Abstract
Background:
Low empathy is one component of affective impairments defining the antisocial youth phenotype callous-unemotional (CU) traits. Research suggests CU traits may be negatively associated with neural networks that are positively associated with cognitive and affective empathy – specifically the default mode (DMN), frontoparietal (FPN), and salience (SAL) networks. Determining which functional network connections are shared between CU traits and empathy could elucidate the extent to which CU traits shares neural substrates with cognitive versus affective empathy. The present study tested whether CU traits and both cognitive and affective empathy share network connections within and between the DMN, FPN, and SAL.
Methods:
Participants (n=112, aged 13–17, 43% female) completed resting-state functional magnetic resonance imaging and self-reports for CU traits and empathy as part of a Nathan-Kline Institute study.
Results:
Analyses revealed inverse associations with shared network connections between CU traits and both cognitive and affective empathy. Specifically, within-DMN connectivity negatively associated with CU traits, but positively associated with cognitive empathy; and between DMN-SAL connectivity positively associated with CU traits, but negatively associated with both cognitive and affective empathy. However, joint models revealed little variance explained by CU traits and empathy overlapped.
Limitations:
The sample was cross-sectional collection with limited participants (n=112) from the community that may not generalize to incarcerated adolescents.
Conclusions:
Results demonstrate CU traits inversely associated with similar connectivity patterns as cognitive and affective empathy though prediction among constructs did not significantly overlap. Further investigation of these connections can inform a mechanistic understanding of empathy impairments in CU traits.
Keywords: empathy, callous-unemotional traits, functional connectivity, adolescents
1. Introduction
Callous-Unemotional (CU) traits are defined by impairments in prosocial emotions of remorse, guilt, and empathy (Frick et al., 2014a, 2014b). Assessing the presence of CU traits has become important in the diagnoses of youth antisocial behavior-related psychiatric disorders including Conduct Disorder and Oppositional Defiant Disorder (American Psychiatric Association, 2013: DSM-5; ICD-11; World Health Organization, 2020). These disorders are associated with aggression, rule breaking and violence, and have a large impact on society (Frick et al., 2014b; Frick & White, 2008). The presence of CU traits within these disorders identifies youth with more severe, stable, and chronic antisocial behavior (Frick & White, 2008). Empathy underlies motivation for prosocial behavior (Decety et al., 2016; Eisenberg et al., 2010; Eisenberg & Miller, 1987) and broadly defines a capacity to understand others by sharing their emotions (Decety et al., 2016). Low empathy is a prominent impairment among a broader set of affective and interpersonal deficits associated with CU traits (Frick et al., 2014a, 2014b; Rijnders et al., 2021). CU traits have an inverse association with empathy (Waller et al., 2020); and recent reviews suggest that empathy and CU traits are associated with activation in similar brain regions across a variety of tasks (for reviews: Seara-Cardoso et al., 2022; Seara-Cardoso & Viding, 2015). Beyond task-based activation of specific regions, recent work highlights the importance of neural connectivity amongst integrated functional networks for empathy (Christov-Moore et al., 2020; Winters, Pruitt, et al., 2021) and that individual differences in network connectivity in these same networks may be associated with CU traits (Pu et al., 2017; Umbach & Tottenham, 2020; Winters, Sakai, et al., 2021; Yoder et al., 2016). Together, these findings suggest that CU traits and empathy may share common neural substrates. However, much of this work has been among adults. Functional connections are important developmental features of adolescent brains (Ernst et al., 2015; Uddin et al., 2011) and understanding the shared functional connections between empathy and CU traits during adolescence can elucidate important developmental features of core impairments associated with CU traits. Thus, the present study examines functional connectivity patterns related to CU traits and empathy and their overlap in a sample of adolescents.
1.2. Neural Correlates of Cognitive and Affective Empathy
Empathy is generally divided into cognitive and affective components, which are characterized by distinct neural correlates. Cognitive empathy involves adopting another’s point of view to understand their thoughts and feelings; whereas affective empathy involves sharing another’s emotional experience, which can involve empathic concern, or feelings of concern for their emotional wellbeing (Decety, 2011; Decety & Cowell, 2015). For affective empathy, the anterior insulae and cingulate cortex work together to facilitate vicarious experiences that support affective empathy (Lockwood, 2016). These regions are activated during tasks eliciting empathy for others pain (For meta-analyses; Fan et al., 2011; Lamm et al., 2011), which further supports recruitment of these regions for vicarious experience of another’s felt state. Together these regions form the core nodes of the salience network (SAL; Menon & Uddin, 2010) that, when active, signals activation of the frontoparietal network (FPN; i.e., dorsolateral prefrontal cortex and posterior parietal cortex; Menon & Uddin, 2010). Functional connectivity studies suggest affective empathy is associated with positive connectivity within the SAL (Cox et al., 2011) and between regions consisting of the SAL and the FPN (Christov-Moore et al., 2020).
Cognitive empathy is commonly measured using theory of mind tasks and these tasks engage the medial prefrontal, posterior cingulate, and temporal parietal junction in adolescents (Blakemore, 2008, 2012) and adults (Saxe, 2009; Young et al., 2010). These regions comprise core areas of the default mode network (DMN; Menon & Uddin, 2010). Functional connectivity has demonstrated cognitive empathy is differentiated from affective empathy by connectivity in the DMN (specifically between the bilateral temporal parietal junction and medial prefrontal cortex) as well as greater anti-correlation between the SAL and DMN (Winters, Pruitt, et al., 2021). Disruptions in these neural associations can negatively impact cognitive functioning and social behavior (Menon & Uddin, 2010).
1.3. Neural Correlates of Callous-Unemotional Traits
Higher levels of CU traits are moderately to strongly associated with lower levels of cognitive and affective empathy (for meta-analysis: Waller et al., 2020). However, some initial evidence suggests that empathy and CU traits may be associated with the same brain networks in inverse ways. For example, during affective empathy eliciting tasks, those with psychopathic traits demonstrate less activity in regions comprising the SAL including the anterior insula, amygdala, and cingulate cortex (Blair, 2013; Decety et al., 2013; Kiehl et al., 2001), which is a pattern observed across the literature (e.g., Seara-Cardoso et al., 2022; Seara-Cardoso & Viding, 2015). Similarly, during cognitive empathy related tasks, regions comprising the DMN (medial prefrontal, cingulate, and temporal parietal junction) are elicited during moral decision making (Harenski et al., 2012; Young et al., 2007) suggesting the consideration of another’s mental state if harm was caused. However, those with psychopathic traits demonstrate less activation of these areas during moral decision making (Harenski & Hamann, 2006; Harenski et al., 2014; Harenski et al., 2010).
Functional connectivity studies on CU traits in adolescents demonstrate similar inverse relationships. For example, those with higher CU traits demonstrate reduced connectivity within the DMN (Umbach & Tottenham, 2020) and SAL, (Yoder et al., 2016) as well as aberrant connectivity within the FPN (Pu et al., 2017), which are inverse to the associations reported for empathy above. Similarly, although in normative samples we expect anticorrelation in functional coupling between task positive and task negative networks (for review see: Menon, 2015), that is suggested to support empathy (Uddin et al., 2009), those with higher CU traits demonstrate with less anticorrelation between DMN-FPN (Pu et al., 2017; Werhahn et al., 2020; Winters, Sakai, et al., 2021) and DMN-SAL (Werhahn et al., 2020). Together these findings suggest reduced efficiency of network function in those with higher CU traits that also support empathy.
1.4. Neural Connectivity Shared Between Callous-Unemotional Traits and Empathy
Impairments in affective empathy (Blair, 2008; Blair et al., 2001; Blair et al., 2014) and cognitive empathy (i.e., theory of mind or mentalizing; Drayton et al., 2018; Sharp et al., 2015; Tillem et al., 2020) that are associated with CU traits may be explained by individual differences in network connectivity that is shared by CU traits and both cognitive and affective empathy (Hamilton et al., 2015). However, shared functional connectivity between these constructs remain understudied. That is, few studies have examined functional connectivity within resting state networks and how differences in connectivity may map simultaneously onto CU traits and empathy. One study on affective empathy did examine coactivation in core regions of the DMN and SAL during emotion eliciting tasks where the participant considered their own and others’ emotions. This study revealed differences in the insula and anterior cingulate cortex at higher CU traits during affective empathy and suggests that empathy differences in CU traits can be examined in the brain (Sethi et al., 2018). For cognitive empathy, two behavioral studies found that individuals higher in CU traits had greater difficulty in cognitive empathy for others complex versus basic emotions (Sharp et al., 2015; Winters & Sakai, 2021). However, though Sharp et al. (2015) speculated these results to be an affective deficit related to amygdala function, Winters and Sakai (2021) found that placing additional demands on cognitive control caused additional decrements in complex cognitive empathy – suggesting the importance of top-down networks for cognitive empathy deficits in CU traits. Top-down networks involving the FPN and between FPN-DMN show differences in connectivity at higher CU traits (Winters, Sakai, et al., 2021), which supports the importance of top-down networks. What remains unknown is which patterns of functional connectivity are shared between empathy and CU traits in adolescents.
