Abstract
Objectives
To find the optimal treatment duration with antibiotics for community-acquired pneumonia (CAP) in adults.
Design
Systematic review and duration-effect meta-analysis.
Data sources
MEDLINE, Embase and CENTRAL through 25 August 2021.
Eligibility criteria
All randomised controlled trials comparing the same antibiotics used at the same daily dosage but for different durations for CAP in adults. Both outpatients and inpatients were included but not those admitted to intensive care units. We imposed no date, language or publication status restriction.
Data extraction and synthesis
Data extraction by two independent reviewers. We conducted a random-effects, one-stage duration-effect meta-analysis with restricted cubic splines. We tested the non-inferiority with the prespecified non-inferiority margin of 10% examined against 10 days. The primary outcome was clinical improvement on day 15 (range 7–45 days). Secondary outcomes: all-cause mortality, serious adverse events and clinical improvement on day 30 (15–60 days).
Results
We included nine trials (2399 patients with a mean (SD) age of 61.2 (22.1); 39% women). The duration-effect curve was monotonic with longer duration leading to a lower probability of improvement, and shorter treatment duration (3–9 days) was likely to be non-inferior to 10-day treatment. Harmful outcome curves indicated no association. The weighted average percentage of the primary outcome in the 10-day treatment arms was 68%. Using that average, the absolute clinical improvement rates of the following durations were: 3-day treatment 75% (95% CI: 68% to 81%), 5-day treatment 72% (95% CI: 66% to 78%) and 7-day treatment 69% (95% CI: 61% to 76%).
Conclusions
Shorter treatment duration (3–5 days) probably offers the optimal balance between efficacy and treatment burden for treating CAP in adults if they achieved clinical stability. However, the small number of included studies and the overall moderate-to-high risk of bias may compromise the certainty of the results. Further research on the shorter duration range is required.
PROSPERO registration number
CRD 42021273357.
Keywords: bacteriology, respiratory medicine (see thoracic medicine), respiratory infections
Strengths and limitations of this study.
We conducted a comprehensive and up-to-date systematic literature review.
The duration-effect meta-analysis treated duration as a continuous variable, which allowed us to estimate the duration-effect relationship with greater resolution than the conventional pairwise meta-analysis that dichotomised duration arbitrarily.
The small number of trials included limited the precision of some study results.
Most of the trials had a moderate-to-high overall risk of bias.
About 80% of the patients had Pneumonia Severity Index class III or less and thus the results may not be generalisable to severely ill patients.
Background
Community-acquired pneumonia (CAP) is a leading cause of morbidity and mortality globally, especially among the elderly.1 In the USA, it is the second most common cause of hospitalisation and the top infectious cause of death.2 3 The initial treatment for CAP is empirical, with guidelines recommending starting several antibiotics depending on patients’ severity and risk factors for certain pathogens.4–6
The optimal duration of antimicrobial therapy remains unclear and controversial. The American and British guidelines recommend a minimum of 5 days of treatment before therapy discontinuation for patients achieving clinical stability.4 5 The European guideline states that the duration of treatment should not exceed 8 days in responding patients.6 In clinical practice, however, antibiotics for pneumonia are often prescribed for 10 to 14 days.7 8 This may mean that many patients are receiving more antibiotics than necessary, with a consequent increase in costs and a higher probability of antimicrobial resistance.9 Finding the optimal duration of antibiotics can facilitate reducing antimicrobial use efficiently. Several meta-analyses have been reported on this topic.10–12 A major limitation of the method used in the previous pairwise meta-analyses is the arbitrary categorisation of duration when the original studies compared different duration, ranging from 3 to 10 days. A pairwise meta-analysis published in 2008,10 for example, categorised a 7-day treatment arm in one trial as short-course and the same in other two trials as long-course.13–15 Another pairwise meta-analysis in 2018 excluded a trial comparing 7-day against 10-day treatment because they defined long-course as 7 days or longer.11 The duration range of short-course therapy defined by a systematic review of systematic reviews and guidelines with pairwise meta-analyses in 2019 was wide (3–7 days) and the duration-effect relationship within that range remains unclear.12 We overcame the limitation of arbitrary dichotomisation of duration by using a novel method called dose-effect meta-analysis.16 It has been used, for example, to examine the effects of potassium intake or sodium reduction on blood pressure.17 18 Unlike conventional categorisation-based meta-analyses,19 dose-effect meta-analysis can reveal more fine-grained optimal dose.20 By treating duration as dose, we aimed to apply this method to obtain a more specific optimal treatment duration.
Methods
We summarised the currently available evidence to find the optimal treatment duration of antibiotics for CAP in adults. We followed the Preferred Reporting Items for Systematic reviews and Meta-Analyses.21 21 The protocol has been prospectively registered in PROSPERO and can be found in the appendix (online supplemental eAppendix 1).
bmjopen-2022-061023supp001.pdf (491KB, pdf)
Data sources
Criteria for considering studies for this review
Types of studies
To examine the duration-effect relationship, we included all trials that compared two or more different durations of the same antibiotic treatment for CAP.
Types of participants
Patients were eligible if they were 18 years or older of both genders with a diagnosis of CAP as defined by the original authors. We included both outpatients and inpatients. We excluded patients who were admitted to the intensive care unit. To focus on individuals at low-to-medium risk, we excluded trials with 20% or more patients meeting one or more of the following criteria: having immunodeficiency; having been treated with another antibiotic within a month.
