Abstract
Our perspectives on aortic stenosis (AS) are changing. Evolving from the traditional thought of a passive degenerative disease, developing a greater understanding of the condition’s mechanistic underpinning has shifted the paradigm to an active disease process. This advancement from the ‘wear and tear’ model is a result of the growing economic and health burden of AS, particularly within industrialised countries, prompting further research. The pathophysiology of calcific AS (CAS) is complex, yet can be characterised similarly to that of atherosclerosis. Progressive remodelling involves lipid-protein complexes, with lipoprotein(a) being of particular interest for diagnostics and potential future treatment options.
There is an unmet clinical need for asymptomatic patient management; no pharmacotherapies are proven to slow progression and intervention timing varies. Novel approaches are developing to address this through: (1) screening with circulating biomarkers; (2) development of drugs to slow disease progression and (3) early valve intervention guided by medical imaging. Existing biomarkers (troponin and brain natriuretic peptide) are non-specific, but cost-effective predictors of ventricular dysfunction. In addition, their integration with cardiovascular MRI can provide accurate risk stratification, aiding aortic valve replacement decision making. Currently, invasive intervention is the only treatment for AS. In comparison, the development of lipoprotein(a) lowering therapies could provide an alternative; slowing progression of CAS, preventing left ventricular dysfunction and reducing reliance on surgical intervention.
The landscape of AS management is rapidly evolving. This review outlines current understanding of the pathophysiology of AS, its management and future perspectives for the condition’s assessment and treatment.
Keywords: Aortic Valve Stenosis, Diagnostic Imaging, Biomarkers, AORTIC VALVE DISEASE
Background
Aortic stenosis (AS) is the most prevalent acquired valvular heart disease. The condition’s incidence increases with age, affecting 2% of adults over 65 years old and rising to 10% in the eighth decade of life.1–3 Disease burden is set to increase due to an ageing population.1 4 AS has a subclinical period which is asymptomatic and does not increase mortality.3 5 However, the prognosis when symptoms arise is poor and untreated patients have an average survival rate of 2–3 years.6
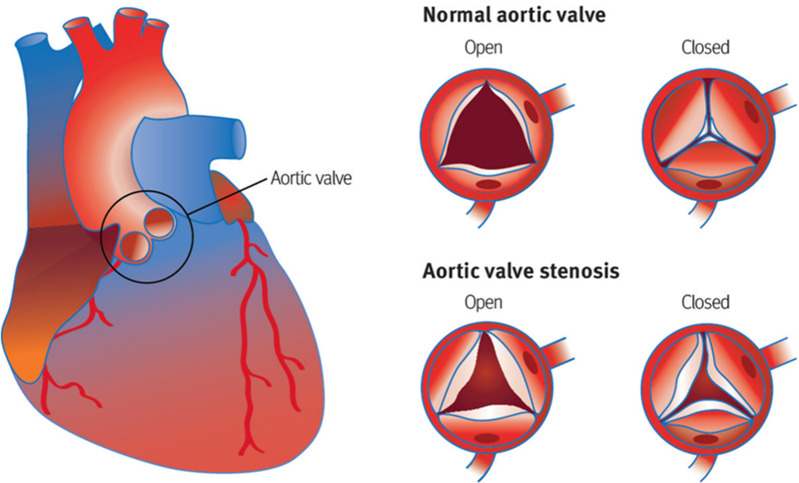
The aetiology of AS can be classified into three groups: congenitally bicuspid aortic valve, inflammatory rheumatic heart disease and degenerative calcification. Chronic rheumatic fever can cause immunologically mediated scarring of the valve, however, its incidence has decreased in developed countries due to better treatment against group A streptococcus.1 7 8 Congenital bicuspid aortic valves have two cusps, impeding blood flow which can increase the risk of early-onset AS and calcification.9 10 It is calcific AS (CAS) which poses the greatest disease burden on developed countries due to its greater prevalence.1 10–12 Symptoms occur in CAS due to the calcification and thickening of the aortic valve reducing the flow of blood into the left ventricular (LV) outflow tract (figure 1).6 The symptoms of severe AS include angina, syncope and, ultimately, heart failure.9 13 14
Figure 1.
Diagram showing the anatomy of the heart and stenosis of the aortic valve during the cardiac cycle. During systole, as the valve opens, the flow of blood from the left ventricle to the aorta is reduced due to the progressive narrowing of the aortic valve. Furthermore, thickening of the valve can reduce its mobility to close fully during diastole, leading to mixed aortic valve disease in some patients. Image from Zakkar et a.6
The European Society of Cardiology (ESC) and European Association for Cardio-Thoracic Surgery Task Force have recently published guidance on the workup and treatment of AS.15 Challenges remain in the timing of surgical intervention, procedural risk, lack of medical treatment options and the unpredictable nature of AS deterioration.3 5 11 15–19 As a result of this, evolving data on the pathophysiology seeks to elucidate novel biomarkers and therapeutics to better patient outcomes. Furthermore, clinical trials such as the Early Valve Replacement guided by Biomarkers of Left Ventricular Decompensation in Asymptomatic Patients with Severe Aortic Stenosis (EVOLVED) and The Early Valve Replacement in Severe Asymptomatic Aortic Stenosis Study aim to influence patient management through emerging imaging modalities, better patient stratification and early intervention.3 11 17 19–24
Outline
This paper will focus on the cellular underpinnings of CAS and future perspectives for the condition’s management. In particular, it will highlight the pathophysiology of CAS, current management of the condition, the use of brain natriuretic peptide (BNP) and troponin as potential biomarkers for assessment; the use of cardiovascular MR imaging (CMR) for assessment and treatments targeting lipoprotein(a) (Lp(a)) to slow disease progression.
Pathophysiology
Cellular pathology
The precise pathological mechanism behind the stenosis of the aortic valve remains unresolved. Nonetheless, advancements in technology and research have changed our insight.25 A once considered passive, degenerative process due to ageing is now understood to be active, similar in its molecular underpinning to atherosclerosis and its associated inflammatory response.26 As a result, the two conditions share common risk factors: old age, elevated Lp(a), hypertension, smoking, raised body mass index, hypercholesterolaemia and diabetes.12 27 Knowledge of this active pathophysiology has the potential to change the paradigm of AS management as pathway biomarkers and advanced imaging techniques aim to identify early signs of significant disease and Lp(a) lowering treatments have the potential to slow progression.3 17 19 26 28–30
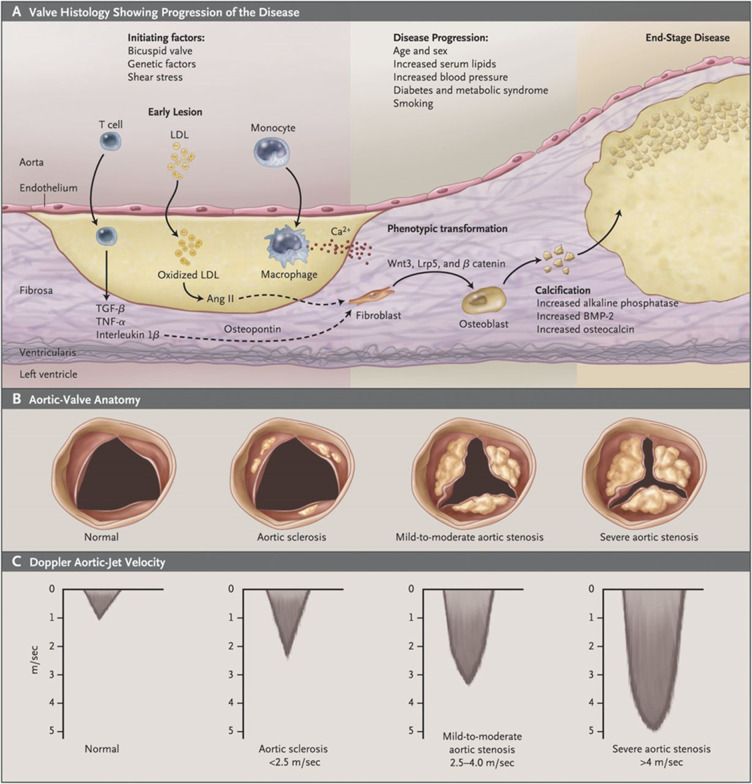
The aortic valve has three leaflets, each leaflet consisting of a three-layer, extracellular matrix structure covered by an endothelial layer (figure 2).21 31 32 The ventricularis layer (on the ventricular side) is rich in circumferentially aligned elastin fibres which allow for flexibility. On the aortic side, the fibrosa layer consists of collagen and fibroblasts. Between these, the spongiosa layer provides lubrication due to its high proteoglycan content. Cumulatively, all layers amalgamate to resist mechanical stress during the cardiac cycle.21 31 33 34 Although the fibrosa is the main load-bearing structure, it is also the most susceptible to fibrocalcification.35 Valve interstitial cells (VICs) form the largest proportion of cells within the valve and their function is to maintain valvular structure.36 Pathologically, this cell type is pivotal in understanding the progression of calcification, particularly the cell phenotype within the fibrosa.35 37
Figure 2.