Functional connectivity represents a particularly important feature of understanding processes associated with adolescent brains (Ernst et al., 2015; Uddin et al., 2011). As opposed to traditional modular task-based activations, functional connections represent distributed function amongst brain regions (Zhang et al., 2021), which are important developmental features of adolescent brains (Ernst et al., 2015; Uddin et al., 2011). Examining functional connectivity is consistent with contemporary theory that differences in connectivity between the DMN, FPN, and SAL may underlie CU traits (Hamilton et al., 2015) and functional connectivity patterns have demonstrated replicability that aid in identifying mechanisms driving behavior (Mišić & Sporns, 2016; Shehzad et al., 2009; Whitfield-Gabrieli et al., 2016). Thus, investigating shared network connections of CU traits with cognitive and affective empathy within and between the DMN, FPN, and SAL can reveal important features of adolescent brains underlying these processes. Although such investigations may reveal mechanisms critical for understanding core impairments in CU traits, less is understood about what network connections are shared between CU traits and both cognitive and affective empathy.
1.5. Current Study
The present study aimed to examine whether specific network connections are shared between CU traits and both cognitive and affective empathy. As suggested in previous research, we hypothesized that higher affective empathy would be associated with higher SAL connectivity and between FPN-SAL connectivity, whereas CU traits would be associated with lower SAL and between FPN-SAL connectivity. We also hypothesized that higher cognitive empathy would be associated with higher DMN connectivity and anticorrelation between SAL-DMN connectivity, whereas CU traits would be associated with lower DMN and less anticorrelation between SAL-DMN connectivity. Given that affective empathy (Blair, 2008; Blair et al., 2001; Blair et al., 2014) and cognitive empathy (Drayton et al., 2018; Sharp et al., 2015; Tillem et al., 2020) are core impairments in CU traits, identifying shared connectivity underlying both empathy and CU traits can help to identify the extent to which CU traits and empathy share neural substrates, which can help to build a better mechanistic understanding of CU traits. CU traits exist on a continuum and are present (although lower) in community samples (e.g., Umbach & Tottenham, 2020; Winters, Sakai, et al., 2021). Similarly, significant evidence suggests that community samples on the continuum of CU traits demonstrate similarities with forensic samples in neurocognitive impairments (Viding & McCrory, 2012) and neurobiological associations (Seara-Cardoso et al., 2022). Thus, the present study investigates shared functional connectivity within and between the DMN, FPN, and SAL between CU traits and both cognitive and affective empathy amongst a community sample of adolescents.
2. Methods
2.1. Sample
Participants, aged 13–17 years old, were drawn from the Nathan Kline Institute’s Rockland study (for study procedures see: Nooner et al., 2012) using the 1000 connectomes project website (www.nitrc.org/projects/fcon_1000/). To ensure integrity of the data we excluded participants that had a WAIS-II IQ score < 80 (α = .96) (Wechsler, 2011). From a total of 122 participants 13–17 years old, we removed 10 for IQ < 80 leaving 112 participants for analysis. Parents reported that the youth in the sample were predominantly White (White= 63%, Black = 24%, Asian = 9%, Indian = 1%, other= 3%) with slightly more boys (female = 43%) and a mean age of 14.52±1.31 years.
2.2. Measures
Interpersonal Reactivity Index (IRI).
Cognitive and affective empathy were assessed using the IRI (Davis, 1980, 1983) perspective taking and empathic concern subscales (respectively). These subscales are commonly used for assessing cognitive and affective empathy (Baron-Cohen & Wheelwright, 2004; Konrath, 2013). Cognitive empathy is defined as the tendency to adopt others psychological point of view (e.g., “I try to look at everybody’s side of a disagreement before I make a decision”; present sample α=.74). Affective empathy is defined as the tendency to experience other’s feelings and have concern for them (e.g., “When I see someone being taken advantage of, I feel kind of protective towards them”; present sample α=.79). These subscales, each consisting of seven items, were rated on a five-point scale from 0 (“does not describe me”) to 4 (“describes me well”).
Inventory of Callous-Unemotional Traits (ICU).
The total score of the 24-item ICU was used to assess CU traits (Frick, 2004). We used the same factor structure that was validated by Kimonis et al. (2008) that removed two items due to poor psychometrics. This factor structure had an adequate reliability in the current sample (present sample α=.72). Items such as “I do not show my emotions to others” are rated on a four-point Likert scale from 0 (“not true at all”) to 3 (“definitely true”), with higher scores meaning greater CU traits.
Covariates.
In our analyses we controlled for sex, pubertal stage, and conduct problems. Because our research question was to examine CU traits, we controlled for conduct problems that are often comorbid with CU traits but are distinct and account for different outcomes (e.g., Baskin-Sommers et al., 2015; Herpers et al., 2012; Hyde et al., 2015). Controlling for conduct problems helps to separate out the impact of CU traits specifically versus their correlation with more severe antisocial behavior. Thus, we used the externalizing subscale of the Achenbach Youth Self-Report (Achenbach & Rescorla, 2001) as a covariate. Validity and reliability of the externalizing measure are acceptable (Achenbach & Rescorla, 2001) and was internally consistent in the present sample (α=.87). We used the raw scores for analysis as suggested by the measurement developers (Achenbach & Rescorla, 2001).
Pubertal stage and sex were measured by the genital and breast development subscales of the Tanner assessment (Petersen et al., 1988). Parents rated pictures representing development of secondary sex characteristics on a scale of 1 (pre-pubertal) to 5 (full maturity), with higher scores indicating greater developmental maturity. The internal consistency of the measure was adequate for the present sample (α = .77). Because the variation in timing of puberty when measured by age (about five years , Parent et al., 2003) and hormonal changes during puberty impact behavior via direct effect on the adolescent brain (Cameron, 2004; Dahl, 2004; Sisk & Foster, 2004), we controlled for pubertal stage instead of age. We included sex as a covariate because it is associated with both empathy and CU traits, and impacts brain structure amongst youth with CU traits (Raschle et al., 2018).
Imaging Acquisition.
During resting state data collection, participants were instructed to keep their eyes closed without falling asleep while images were collected with a Siemens TimTrio 3T scanner using a blood oxygen level dependent (BOLD) contrast with an interleaved multiband echo planar imaging (EPI) sequence. Each participant received a functional magnetic resonance imaging scan during resting state (260 EPI volumes; repetition time (TR) 1400ms; echo time (TE) 30ms; flip angle 65°; 64 slices, Field of view (FOV) = 224mm, voxel size 2mm isotropic, duration = 10 minutes) and a magnetization prepared rapid gradient echo (MPRAGE) anatomical image (TR= 1900ms, flip angle 9°, 176 slices, FOV= 250mm, voxel size= 1mm isotropic). Removing scans for T1 stabilization was not necessary given that the Siemens sequence collects images after saturation is achieved.
Imaging Preprocessing.
We downloaded the raw data and used the standard preprocessing pipeline in the CONN toolbox (version 18b; Whitfield-Gabrieli & Nieto-Castanon, 2012) using Statistical Parametric Mapping (SPM version 12; Penny et al., 2011). The Artifact Detection Tools (ART; http://www.nitrc.org/projects/artifact_detect) identified motion outliers at each timepoint and flagged them for correction with de-spiking if framewise displacement > 0.5mm or if global BOLD signal change > 3 standard deviations. Additionally, motion was regressed out of each individual timeseries using 6 motion parameters (x, y, z translations and rotations). Physiologic, CSF, and white matter noise was regressed out of the BOLD signal using anatomic component-based noise correction method (aCompCor; Whitfield-Gabrieli & Nieto-Castanon, 2012). Because imaging collection used a fast multiband sequence, no slice timing correction was applied (Glasser et al., 2013; Wu et al., 2011). Co-registered MPRAGE and EPI images were normalized to an MNI template and a 6mm Gaussian kernel was applied to smooth images. Finally, a 0.009 – 0.08Hz bandpass filter was used to retain resting state signals (Satterthwaite et al., 2013).
From this preprocessing we found that 24 participants had motion > 3mm and four had >20% of invalid scans. Because this impacts the integrity of the imaging data, we did not retain the time series of these participants. This left a total of 84 participants with full imaging data and 28 participants (25%) without imaging data.
Region of Interest Selection.
The focus on the DMN, FPN, and SAL was supported by studies on empathy (Decety & Michalska, 2010; Decety et al., 2008; Fan et al., 2011; Kral et al., 2017; Lamm et al., 2011) and CU traits (Cohn et al., 2015; Pu et al., 2017; Umbach & Tottenham, 2020; Yoder et al., 2016). Regions representing these networks were defined anatomically using the Harvard-Oxford atlas within the CONN toolbox. The Harvard-Oxford atlas defined the DMN as the medial prefrontal cortex, posterior cingulate cortex, and angular gyri (part of the temporal parietal junction); the FPN as the bilateral lateral prefrontal and posterior parietal cortices for the FPN; and the SAL as the bilateral anterior insula, anterior cingulate, and bilateral rostral prefrontal cortices (MNI coordinates: Supplementary Table 1).
Extracting Connectivity Parameters.