Types of interventions
We included trials examining any antibiotics, administered orally or intravenously. We evaluated antibiotics as a class because clinical guidelines recommend treatment duration irrespective of the antibiotic used,4–6 and because recent meta-analyses of antibiotics for CAP have not shown efficacy differences among antibiotics.22 23 Oral and intravenous antibiotics were merged because they have been shown equally effective in many infectious conditions within the same time frame.24–26 We included trials comparing the same agents used at the same daily dosage but for different durations. We used the predefined duration for fixed-duration arms. If some studies did not prespecified the duration (eg, left it to clinicians’ judgement27), we used the median duration actually prescribed.
Primary outcome and secondary outcomes
The primary outcome of interest in this study was the clinical improvement as defined by the original authors at a time point as close to 15 days (range 7–45 days) as possible in each included study.28 Secondary outcomes of interest were: all-cause mortality on day 15 (range 7–45 days), serious adverse events as defined by the original study on day 15 (range 7–45 days) and clinical improvement as defined by the original study on day 30 (range 15–60). We used the number of randomised patients as the denominator for the intention-to-treat (ITT) data set. When only clinical failure was reported, clinical improvement was calculated by subtracting clinical failure from the total number randomised. We used ITT for the primary analysis and the per-protocol (PP) data set for a sensitivity analysis.29 30 We used the odds ratio (OR) of each outcome to synthesise data.31 32
Search methods for identification of studies
Electronic searches
We systematically searched the following electronic bibliographic databases from inception through 25 August 2021: MEDLINE, Embase and CENTRAL. We used search terms for CAP in conjunction with the names of individual antibiotics as well as the names of antibiotic classes. Detailed search formulas are presented in the appendix (online supplemental eAppendix 2). We imposed no date, language or publication status restriction.
Reference lists
We checked the reference lists of all the included studies and review articles for additional references.
Data collection and analysis
Selection of studies
Two review authors independently screened and selected the included studies (YF and one of AO, EO, SF or YL). Two review authors extracted data independently from the included studies (YF and one of AO, EO, SF or YL). We used the Cochrane risk of bias tool V.233 to assess and summarise the risk of bias. Disagreements were resolved through discussion.
Statistical analysis
To perform our analyses, we used the dosresmeta package (V.2.0.1) and meta package (V.5.0–1) for R (V.4.1.0. R foundation, Wien, Austria).34–36
Assessment of heterogeneity
We investigated the heterogeneity between studies by the variance partition coefficient (VPC).16 VPC represents the percentage of variation attributed to heterogeneity rather than sampling error and can be interpreted similarly to the I2.
Duration-effect meta-analysis
In the duration-effect meta-analysis, we assumed that the relative efficacy of a certain treatment duration () against another () can be expressed in the log-OR () and that it is a function of both durations (). We fitted restricted cubic splines with three knots to the data set obtained by the systematic review because this model has shown sufficient flexibility to capture different shapes.37 Given the clinical and methodological heterogeneity likely present in the included studies, we used the random effects model. We used three knots, equally spaced across the duration range (25%, 50% and 75%). Typically, in dose-effect meta-analyses, the reference dose is assigned to the zero or the minimal dose to make interpretation easier.37 As this duration-effect meta-analysis aimed to test the non-inferiority of the shorter treatment duration, we decided to use the maximum duration as the reference to make interpretation easier. Also, the reference we set (10-day treatment) can be regarded as the current practice.7 8 27 We tested the non-inferiority with the non-inferiority margin of 10%, as previously proposed,28 and the superiority of the shorter duration examined against 10-day treatment using the ITT data set.
Sensitivity analyses
To ascertain the robustness of the primary analyses, we conducted the following sensitivity analyses. To test the stability of the shape of the spline curves, we used different locations of knots (10%, 50% and 90%). To test the influence of trials included, we conducted sensitivity analyses excluding trials with an overall high risk of bias and excluding trials with outpatients. To test the robustness of the analytical method, we used the PP data set. To test the influence of antibiotics examined, we conducted sensitivity analyses restricting eligible antibiotics only to those recommended by clinical guidelines for empirical treatment of CAP.4 5 In addition to the predefined sensitivity analyses, we conducted exploratory sensitivity analyses including only trials that randomised before the initial antibiotic treatment to test the influence of randomisation timing. We further conducted sensitivity analyses excluding trials with substantial deviation from the day 15 measurement time and analyses imputing missing data as improved outcomes.
Amendments
We report amendments with the date and the rationale in the appendix (online supplemental eAppendix 3).
