Abstract
Background
The prostate tumor microenvironment (TME) is immunosuppressive, with few effector T cells and enrichment of inhibitory immune populations, leading to limited responses to treatments such as immune checkpoint therapies (ICTs). The immune composition of the prostate TME differs across soft tissue and bone, the most common site of treatment-refractory metastasis. Understanding immunosuppressive mechanisms specific to prostate TMEs will enable rational immunotherapy strategies to generate effective antitumor immune responses. Daratumumab (anti-CD38 antibody) and edicotinib (colony-stimulating factor-1 receptor (CSF-1R) inhibitor) may alter the balance within the prostate TME to promote antitumor immune responses.
Hypothesis
Daratumumab or edicotinib will be safe and will alter the immune TME, leading to antitumor responses in localized prostate cancer.
Patients and methods
In this presurgical study, patients with localized prostate cancer received 4 weekly doses of daratumumab or 4 weeks of daily edicotinib prior to radical prostatectomy (RP). Treated and untreated control (Gleason score ≥8 in prostate biopsy) prostatectomy specimens and patient-matched pre- and post-treatment peripheral blood mononuclear cells (PBMCs) and bone marrow samples were evaluated. The primary endpoint was incidence of adverse events (AEs). The secondary endpoint was pathologic complete remission (pCR) rate.
Results
Twenty-five patients were treated (daratumumab, n=15; edicotinib, n=10). All patients underwent RP without delays. Grade 3 treatment-related AEs with daratumumab occurred in 3 patients (12%), and no ≥grade 3 treatment-related AEs occurred with edicotinib. No changes in serum prostate-specific antigen (PSA) levels or pCRs were observed. Daratumumab led to a decreased frequency of CD38+ T cells, natural killer cells, and myeloid cells in prostate tumors, bone marrow, and PBMCs. There were no consistent changes in CSF-1R+ immune cells in prostate, bone marrow, or PBMCs with edicotinib. Neither treatment induced T cell infiltration into the prostate TME.
Conclusions
Daratumumab and edicotinib treatment was safe and well-tolerated in patients with localized prostate cancer but did not induce pCRs. Decreases in CD38+ immune cells were observed in prostate tumors, bone marrow, and PBMCs with daratumumab, but changes in CSF-1R+ immune cells were not consistently observed with edicotinib. Neither myeloid-targeted agent alone was sufficient to generate antitumor responses in prostate cancer; thus, combinations with agents to induce T cell infiltration (eg, ICTs) will be needed to overcome the immunosuppressive prostate TME.
Keywords: Prostatic Neoplasms, Immunotherapy, Tumor Microenvironment, Immunomodulation
WHAT IS ALREADY KNOWN ON THIS TOPIC
Prostate cancer has limited responses to treatments such as immune checkpoint therapies due to an immunosuppressive prostate tumor microenvironment (TME) that differs across soft tissue and bone, the most common site of treatment-refractory metastasis.
Understanding immunosuppressive mechanisms specific to prostate TMEs will enable rational immunotherapy strategies to generate antitumor immune responses.
WHAT THIS STUDY ADDS
We present findings from the first presurgical trial of an anti-CD38 antibody (daratumumab) or colony-stimulating factor-1 receptor (CSF-1R) inhibitor (edicotinib), with analysis of primary tumor, bone marrow, and peripheral blood.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Decreases in CD38+ immune cells were observed in prostate tumors, bone marrow, and peripheral blood mononuclear cells with daratumumab, but changes in CSF-1R+ immune cells were not consistently observed with edicotinib.
Although treatment was safe and well-tolerated, neither agent alone was sufficient to generate antitumor responses in prostate cancer; thus, combinations with agents to induce T cell infiltration will be needed to overcome the immunosuppressive prostate TME.
Introduction
Prostate cancer harbors an immunosuppressive tumor microenvironment (TME), characterized by low tumor mutational burden and neoantigen load associated with few intratumoral T cells.1 2 In keeping with this, the prostate TME is enriched in anti-inflammatory myeloid cells (eg, myeloid-derived suppressor cell (MDSC) subsets) and immunosuppressive cytokines and pathways (eg, adenosine signaling).3 4 Our group has shown that presurgical (‘window of opportunity’) trials can reveal biologically relevant mechanisms of immune responsiveness within this immunosuppressive prostate TME.5–7 However, the prostate TME differs across organ sites, including primary prostate tissue, lymph nodes (ie, soft tissue) and bone, the most common site of treatment-refractory metastases in prostate cancer.8 Through evaluation of patient-matched pre- and post-treatment bone marrow samples, our group has previously identified candidate mechanisms of resistance to immunotherapies within the bone TME, such as immunosuppressive myeloid cell subsets.8 9 We therefore designed a presurgical trial in patients with prostate cancer, incorporating matched evaluation of the primary tumor, bone marrow and peripheral blood microenvironments, testing two therapies targeting immunosuppressive populations within the TME: daratumumab (an anti-CD38 antibody) and edicotinib (a small-molecule inhibitor of colony-stimulating factor-1 receptor (CSF-1R)).
CD38 is widely expressed on immune cells, including myeloid cells (macrophages, monocytes, neutrophils, and dendritic cells), T cells, natural killer (NK) cells, and plasma B cells.10 11 Functionally, CD38 catalyzes the conversion of nicotinamide adenine dinucleotide (NAD+) to ADP-ribose (ADPR) and cyclic ADPR (cADPR), substrates for non-canonical adenosine production.10 11 Adenosine receptor signaling promotes differentiation of myeloid cells such as tumor-associated macrophages (TAMs) into a suppressive phenotype, inhibits NK cell maturation and function, and limits effector T cell activation and proliferation, supporting an immunosuppressive TME.4 Adenosine receptor antagonists are currently being evaluated clinically with evidence of durable responses in subsets of patients.12 13 Daratumumab is a fully human monoclonal antibody targeting CD38 that prolonged overall survival in multiple myeloma, a plasma cell dyscrasia arising from bone marrow, which led to its approval by the U.S. Food and Drug Administration (FDA).10 14 Daratumumab has been shown to deplete immunosuppressive cell populations, including CD38+ myeloid-derived suppressor cells (MDSCs), regulatory T (Treg) cells, and regulatory B (Breg) cells.15 16 In the bone marrow and peripheral blood of patients with multiple myeloma, daratumumab led to increased frequency of CD3+, CD4+ and CD8+ T cells.15 Furthermore, it increased T cell receptor clonality and the frequency of effector memory CD8+ T (TEM) cells in PBMCs of patients with multiple myeloma.15
Edicotinib (JNJ-40346527) is an orally bioavailable inhibitor of CSF-1R, a receptor tyrosine kinase that is primarily expressed on the myeloid lineage (on macrophages and granulocytes) and controls macrophage differentiation and function.17 18 CSF-1R promotes polarization of TAMs into a suppressive phenotype and restrains antitumor Th1 cells.19 In preclinical models (including the autochthonous transgenic TRAMP model of prostate cancer), edicotinib reduced CD11b+Gr-1-F4/80hi tumor-associated macrophages within the TME.20 Edicotinib has shown evidence of safety and target engagement in humans, with >80% inhibition of CSF-1R phosphorylation in a phase I/II trial in patients with relapsed or refractory Hodgkin lymphoma.21
Based on these preclinical and clinical data, we hypothesized that daratumumab or edicotinib would be safe and well-tolerated as presurgical therapy for prostate cancer and deplete suppressive immune populations within the TME, leading to antitumor responses. Here, we present findings from the first presurgical trial of an anti-CD38 antibody or CSF-1R inhibitor, with analysis of the immune microenvironments within the primary tumor, bone marrow, and peripheral blood.