BOLD time-series of each ROI was extracted from the 4D preprocessed resting state scan. Then all participant-level pairwise within- and between-network time series were averaged, converted to a Z value using Fisher’s r-to-z transformation, and extracted, which represented a connectivity value for each within and between network connection. These connectivity values were used in subsequent analyses.
2.3. Analysis
After extracting connectivity parameters, we conducted analyses using R (Version 4.02; R Core Team, 2021). To improve estimation of multiple dependent variables by doing so simultaneously in one model (as opposed to multiple models and raising concerns for multiple comparisons) we ran a series of path analyses using the ‘lavaan’ package (Rosseel, 2012). These path analyses were estimated using maximum likelihood with Huber-White robust standard errors to correct asymptotic standard errors and improve confidence interval estimation (Maas & Hox, 2004).
Missing data analysis.
Prior to analysis we assessed data missingness using the Visualization and Imputation of Missing Values ‘VIF’ package (Kowarik & Templ, 2016); and conducted test for Missing Completely at Random (MCAR) described by Jamshidian and Jalal (2010) using the ‘MissMech’ package in R (Mortaza et al., 2014). This MCAR test uses the Hawkins (1981) test statistic to quantitate homoscedasticity across different patterns of missingness between groups with and without missing to assert whether the missing data has a systematic bias (Jamshidian & Jalal, 2010). This method demonstrates reliability with smaller sample sizes (Jamshidian & Jalal, 2010). We then did additional testing for systematic missingness by creating a dichotomous variable for missing values (missing = 1 and not missing = 0) and conducting chi-square or t tests to quantitate any explanations for missing values present (Little & Rubin, 2019).
No behavioral data was missing and 25% of participants were missing connectivity values (n=28). The test for MCAR suggested no homoscedasticity that accounted for missing data and, thus, could not rule out MCAR (p= 0.332). Further investigation with t-tests did not detect any systematic reasons for missingness. Therefore, we concluded that estimating missing values would not introduce bias into our analysis but, instead, allow us to retain power while improving confidence in our estimates. Simulations demonstrate that full-information maximum likelihood produced unbiased estimates with over 50% of missing data when missing at random (Schafer & Graham, 2002). Moreover, modern missing data approaches, such as full information maximum likelihood, reduce bias when compared to removing cases or listwise deletion (Little & Rubin, 2019). Thus, we used full information maximum likelihood to retain all 112 participants.
Path analyses.
We then ran path models to examine network connectivity of the DMN, FPN, and SAL in relation to CU traits and both cognitive and affective empathy. We first ran separate models for each independent variable of interest (cognitive empathy, affective empathy, CU traits) including covariates (conduct problems only included in analyses with CU traits). To ensure CU traits estimates were not a result of suppression effects when including conduct problems (e.g., Hyde et al., 2016; Lozier et al., 2014) we ran models without conduct problems to assess if path estimates changed. Because all path estimates were the same and did not evidence a suppression effect, we only report on CU trait models with conduct problems included as a covariate. Because sex is associated with both CU traits and empathy, we assessed if sex was a moderator using multigroup models separated by sex. With these multigroup models, we compared a model with constrained intercepts and regression parameters to an unconstrained model using a Satorra-Bentler x2 difference test to determine whether model parameters are significantly different across sexes (Satorra, 2000). Finally, we examined whether the CU traits and empathy explained overlapping variance in the neural phenotypes. Because tests of mediation and confounding are equivalent statistically (MacKinnon et al., 2000), we tested indirect effects to examine whether CU traits and empathy were explaining the same variance in the neural outcomes (indirect effect) or different variance (remaining direct effects). We used the product of coefficients to calculate indirect effects and used 5000 resamples to simulate confidence intervals using bias corrected bootstraps to verify indirect effects (MacKinnon, 2000). We used criteria by MacKinnon et al. (2000) to evaluate indirect effects for the impact on x – y relationship and then calculated the relative magnitude of the total effects accounted for by the indirect and direct effects using equations outlined by Preacher and Kelley (2011). All analyses were controlled for multiple comparisons using a false discovery rate correction (Benjamini & Hochberg, 1995) for each analysis using ‘p.adjust’ command (R Core Team, 2021).
3. Results
3.1. Descriptives
A total of 12 participants (11%) met the clinical cut-off (Kemp et al., 2019) for CU traits (6 male and 6 female) and a total of 4 (3%) met the clinical cut-off (Sandoval et al., 2006) for externalizing symptoms (2 male and 2 female). Correlations were significant between CU traits and both cognitive (r= − 0.33, p< 0.001) and affective (r= − 0.67, p< 0.001) as well as between cognitive and affective empathy (r= 0.28, p= 0.003). In comparing the results to the meta-analysis by Waller et al. (2020), CU traits association with cognitive empathy matched what was expected, but CU traits association with affective empathy was stronger than expected in the present sample.
3.2. Separate Analysis: Shared Network Associations between Empathy and CU Traits.
CU Traits.
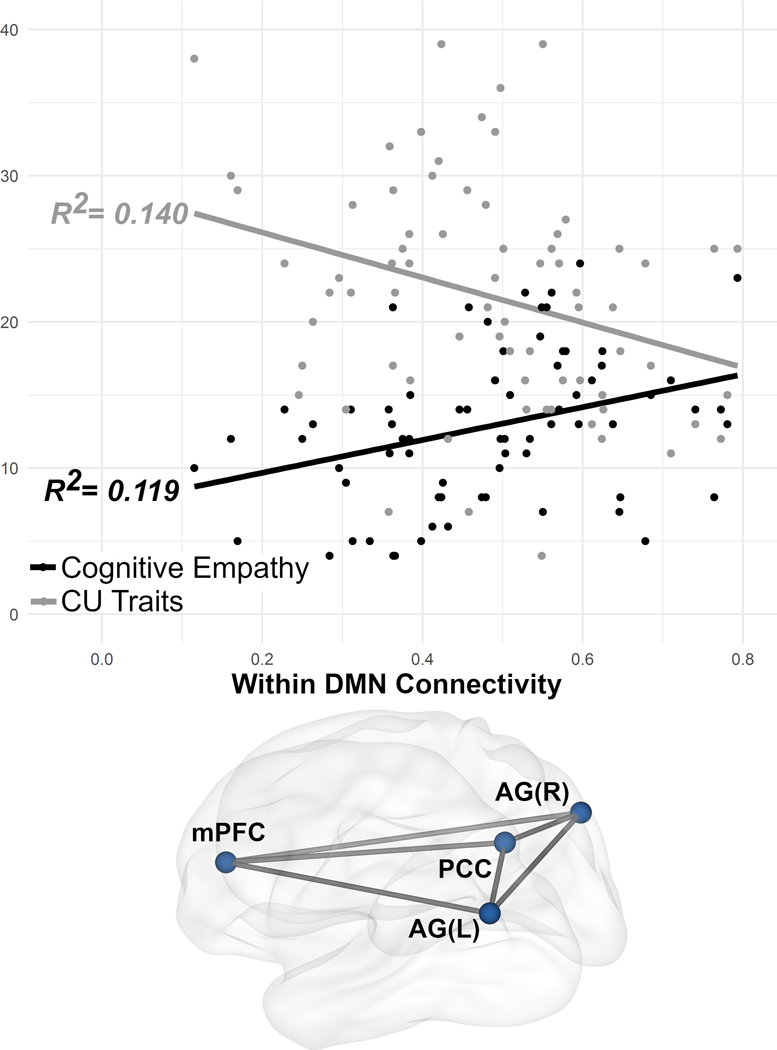
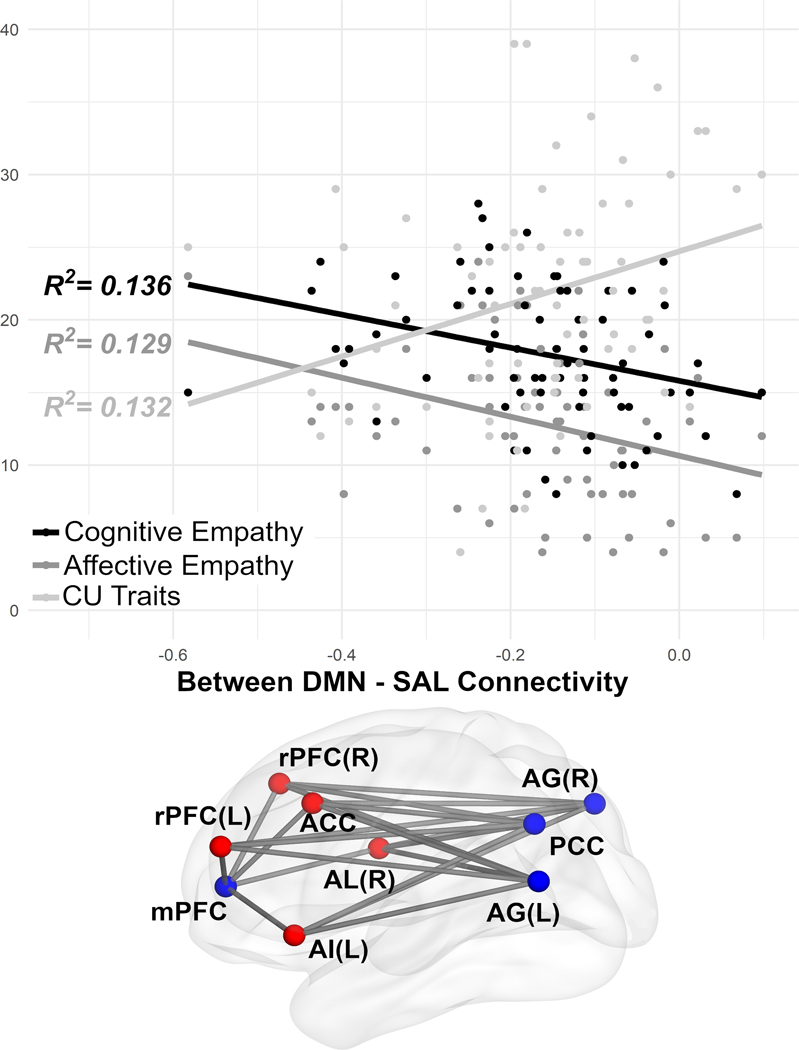
Separate analyses indicated higher CU traits were associated with less within DMN connectivity (β= – 0.005, q (FDR Corrected p) = 0.038, R2= 0.140) and greater between DMN-SAL connectivity (β= 0.004, q (FDR Corrected p) = 0.029, R2= 0.132). CU traits were not associated with the SAL or FPN (Table 1, Supplementary Figure 1).
