Abstract
Objective:
Reports of smell loss following traumatic brain injury (TBI) are a well-documented but understudied phenomenon. Given the broad consequences of olfactory loss, we characterized psychophysical olfactory dysfunction in individuals with moderate to severe TBI using systematic review and meta-analytic methods.
Methods:
Following Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) protocol, five databases (PubMed, EMBASE, Cochrane Library, Web of Science, Scopus) were reviewed for studies investigating olfactory dysfunction in persons with moderate to severe TBI. Of the 5,223 studies reviewed, 19 met our inclusion criteria for the systematic review and 11 met inclusion criteria for meta-analysis. We calculated effect sizes (Hedges’ g) to characterize the degree of olfactory dysfunction between patients with moderate to severe TBI and controls.
Results:
A total of 951 moderate-severe TBI patients from 19 studies were included in the systematic review, which largely demonstrated poorer olfactory psychophysical performances in this patient population. Meta-analysis demonstrated a large effect size for olfactory dysfunction in moderate-severe TBI relative to healthy controls (g=−2.43, 95%CI: −3.16<δ<−1.69). The magnitude of the effect was moderated by age and patient sex, with larger effect sizes associated with older age (following exclusion of a pediatric population) and larger compositions of women in the patient group.
Conclusions:
Moderate to severe TBI is associated with prominent olfactory dysfunction. Significant research gaps remain regarding the mechanism, recovery and natural history of olfactory dysfunction following moderate to severe TBI, which has significant clinical implications for the identification and treatment for those with post-traumatic olfactory dysfunction.
Keywords: head injury, smell, anosmia, hyposmia, head trauma
Introduction
The olfactory nerve is the only cranial nerve with direct exposure to the environment, making it especially vulnerable to illness, disease, environmental pathogens, and traumatic injury. Head injury is a common cause of dysosmia, accounting for 10–20% of patients with smell loss (Kim et al., 2017; Schafer et al., 2021; Temmel et al., 2002). The reported incidence of olfactory dysfunction following mild TBI ranges from 0 to 13% and these rates can increase to 15 to 30% following moderate to severe TBI (Haxel et al., 2008; Yousem et al., 1996). The clinical manifestation of post-traumatic olfactory dysfunction is believed to arise from different mechanisms, including shearing of the olfactory nerve at the level of the cribriform plate, disruption of the sinonasal tract, and focal damage within the olfactory bulb or cortical brain regions that subserve olfactory processing (Howell et al., 2018).
A 1964 clinical series of 1,167 head injury cases indicated an incidence of 5–7% for olfactory loss, assessed through self-report and non-standardized olfactory testing (Sumner, 1964). Since then, multiple studies, bolstered by the creation of standardized psychometrically-validated olfactory assessments and advanced neuroimaging techniques, have provided a better understanding of post-traumatic olfactory dysfunction and its underlying pathophysiology (Doty et al., 1984; Kobal et al., 1996). Indeed, studies have evaluated the influence of demographic factors, injury characteristics, and time since trauma on olfactory dysfunction in TBI patients who presented with olfactory complaints (Doty et al., 1997). One group found that anosmia following TBI is associated with abnormalities of the peripheral and central olfactory system (Yousem et al., 1999; 1996). These developments, coupled with improved definitions of TBI severity, incorporating factors such as the Glasgow Coma Scale (GCS), loss of consciousness (LOC), and post-traumatic amnesia (PTA), have advanced our understanding of post-traumatic olfactory dysfunction.
Though prior systematic reviews on olfactory dysfunction in TBI have been conducted (Proskynitopoulos et al., 2016; Schofield et al., 2014), the effect of TBI severity has not been completely examined. To our knowledge, no study has quantified the degree of olfactory dysfunction in moderate to severe TBI patients. Prior reviews demonstrate that there is limited data from prospective, controlled studies of olfactory psychophysical functioning in mild TBI, which can be difficult to interpret due to varied injury definitions and small sample sizes. For this reason, the current study focused on the moderate-severe TBI population with high-level evidence. We addressed knowledge gaps through a systemic review of the extant literature and employed meta-analytic methods to characterize the degree of olfactory impairment in moderate to severe TBI using more rigorous inclusion criteria. Meta-regression methods were employed to examine the influence of age, sex and duration since injury on effect size magnitude.
Methods
Literature Search
A comprehensive literature review using five databases (PubMed, EMBASE, Cochrane Library, Web of Science, and SCOPUS) was performed in October 2021 following Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines (Liberati et al., 2009). Records were obtained by a qualified data scientist. Search terms including “olfaction disorders,” “anosmia,” and “smell disorder” were combined with “brain injuries,” “traumatic brain injury,” and “head injury.” For our inquiry on PubMed, we employed the medical subject headings (MeSH), a hierarchical vocabulary system organized by the National Library of Medicine. The full search terms for each database are presented in Online Resource 1. The overall search yielded 5,223 records. Following removal of 2,051 duplicates, the remaining 3,172 studies were imported and managed in Covidence (Veritas Health Innovation Ltd, Melbourne, Australia) for title/abstract screening. Following abstract review by two authors, 244 articles remained for full text review. Disagreement on inclusion criteria was reached by consensus with another author. No additional records were obtained following review of full text article reference lists. A flowchart of the literature search and study selection is presented in Figure 1.
Fig. 1.