Schematic diagram portraying the pathophysiology of CAS. (A) Diagram showing the histological structure of the aortic valve. Initial endothelial damage promotes the uptake of LDLs which in turn activate an inflammatory cascade leading to subsequent calcification. Of note is the abundance of pathological processes occurring within the fibrosa microenvironment The two stages of CAS progression are initiation and propagation. Initiation is associated with inflammation, mediated by immune cells, whereas propagation involves fibrocalcification. (B) On a local, valvular level, morphological change is visualised by calcium deposits narrowing the valve. (C) As a result of valvular narrowing, Doppler velocities increase leading to maladaptive ventricular remodelling. Image from Iung and Vahanian.32 Ang II, angiotensin II; BMP-2, bone morphogenic protein 2; CAS, calcific aortic stenosis; LDL, low-density lipoprotein; Lrp5: low-density lipoprotein receptor-related protein 5; TGF- β: transforming growth factor beta; TNF- α: tumour necrosis factor alpha.
On a cellular level, CAS progression can be divided into two phases: initiation and propagation (figure 2).26 32 33 The initiation stage is analogous to that found in atherosclerosis. Initial endothelial insult results in the infiltration of low-density lipoproteins (LDLs) and Lp(a) into the valve, depositing within the fibrosa. Haemodynamic stress exerted during aortic cusp movement is the likely cause of endothelial damage; the altered blood flow through bicuspid valves intensifies this stress.10 38 39 Following this, reactive oxygen species modify the lipids into oxidised LDLs (OxLDLs).33 OxLDLs stimulate the extravasation of monocytes into the valve interstitium which consequently differentiate into macrophages. At this stage, the inflammatory cascade initiates; macrophages capture the OxLDLs to form foam cells, enhancing the influx of immune cells through a greater expression of adhesion molecules E-selectin and intercellular adhesion molecule 1, thus perpetuating the cycle.34 38 40 Furthermore, Lp(a) acts as a carrier for oxidised phospholipids, potentiating inflammatory pathways.41 The activated macrophages release proinflammatory cytokines: interleukin 1 beta (IL-1β), interleukin 6 (IL-6), receptor activator of nuclear factor kappa-Β ligand (RANKL) and tumour necrosis factor alpha (TNF-α) which activates nuclear factor-κB (NF-κB). This recruits T lymphocytes, promotes extracellular matrix remodelling leading to fibrosis and activates VICs, propagating CAS.31
The propagation phase of CAS is characterised by repeated fibrosis and calcification.26 38 Inflammation activated VICs induce fibrosis by the secretion of matrix metalloproteinases through a myofibroblastic phenotype.38 42 This scarred tissue acts as a nidus for calcification in which inflammation-induced apoptosis of VICs leads to diffuse microcalcification through the release of apoptotic bodies.33 Microcalcification is potentiated by the release of calcifying microvesicles by VICs and macrophages.33 43 Following this, VICs drive macrocalcification by switching to an osteoblast-like phenotype, promoted by the dysregulation of osteogenic mediators such as bone morphogenic protein 2 and NOTCH1.21 26 31 38 44 Lp(a) has also been shown to stimulate VIC differentiation.45 The multifactorial nature of CAS progression is demonstrated as both Lp(a) levels and NOTCH1 function are influenced by genetic variance.44 46 47 As the disease progresses, stiffening of the valve prompts further apoptosis, resulting in the calcific mechanisms superseding the immunological pathway in propagating CAS.33 48 Furthermore, morphological differences of the aortic valve become more apparent.
Morphological pathology
The macroscopic manifestations of CAS, as a result of cellular pathological processes, affect the aortic valve and surrounding myocardium (figure 2). Initially, valve disease is difficult to detect. This subclinical phase, aortic sclerosis, involves valve thickening without impeding blood flow.49 50 Within 7 years, 10% of these patients would progress to CAS,50 51 distinguished by restricted valve motion and haemodynamic obstruction.6 The degree of calcium deposition is visualised by CT, although clinical severity is more frequently determined with echocardiograms assessing valve narrowing.15 52
Valvular narrowing increases myocardial burden, precipitating morphological maladaptation. Frank-Starling forces demonstrate that stenosis increases afterload and subsequently, greater ventricular pressures are needed to maintain cardiac output.53 As a result, there is compensatory concentric hypertrophy of the LV myocardium. Hypertrophy itself can be maladaptive, contributing to diastolic dysfunction.54 Chronically, this mechanism decompensates due to fibrosis and myocyte death. Dilation of the myocardium results in systolic dysfunction and heart failure, which is the main driver of adverse outcomes.23 The dilated heart can be visualised by multiple imaging modalities however CMR imaging can identify the underlying fibrosis at an earlier stage.3 23
Current management
AS is detected following a physical examination. The clinical signs include a slow rising pulse, palpable precordial thrill, narrow pulse pressure, ejection-systolic murmur radiating to the carotids, soft second heart second due to valve restriction and a fourth heart sound.55 Currently, transthoracic echocardiography remains the initial imaging modality to assess valve morphology and haemodynamics.15 Following diagnosis, patients are stratified based on symptom status, valvular anatomy and haemodynamics. Severe AS is defined by an aortic valve area (AVA) ≤1 cm2 (or indexed AVA of ≤0.6 cm2m2), peak transvalvular velocity ≥4.0 m/s, mean pressure gradient ≥40 mm Hg and velocity time integral of <0.25.1 15 Severity guides intervention timing, valve replacement being the gold standard in symptomatic patients.10 15 55 Transcatheter aortic valve implantation is preferred in high-risk patients over surgery and its indications are expanding.4 16 56
However, limitations around the timing of intervention arise; the balance between early intervention risk and irreversible cardiac damage is difficult to evaluate within current diagnostic parameters.21 57 Compounding this is the lack of robust evidence in treating severe asymptomatic AS. As a result, standard recommendations are passive and suggest watchful waiting.15 Interpretation of symptom severity is challenging in an elderly, comorbid, sedentary population and the rapid deterioration of symptomatic AS necessitates the need for advanced assessment techniques3 11 55 57 Current research is ongoing assessing evaluating earlier valve intervention in the asymptomatic patient.19 57
AHA and ESC guidelines currently do not recommend intervention in patients with moderate AS but it can be considered if the patient is undergoing CABG or surgical intervention on the ascending aorta or another valve.15 58 Large cohort studies do, however, show that moderate AS is not a benign condition and that these patients have poor survival rates and that AVR in this population group is associated with better outcomes.59 Further randomised controlled trials (RCTs) are required to guide future recommendations. The PROGRESS60 and Evolut EXPAND TAVR II Pivotal61 trials are aiming to evaluate the efficacy and safety of TAVR in moderate AS and the TIAMAR62 and TAVR UNLOAD63 studies are also investigating intervention in patients with concomitant moderate mitral regurgitation and heart failure, respectively.