Table 1.
Individual models association with functional connectivity parameters
| Individual models | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| Callous-Unemotional traits | Cognitive empathy | Affective empathy | |||||||
|
|
|
|
|||||||
| Outcomes | β | q | R2 | β | q | R2 | β | q | R2 |
| DMN | −0.005* | 0.038 | 0.140 | 0.008* | 0.015 | 0.119 | 0.008 | 0.057 | 0.122 |
| FPN | −0.001 | 0.969 | 0.001 | 0.001 | 0.927 | 0.016 | 0.000 | 0.972 | 0.076 |
| SAL | −0.002 | 0.694 | 0.036 | 0.002 | 0.709 | 0.002 | 0.009 | 0.082 | 0.001 |
| DMN-FPN | −0.003 | 0.136 | 0.124 | 0.001 | 0.659 | 0.062 | 0.000 | 0.694 | 0.062 |
| DMN-SAL | 0.004* | 0.002 | 0.132 | −0.007* | 0.006 | 0.129 | −0.008* | 0.003 | 0.136 |
| FPN-SAL | 0.003 | 0.152 | 0.669 | 0.002 | 0.627 | 0.015 | −0.005 | 0.440 | 0.042 |
Note: all path models adjusted for sex and pubertal development, callous-unemotional traits model additionally adjusted for conduct problems.
DMN= default mode network, SAL= salience network; Network abbreviations separated by a hyphen indicate between network connectivity whereas standalone abbreviations indicate within network.
Predictor at the top was a separate path model estimates on the multiple outcomes in the left column.
See Supplementary Figure 1 for depiction of all models ran.
q= FDR corrected p value
q < 0.05
Cognitive empathy.
In parallel to the CU traits findings (though with an inverse association), higher cognitive empathy was associated with greater within DMN connectivity (β= 0.009, q (FDR Corrected p) = 0.11, R2= 0.119) and less between DMN-SAL connectivity (β= − 0.008, q (FDR Corrected p) = 0.002, R2= 0.129). Cognitive empathy was not associated with the SAL or FPN (Table 1, Supplementary Figure 1).
Affective empathy.
Affective empathy was associated with less between DMN-SAL connectivity (β= − 0.008, q (FDR Corrected p) = 0.004, R2= 0.136). No other associations were found with affective empathy. Affective empathy was not associated with the DMN, FPN, or SAL (Table 1, Supplementary Figure 1).
3.3. Test for Indirect Effects: No Evidence for Shared Variance
There were no significant indirect effects between CU traits, cognitive empathy, or affective empathy and brain parameters with either CU traits or empathy as the indirect parameter (Table 2, Supplementary Figure 2). The magnitude of total effects for all indirect effects were lower (0.22 – 0.44) than direct effects (0.56– 0.78, see Table 2, Supplementary Figure 2).
Table 2.
Results of tests for indirect effects
| Estimate | p | q | 95% CI bootstrapped | Decision | ||
|---|---|---|---|---|---|---|
|
|
||||||
| Low | High | |||||
| CU traits as the indirect parameter | ||||||
| DMN ~ Cognitive Empathy | No Effect | |||||
| Direct Effect | 0.006 | 0.132 | 0.133 | −0.002 | 0.013 | |
| Indirect Effect | 0.002 | 0.113 | 0.164 | −0.001 | 0.005 | |
| Total Effect | 0.008* | 0.024 | 0.053 | 0.001 | 0.015 | |
| % Total Effect – Direct | 72.9%* | 30.7% | 115.1% | |||
| % Total Effect – Indirect | 27.1% | −15.1% | 69.3% | |||
| DMN-SAL~ Cognitive Empathy | No Effect | |||||
| Direct Effect | −0.006 | 0.073 | 0.109 | −0.012 | 0.001 | |
| Indirect Effect | −0.002 | 0.147 | 0.147 | −0.004 | 0.001 | |
| Total Effect | −0.007* | 0.012 | 0.035 | −0.013 | −0.002 | |
| % Total Effect – Direct | 78.1%* | 42.8% | 113.4% | |||
| % Total Effect – Indirect | 21.9% | −13.4% | 57.2% | |||
| DMN-SAL~ Affective Empathy | No Effect | |||||
| Direct Effect | −0.005 | 0.223 | 0.285 | −0.013 | 0.003 | |
| Indirect Effect | −0.003 | 0.285 | 0.285 | −0.008 | 0.002 | |
| Total Effect | −0.008* | 0.008 | 0.023 | −0.014 | −0.002 | |
| % Total Effect – Direct | 63.6% | −9.7% | 137.0% | |||
| % Total Effect – Indirect | 36.3% | −37.0% | 109.7% | |||
| Cognitive Empathy as the indirect parameter | ||||||
| DMN~ CU Traits | No Effect | |||||
| Direct Effect | −0.004 | 0.108 | 0.143 | −0.010 | 0.0002 | |
| Indirect Effect | −0.001 | 0.144 | 0.144 | −0.003 | 0.001 | |
| Total Effect | −0.005* | 0.021 | 0.062 | −0.010 | −0.001 | |
| % Total Effect – Direct | 77.8% | −2.6% | 110.4% | |||
| % Total Effect – Indirect | 22.2% | −10.4% | 102.6% | |||
| DMN-SAL~ CU Traits | No Effect | |||||
| Direct Effect | 0.003 | 0.114 | 0.128 | −0.001 | 0.007 | |
| Indirect Effect | 0.001 | 0.129 | 0.129 | −0.0002 | 0.003 | |
| Total Effect | 0.004* | 0.007 | 0.021 | 0.001 | 0.007 | |
| % Total Effect – Direct | 72.5% | −44.5% | 105.2% | |||
| % Total Effect – Indirect | 27.5% | −5.2% | 144.5% | |||
| Affective Empathy as the indirect parameter | ||||||
| DMN-SAL~CU Traits | No Effect | |||||
| Direct Effect | 0.003 | 0.026 | 0.276 | −0.002 | 0.007 | |
| Indirect Effect | 0.002 | 0.250 | 0.275 | −0.001 | 0.005 | |
| Total Effect | 0.005* | 0.002 | 0.006 | 0.002 | 0.007 | |
| % Total Effect – Direct | 55.9% | −83.3% | 128.9% | |||
| % Total Effect – Indirect | 44.1% | −28.9% | 183.3% | |||
Note: Bootstrapped confidence intervals are bias corrected and used 500 resamples; CU = callous-unemotional; DMN= default mode network, SAL= salience network, CI= Confidence interval, p = uncorrected p value, q = FDR corrected p value, FDR = false discovery rate.
See Supplementary Figure 2 for depiction of all indirect models ran
bootstrapped confidence intervals do not cross 0
3.4. No Sex Differences Detected
No significant sex differences were detected for individual parameters in the formal analyses nor multigroup model comparisons (Table 3).
Table 3.
Likelihood ratio test results of multigroup comparisons by sex
| Models compared | Chi-square Δ | P value |
|---|---|---|
| Cognitive empathy unconstrained and constrained by sex | 13.038 | 0.789 |
| Affective empathy unconstrained and constrained by sex | 16.839 | 0.534 |
| Callous-unemotional traits unconstrained and constrained by sex | 22.154 | 0.225 |
4. Discussion
The present study identified shared network patterns for CU traits and both cognitive and affective empathy amongst a community sample of adolescents. Generally, CU traits had network associations that were inverse to cognitive and affective empathy. Specifically, cognitive empathy was inversely associated with within DMN connectivity and both cognitive and affective empathy were inversely associated with between DMN-SAL connectivity, a pattern similar to CU traits association with network connectivity. Though these associations would imply overlapping neural networks for CU traits and empathy, quantitative tests showed relatively more unique versus shared/overlapping variance in multivariate models, implying that, though these constructs appear to have similar neural correlates, the variance explained in neural connectivity by each construct appears to be relatively independent.