Methods
Study design and patients
This study (NCT03177460) was an open-label, single-center phase I trial in patients with high-risk, localized prostate cancer. Key inclusion criteria included histologic evidence of prostate adenocarcinoma (small cell, neuroendocrine, or transitional cell histologies were excluded), high-risk disease defined by at least one prostate biopsy core with Gleason score ≥8 (with at least three core biopsies involved with cancer), no radiographic evidence of distant metastatic disease as assessed by CT scan and technetium-99m-MDP bone scintigraphy, and localized or locally advanced disease deemed by the treating surgeon to be resectable and appropriate for radical prostatectomy. Full inclusion and exclusion criteria are provided in the clinical protocol (online supplemental appendix 1). Patients were consented to the MD Anderson Institutional Review Board (IRB)-approved laboratory protocol PA13-0291 for immune monitoring analyses on peripheral blood, bone marrow, and tumor tissue. A separate cohort of patients with Gleason sum score ≥8 on prostate biopsy who proceeded directly to standard of care prostatectomy without systemic therapy were consented to PA13-0291 and provided prostate tumor specimens as untreated controls.
jitc-2022-006262supp001.pdf (1.1MB, pdf)
Treatment
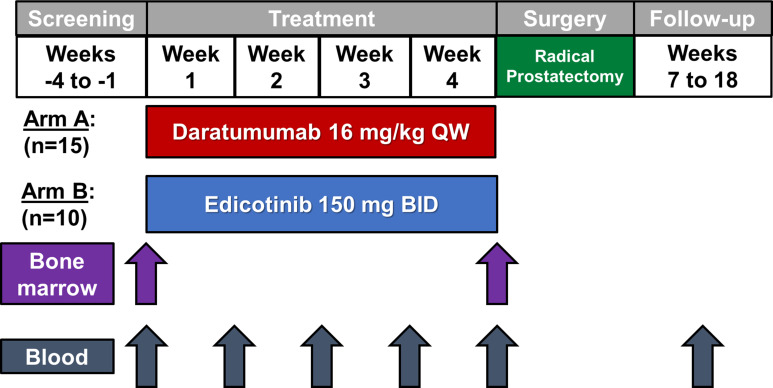
For the open-label treatment phase, patients were enrolled into either Arm A (daratumumab 16 mg/kg as per the FDA-approved dosing for multiple myeloma for 4 weekly doses prior to surgery)22–24 or Arm B (edicotinib 150 mg two times per day based on a prior clinical trial indicating pharmacodynamic activity at this dose for 4 weeks prior to surgery)21 (figure 1). Patients were assigned alternating treatments in cohorts of five, with patients 1–5 treated with daratumumab, patients 6–10 treated with edicotinib, patient 11–15 treated with daratumumab, and so on. Daratumumab was discontinued after week 4 of the open-label treatment phase. Edicotinib was discontinued at the end of week 4 with a 3-day wash-out prior to surgery. In the surgical phase, radical prostatectomy (open or robotic-assisted laparoscopic) was performed on or after week 6. The end of study visit occurred at week 18. Complete discontinuation criteria and adverse event management guidelines are provided in the protocol (online supplemental appendix 1).
Figure 1.
Study schema. BID, two times per day; QW, weekly.
Assessments and end points
The primary objective was to evaluate safety and tolerability of the study drugs in men with high-risk localized prostate cancer. The primary endpoint was incidence of adverse events (AEs). The secondary objective was to assess the proportion of patients who achieved pathological complete response (pCR) with the study drugs. The secondary endpoint was pCR rate (pT0). The exploratory objective was to study immunological changes in tumor tissue, bone, and peripheral blood in response to the study drugs. The exploratory endpoints were immunological variables measured in tumor tissue, bone marrow, and peripheral blood. Treated and untreated (Gleason-matched control) prostate tumors and patient-matched pre- and post-treatment peripheral blood mononuclear cells (PBMCs) and iliac crest bone marrow biopsies and aspirates were obtained. Detailed end point definitions are provided in the clinical protocol (online supplemental appendix 1).
Statistical analyses and safety monitoring
The trial was designed as a pilot study with an intended size of 15 patients in each treatment arm to establish safety and allow descriptive analysis of secondary endpoints to plan future trials (operating characteristics are presented in the protocol in online supplemental appendix 1). No formal hypotheses were planned as these patients were intended to form the basis for designing a future hypothesis-driven study. Interim safety monitoring rules were implemented with enrollment alternating between arms to accommodate the AE assessment period. The following AEs at least possibly related to study therapy were considered a trial limiting toxicity (TOX): grade 3 infusion reactions lasting >6 hours; grade 2 toxicity lasting >14 days; any grade 3–4 events except asymptomatic grade 3–4 neutropenia or anemia lasting ≤5 days or asymptomatic grade 3 thrombocytopenia lasting ≤5 days; major surgical complications; or surgery delay >4 weeks. A Bayesian sequential monitoring design was used to monitor the trial for toxicity separately for each treatment group in cohorts of 5 for each arm.25 26 Each arm would be independently terminated if Prob(TOX>0.10|data) >0.80, which equated to stopping if ≥2/5 or ≥3/10 patients had a TOX.
Patient characteristics were tabulated at the time of starting initial therapy. AEs were tabulated for all grades and high grades (grade 3 or higher) for events related to study treatment based on the final determination of relation for each event in each patient. TOX events were tabulated by arm. Pathologic response was tabulated with 90% credible intervals assuming an uninformative prior of beta(0.5, 0.5). Tables were prepared in SAS V.9.4 (SAS Institute). Posterior estimates were calculated in Parameter Solver V.3.0 (MDACC Department of Biostatistics, Houston, Texas, USA).