Emerging assessment techniques
Biomarkers
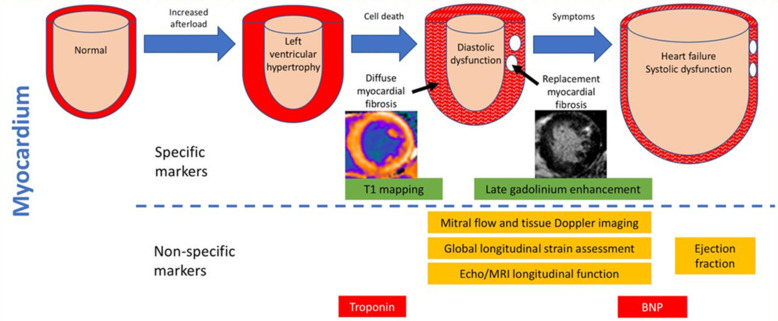
A range of CAS biomarkers have been identified with the potential to monitor asymptomatic patients and predict postprocedural outcomes.17 64–66 BNP and highly sensitive troponin I (hsTnI) hold promise due to their accessibility, simple analysis and cost-effectiveness (figure 3).17 57
Figure 3.
Diagram illustrating the morphological pathology and use of multiple markers within the pathological timeline of CAS. Morphological change is demonstrated by myocardial remodelling. Hypertrophy is followed by fibrosis which leads to the decompensatory dilation of the heart, visualised through imaging modalities. Specific markers include the CMR techniques of T1 mapping, which can quantitatively analyse diffuse fibrosis and late gadolinium enhancement. The red arrow illustrates an area of irreversible, replacement myocardial fibrosis. Both troponin and BNP are non-specific biomarkers for the assessment of CAS. Troponin detects myocyte injury and fibrosis. Whereas BNP measures the degree of ventricular stretch as a result of the fluid overload exhibited in heart failure. Image adapted from Everett et al.57 BNP, brain natriuretic peptide; CAS, calcific aortic stenosis; CMR, cardiovascular MR.
BNP is a hormone secreted in response to cardiomyocyte stretch commonly used to assess heart failure severity.67 68 With regard to AS, BNP can approximate the point of ventricular dysfunction, thus predicting symptom-free survival and improving interventional timing.57 69 70 The ESC guidelines suggest considering valve replacement in asymptomatic patients with a BNP of over three times the normal.15 However, the attributed recommendation class of IIb indicates the need for more RCTs. Nonetheless, a recent systematic review of 21 biomarker studies associated BNP level rise with all-cause mortality. Importantly, BNP was the strongest predictor, with risk of death more than doubling.17 Due to the overlap in mechanisms behind BNP release, it is non-specific for AS in isolation.57 71 As a result, an approach with multiple biomarkers may provide better insight.64 65 72
Troponin acts as a surrogate for myocardial damage. Mechanistically, it is associated with maladaptive remodelling and fibrosis within AS. Currently, it is the preferred marker for assessing acute coronary syndromes, increasing its accessibility and cost-effectiveness.57 73 In a systematic review of AS biomarkers, elevated troponin predicted increased risk of death.17 However, three studies did not find significance; and the negative finding was supported by a large (n=708) retrospective cohort study.66 This variance further demonstrates the need for RCTs to determine causality and reduce our reliance on observational biomarker studies. A multimarker approach would likely increase the specificity of prognosis. EVOLVED, a multicentre RCT, seeks to evaluate these limitations by screening asymptomatic patients with hsTnI.19 Measurements of hsTnI are more sensitive than BNP as it identifies low-level myocyte death.11 74 Moreover, prior to early valve replacement, CMR is used to assess myocardial fibrosis as a surrogate of myocardial strain.
Imaging modalities
AS is a disease with isolated valvular and subsequent global myocardial dysfunction. CMR can quantify both parameters with a high degree of specificity, allowing for enhanced risk stratification and treatment timing optimisation.3 11 23 Myocardial fibrosis with cardiac biopsy is not routinely assessed due to the complication rates75 and sampling error.23 As the gold standard in measuring ventricular function,76 CMR’s growing use in the non-invasive tissue characterisation of AS gives it the potential to revolutionise management especially in the asymptomatic population.
Aortic compliance and flow are CMR markers which can predict morbidity in patients with AS. Arterial load consists of resistive load and pulsatile load, the latter determined by arterial wave reflections and aortic stiffness. In addition to the increased ventricular pressures exerted due to valvular stenosis, increased arterial load can further drive maladaptive remodelling and decompensation. In particular, greater magnitudes of wave reflections and reduced arterial compliance are associated with decompensation and a poorer clinical course following valve replacement.77 Measurements of LV blood flow kinetic energy have also been associated with ventricular remodelling and an inverse correlation to exercise capacity.78 The ability of CMR to assess these components can lead to greater risk stratification and prediction of clinical outcomes, guiding management prior to and following treatment. Although these findings are derived from small sample sizes,77 78 larger clinical studies are warranted to evaluate the prognostic capabilities of CMR and to therapeutically target these biomarkers to improve patient quality of life.
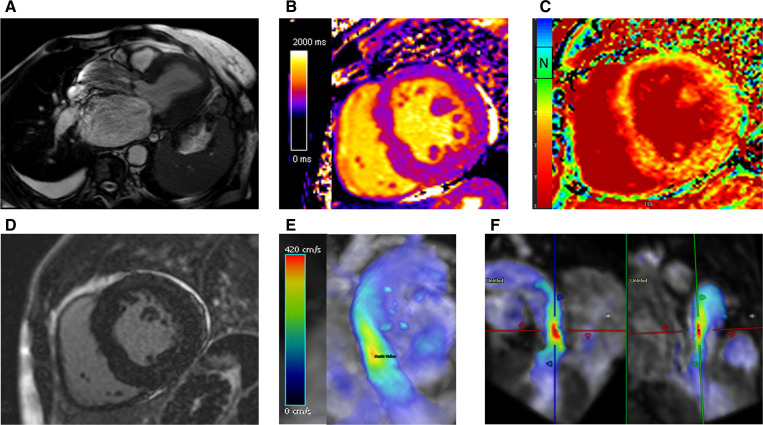
The pathology of CAS fibrosis can be visualised using late gadolinium enhancement (LGE) and T1 mapping (figure 3).57 LGE involves the accumulation of gadolinium chelate within an expanded extracellular matrix, qualitatively detecting replacement fibrosis.79 Multiple studies support LGE’s prognostic capabilities in AS.80–83 In addition, the emergence of quantitatively assessing diffuse myocardial fibrosis through T1 mapping is of interest23 84 (figure 4). This technique analyses CMR maps on a voxel by voxel basis and quantifies fibrosis by measuring T1 relaxation time.84 The strength of T1 mapping is its ability to detect early AS pathology prior to decompensation; there is potential to accurately monitor patients and intervene to prior to fibrosis.23 85 Standardised protocols have redressed initial concerns with result reproducibility, however, limitations due to variance between patients still exist.23 86 As a result, the ongoing EVOLVED trial will test the clinical efficacy of CMR and provide insight into potential thresholds for T1 mapping. Moreover, a greater understanding of early patient stratification will assist in developing targeted novel therapeutics for CAS.
Figure 4.
(A) Three-chamber cine demonstrating restrictive aortic valve at peak systole with a dephasing artefact at the level of accelerated flow through the restrictive aortic valve. (B, C) T1-mapping and extracellular volume (ECV) mapping demonstrating a rise in ECV with increased afterload associated fibrotic changes. (D) Late gadolinium enhancement imaging shows no evidence of any ischaemic scar. (E, F) Four-dimensional flow mapping demonstrating in 3D the peak velocity (red zones) in 3D (E) and in two orthogonal planes (F). The peak velocity was 4.3 m/s, which is consistent with severe AS. 3D, three dimensions; AS, aortic stenosis.
Novel treatments
There are no efficacious pharmacological treatments proven to slow CAS progression.15 21 26 87–89 However, multiple therapeutics have been repurposed or developed to decrease mortality without the associated complications of valve replacement (table 1).56 90 Novel treatments targeting Lp(a) show promise due to the molecule’s known structure and role in CAS pathophysiology; associated genetics and role as a monitorable biomarker (figure 5).28 34 87–89 91 92
Table 1.