Results
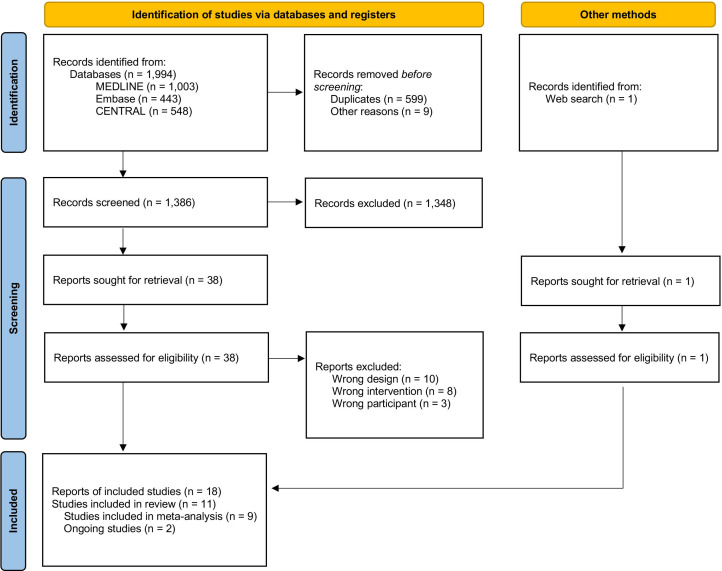
We identified 1994 records via database and 1 record via searching websites, which revealed that some different records refer to the same clinical trial. We assessed 38 full-text records for eligibility and included eleven eligible studies (figure 1). Of these, eight were published,13–15 27 38–41 one was unpublished42 and two studies were still ongoing,43 44 resulting in nine trials for the primary outcome analysis. The lists of included and excluded studies are provided in the appendix (online supplemental eAppendies 4 and 5). The nine studies with 2399 participants in total included 18 eligible arms. Treatment duration ranged from 3 to 10 days. The study year ranged between 1999 and 2021. Table 1 presents the characteristics of the included studies (more details can be found in online supplemental eAppendix 4).
Figure 1.
Preferred Reporting Items for Systematic reviews and Meta-Analyses flow diagram.
Table 1.
Characteristics of included studies
| Study | Age, mean (SD), years | Female, % | PSI IV+V, % | Setting | Duration, day, median | Antibiotics | No. of participants | No. of clinical improvement on day 15 |
| Siegel et al13 | 61.1 (15.1) | NA | NA | Inpatient | 7 | CXM | 25 | 21 |
| 10 | 27 | 20 | ||||||
| Léophonte et al38 | 64.0 (18.7) | 25 | NA | Inpatient | 5 | CRO | 125 | 93 |
| 10 | 119 | 85 | ||||||
| Tellier et al14 | 45.8 (18–87*) | 42 | 7 | Both | 5 | TEL | 193 | 154 |
| 7 | 195 | 157 | ||||||
| El Moussaoui et al39 | 57.2† (23.9†) | 40 | 12 | Inpatient | 3 | AMX | 57 | 50 |
| 8 | 64 | 56 | ||||||
| File et al15 | 45.4 (16.8) | 42 | 3 | Outpatient | 5 | GMI | 256 | 240 |
| 7 | 256 | 234 | ||||||
| Strålin et al42 | NA (NA) | NA | NA | Inpatient | 5 | β-lactam | 103 | 79 |
| 10 | 104 | 81 | ||||||
| Uranga et al27 | 65.4 (18.3) | 37 | 39 | Inpatient | 5 | Various | 162 | 90 |
| 10 | 150 | 71 | ||||||
| Aliberti et al40 | 60.6† (24.8†) | 40 | 24 | Inpatient | 6 | Various | 125 | 111 |
| 8 | 135 | 125 | ||||||
| Dinh et al41 | 73.2† (21.0†) | 41 | 39 | Inpatient | 3 | β-lactum+placebo | 152 | 117 |
| 8 | β-lactum+AMC | 151 | 102 |
*Range.
†Calculated using median and IQR.
AMC, amoxicillin-clavulanic acid; AMX, amoxicillin; CRO, ceftriaxone; CXM, cefuroxime; GMI, gemifloxacin; PSI, Pneumonia Severity Index; SAE, serious adverse events; TEL, telithromycin.
The included studies were all parallel-group and individually randomised. Seven out of nine were reported as non-inferiority trials. In total, 1199 participants were randomly assigned to the shorter duration arm and 1200 to the longer duration arm. The mean age was 61.2 years (SD 22.1); 831 (39%) of 2140 reported were women. Six were conducted in a single European country, one in the USA and the two were cross-continental. CAP was defined as newly confirmed clinical symptoms (eg, dyspnoea, cough, purulent sputum or crackles), and radiological findings. Antibiotic treatment was discontinued when the patient was clinically stable, and the predetermined treatment period was completed. Clinical stability was often defined as without fever (temperature ≤37.8°C) for 48 hours, heart rate below 100 beats per min, a respiratory rate below 24 breaths per min, arterial oxygen saturation of 90% or higher, systolic blood pressure of 90 mm Hg or higher and normal mental status.45 Clinical improvement was often described as ‘clinical cure’ or ‘clinical success’ and was often defined as the resolution of fever and improvement of symptoms related to pneumonia without further antibiotics. More detailed definitions of clinical improvement in each included study are listed in the appendix (online supplemental eAppendix 6). The percentage of Pneumonia Severity Index class IV or V was on average 19% (362 of 1896 reported; ranging from 2% to 41%). Seven studies focused on inpatients, whereas one study focused on outpatients and one included both. Antibiotics used included β-lactams (amoxicillin, amoxicillin/clavulanate, ampicillin/sulbactam, ceftazidime, ceftriaxone, cefuroxime, piperacillin/tazobactam), macrolides (azithromycin, clarithromycin), quinolones (ciprofloxacin, gemifloxacin, levofloxacin, telithromycin), amikacin, doxycycline and meropenem. Pharmaceutical companies funded four studies.13–15 38 Four studies had a high overall risk of bias, four some concerns and only one had a low overall risk of bias (online supplemental eAppendix 7).