4.1. Inverse Associations Within DMN Connectivity
Within DMN connectivity was lower at higher CU traits, whereas DMN connectivity was higher at higher levels of cognitive empathy. These findings reflect separate studies that have shown that CU traits are negatively associated with DMN connectivity (Umbach & Tottenham, 2020) and that cognitive empathy is positively associated (Esménio et al., 2019; Winters, Pruitt, et al., 2021) with DMN connectivity. We extend this work to demonstrate these similar inverse association with the DMN within the same sample of adolescents.
Cognitive empathy involves self-referential cognitive processes when taking another’s perspective, which are some of the processes the DMN is consistently recruited for (Buckner et al., 2008; Buckner & Carroll, 2007; Uddin et al., 2009). Previous studies demonstrate that perspective taking is impaired as CU traits increase (Lui et al., 2016). This may explain why only cognitive empathy had a similar an inverse association in the DMN with CU traits. The negative association of CU traits with both cognitive empathy and DMN connectivity suggests differences in trait-like network connectivity in the DMN may explain impairments in self-referential processes necessary for cognitive empathy.
4.2. Inverse Associations Between DMN-SAL
Between DMN-SAL connectivity was higher with higher CU traits, whereas between DMN-SAL connectivity was lower at higher levels of both cognitive and affective empathy. This finding extends the extant literature on CU traits (Werhahn et al., 2020) and both cognitive and affective empathy (Winters, Pruitt, et al., 2021) by demonstrating their similar, and inverse, associations with between DMN-SAL connectivity amongst the same sample of adolescents.
The SAL integrates multiple sources of information to signal the SAL to downregulate task-negative networks (i.e., DMN) and switch to task-positive networks (for review see: Menon, 2015), which supports empathic feelings (Craig & Craig, 2009; Singer et al., 2009). Higher levels of cognitive and affective empathy are associated with switching between task-positive and task-negative networks indicated by greater between DMN-SAL anticorrelations (Winters, Pruitt, et al., 2021). Lower between DMN-SAL anticorrelation suggests signals from task negative cognitive processes are more difficult to regulate during task positive processes. Given that cognitive and affective empathy are associated with a greater anticorrelation between these networks, reduced anticorrelation of these networks at higher CU traits suggests an inefficiency of functional coupling between networks that could make empathy more difficult.
4.3. Overlap of CU Traits and Empathy in Association with Neural Phenotypes
Interestingly, though findings between empathy and neural phenotypes and CU traits and neural phenotypes were highly similar (though in the expected opposite directions), when tested quantitatively, we found no significant overlap in the variance empathy and CU traits were explaining in each neural phenotype. Given the inverse associations in neural networks, we anticipated a high level of shared variance between empathy and CU traits on related networks. However, we were unable to detect shared variance, which could indicate similar neural correlates that do not actually overlap in terms of variance explained. Other possible explanations are that the current study was under-powered to parse unique versus shared variance; another is that shared methods of assessment could bias associations (Baumgartner et al., 2021). This result is surprising and requires further investigation with larger samples.
4.4. Null Results
No associations with the SAL.
Although we hypothesized both affective empathy and CU traits would associate with SAL connectivity, this hypothesis was not supported. This finding is contrary to some of the task-based literature and theoretical understandings of both constructs (Downar et al., 2003; Saarela et al., 2007; Sethi et al., 2018; Wicker et al., 2003). However, other studies also found that CU traits (Umbach & Tottenham, 2020) and empathy (Winters, Pruitt, et al., 2021; Winters, Sakai, et al., 2021) were not associated with connectivity in the SAL in youth. It may be that there are certain conditions in which the SAL is involved in these processes at this age (e.g., during specific tasks) or that specific regions involved with the SAL not included in the present analysis (i.e., the amygdala) are most relevant. There does not appear to be a clear consensus across the literature on the SAL’s involvement in affective empathy and CU traits, which highlights an important discrepancy for future investigations.
No Sex Differences Detected.
The present analysis revealed no sex differences. Although the literature supports mean level sex differences via self-report for empathy (Baron-Cohen & Wheelwright, 2004; Cohen & Strayer, 1996; Davis, 1983) and CU traits (Fragkaki et al., 2016; Wymbs et al., 2012) as well as general functional connectivity in adolescents (Satterthwaite et al., 2015), prior studies also found no sex differences when specifically examining neural associations of either empathy (Decety & Michalska, 2010; Kral et al., 2017; Michalska et al., 2013; Winters, Pruitt, et al., 2021) or CU traits (Dotterer et al., 2017).
4.5. Limitations
The present results must be interpreted under some limitations. First, the data analyzed is cross-sectional and cannot determine causality. Second, the study relied exclusively on self-report measures. This likely introduced shared method variance (i.e., the same person and reporting style influenced measures of CU traits and empathy). Ironically, though shared method variance would be expected to increase (or inflate) the associations and overlap among the self-reported constructs (i.e., CU traits, empathy), here we found little evidence that these self-reported constructs explained overlapping variance in brain network structure. Examining these associations with behavioral measurements would be important to extend this line of work in future studies. Third, the self-report measures capture perceived cognitive and affective empathy abilities instead of measuring actual empathic performance. Studies demonstrate that the Interpersonal Reactivity Index only accounts for roughly 1% of the variance in performance on affective and cognitive empathy tasks (Melchers et al., 2015; Murphy & Lilienfeld, 2019); and measuring perception versus actual behavior, particularly among those higher on CU traits may undermine the results of this study. There is a need for further investigation into self-reported empathy and performance on empathy or theory of mind tasks in relation to CU traits. Fourth, shared method variance may have biased results and it would be important for future investigations to include multiple assessment modalities. Fifth, the sample size was quite modest which may undermine power (i.e., meaning null findings may be due to small effect sizes and low power) and increase the risk of spurious findings (Turner et al., 2018). Finally, the sample analyzed is a community sample and did not examine comorbidity of mental health conditions. Although substantial evidence exists supporting CU traits are dimensional and present in community samples (Viding & McCrory, 2012), and that there are similarities in neurocognitive impairments (Viding & McCrory, 2012) as well as neurobiological associations (Seara-Cardoso et al., 2022; Seara-Cardoso & Viding, 2015) between community and forensic samples, CU traits may present differently or have a different etiology at high levels of frequency and intensity of antisocial behavior, which distinguishes those in clinical or forensic settings (LeBreton et al., 2006).
4.6. Conclusions
The present study demonstrated similar network connectivity between CU traits and both cognitive and affective empathy among a community sample of adolescents. Higher CU traits were associated with decreased within DMN connectivity and increased between DMN-SAL connectivity. Greater cognitive empathy was associated with higher within DMN connectivity, whereas greater cognitive and affective empathy was associated with decreases in between DMN-SAL connectivity. However, multivariate analyses indicated that, though the pattern of findings was similar for CU traits versus empathy, these constructs did not explain overlapping variance in the neural connectivity outcomes. Future studies could examine the developmental underpinnings of these differences in network connectivity and network inefficiency associated with CU traits in relation to empathy.
Supplementary Material
Figure 1.
association of callous-unemotional traits and cognitive empathy with within default mode network connectivity. Brain image depicts all network connections within the DMN
Figure 2.
Association of callous-unemotional traits, cognitive empathy, and affective empathy with between default mode-salience network connectivity. Brain image depicts all pairwise connections between DMN and FPN
Highlights.
Callous-unemotional (CU) traits and cognitive empathy inversely associate with DMN
Cognitive and affective empathy associate with between DMN—SAL inversely to CU traits
Statistically, empathy and CU traits show low shared variance with brain connectivity
Acknowledgements
Authors have no acknowledgements to report.
Drew E Winters, PhD. was supported by a training grant from National Institutes of Mental Health, T32MH015442
Footnotes
CRediT authorship contribution statement
Drew E. Winters: Conceptualization, Methodology, Software, Formal analysis, Data curation, Writing - original draft, Writing - review & editing, Visualization, Funding Acquisition Luke W. Hyde: Conceptualization, Methodology, Supervision, Writing - review & editing
Conflict of interest statement All authors declare no conflicts.
