Abstract
Background
To determine the efficacy and toxicity of nivolumab monotherapy in treatment-naïve patients with non-clear cell renal cell carcinoma (nccRCC) and the efficacy of nivolumab/ipilimumab salvage therapy in patients with tumors unresponsive to initial nivolumab monotherapy.
Methods
Eligible patients with treatment-naïve nccRCC received nivolumab until progressive disease (PD), toxicity, or completion of 96 weeks of treatment (Part A). Patients with PD prior to, or stable disease (SD) at 48 weeks (prolonged SD) were potentially eligible to receive salvage nivolumab/ipilimumab (Part B). Patients were required to submit tissue from a metastatic lesion obtained within 12 months prior to study entry and prior to Part B for correlative studies.
Results
35 patients with nccRCC were enrolled: 19 (54%) had papillary, 6 (17%) had chromophobe and 10 (29%) had unclassified histology. At median follow-up of 22.9 months, RECIST-defined objective response rate (ORR) was 5 of 35 (14.3% 95% CI 4.8% to 30.3%) (complete response (CR) 2 (5.7%) and partial response (PR) 3 (8.6%)). ORR by histology was: papillary—1/19 (5%); chromophobe—1/6 (17%); and unclassified—3/10 (30%). Nine patients (26%) had tumors with sarcomatoid features with 3 (33%) (2 unclassified and 1 papillary) responding. ORR was 0/18, 3/11 (27%) and 2/6 (33%) for patients with tumor progammed death ligand 1 (PD-L1) expression of <5%, ≥5% or not measured, respectively. Median progression-free survival was 4.0 (2.7–4.3) months. Two of five responders have progressed. Thirty-two patients had PD or prolonged SD and therefore, were potentially eligible for salvage nivolumab/ipilimumab (Part B), but 15 patients did not enroll due to grade 2–3 toxicity (6) on nivolumab, symptomatic disease progression (5), or other reasons including no biopsy tissue (4). In the 17 Part B patients, there was one PR (6%) (unclassified/non-sarcomatoid). Grade >3 treatment-related adverse events were seen in 7/35 (20%) on nivolumab and 7/17 (41%) on salvage nivolumab/ipilimumab with one patient experiencing sudden death.
Conclusions
Nivolumab monotherapy has limited activity in treatment-naïve nccRCC with most responses (4 of 5) seen in patients with sarcomatoid and/or unclassified tumors. Toxicity is consistent with prior nivolumab studies. Salvage treatment with nivolumab/ipilimumab was provided in half of these patients with minimal activity.
Trial registration number
Keywords: clinical trials, phase II as topic; immunotherapy; kidney neoplasms
WHAT IS ALREADY KNOWN ON THIS TOPIC
At the time this study was initiated, no clinical trial had examined the activity of nivolumab monotherapy, or the role of tumor programmed death ligand 1 (PD-L1) expression in predicting efficacy, in treatment-naïve patients with non-clear cell renal cell carcinoma (nccRCC), or the value of combination nivolumab/ipilimumab salvage in patients not responding to upfront nivolumab.
WHAT THIS STUDY ADDS
Nivolumab monotherapy has limited activity in treatment-naïve nccRCC with most responses occurring in patients with sarcomatoid and/or unclassified tumors and no responses occurring in patients with tumor PD-L1 expression <5%. Salvage nivolumab/ipilimumab had minimal activity in patients not responding to first-line nivolumab.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
New treatment options are needed for patients with nccRCC, particularly those with non-sarcomatoid papillary or chromophobe histology or with low tumor PD-L1 staining. Additional correlative biomarker studies to be performed on tumor biopsies obtained as part of this study may further characterize mechanisms of resistance and identify alternative therapeutic targets.
Introduction
Nivolumab monotherapy received US Food and Drug Administration (FDA) approval in 2015 for treatment of patients with vascular endothelial growth factor receptor tyrosine kinase inhibitor-resistant clear cell renal cell carcinoma (ccRCC) based on the results of the CheckMate 025 study.1 In this study, nivolumab demonstrated a statistically significant improvement in overall survival (OS) compared with everolimus, with a median survival of 25.0 versus 19.6 months (HR 0.73, p<0.002) and confirmed objective response rates (ORR) of 21.5% versus 3.9%, respectively. These benefits were sustained at 5 years.2
Combination nivolumab/ipilimumab received FDA approval in 2018 for patients with treatment-naïve International Metastatic RCC Database Consortium (IMDC) intermediate-risk and poor-risk advanced ccRCC, based on the results of the CheckMate 214 study comparing the combination to sunitinib.3 In this study nivolumab/ipilimumab showed improved median progression-free survival (PFS), ORR and median OS relative to sunitinib in patients with IMDC intermediate-risk and poor-risk ccRCC. This benefit has been maintained out to 67.7 months median follow-up4: HR for OS of 0.68; (0.58 to 0.81). In addition, 5-year PFS was 31% for the combination versus 11% for sunitinib reflecting the durability of benefit seen with nivolumab/ipilimumab. Similar benefits were also seen in the intent-to-treat population.4
HCRN GU16-260 Cohort A explored the efficacy and toxicity of nivolumab monotherapy with nivolumab/ipilimumab salvage in patients with metastatic ccRCC showing a 34.6% ORR within the first 48 weeks of treatment, including 57% ORR in IMDC favorable-risk patients, and a positive association of ORR and 1-year PFS with increasing tumor programmed death ligand 1 (PD-L1) expression.5 Salvage nivolumab/ipilimumab showed 11.4% ORR in patients with tumors that failed to respond to upfront nivolumab.5
Despite several approvals of anti-PD-1-based immunotherapies for patients with ccRCC, little progress has been made with immunotherapy in patients with non(n)-ccRCC. National Comprehensive Cancer Network (NCCN) guidelines list cabozantinib and sunitinib as ‘preferred regimens’ in this patient population with lenvatinib+everolimus and nivolumab and pembrolizumab listed as ‘other recommended regimens’. (NCCN CPG for Kidney Cancer V.4.2022). Similarly, the European Society For Medical Oncology (ESMO) guidelines list sunitinib and pazopanib as ‘standard therapies’ with everolimus and cabozantinib as options.6 A retrospective analysis of nivolumab monotherapy in patients with nccRCC showed an ORR of 20% (7 of 35 evaluable patients) with responses seen in all histologic subtypes and 5 of 7 responses ongoing.7 While Keynote-427 examined the role of pembrolizumab in nccRCC,8 no clinical trial has examined the activity of nivolumab monotherapy, or the role of tumor PD-L1 expression in predicting efficacy, in treatment-naïve patients with nccRCC or the value of nivolumab/ipilimumab salvage in patients not responding to upfront nivolumab. Given this limited data, the HCRN GU16-260 trial Cohort B was launched to prospectively fill these knowledge gaps.