Summary of the potential medical therapies in aortic stenosis
| Treatment | Target | Mechanism of action | Current evidence |
| Antisense oligonucleotide | Lp(a) synthesis | RNA therapeutic drug which decreases hepatic synthesis of Lp(a), thus inhibiting inflammatory cascade of CAS.88 | Reduced Lp(a) concentrations in two RCTs.101 |
| PCSK9 inhibitor | LDL and Lp(a) concentration through PCSK9 inhibition | Monoclonal antibodies or siRNA indirectly decrease Lp(a) infiltration through inhibition of PCSK9. Less circulating PCSK9 increases LDL-R on hepatocytes, decreasing circulating LDL and Lp(a) concentration.88 | Reduced Lp(a) concentrations in FOURIER clinical trial.93 102 An ongoing RCT with placebo is assessing its effect on mild-moderate CAS progression.103 |
| CETP inhibitor | LDL synthesis inhibition; Lp(a) synthesis inhibition to a lesser extent | The drug inhibits cholesterol ester transfer from HDL to LDL. This decreases LDL and increases HDL levels. It also decreases Lp(a) synthesis by decreasing apo(a) production.107 108 | Multiple RCTs confirmed a reduction of LDL, Lp(a) and cardiovascular events.109–111 However, data from previous trials demonstrated an increase in cardiovascular events.112 Moreover, the development of anacetrapib, treatment arm of the REVEAL study, has stopped.87 |
| Statin | LDL cholesterol synthesis | Inhibition of HmG-CoA reductase reduces intracellular cholesterol. Hepatocytes upregulate LDL-R to increase cholesterol uptake, decreasing circulating LDL levels. This is theorised to limit CAS pathogenesis and calcification.113 | Two large RCTs and current guidelines demonstrate that statins do not prevent CAS progression.15 105 106 However, secondary analysis of an RCT found a reduction in aortic valve replacement rate in mild AS.114 |
| Niacin | LDL synthesis; Lp(a) indirectly | Niacin inhibits hepatic triglyceride synthesis. This increases hepatic apo(b) degradation, thus reducing LDL and Lp(a) levels.115 | Reduced Lp(a) in a systematic review of RCTs.116 Niacin is not currently recommended due to the risk of serious adverse events.89 The ongoing EAVaLL RCT is testing its use in mild AS patients screened for high Lp(a).117 |
| Vitamin K2/menaquinone-7 | Calcium metabolism | Vitamin K2 carboxylates, thus potentiates, proteins which inhibit calcification.118 | The recent AVADEC RCT demonstrated that vitamin K supplementation does not influence CAS progression. However, the results may not be generalisable due to limited patient diversity.118 119 Moreover, an RCT in bicuspid patients is ongoing.120 |
| Anti-osteoporotic drugs (bisphosphonates and denosumab) | Calcium metabolism and osteogenesis | Paradoxical to bone disease, bisphosphonates prevent the differentiation of osteoblasts within the valve. Denosumab inhibits RANKL, attenuating the CAS pathological cascade.26 121 | Retrospective studies demonstrate a conflicted view on efficacy with concomitant osteoporosis confounding results.122 123 A recent RCT confirmed that neither drug affected CAS.124 |
| NOACs | Coagulation and inflammatory cascades | Limits valvular inflammation through inhibition of the coagulation cascade, thus attenuating atherosclerosis and VIC activation.34 | VICs in culture demonstrate the downregulation of pro-calcification proteins. Expansion to in vivo studies is necessary.125 |
apo(a), apoprotein(a); apo(b), apoprotein(b); AS, aortic stenosis; AVADEC, The Aortic Valve Decalcification; CAS, calcific aortic stenosis; CETP, cholesteryl ester transfer protein; EAVaLL, Early Aortic Valve Lipoprotein(a) Lowering Trial; FOURIER, Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk; HDL, high-density lipoprotein; HmG-CoA, 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase; LDL, low-density lipoprotein; LDL-R, LDL receptor; Lp(a), lipoprotein(a); NOAC, novel oral anticoagulant; PCSK9, proprotein convertase subtilisin/kexin type 9; RANKL, receptor activator of nuclear factor kappa-Β ligand; RCT, randomised controlled trial; REVEAL, Randomised Evaluation of the Effects of Anacetrapib Through Lipid-modification; RNA, ribonucleic acid; siRNA, small interfering ribonucleic acid; VIC, valve interstitial cell.
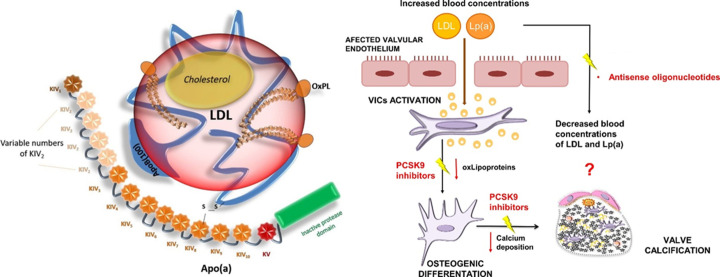
Figure 5.
Diagram illustrating the structure of Lp(a) and drug mechanisms for its treatment. Left panel: The characteristic disulphide bridge between apolipoprotein(b) and apolipoprotein(a) in Lp(a) differentiates it from low-density lipoprotein. Variance within the KIV-2 domain is due to genetics and this affects its molecular weight. Individuals with smaller isoforms are thought to have a greater amount of circulating Lp(a). Right panel: Proposed mechanisms for the action of Lp(a) lowering drugs. PCKS9 reduces both Lp(a) and LDL concentrations (with a greater impact on the latter) whereas antisense oligonucleotides decrease Lp(a) by targeting apolipoprotein(a). Images adapted from Fusco et al92 and Natorska et al.34 Apo(a), apolipoprotein(a); ApoB, apolipoprotein(b); KIV, kringle IV domain; KV, kringle V domain; LDL, low-density lipoprotein; oxLipoproteins, oxidised lipoproteins; PCSK9, proprotein convertase subtilisin/kexin type 9; S-S, disulphide bridge.
Elevated Lp(a) potentiates the atherosclerotic process of CAS, with an approximated one billion people having high levels.93–95 Furthermore, the identified polymorphism rs1045872 in genetically susceptible patients supports the causal role of Lp(a) concentration on calcification progression.47 Genetics also provides a platform from which screening and quantification of circulating Lp(a) could stratify at-risk patients for medical intervention.33 96 97 The viability of genetic screening is simplified as Lp(a) is the only monogenic risk factor for AS.41 Following potential screening, lipoprotein apheresis is the current treatment for raised Lp(a). However, its clinical viability is limited to severe dyslipidaemia due to its costs and inherent extracorporeal risks.89 91 98 Consequently, the development of proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors and RNA-based antisense oligonucleotides (ASOs), as Lp(a) lowering therapies, is necessitated (figure 5). Although unable to reverse CAS, these drugs aim to slow disease progression by targeting the initiation phase.28 As a result, it is hypothesised that their use is most effective in mild-moderate AS,99 a population with no current therapeutics of significant benefit.
PCSK9 inhibitors function to lower LDL and Lp(a) through upregulation of LDL receptors on hepatocytes, preventing the progression of CAS pathology.88 100 In comparison, ASOs use RNA to target apolipoprotein(a) overexpression, reducing Lp(a). Both drug classes are supported by robust RCTs showing an ability to decrease Lp(a)93 101 102 and a study is ongoing to test PCSK9 inhibitors’ effect on CAS.103 ASOs may prove to be superior for populations with genetic overexpression and very high Lp(a) levels.104 This is, in part, due to ASOs greater ability to reduce Lp(a): a 99% decrease in comparison to 25% with PSCK9 inhibitors.89 Within a wider population, this increased effect of ASOs may also prove beneficial.104 In addition to this, the failure of statins to affect CAS outcome suggests therapeutics targeting LDLs are less effective than anticipated.105 106 Nevertheless, large RCTs testing the clinical efficacy of these drugs on the progression of CAS are warranted. This, coupled with improved in vitro techniques to understand the disease’s pathophysiology and develop advanced treatments,33 will validate the use of pharmacotherapies in addressing the burden of AS.