Immunohistochemistry
Representative formalin-fixed paraffin-embedded blocks from daratumumab-treated and edicotinib-treated prostatectomy specimens and untreated prostatectomy control specimens (Gleason sum score ≥8 on prostate biopsy) were sectioned at 4 µm. Pretreatment and post-treatment bone marrow biopsy cores were decalcified in a working solution of equal parts 8% hydrochloric acid and 8% formic acid at 20× volume, with daily solution changes until decalcification was completed. The specimens were rinsed in water, neutralized with a concentrated ammonia solution, and washed. The bone marrow biopsy cores and clots were fixed in 10% formalin, embedded in paraffin and cut into 4 µm serial sections for downstream staining and analysis. H&E and immunohistochemical staining was performed using CD38, CSF-1R, CD3, CD8, CD20, CD68, CD45RO, FOXP3, PD-1 and PD-L1 antibodies (online supplemental table 1). Slides were scanned and digitized using the Aperio Scan Scope XT system (Leica Technologies). Quantification analysis was done using Halo software (V.3.2.1851) and cell densities (cells/mm2) were plotted. Statistical significance was determined by the Mann-Whitney test for prostate tissue samples (untreated vs treated) and Wilcoxon test for bone marrow specimens (pre- and post-treatment) (GraphPad Prism software V.8.0). P value <0.05 was considered statistically significant.
jitc-2022-006262supp002.pdf (185KB, pdf)
Flow cytometry
Flow cytometry was performed as previously described7 on daratumumab or edicotinib-treated prostatectomy specimens, untreated prostatectomy control specimens (Gleason sum score ≥8 on prostate biopsy), and pre- and post-treatment bone marrow aspirates and PBMCs. Prostatectomy samples were dissociated with the gentleMACS Dissociator (Miltenyi Biotec) and cultured overnight in complete RPMI media. Single cell suspensions were processed for the flow cytometry assay. Expression of the following markers was assessed: CD45, CD3, CD4, CD8, CD127, CD25, CD19, CD11b, CD14, HLA-DR, FoxP3, CD56, CD38 and CSF1-R (online supplemental table 2). Flow analysis was performed using FlowJo V.10 (BD). The flow cytometry gating strategy is shown in online supplemental figure 1. Data were analyzed with GraphPad Prism software V.8.0. Statistical significance was determined by the Mann-Whitney test for prostate tissue samples and Wilcoxon sign rank test for bone marrow aspirate and PBMC samples. P value <0.05 was considered statistically significant.
jitc-2022-006262supp003.pdf (2MB, pdf)
NanoString transcriptional profiling
Stored cells from prostatectomy specimens were processed with RiboPure RNA Purification Kit (Thermo Fisher Scientific) according to the manufacturer’s protocol. Extracted RNA was quantified by ND Nanodrop1000 spectrometer (Thermo Scientific, Wilmington, Massachusetts, USA). For NanoString assay, 100 ng of RNA was used to detect immune gene expression using nCounter PanCancer Immune Profiling panel along with custom CodeSet. Counts of the reporter probes were tabulated for each sample by the nCounter Digital Analyzer and raw data output was imported into nSolver (http://www.nanostring.com/products/nSolver, V.4.0). nSolver data analysis package was used for normalization, cell type analysis and differential gene expression analysis, and Gene set enrichment analyses (GSEA) were performed with Qlucore Omics Explorer V.3.5 software (Qlucore, New York, USA).27 28 Data were plotted using GraphPad Prism V.8 (GraphPad Software V.8.4.3). Statistical analysis was performed using the two-tailed Student’s t-test to compare two groups. P values <0.05 denote significant differences.
Results
Patients
Twenty-five patients (Arm A, daratumumab, n=15; Arm B, edicotinib, n=10) were enrolled between June 2017 and November 2019 and received at least 1 dose of protocol therapy (figure 1). Interim analysis was performed after 10 patients, and due to an observed absence of immunological changes in the edicotinib arm, further enrollment was halted in Arm B. Baseline demographics were similar in the two arms, with a median age of 63 years (range 47–69) and primarily white/non-Hispanic descent (table 1).
Table 1.
Baseline characteristics by treatment arm
| Patient characteristics | All | Arm A: Daratumumab | Arm B: Edicotinib | |
| n (%) | n (%) | n (%) | ||
| All | 25 (100%) | 15 (100%) | 10 (100%) | |
| Age, median (min, max) | 63.0 (47.0, 69.0) | 63.0 (47.0, 69.0) | 63.5 (51.0, 69.0) | |
| Race/ethnicity | ||||
| Black/non-Hispanic | 2 (8%) | 2 (13%) | 0 (0%) | |
| Other/Hispanic | 1 (4%) | 0 (0%) | 1 (10%) | |
| White/non-Hispanic | 22 (88%) | 13 (87%) | 9 (90%) | |
| Gleason score at biopsy | 8 (4+4) | |||
| 9 (4+5 or 5+4) | ||||
| 10 (5+5) | ||||
Safety and efficacy
AEs related to daratumumab and edicotinib treatment are listed in table 2.
Table 2.
