6.8[5.3,8.7], Table 6).
Table 5.
Impact of Drive on Flow and Muscle Activity
|
|
||||
|---|---|---|---|---|
| Flow | Genioglossus | |||
|
|
||||
| Baseline “Collapsibility” (%) | Slope “Effectiveness” (%/drive) | Baseline “Tone” (%max) | Slope “Responsiveness” (%max/drive) | |
| All patients | 60[53,69] | 17[14, 20] P<1×10−9 | 5.9[4.2, 8.0] | 3.5[3.3, 3.8] P<1×10−9 |
|
| ||||
| Phenotypes | ||||
| Classic | 56[55, 74] | 4[2, 7] | 6.7[4.0,10.5] | 3.9[3.6, 4.3] |
| Drive-Dependent | 65[55, 74] | 37[34, 40] P<1×10−9 | 5.5[3.5, 8.1] | 3.0[2.4, 3.5] |
| Difference | +9[−6, 24] | +33[29, 37] P<1×10−9 | −1.3[−4.2, 3.2] | −0.4[−0.9, 0.1] |
Mixed model analysis of the effect of drive on flow and genioglossus activity during sleep (values are estimates[95%CI]). Models illustrate significant increases in flow and genioglossus activity with increasing Drive in all patients (“effectiveness” and “responsiveness” respectively, P-values in table). Models also illustrate significantly greater increases in flow wth drive in those with a drive-dependent phenotype of OSA (v. classic OSA who did not increase flow with drive). Flow refers to ventilation (tidal volume × respiratory rate) expressed as a percentage of eupnea. Mixed-model equations followed the forms: 1) For all patients: Flow ~ Drive + (1|Subject) and 2) For separate phenotypes: Flow ~ Drive + Group + Drive×Group + (1|Subject). Collapsibility represents the value of Flow at Drive=100%. Effectiveness represents the increase in flow for a 100% increase in drive (equivalent to a 2SD increase). Analysis for genioglossus activity (peak genioglossus activity) follows the same form as for flow. Models are built from decile data: each subject contributed 10 values of drive, flow and genioglossus activity. Data were exclusively taken during sleep (arousals excluded). Genioglossus activity data were transformed for normality (exponent=0.33); results were back transformed for presentation.
Table 6.
Impact of Drive on Respiratory Event Likelihood
|
|
|||||||
|---|---|---|---|---|---|---|---|
| Model | Odds Ratio | Event Probability | Interpretation | ||||
|
|
|||||||
| at Baseline Drive (Probability) | Drive dependence (Probability/drive) | Per 100% reduction in ventilatory drive* | Reduced Drive (half eupnea) | Eupneic Drive (eupnea) | Elevated Drive (double eupnea) | ||
| All patients | −0.11[−0.52,0.30] | −0.77[−0.92,−0.60] P<1×10−9 | 2.2[1.8,2.5] | 0.57[0.46,0.67] | 0.47[0.37,0.57] | 0.29[0.21,0.39] | Higher drive → Reduced risk of events |
| Phenotypes | |||||||
| Classic | 0.10[−0.46,0.66] | −0.10[−0.23,0.03] P=0.14 | 1.1[0.97,1.3] | 0.54[0.40,0.67] | 0.52[0.39,0.66] | 0.50[0.36,0.64] | Higher drive → Unchanged risk |
| Drive-Dependent | −0.38[−0.86,0.10] | −1.91[−2.16,−1.66] P<1×10−9 | 6.8[5.3,8.7] | 0.64[0.52,0.74] | 0.41[0.30,0.52] | 0.09[0.06,0.15] | Higher drive → Substantially-reduced risk of events |
| Difference | −0.48[−1.22,0.26] | −1.81[−2.09,−1.52] P<1×10−9 | |||||
Mixed-model logistic regression analysis of the effect of drive on respiratory event likelihood (risk) during sleep. Models illustrate significant reduction in event likelihood with increasing drive in all patients (P-values in table). Models also illustrate significantly greater reduction in event likelihood wth drive in those with a drive-dependent phenotype of OSA (v. classic OSA who did not reduce event likelihood flow with drive). Equations followed the forms: 1) For all patients: Event ~ Drive + (1|Subject) and 2) For separate phenotypes: Event ~ Drive + Group + Drive×Group + (1|Subject).