Conclusion
Our perspectives are shifting from passive monitoring to active management for the assessment and treatment of those with AS. Insight into the two stages of CAS, initiation and propagation, have proven to be invaluable, providing novel management options. Of particular interest is Lp(a), which can play a multifaceted role in genetic screening, biomarker measurement and targeted treatment. Further studies into cellular pathology are warranted to contextualise current research and identify additional biomarkers, increasing the specificity of a multimarker screening approach. In addition to this, asymptomatic management has the potential to be revolutionised through the use of therapeutics to slow disease progression and CMR to guide early valve replacement. Validation of advanced patient stratification and diagnostic workup could propagate the widespread adoption of personalised AS therapy. Although many pharmacotherapies have exhibited potential, an increase in the number of prospective RCTs, with the larger cohort sizes of retrospective studies, is necessary to validate their clinical efficacy in reducing CAS burden.
Footnotes
Contributors: SMS, JS, SML and DPR contributed to the final version of the manuscript. PG provided CMR images. SML, PG and DPR reviewed article and provided oversight.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
No data are available.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Baumgartner H, Falk V, Bax JJ, et al. 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J 2017;38:2739–91. 10.1093/eurheartj/ehx391 [DOI] [PubMed] [Google Scholar]
- 2.Stewart RL, Chan KL. Management of asymptomatic severe aortic stenosis. Curr Cardiol Rev 2009;5:29–35. 10.2174/157340309787048103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bohbot Y, Renard C, Manrique A, et al. Usefulness of cardiac magnetic resonance imaging in aortic stenosis. Circ: Cardiovascular Imaging 2020;13. 10.1161/CIRCIMAGING.119.010356 [DOI] [PubMed] [Google Scholar]
- 4.Danielsen R, Aspelund T, Harris TB, et al. The prevalence of aortic stenosis in the elderly in iceland and predictions for the coming decades: the AGES-reykjavík study. Int J Cardiol 2014;176:916–22. 10.1016/j.ijcard.2014.08.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Joseph J, Naqvi SY, Giri J, et al. Aortic stenosis: pathophysiology, diagnosis, and therapy. Am J Med 2017;130:253–63. 10.1016/j.amjmed.2016.10.005 [DOI] [PubMed] [Google Scholar]
- 6.Zakkar M, Bryan AJ, Angelini GD. Aortic stenosis: diagnosis and management. BMJ 2016;355:i5425. 10.1136/bmj.i5425 [DOI] [PubMed] [Google Scholar]
- 7.Tal R, Hamad Saied M, Zidani R, et al. Rheumatic fever in a developed country-is it still relevant? A retrospective, 25 years follow-up. Pediatr Rheumatol Online J 2022;20:20. 10.1186/s12969-022-00678-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iung B, Vahanian A. Epidemiology of acquired valvular heart disease. Can J Cardiol 2014;30:962–70. 10.1016/j.cjca.2014.03.022 [DOI] [PubMed] [Google Scholar]
- 9.Carabello BA. Introduction to aortic stenosis. Circ Res 2013;113:179–85. 10.1161/CIRCRESAHA.113.300156 [DOI] [PubMed] [Google Scholar]
- 10.Schwarz NG, Kemp WL. Educational case: aortic valve stenosis. Acad Pathol 2020;7:2374289520961765. 10.1177/2374289520961765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lindman BR, Dweck MR, Lancellotti P, et al. Management of asymptomatic severe aortic stenosis: evolving concepts in timing of valve replacement. JACC Cardiovasc Imaging 2020;13:481–93. 10.1016/j.jcmg.2019.01.036 [DOI] [PubMed] [Google Scholar]
- 12.Lindman BR, Clavel M-A, Mathieu P, et al. Calcific aortic stenosis. Nat Rev Dis Primers 2016;2:16006. 10.1038/nrdp.2016.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kemp CD, Conte JV. The pathophysiology of heart failure. Cardiovasc Pathol 2012;21:365–71. 10.1016/j.carpath.2011.11.007 [DOI] [PubMed] [Google Scholar]
- 14.Elmariah S. Patterns of left ventricular remodeling in aortic stenosis: therapeutic implications. Curr Treat Options Cardiovasc Med 2015;17:391. 10.1007/s11936-015-0391-0 [DOI] [PubMed] [Google Scholar]
- 15.Vahanian A, Beyersdorf F, Praz F, et al. 2021 ESC/EACTS guidelines for the management of valvular heart disease: developed by the task force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2022;43:561–632. 10.1093/eurheartj/ehab395 [DOI] [PubMed] [Google Scholar]
- 16.Makkar RR, Thourani VH, Mack MJ, et al. Five-year outcomes of transcatheter or surgical aortic-valve replacement. N Engl J Med 2020;382:799–809. 10.1056/NEJMoa1910555 [DOI] [PubMed] [Google Scholar]
- 17.White M, Baral R, Ryding A, et al. Biomarkers associated with mortality in aortic stenosis: a systematic review and meta-analysis. Med Sci (Basel) 2021;9:29. 10.3390/medsci9020029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pellikka PA, Sarano ME, Nishimura RA, et al. Outcome of 622 adults with asymptomatic, hemodynamically significant aortic stenosis during prolonged follow-up. Circulation 2005;111:3290–5. 10.1161/CIRCULATIONAHA.104.495903 [DOI] [PubMed] [Google Scholar]
- 19.Bing R, Everett RJ, Tuck C, et al. Rationale and design of the randomized, controlled early valve replacement guided by biomarkers of left ventricular decompensation in asymptomatic patients with severe aortic stenosis (evolved) trial. Am Heart J 2019;212:91–100. 10.1016/j.ahj.2019.02.018 [DOI] [PubMed] [Google Scholar]
- 20.Dahou A, Clavel M-A, Capoulade R, et al. B-Type natriuretic peptide and high-sensitivity cardiac troponin for risk stratification in low-flow, low-gradient aortic stenosis: a substudy of the TOPAS study. JACC Cardiovasc Imaging 2018;11:939–47. 10.1016/j.jcmg.2017.06.018 [DOI] [PubMed] [Google Scholar]
- 21.Mazur P, Kopytek M, Ząbczyk M, et al. Towards personalized therapy of aortic stenosis. J Pers Med 2021;11:1292. 10.3390/jpm11121292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levy F, Iacuzio L, Civaia F, et al. Usefulness of 3-tesla cardiac magnetic resonance imaging in the assessment of aortic stenosis severity in routine clinical practice. Arch Cardiovasc Dis 2016;109:618–25. 10.1016/j.acvd.2016.04.006 [DOI] [PubMed] [Google Scholar]
- 23.Bing R, Cavalcante JL, Everett RJ, et al. Imaging and impact of myocardial fibrosis in aortic stenosis. JACC Cardiovasc Imaging 2019;12:283–96. 10.1016/j.jcmg.2018.11.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.University of Leicester . A randomised controlled trial of early valve replacement in severe asymptomatic aortic stenosis. 2021. Available: https://clinicaltrials.gov/ct2/show/NCT04204915 [Accessed 23 May 2022].