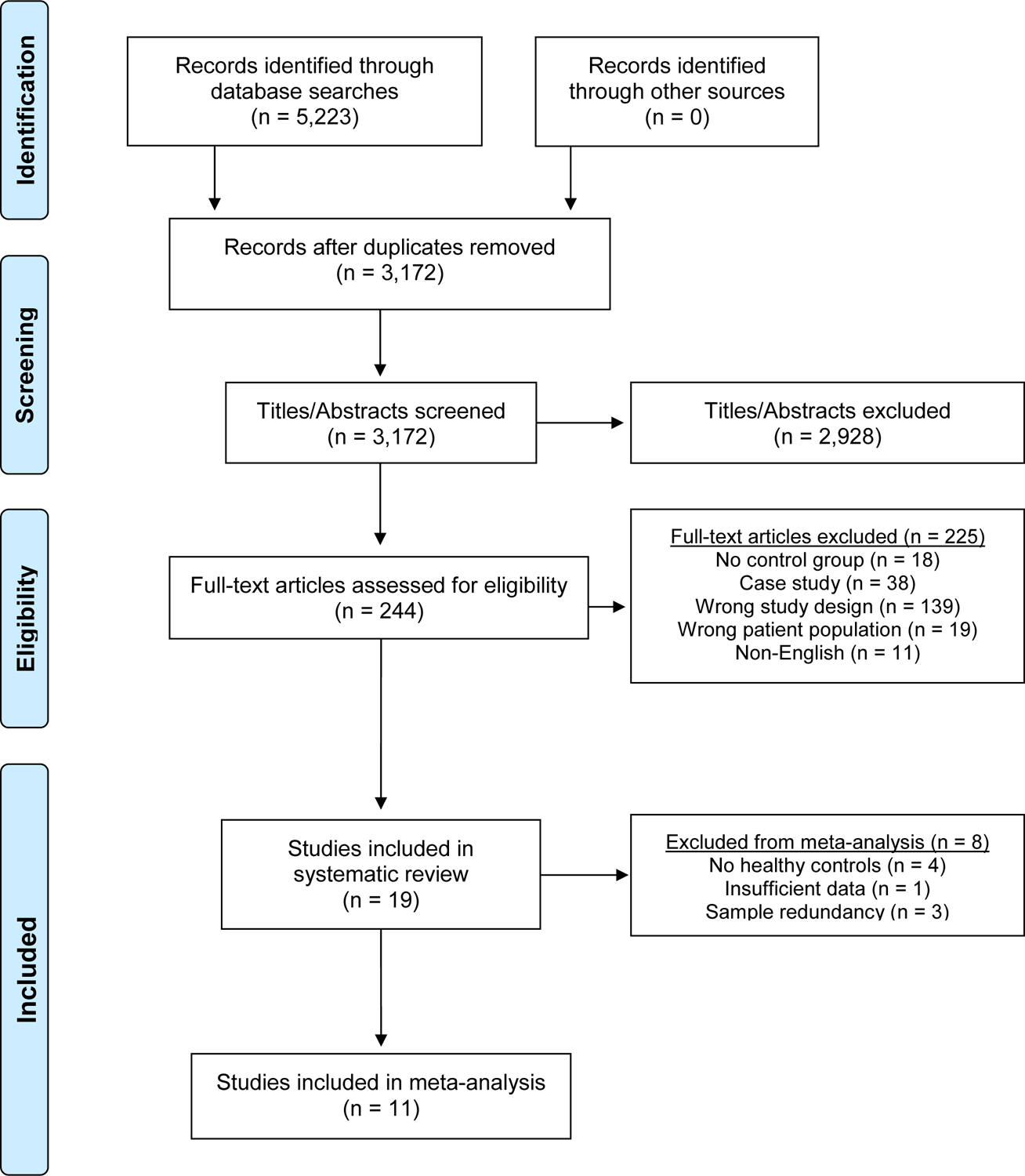
PRISMA Flow Diagram of the literature search and study selection
Study Selection
Two authors independently reviewed full text articles for the following systematic review inclusion criteria:
Availability of results in English.
Presence of an adult or pediatric sample with a moderate to severe TBI cohort.
Presence of a comparison group without subjective chemosensory complaints. Of note, control groups with prior TBI but without olfactory complaints were satisfactory to meet inclusion criteria for the systematic review but not meta-analysis, as described below.
Formal measurement of psychophysical olfactory functioning with a psychometrically-validated assessment of odor detection threshold, odor discrimination, odor identification, or odor memory (described in Olfactory Domain and Task Type).
Sufficient information on injury characteristics, including imaging abnormalities, GCS, PTA, LOC or alteration of consciousness (AOC), to classify TBI severity as moderate, severe, or moderate / severe, mixed (including >50% moderate/severe) based on the 2021 Department of Veterans Affairs / Department of Defense Clinical Practice Guideline criteria for TBI classification (VA/DoD, 2021) or by the traditional GCS definition (Table 1) (Teasdale & Jennett, 1974).
Nineteen studies were included in the qualitative systematic review following application of these criteria. For meta-analysis, additional inclusion criteria were applied:
Inclusion of a healthy comparison group without subjective chemosensory, cognitive, or neurologic complaints and without history of head injury. Four studies were excluded on the basis of this criteria (Bratt et al., 2018; Haxel et al., 2008; Neumann et al., 2012; Sigurdardottir et al., 2016).
Sufficient olfactory data to generate an effect size (i.e., p-values, means and standard deviations). One study was excluded on the basis of this criteria (Osborne-Crowley & McDonald, 2016).
To address instances when an author group published more than one relevant study on the same sample, one representative publication with the largest sample or most detailed clinical assessment was retained. Three publications were excluded from meta-analysis based on this criteria (Green et al., 2003; Han et al., 2018b; Yousem et al., 1996).
Eleven studies met inclusion criteria for meta-analysis (see Table 2).
Table 1:
Classification of TBI Severity Based on Veteran’s Affairs / Department of Defense Clinical Practice Guideline TBI Criteria and GCS scale
| Criteria | Mild* | Moderate | Severe |
|---|---|---|---|
| Structural imaging | Normal | Normal or abnormal | Normal or abnormal |
| LOC | 0–30min | <30 min and <24 hours | >24 hours |
| AOC / mental state | Up to 24 hours | >24 hours, severity based on other criteria | |
| PTA | 0–1 day | >1 day and >7 days | >7 days |
| GCS (best in first 24 hours) | 13–15 | 9–12 | 3–8 |
If a patient meets criteria for more than one category of severity, the higher severity level is assigned. Note: AOC: alteration of consciousness; GCS: Glasgow Coma Scale; LOC: loss of consciousness; PTA: post traumatic amnesia; TBI: traumatic brain injury;
Studies assessing individuals with mild TBI were excluded
Table 2:
Descriptive characteristics of studies included in systematic review and meta-analysis
| Study | Patient Number | TBI Metric | Olfactory Tests | Recruitment location | Olfactory Scale | Conclusions |
|---|---|---|---|---|---|---|
| TBI Severity | ||||||
| Bratt (2018) | 28 TBI (25 OD, 3 normosmic) |
GCS, Imaging | Sniffin’ Sticks | Level 1 neurosurgical trauma referral center | TDI 1–48 Normosmia: >30.5 Hyposmia: >16.5 - ≤30.5 Anosmia: ≤16.5 |
Patients were initially evaluated based on self-reported olfactory status. Patients reporting no olfactory dysfunction were considered “normosmic” (154/182, 84.6%). Psychophysical testing performed in 28 patients with positive self-screening. OD identified in 25/28 (25/182, 13.7%), 15 of which had anosmia. Averaged TDI score was 17.4±9.3. Anosmia present in 8.2% in chronic phase after trauma (9–104 months post injury). |
| Mod/Sev | ||||||
| Green (2003) | 35 TBI OD 196 Control |
GCS, LOC, PTA, imaging | AST | Referred to private practice for psychological or neuropsychological assessment | AST 0–10 per nostril Normosmic: >2.4 OD: ≤2.4 |
Data provided for severity based on GCS and PTA (as well as when both were considered together), and stratified by abnormal imaging. Based on GCS: Both Mod TBI (2.92±2.9) and Sev TBI (3.41±27) patients had lower AST scores than control (5.8±2.1) (p<0.001). Greater OD with increased severity of head injury. |
| Mod/Sev | ||||||
| Han (2018b) | 19 TBI hyposmic 21 TBI anosmic 19 Control |
Imaging | Sniffin’ Sticks | Smell and Taste outpatient clinic | TDI 1–48 Normosmia: >30.5 Hyposmia: >16.5 - ≤30.5 Anosmia: ≤16.5 |
Imaging study evaluating gray matter density in setting of smell. Participant groups dictated by outcome of Sniffin’ Sticks scores. Olfactory function was impaired in all smell domains in patients compared to controls. TDI: control: 32.0±3.2, Hyposmia 22.3±4.1, Anosmia 11.0±2.7 (p<0.001) Threshold: control: 7.9±2.3, hyposmia 3.7±2.3, anosmia 1.1±0.1 (p<0.001) Discrimination: control 12.6±1.6, hyposmia 9.9±2.0, anosmia 6.2±2.2 (p<0.001) Identification: control 13.5±1.2, hyposmia 8.8±2.4, anosmia 3.7±1.6 (p<0.001) |
| Mixed | ||||||
| Osborne-Crowley (2016) | 23 TBI OD 15 Control |
PTA | B-SIT | Outpatient records from three brain surgery units | NR | OD identified in 8/23 (35%) TBI patients and 2/15 (13%) controls. Of the 8 OD patients, only 3 were aware of their dysfunction. |
| Severe | ||||||
| Sigurdardottir (2016) | 132 TBI (~30–80% OD) |
GCS, GOAT | UPSIT (65 patients) B-SIT (64 patients) |
Admitted to neurosurgical departments | UPSIT 0–40 normosmia: 34–40 (males), 35 to 40 (females) mild hyposmia: 26–33 (males), 26–34 (females) severe hyposmia: 19–25 (males, females) anosmia: 6–18 (males, females). possible malingering: 0–5 B-SIT 0–12 normosmia: 9 −12 hyposmia: 7–8 anosmia: 2–6 possible malingering: 0–1 |
UPSIT: OD overall 58/67 (89.2%) - hyposmia 35/67 (53.8%), anosmia 23/67(35.4%), malingering 2/67 B-SIT: OD overall 19/65 (29.7%) - hyposmia 6/65 (9.4%), anosmia 13/65 (20.3%), malingering 1/65 Significantly different results based on testing method (p<0.001), but concluded ~30% of severe TBI patients had anosmia. |