Methods
Study design
HCRN-GU16-260 was a single-arm, open-label, non-randomized, multicenter (13 sites), phase 2 trial. The study was conducted in accordance with International Conference on Harmonization guidelines for Good Clinical Practice and the principles of the Declaration of Helsinki. The protocol was approved by the institutional review board for each participating institution. All patients provided written informed consent.
Treatment
Treatment schema is described in online supplemental figure 1. In Part A, patients received nivolumab monotherapy for up to 96 weeks of total treatment. If patients experienced disease progression (PD) at any time or had a best response of stable disease (SD) at 48 weeks of treatment (prolonged SD), they were potentially eligible for Part B, which involved the combination of nivolumab/ipilimumab for 12 weeks followed by nivolumab monotherapy for up to 48 weeks. Imaging was performed at baseline and then every 12 weeks. Tumor responses were investigator-assessed and confirmation of response was required on subsequent imaging obtained at least 4 weeks after the initial measurement. Patients with asymptomatic disease progression could continue therapy until a repeat scan six or more weeks later confirmed disease progression.
jitc-2022-004780supp001.pdf (45.2KB, pdf)
For Part A, patients were enrolled into either Cohort A (ccRCC) or Cohort B (nccRCC). This manuscript describes only those patients enrolled in Cohort B who must have had advanced nccRCC with either papillary, chromophobe or unclassified histology. Pathology reports for each enrolled patient were reviewed retrospectively by study leadership and when histology was ambiguous, pathology specimens were re-reviewed by local pathologists to ensure both study eligibility and assignment of the patient to the appropriate cohort. Patients were required to have at least one RECIST V.1.1-defined measurable site of disease that had not been previously irradiated, or exhibited evidence of progression since the radiation. Patients were required to have Eastern Cooperative Oncology Group (ECOG) performance status 0–2, age ≥18 years, adequate organ and bone marrow function within 14 days prior to study entry, and a tumor biopsy (core or excisional) of a metastatic lesion obtained within 1-year prior to study registration.
Patients were excluded if they had major surgery or radiation therapy within 14 days of starting study treatment, active autoimmune disease, a concurrent medical condition requiring use of systemic corticosteroids with prednisone >10 mg per day, uncontrolled brain metastases, or prior systemic therapy for Stage IV RCC.
For enrollment into Part B, patients must have met the eligibility for Part A, must not have experienced a grade >3 immune-related adverse event (irAE) on nivolumab, excepting an endocrine irAE managed with hormone replacement therapy, and were required to have a repeat tumor biopsy establishing the existence of residual and/or PD. Patients with symptomatic disease progression on nivolumab for whom the investigator, in consultation with the patient, felt an alternative therapy was more appropriate were also not enrolled in Part B.
Tissue analysis
Analysis of PD-L1 expression was performed on formalin-fixed paraffin-embedded tumor tissue sections from metastatic lesions or primary tumors (if sufficient metastatic tissue was unavailable). The tumor sections were stained with a validated anti-PD-L1 antibody (1:100; E1L3N rabbit monoclonal antibody; Cell Signaling Technology, according to a standard protocol),9 10 using a Bond RX Autostainer (Leica Biosystems) and a Polymer Refine Detection kit (DS9800; Leica Biosystems). Antigen retrieval was carried out using the Bond Epitope Retrieval Solution 2 (EDTA, pH=9.0) for 30 min. Slides were counterstained with hematoxylin, dehydrated in graded ethanol and xylene, and cover-slipped. The percentage of PD-L1 positive tumor cells was independently scored by three pathologists who were blinded to clinical outcomes. Discrepancies in scores were resolved by consensus review. For patients with PD-L1 measures from both metastatic and primary lesions, preference was given to the metastatic lesion score. For statistical analysis, PD-L1 scores were categorized as either 0%, 1–5%, >5%–20% or >20% to study for association with clinical outcomes. The baseline PD-L1 score was used for analysis of efficacy in Part B.