Treatment-related adverse events
| Arm A: Daratumumab (n=15) | Arm B: Edicotinib (n=10) |
Total (n=25) | ||||
| All | Grade 3+ | All | Grade 3+ | All | Grade 3+ | |
| Adverse event | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) |
| Any event | 13 (87%) | 3 (20%) | 8 (80%) | 0 (0%) | 21 (80%) | 3 (12%) |
| Infusion reaction | 5 (33%) | 2 (13%) | 0 (0%) | 0 (0%) | 5 (20%) | 2 (8%) |
| Urticaria | 1 (7%) | 1 (7%) | 0 (0%) | 0 (0%) | 1 (4%) | 1 (4%) |
| AST increased | 0 (0%) | 0 (0%) | 4 (40%) | 0 (0%) | 4 (16%) | 0 (0%) |
| Fatigue/malaise | 3 (20%) | 0 (0%) | 1 (10%) | 0 (0%) | 4 (16%) | 0 (0%) |
| White blood cell decreased | 2 (13%) | 0 (0%) | 2 (20%) | 0 (0%) | 4 (16%) | 0 (0%) |
| Anemia | 1 (7%) | 0 (0%) | 2 (20%) | 0 (0%) | 3 (12%) | 0 (0%) |
| Constipation | 2 (13%) | 0 (0%) | 1 (10%) | 0 (0%) | 3 (12%) | 0 (0%) |
| Creatinine increased | 1 (7%) | 0 (0%) | 2 (20%) | 0 (0%) | 3 (12%) | 0 (0%) |
| Hyperglycemia | 1 (7%) | 0 (0%) | 2 (20%) | 0 (0%) | 3 (12%) | 0 (0%) |
| Paresthesia | 2 (13%) | 0 (0%) | 1 (10%) | 0 (0%) | 3 (12%) | 0 (0%) |
| ALT increased | 1 (7%) | 0 (0%) | 1 (10%) | 0 (0%) | 2 (8%) | 0 (0%) |
| Chloride decreased | 2 (13%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (8%) | 0 (0%) |
| Concentration impairment | 1 (7%) | 0 (0%) | 1 (10%) | 0 (0%) | 2 (8%) | 0 (0%) |
| Dyspepsia/acid reflux | 2 (13%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (8%) | 0 (0%) |
| Headache | 2 (13%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (8%) | 0 (0%) |
| Myalgia | 2 (13%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (8%) | 0 (0%) |
| Nausea | 2 (13%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (8%) | 0 (0%) |
| Rash | 2 (13%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (8%) | 0 (0%) |
| Rhinitis | 2 (13%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (8%) | 0 (0%) |
| Sore throat | 2 (13%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (8%) | 0 (0%) |
| Abdominal pain | 1 (7%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (4%) | 0 (0%) |
| Alk phos increased | 0 (0%) | 0 (0%) | 1 (10%) | 0 (0%) | 1 (4%) | 0 (0%) |
| Alopecia | 1 (7%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (4%) | 0 (0%) |
| Bloating/abdominal distension | 0 (0%) | 0 (0%) | 1 (10%) | 0 (0%) | 1 (4%) | 0 (0%) |
| Blurred vision | 1 (7%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (4%) | 0 (0%) |
| CO2 decreased | 1 (7%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (4%) | 0 (0%) |
| Confusion | 1 (7%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (4%) | 0 (0%) |
| Cough | 1 (7%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (4%) | 0 (0%) |
| Diarrhea | 1 (7%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (4%) | 0 (0%) |
| Dysphagia | 1 (7%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (4%) | 0 (0%) |
| Dysuria | 1 (7%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (4%) | 0 (0%) |
| Ear pain | 1 (7%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (4%) | 0 (0%) |
| Influenza-like symptoms | 1 (7%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (4%) | 0 (0%) |
| Hyperkalemia | 0 (0%) | 0 (0%) | 1 (10%) | 0 (0%) | 1 (4%) | 0 (0%) |
| Hyponatremia | 1 (7%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (4%) | 0 (0%) |
| Hypophosphatemia | 0 (0%) | 0 (0%) | 1 (10%) | 0 (0%) | 1 (4%) | 0 (0%) |
| Lymphocyte count decreased | 0 (0%) | 0 (0%) | 1 (10%) | 0 (0%) | 1 (4%) | 0 (0%) |
| Nocturia | 0 (0%) | 0 (0%) | 1 (10%) | 0 (0%) | 1 (4%) | 0 (0%) |
| Pain | 1 (7%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (4%) | 0 (0%) |
| Platelet count decreased | 1 (7%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (4%) | 0 (0%) |
| Pruritus | 1 (7%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (4%) | 0 (0%) |
| Shingles | 1 (7%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (4%) | 0 (0%) |
| Sinus pain | 1 (7%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (4%) | 0 (0%) |
| T3 decreased | 1 (7%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (4%) | 0 (0%) |
| Tremor | 1 (7%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (4%) | 0 (0%) |
| Wheezing | 1 (7%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (4%) | 0 (0%) |
| Yellow skin tone | 1 (7%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (4%) | 0 (0%) |
ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Grade 3 or higher treatment-related AEs (TRAEs) occurred in 20% of patients (n=3) in the daratumumab arm and none in the edicotinib arm. In the daratumumab arm, the most common grade 3 or higher TRAEs were infusion reaction (n=2, 13%) and urticaria (n=1, 7%). The most common TRAE of any grade in patients receiving daratumumab was infusion reaction (n=5, 33%), in line with previously published studies of daratumumab in multiple myeloma.29 No grade 3 or higher TRAEs were observed on the edicotinib arm, consistent with previously reported phase I trials with this agent.21 The most common TRAE among patients on the edicotinib arm was grade 1 increase in aspartate aminotransferase (n=4, 40%). Online supplemental table 3 provides all treatment-emergent events regardless of attribution to study therapy. The trial did not stop for TOX events. Two patients on each arm experienced TOX events (Arm A: 13%, Arm B, 20%) (table 3), and it should be noted that no patients experienced a delay in surgery. One patient receiving daratumumab experienced grade 2 infection lasting greater than 14 days and one experienced grade 3 urticaria (table 3). Another patient receiving edicotinib experienced grade 2 lymphocyte count decrease exceeding 14 days (table 3). No patients achieved pCRs in either arm (table 3). There was no evidence of effect on serum prostate-specific antigen (PSA) levels or pathologic downstaging, although this study was not designed to formally evaluate these endpoints (online supplemental tables 4-6).
Table 3.
Toxicity (TOX) events and clinical outcomes
| Outcome | Arm A: Daratumumab (n=15) |
Arm B: Edicotinib (n=10) |
| TOX | ||
| Patients with any TOX event | 2 (13%) | 2 (20%) |
| Surgery delay | 0 (0%) | 0 (0%) |
| Surgical complications | 0 (0%) | 1 (10%) |
| Grade 3 infusion reactions >6 hours | 0 (0%) | NA |
| Related grade 2 lasting >14 days | 1 (7%)—infection | 1 (10%)—lymphocyte count decrease |
| Related grade 3, not excepted | 1 (7%)—urticaria | 0 (0%) |
| pCR | ||
| pCR empirical estimate N (%) | 0 (0%) | 0 (0%) |
pCR, pathologic complete remission.