Odds ratio describes increased event likelihood per reduction in drive by 100%eupnea (equivalent to a 2SD reduction); thus, a change from 200%eupnea to 100%eupnea provides a 2.2-fold greater odds of events (all patients). Overall, classic patients have a similar likelihood of being in an event regardless of drive. Drive-dependent patients exhibit marked reduction in event likelihood with elevated drive.
Greater Reduction in Drive in Apneas v. Hypopneas
Based on the drive-dependent model of OSA, we would expect more severe events to exhibit a greater loss of ventilatory drive: Overall we found a significantly greater reduction in drive during apneas v. hypopneas (all patients: 24.9[19.7,30.2] v. 12.4[7.4,17.5]%baseline, P<1×10−9; Table E2).
Discussion
In stark contrast to the prevailing view on OSA pathogenesis, the current study showed that the drive-dependent model is best supported by direct physiological measurement. Unexpectedly, we found that 1) ventilatory drive typically falls—rather than rises—during respiratory events in OSA, 2) a strong temporal correlation exists between drive and flow during events (average R=0.78), 3) spontaneous reductions in drive during sleep were significantly associated with reduced flow, reduced genioglossus activity, and increased risk of respiratory events, and 4) apneas exhibit greater reductions in ventilatory drive compared with hypopneas. Together these findings provide overwhelming support for the drive-dependent v. classic model of OSA. Drive-dependence of OSA, however, was concentrated in a majority subgroup of patients (60%, N=30/50); while a minority of patients exhibited classic pathophysiology (40%, N=20/50).
Physiological Insight
Why was classic OSA the prevailing model?
Despite significant physiological evidence for the role of lowered drive in worsening upper airway patency, until now it has remained widely accepted that the classic model best describes OSA event pathophysiology. We highlight two main reasons for this: 1) While OSA characterized by prolonged apneas (>20 s) is atypical [34 ,35], these patients—who do exhibit classic event pathophysiology (flow is absent regardless of drive)—have been commonly used in illustrative example traces [4 ,25 ,31]. 2) Drive—per intra-esophageal diaphragm EMG—is not measured clinically, and rarely in physiology studies [8 ,19 ,25]. Rather, in prior work, our laboratory and others have routinely measured esophageal pressure swings as a marker of respiratory effort, which paint a very different picture of OSA pathogenesis [3]: While esophageal pressures and diaphragm EMG signals track proportionally during unobstructed breathing [16 ,25], pressure swings become markedly augmented with sudden onset of obstruction[36] and are diminished again at event termination with the release of pharyngeal obstruction. Mechanistically, occlusion and loss of tidal volume provides for an absence of (positive) volume-related chest wall recoil pressure, and diaphragm mechanical efficiency is increased via length-tension and force-velocity effects [25 ,27]. Such volume-dependence is so strong that when neural drive is at its highest level during post-event recovery (when obstructive load is released), respiratory effort per pressure swings is reduced rather than elevated [25], which provides a false impression that drive is maximal during events. Diaphragm EMG is not subject to such mechanical confounding [8 ,26 ,37]. See Supplemental Figures 2 and 8 for further analyses.
Drive-dependent OSA vs. central sleep apnea (CSA).
Drive-dependent OSA, unlike CSA, is characterized by the confluence of an anatomically susceptible (collapsible) airway and a tendency to exhibit more severe flow limitation when neural drive is diminished (see Figure 1D, right). Accompanying diminished drive in drive-dependent OSA, there were clear signs of worsening upper airway obstruction during events: Typically, flow was limited to a third of eupneic levels (individual events, Table 2) and flow:drive fell by ~44% from pre-event baseline (Table 3); unobstructed events were rare (7%, Supplemental Figure 3). Thus, events in drive-dependent OSA are characterized by both diminished drive and worsening pharyngeal obstruction. Indeed, CPAP is highly efficacious for the majority of OSA patients, which may appear at odds with the identified prevalence of drive-dependent behavior. However, by fully resolving flow limitation, CPAP acts to prevent any loss of flow (and ensuing obstructive events) that would normally accompany any (drive related) reduction in upper airway muscle activity; in the absence of a vulnerable/collapsible airway, the mechanism of obstruction described in the current study is no longer possible. The typical reductions in drive (average 20%baseline) would also be insufficient to yield central hypopneas.
Novelty.