Statistical analysis
The planned number of eligible and treated patients to be enrolled was 160 with up to 40 in Cohort B (nccRCC).
Part A
The primary objective of Cohort B, Part A of this trial was to use investigator-assessed RECIST V.1.1 measurements to determine the ORR (CR or PR) for front-line nivolumab monotherapy for all enrolled subjects. With 40 patients, the 95% CI on the true objective response proportion will be no wider than 32.4% points and the probability of observing five or more responses under the hypothesis that the true response rate is 0.20 was at least 92%; hence there was a high power to detect a true response rate of interest in this cohort. Other endpoints of interest in this group of patients included rates of SD and PFS at 1-year, ORR based on histologic type, presence of a sarcomatoid component or tumor PD-L1 expression as well as ORR by immune-related (ir)RECIST, duration of response, median PFS and safety of nivolumab monotherapy in the front-line setting.
Part B
Patients who exhibited a best response of SD at 48 weeks or who experienced disease progression were considered for Part B. The primary endpoint for Part B was ORR using tumor measurements at the start of Part B as baseline. It was expected that roughly one-half of the Part A patients would enroll in Part B. If no responses were seen in the first 14 patients, then the response rate was deemed to be uninteresting. If any confirmed responses were seen, accrual was to continue for all eligible patients completing Part A.
Results
Patient characteristics
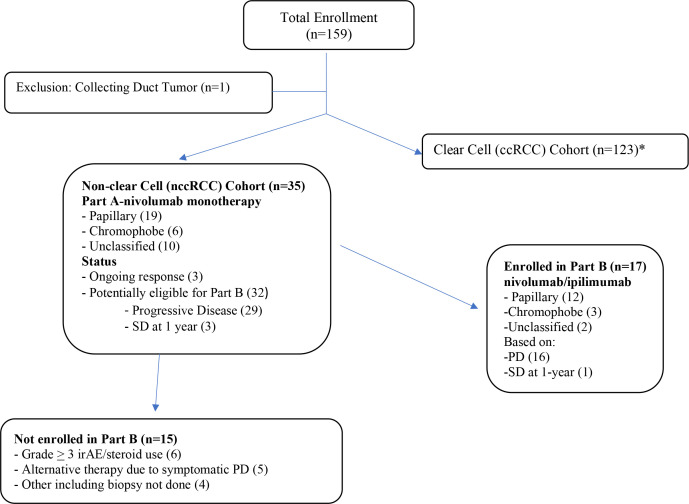
The disposition of subjects is shown in the Consolidated Standards of Reporting Trials diagram (figure 1). Thirty-five subjects with nccRCC were enrolled from May 2017 until December 2019. At the time of data lock (April 07, 2021) and at a median (range) follow-up of 22.9 (1.9–38.2) months, 17 subjects had gone onto Part B. Of the 18 other subjects: three were still in response and all 18 were off protocol therapy.
Figure 1.
Consolidated Standards of Reporting Trials diagram. *Published separately. irAE, immune-related adverse event; PD, progressive disease; RCC, renal cell carcinoma; SD, stable disease.
Thirty-two patients were potentially eligible for salvage nivolumab/ipilimumab (Part B), but 15 did not enroll due to symptomatic PD (5), grade 3–4 toxicity on nivolumab (6), or other reasons (including no biopsy tissue) (4).
Demographics are displayed in table 1. Median age 63 (range 35–84 years); 89% men. IMDC favorable 8 (23%), intermediate 18 (51%) and poor-risk 9 (26%). Nineteen (54%) had papillary, 6 (17%) chromophobe and 10 (29%) unclassified histology. Tumor PD-L1 expression was <5% in 18 (51%), ≥5% in 11 (31%) and not available for 6 patients (17%).
Table 1.
Patientdemographic and baseline characteristics
| Characteristic | N=35 |
| Age, median (range), years | 63 (35–84) |
| ECOG PS (0, 1, 2) | 16 (46%), 17 (49%), 2 (6%) |
| Male, n (%) | 31 (89) |
| Histology, n (%) | |
| Papillary | 19 (54) |
| Chromophobe | 6 (17) |
| Unclassified | 10 (29) |
| IMDC risk category, n (%) | |
| Favorable | 8 (23) |
| Intermediate | 18 (51) |
| Poor | 9 (26) |
| Sarcomatoid features | 9 (26) |
| PD-L1 status—missing | 6 (17) |
| %+ (0, 1–5, 5–20,>20) | 14 (40), 4 (11), 3 (9), 8 (23) |
ECOG, Eastern Cooperative Oncology Group; IMDC, International Metastatic RCC Database Consortium; PD-L1, programmed death ligand 1; PS, performance status.