Evaluation of target modulation within the primary tumor, bone, and peripheral blood microenvironments
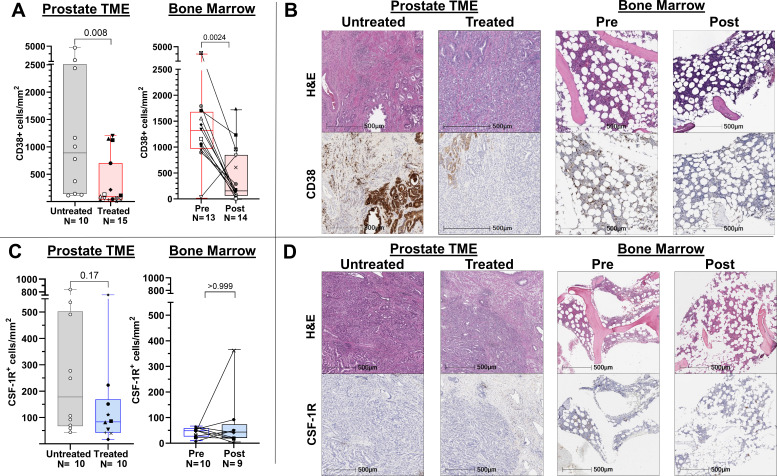
To evaluate the pharmacodynamic activity of daratumumab and edicotinib, respectively, we first performed quantitative immunohistochemistry (IHC) of treated versus untreated prostate tumors and patient-matched post- versus pre-treatment bone marrow biopsies for CD38 and CSF-1R. A lower density of CD38+ cells was observed in both the primary tumor and bone microenvironments following treatment with daratumumab (figure 2A, B). By contrast, there was no statistically significant difference in density of CSF-1R+ cells in edicotinib-treated versus untreated prostate tumors or post- and pre-treatment bone marrow specimens (figure 2C, D). Minimal differences in overall immune cell density were observed in prostate tumors or bone marrow specimens in the daratumumab (online supplemental figure 2) or edicotinib (online supplemental figure 3) arms.
Figure 2.
Target modulation in evaluable prostate tumors and bone marrow by daratumumab but not edicotinib as assessed by immunohistochemistry (IHC). (A) Quantitative CD38 IHC. (B) Representative H&E and CD38 IHC. (C) Quantitative CSF-1R IHC. (D) Representative H&E and CSF-1R IHC. Daratumumab-treated versus untreated prostate tumors and patient-matched pre-daratumumab and post-daratumumab bone marrow cores were assessed. CSF-1R, colony-stimulating factor-1 receptor; TME, tumor microenvironment.
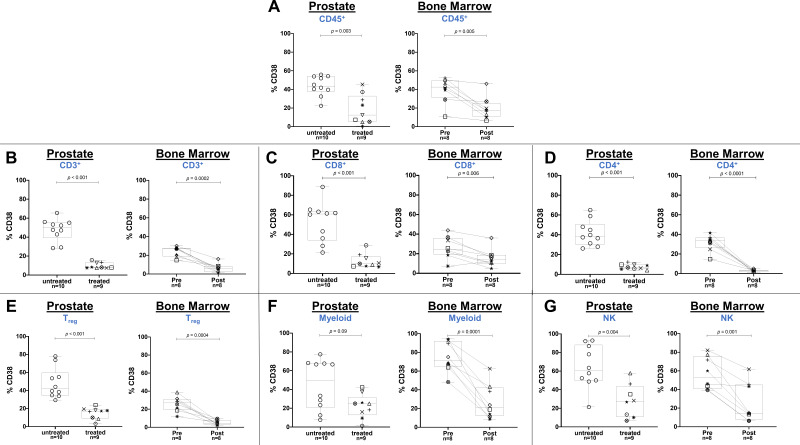
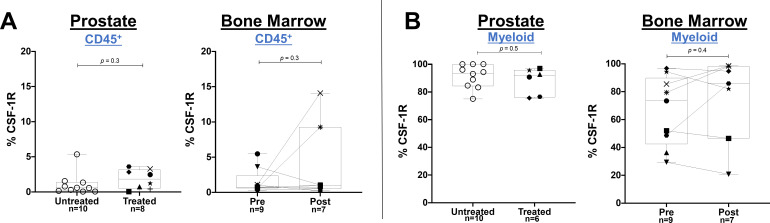
To determine if specific CD38+ or CSF-1R+ immune cell subsets were affected by daratumumab or edicotinib, respectively, we performed flow cytometry on prostate tumors, bone marrow aspirates, and PBMCs. Daratumumab treatment was consistently associated with a decreased frequency of CD38+ immune cells, including CD8+ T cells, CD4+ T cells, CD4+CD25+FoxP3+ Treg cells, CD11b+CD14+HLA-DR+ myeloid cells, and CD3-CD19-CD56+ NK cells in prostate tumors, bone marrow aspirates, and PBMCs (figure 3A–G & online supplemental figure 4). No consistent changes were observed in the frequency of total (CD38+ and CD38- combined) immune cell subsets in prostate tumors, bone marrow, and PBMCs at the protein or transcriptional levels, with the exception of a decrease in NK cells in blood, which is a known pharmacodynamic biomarker for daratumumab treatment (online supplemental figure 5–7).15 Among the most highly differentially expressed genes in daratumumab-treated prostate tumors was CD80, consistent with prior data from co-culture experiments indicating that daratumumab induced upregulation of costimulatory T cell surface antigens (CD80 and CD86) on monocytes in the presence of NK cells (online supplemental figure 6).30 These data suggest that daratumumab may promote a stimulatory effect within the prostate TME, but is insufficient to generate antitumor activity in the absence of infiltrating effector T cells. In edicotinib-treated patients, no changes in the frequency of CSF-1R+ immune cells were observed in edicotinib-treated prostate tumors or bone marrow specimens, including in CD11b+CD14+HLA-DR+ myeloid cells, a high frequency of which expressed CSF-1R (figure 4A, B &online supplemental figures 8,9). Consistent with these findings, no significant changes were observed in the frequency of total (CSF-1R+ and CSF-1R- combined) immune cell subsets, in prostate tumors, bone marrow, or PBMCs with edicotinib (online supplemental figure 10,11).
Figure 3.
Target modulation by daratumumab in evaluable prostate tumors and bone marrow as assessed by flow cytometry. Flow cytometric analysis gated on CD45+ live cells showing frequency of CD38+ cells in (A) CD45+ immune cells; (B) CD3+ T cells; (C) CD8+ T cells; (D) CD4+ T cells; (E) CD4+CD25+FoxP3+ Treg cells; (F) CD11b+CD14+HLA-DR+ myeloid cells; (G) CD3-CD19-CD56+ NK cells. Daratumumab-treated versus untreated prostate tumors and patient-matched pre-daratumumab and post-daratumumab bone marrow aspirates were assessed. NK, natural killer.
Figure 4.
Absence of target modulation by edicotinib in evaluable prostate tumors and bone marrow as assessed by flow cytometry. Flow cytometric analysis gated on CD45+ live cells showing frequency of CSF-1R+ cells in (A) CD45+ immune cells; (B) CD11b+CD14+HLA-DR+ myeloid cells. Edicotinib-treated versus untreated prostate tumors and patient-matched pre-edicotinib and post-edicotinib bone marrow aspirates were assessed. CSF-1R colony-stimulating factor-1 receptor.
To ensure that these findings were not skewed by differences in Gleason score assessed at prostatectomy versus at the time of biopsy (due to limited sampling by biopsy), we stratified by Gleason score (Gleason 7 vs Gleason 8–10) and observed no changes in the overall findings (online supplemental figure 12).