Physiological studies have established that experimentally-raising ventilatory drive can improve dilator muscle activity, respiratory mechanics, and obstructive events, in many individuals [9 ,22 ,38], but it has been unclear whether spontaneous reductions in drive are responsible for the observed events in OSA. During spontaneous events, Cori et al [39] demonstrated an association between greater pre-event (arousal-related) hypocapnia and a greater subsequent (event-related) loss of flow and epiglottic pressure swings, but direct measurements of drive have been lacking. For the first time, we used measurements of ventilatory drive during the spontaneous undisturbed obstructive events that characterize OSA to provide evidence supporting a predominant drive-dependent nature of OSA.
Implications for OSA pathophysiology.
First, a major implication of the drive-dependent model is the concept that one respiratory event can positively reinforce another: greater post-event ventilatory “overshoot” and accompanying transient hypocapnia can promote a greater subsequent secondary ventilatory drive “undershoot” with ensuing drive-related pharyngeal collapse [9]. Although airway occlusion is seen at the nadir of drive in central sleep apnea [11], until now this concept lacked supporting data in OSA. Second, an unresolved discrepancy in OSA pathophysiology is the perplexing observation that genioglossus activity typically falls from pre-event levels (Figure 4B) to promote pharyngeal obstruction despite progressively increasing negative esophageal pressure stimuli. Falling then rising drive, described here, provides an explanation for the typical time-course of genioglossus activity during events (Supplemental Figure 4).
Mechanistic differences between drive-dependent and classic OSA.
Compared to classic OSA, drive-dependent OSA was characterized by greater muscle effectiveness and a lower arousal threshold (Supplemental Figure 5). Intuitively, a greater muscle effectiveness with a high arousal threshold should theoretically be sufficient to prevent OSA [40]. Unexpectedly, loop gain was similar between groups, which contrasts with the model that drive-dependent events should be driven by a more unstable chemical ventilatory control system [10]. However, we still believe that greater ventilatory instability would have the most impact on OSA severity in those with drive-dependent OSA. The absence of differences between groups in pharyngeal collapsibility (Vpassive, Table 4), AHI, event duration (Table 1), or event depth (Table 2) suggests that the groups represent distinct pathophysiology rather than different severities of disease.
The higher muscle effectiveness (flow v. drive slope) but similar genioglossus responsiveness in drive-dependent v. classic subgroups suggests that neuromechanical inefficiency may be a key characteristic of classic OSA behaviour. Indeed, further analysis (Supplemental Figure 6, Supplemental Table 3–4) revealed that some individuals failed to raise flow with drive during events via low genioglossus responsiveness (“won’t respond”, N=7/16), while others failed to translate a genioglossus response into an improvement in pharyngeal mechanics (neuromechanical inefficiency, “can’t respond”, N=9/16). In the latter, genioglossus responsiveness was actually augmented (Supplemental Table 3). Further investigation is needed to determine whether improving aspects of neuromechanical efficiency (contractile function, mechanical coupling/coordination) may have therapeutic potential for this minority subgroup of patients. Such inefficiency might be explained if these patients exhibited pharyngeal collapse that was minimally related to the genioglossus (e.g. lateral walls rather than tongue-base).
Clinical Implications
Drive-dependence as a therapeutic target.
The predominance of drive-dependent events in OSA suggests that strategies focused on stabilizing ventilatory drive or preventing transient reductions in drive could benefit more individuals than previously appreciated. So far, oxygen therapy (to prevent dips in drive via lowered loop gain) has shown acute benefit for patients with greater muscle responsiveness, milder airway collapsibility, and higher loop gain [13]. However, the response rate (AHI reduced by >50% in 25% [13]) is considerably lower than the prevalence of drive-dependent OSA found in this study; this may be a result of oxygen lowering average drive, offsetting the expected benefits of stabilized drive. Increasing ventilatory drive via induced hypercapnia resolved OSA in ~two-thirds of patients [22] a similar rate to the observed predominance of drive-dependent behaviour here. Therapies that achieve this goal without augmentation of sympathetic activity are of interest. Acetazolamide reduces AHI by approximately 30-40% in OSA [41]; this agent not only stabilizes ventilatory control but also increases resting ventilatory drive [14 ,42], which may explain its apparent benefit over supplemental oxygen. Agents designed to raise the arousal threshold are also expected to have a preferential benefit in drive-dependent OSA [40] and potentially be deleterious in those with classic OSA. Indeed, a combination of oxygen and a hypnotic used recently showed promising efficacy (response rate ~45%), particularly in those with more effective upper airway muscle responses and milder collapsibility [15].