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achenbach TM, & Rescorla LA (2001). Manual for ASEBA schoolage forms & profiles. University of Vermont, Research Center for Children, Youth, & Families. http://www.aseba.org/ [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and Statistical Manual of Mental Disorders (5th ed.). American Psychiatric Publishing. https://books.google.com/books?id=EIbMlwEACAAJ [Google Scholar]
- Baron-Cohen S, & Wheelwright S. (2004). The empathy quotient: an investigation of adults with Asperger syndrome or high functioning autism, and normal sex differences. J Autism Dev Disord, 34(2), 163–175. [DOI] [PubMed] [Google Scholar]
- Baskin-Sommers AR., Waller R., Fish AM., & Hyde LW. (2015, Nov). Callous-unemotional traits trajectories interact with earlier conduct problems and exec-utive control to predict violence and substance use among high risk male adolescents. J Abnorm Child Psychol, 43(8), 1529–1541. 10.1007/s10802-015-0041-8 [DOI] [PubMed] [Google Scholar]
- Baumgartner H, Weijters B, & Pieters R. (2021, 2021/03/01). The biasing effect of common method variance: some clarifications. Journal of the academy of marketing science, 49(2), 221–235. 10.1007/s11747-020-00766-8 [DOI] [Google Scholar]
- Benjamini Y, & Hochberg Y. (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal statistical society: series B (Methodological), 57(1), 289–300. [Google Scholar]
- Blair RJ (2008). Fine cuts of empathy and the amygdala: dissociable deficits in psychopathy and autism. The Quarterly Journal of Experimental Psychology, 61(1), 157–170. [DOI] [PubMed] [Google Scholar]
- Blair RJ, Colledge E, & Mitchell DG (2001, Dec). Somatic markers and response reversal: Is there orbitofrontal cortex dysfunction in boys with psychopathic tendencies? J Abnorm Child Psychol, 29(6), 499–511. [DOI] [PubMed] [Google Scholar]
- Blair RJ, Leibenluft E, & Pine DS (2014, Dec 04). Conduct disorder and callous-unemotional traits in youth. N Engl J Med, 371(23), 2207–2216. 10.1056/NEJMra1315612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RJR (2013). The neurobiology of psychopathic traits in youths. Nature Reviews Neuroscience, 14(11), 786–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore S. (2008, Apr). The social brain in adolescence. Nat Rev Neurosci, 9(4), 267–277. 10.1038/nrn2353 [DOI] [PubMed] [Google Scholar]
- Blakemore S. (2012, Mar). Development of the social brain in adolescence. J R Soc Med, 105(3), 111–116. 10.1258/jrsm.2011.110221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, & Schacter DL (2008). The brain’s default network: anatomy, function, and relevance to disease. [DOI] [PubMed] [Google Scholar]
- Buckner RL, & Carroll DC (2007). Self-projection and the brain. Trends in cognitive sciences, 11(2), 49–57. [DOI] [PubMed] [Google Scholar]
- Cameron JL (2004). Interrelationships between hormones, behavior, and affect during adolescence: understanding hormonal, physical, and brain changes occurring in association with pubertal activation of the reproductive axis. Introduction to part III. Annals of the New York Academy of Sciences, 1021(1), 110–123. [DOI] [PubMed] [Google Scholar]
- Christov-Moore L, Reggente N, Douglas PK, Feusner JD, & Iacoboni M. (2020, 2020-February-14). Predicting Empathy From Resting State Brain Connectivity: A Multivariate Approach [Original Research]. Frontiers in Integrative Neuroscience, 14(3). 10.3389/fnint.2020.00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen D, & Strayer J. (1996). Empathy in conduct-disordered and comparison youth. Developmental psychology, 32(6), 988. [Google Scholar]
- Cohn MD, Pape LE, Schmaal L, van den Brink W, van Wingen G, Vermeiren RR, Doreleijers TA, Veltman DJ, & Popma A. (2015). Differential relations between juvenile psychopathic traits and resting state network connectivity. Human Brain Mapping, 36(6), 2396–2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox CL., Uddin LQ., Di Martino A., Castellanos FX., Milham MP., & Kell C. (2011). The balance between feeling and knowing: Affective and cognitive empathy are reflected in the brain’s intrinsic functional dynamics. Social Cognitive and Affective Neuroscience, 7(6), 727–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD, & Craig A. (2009). How do you feel--now? The anterior insula and human awareness. Nature Reviews Neuroscience, 10(1). [DOI] [PubMed] [Google Scholar]
- Dahl RE (2004). Adolescent brain development: a period of vulnerabilities and opportunities. Keynote address. Annals of the New York Academy of Sciences, 1021(1), 1–22. [DOI] [PubMed] [Google Scholar]
- Davis MH (1980). A multidimensional approach to individual differences in empathy. Journal of personality and social psychology, 10(85). [Google Scholar]
- Davis MH (1983). Measuring individual differences in empathy: Evidence for a multidimensional approach. Journal of personality and social psychology, 44(1), 113–126. [Google Scholar]
- Decety J. (2011). Dissecting the neural mechanisms mediating empathy. Emotion Review, 3(1), 92–108. 10.1177/1754073910374662 [DOI] [Google Scholar]
- Decety J, Bartal IB-A, Uzefovsky F, & Knafo-Noam A. (2016, 09/18/accepted). Empathy as a driver of prosocial behaviour: Highly conserved neurobehavioural mechanisms across species. Philosophical Transactions of the Royal Society B: Biological Sciences, 371(1686), 20150077. 10.1098/rstb.2015.0077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J, & Cowell JM (2015, 07/30). Empathy, justice, and moral behavior. AJOB neuroscience, 6(3), 3–14. 10.1080/21507740.2015.1047055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J, & Michalska KJ (2010). Neurodevelopmental changes in the circuits underlying empathy and sympathy from childhood to adulthood. Developmental Science, 13(6), 886–899. [DOI] [PubMed] [Google Scholar]
- Decety J, Michalska KJ, & Akitsuki Y. (2008). Who caused the pain? An fMRI investigation of empathy and intentionality in children. Neuropsychologia, 46(11), 2607–2614. [DOI] [PubMed] [Google Scholar]
- Decety J, Skelly LR, & Kiehl KA (2013). Brain Response to Empathy-Eliciting Scenarios Involving Pain in Incarcerated Individuals With Psychopathy. JAMA Psychiatry, 70(6), 638–645. 10.1001/jamapsychiatry.2013.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotterer HL, Hyde LW, Swartz JR, Hariri AR, & Williamson DE (2017). Amygdala reactivity predicts adolescent antisocial behavior but not callous-unemotional traits. Developmental cognitive neuroscience, 24, 84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downar J, Mikulis DJ, & Davis KD (2003). Neural correlates of the prolonged salience of painful stimulation. NeuroImage, 20(3), 1540–1551. [DOI] [PubMed] [Google Scholar]
- Drayton LA., Santos LR., & Baskin-Sommers A. (2018). Psychopaths fail to automatically take the perspective of others. Proceedings of the National Academy of Sciences, 115(13), 3302–3307. 10.1073/pnas.1721903115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg N, Eggum ND, & Di Giunta L. (2010). Empathy-related responding: Associations with prosocial behavior, aggression, and inter-group relations. Social issues and policy review, 4(1), 143–180. 10.1111/j.1751-2409.2010.01020.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg N, & Miller P. (1987). The relation of empathy to prosocial and related behaviors (Vol. 101). 10.1037/0033-2909.101.1.91 [DOI] [PubMed]
- Ernst M, Torrisi S, Balderston N, Grillon C, & Hale EA (2015, 01/02). fMRI functional connectivity applied to adolescent neurodevelopment. Annu Rev Clin Psychol, 11, 361–377. 10.1146/annurev-clinpsy-032814-112753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esménio S, Soares JM, Oliveira-Silva P, Zeidman P, Razi A, Gonçalves ÓF, Friston K, & Coutinho J. (2019, 2019/02/22). Using resting-state DMN effective connectivity to characterize the neurofunctional architecture of empathy. Scientific Reports, 9(1), 2603. 10.1038/s41598-019-38801-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Duncan NW, de Greck M, & Northoff G. (2011). Is there a core neural network in empathy? An fMRI based quantitative meta-analysis. Neuroscience & Biobehavioral Reviews, 35(3), 903–911. [DOI] [PubMed] [Google Scholar]
- Fragkaki I, Cima M, & Meesters C. (2016). The association between callous–unemotional traits, externalizing problems, and gender in predicting cognitive and affective morality judgments in adolescence. J Youth Adolesc, 45(9), 1917–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick PJ (2004). The inventory of callous-unemotional traits. Unpublished rating scale. [Google Scholar]
- Frick PJ, Ray JV, Thornton LC, & Kahn RE (2014a). Annual research review: A developmental psychopathology approach to understanding callous-unemotional traits in children and adolescents with serious conduct problems. Journal of child psychology and psychiatry, 55(6), 532–548. [DOI] [PubMed] [Google Scholar]
- Frick PJ, Ray JV, Thornton LC, & Kahn RE (2014b). Can callous-unemotional traits enhance the understanding, diagnosis, and treatment of serious conduct problems in children and adolescents? A comprehensive review. Psychol Bull, 140(1), 1. [DOI] [PubMed] [Google Scholar]