| Severe | ||||||
| Neumann (2012) | 106 TBI (59 OD, 47 normosmic) |
GCS, LOC, PTA | B-SIT | Outpatient brain injury rehabilitation centers, local brain injury support groups | B-SIT 0–12 Normative data used for classification. |
Dysosmia was present in 59/106 (56%) of patients. People with dysosmia had higher rates of self-reported OD than normosmics (p=0.016), but only 36% of dysosmics were aware of OD. B-SIT did not significantly correlate with GCS (p=0.128), LOC (p=0.058), or PTA duration (p=0.219). |
| Mod/Sev | ||||||
| Haxel (2008) | 82 TBI (20 OD, 62 normosmic) |
GCS, Imaging | B-SIT (82 patients) Sniffin’ Sticks (19 patients) |
Admitted to hospital for head injury / trauma | B-SIT 0–12 Normosmia: ≥9 OD: <9 TDI 1–48 Normosmia: >27 Hyposmia: >16 - ≤27 Anosmia: ≤15.5 |
B-SIT: OD 14/82 (17.1%). Only 8/14 (57%) of OD patients self-reported OD. Sniffin’ Sticks: hyposmia 3/19 (15.8%), anosmia 7/19 (36.8%). Average TDI score of reported data was 23.0±9.7. GCS and LOC were not correlated with OD after head injury. |
| Mixed | ||||||
| Yousem (1996) | 25 TBI OD 8 Control |
Imaging | UPSIT 12-item memory test Single-staircase odor detection threshold |
Smell and Taste center | UPSIT: 0–40 (0–20 each nostril) Normosmic: >34 Mild hyposmic ≥27-≤34 Severe hyposmic: ≥18-≤25 Anosmic: <18 Odor memory: 0–12 per nostril PEA threshold: Anosmia: −2.0 |
UPSIT: OD 24/25 (96%); anosmia 12/25 (48%), severe OD 8/25 (32%), mild OD 4/25 (16%). Odor Memory: Control 16±2.8; 19/25 (76%) of TBI patients scored below 10 Odor Discrimination: Control 21.8±2.8; 22/25 (88%) of TBI patients scored below 20 Odor Detection Threshold: Control −6.3±1.6; 17/25 (68%) of TBI patients scored −2.0 MRI abnormalities: 22/25 (88%) OBs and tracts, 15/25 (60%) subfrontal regions, and 8/25 (32%) temporal lobes. |
| Mod/Sev | ||||||
| Meta-Analysis Inclusion Criteria Met | ||||||
| Yamaki (2020) | 31 TBI OD 10 Control |
GCS, Imaging |
OSIT-J | Admitted to hospital for severe TBI | OSIT-J 0–12 Normosmic: ≥8 OD: <8 |
OD identified in 28/31 (90.3%) of patients and 0/10 controls. Anosmia in 8/31 (25.8%), parosmia in 14/31 (45.2%), and both anosmia and parosmia in 6/31 (19.4%). OSIT-J scores were 2.9±2.8 in patients and 10.1±1.4 in controls (p<0.0001). |
| Severe | ||||||
| Green (2001) | 133 TBI OD 126 Control |
GCS, LOC, PTA, imaging | AST | Referred to private practice for psychological or neuropsychological assessment | AST 0–10 per nostril | AST score worse in “Definitive” TBI (7.3±5.4, p<0.0001) and severe TBI (7.0±5.9, p<0.0001) when compared to controls (11.4±4.1). After removing patients with low effort, there was modest correlations between smell test total scores and PTA duration (r = −0.23, p = 0.001), GCS (r = 0.30, p < 0.001), and abnormality on CT (r = −0.40, p < 0.001, n = 143). Patients with more severe TBI injuries were 10–12 times more likely to have OD than mild TBI. |
| Mod/Sev | ||||||
| Han (2018a) | 22 TBI hyposmic 24 TBI anosmic 22 Control |
Imaging | Sniffin’ Sticks | History of head injury | TDI 1–48 Normosmia: >30.5 Hyposmia: >16.5 - ≤30.5 Anosmia: ≤16.5 |
Imaging study evaluating gray matter density in setting of smell. Participant groups dictated by outcome of Sniffin’ Sticks scores. Olfactory function was impaired in all smell domains in patients compared to controls. TDI: control: 33.8±3.1, Hyposmia 22.0±4.0, Anosmia 11.3±2.7 (p<0.001) Odor Detection Threshold: control: 7.8±2.2, hyposmia 3.5±2.3, anosmia 1.1±0.3 (p<0.001) Odor Discrimination: control 12.4±1.7, hyposmia 10.1±1.9, anosmia 6.3±2.3 (p<0.001) Odor Identification: control 13.6±1.3, hyposmia 8.4±2.5, anosmia 3.9±1.6 (p<0.001) Compared to controls, hyposmic and anosmic patients had lower left (p-0.002, p<0.001)), right (p=0.01, p<0.001), and whole OB (p=0.002, p<0.001) volumes. |
| Mixed | ||||||
| Miao (2015) | 21 TBI OD 26 Control |
LOC, Imaging | Sniffin’ Sticks T&T | Department of Otolaryngology, patients complaining of OD | Normative data used for classification. | Study evaluating MRI and oERP in traumatic anosmic patients. TDI scores worse in cases (5.38±2.826) versus controls (32.05±2.89) (p=0.001). T&T scores worse in cases (5.92±0.13) versus controls (−0.99±0.97) (p=0.001). MRI: In patients with measurable olfactory bulbs (OB), OB volume was lower than controls on right (p=0.005) and left (p=0.012) sides. oERP: oERPs were detectable in 17 patients, but had longer latencies and lower amplitudes than in controls (p<0.05). Nine anosmic patients had no detectable oERPs. |
| Mixed (primarily Mod/Sev based off abnormal imaging) | ||||||
| Xydakis (2015) | 40 TBI OD 47 Control (normal neuroimaging) 8 Control (no neuroimaging) |
Imaging, Injury Severity Score | UPSIT | US service members with blast-related injuries requiring transfer to the US | UPSIT: 0–40 Normosmic: ≥33 Hyposmic: ≥25–33 Anosmic: <25 |
OD reported in 14/40 (35.0%) of patients with moderate/severe TBI. Olfactory testing predicted abnormal neuroimaging better than chance alone (AUC 0.78, p<0.001). |
| Mod/Sev | ||||||
| Parma (2012) | 12 TBI OD 12 Control |
GCS | UPSIT | History of head injury | NR | Significantly worse UPSIT scores in cases (21.17±8.65) versus controls (32.27±3.77) (p<0.001). |
| Severe | ||||||
| Fujiwara (2008) | 46 TBI OD 25 Control |
GCS | Smell ID Test | 1 year post injury from consecutive admission lists | NR | Both Mod TBI (31.93±6.78, p<0.01) and Sev TBI (28.16±7.78, p<0.0001) demonstrated worse olfactory scores than controls (35.92±2.78). |
| Mod/Sev | ||||||
| Sandford (2006) | 7 TBI OD 36 Control |
GCS, LOC, Imaging | SDCOIT | Presenting to peds ED with blunt head trauma | Scored based on percentage of correctly identified odorants – 0–100% Hyposmia: ≤75% Anosmia: 0% |
Pediatric population, scores from mod/sev subset of TBI were used to generate study effect size Hyposmia was identified in 2/7 (28.6%) of patients. No patients were anosmic. Olfactory function was predicted by both GCS (p<0.05) and head CT abnormality (p<0.01). |
| Mod/Sev | ||||||
| Savage (2002) | 13 TBI OD 13 Control |
LOC, PTA, Imaging | CCCRC – threshold and olfactory-word ID Delayed Odor Recognition Memory Test |