Strengths and Limitations
This study is unique in the number of OSA patients with highly instrumented physiological sleep recordings, providing detailed ventilatory drive and ventilation data from naturally occurring sleep. Our study has several limitations. 1) While our sample size may appear relatively modest, our main conclusions appear robust from the perspective of statistical power: 95% confidence intervals (per bootstrapping) indicate that drive typically falls rather than rises during events (individual events (20[19,21]; ensemble averages (16[12,20]; median[95%CI on median]). Similarly, confidence intervals of the correlation between flow and drive indicates a clear positive correlation (0.78[0.54,0.90]). Where sample size is the smallest (in exploratory subphenotype analysis), the subphenotypes still clearly differ in drive-related event risk (per 95%CI, Supplemental Table 4, see Supplemental Methods–Statistical Analysis). Nonetheless, increased sample size would further improve generalizability. 2) Patients were recruited primarily from sleep clinics and thus likely reflect a clinical population rather than community population. 3) Our study provides insights based on gold standard invasive physiological signals. However, if there are treatment implications of drive-dependent v. classic OSA, non-invasive means to identify these subgroups will be required. Several techniques are now available and will be the subject of further investigation [19 ,29 ,32]. Precise thresholds for drive-dependence (e.g. Δdrive/Δflow>25% or R>0.5, Figure 3) will require validation for clinical use. 4) We did not experimentally-raise ventilatory drive to confirm that rising drive improves flow (and OSA) preferentially in drive-dependent patients, which will be subject to future investigation now that drive-dependence appears common. 5) Our data do not describe the source of the lowered drive—i.e. chemical drive (hypocapnia) v. state-related input (“wakefulness” drive)—but additional analysis (demonstrates that the deleterious effect of lowered drive on pharyngeal patency (per flow) is consistently observed no matter whether drive is falling (i.e. after sleep onset, with declining chemical and wakefulness drives) or rising (with rising chemical drive; see Supplemental Figure 7, Supplemental Table 5) suggests a chemical drive origin. 6) One might consider that lowered drive during events could occur secondary to airway collapse via reflex inhibition, however acute resistive loading does not generally lower diaphragm EMG [26 ,37]; previously observed reflex inhibition has been transient (<100ms timescale)[43]. Reflex inhibition was not visually evident during experimentally-induced obstruction (CPAP manipulation) in the current study (see Supplemental Figure 8). 7) The assumption that falling drive leads to reduced upper airway dilator muscle activity was based on falling genioglossus EMG but may not apply equally to all upper airway dilator muscles and hence might not be as relevant for non-tongue related sites of collapse. 8) Our measure of neural drive was based on activity of the diaphragm—albeit the primary generator of flow [44]—without recordings from extra-diaphragmatic inspiratory muscles e.g. external intercostals. We note that a sustained reduction in extra-diaphragmatic drive (e.g. in REM) would lead to overestimation of drive and obstruction, but changes in drive and obstruction (%baseline) would be unaffected. Intercostal muscles are typically thought to rise and fall in parallel with diaphragm EMG with changing chemical drive [45]. 9) Our study implies an absence of an important role for ventilatory drive in classic OSA, and the characteristic ‘rising drive’ could be considered an oversimplification; drive can still fall in these individuals (e.g. earlier in events) and may have meaningful consequences (e.g. prolonged event duration).
Conclusions
In contrast to the prevailing view that ventilatory drive rises during respiratory events in OSA, direct measures of diaphragm EMG indicate events are associated with a clear reduction in drive. In most patients, flow and drive remain closely correlated for the duration of the respiratory event. Interventions that either elevate drive or prevent falls in drive may be of value to a majority of OSA patients.
Supplementary Material
Key Messages.
What is the key question?
What is the role of ventilatory drive in the pathophysiology of obstructive sleep apnea?
What is the bottom line?
Contrary to the prevailing view, obstructive events in patients with sleep apnea are typically characterized by a loss rather than rise in ventilatory drive.
Why read on?
Our data refute entrenched notions about the causes of events in OSA and demonstrate that the ventilatory control contribution to OSA is greater than previously appreciated.
Support:
This work was supported financially by the American Heart Association (15SDG25890059) and the National Institute of Health NHLBI (R01HL146697, R01HL102321). The authors are grateful to Maquet Getinge Group for the loan of the Servo-i ventilator to measure intra-esophageal diaphragm EMG. AA was supported by the National Institutes of Health (R01HL153874), American Heart Association (19CDA34660137) and the American Academy of Sleep Medicine Foundation (188-SR-17).