- Frick PJ., & White SF. (2008). Research review: The importance of callous-unemotional traits for developmental models of aggressive and antisocial behavior. Journal of child psychology and psychiatry, 49(4), 359–375. [DOI] [PubMed] [Google Scholar]
- Glasser MF, Sotiropoulos SN, Wilson JA, Coalson TS, Fischl B, Andersson JL, Xu J, Jbabdi S, Webster M, & Polimeni JR (2013). The minimal preprocessing pipelines for the Human Connectome Project. NeuroImage, 80, 105–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton RK, Hiatt Racer K, & Newman JP (2015, Oct). Impaired integration in psychopathy: A unified theory of psychopathic dysfunction. Psychol Rev, 122(4), 770–791. 10.1037/a0039703 [DOI] [PubMed] [Google Scholar]
- Harenski CL, & Hamann S. (2006). Neural correlates of regulating negative emotions related to moral violations. NeuroImage, 30(1), 313–324. [DOI] [PubMed] [Google Scholar]
- Harenski CL, Harenski KA, & Kiehl KA (2014). Neural processing of moral violations among incarcerated adolescents with psychopathic traits. Developmental cognitive neuroscience, 10, 181–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harenski CL, Harenski KA, Shane MS, & Kiehl KA (2010). Aberrant neural processing of moral violations in criminal psychopaths. J Abnorm Psychol, 119(4), 863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harenski CL, Harenski KA, Shane MS, & Kiehl KA (2012). Neural development of mentalizing in moral judgment from adolescence to adulthood. Developmental cognitive neuroscience, 2(1), 162–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins DM (1981). A new test for multivariate normality and homoscedasticity. Technometrics, 23(1), 105–110. [Google Scholar]
- Herpers PCM, Rommelse NNJ, Bons DMA, Buitelaar JK, & Scheepers FE (2012). Callous-unemotional traits as a cross-disorders construct. Social psychiatry and psychiatric epidemiology, 47(12), 2045–2064. 10.1007/s00127-012-0513-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde LW, Burt SA, Shaw DS, Donnellan MB, & Forbes EE (2015). Early starting, aggressive, and/or callous–unemotional? Examining the overlap and predictive utility of antisocial behavior subtypes. J Abnorm Psychol, 124(2), 329–342. 10.1037/abn0000029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde LW, Shaw DS, Murray L, Gard A, Hariri AR, & Forbes EE (2016, May). Dissecting the role of amygdala reactivity in antisocial behavior in a sample of young, low-income, urban men. Clin Psychol Sci, 4(3), 527–544. 10.1177/2167702615614511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamshidian M, & Jalal S. (2010). Tests of homoscedasticity, normality, and missing completely at random for incomplete multivariate data. Psychometrika, 75(4), 649–674. [Record #3532 is using a reference type undefined in this output style.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehl KA, Smith AM, Hare RD, Mendrek A, Forster BB, Brink J, & Liddle PF (2001). Limbic abnormalities in affective processing by criminal psychopaths as revealed by functional magnetic resonance imaging. Biological Psychiatry, 50(9), 677–684. [DOI] [PubMed] [Google Scholar]
- Kimonis ER., Frick PJ., Skeem JL., Marsee MA., Cruise K., Munoz LC., Aucoin KJ., & Morris AS. (2008). Assessing callous–unemotional traits in adolescent offenders: Validation of the Inventory of Callous–Unemotional Traits. International journal of law and psychiatry, 31(3), 241–252. [DOI] [PubMed] [Google Scholar]
- Konrath SH (2013). Critical Synthesis Package: Interpersonal Reactivity Index (IRI). Mededportal Publications, 9. 10.15766/mep_2374-8265.9596 [DOI] [Google Scholar]
- Kowarik A, & Templ M. (2016). Imputation with the R Package VIM. Journal of Statistical Software, 74(7), 1–16. [Google Scholar]
- Kral TRA, Solis E, Mumford JA, Schuyler BS, Flook L, Rifken K, Patsenko EG, & Davidson RJ (2017, Nov 1). Neural correlates of empathic accuracy in adolescence. Soc Cogn Affect Neurosci, 12(11), 1701–1710. 10.1093/scan/nsx099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm C, Decety J, & Singer T. (2011). Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. NeuroImage, 54(3), 2492–2502. [DOI] [PubMed] [Google Scholar]
- LeBreton JM, Binning JF, & Adorno AJ (2006). Subclinical psychopaths. Comprehensive handbook of personality and psychopathology, 1, 388–411. [Google Scholar]
- Little RJA, & Rubin DB (2019). Statistical Analysis with Missing Data. Wiley. https://books.google.com/books?id=BemMDwAAQBAJ [Google Scholar]
- Lockwood PL (2016). The anatomy of empathy: Vicarious experience and disorders of social cognition. Behavioural Brain Research, 311, 255–266. 10.1016/j.bbr.2016.05.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozier LM, Cardinale EM, VanMeter JW, & Marsh AA (2014, Jun). Mediation of the relationship between callous-unemotional traits and proactive aggression by amygdala response to fear among children with conduct problems. JAMA Psychiatry, 71(6), 627–636. 10.1001/jamapsychiatry.2013.4540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui JH., Barry CT., & Sacco DF. (2016, Sep). Callous-unemotional traits and empathy deficits: Mediating effects of affective perspective-taking and facial emotion recognition. Cogn Emot, 30(6), 1049–1062. 10.1080/02699931.2015.1047327 [DOI] [PubMed] [Google Scholar]
- Maas CJ, & Hox JJ (2004). Robustness issues in multilevel regression analysis. Statistica Neerlandica, 58(2), 127–137. [Google Scholar]
- MacKinnon DP (2000). Contrasts in multiple mediator models. Multivariate applications in substance use research: New methods for new questions, 141, 160. [Google Scholar]
- MacKinnon DP, Krull JL, & Lockwood CM (2000). Equivalence of the mediation, confounding and suppression effect. Prevention science, 1(4), 173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchers M, Montag C, Markett S, & Reuter M. (2015). Assessment of empathy via self-report and behavioural paradigms: data on convergent and discriminant validity. Cognitive Neuropsychiatry, 20(2), 157–171. [DOI] [PubMed] [Google Scholar]
- Menon V. (2015). Salience network. In Toga AW (Ed.), Brain Mapping: An Encyclopedic Reference (Vol. 2, pp. 597–611). Elsevier. [Google Scholar]
- Menon V, & Uddin LQ (2010, 05/29). Saliency, switching, attention and control: a network model of insula function. Brain Structure & Function, 214(5–6), 655–667. 10.1007/s00429-010-0262-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalska KJ, Kinzler KD, & Decety J. (2013, 2013/01/01/). Age-related sex differences in explicit measures of empathy do not predict brain responses across childhood and adolescence. Developmental cognitive neuroscience, 3(Supplement C), 22–32. 10.1016/j.dcn.2012.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mišić B, & Sporns O. (2016, 2016/10/01/). From regions to connections and networks: new bridges between brain and behavior. Current opinion in neurobiology, 40, 1–7. 10.1016/j.conb.2016.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortaza J, Siavash J, & Camden J. (2014). MissMech: An R Package for Testing Homoscedasticity, Multivariate Normality, and Missing Completely at Random (MCAR). Journal of Statistical Software, 56(6), 1–31. [Google Scholar]
- Murphy BA, & Lilienfeld SO (2019). Are self-report cognitive empathy ratings valid proxies for cognitive empathy ability? Negligible meta-analytic relations with behavioral task performance. Psychological Assessment, 31(8), 1062. [DOI] [PubMed] [Google Scholar]
- Nooner KB., Colcombe SJ., Tobe RH., Mennes M., Benedict MM., Moreno AL., & … Milham MP (2012). The NKI-Rockland Sample: A Model for Accelerating the Pace of Discovery Science in Psychiatry. Frontiers in Neuroscience, 6(152). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent A-S, Teilmann G, Juul A, Skakkebaek NE, Toppari J, & Bourguignon J-P (2003). The Timing of Normal Puberty and the Age Limits of Sexual Precocity: Variations around the World, Secular Trends, and Changes after Migration. Endocrine Reviews, 24(5), 668–693. 10.1210/er.2002-0019 [DOI] [PubMed] [Google Scholar]
- Penny WD, Friston KJ, Ashburner JT, Kiebel SJ, & Nichols TE (2011). Statistical parametric mapping: the analysis of functional brain images. Elsevier. [Google Scholar]
- Petersen AC, Crockett L, Richards M, & Boxer A. (1988). A self-report measure of pubertal status: Reliability, validity, and initial norms. J Youth Adolesc, 17(2), 117–133. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, & Kelley K. (2011, Jun). Effect size measures for mediation models: quantitative strategies for communicating indirect effects. Psychol Methods, 16(2), 93–115. 10.1037/a0022658 [DOI] [PubMed] [Google Scholar]
- Pu W, Luo Q, Jiang Y, Gao Y, Ming Q, & Yao S. (2017, Sep 12). Alterations of Brain Functional Architecture Associated with Psychopathic Traits in Male Adolescents with Conduct Disorder. Sci Rep, 7(1), 11349. 10.1038/s41598-017-11775-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. (2021). R: A language and environment for statistical computing. URL https://www.R-project.org/.