2 medical rehab facilities, outpatient neuropsychology clinic | Threshold 0–10, higher score indicating more sensitive olfactory threshold ID: 0–8, higher score indicating better performance Recognition: 0–7, higher score indicating better performance |
Odor Identification: TBI patients had impaired odor-word ID scores on left nostril (p=0.001) and right nostril (p<0.00001) as compared to controls. Odor Detection Threshold: No difference between controls and TBI group. Odor Recognition: TBI patients had impaired recognition scores on left nostril (p=0.0007) and right nostril (p=0.0001) as compared to controls. |
| Mod/Sev | ||||||
| Yousem (1999) | 36 TBI OD 24 Control |
Imaging | UPSIT 12-item memory test Single-staircase odor detection threshold |
Smell and Taste center | UPSIT: 0–40 (0–20 each nostril) Odor memory: 0–12 per nostril PEA threshold: −1.9 - −10.0 |
UPSIT scores were significantly worse in patients (21.9±10.5) compared to controls (26.6±2.6) (p<0.0001). Anosmia was identified in 16/36 (44.4%) of patients. Patients had worse function on all olfactory domains after controlling for age (p<0.001). Left OB and tract volumes were correlated with left and total UPSIT scores. There was a significant difference in right and left OB and tract volumes between TBI and control patients, as well as between anosmic and hyposmic patients. |
| Mod/Sev | ||||||
| Levin (1985) | 45 TBI OD 19 Control |
GCS, LOC, PTA | Olfactory ID Test | History of head injury | ID test: 0–12 | Data provided for severity based on GCS, LOC, and PTA. GCS: In comparison with age-matched controls, olfactory naming and recognition were impaired in TBI patients. No difference noted in patients with mild TBI. Recognition: Mod TBI (median 8.3), Sev TBI (median 8.1), controls (median 9.9) (p<0.01) Naming: Mod TBI (median 2.0), Sev TBI (median 07), controls (median 4.1) (p<0.01) |
| Mod/Sev | ||||||
Note: AST: Alberta Smell Test; B-SIT: Brief Smell Identification Test; CCCRC: Connecticut Chemosensory Clinical Research Center; GCS: Glasgow Coma Scale; GOAT: Galveston Orientation and Amnesia Test; ID: Identification; LOC: loss of consciousness; Mod: Moderate; MRI: magnetic resonance imaging; NR: not reported; OD: olfactory dysfunction; oERP: olfactory event-related potentials; OSIT-J: odor stick identification test for the Japanese; PTA: post-traumatic amnesia; SDCOIT: San Diego Children’s Olfaction Identification Test; Sev: Severe; TBI: traumatic brain injury; TDI: Threshold, Discrimination, Identification; UPSIT: University of Pennsylvania Smell Identification Test
Data Extraction
Data was extracted and entered from included studies. Available information on sample characteristics were entered separately for patients and controls, including sample size, mean age, and % men. We attempted to extract information on other factors such as race, ethnicity, education level, and smoking history; however, these variables were not reported consistently in included studies to be assessed as effect size moderators. Injury severity (moderate, severe, moderate-severe), PTA, duration of LOC, GCS score, location of brain injury and time since injury were also extracted. Of these variables, only time since injury was reported consistently enough to be analyzed as an effect size moderator. Olfactory outcome measures and associations with evoked potentials, neuroimaging findings and behavioral outcomes were also extracted for systematic review.
Olfactory Domain and Task Type
Odor identification tasks assess the ability to attach the correct semantic label to an odorant. The most popular tests used in the clinic setting include the 40-item Smell Identification Test, 12-item Brief Smell Identification Test and the 12- or 16-item Sniffin’ Sticks Odor Identification Test (Doty et al., 1996; Doty et al., 1984; Kobal et al., 1996). Odor discrimination tests typically assess the ability to distinguish an odor from two identical foils, such as the 16-item Sniffin’ Sticks’ odor discrimination test (Kobal et al., 1996). Odor detection threshold tests assess the minimum concentration at which a person can reliably detect an odor. One example is the T&T olfactometer, which averages the concentration that five unique odorants are detected to generate an odor threshold score (Kondo et al., 1998). An odor memory test requires presentation of odors and recognition of these odors following a delay (Yousem et al., 1999). In addition, instruments have been developed that assess more than one olfactory domain, such as the Connecticut Chemosensory Clinical Research Center (CCCRC) olfactory battery which consists of odor detection threshold and odor identification tasks. The Sniffin’ Sticks olfactory battery yields an aggregated TDI score, which reflects the sum of a person’s threshold, discrimination and identification scores. Individual performance across olfactory tests can be assessed continuously or categorically using established clinical cutoffs to categorize participants as normosmic (intact smell), hyposmic (mild smell loss) or functionally anosmic (significant smell loss).
Meta-Analysis
Comprehensive Meta-Analysis Version 3.0 was used for effect size generation. We used a random-effects model to account for within- and between-study variation in effect size estimates. The model estimates any variability beyond sampling error variance, this excess variability is often thought to be substantive and unique to studies. Studies were weighted according to their inverse variance estimates to account for sample size differences. We calculated effect sizes (Hedges’ g) to standardize group differences. A negative g reflects poorer TBI patient performance relative to controls. Effect size directions were inverted for tasks in which larger scores indicated greater impairment. Effect sizes were categorized as small (g = −0.2), medium (g = −0.5), or large (g ≥ −0.8). Sensitivity analyses were applied to identify potential outliers in the dataset using the “one study removed” module in Comprehensive Meta-Analysis Version 3.0, which calculates a random-effects mean and standard error as each study is removed one at a time from the analysis (Tobias, 1999). This method was used to examine the effect of a single study on the overall study effect size.