Conflict statement:
SS received grant support from Apnimed, Prosomnus, and Dynaflex, and has served as a consultant for Apnimed, Nox Medical, and Merck. DPW is a distinguished scientist with Apnimed and a consultant for RBNC Therateutics, Philips Respironics, Cryosa, and Cerebra Health.AA serves as consultant for Somnifix and Apnimed and receives grant support from Somnifix. AW works as a consultant for Apnimed, Nox, Inspire, and Somnifix and has received grants from Sanofi and Somnifix. LTM works as medical director at Apnimed. AW and LTM have a financial interest in Apnimed Inc., a company developing pharmacologic therapies for sleep apnea; their interests were reviewed and are managed by Brigham and Women’s Hospital and Partners HealthCare in accordance with their conflict of interest policies.
References
- 1.Azarbarzin A, Sands SA, Stone KL, et al. The hypoxic burden of sleep apnoea predicts cardiovascular disease-related mortality: the Osteoporotic Fractures in Men Study and the Sleep Heart Health Study. Eur Heart J 2019;40(14):1149–57. doi: 10.1093/eurheartj/ehy624 [published Online First: 2018/10/31] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wellman A, Edwards BA, Sands SA, et al. A simplified method for determining phenotypic traits in patients with obstructive sleep apnea. J Appl Physiol 2013;114(7):911–22. doi: 10.1152/japplphysiol.00747.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eckert DJ, Malhotra A. Pathophysiology of adult obstructive sleep apnea. Proc Am Thorac Soc 2008;5(2):144–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Remmers JE, deGroot WJ, Sauerland EK, et al. Pathogenesis of upper airway occlusion during sleep. J Appl Physiol 1978;44(6):931–8. [DOI] [PubMed] [Google Scholar]
- 5.Onal E, Lopata M, O’Connor TD. Diaphragmatic and genioglossal electromyogram responses to isocapnic hypoxia in humans. Am Rev Respir Dis 1981;124(3):215–7. [published Online First: 1981/09/01] [DOI] [PubMed] [Google Scholar]
- 6.Warner G, Skatrud JB, Dempsey JA. Effect of hypoxia-induced periodic breathing on upper airway obstruction during sleep. J Appl Physiol 1987;62(6):2201–11. [DOI] [PubMed] [Google Scholar]
- 7.Longobardo GS, Gothe B, Goldman MD, et al. Sleep apnea considered as a control system instability. Respir Physiol 1982;50(3):311–33. [DOI] [PubMed] [Google Scholar]
- 8.Onal E, Lopata M, O’Connor T. Pathogenesis of apneas in hypersomnia-sleep apnea syndrome. Am Rev Respir Dis 1982;125(2):167–74. doi: 10.1164/arrd.1982.125.2.167 [published Online First: 1982/02/01] [DOI] [PubMed] [Google Scholar]
- 9.Younes M Role of respiratory control mechanisms in the pathogenesis of obstructive sleep disorders. J Appl Physiol 2008;105(5):1389–405. [DOI] [PubMed] [Google Scholar]
- 10.Dempsey JA, Xie A, Patz DS, et al. Physiology in medicine: obstructive sleep apnea pathogenesis and treatment--considerations beyond airway anatomy. J Appl Physiol (1985) 2014;116(1):3–12. doi: 10.1152/japplphysiol.01054.2013 [published Online First: 2013/11/10] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Badr MS, Toiber F, Skatrud JB, et al. Pharyngeal narrowing/occlusion during central sleep apnea. J Appl Physiol 1995;78(5):1806–15. [published Online First: 1995/05/01] [DOI] [PubMed] [Google Scholar]
- 12.Eckert DJ, White DP, Jordan AS, et al. Defining phenotypic causes of obstructive sleep apnea. Identification of novel therapeutic targets. Am J Respir Crit Care Med 2013;188(8):996–1004. doi: 10.1164/rccm.201303-0448OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sands SA, Edwards BA, Terrill PI, et al. Identifying obstructive sleep apnoea patients responsive to supplemental oxygen therapy. Eur Respir J 2018;52(3) doi: 10.1183/13993003.00674-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edwards BA, Sands SA, Eckert DJ, et al. Acetazolamide improves loop gain but not the other physiological traits causing obstructive sleep apnoea. J Physiol 2012;590(Pt 5):1199–211. doi: 