Efficacy and safety results-nivolumab monotherapy: part A
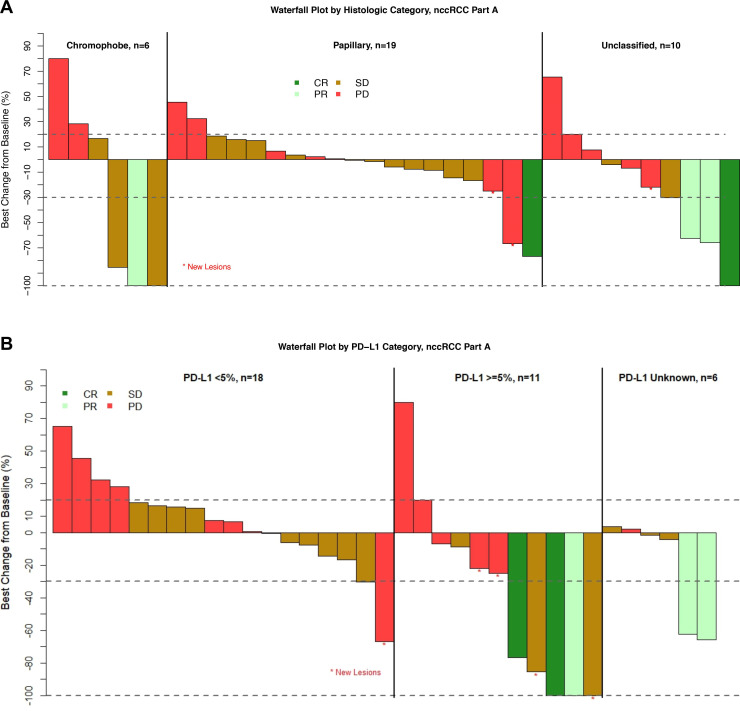
Response data by histology for Part A are shown in table 2. There were five responses observed in 35 subjects for a RECIST ORR of 14.3% (95% CI 4.8% to 30.3%) with two CRs. The immune-related ORR was 22.9% (95% CI 10.4% to 40.1%). Response rates for patients with papillary, chromophobe and unclassified tumors were 5.3%, 17% and 30%, respectively. For those with sarcomatoid tumors ORR was 3/9 (33%) with two patients with unclassified and one patient with a papillary tumor responding. There were no responses in the 18 patients with PD-L1 expression <5%, while 27% of patients with PD-L1 >5% and 33% of patients with missing PD-L1 status responded. All responses were initially evident at the initial (12-week) CT scan and confirmed at the 18-week scan. Median duration of response was 20.3 months (range 17.8–NA) (online supplemental figure 2) with three responses ongoing at the time of data lock (online supplemental figure 3).
Table 2.
Objective response rate (ORR) and ir(ORR) by histology and Programmed Death Ligand 1 (PD-L1) categories
| Best response N (%) (95% CI) |
Histologic type (N) | Total (N=35) N (%) |
||
| Papillary (19) | Chromophobe (6) | Unclassified (10) | ||
| CR | 1 (5.3)* | – | 1 (10) | 2 (5.7) |
| PR | – | 1 (16.7) | 2 (20)* | 3 (8.6) |
| SD | 11 (57.9) | 3 (50) | 2 (20) | 16 (45.7) |
| PD | 7 (36.8) | 2 (33.3) | 5 (50) | 14 (40) |
| ORR | 1 (5.3) | 1 (17) | 3 (30) |
5 (14.3) (4.8 to 30.3) |
| irORR | 1 (5.3) | 3 (50) | 4 (40) | 8 (22.9) (10.4 to 40.1) |
| PD-L1 >5% (N=11) | 1/3 | 1/4 | 1/4 | 3/11 (27.2) |
| PD-L1 <5% (N=18) | 0/13 | 0/2 | 0/3 | 0/18 (0) |
| PD-L1 NA (N=6) | 0/3 | – | 2/3 | 2/6 (33) |
*Sarcomatoid histology.
CR, complete response; irORR, immune-related ORR; NA, not available or missing; PD, progressive disease; PR, partial response; SD, stable disease.
jitc-2022-004780supp002.pdf (12.3KB, pdf)
jitc-2022-004780supp003.pdf (14KB, pdf)
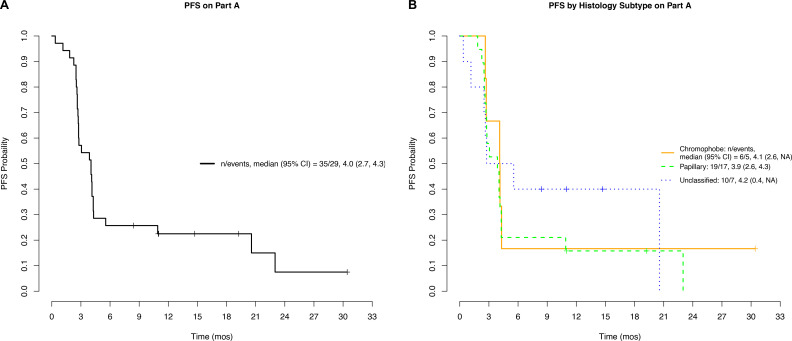
Figure 2 displays a waterfall plot of maximum depth of response in target lesions separated by histology and PD-L1 status. Several patients exhibited tumor shrinkage without having a confirmed response. Median (range) PFS was 4.0 (0.4–30.4) months (figure 3A) and similar across all histologic types (figure 3B). Grade ≥3 treatment-related adverse events were seen in 7/35 (20%) on nivolumab monotherapy (table 3).
Figure 2.
Waterfall plots of maximum tumor shrinkage by histology (A) and PD-L1 status (B). CR, complete response; nccRCC, non-clear cell renal cell carcinoma; PD, progressive disease; PR, partial response; SD, stable disease; PD-L1, programmed death ligand 1.
Figure 3.
Progression free survival (PFS) for Part A: overall (A); by histology (B). mos, months.
Table 3.