To examine the effect of publication bias, analyses were conducted using previously established approaches (Begg & Mazumdar, 1994; Egger et al., 1997). A funnel plot was generated for graphical representation as were adjusted rank-correlation tests (Online Resource 3). This analysis generates the number of studies needed with null effects residing in file drawers to reduce the mean effect size to a negligible level. The overall effect size homogeneity was assessed using the Cochran’s Q statistic. We explored the influence of age, sex, and duration since injury on effect size magnitude using meta-regression.
Quality Assessment
Study quality and risk of bias was evaluated using the Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses (Wells et al., 2000). Studies were scored on a scale (0–9) by two raters; a higher score indicated lower risk of bias. Complete NOS scoring can be found in the Online Resource 2. Using the NOS, a standard scale for what constitutes a “high quality” versus a “low quality” study has not been routinely established. The mean NOS score, calculated by averaging the total scores from two reviewers, was 7.0 (SD=1.5) for studies included in meta-analysis.
Results
SYSTEMATIC REVIEW of Olfactory Dysfunction in Moderate to Severe TBI
Age and sex
A total of 951 TBI patients were included in the systematic review (14.84% moderate, 30.21% severe, 54.95% combined moderate/severe injuries). In the meta-analysis, 429 TBI patients were included (33.82% moderate, 34.56% severe, 31.62% combined moderate/severe injuries), along with 559 non-TBI controls without olfactory complaints. Men comprised approximately 75% of the patient populations (range 45–97%). In adult studies, the average age was 36 years (range of mean 28–54). Only one study evaluated pediatric populations with moderate to severe TBI with a mean age of 9 years (Sandford et al., 2006). The prevalence of TBI in the general population is highest among older adults (Peterson et al., 2021), whereas the study populations assessed in this review skewed towards younger cohorts. However, the study populations paralleled general trends with predominantly male cohorts (Summers et al., 2009). No studies investigated differences by education or race.
Injury Characteristics
This review focuses on the moderate to severe TBI patient population. Three studies focused on patients with severe TBI (Osborne-Crowley & McDonald, 2016; Parma et al., 2012; Sigurdardottir et al., 2016), but others separated their cohorts by degree of TBI severity (Fujiwara et al., 2008; Green & Iverson, 2001; Levin et al., 1985; Yamaki et al., 2020). The most common metric for measuring TBI severity was GCS, with 11 of 19 papers utilizing GCS. Other commonly utilized metrics included PTA and LOC. Four studies provided details about TBI severity solely through abnormal neuroimaging results, which meets inclusion criteria for moderate/severe TBI based on the VA/DoD clinical practice guidelines for classification of TBI severity (Table 1) (Han et al., 2018a, 2018b; Yousem et al., 1999; Yousem et al., 1996). Levin et al. (1985) found that patients with GCS scores, duration of LOC, and length of PTA reflective of moderate to severe TBI demonstrated greater olfactory psychophysical dysfunction compared to healthy controls. Green et al. (2003) also evaluated several markers of injury severity and found that decreased olfactory scores correlated with longer PTA duration, lower GCS scores and the presence of CT abnormalities. However, two studies found that GCS, LOC, or PTA individually did not significantly correlate with olfactory dysfunction (Haxel et al., 2008; Neumann et al., 2012).
Olfactory Task Type
Despite differences in sample characteristics and methods of olfactory assessment, most studies reviewed showed reduced olfactory psychophysical performances in patients moderate to severe TBI. Odor identification measures were the most common olfactory domain tested, appearing in all included studies. All studies reported poorer odor identification accuracy in moderate to severe TBI. Of note, Savage et al. (2002) examined odor identification performance unirhinally, in which each nostril is tested separately, and found patient-control differences for each side assessed. In addition, the sole study in children using the San Diego Children’s Odor Identification test found that children with moderate to severe TBI had poorer olfactory scorers compared to children without TBI (Sandford et al., 2006). Five of the six studies assessing odor detection threshold scores reported significant patient-control differences. Of these five studies reporting differences, one study utilized the Sniffin’ Sticks n-butanol odor detection threshold test (Miao et al., 2015) and two used the phenyl ethyl alcohol (PEA) Sniffin’ Sticks odor detection threshold test (Han et al., 2018a, 2018b). The two remaining studies by Yousem et al. (1999; 1996) used a single-staircase PEA detection threshold test described in Deems and Doty (1987). Conversely, one study utilized the CCCRC n-butanol smell threshold test and found no significant difference between the TBI and control groups (Savage et al., 2002). Four studies examined odor discrimination accuracy in TBI patients and controls, with significant differences observed across groups (Han et al., 2018a, 2018b; Yousem et al., 1999; 1996). Yousem et al. (1996, 1999) utilized a 16-item odor discrimination test and 12-item odor memory test; significant differences between patients and controls were observed on both tasks. Savage et al. (2002) found significantly lower odor recognition memory scores in TBI patients as compared to controls.
Five studies employed the full Sniffin’ Sticks olfactory battery (Bratt et al., 2018; Han et al., 2018a, 2018b; Haxel et al., 2008; Miao et al., 2015). In addition, five studies employed more than one test to assess olfactory function (Haxel et al., 2008; Miao et al., 2015; Savage et al., 2002; Sigurdardottir et al., 2016; Yousem et al., 1996). Haxel et al. (2008) utilized the BSIT test as a screening test, followed by further testing with the full Sniffin’ Sticks olfactory battery to generate a TDI score. Of the original 8 individuals that demonstrated olfactory dysfunction with the BSIT, 6 demonstrated olfactory dysfunction with Sniffin’ Sticks (Haxel et al., 2008). The authors attributed the discrepancy to the higher sensitivity of the comprehensive Sniffin’ Sticks evaluation. Similarly, another study administered the BSIT or the UPSIT to two subsamples of patients with severe TBI. The authors found higher rates of olfactory dysfunction in the subsample tested with the UPSIT (29.7% versus 89.2%), which was attributed to the UPSIT’s higher sensitivity for detecting olfactory dysfunction in TBI (Sigurdardottir et al., 2016).
Self-Awareness of Olfactory Dysfunction
Prior studies have described discordance between self-report of olfactory functioning and psychophysical assessment of olfactory performance, with unawareness of olfactory loss noted in older adults and individuals with sinonasal and neurodegenerative conditions (Adams et al., 2017; Doty et al., 1988; Murphy et al., 2002; Nordin et al., 1995; Wehling et al., 2011). In TBI populations, three studies commented on the frequency with which patients recognized and self-reported their own olfactory dysfunction. Two studies characterized olfactory dysfunction using the BSIT (Neumann et al., 2012; Osborne-Crowley & McDonald, 2016), and found that 37.5% (3/8) and 36% (21/59) of their populations were aware of their dysfunction. Using BSIT as a screening test, a third study reported a 57% sensitivity and 91% specificity for self-awareness of olfactory dysfunction among patients with olfactory dysfunction (Haxel et al., 2008).