Discussion
Here, we present data from the first presurgical trial of an anti-CD38 antibody (daratumumab) or a CSF-1R inhibitor (edicotinib) in patients with prostate cancer with matched pre- and post-treatment evaluation of the primary tumor, bone marrow, and peripheral blood microenvironments. We confirmed that the presurgical treatments were safe, and all patients completed surgery without delay. Daratumumab demonstrated evidence of target modulation, with consistent depletion of CD38+ immune cell subsets (CD4+ and CD8+ T cells, CD4+CD25+FoxP3+ Treg cells, CD11b+CD14+HLA-DR+ myeloid cells, and CD3-CD19-CD56+ NK cells) in prostate tumors, bone marrow, and peripheral blood. No consistent changes in CSF-1R+ immune cells were observed with edicotinib treatment. We did not observe pCRs with either treatment. No changes in overall immune cell density were observed in prostate tumors and bone marrow specimens with either treatment, with the exception of a decrease in PD-1+ cells in the prostate following both treatments and lower levels of CD45RO in the prostate tumor of patients treated with edicotinib. Given that changes were only observed in the tumor samples and not the bone marrow, this could be due to baseline cohort differences.
At the time this study was designed, the distribution of CD38 expression and the consequences of CD38+ cell depletion within the human prostate TME had not been defined. Recently, increased CD38 expression within the prostate TME has been shown to associate with poorer survival in prostate cancer.31 Consistent with this negative prognostic association, CD38 mRNA expression in metastatic castration-resistant prostate cancer (CRPC) tumor biopsies correlated with immunosuppressive transcriptional signatures, including adenosine signaling (currently being targeted clinically in prostate cancer via the CD39, CD73, A2A and A2B receptors), IL-23 signaling, and T cell exhaustion.31 32 These findings are consistent with prior studies correlating CD38 expression with PSA-specific CD8+ T cells expressing TIM-3 (a marker of exhaustion) in peripheral blood from patients with prostate cancer.33 In patient-matched biopsies from castration-sensitive prostate cancer (CSPC) primary tumors and CRPC metastatic tumors, CD38 was expressed on myeloid cells (CD15+ or CD33+) and B cells (CD19+, CD20+, or CD138+).31 The lack of pathologic responses in our study despite effective target modulation by daratumumab indicates that depletion of immunosuppressive CD38+ lymphoid and myeloid cells alone is insufficient to induce effective antitumor responses. Therefore, a combinatorial or sequential approach with a drug to induce T cell infiltration into the prostate TME, such as vaccines (eg, sipuleucel-T34), immune checkpoint therapies (eg, anti-CTLA-4),7 or T cell bispecifics, is likely to be necessary to generate effective antitumor immune responses with daratumumab. This strategy may warrant further evaluation in the CRPC setting, which has been shown to have higher CD38 expression on immune cells compared with CSPC primary tumors.31
For edicotinib, as changes in CSF-1R levels were not observed in the prostate tumor, bone marrow, or peripheral blood microenvironments (including in CSF-1R+ CD11b+CD14+HLA-DR+ myeloid cells), the treatment had limited impact on TME remodeling. Based on previous data that edicotinib 150 mg two times per day demonstrated pharmacodynamic activity in patients (as assessed by CSF-1R phosphorylation), we selected this dose for our study and directly evaluated depletion of CSF-1R+ cells (rather than CSF-1R phosphorylation or soluble CSF-1 levels) as this was anticipated to be the most biologically relevant consequence of treatment with edicotinib.21 35 We also chose the timing of the wash-out period of edicotinib prior to surgery (72 hours) based on the apparent elimination half-life of edicotinib of approximately 60 hours (unpublished data, Janssen Research & Development, Edicotinib Investigator’s Brochure). A potential explanation is that inhibition of CSF-1R kinase by edicotinib is sufficient to deplete CSF-1R-expressing myeloid cells (eg, CD11b+Gr-1-F4/80hi) in murine models, but not in humans; although it should be noted that the preclinical study used a higher dose of 20 mg/kg of edicotinib than the 150 mg flat dose in our trial.20
These findings are consistent with the overall limited clinical benefit of CSF-1R-targeted agents observed to date.36 Currently, pexidartinib is the only FDA-approved CSF-1R inhibitor for the treatment of tenosynovial giant cell tumors, which are characterized by overexpression of CSF-1R.36 A recent study of an anti-CSF-1R monoclonal antibody that decreased CD14DIMCD16BRIGHT cells in the peripheral blood of patients with CRPC did not lead to clinical responses. Data from post-treatment biopsies from the patients with CRPC were not reported; therefore, it is unknown if these cells were depleted within the prostate TME.37 The differences in markers for mouse and human myeloid cell subsets and the heterogeneity of these populations may have also contributed to the challenges in translating preclinical observations with CSF-1R blockade to patients.38 For example, a study in colorectal cancer demonstrated that CSF-1R blockade depleted proinflammatory TAMs, but spared certain protumorigenic TAMs.39 CSF-1R inhibition was also shown to lead to upregulation compensatory mechanisms, such as increased recruitment of MDSCs into the TME.20 Future studies with CSF-1R inhibition should therefore account for these potential redundant mechanisms as well as ensure that myeloid subsets evaluated in preclinical models are relevant to human tumor biology.
A potential limitation to our study was that although patient-matched pre- and post-treatment samples were used for bone marrow and peripheral blood assessments, treated prostate tumors were compared with non-patient-matched untreated prostate tumors. This was done because prostate tumors harbor few infiltrating CD45+ immune cells and therefore pre-treatment biopsies provide insufficient cells for flow cytometric analyses. Nevertheless, the findings in prostate tumors were consistent with those in bone marrow and peripheral blood.
In conclusion, daratumumab and edicotinib were safe and well-tolerated prior to surgery for primary prostate cancer. This study is the first presurgical trial of an anti-CD38 antibody or CSF-1R inhibitor in prostate cancer with evaluation of the immune microenvironments within the primary prostate tumor, bone marrow, and peripheral blood. Target modulation was consistently observed with daratumumab, but not edicotinib. Myeloid-targeted agents such as daratumumab alone are insufficient to generate effective antitumor responses in prostate cancer, and combinations with agents to induce T cell infiltration (such as anti-CTLA-4 immune checkpoint therapy, T cell bispecifics, or vaccines) will be needed to overcome the immunosuppressive TME. In immune-sensitive tumors like clear cell renal cell carcinoma (RCC) and urothelial cancer (UC), daratumumab monotherapy may be more effective. Analyses of the TME in patients with RCC and UC treated with daratumumab are ongoing from a recently completed partner clinical trial (NCT03473730).