- 25.Dweck MR, Khaw HJ, Sng GKZ, et al. Aortic stenosis, atherosclerosis, and skeletal bone: is there a common link with calcification and inflammation? Eur Heart J 2013;34:1567–74. 10.1093/eurheartj/eht034 [DOI] [PubMed] [Google Scholar]
- 26.Peeters FECM, Meex SJR, Dweck MR, et al. Calcific aortic valve stenosis: hard disease in the heart. Eur Heart J 2018;39:2618–24. 10.1093/eurheartj/ehx653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stewart BF, Siscovick D, Lind BK, et al. Clinical factors associated with calcific aortic valve disease. cardiovascular health study. J Am Coll Cardiol 1997;29:630–4. 10.1016/s0735-1097(96)00563-3 [DOI] [PubMed] [Google Scholar]
- 28.Zheng K, Dweck M. Lipoprotein (a): marker and target in calcific aortic valve disease. Br J Cardiol 2022:S11–4. 10.5837/bjc.2022.s03 [DOI] [Google Scholar]
- 29.Liu Q, Yu Y, Xi R, et al. Association between lipoprotein (a) and calcific aortic valve disease: a systematic review and meta-analysis. Front Cardiovasc Med 2022;9:877140. 10.3389/fcvm.2022.877140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abdelbaky A, Corsini E, Figueroa AL, et al. Early aortic valve inflammation precedes calcification: a longitudinal FDG-PET/CT study. Atherosclerosis 2015;238:165–72. 10.1016/j.atherosclerosis.2014.11.026 [DOI] [PubMed] [Google Scholar]
- 31.Zebhi B, Lazkani M, Bark D. Calcific aortic stenosis-a review on acquired mechanisms of the disease and treatments. Front Cardiovasc Med 2021;8:734175. 10.3389/fcvm.2021.734175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iung B, Vahanian A. Degenerative calcific aortic stenosis: a natural history. Heart 2012;98 Suppl 4:iv7–13. 10.1136/heartjnl-2012-302395 [DOI] [PubMed] [Google Scholar]
- 33.Goody PR, Hosen MR, Christmann D, et al. Aortic valve stenosis: from basic mechanisms to novel therapeutic targets. Arterioscler Thromb Vasc Biol 2020;40:885–900. 10.1161/ATVBAHA.119.313067 [DOI] [PubMed] [Google Scholar]
- 34.Natorska J, Kopytek M, Undas A. Aortic valvular stenosis: novel therapeutic strategies. Eur J Clin Invest 2021;51:e13527. 10.1111/eci.13527 [DOI] [PubMed] [Google Scholar]
- 35.Yip CYY, Simmons CA. The aortic valve microenvironment and its role in calcific aortic valve disease. Cardiovasc Pathol 2011;20:177–82. 10.1016/j.carpath.2010.12.001 [DOI] [PubMed] [Google Scholar]
- 36.Mulholland DL, Gotlieb AI. Cell biology of valvular interstitial cells. Can J Cardiol 1996;12:231–6. [PubMed] [Google Scholar]
- 37.Liu AC, Joag VR, Gotlieb AI. The emerging role of valve interstitial cell phenotypes in regulating heart valve pathobiology. Am J Pathol 2007;171:1407–18. 10.2353/ajpath.2007.070251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zheng KH, Tzolos E, Dweck MR. Pathophysiology of aortic stenosis and future perspectives for medical therapy. Cardiol Clin 2020;38:1–12. 10.1016/j.ccl.2019.09.010 [DOI] [PubMed] [Google Scholar]
- 39.Sun L, Chandra S, Sucosky P. Ex vivo evidence for the contribution of hemodynamic shear stress abnormalities to the early pathogenesis of calcific bicuspid aortic valve disease. PLoS One 2012;7:e48843. 10.1371/journal.pone.0048843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Passos LSA, Lupieri A, Becker-Greene D, et al. Innate and adaptive immunity in cardiovascular calcification. Atherosclerosis 2020;306:59–67. 10.1016/j.atherosclerosis.2020.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yeang C, Wilkinson MJ, Tsimikas S. Lipoprotein (a) and oxidized phospholipids in calcific aortic valve stenosis. Curr Opin Cardiol 2016;31:440–50. 10.1097/HCO.0000000000000300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Latif N, Quillon A, Sarathchandra P, et al. Modulation of human valve interstitial cell phenotype and function using a fibroblast growth factor 2 formulation. PLoS ONE 2015;10:e0127844. 10.1371/journal.pone.0127844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hutcheson JD, Goettsch C, Bertazzo S, et al. Genesis and growth of extracellular-vesicle-derived microcalcification in atherosclerotic plaques. Nat Mater 2016;15:335–43. 10.1038/nmat4519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Irtyuga O, Malashicheva A, Zhiduleva E, et al. Notch1 mutations in aortic stenosis: association with osteoprotegerin/RANK/RANKL. Biomed Res Int 2017;2017:6917907. 10.1155/2017/6917907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yu B, Hafiane A, Thanassoulis G, et al. Lipoprotein (a) induces human aortic valve interstitial cell calcification. JACC Basic Transl Sci 2017;2:358–71. 10.1016/j.jacbts.2017.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cairns BJ, Coffey S, Travis RC, et al. A replicated, genome-wide significant association of aortic stenosis with a genetic variant for lipoprotein (a): meta-analysis of published and novel data. Circulation 2017;135:1181–3. 10.1161/CIRCULATIONAHA.116.026103 [DOI] [PubMed] [Google Scholar]
- 47.Thanassoulis G, Campbell CY, Owens DS, et al. Genetic associations with valvular calcification and aortic stenosis. N Engl J Med 2013;368:503–12. 10.1056/NEJMoa1109034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dweck MR, Pawade TA, Newby DE. Aortic stenosis begets aortic stenosis: between a rock and a hard place? Heart 2015;101:919–20. 10.1136/heartjnl-2015-307519 [DOI] [PubMed] [Google Scholar]
- 49.Freeman RV, Otto CM. Spectrum of calcific aortic valve disease. Circulation 2005;111:3316–26. 10.1161/CIRCULATIONAHA.104.486738 [DOI] [PubMed] [Google Scholar]
- 50.Nightingale AK, Horowitz JD. Aortic sclerosis: not an innocent murmur but a marker of increased cardiovascular risk. Heart 2005;91:1389–93. 10.1136/hrt.2004.057117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Otto CM. Aortic stenosis: even mild disease is significant. Eur Heart J 2004;25:185–7. 10.1016/j.ehj.2003.12.010 [DOI] [PubMed] [Google Scholar]
- 52.Pawade T, Clavel M-A, Tribouilloy C, et al. Computed tomography aortic valve calcium scoring in patients with aortic stenosis. Circ Cardiovasc Imaging 2018;11:e007146. 10.1161/CIRCIMAGING.117.007146 [DOI] [PubMed] [Google Scholar]
- 53.Delicce AV, Makaryus AN. Physiology, frank starling law. Treasure Island (FL): StatPearls Publishing, 2022. [PubMed] [Google Scholar]
- 54.Chambers J. The left ventricle in aortic stenosis: evidence for the use of ACE inhibitors. Heart 2006;92:420–3. 10.1136/hrt.2005.074112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maganti K, Rigolin VH, Sarano ME, et al. Valvular heart disease: diagnosis and management. Mayo Clin Proc 2010;85:483–500. 10.4065/mcp.2009.0706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Howard C, Jullian L, Joshi M, et al. Tavi and the future of aortic valve replacement. J Card Surg 2019;34:1577–90. 10.1111/jocs.14226 [DOI] [PubMed] [Google Scholar]
- 57.Everett RJ, Clavel M-A, Pibarot P, et al. Timing of intervention in aortic stenosis: a review of current and future strategies. Heart 2018;104:2067–76. 10.1136/heartjnl-2017-312304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Otto CM, Nishimura RA, Bonow RO, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American College of cardiology/American heart association joint committee on clinical practice guidelines. Circulation 2021;143:e72–227. 10.1161/CIR.0000000000000923 [DOI] [PubMed] [Google Scholar]
- 59.Strange G, Stewart S, Celermajer D, et al. Poor long-term survival in patients with moderate aortic stenosis. J Am Coll Cardiol 2019;74:1851–63. 10.1016/j.jacc.2019.08.004 [DOI] [PubMed] [Google Scholar]
- 60.Edwards Lifesciences . The PROGRESS trial: A prospective, randomized, controlled trial to assess the management of moderate aortic stenosis by clinical surveillance or transcatheter aortic valve replacement. 2023. Available: https://clinicaltrials.gov/ct2/show/NCT04889872 [Accessed 26 Feb 2023].
- 61.Medtronic Cardiovascular . EvolutTM Expand TAVR II pivotal trial. 2023. Available: https://clinicaltrials.gov/ct2/show/NCT05149755 [Accessed 26 Feb 2023].
- 62.University Hospital Inselspital, Berne . Early versus deferred aortic valve replacement in patients with moderate aortic stenosis combined with mitral regurgitation: a randomized clinical trial. 2023. Available: https://clinicaltrials.gov/ct2/show/NCT05310461 [Accessed 26 Feb 2023].
- 63.Cardiovascular Research Foundation, New York . Transcatheter aortic valve replacement to UNLOAD the left ventricle in patients with advanced heart failure: A randomized trial (TAVR UNLOAD). 2023. Available: https://clinicaltrials.gov/ct2/show/NCT02661451 [Accessed 26 Feb 2023].