Association with Cognitive and Psychosocial Functioning
Given the neuroanatomic overlap between olfactory and orbitofrontal-limbic neurocircuitry, researchers have previously investigated the association between olfactory performance, behavioral disturbance and executive functioning (Levin et al., 1985). Osbourne-Crowley et al. (2016) evaluated 23 severe TBI patients and found that hyposmia, as characterized by the BSIT, was a significant predictor of interpersonal relationship changes, but not social disinhibition. Sigurdardottir et al. (2016) examined two demographically- and clinically-comparable subgroups with severe TBI, of which one subgroup completed the 12-item BSIT and the other completed the 40-item UPSIT. All patients also completed measures of executive functioning and the Glasgow Outcome Scale Extended (GOSE), a well-studied metric of TBI outcome. Patients in each subgroup were defined as normosmic, hyposmia and anosmia based on their BSIT or UPSIT score. Of note, patient groups with BSIT-defined olfactory dysfunction had greater executive dysfunction and disability on the GOSE when compared to the BSIT-defined normosmia group. In contrast, the UPSIT-defined groups did not differ on executive measures. These discrepant findings were hypothesized by the authors to reflect differences in the psychometric properties of each olfactory test, which differ in their sensitivity to capturing olfactory dysfunction. However, the authors also noted that the 12 BSIT items were specifically selected based on their broader cross-cultural application compared to the 40 UPSIT items (Sigurdardottir et al., 2016). As this study was conducted in Norway, it is possible that cultural differences led to overestimation of olfactory dysfunction in the UPSIT-defined group. Finally, in both BSIT- and UPSIT-defined subgroups, different normative groups were used to define olfactory impairment status and two of the resulting subgroups used to compare scores were very small (n ≤ 7). These factors likely also contributed to the discrepancies observed between tests.
Olfactory Event-Related Potentials
Olfactory event-related potentials (oERPs) are non-invasive recordings of neuroelectric activity via scalp electrodes following the presentation of an olfactory stimulus (Kobal & Hummel, 1998). A delay or lack of measurable oERP waveforms following stimulus presentation is believed to represent olfactory dysfunction. Two studies measured psychophysical olfactory functioning and oERPs in their moderate to severe TBI patients (Haxel et al., 2008; Miao et al., 2015). In Miao et al. (2015), approximately 33% of the patient sample did not have identifiable oERPs, which was interpreted to reflect their complete anosmia. Of the remaining 66% with detectable oERPs, TBI patients had longer oERP latencies and smaller amplitudes than controls (Miao et al., 2015). The authors suggested that injury to the olfactory bulb and gyrus rectus produced decreased and delayed oERPs, while frontal lobe damage may explain complete anosmia and absence of oERPs. Haxel et al. (2008) evaluated oERPs following unilateral presentation of PEA and hydrogen sulfide (i.e., presentation to the left and right nostril in isolation). In this study, 43% (3/7) of patients with anosmia, as defined by comprehensive Sniffin’ Sticks testing (TDI score<16), did not have identifiable oERPs on either side. The remaining four patients demonstrated unilateral odor evoked potentials following presentation to at least one nostril side.
Neuroimaging Correlates of TBI Sequelae
Several investigators also utilized psychophysical olfactory testing to examine the relationship between post-traumatic olfactory dysfunction and structural brain imaging (e.g., computed tomography and MRI) findings (Fujiwara et al., 2008; Han et al., 2018a, 2018b; Miao et al., 2015; Savage et al., 2002; Xydakis et al., 2015; Yousem et al., 1999; Yousem et al., 1996). After olfactory dysfunction was established with psychophysical testing, abnormalities were observed in both peripheral and central aspects of the olfactory system, including the olfactory bulbs, olfactory tract, inferior frontal, orbitofrontal and temporal brain regions. TBI patients with hyposmia or anosmia, as defined on psychophysical testing, had smaller olfactory bulb (OB) volumes compared to the comparison group in three separate studies (Miao et al., 2015; Yousem et al., 1999; Yousem et al., 1996). Furthermore, decreased gray matter density was observed in the primary olfactory cortex, but not temporal lobes, in these TBI patients relative to controls (Yousem et al., 1999; Yousem et al., 1996). Sigurdardottir et al. (2016) utilized the Rotterdam CT classification to indicate severity of pathology (Maas et al., 2005). They found a significant difference between the anosmic (as defined by B-SIT and UPSIT) and comparison groups on Rotterdam scores. In addition, subarachnoid hemorrhage was present in 80% of individuals in the anosmia group compared to 45% in the TBI comparison group without olfactory dysfunction (Sigurdardottir et al., 2016).
META-ANALYSIS OF OLFACTORY DYSFUNCTION IN MODERATE TO SEVERE TBI
Studies with enough information to calculate effect sizes were included in the meta-analysis (n=11). Across these 11 studies of post-traumatic olfactory dysfunction, the overall effect size was large (g=−2.43, 95%CI: −3.16<δ<−1.69) and significantly heterogeneous (QB [31]=186.57, p<0.001; see Figure 2). The “one study removed” sensitivity analysis revealed that the smallest (g=−1.94) and largest (g=−2.69) effect sizes fell within the confidence interval of the mean study effect size, indicating minimal influence from a single study. Assessment of publication bias found that the Begg and Mazumdar rank correlation and one-tailed Egger tests were statistically significant (p=0.001), suggesting risk of publication bias. Calculation of the classic fail-safe N indicated that 1,103 null studies would be needed to render the study effect size statistically non-significant. Taken together, these findings indicate that publication bias imposed a negligible influence on the results.
Fig. 2.
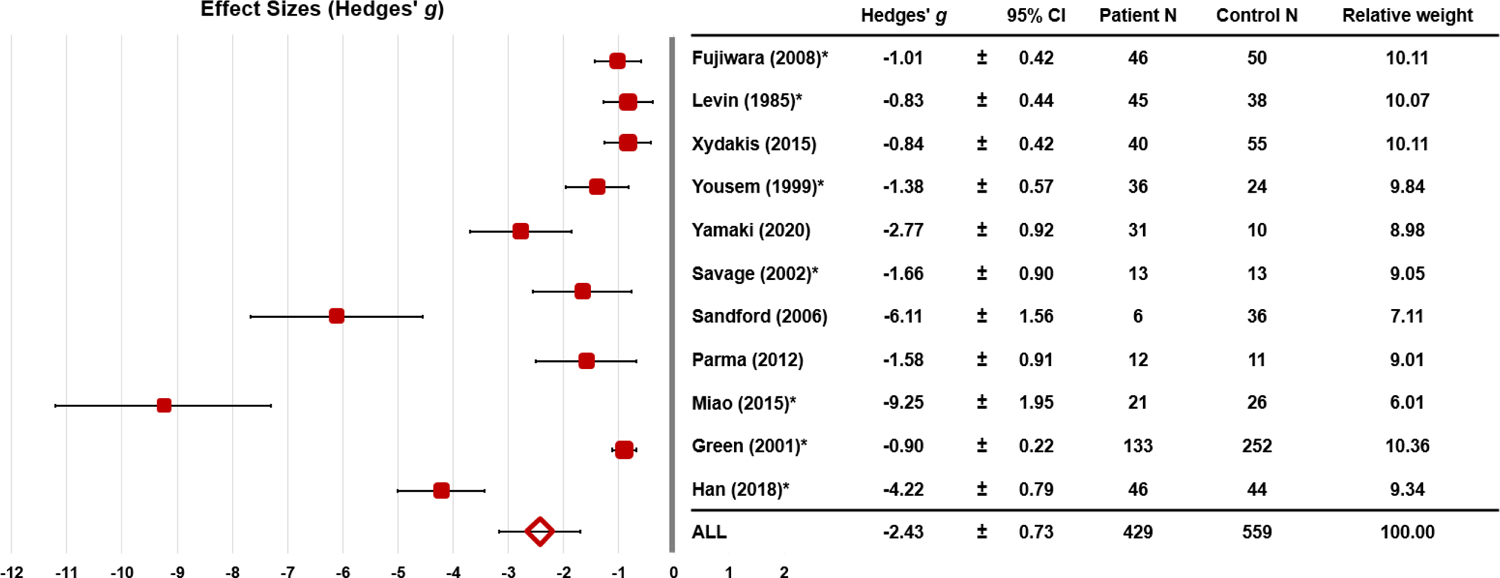
Effect sizes (± 95% CI) for olfactory performances by study.