10.1113/jphysiol.2011.223925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edwards BA, Sands SA, Owens RL, et al. The Combination of Supplemental Oxygen and a Hypnotic Markedly Improves Obstructive Sleep Apnea in Patients with a Mild to Moderate Upper Airway Collapsibility. Sleep 2016;39(11):1973–83. doi: 10.5665/sleep.6226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Onal E, Leech JA, Lopata M. Dynamics of respiratory drive and pressure during NREM sleep in patients with occlusive apneas. J Appl Physiol (1985) 1985;58(6):1971–4. doi: 10.1152/jappl.1985.58.6.1971 [published Online First: 1985/06/01] [DOI] [PubMed] [Google Scholar]
- 17.Malhotra A, Pillar G, Fogel RB, et al. Genioglossal but not palatal muscle activity relates closely to pharyngeal pressure. Am J Respir Crit Care Med 2000;162(3 Pt 1):1058–62. doi: 10.1164/ajrccm.162.3.9912067 [published Online First: 2000/09/16] [DOI] [PubMed] [Google Scholar]
- 18.Sands SA, Eckert DJ, Jordan AS, et al. Enhanced Upper-airway Muscle Responsiveness is a Distinct Feature of Overweight/Obese Individuals Without Sleep Apnea. Am J Respir Crit Care Med 2014. doi: 10.1164/rccm.201404-0783OC [published Online First: 2014/09/06] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sands SA, Edwards BA, Terrill PI, et al. Phenotyping Pharyngeal Pathophysiology using Polysomnography in Patients with Obstructive Sleep Apnea. Am J Respir Crit Care Med 2018;197(9):1187–97. doi: 10.1164/rccm.201707-1435OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McGinley BM, Schwartz AR, Schneider H, et al. Upper airway neuromuscular compensation during sleep is defective in obstructive sleep apnea. J Appl Physiol (1985) 2008;105(1):197–205. doi: 10.1152/japplphysiol.01214.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patil SP, Schneider H, Marx JJ, et al. Neuromechanical control of upper airway patency during sleep. J Appl Physiol 2007;102(2):547–56. doi: 00282.2006 [pii] 10.1152/japplphysiol.00282.2006 [published Online First: 2006/09/30] [DOI] [PubMed] [Google Scholar]
- 22.Xie A, Teodorescu M, Pegelow DF, et al. Effects of stabilizing or increasing respiratory motor outputs on obstructive sleep apnea. J Appl Physiol 2013;115(1):22–33. doi: 10.1152/japplphysiol.00064.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Melo CM, Taranto-Montemurro L, Butler JP, et al. Stable Breathing in Patients With Obstructive Sleep Apnea Is Associated With Increased Effort but Not Lowered Metabolic Rate. Sleep 2017;40(10) doi: 10.1093/sleep/zsx128 [published Online First: 2017/10/05] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ratnavadivel R, Chau N, Stadler D, et al. Marked reduction in obstructive sleep apnea severity in slow wave sleep. J Clin Sleep Med 2009;5(6):519–24. [published Online First: 2010/05/15] [PMC free article] [PubMed] [Google Scholar]
- 25.Luo YM, Wu HD, Tang J, et al. Neural respiratory drive during apnoeic events in obstructive sleep apnoea. Eur Respir J 2008;31(3):650–7. doi: 10.1183/09031936.00049907 [published Online First: 2007/11/23] [DOI] [PubMed] [Google Scholar]
- 26.Badr MS, Skatrud JB, Dempsey JA, et al. Effect of mechanical loading on expiratory and inspiratory muscle activity during NREM sleep. J Appl Physiol (1985) 1990;68(3):1195–202. doi: 10.1152/jappl.1990.68.3.1195 [published Online First: 1990/03/01] [DOI] [PubMed] [Google Scholar]
- 27.Younes M, Riddle W, Polacheck J. A model for the relation between respiratory neural and mechanical outputs. III. Validation. J Appl Physiol 1981;51(4):990–1001. [DOI] [PubMed] [Google Scholar]