Acknowledgments
We are grateful to our patients and their families for their participation in this study. We thank Janssen for their support of this study.
Footnotes
Twitter: @BeckyTidwell1
Contributors: The first draft of the manuscript was written by BAS. All authors contributed to the final manuscript and approved the decision to submit the manuscript for publication. The corresponding authors (SKS and PS) and principal investigator (SKS) had access to all data in the study, accept full responsibility for the conduct of the study, and had final responsibility for the decision to submit for publication.
Funding: These studies were supported in part by Janssen Research & Development. The research work was also supported by The University of Texas MD Anderson Cancer Center Prostate Cancer Moon Shot Program (PS and SS); National Institutes of Health (NIH)/National Cancer Institute (NCI) P50 CA140388 (Prostate Cancer SPORE) (SS, CJL, PS); and The V Foundation for Cancer Research’s Lloyd Family Clinical Oncology Scholar Award D2018-014 (SS). The Genitourinary Cancers Program of the Cancer Center Support Grant shared resources at The University of Texas M.D. Anderson Cancer Center. PS is a member of the Parker Institute for Cancer Immunotherapy at The University of Texas M.D. Anderson Cancer Center.
Competing interests: BAS, FD, SB, SJ, ABE, A-DG, CAP, PCJ, JW, SSY, and RSST report no competing interests. BFC reports Blue Earth Diagnostics - Scientific study/Trial. Johnson and Johnson - Consultant/Advisor. CJL reports Honoraria: Merck, Sharp & Dohme, Bayer, Amgen; Consulting/Advisory: Merck, Sharp & Dohme, Exelixis, Bayer, Amgen; Clinical Grants: Janssen, ORIC Pharmaceuticals, Novartis, Aragon Pharmaceuticals. CG and NH are employees of Janssen Research and Development and report no other relevant disclosures. PS reports Scientific/Advisory Committee Member: Achelois, BioAlta LLC, Candel Therapeutics, Codiak Biosciences, Inc, C-Reveal Therapeutics, Dragonfly Therapeutics, formerly called Equipoise Therapeutics Corporation, Earli Inc, InterVenn Biosciences, LAVA Therapeutics, Lytix Biopharma, PBM Capital, Phenomic Al, Trained Therapeutix Discovery, Xilis, Inc., Two Bear, Henlius/Hengenix. SKS reports Consulting or Advisory Role: Amgen, Apricity Health LLC, AstraZeneca, Bayer, Bristol-Myers Squibb, Cancer Expert Now, Dava Oncology, Dendreon, Exelixis, Kahr Medical Ltd., Janssen Oncology, Javelin Oncology, and MD Education Limited, OncLive (owned by Intellisphere, LLC); Research Funding: AstraZeneca, Bristol-Myers Squibb, and Janssen Oncology; Other (Joint Scientific Committee): Amgen, Janssen Oncology, and Polaris.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The trial was conducted in accordance with the Declaration of Helsinki Good Clinical Practice guidelines. The protocol and its amendments were approved by the Institutional Review Board (IRB) at MD Anderson, approval number 2017-0103. Written informed consent for participation in the study was obtained from all participants. Participants gave informed consent to participate in the study before taking part.
References
- 1.Abida W, et al. Analysis of the prevalence of microsatellite instability in prostate cancer and response to immune checkpoint blockade. JAMA Oncol 2018;5:471–8. 10.1001/jamaoncol.2018.5789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017;357:409–13. 10.1126/science.aan6733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu X, Horner JW, Paul E, et al. Effective combinatorial immunotherapy for castration-resistant prostate cancer. Nature 2017;543:728–32. 10.1038/nature21676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allard B, Allard D, Buisseret L, et al. The adenosine pathway in immuno-oncology. Nat Rev Clin Oncol 2020;17:611–29. 10.1038/s41571-020-0382-2 [DOI] [PubMed] [Google Scholar]
- 5.Carthon BC, Wolchok JD, Yuan J, et al. Preoperative CTLA-4 blockade: tolerability and immune monitoring in the setting of a presurgical clinical trial. Clin Cancer Res 2010;16:2861–71. 10.1158/1078-0432.CCR-10-0569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gao J, Navai N, Alhalabi O, et al. Neoadjuvant PD-L1 plus CTLA-4 blockade in patients with cisplatin-ineligible operable high-risk urothelial carcinoma. Nat Med 2020;26:1845–51. 10.1038/s41591-020-1086-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao J, Ward JF, Pettaway CA, et al. Vista is an inhibitory immune checkpoint that is increased after ipilimumab therapy in patients with prostate cancer. Nat Med 2017;23:551–5. 10.1038/nm.4308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiao S, Subudhi SK, Aparicio A, et al. Differences in tumor microenvironment dictate T helper lineage polarization and response to immune checkpoint therapy. Cell 2019;179:1177–90. 10.1016/j.cell.2019.10.029 [DOI] [PubMed] [Google Scholar]
- 9.Subudhi SK, Siddiqui BA, Aparicio AM, et al. Combined CTLA-4 and PD-L1 blockade in patients with chemotherapy-naïve metastatic castration-resistant prostate cancer is associated with increased myeloid and neutrophil immune subsets in the bone microenvironment. J Immunother Cancer 2021;9:e002919. 10.1136/jitc-2021-002919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van de Donk N. Immunomodulatory effects of CD38-targeting antibodies. Immunol Lett 2018;199:16–22. 10.1016/j.imlet.2018.04.005 [DOI] [PubMed] [Google Scholar]
- 11.Piedra-Quintero ZL, Wilson Z, Nava P, et al. Cd38: an immunomodulatory molecule in inflammation and autoimmunity. Front Immunol 2020;11:597959. 10.3389/fimmu.2020.597959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harshman LC, Chu M, George S, et al. Adenosine receptor blockade with ciforadenant +/- atezolizumab in advanced metastatic castration-resistant prostate cancer (mcrpc). JCO 2020;38:129. 10.1200/JCO.2020.38.6_suppl.129 [DOI] [Google Scholar]
- 13.Subudhi SK, Bendell JC, Carducci MA, et al. ARC-6: a phase 1b/2, open-label, randomized platform study to evaluate efficacy and safety of etrumadenant (AB928) -based treatment combinations in patients with metastatic castrate-resistant prostate cancer (mcrpc). JCO 2021;39:5039. 10.1200/JCO.2021.39.15_suppl.5039 [DOI] [Google Scholar]
- 14.Facon T, Kumar SK, Plesner T, et al. Daratumumab, lenalidomide, and dexamethasone versus lenalidomide and dexamethasone alone in newly diagnosed multiple myeloma (maia): overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol 2021;22:1582–96. 10.1016/S1470-2045(21)00466-6 [DOI] [PubMed] [Google Scholar]