Note: Studies in which multiple groups (moderate, severe TBI) and/or multiple tasks were administered (odor identification, discrimination) are indicated with an asterisk. For these studies, the mean effect size across all subgroups and olfactory outcomes is presented
Meta-regression analyses demonstrated that patient sex composition was a significant moderator of study effect size (Z=2.31, p=0.02). Contrary to expectation, a larger percentage of men in the patient group was associated with a smaller effect size magnitude. Sex composition of the control population was not a statistically significant moderator (Z=0.99, p=0.32). Mean control age was not a statistically significant moderator of study effect size (Z=−1.63, p=0.102). Mean patient age was not associated with effect size magnitude (Z=−1.88, p=0.06). However, following exclusion of the pediatric study, patient (Z=−4.27, p<0.001) and control age (Z=−4.38, p<0.001) were significant modifiers, with larger effect size associated with older age. Duration since injury was not a significant moderator of study effect size (Z=−1.35, p=0.18).
Due to the limited number of olfactory domains assessed across studies, an overall effect size could not be generated and compared between olfactory task types (i.e., odor identification vs. discrimination vs. threshold). Odor identification was the only olfactory task type in which an effect size could be generated. Across 10 studies, there was a large effect size (g=−1.88, 95% CI: −2.40<δ<−1.36, p<0.001).
Discussion
Post-traumatic olfactory loss remains a poorly understood phenomenon. In this study, we systematically reviewed and quantified the impact of moderate to severe TBI on olfactory function. Our meta-analysis of 11 studies found a large effect (g=−2.43, 95%CI: −3.16<δ<−1.69) for olfactory dysfunction in moderate to severe TBI. Indeed, all studies included in our meta-analysis demonstrated significant patient-control differences across all olfactory domains assessed, including odor detection threshold, identification, discrimination and memory. In our systematic review, both peripheral olfactory structures (Fujiwara et al., 2008; Miao et al., 2015; Yousem et al., 1999; Yousem et al., 1996) and orbitofrontal brain regions (Levin et al., 1985; Savage et al., 2002; Yousem et al., 1999; Yousem et al., 1996) emerged as correlates of olfactory dysfunction in moderate to severe TBI. Though higher age and a larger composition of women in the patient group were associated with a larger magnitude of patient-control differences, duration since injury was not a significant moderator of study effect size.
There are several implications of the current work. Olfactory testing is underutilized in TBI patients. It is well known that chemosensory disturbance is associated with other complications, including depression, frailty, and even mortality risk (Bernstein et al., 2021; Choi et al., 2021; Ekstrom et al., 2017; Merkonidis et al., 2015). As up to 33% of patients with olfactory dysfunction may not recognize their deficits, a large post-TBI population may be missed if recruitment is limited to patients self-reporting olfactory dysfunction (Neumann et al., 2012; Osborne-Crowley & McDonald, 2016). By formally assessing olfactory loss post-TBI, patients at risk for olfactory dysfunction can be identified and managed appropriately. In addition, systematic efforts in the acute care setting, as well as during the rehabilitation process, could be implemented to improve olfactory function in patients with TBI. Prior studies have shown that implementing treatment earlier may be associated with better outcomes for those with post-traumatic olfactory dysfunction (Hura et al., 2020; Konstantinidis et al., 2013). Multiple studies have investigated the use of olfactory training in this patient population. Although not a panacea, a notable proportion of patients with TBI may achieve clinically meaningful improvement in TDI scores with olfactory training (Huang et al., 2021). From a medical-legal perspective, an increased understanding of olfaction in TBI patients may help identify cases of malingering and help establish disability compensation more expediently for patients in need (Doty, 2015). Psychophysical olfactory testing has been useful for detecting malingering through improbable responding on validated olfactory assessment methods (Doty, 2015). For example, it would be expected that one fourth of responses in a four-alternative test would be identified accurately by chance alone. As such, Doty (2006) estimated that the probability of scoring 0 out of 40 on the UPSIT is approximately 1 in 100,000.
The magnitude of olfactory dysfunction following TBI has been noted to vary as a function of injury severity (Schofield & Doty, 2019). To date, the existing literature on the effect of mild TBI on olfactory functioning remains mixed, with multiple studies noting intact psychophysical olfactory scores (de Kruijk et al., 2003; Foster et al., 2022; Green & Iverson, 2001; Green et al., 2003) and others noting reduced olfaction in mild TBI (Charland-Verville et al., 2012; Giguere et al., 2019). Of note, two of three studies that assessed olfactory performance in the acute phase of mild TBI reported equivocal findings (de Kruijk et al., 2003; Foster et al., 2022). Children with mild TBI were noted to have equivocal odor identification scores compared to healthy children but poorer olfactory scores compared to children with moderate to severe TBI (Sandford et al., 2006). In contrast, Fortin et al. (2010) did not observe differences in olfactory performance between mild, moderate and severe TBI groups after controlling for age. These discrepancies across studies may be driven by the evolving definition of mild TBI, the heterogeneity within the mild TBI population and an inadequate assessment of performance validity across most mild TBI studies (McCrea, 2008; Nelson et al., 2019). In patients seeking compensation claims, for example, Green and Iverson (2001) found that performance validity scores influenced the dose-response relationship between head injury severity and olfactory dysfunction. After accounting for suboptimal performance validity, patients with trivial to mild head injury had comparable olfactory scores to those of a non-head injured orthopedic control group. Given that patients with mild TBI can span mild concussion without neuroimaging evidence of injury to injuries with intracranial abnormalities, there is also growing appreciation that outcomes can vary considerably. Taken together, prospective and controlled studies of olfactory psychophysical functioning in TBI are needed, in which non-injury-related factors such as premorbid psychosocial difficulties, co-morbid psychiatric conditions, post-injury stressors, substance use disorders and litigation status are evaluated.