- 64.Lindman BR, Clavel M-A, Abu-Alhayja’a R, et al. Multimarker approach to identify patients with higher mortality and rehospitalization rate after surgical aortic valve replacement for aortic stenosis. JACC Cardiovasc Interv 2018;11:2172–81. 10.1016/j.jcin.2018.07.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Spampinato RA, Bochen R, Sieg F, et al. Multi-biomarker mortality prediction in patients with aortic stenosis undergoing valve replacement. J Cardiol 2020;76:154–62. 10.1016/j.jjcc.2020.02.019 [DOI] [PubMed] [Google Scholar]
- 66.Vidula MK, Orlenko A, Zhao L, et al. Plasma biomarkers associated with adverse outcomes in patients with calcific aortic stenosis. Eur J Heart Fail 2021;23:2021–32. 10.1002/ejhf.2361 [DOI] [PubMed] [Google Scholar]
- 67.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of cardiology (ESC) developed with the special contribution of the heart failure association (HFA) of the ESC. Eur Heart J 2016;37:2129–200. 10.1093/eurheartj/ehw128 [DOI] [PubMed] [Google Scholar]
- 68.Taylor CJ, Lay-Flurrie SL, Ordóñez-Mena JM, et al. Natriuretic peptide level at heart failure diagnosis and risk of hospitalisation and death in England 2004-2018. Heart 2022;108:543–9. 10.1136/heartjnl-2021-319196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Clavel M-A, Malouf J, Michelena HI, et al. B-Type natriuretic peptide clinical activation in aortic stenosis: impact on long-term survival. J Am Coll Cardiol 2014;63:2016–25. 10.1016/j.jacc.2014.02.581 [DOI] [PubMed] [Google Scholar]
- 70.Stein EJ, Fearon WF, Elmariah S, et al. Left ventricular hypertrophy and biomarkers of cardiac damage and stress in aortic stenosis. J Am Heart Assoc 2022;11:e023466. 10.1161/JAHA.121.023466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Madamanchi C, Alhosaini H, Sumida A, et al. Obesity and natriuretic peptides, BNP and NT-proBNP: mechanisms and diagnostic implications for heart failure. Int J Cardiol 2014;176:611–7. 10.1016/j.ijcard.2014.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Elmariah S, McCarthy C, Ibrahim N, et al. Multiple biomarker panel to screen for severe aortic stenosis: results from the Casablanca study. Open Heart 2018;5:e000916. 10.1136/openhrt-2018-000916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Collet J-P, Thiele H, Barbato E, et al. 2020 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: the task force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the european society of cardiology (ESC). Eur Heart J 2021;42:1289–367. 10.1093/eurheartj/ehaa909 [DOI] [PubMed] [Google Scholar]
- 74.Ferrer-Sistach E, Lupón J, Cediel G, et al. High-sensitivity troponin T in asymptomatic severe aortic stenosis. Biomarkers 2019;24:334–40. 10.1080/1354750X.2019.1567818 [DOI] [PubMed] [Google Scholar]
- 75.Yilmaz A, Kindermann I, Kindermann M, et al. Comparative evaluation of left and right ventricular endomyocardial biopsy: differences in complication rate and diagnostic performance. Circulation 2010;122:900–9. 10.1161/CIRCULATIONAHA.109.924167 [DOI] [PubMed] [Google Scholar]
- 76.Rigolli M, Anandabaskaran S, Christiansen JP, et al. Bias associated with left ventricular quantification by multimodality imaging: a systematic review and meta-analysis. Open Heart 2016;3:e000388. 10.1136/openhrt-2015-000388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chirinos JA, Akers SR, Schelbert E, et al. Arterial properties as determinants of left ventricular mass and fibrosis in severe aortic stenosis: findings from ACRIN PA 4008. J Am Heart Assoc 2019;8:e010271. 10.1161/JAHA.118.010271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Elhawaz A, Archer GT, Zafar H, et al. Left ventricular blood flow kinetic energy is associated with the six-minute walk test and left ventricular remodelling post valvular intervention in aortic stenosis. Quant Imaging Med Surg 2021;11:1470–82. 10.21037/qims-20-586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kim RJ, Fieno DS, Parrish TB, et al. Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation 1999;100:1992–2002. 10.1161/01.cir.100.19.1992 [DOI] [PubMed] [Google Scholar]
- 80.Chin CWL, Everett RJ, Kwiecinski J, et al. Myocardial fibrosis and cardiac decompensation in aortic stenosis. JACC Cardiovasc Imaging 2017;10:1320–33. 10.1016/j.jcmg.2016.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dweck MR, Joshi S, Murigu T, et al. Midwall fibrosis is an independent predictor of mortality in patients with aortic stenosis. J Am Coll Cardiol 2011;58:1271–9. 10.1016/j.jacc.2011.03.064 [DOI] [PubMed] [Google Scholar]
- 82.Vassiliou VS, Perperoglou A, Raphael CE, et al. Midwall fibrosis and 5-year outcome in moderate and severe aortic stenosis. J Am Coll Cardiol 2017;69:1755–6. 10.1016/j.jacc.2017.01.034 [DOI] [PubMed] [Google Scholar]
- 83.Balciunaite G, Skorniakov V, Rimkus A, et al. Prevalence and prognostic value of late gadolinium enhancement on CMR in aortic stenosis: meta-analysis. Eur Radiol 2020;30:640–51. 10.1007/s00330-019-06386-3 [DOI] [PubMed] [Google Scholar]
- 84.Everett RJ, Stirrat CG, Semple SIR, et al. Assessment of myocardial fibrosis with T1 mapping MRI. Clin Radiol 2016;71:768–78. 10.1016/j.crad.2016.02.013 [DOI] [PubMed] [Google Scholar]
- 85.Bing R, Dweck MR. Myocardial fibrosis: why image, how to image and clinical implications. Heart 2019;105:1832–40. 10.1136/heartjnl-2019-315560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kramer CM, Barkhausen J, Bucciarelli-Ducci C, et al. Standardized cardiovascular magnetic resonance imaging (CMR) protocols: 2020 update. J Cardiovasc Magn Reson 2020;22:17. 10.1186/s12968-020-00607-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Iannuzzo G, Tripaldella M, Mallardo V, et al. Lipoprotein(a) where do we stand? from the physiopathology to innovative terapy. Biomedicines 2021;9:838. 10.3390/biomedicines9070838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Donato M, Ferri N, Lupo MG, et al. Current evidence and future perspectives on pharmacological treatment of calcific aortic valve stenosis. Int J Mol Sci 2020;21:8263. 10.3390/ijms21218263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cegla J, Neely RDG, France M, et al. HEART UK consensus statement on lipoprotein(a): a call to action. Atherosclerosis 2019;291:62–70. 10.1016/j.atherosclerosis.2019.10.011 [DOI] [PubMed] [Google Scholar]
- 90.Bhatia N, Basra SS, Skolnick AH, et al. Aortic valve disease in the older adult. J Geriatr Cardiol JGC 2016;13:941–4. 10.11909/j.issn.1671-5411.2016.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Khan T. Lipoprotein(a): mechanisms of pathogenicity. Br J Cardiol 2022;29:S7–10. 10.5837/bjc.2022.s02 [DOI] [Google Scholar]
- 92.Di Fusco SA, Arca M, Scicchitano P, et al. Lipoprotein (a): a risk factor for atherosclerosis and an emerging therapeutic target. Heart 2022;109:18–25. 10.1136/heartjnl-2021-320708 [DOI] [PubMed] [Google Scholar]
- 93.Bergmark BA, O’Donoghue ML, Murphy SA, et al. An exploratory analysis of proprotein convertase subtilisin/kexin type 9 inhibition and aortic stenosis in the Fourier trial. JAMA Cardiol 2020;5:709–13. 10.1001/jamacardio.2020.0728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tsimikas S, Fazio S, Ferdinand KC, et al. NHLBI working group recommendations to reduce lipoprotein(a)-mediated risk of cardiovascular disease and aortic stenosis. J Am Coll Cardiol 2018;71:177–92. 10.1016/j.jacc.2017.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Capoulade R, Chan KL, Yeang C, et al. Oxidized phospholipids, lipoprotein(a), and progression of calcific aortic valve stenosis. J Am Coll Cardiol 2015;66:1236–46. 10.1016/j.jacc.2015.07.020 [DOI] [PubMed] [Google Scholar]
- 96.Marcovina SM, Shapiro MD. Measurement of lipoprotein(a). J Am Coll Cardiol 2022;79:629–31. 10.1016/j.jacc.2021.11.053 [DOI] [PubMed] [Google Scholar]
- 97.Dermot R, Neely G. Lipoprotein (a): a historical perspective. Br J Cardiol 2022:S3–6. 10.5837/bjc.2022.s01 [DOI] [Google Scholar]
- 98.Boot C. How to measure lipoprotein (a) and in whom. Br J Cardiol 2022:S15–9. 10.5837/bjc.2022.s04 [DOI] [Google Scholar]
- 99.Capoulade R, Yeang C, Chan KL, et al. Association of mild to moderate aortic valve stenosis progression with higher lipoprotein (a) and oxidized phospholipid levels: secondary analysis of a randomized clinical trial. JAMA Cardiol 2018;3:1212–7. 10.1001/jamacardio.2018.3798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Page MM, Watts GF. PCSK9 inhibitors-mechanisms of action. Aust Prescr 2016;39:164–7. 10.18773/austprescr.2016.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Viney NJ, van Capelleveen JC, Geary RS, et al. Antisense oligonucleotides targeting apolipoprotein (a) in people with raised lipoprotein (a): two randomised, double-blind, placebo-controlled, dose-ranging trials. Lancet 2016;388:2239–53. 10.1016/S0140-6736(16)31009-1 [DOI] [PubMed] [Google Scholar]
- 102.Qamar A, Giugliano RP, Keech AC, et al. Interindividual variation in low-density lipoprotein cholesterol level reduction with evolocumab: an analysis of Fourier trial data. JAMA Cardiol 2019;4:59–63. 10.1001/jamacardio.2018.4178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kim H-S. Proprotein convertase subtilisin kexin type 9 inhibitor in aortic stenosis. 2017. Available: https://clinicaltrials.gov/ct2/show/NCT03051360 [Accessed 19 May 2022].