Assessment of heterogeneity and publication bias
We assessed the heterogeneity in the efficacy outcome across the duration range (nine studies). VPC values were constantly below 10% which suggests low levels of heterogeneity. Visual inspection of the funnel plot suggested no significant publication bias. However, these assessments need to be carefully interpreted due to the small number of included studies (online supplemental eAppendies 8 and 9).
Duration-effect meta-analysis
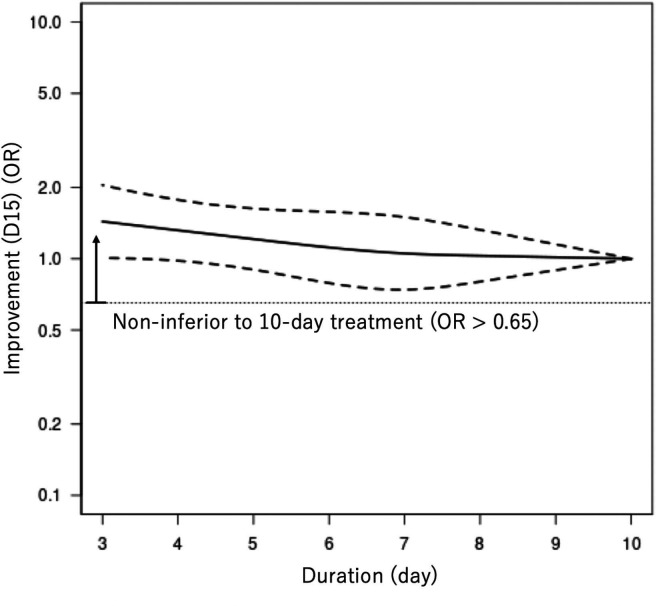
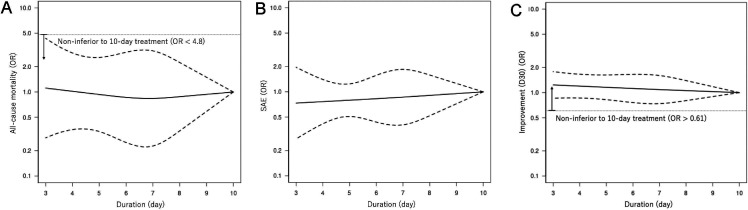
We present the duration-effect curves in figures 2 and 3, and the tabulation of results in table 2. The x-axis of the figures represents the treatment duration in days. The y-axis represents the OR of the outcome on a logarithmic scale, just as in the forest plot of conventional pairwise meta-analysis using binary outcomes. The thin dotted horizontal line in the clinical improvement figures and the all-cause mortality figure corresponds to the non-inferiority margin translated into OR. (The weighted average percentage of clinical improvement rate on day 15 in the 10-day treatment arms was 68%. The non-inferiority margin was therefore 58% and the corresponding OR was 0.65. For all-cause mortality, the numbers were 3%, 13% and OR 4.8, respectively. For clinical improvement on day 30, the numbers were 77%, 67% and OR 0.61, respectively. We did not show the non-inferiority margin in the figures for severe adverse events, because the position paper did not provide any margin for this outcome.28) The thick solid line represents the duration-effect curve and the thick dotted lines represent its 95% CI. The 95% CI band becomes narrower when the duration range was examined by many trials or when it gets closer to the reference point. For the beneficial outcomes (clinical improvement), OR >1 means more effective. For the harmful outcomes (all-cause mortality and serious adverse events), OR <1 means safer.
Figure 2.
Duration-effect relationship of antibiotics for community-acquired pneumonia in adults. Clinical improvement on day 15. D15, day 15. The dotted lines represent 95% CIs. The thin horizontal dotted line represents the non-inferiority margin, corresponding with 10% absolute risk difference given the control event rate of 68% (OR 0.65). ORs greater than the non-inferiority threshold signifies that the treatment is non-inferior to the 10-day treatment.
Figure 3.
Duration-effect relationships of antibiotics for community-acquired pneumonia in adults. (A) All-cause mortality. (B) Severe adverse events. (C) Clinical improvement on day 30. D30, day 30. The dotted lines represent 95% CIs. The thin horizontal dotted line represents the non-inferiority margin, corresponding with 10% absolute risk difference given the control event rate of 3% (OR 4.8) in all-cause mortality and 77% (OR 0.61) in clinical improvement on day 30. SAE, serious adverse event.
Table 2.
Primary and secondary outcomes for 3, 5, 7 and 10-day treatment
| Outcome | Treatment duration (days) | |||||||
| 3 | 5 | 7 | 10 | |||||
| Clinical improvement on day 15 | OR | 1.44 | (1.01–2.05) | 1.21 | (0.90–1.63) | 1.05 | (0.74–1.50) | 1.00 |
| Rate | 75% | (68–81%) | 72% | (66–78%) | 69% | (61–76%) | 68% | |
| All-cause mortality | OR | 1.11 | (0.28–4.35) | 0.93 | (0.34–2.58) | 0.84 | (0.23–3.09) | 1.00 |
| Rate | 3% | (1–11%) | 3% | (1–7%) | 2% | (1–8%) | 3% | |
| Serious adverse events | OR | 0.73 | (0.27–1.96) | 0.80 | (0.51–1.24) | 0.86 | (0.40–1.85) | 1.00 |
| Rate | 15% | (6–31%) | 16% | (11–22%) | 17% | (9–30%) | 19% | |
| Clinical improvement on day 30 | OR | 1.24 | (0.86–1.78) | 1.16 | (0.82–1.63) | 1.09 | (0.74–1.60) | 1.00 |
| Rate | 81% | (74–86%) | 80% | (74–85%) | 79% | (73–84%) | 77% | |
The duration-effect curve is monotonic with a longer duration leading to a lower probability of improvement. The lower 95% CI curve was constantly above the prespecified non-inferiority margin, meaning that a shorter treatment duration (3–9 days) was likely to be non-inferior to the standard treatment duration (10 days). It was slightly above the OR=1 around 3-day treatment, suggesting 3-day treatment may be superior to 10-day treatment. Harmful outcome curves (all-cause mortality and severe adverse events) were almost flat and 95% CI curves did not cross the OR=1, indicating no association. Although the CI curves were wide for all-cause mortality, shorter treatment duration (3–9 days) was likely to be non-inferior to 10-day treatment. Clinical improvement on day 30 showed a similar trend with the primary outcome with the lower 95% CI curve constantly above the prespecified non-inferiority margin. We made a league table (online supplemental eAppendix 10), which showed that shorter treatment duration was likely to be non-inferior to longer treatment duration, regardless of the reference duration.