Incidence of treatment-related adverse events—Parts A and B
| Part A—N=35 | Part B—N=17 | |||
| Category | Any grade (>2 patients) n (%) |
Grade 3 n (%) |
Any grade (>2 Patients) n (%) |
Grade 3* n (%) |
| Rash | 15 (43) | 2 (6)† | 5 (29) | 1 (6) |
| Fatigue | 14 (40) | 0 (–) | 6 (35) | 0 (–) |
| Pruritus | 6 (17) | 0 (–) | 2 (12) | 1 (6) |
| Diarrhea | 4 (11)† | 0 (–) | 2 (12) | 1 (6) |
| Fever | 3 (9) | 0 (–) | 2 (12) | 0 (–) |
| Amylase increased | 3 (9) | 1 (6) | – | – |
| Alk Phos | 3 (9) | 0 (–) | – | – |
| Arthralgias/arthritis | 2 (6) | 0 (–) | – | – |
| Lipase increased | 2 (6) | 1 (6) | 2 (12) | 0 (–) |
| Creatinine increased | 2 (6) | 1 (3) | – | – |
| Dyspnea | 2 (6) | 0 (–) | – | – |
| Myalgia | 2 (6) | 0 (–) | – | – |
| Dry Mouth | 2 (6) | 0 (–) | – | – |
| Hyperuricemia | 2 (6) | 1 (1) | – | – |
| Hypothyroidism | – | – | 4 (24) | 0 (–) |
| AST | – | – | 2 (12) | 1 (6) |
| Weight Loss | – | – | 2 (12) | 0 (–) |
| Anorexia | – | – | 2 (12) | 0 (–) |
| Headache | – | – | 2 (12) | 1 (–) |
| Total | 29 (82) | 7 (20) | 14 (82) | 7 (41) |
*Grade 5 sudden death ×1.
†Prevented enrollment in Part B.
Alk Phos, alkaline phosphatase; AST, aspartate aminotransferase.
Efficacy and safety results-salvage nivolumab/ipilimumab therapy: part B
One of the first 14 patients responded to salvage nivolumab and ipilimumab enabling this arm to proceed to full enrollment which turned out to be 17 subjects. This patient was the only responder for an ORR of 1/17 (6%). The lone responder had unclassified/non-sarcomatoid RCC and tumor PD-L1 expression of 1–5%. Median (range) PFS was 2.8 (0.03–18.9) months. Toxicity was seen in 7 of 17 (41.2%) patients with one patient experiencing sudden death on treatment of unclear etiology.
Discussion
In this trial, nivolumab monotherapy had limited efficacy in patients with nccRCC with an ORR of 14.3%. Most responses (4 of 5) were seen in patients with sarcomatoid and/or unclassified tumors. The approximately 30% response rates in patients with unclassified or sarcomatoid tumors is consistent with what has been seen with single-agent pembrolizumab11 and the combination atezolizumab+bevacizumab study.12 These data call into question the underlying biology of unclassified tumors and also suggest that tumors with sarcomatoid differentiation are more immunogenic regardless of their underlying histology.13
We observed only one RECIST-defined response in six patients with chromophobe histology. While two additional patients with chromophobe histology had responses by irRECIST, they were not durable. This efficacy is similar to the 10% ORR reported with pembrolizumab and combination atezolizumab+bevacizumab in the chromophobe population.11 12 This suggests that only rare individuals with chromophobe histology will respond well to anti-PD-(L)1-based therapy and that further efforts to identify the unique features of these rare responders might be worthwhile.
Notably, only 1 of 19 patients (5.3%) with papillary histology responded to nivolumab in this trial and this one responder had a tumor with sarcomatoid features. This appeared to be distinct from what was reported for pembrolizumab in the Keynote-427 trial where 34 of 118 patients (28.8%—95% CI 20.8% to 37.9%) with papillary RCC responded including seven CRs.11 Whether this difference is due to chance and the relatively small number of patients on our trial or actually represents a distinction in the function of pembrolizumab compared with nivolumab in this population is uncertain. Of concern, in the CheckMate 374 study involving nivolumab monotherapy for patients with nccRCC with 0–3 prior treatments, only 2 of 24 (8.3%) patients with papillary histology responded.14 If this difference is indeed real, the mechanism by which pembrolizumab is selectively more active than nivolumab in patients with papillary RCC, despite having comparable antitumor efficacy for clear cell, unclassified and chromophobe RCC subsets of their respective studies,5 11 15 needs to be identified. Perhaps the ongoing correlative studies will help to explain these differences.
Tumor PD-L1 expression (≥5%) was observed in 11 of 29 patients (38%) tested. This rate is numerically higher than the 26% noted in the ccRCC cohort of this study using the same assay. As with the ccRCC cohort, ORR appeared to correlate with PD-L1 expression with 27% of patients with PD-L1 >5% responding in the nccRCC cohort. However, while 28 of 91 patients (31%) with ccRCC and tumor PD-L1 expression <5% responded,5 there were no responses in patients with nccRCC and tumor PD-L1 <5%. This data suggests that PD-L1 expression may be more useful, particularly as a negative predictive biomarker, for nivolumab monotherapy in patients with nccRCC than those with ccRCC. This observation is supported by CheckMate 920 study involving combination nivolumab/ipilimumab in patients with nccRCC where the ORR was 14.3% in the 21 patients with PD-L1-negative tumors and 30.8% in the 13 patients with tumor PD-L1 expression >1%.16 However, given the small number of patients with PD-L1 assessment and the mixed nccRCC histologies in this trial, further study is necessary before assessing the relative value of tumor PD-L1 expression as a predictive biomarker in patients with nccRCC vs ccRCC.