- 28.Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med 2012;8(5):597–619. doi: 10.5664/jcsm.2172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mann DL, Terrill PI, Azarbarzin A, et al. Quantifying the magnitude of pharyngeal obstruction during sleep using airflow shape. Eur Respir J 2019;54(1) doi: 10.1183/13993003.02262-2018 [published Online First: 2019/04/20] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vena D, Azarbarzin A, Marques M, et al. Predicting sleep apnea responses to oral appliance therapy using polysomnographic airflow. Sleep 2020. doi: 10.1093/sleep/zsaa004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jordan AS, White DP, Lo YL, et al. Airway dilator muscle activity and lung volume during stable breathing in obstructive sleep apnea. Sleep 2009;32(3):361–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Terrill PI, Edwards BA, Nemati S, et al. Quantifying the ventilatory control contribution to sleep apnoea using polysomnography. Eur Respir J 2015;45(2):408–18. doi: 10.1183/09031936.00062914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sands SA, Terrill PI, Edwards BA, et al. Quantifying the Arousal Threshold Using Polysomnography in Obstructive Sleep Apnea. Sleep 2018;41(1) doi: 10.1093/sleep/zsx183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Butler MP, Emch JT, Rueschman M, et al. Apnea-Hypopnea Event Duration Predicts Mortality in Men and Women in the Sleep Heart Health Study. Am J Respir Crit Care Med 2019;199(7):903–12. doi: 10.1164/rccm.201804-0758OC [published Online First: 2018/10/20] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Edwards BA, Eckert DJ, McSharry DG, et al. Clinical Predictors of the Respiratory Arousal Threshold in Patients with Obstructive Sleep Apnea. Am J Respir Crit Care Med 2014. doi: 10.1164/rccm.201404-0718OC [published Online First: 2014/10/17] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gell LK, Stadler DL, Reynolds KJ, et al. Exaggerated ventilatory drive estimates from epiglottic and esophageal pressure deflections in the presence of airway occlusion. Journal of Applied Physiology 2021;131(2):760–67. doi: 10.1152/japplphysiol.00896.2020 [DOI] [PubMed] [Google Scholar]
- 37.Hudgel DW, Mulholland M, Hendricks C. Neuromuscular and mechanical responses to inspiratory resistive loading during sleep. J Appl Physiol 1987;63(2):603–8. [published Online First: 1987/08/01] [DOI] [PubMed] [Google Scholar]
- 38.Onal E, Lopata M, O’Connor TD. Diaphragmatic and genioglossal electromyogram responses to CO2 rebreathing in humans. J Appl Physiol 1981;50(5):1052–5. [published Online First: 1981/05/01] [DOI] [PubMed] [Google Scholar]
- 39.Cori JM, Thornton T, O’Donoghue FJ, et al. Arousal-Induced Hypocapnia Does Not Reduce Genioglossus Activity in Obstructive Sleep Apnea. Sleep 2017;40(6) doi: 10.1093/sleep/zsx057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jordan AS, O’Donoghue FJ, Cori JM, et al. Physiology of Arousal in OSA and Potential Impacts for Sedative Treatment. Am J Respir Crit Care Med 2017. doi: 10.1164/rccm.201612-2511PP [DOI] [PubMed] [Google Scholar]
- 41.Schmickl CN, Landry SA, Orr JE, et al. Acetazolamide for OSA and Central Sleep Apnea: A Comprehensive Systematic Review and Meta-Analysis. Chest 2020. doi: 10.1016/j.chest.2020.06.078 [published Online First: 2020/08/10] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Edwards BA, Connolly JG, Campana LM, et al. Acetazolamide attenuates the ventilatory response to arousal in patients with obstructive sleep apnea. Sleep 2013;36(2):281–5. doi: 10.5665/sleep.2390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jeffery S, Butler JE, McKenzie DK, et al. Brief airway occlusion produces prolonged reflex inhibition of inspiratory muscles in obstructive sleep apnea. Sleep 2006;29(3):321–8. doi: 10.1093/sleep/29.3.321 [published Online First: 2006/03/24] [DOI] [PubMed] [Google Scholar]
- 44.Butler JE. Drive to the human respiratory muscles. Respir Physiol Neurobiol 2007;159(2):115–26. doi: 10.1016/j.resp.2007.06.006 [published Online First: 2007/07/31] [DOI] [PubMed] [Google Scholar]
- 45.van Lunteren E, Cherniack NS. Electrical and mechanical activity of respiratory muscles during hypercapnia. J Appl Physiol (1985) 1986;61(2):719–27. doi: 10.1152/jappl.1986.61.2.719 [published Online First: 1986/08/01] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