ORs need to be translated into absolute event rates so that the results can be interpreted from the clinical point of view. The weighted average percentage of clinical improvement rate on day 15 in the 10-day treatment arms was 68%, based on a single proportion meta-analysis of the included studies. Using this average, we computed the absolute clinical improvement rates at the following durations as follows: 3-day treatment 75% (95% CI: 68% to 81%), 5-day treatment 72% (95% CI: 66% to 78%) and 7-day treatment 69% (95% CI: 61% to 76%) (table 2).
Sensitivity analyses
Sensitivity analyses were in line with the primary analyses. Sensitivity analyses using different locations of knots confirmed the stability of the shape of the spline curves (online supplemental eAppendix11, figure S1). Sensitivity analyses excluding trials with an overall high risk of bias were also in agreement with the primary analyses (online supplemental eAppendix11, figure S2.1). Sensitivity analyses excluding trials with outpatients also confirmed the main findings, suggesting the results are generalisable to inpatients, except for those admitted to the intensive care unit (online supplemental eAppendix11, figure S2.2). Sensitivity analyses using the PP data set and those including only trials that used antibiotics recommended for empirical treatment of CAP by clinical guidelines also confirmed the results (online supplemental eAppendix11, figures S3 and S4). Exploratory sensitivity analyses showed that non-inferiority of the shorter duration was more likely to be the case in studies that randomised patients who had reached clinical stability early (online supplemental eAppendix11, figure S5.1 and 5.2). Furthermore, post hoc sensitivity analyses which excluded trials with substantial deviation from the day 15 measurement time (online supplemental eAppendix11, figure S5.3) and those which imputed missing data as clinically improved (online supplemental eAppendix11, figure S5.4) also aligned with the primary analyses.
Discussion
To our knowledge, this is the first systematic review and duration-effect meta-analysis of antibiotics treatment for CAP in adults. The results showed that shorter treatment duration (3–9 days) was likely to be non-inferior to the standard treatment duration (10 days) for CAP in adults if they achieved clinical stability. There may be no significant difference in all-cause mortality or serious adverse events. Shorter treatment duration (3–5 days) probably achieves the optimal balance between efficacy and treatment burden. Multiple sensitivity analyses confirmed the primary findings.
This is in line with the previous pairwise meta-analyses that showed shorter duration was non-inferior to longer duration.10–12 We updated the systematic review and found four trials that were not included in the previous studies. This allowed us to focus on trials that used the same antibiotics with the same daily dosage. The previous studies included trials using different antibiotics or different daily dosages, so the results may not have reflected the differences in treatment durations alone. Moreover, they subcategorised the treatment durations and may have thus lost some statistical power to detect meaningful differences among durations. We overcame this limitation by examining the duration of antibiotic treatment range in days as a continuous variable and found that 3 to 9-day treatment is likely to be non-inferior to 10-day treatment. Our results are in line with the guidelines for CAP recommending antibiotics to be prescribed for a duration shorter (5–8 days) than current clinical standard practice (10 days).4–6 Our results suggest that an even shorter duration (3–5 days) may be considered, which is in line with the trials that found 3-day treatment was non-inferior to 8-day treatment.39 41 Possibility of 3-day treatment being superior to 10-day treatment should be carefully interpreted, as none of the included trials, previous meta-analyses11 12 or the pairwise meta-analysis of the included trials (online supplemental eAppendix 12, post hoc analysis) showed the superiority of shorter treatment duration. This could be explained by the fact that most of the combinations of treatment durations examined (7 days vs 10 days, 5 days vs 10 days, 5 days vs 7 days, 3 days vs 8 days) suggested better efficacy of shorter durations, if not statistically significant alone (online supplemental eAppendix 12, post hoc analysis). The duration-effect meta-analysis combined these findings, leading to the possible superiority of the shortest duration examined (3 days) over the longest duration examined (10 days). Further research focusing on the shorter duration range is warranted to confirm this finding.