In this trial, nivolumab/ipilimumab boost was provided to only 17/30 non-responders and offered limited ability to salvage them (6% ORR). While this is disappointing, it is not dramatically different from the ccRCC population (Cohort A) of this study where only 43% (35 of 81) of non-responding patients enrolled in Part B and an 11.4% ORR to salvage nivolumab/ipilimumab was seen.5 While omitting the biopsy requirement for enrollment in Part B or providing nivolumab/ipilimumab salvage earlier in patients before nivolumab monotherapy toxicity or symptomatic progression was observed might have increased this number it is unlikely to have changed the overall results.
For example, similar limited efficacy for nivolumab/ipilimumab salvage in patients with ccRCC was noted in the OMNIVORE and TITAN-RCC trials, despite the fact the addition of ipilimumab was provided earlier and biopsy requirement was omitted.17 18 In OMNIVORE, nivolumab/ipilimumab salvage was provided to patients with PD or with SD at 6 months with responses observed in 2 of 57 patients (4%) and in TITAN-RCC it was provided to patients with PD as early as 8 weeks or SD at 16 weeks with ORR of 12%.
Of note, while the median time to response in CheckMate 214 study was 2.8 months (identical to this study) the time to response range in the CheckMate 214 study extended out to 35 months. Therefore, it is conceivable that the design of the study in which patients with SD at 48 weeks were considered for crossover to Part B, might have led to an underestimation of nivolumab monotherapy efficacy; however, the fact that only three patients had SD at 48 weeks and none of these patients responded to nivolumab/ipilimumab salvage therapy mitigates this concern. Other limitations to this study included lack of central pathology or radiology review, the small number of patients accrued and the fact that only half of the patients were enrolled in Part B.
New treatment options are needed for patients with nccRCC, particularly those with non-sarcomatoid papillary or chromophobe histology or with low tumor PD-L1 staining. In addition to the pembrolizumab data noted above, efforts to improve the efficacy of immunotherapy-based regimens in patients with papillary RCC have included combinations of cabozantinib and PD-1-pathway blockers. Both cabozantinib+nivolumab (15 of 32)19 and cabozantinib+atezolizumab (7 of 15) showed ORRs of 47% in this population.20 These data compare favorably to the 24% ORR (10 of 41) seen with cabozantinib alone in patients with papillary RCC in the PAPMET trial21 suggesting that nivolumab activity is similar to atezolizumab in this patient population and potentially at least additive with cabozantinib.
Other approaches currently being explored include the triple combination of cabozantinib+nivolumab + ipilimumab (NCT04413123 and NCT03866382), and the combinations of lenvatinib+pembrolizumab (NCT04704219) and savolitinib+durvalumab (NCT05043090). Hopefully, these mixed combination regimens will produce more efficacy in patients with nccRCC than has been observed with immunotherapy-alone regimens. Additional correlative biomarker studies to be performed on tumor biopsies obtained prior to Part A and Part B treatment may also further characterize mechanisms of resistance and identify alternative therapeutic targets.
jitc-2022-004780supp004.pdf (1.5MB, pdf)
Acknowledgments
We acknowledge the contributions to this effort of the patients and their families; the hard work of the team at the HCRN including Robyn Lillie, the Study Coordinator, and the research coordinators and data managers at the investigational sites.
Footnotes
Twitter: @bilenma, @BraunMDPhD
Presented at: This study was presented as Part of a Poster Discussion Session at the 2021 ASCO (American Society of Clinical Oncology) Virtual Annual Meeting; June 4–8, 2021.
Contributors: MBA and HH conceived of and designed the study with the assistance of DE, SS and CW. MBA, NBH, DFM, MAB, MS, JS, RA, ERP, MCO, MH, DJP, SS, TD, AC, CW, DB and HH collected the data. OAJ, PJC and MBA analyzed the data. MBA wrote the manuscript and is the guarantor. All authors reviewed and revised the manuscript and approved the submission.
Funding: The investigator-initiated study was supported by Bristol-Myers Squibb via a contract with the Hoosier Cancer Research Network (CheckMate-669). Funds for correlative work was provided by a Department of Defense Translational Team Science Grant (KC170216) to MBA and CW, the Dana Farber Harvard Kidney Cancer SPORE Grant (NCI P50CA101942) to DFM, and NCI P30 CA051008 to the Georgetown Lombardi Comprehensive Cancer Center.