- Raschle NM, Menks WM, Fehlbaum LV, Steppan M, Smaragdi A, Gonzalez-Madruga K, Rogers J, Clanton R, Kohls G, Martinelli A, Bernhard A, Konrad K, Herpertz-Dahlmann B, Freitag CM, Fairchild G, De Brito SA, & Stadler C. (2018, 2018/01/01/). Callous-unemotional traits and brain structure: Sex-specific effects in anterior insula of typically-developing youths. NeuroImage: Clinical, 17, 856–864. 10.1016/j.nicl.2017.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijnders RJ, Terburg D, Bos PA, Kempes MM, & van Honk J. (2021). Unzipping empathy in psychopathy: Empathy and facial affect processing in psychopaths. Neuroscience & Biobehavioral Reviews. [DOI] [PubMed] [Google Scholar]
- Rosseel Y. (2012). Lavaan: An R Package for Structural Equation Modeling. Journal of Statistical Software, 48(2), 1–36. [Google Scholar]
- Saarela MV, Hlushchuk Y, Williams A. C. d. C., Schürmann M, Kalso E, & Hari R. (2007). The compassionate brain: humans detect intensity of pain from another’s face. Cerebral Cortex, 17(1), 230–237. [DOI] [PubMed] [Google Scholar]
- Sandoval M, Lemos S, & Vallejo G. (2006). Self-reported competences and problems in Spanish adolescents: A normative study of the YSR. Psicothema, 804–809. [PubMed] [Google Scholar]
- Satorra A. (2000). Scaled and adjusted restricted tests in multi-sample analysis of moment structures. In Innovations in multivariate statistical analysis (pp. 233–247). Springer. [Google Scholar]
- Satterthwaite TD, Elliott MA, Gerraty RT, Ruparel K, Loughead J, Calkins ME, Eickhoff SB, Hakonarson H, Gur RC, & Gur RE (2013). An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. NeuroImage, 64, 240–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD., Wolf DH., Roalf DR., Ruparel K., Erus G., Vandekar S., Gennatas ED., Elliott MA., Smith A., Hakonarson H., Verma R., Davatzikos C., Gur RE., & Gur RC. (2015). Linked Sex Differences in Cognition and Functional Connectivity in Youth. Cerebral cortex (New York, N.Y. : 1991), 25(9), 2383–2394. 10.1093/cercor/bhu036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe R. (2009). Theory of mind (neural basis). Encyclopedia of consciousness, 2, 401–410. [Google Scholar]
- Schafer JL, & Graham JW (2002, Jun). Missing data: our view of the state of the art. Psychol Methods, 7(2), 147–177. [PubMed] [Google Scholar]
- Seara-Cardoso A, Vasconcelos M, Sampaio A, & Neumann CS (2022). Neural correlates of psychopathy: A comprehensive review. Psychopathy and Criminal Behavior, 43–73. [Google Scholar]
- Seara-Cardoso A, & Viding E. (2015). Functional neuroscience of psychopathic personality in adults. J Pers, 83(6), 723–737. [DOI] [PubMed] [Google Scholar]
- Sethi A, O’Nions E, McCrory E, Bird G, & Viding E. (2018). An fMRI investigation of empathic processing in boys with conduct problems and varying levels of callous-unemotional traits. NeuroImage. Clinical, 18, 298–304. 10.1016/j.nicl.2018.01.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp C, Vanwoerden S, Van Baardewijk Y, Tackett J, & Stegge H. (2015). Callous-unemotional traits are associated with deficits in recognizing complex emotions in preadolescent children. Journal of personality disorders, 29(3), 347–359. [DOI] [PubMed] [Google Scholar]
- Shehzad Z, Kelly AMC, Reiss PT, Gee DG, Gotimer K, Uddin LQ, Lee SH, Margulies DS, Roy AK, Biswal BB, Petkova E, Castellanos FX, & Milham MP (2009). The Resting Brain: Unconstrained yet Reliable. Cerebral Cortex, 19(10), 2209–2229. 10.1093/cercor/bhn256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer T, Critchley HD, & Preuschoff K. (2009, Aug). A common role of insula in feelings, empathy and uncertainty. Trends Cogn Sci, 13(8), 334–340. 10.1016/j.tics.2009.05.001 [DOI] [PubMed] [Google Scholar]
- Sisk CL, & Foster DL (2004). The neural basis of puberty and adolescence. Nature Neuroscience, 7(10), 1040–1047. [DOI] [PubMed] [Google Scholar]
- Tillem S, Chang SA, & Baskin-Sommers AR (2020). Comparision of Socio-Affective Processing Across Subtypes of Antisocial Psychopathology. In Focquaert F, Shaw E, & Waller BN (Eds.), The Routledge Handbook of the Philosophy and Science of Punishment. Routledge. [Google Scholar]
- Turner BO, Paul EJ, Miller MB, & Barbey AK (2018). Small sample sizes reduce the replicability of task-based fMRI studies. Communications biology, 1(1), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ., Kelly AM., Biswal BB., Castellanos FX., & Milham MP. (2009). Functional connectivity of default mode network components: correlation, anticorrelation, and causality. Human Brain Mapping, 30(2), 625–637. 10.1002/hbm.20531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Supekar KS, Ryali S, & Menon V. (2011). Dynamic reconfiguration of structural and functional connectivity across core neurocognitive brain networks with development. Journal of Neuroscience, 31(50), 18578–18589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbach RH, & Tottenham N. (2020). Callous-unemotional traits and reduced default mode network connectivity within a community sample of children. Development and psychopathology, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viding E, & McCrory EJ (2012, Aug). Genetic and neurocognitive contributions to the development of psychopathy. Dev Psychopathol, 24(3), 969–983. 10.1017/s095457941200048x [DOI] [PubMed] [Google Scholar]
- Waller R, Wagner NJ, Barstead MG, Subar A, Petersen JL, Hyde JS, & Hyde LW (2020). A meta-analysis of the associations between callous-unemotional traits and empathy, prosociality, and guilt. Clinical Psychology Review, 75, 101809. [DOI] [PubMed] [Google Scholar]
- Wechsler D. (2011). Wechsler abbreviated scale of intelligence-(WASI-II) (Vol. 4). NCS Pearson. [Google Scholar]
- Werhahn JE, Mohl S, Willinger D, Smigielski L, Roth A, Hofstetter C, Stämpfli P, Naaijen J, Mulder LM, & Glennon JC (2020). Aggression subtypes relate to distinct resting state functional connectivity in children and adolescents with disruptive behavior. European Child & Adolescent Psychiatry, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Ghosh S, Nieto-Castanon A, Saygin Z, Doehrmann O, Chai X, Reynolds G, Hofmann S, Pollack M, & Gabrieli J. (2016). Brain connectomics predict response to treatment in social anxiety disorder. Molecular Psychiatry, 21(5), 680. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, & Nieto-Castanon A. (2012). Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect, 2(3), 125–141. 10.1089/brain.2012.0073 [DOI] [PubMed] [Google Scholar]
- Wicker B., Keysers C., Plailly J., Royet J-P., Gallese V., & Rizzolatti G. (2003). Both of us disgusted in My insula: the common neural basis of seeing and feeling disgust. Neuron, 40(3), 655–664. [DOI] [PubMed] [Google Scholar]
- Winters DE, Pruitt PJ, Fukui S, Cyders MA, Pierce BJ, Lay K, & Damoiseaux JS (2021, Jun 18). Network functional connectivity underlying dissociable cognitive and affective components of empathy in adolescence. Neuropsychologia, 156, 107832. 10.1016/j.neuropsychologia.2021.107832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters DE, & Sakai JT (2021). Affective theory of mind impairments underlying callous-unemotional traits and the role of cognitive control: A pilot study. NeuroImage: Clinical, 32. 10.31234/osf.io/stwj8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters DE, Sakai JT, & Carter MR (2021). Resting-state network topology characterizing callous-unemotional traits in adolescence. NeuroImage: Clinical, 32, 102878. 10.1016/j.nicl.2021.102878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. (2020). International statistical classification of diseases and related health problems (11th ed.). https://icd.who.int/browse11/l-m/en
- Wu CW, Chen C-L, Liu P-Y, Chao Y-P, Biswal BB, & Lin C-P (2011). Empirical evaluations of slice-timing, smoothing, and normalization effects in seed-based, resting-state functional magnetic resonance imaging analyses. Brain connectivity, 1(5), 401–410. [DOI] [PubMed] [Google Scholar]
- Wymbs BT, McCarty CA, King KM, McCauley E, Vander Stoep A, Baer JS, & Waschbusch DA (2012, Oct). Callous-unemotional traits as unique prospective risk factors for substance use in early adolescent boys and girls. J Abnorm Child Psychol, 40(7), 1099–1110. 10.1007/s10802-012-9628-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder KJ, Lahey BB, & Decety J. (2016). Callous traits in children with and without conduct problems predict reduced connectivity when viewing harm to others. Scientific Reports, 6(1), 20216. 10.1038/srep20216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young L, Cushman F, Hauser M, & Saxe R. (2007). The neural basis of the interaction between theory of mind and moral judgment. Proceedings of the National Academy of Sciences, 104(20), 8235–8240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young L, Dodell-Feder D, & Saxe R. (2010). What gets the attention of the temporo-parietal junction? An fMRI investigation of attention and theory of mind. Neuropsychologia, 48(9), 2658–2664. [DOI] [PubMed] [Google Scholar]
- Zhang J., Kucyi A., Raya J., Nielsen AN., Nomi JS., Damoiseaux JS., Greene DJ., Horovitz SG., Uddin LQ., & Whitfield-Gabrieli S. (2021, 2021/11/15/). What have we really learned from functional connectivity in clinical populations? NeuroImage, 242, 118466. 10.1016/j.neuroimage.2021.118466 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.