Limitations
Our study has several limitations. First, most of the included studies presented a moderate-to-high overall risk of bias, which compromises the validity of this meta-analysis. Second, the number of studies was small, leaving CIs for secondary outcomes wide. Third, original studies excluded patients with complications of CAP and therefore the results of this study may not be generalisable to those patients. Fourth, baseline severity of the included studies varied. We included both the outpatients and inpatients, which may have concealed important heterogeneity in the study results. However, sensitivity analyses excluding trials with outpatients generally confirmed the primary analyses (online supplemental eAppendix 11) and the overall statistical heterogeneity was low. Fifth, we did not include patients admitted to the intensive care units and the results of this study may not be generalisable to those patients. Sixth, the actual measurement day for the primary outcome in each included study varied (7–44 days) and this may have introduced between-study heterogeneity. However, post hoc sensitivity analyses excluding trials with large deviation from the day 15 measurement time were in line with the primary analyses.
Strengths
First, we did a comprehensive systematic review and found four studies that were not included in the previous systematic reviews. Second, we treated duration as a continuous variable, which allowed us to estimate the duration-effect relationship with greater resolution of change points. Third, we examined the impacts of treatment duration not only for clinical improvement but also for all-cause mortality and severe adverse events and made sure that a shorter treatment duration would not translate into more harmful events. Finally, the very nature of shortened duration treatment offers a unique opportunity for interpretation. Shorter treatment duration has been examined by non-inferiority trials. The underlying assumption has been that there was a trade-off between a loss in the efficacy of standard treatment duration and other benefits of shortened treatment duration,46 47 such as less time, less cost and probably a diminished rate of antimicrobial resistance. This study suggests that there may be even no trade-off for antibiotic treatments of 3–5 days. The shorter treatment duration reduces the burden on patients, the healthcare system and the risk of antimicrobial resistance and might even offer better clinical outcomes at the same time.
Conclusions
Short treatment duration (3–9 days) was likely to be non-inferior to the standard treatment duration (10 days) for adults with CAP if they achieved clinical stability. Shorter range (3–5 days) probably results in an optimal balance between efficacy and treatment burden. However, the small number of included studies and the overall moderate-to-high risk of bias may compromise the certainty of the results. Further research focusing on the shorter duration range is required.
Supplementary Material
Footnotes
Twitter: @funada_satoshi, @Toshi_FRKW
Contributors: All authors had full access to all of the data (including statistical reports and tables) in this study and take full responsibility for the integrity of the data and the accuracy of the data analysis. Conception and design: YF, YL, SF, AO, EO, TAF and YK. Analysis and interpretation of the data: YF, YL, SF, AO, EO, TH, TAF and YK. Drafting of the article: YF. Critical revision of the article for important intellectual content: YL, SF, AO, EO, TH, TAF and YK. Final approval of the article: YF, YL, SF, AO, EO, TH, TAF and YK. Obtaining of funding: None. Administrative, technical or logistical support: YF and TH. Collection and assembly of data: YF, YL, SF, AO and EO. Guarantor: YF. Transparency declaration: As guarantor, YF affirms that the manuscript is an honest, accurate and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Funding: This study has been supported in part by JSPS KAKENHI (22K19688) to TAF.
Disclaimer: The views expressed are those of the authors and not necessarily those of affiliated organisations.
Competing interests: YL is receiving a Grant-in-Aid for JSPS Fellow (KAKENHI Grant Number 21J15050). SF has a research grant from JSPS KAKENHI Grant Number JP 20K18964 and the KDDI Foundation. AO obtained speakers fees from Chugai Pharmaceutical, Asahi Kasei Corporation, Eli Lilly, AbbVie GK, Pfizer, Mitsubishi Tanabe Pharma Corporation and GlaxoSmithKline, and research grants from Advantest and JSPS KAKENHI outside the submitted work. EO has received research and consultancy fees from Angelini Pharma. EO is supported by the National Institute for Health Research (NIHR) Research Professorship to Professor Andrea Cipriani (grant RP-2017-08-ST2-006), by the NIHR Applied Research Collaboration (ARC) Oxford and Thames Valley, by the NIHR Oxford Cognitive Health Clinical Research Facility and by the NIHR Oxford Health Biomedical Research Centre (grant BRC-1215-20005). TAF reports grants and personal fees from Mitsubishi-Tanabe, personal fees from MSD, personal fees from Shionogi, personal fees from Sony, outside the submitted work; in addition, TAF has a patent 2018-177688 concerning smartphone CBT applications pending, and intellectual properties for Kokoro-application licensed to Mitsubishi-Tanabe. YK received a research grant from the Systematic Review Workshop Peer Support Group, the Japan Osteoporosis Foundation and Yasuda Memorial Medical Foundation for other research purposes. YF and TH declare no conflicts of interest.