Competing interests: MBA, has/had an advisory role for Bristol-Myers Squibb, Merck, Novartis, Eisai, Aveo, Pfizer, Werewolf, Fathom, Pneuma, Leads, Pyxis Oncology, PACT, Elpis, X4Pharma, ValoHealth, ScholarRock, Surface, Takeda, Simcha, Roche, SAB Bio and GSK and has served as a consultant: Bristol-Myers Squibb, Merck, Novartis, Pfizer, Roche, Exelixis, Iovance, COTA, Idera, Agenus, Apexigen, Asher Bio, Neoleukin, AstraZeneca, Calithera, SeaGen, and Sanofi. He reports research support to his institution from Bristol-Myers Squibb, Merck and Pfizer. He holds stock/stock options in Pyxis Oncology, Werewolf and Elpis. NBH has/had an advisory role for Merck, Eisai, Aveo, and Roche. DFM has acted as a paid consultant for and/or as a member of the advisory boards of BMS, Pfizer, Merck, Alkermes, EMD Serono, Eli Lilly and Company, Iovance, Eisai, Werewolf Technologies, Calithera Biosciences, Synthekine, Johnson & Johnson, and Aveo. He has received support to his institution from BMS, Merck, Genentech, Pfizer, Exelixis, X4 Pharma, Alkermes, Checkmate Pharmaceuticals, and CRISPR Therapeutics for work performed outside of the current study. MAB has acted as a paid consultant for and/or as a member of the advisory boards of Exelixis, Bayer, BMS, Eisai, Pfizer, AstraZeneca, Janssen, Calithera Biosciences, Genomic Health, Nektar, EMD Serono, SeaGen, and Sanofi and has received grants to his institution from Merck, Xencor, Bayer, Bristol-Myers Squibb, Genentech/Roche, SeaGen, Incyte, Nektar, AstraZeneca, Tricon Pharmaceuticals, Genome & Company, AAA, Peloton Therapeutics, and Pfizer for work performed outside of the current study. MS has consulted for Exelixis, Xencor, Janssen, Vaccitech, Bristol-Myers Squibb and reports research funding to his institution from Bellicum, Merck, Janssen, Medivation/Astellas, Advaxis, Harpoon, Bristol-Myers Squibb, Genocea, Lilly, Nektar, SeaGen, Xencor, Tmunity, Exelixis. JS serves on Advisory Boards for Eisai, Apexigen, Iovance, Covus. He reports research funding to his institution from Bristol Myers Squibb, PACT and Corvus. RA has consulted for Eisai, Bayer and Janssen Oncology and participated in Speakers' Bureaus for Astellas Pharma, Janssen Oncology, Bayer, Pfizer, Exelixis, Gilead Sciences, Aveo and Bristol-Myers Squibb. ERP reports consulting or advisory roles for Astellas, AstraZeneca, Aveo, Bristol Myers Squibb, Exelixis, Merck, Natera, Pfizer, Regeneron, SeaGen and research support to institution from Astella, Bristol Myers Squibb, Genentech and Merck. MCO has served in consulting or advisory roles for Eisai, Exelixis, Pfizer, Aveo, Merck, and Bristol-Myers Squibb; served on speakers’ bureaus for Bristol Myers Squibb; received institutional research funding from Bristol Myers Squibb, Pfizer, Merck, AstraZeneca, Astellas, Aravive, and Surface Oncology; has received reimbursement for travel and accommodations expenses from Bristol Myers Squibb, Pfizer, and Exelixis. MH has served on Advisory Boards for Bristol Myers Squibb, CRISPR Therapeutics, Exelixis, Nektar Therapeutics, Janssen and has received research support to his institution from Alpine, Achilles Therapeutics, Apexigen, Arrowhead, Astellas, AstraZeneca, Bayer, Bristol Myer Squibb, CRISPR Therapeutics, Corvus, Eli Lilly, Endocyte, Fate Therapeutics, Genentech, Genmab, GSK, Innocrin, Iovance, KSQ, Merck, Nektar Therapeutics, Novartis, Pfizer, Progenics, Sanofi Aventis, SeaGen, Tmunity, Torque, Unum He also reports Spouse salary from Gamida Cell, Arvinas. SS reports receiving commercial research grants from Bristol-Myers Squibb, AstraZeneca, Exelixis and Novartis; is a consultant/advisory board member for Merck, AstraZeneca, Bristol-Myers Squibb, CRISPR Therapeutics AG, AACR, and NCI; and receives royalties from Biogenex. CW reports receiving research funding from Pharmacyclics and being an equity holder of BionTech. DB reports personal fees from LM Education and Exchange, Adnovate Strategies, MDedge, Cancer Network, Cancer Expert Now, OncLive, Catenion, AVEO, and grants and personal fees from Exelixis, outside the submitted work. DE reports research funding to institution from Bristol-Myers Squibb, Cardiff Oncology, and Puma Biotechnology, as well as discounted research sequencing from Foundation Medicine and honorarium from OncLive. HH has acted as a paid consultant for and/or as a member of the advisory boards of Exelixis, BMS, Pfizer, Merck, Corvus, Armo Biosciences, Eisai, Eli Lilly, Surface Oncology, Aveo and Novartis and has received grants to his institution from Merck, Bristol-Myers Squibb, Surface Oncology and Aravive for work performed as outside of the current study.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. The clinical trial was opened prior to January 1, 2019. The final database for the clinical study will be available upon reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by MedStar-Georgetown Cancer IRB IRB# 017-0010. Participants gave informed consent to participate in the study before taking part.