- 15.Krejcik J, Casneuf T, Nijhof IS, et al. Daratumumab depletes CD38+ immune regulatory cells, promotes T-cell expansion, and skews T-cell repertoire in multiple myeloma. Blood 2016;128:384–94. 10.1182/blood-2015-12-687749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krejcik J, Frerichs KA, Nijhof IS, et al. Monocytes and granulocytes reduce CD38 expression levels on myeloma cells in patients treated with daratumumab. Clin Cancer Res 2017;23:7498–511. 10.1158/1078-0432.CCR-17-2027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rojo R, Raper A, Ozdemir DD, et al. Deletion of a CSF1R enhancer selectively impacts CSF1R expression and development of tissue macrophage populations. Nat Commun 2019;10:3215. 10.1038/s41467-019-11053-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guleria I, Pollard JW. Aberrant macrophage and neutrophil population dynamics and impaired th1 response to listeria monocytogenes in colony-stimulating factor 1-deficient mice. Infect Immun 2001;69:1795–807. 10.1128/IAI.69.3.1795-1807.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cannarile MA, Weisser M, Jacob W, et al. Colony-stimulating factor 1 receptor (CSF1R) inhibitors in cancer therapy. J Immunother Cancer 2017;5:53. 10.1186/s40425-017-0257-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar V, Donthireddy L, Marvel D, et al. Cancer-associated fibroblasts neutralize the anti-tumor effect of CSF1 receptor blockade by inducing PMN-MDSC infiltration of tumors. Cancer Cell 2017;32:654–68. 10.1016/j.ccell.2017.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.von Tresckow B, Morschhauser F, Ribrag V, et al. An open-label, multicenter, phase I/II study of JNJ-40346527, a CSF-1R inhibitor, in patients with relapsed or refractory Hodgkin lymphoma. Clin Cancer Res 2015;21:1843–50. 10.1158/1078-0432.CCR-14-1845 [DOI] [PubMed] [Google Scholar]
- 22.Palumbo A, Chanan-Khan A, Weisel K, et al. Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N Engl J Med 2016;375:754–66. 10.1056/NEJMoa1606038 [DOI] [PubMed] [Google Scholar]
- 23.Facon T, Kumar S, Plesner T, et al. Daratumumab plus lenalidomide and dexamethasone for untreated myeloma. N Engl J Med 2019;380:2104–15. 10.1056/NEJMoa1817249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mateos M-V, Dimopoulos MA, Cavo M, et al. Daratumumab plus bortezomib, melphalan, and prednisone for untreated myeloma. N Engl J Med 2018;378:518–28. 10.1056/NEJMoa1714678 [DOI] [PubMed] [Google Scholar]
- 25.Thall PF, Simon RM, Estey EH. Bayesian sequential monitoring designs for single-arm clinical trials with multiple outcomes. Stat Med 1995;14:357–79. 10.1002/sim.4780140404 [DOI] [PubMed] [Google Scholar]
- 26.Thall PF, Sung HG. Some extensions and applications of a Bayesian strategy for monitoring multiple outcomes in clinical trials. Stat Med 1998;17:1563–80. [DOI] [PubMed] [Google Scholar]
- 27.Waks AG, Stover DG, Guerriero JL, et al. The immune microenvironment in hormone receptor-positive breast cancer before and after preoperative chemotherapy. Clin Cancer Res 2019;25:4644–55. 10.1158/1078-0432.CCR-19-0173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Danaher P, Warren S, Dennis L, et al. Gene expression markers of tumor infiltrating leukocytes. J Immunother Cancer 2017;5:18. 10.1186/s40425-017-0215-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Usmani SZ, Nahi H, Plesner T, et al. Daratumumab monotherapy in patients with heavily pretreated relapsed or refractory multiple myeloma: final results from the phase 2 GEN501 and sirius trials. Lancet Haematol 2020;7:e447–55. 10.1016/S2352-3026(20)30081-8 [DOI] [PubMed] [Google Scholar]
- 30.Viola D, Dona A, Caserta E, et al. Daratumumab induces mechanisms of immune activation through CD38+ NK cell targeting. Leukemia 2021;35:189–200. 10.1038/s41375-020-0810-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guo C, Crespo M, Gurel B, et al. Cd38 in advanced prostate cancers. Eur Urol 2021;79:736–46. 10.1016/j.eururo.2021.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Calcinotto A, Spataro C, Zagato E, et al. Il-23 secreted by myeloid cells drives castration-resistant prostate cancer. Nature 2018;559:363–9. 10.1038/s41586-018-0266-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Japp AS, Kursunel MA, Meier S, et al. Dysfunction of PSA-specific CD8+ T cells in prostate cancer patients correlates with CD38 and Tim-3 expression. Cancer Immunol Immunother 2015;64:1487–94. 10.1007/s00262-015-1752-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fong L, Carroll P, Weinberg V, et al. Activated lymphocyte recruitment into the tumor microenvironment following preoperative sipuleucel-T for localized prostate cancer. J Natl Cancer Inst 2014;106:dju268. 10.1093/jnci/dju268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Genovese MC, Hsia E, Belkowski SM, et al. Results from a phase IIA parallel group study of JNJ-40346527, an oral CSF-1R inhibitor, in patients with active rheumatoid arthritis despite disease-modifying antirheumatic drug therapy. J Rheumatol 2015;42:1752–60. 10.3899/jrheum.141580 [DOI] [PubMed] [Google Scholar]
- 36.Lin CC. Clinical development of colony-stimulating factor 1 receptor (CSF1R) inhibitors. J Immunother Precis Oncol 2021;4:105–14. 10.36401/JIPO-20-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Autio KA, Klebanoff CA, Schaer D, et al. Immunomodulatory activity of a colony-stimulating factor-1 receptor inhibitor in patients with advanced refractory breast or prostate cancer: a phase I study. Clin Cancer Res 2020;26:5609–20. 10.1158/1078-0432.CCR-20-0855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goswami S, Anandhan S, Raychaudhuri D, et al. Myeloid cell-targeted therapies for solid tumours. Nat Rev Immunol 2023;23:106–20. 10.1038/s41577-022-00737-w [DOI] [PubMed] [Google Scholar]
- 39.Zhang L, Li Z, Skrzypczynska KM, et al. Single-cell analyses inform mechanisms of myeloid-targeted therapies in colon cancer. Cell 2020;181:442–59. 10.1016/j.cell.2020.03.048 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2022-006262supp001.pdf (1.1MB, pdf)
jitc-2022-006262supp002.pdf (185KB, pdf)
jitc-2022-006262supp003.pdf (2MB, pdf)
Data Availability Statement
Data are available upon reasonable request.