Patient and public involvement: Patients or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.GBD 2016 Lower Respiratory Infections Collaborators . Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990-2016: a systematic analysis for the global burden of disease study 2016. Lancet Infect Dis 2018;18:1191–210. 10.1016/S1473-3099(18)30310-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Most frequent conditions in U.S. hospitals. 2011. Available: https://www.hcup-us.ahrq.gov/reports/statbriefs/sb162.pdf
- 3.Xu J, Murphy SL, Kochanek KD, et al. Deaths: final data for 2013. Natl Vital Stat Rep 2016;64:1–119. [PubMed] [Google Scholar]
- 4.Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American thoracic Society and infectious diseases Society of America. Am J Respir Crit Care Med 2019;200:e45–67. 10.1164/rccm.201908-1581ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Institute of Health and Care Excellence (NICE) . Pneumonia (community-acquired): antimicrobial prescribing. n.d. Available: https://www.nice.org.uk/guidance/NG138
- 6.Woodhead M, Blasi F, Ewig S, et al. Guidelines for the management of adult lower respiratory tract infections--full version. Clin Microbiol Infect 2011;17:E1–59. 10.1111/j.1469-0691.2011.03672.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aliberti S, Blasi F, Zanaboni AM, et al. Duration of antibiotic therapy in hospitalised patients with community-acquired pneumonia. Eur Respir J 2010;36:128–34. 10.1183/09031936.00130909 [DOI] [PubMed] [Google Scholar]
- 8.Yi SH, Hatfield KM, Baggs J, et al. Duration of antibiotic use among adults with uncomplicated community-acquired pneumonia requiring hospitalization in the United States. Clin Infect Dis 2018;66:1333–41. 10.1093/cid/cix986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guillemot D, Carbon C, Balkau B, et al. Low dosage and long treatment duration of beta-lactam: risk factors for carriage of penicillin-resistant Streptococcus pneumoniae. JAMA 1998;279:365–70. 10.1001/jama.279.5.365 [DOI] [PubMed] [Google Scholar]
- 10.Dimopoulos G, Matthaiou DK, Karageorgopoulos DE, et al. Short- versus long-course antibacterial therapy for community-acquired pneumonia: a meta-analysis. Drugs 2008;68:1841–54. 10.2165/00003495-200868130-00004 [DOI] [PubMed] [Google Scholar]
- 11.Tansarli GS, Mylonakis E. Systematic review and meta-analysis of the efficacy of short-course antibiotic treatments for community-acquired pneumonia in adults. Antimicrob Agents Chemother 2018;62:e00635-18. 10.1128/AAC.00635-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Furlan L, Erba L, Trombetta L, et al. Short- vs long-course antibiotic therapy for pneumonia: a comparison of systematic reviews and guidelines for the SIMI choosing wisely campaign. Intern Emerg Med 2019;14:377–94. 10.1007/s11739-018-1955-2 [DOI] [PubMed] [Google Scholar]
- 13.Siegel RE, Alicea M, Lee A, et al. Comparison of 7 versus 10 days of antibiotic therapy for hospitalized patients with uncomplicated community-acquired pneumonia: a prospective, randomized, double-blind study. Am J Ther 1999;6:217–22. 10.1097/00045391-199907000-00007 [DOI] [PubMed] [Google Scholar]
- 14.Tellier G, Niederman MS, Nusrat R, et al. Clinical and bacteriological efficacy and safety of 5 and 7 day regimens of telithromycin once daily compared with a 10 day regimen of clarithromycin twice daily in patients with mild to moderate community-acquired pneumonia. J Antimicrob Chemother 2004;54:515–23. 10.1093/jac/dkh356 [DOI] [PubMed] [Google Scholar]
- 15.File TM, Mandell LA, Tillotson G, et al. Gemifloxacin once daily for 5 days versus 7 days for the treatment of community-acquired pneumonia: a randomized, multicentre, double-blind study. J Antimicrob Chemother 2007;60:112–20. 10.1093/jac/dkm119 [DOI] [PubMed] [Google Scholar]
- 16.Crippa A, Discacciati A, Bottai M, et al. One-Stage dose-response meta-analysis for aggregated data. Stat Methods Med Res 2019;28:1579–96. 10.1177/0962280218773122 [DOI] [PubMed] [Google Scholar]
- 17.Filippini T, Naska A, Kasdagli M-I, et al. Potassium intake and blood pressure: a dose-response meta-analysis of randomized controlled trials. J Am Heart Assoc 2020;9:e015719. 10.1161/JAHA.119.015719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Filippini T, Malavolti M, Whelton PK, et al. Blood pressure effects of sodium reduction. Circulation 2021;143:1542–67. 10.1161/CIRCULATIONAHA.120.050371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Højlund M, Kemp AF, Haddad PM, et al. Standard versus reduced dose of antipsychotics for relapse prevention in multi-episode schizophrenia: a systematic review and meta-analysis of randomised controlled trials. Lancet Psychiatry 2021;8:471–86. 10.1016/S2215-0366(21)00078-X [DOI] [PubMed] [Google Scholar]
- 20.Leucht S, Bauer S, Siafis S, et al. Examination of dosing of antipsychotic drugs for relapse prevention in patients with stable schizophrenia: a meta-analysis. JAMA Psychiatry 2021;78:1238–48. 10.1001/jamapsychiatry.2021.2130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Montes-Andujar L, Tinoco E, Baez-Pravia O, et al. Empiric antibiotics for community-acquired pneumonia in adult patients: a systematic review and a network meta-analysis. Thorax 2021;76:1020–31. 10.1136/thoraxjnl-2019-214054 [DOI] [PubMed] [Google Scholar]
- 23.Pakhale S, Mulpuru S, Verheij TJM, et al. Antibiotics for community-acquired pneumonia in adult outpatients. Cochrane Database Syst Rev 2014;2014:CD002109. 10.1002/14651858.CD002109.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keren R, Shah SS, Srivastava R, et al. Comparative effectiveness of intravenous vs oral antibiotics for postdischarge treatment of acute osteomyelitis in children. JAMA Pediatr 2015;169:120–8. 10.1001/jamapediatrics.2014.2822 [DOI] [PubMed] [Google Scholar]