References
- 1.Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med 2015;373:1803–13. 10.1056/NEJMoa1510665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Motzer RJ, Escudier B, George S, et al. Nivolumab versus everolimus in patients with advanced renal cell carcinoma: updated results with long-term follow-up of the randomized, open-label, phase 3 checkmate 025 trial. Cancer 2020;126:4156–67. 10.1002/cncr.33033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med 2018;378:1277–90. 10.1056/NEJMoa1712126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Motzer RJ, McDermott DF, Escudier B, et al. Conditional survival and long-term efficacy with nivolumab plus ipilimumab versus sunitinib in patients with advanced renal cell carcinoma. Cancer 2022;128:2085–97. 10.1002/cncr.34180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atkins MB, Jegede OA, Haas NB, et al. Phase II study of nivolumab and salvage nivolumab/ipilimumab in treatment-naive patients with advanced clear cell renal cell carcinoma (HCRN GU16-260-cohort a). J Clin Oncol 2022;40:2913–23. 10.1200/JCO.21.02938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Escudier B, Porta C, Schmidinger M, et al. Renal cell carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up†. Ann Oncol 2019;30:706–20. 10.1093/annonc/mdz056 [DOI] [PubMed] [Google Scholar]
- 7.Koshkin VS, Barata PC, Zhang T, et al. Clinical activity of nivolumab in patients with non-clear cell renal cell carcinoma. J Immunother Cancer 2018;6:9.:9. 10.1186/s40425-018-0319-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Regan MM, Werner L, Rao S, et al. Treatment-free survival: a novel outcome measure of the effects of immune checkpoint inhibition-a pooled analysis of patients with advanced melanoma. J Clin Oncol 2019;37:3350–8. 10.1200/JCO.19.00345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cogswell J, Inzunza HD, Wu Q, et al. An analytical comparison of dako 28-8 pharmdx assay and an E1L3N laboratory-developed test in the immunohistochemical detection of programmed death-ligand 1. Mol Diagn Ther 2017;21:85–93. 10.1007/s40291-016-0237-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rimm DL, Han G, Taube JM, et al. A prospective, multi-institutional, pathologist-based assessment of 4 immunohistochemistry assays for PD-L1 expression in non-small cell lung cancer. JAMA Oncol 2017;3:1051–8. 10.1001/jamaoncol.2017.0013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McDermott DF, Lee J-L, Ziobro M, et al. Open-Label, single-arm, phase II study of pembrolizumab monotherapy as first-line therapy in patients with advanced non-clear cell renal cell carcinoma. J Clin Oncol 2021;39:1029–39. 10.1200/JCO.20.02365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McGregor BA, McKay RR, Braun DA, et al. Results of a multicenter phase II study of atezolizumab and bevacizumab for patients with metastatic renal cell carcinoma with variant histology and/or sarcomatoid features. J Clin Oncol 2020;38:63–70. 10.1200/JCO.19.01882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rini BI, Motzer RJ, Powles T, et al. Atezolizumab plus bevacizumab versus sunitinib for patients with untreated metastatic renal cell carcinoma and sarcomatoid features: a prespecified subgroup analysis of the immotion151 clinical trial. Eur Urol 2021;79:659–62. 10.1016/j.eururo.2020.06.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vogelzang NJ, Olsen MR, McFarlane JJ, et al. Safety and efficacy of nivolumab in patients with advanced non-clear cell renal cell carcinoma: results from the phase iiib/IV checkmate 374 study. Clin Genitourin Cancer 2020;18:461–8. 10.1016/j.clgc.2020.05.006 [DOI] [PubMed] [Google Scholar]
- 15.McDermott DF, Lee J-L, Bjarnason GA, et al. Open-label, single-arm phase II study of pembrolizumab monotherapy as first-line therapy in patients with advanced clear cell renal cell carcinoma. J Clin Oncol 2021;39:1020–8. 10.1200/JCO.20.02363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tykodi SS, Gordan LN, Alter RS, et al. Nivolumab plus ipilimumab in patients with advanced non-clear cell renal cell carcinoma (nccrcc): safety and efficacy from checkmate 920. JCO 2021;39:309. 10.1200/JCO.2021.39.6_suppl.309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McKay RR, McGregor BA, Xie W, et al. Optimized management of nivolumab and ipilimumab in advanced renal cell carcinoma: a response-based phase II study (OMNIVORE). J Clin Oncol 2020;38:4240–8. 10.1200/JCO.20.02295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grimm M-O, Esteban E, Barthélémy P, et al. Efficacy of nivolumab/ipilimumab in patients with initial or late progression with nivolumab: updated analysis of a tailored approach in advanced renal cell carcinoma (TITAN-RCC). JCO 2021;39:4576. 10.1200/JCO.2021.39.15_suppl.4576 [DOI] [Google Scholar]
- 19.Lee CH, Voss MH, Carlo MI, et al. Nivolumab plus cabozantinib in patients with non-clear cell renal cell carcinoma: results of a phase 2 trial. J Clin Oncol 2021;39:4509. [Google Scholar]
- 20.Pal SK, McGregor B, Suárez C, et al. Cabozantinib in combination with atezolizumab for advanced renal cell carcinoma: results from the COSMIC-021 study. J Clin Oncol 2021;39:3725–36. 10.1200/JCO.21.00939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pal SK, Tangen C, Thompson IM, et al. A comparison of sunitinib with cabozantinib, crizotinib, and savolitinib for treatment of advanced papillary renal cell carcinoma: a randomised, open-label, phase 2 trial. Lancet 2021;397:695–703. 10.1016/S0140-6736(21)00152-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2022-004780supp001.pdf (45.2KB, pdf)
jitc-2022-004780supp002.pdf (12.3KB, pdf)
jitc-2022-004780supp003.pdf (14KB, pdf)
jitc-2022-004780supp004.pdf (1.5MB, pdf)
Data Availability Statement
Data are available upon reasonable request. The clinical trial was opened prior to January 1, 2019. The final database for the clinical study will be available upon reasonable request.