- 104.Tsimikas S. Potential causality and emerging medical therapies for lipoprotein (a) and its associated oxidized phospholipids in calcific aortic valve stenosis. Circ Res 2019;124:405–15. 10.1161/CIRCRESAHA.118.313864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rossebø AB, Pedersen TR, Boman K, et al. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med 2008;359:1343–56. 10.1056/NEJMoa0804602 [DOI] [PubMed] [Google Scholar]
- 106.Chan KL, Teo K, Dumesnil JG, et al. Effect of lipid lowering with rosuvastatin on progression of aortic stenosis: results of the aortic stenosis progression observation: measuring effects of rosuvastatin (ASTRONOMER) trial. Circulation 2010;121:306–14. 10.1161/CIRCULATIONAHA.109.900027 [DOI] [PubMed] [Google Scholar]
- 107.Yang W, Cegla J. Current management of the patient with high lipoprotein(a). Br J Cardiol 2022;29:S20–3. 10.5837/bjc.2022.s05 [DOI] [Google Scholar]
- 108.Thomas T, Zhou H, Karmally W, et al. CETP (cholesteryl ester transfer protein) inhibition with anacetrapib decreases production of lipoprotein(a) in mildly hypercholesterolemic subjects. Arterioscler Thromb Vasc Biol 2017;37:1770–5. 10.1161/ATVBAHA.117.309549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cannon CP, Shah S, Dansky HM, et al. Safety of anacetrapib in patients with or at high risk for coronary heart disease. N Engl J Med 2010;363:2406–15. 10.1056/NEJMoa1009744 [DOI] [PubMed] [Google Scholar]
- 110.Nicholls SJ, Ruotolo G, Brewer HB, et al. Evacetrapib alone or in combination with statins lowers lipoprotein(a) and total and small LDL particle concentrations in mildly hypercholesterolemic patients. J Clin Lipidol 2016;10:519–27. 10.1016/j.jacl.2015.11.014 [DOI] [PubMed] [Google Scholar]
- 111.Bowman L, Hopewell JC, Chen F, et al. Effects of anacetrapib in patients with atherosclerotic vascular disease. N Engl J Med 2017;377:1217–27. 10.1056/NEJMoa1706444 [DOI] [PubMed] [Google Scholar]
- 112.Barter PJ, Caulfield M, Eriksson M, et al. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med 2007;357:2109–22. 10.1056/NEJMoa0706628 [DOI] [PubMed] [Google Scholar]
- 113.Thiago L, Tsuji SR, Nyong J, et al. Statins for aortic valve stenosis. Cochrane Database Syst Rev 2016;9:CD009571. 10.1002/14651858.CD009571.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Greve AM, Bang CN, Boman K, et al. Relation of lipid-lowering therapy to need for aortic valve replacement in patients with asymptomatic mild to moderate aortic stenosis. Am J Cardiol 2019;124:1736–40. 10.1016/j.amjcard.2019.08.037 [DOI] [PubMed] [Google Scholar]
- 115.Kamanna VS, Kashyap ML. Mechanism of action of niacin. Am J Cardiol 2008;101:20B–26B. 10.1016/j.amjcard.2008.02.029 [DOI] [PubMed] [Google Scholar]
- 116.Sahebkar A, Reiner Ž, Simental-Mendía LE, et al. Effect of extended-release niacin on plasma lipoprotein (a) levels: a systematic review and meta-analysis of randomized placebo-controlled trials. Metabolism 2016;65:1664–78. 10.1016/j.metabol.2016.08.007 [DOI] [PubMed] [Google Scholar]
- 117.Thanassoulis G. A pilot randomized controlled-trial of lipoprotein(A) lowering for the prevention of aortic valve disease-translating genomic knowledge for cardiovascular prevention. 2016. Available: https://clinicaltrials.gov/ct2/show/NCT02109614 [Accessed 19 May 2022].
- 118.Diederichsen ACP, Lindholt JS, Möller S, et al. Vitamin K2 and D in patients with aortic valve calcification: a randomized double-blinded clinical trial. Circulation 2022;145:1387–97. 10.1161/CIRCULATIONAHA.121.057008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Diederichsen A. The effects of menaquinone-7 supplementation in patients with aortic valves calcification. 2021. Available: https://clinicaltrials.gov/ct2/show/NCT03243890 [Accessed 19 May 2022]. [DOI] [PMC free article] [PubMed]
- 120.Maastricht University Medical Center . Bicuspid aortic valve stenosis and the effect of vitamin K2 on calciummetabolism on 18F-naf PET/MRI (BASIK2): a pilot study. 2018. Available: https://clinicaltrials.gov/ct2/show/NCT02917525 [Accessed 19 May 2022].
- 121.Persy V, D’Haese P. Vascular calcification and bone disease: the calcification paradox. Trends Mol Med 2009;15:405–16. 10.1016/j.molmed.2009.07.001 [DOI] [PubMed] [Google Scholar]
- 122.Aksoy O, Cam A, Goel SS, et al. Do bisphosphonates slow the progression of aortic stenosis? J Am Coll Cardiol 2012;59:1452–9. 10.1016/j.jacc.2012.01.024 [DOI] [PubMed] [Google Scholar]
- 123.Dweck MR, Newby DE. Osteoporosis is a major confounder in observational studies investigating bisphosphonate therapy in aortic stenosis. J Am Coll Cardiol 2012;60:1027. 10.1016/j.jacc.2012.04.048 [DOI] [PubMed] [Google Scholar]
- 124.Pawade TA, Doris MK, Bing R, et al. Effect of denosumab or alendronic acid on the progression of aortic stenosis. Circulation 2021;143:2418–27. 10.1161/CIRCULATIONAHA.121.053708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wypasek E, Natorska J, Mazur P, et al. Effects of rivaroxaban and dabigatran on local expression of coagulation and inflammatory factors within human aortic stenotic valves. Vascul Pharmacol 2020;130:106679. 10.1016/j.vph.2020.106679 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data are available.