- 25.Li H-K, Rombach I, Zambellas R, et al. Oral versus intravenous antibiotics for bone and joint infection. N Engl J Med 2019;380:425–36. 10.1056/NEJMoa1710926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iversen K, Ihlemann N, Gill SU, et al. Partial oral versus intravenous antibiotic treatment of endocarditis. N Engl J Med 2019;380:415–24. 10.1056/NEJMoa1808312 [DOI] [PubMed] [Google Scholar]
- 27.Uranga A, España PP, Bilbao A, et al. Duration of antibiotic treatment in community-acquired pneumonia: a multicenter randomized clinical trial. JAMA Intern Med 2016;176:1257–65. 10.1001/jamainternmed.2016.3633 [DOI] [PubMed] [Google Scholar]
- 28.Spellberg B, Talbot GH, Brass EP, et al. Position paper: recommended design features of future clinical trials of antibacterial agents for community-acquired pneumonia. Clin Infect Dis 2008;47:S249–65. 10.1086/591411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bai AD, Komorowski AS, Lo CKL, et al. Intention-To-Treat analysis may be more conservative than per protocol analysis in antibiotic non-inferiority trials: a systematic review. BMC Med Res Methodol 2021;21:75. 10.1186/s12874-021-01260-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aberegg SK, Hersh AM, Samore MH. Empirical consequences of current recommendations for the design and interpretation of noninferiority trials. J Gen Intern Med 2018;33:88–96. 10.1007/s11606-017-4161-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bakbergenuly I, Hoaglin DC, Kulinskaya E. Pitfalls of using the risk ratio in meta-analysis. Res Synth Methods 2019;10:398–419. 10.1002/jrsm.1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Doi SA, Furuya-Kanamori L, Xu C, et al. Controversy and debate: questionable utility of the relative risk in clinical research: paper 1: a call for change to practice. J Clin Epidemiol 2022;142:271–9. 10.1016/j.jclinepi.2020.08.019 [DOI] [PubMed] [Google Scholar]
- 33.Sterne JAC, Savović J, Page MJ, et al. Rob 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898. 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 34.R Core Team . R: A language and environment for statistical computing. R foundation for statistical computing. 2020. Available: https://www.R-project.org/
- 35.Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health 2019;22:153–60. 10.1136/ebmental-2019-300117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crippa A, Orsini N. Multivariate dose-response meta-analysis: the dosresmeta R package. 2016. 10.18637/jss.v072.c01 [DOI]
- 37.Hamza T, Furukawa TA, Orsini N, et al. Dose-Effect meta-analysis for psychopharmacological interventions using randomised data. Evid Based Ment Health 2022;25:1–6. 10.1136/ebmental-2021-300278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Léophonte P, Choutet P, Gaillat J, et al. Efficacité comparée de la ceftriaxone dans un traitement de DIX jours versus un traitement raccourci de cinq jours des pneumonies aigues communautaires de l’Adulte hospitalisé avec facteur de Risque. Médecine et Maladies Infectieuses 2002;32:369–81. 10.1016/S0399-077X(02)00384-0 [DOI] [Google Scholar]
- 39.el Moussaoui R, de Borgie CAJM, van den Broek P, et al. Effectiveness of discontinuing antibiotic treatment after three days versus eight days in mild to moderate-severe community acquired pneumonia: randomised, double blind study. BMJ 2006;332:1355. 10.1136/bmj.332.7554.1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aliberti S, Ramirez J, Giuliani F, et al. Individualizing duration of antibiotic therapy in community-acquired pneumonia. Pulm Pharmacol Ther 2017;45:191–201. 10.1016/j.pupt.2017.06.008 [DOI] [PubMed] [Google Scholar]
- 41.Dinh A, Ropers J, Duran C, et al. Discontinuing β-lactam treatment after 3 days for patients with community-acquired pneumonia in non-critical care wards (PTC): a double-blind, randomised, placebo-controlled, non-inferiority trial. Lancet 2021;397:1195–203. 10.1016/S0140-6736(21)00313-5 [DOI] [PubMed] [Google Scholar]
- 42.Strålin K, Rubenson A, Lindroth H, et al. Betalactam treatment until no feve for 48 hours (at least 5 days) versus 10 days in community-acquired pneumonia: randomised, non-inferiority, open study. Pneumonia 2014;3:246–81. 10.1007/BF03399446 [DOI] [Google Scholar]
- 43.Adequate duration of antibiotic treatment in community-acquired pneumonia with high risk class and adequate initial clinical response (2017-001406-15). NCT03609099. n.d. Available: https://clinicaltrials.gov/ct2/show/NCT03609099
- 44.Shortened antibiotic treatment of 5 days in community-acquired pneumonia (CAP5). NCT04089787. n.d. Available: https://clinicaltrials.gov/ct2/show/NCT04089787
- 45.Halm EA, Fine MJ, Marrie TJ, et al. Time to clinical stability in patients hospitalized with community-acquired pneumonia: implications for practice guidelines. JAMA 1998;279:1452–7. 10.1001/jama.279.18.1452 [DOI] [PubMed] [Google Scholar]
- 46.Mulla SM, Scott IA, Jackevicius CA, et al. How to use a noninferiority trial: users’ guides to the medical literature. JAMA 2012;308:2605–11. 10.1001/2012.jama.11235 [DOI] [PubMed] [Google Scholar]
- 47.Acuna SA, Chesney TR, Baxter NN. Incorporating patient preferences in noninferiority trials. JAMA 2019;322:305–6. 10.1001/jama.2019.7059 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-061023supp001.pdf (491KB, pdf)
Data Availability Statement
Data are available upon reasonable request.