Historically, women have been noted to perform better on olfactory tasks than men. Differences in cognitive abilities, environmental odor exposure, sex hormones, and neuroendocrine influences on olfactory-eloquent brain areas have been put forth to explain this advantage (for a review, see: Sorokowski et al., 2019). As such, we hypothesized that samples with higher proportions of women would have smaller effect size magnitudes. Contrary to expectation, the effect magnitude was larger in TBI samples with a higher percentage of women. These findings contrast included studies that found more severe olfactory dysfunction in men with TBI compared to women (Green et al., 2003; Sigurdardottir et al., 2016). Interestingly, biological sex has been noted to interact with factors ranging from injury severity, genetics, race, baseline cognitive functioning, and mitochondrial dysfunction in determining TBI outcomes (for a review, see: Gupte et al., 2019). In this review, 41 studies examined outcomes in moderate to severe TBI, of which 34% of studies reported poorer outcomes in women with TBI than men. Furthermore, in smaller prospective studies that specifically assessed social-behavioral outcomes, women were found to have poorer outcomes than men. It is also notable that a high proportion of women experiencing intimate partner violence (IPV) may not report IPV-related TBI and are systematically underrepresented in prevalence estimates (Biegon, 2021; St Ivany & Schminkey, 2016). Collectively, these findings highlight the heterogeneity observed across TBI studies and raise the opportunity for further scrutiny on the impact of sex in TBI-associated olfactory dysfunction.
Similar to studies in olfaction, the literature regarding the effect of moderate to severe TBI on other sensory functions is limited. A systematic review examined 12 studies of hearing impairment post-TBI without fracture to the temporal bone (Chen et al., 2018). The high number of case reports with few prospective case-controlled studies precluded the authors from conducting a meta-analysis or examining studies as a function of injury severity. As such, the prevalence of reduced hearing ranged considerably from 1 to 58%. Visual changes are also observed post-TBI, including changes in visual acuity, convergence insufficiency, visual field loss, and accommodative dysfunction (Merezhinskaya et al., 2019). When compared to mild TBI, a higher prevalence of visual field loss was noted in the moderate to severe TBI population. Furthermore, moderate to severe TBI patients were noted to have increased latency of eye movements and decreased accuracy of visually-guided saccades compared to controls (Kraus et al., 2007). To our knowledge, formal assessment of gustatory functions has yet to be comprehensively examined in moderate to severe TBI. In the National Health and Nutrition Examination Survey (NHANES) of community-dwelling middle-aged and older adults, head injury was not associated with taste performance; however, participants were not separately examined by injury severity and head injury was not examined as the main exposure with appropriate correction for confounding variables (Liu et al., 2016). Future studies examining changes in olfaction, hearing, vision and taste in moderate to severe TBI may help disentangle the occurrence of dual or multisensory dysfunction post-TBI.
Our review has several strengths, including the focus on more rigorous studies of moderate to severe TBI and the application of meta-analytic methods to characterize the magnitude of TBI-related olfactory dysfunction. The use of meta-regression allowed us to make preliminary inferences about the influence of age, sex and duration since injury on the overall study effect size. Though post-injury times varied from a few days to several years across studies, duration since time of injury was not a significant effect size moderator. This finding raises questions about olfactory recovery following head trauma. Further study of the factors associated with improved olfactory functioning versus persistent olfactory dysfunction will be helpful.
Limitations of the current investigation include the limited number of patient-control studies of olfaction in moderate to severe TBI and the heterogeneity in how demographic, clinical and olfactory task information was reported, which made it challenging to draw detailed conclusions. For example, the lack of information on race and education or uniform reporting on injury characteristics precluded our ability to examine how these factors influence effect size magnitude. Additional patient demographics and clinical risk factors, such as medical co-morbidities, psychiatric symptoms, post-traumatic seizures, and medications, may have confounding effects on olfaction and have yet to be disentangled (Doty et al., 1997; Fortin et al., 2010; Ghanizadeh, 2009; Gupta et al., 2014). Studies also varied in whether they reported composite TDI scores or subtest scores and most studies solely assessed odor identification. Although we ensured that included studies focused on majority (i.e., at least >50%) moderate/severe populations, the inclusion of a subset of mild TBI patients may bias results. Studies varied with respect to recruitment location (e.g., TBI rehabilitation centers, ENT clinics) and several studies limited enrollment to patients complaining of olfactory dysfunction or presenting to an ENT clinic, thereby leading to potential selection bias in the study population. Given the discrepancies noted between self-report of olfactory abilities and formal psychophysical assessment, future studies would benefit from assessing olfactory functioning in larger TBI cohorts irrespective of self-report. Finally, patients with TBI can experience olfactory distortions following TBI, including phantosmia and parosmia (Yamaki et al., 2020), and reduced quality of life related to smell loss (Ahmedy et al., 2020). Results from NHANES indicate a 23% prevalence of self-reported olfactory alterations, including 6% reporting phantosmia in a general community-dwelling sample (Rawal et al., 2016). However, prevalence estimates in TBI populations have yet to be examined systematically. Multiple self-report assessments have been developed to examine the presence, degree and duration of odor sensitivity, parosmia and phantosmia (Han et al., 2021). These olfactory changes can have an adverse impact on a person’s quality of life and nutritional intake, and would be useful to assess in relation to olfactory psychophysical performance in future TBI studies.
Conclusion
Moderate to severe TBI has a profound impact on multiple domains of olfactory functioning. Our meta-analysis of 11 studies demonstrated that the effect size of olfactory dysfunction in moderate to severe TBI is large and nearly all studies included in our systematic review demonstrated olfactory performance deficits in patients with moderate to severe TBI. However, there remains significant research gaps regarding the mechanism, recovery, and natural history of post-TBI olfactory dysfunction. Increased awareness of post-TBI olfactory dysfunction and future prospective controlled longitudinal studies across injury severities will better determine not only the incidence but impact and treatment of olfactory dysfunction after TBI.
Supplementary Material
Footnotes
Conflict of Interest: The authors declare no conflicts of interest.
References
- Adams DR, Wroblewski KE, Kern DW, Kozloski MJ, Dale W, McClintock MK, & Pinto JM (2017). Factors associated with inaccurate self-reporting of olfactory dysfunction in older us adults. Chem Senses, 42(3), 223–231. 10.1093/chemse/bjw108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmedy F, Mazlan M, Danaee M, & Abu Bakar MZ (2020). Post-traumatic brain injury olfactory dysfunction: Factors influencing quality of life. European Archives of Oto-Rhino-Laryngology, 277(5), 1343–1351. 10.1007/s00405-020-05823-0 [DOI] [PubMed] [Google Scholar]
- Begg CB, & Mazumdar M (1994). Operating characteristics of a rank correlation test for publication bias. Biometrics, 50(4), 1088–1101. [PubMed] [Google Scholar]
- Bernstein IA, Roxbury CR, Lin SY, & Rowan NR (2021). The association of frailty with olfactory and gustatory dysfunction in older adults: A nationally representative sample. Int Forum Allergy Rhinol, 11(5), 866–876. 10.1002/alr.22718 [DOI] [PubMed] [Google Scholar]
- Biegon A (2021). Considering biological sex in traumatic brain injury. Frontiers in Neurology, 12, 576366. 10.3389/fneur.2021.576366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bratt M, Skandsen T, Hummel T, Moen KG, Vik A, Nordgard S, & Helvik AS (2018). Frequency and prognostic factors of olfactory dysfunction after traumatic brain injury. Brain Injury, 32(8), 1021–1027. 10.1080/02699052.2018.1469043 [DOI] [PubMed] [Google Scholar]
- Charland-Verville V, Lassonde M, & Frasnelli J (2012). Olfaction in athletes with concussion. Am J Rhinol Allergy, 26(3), 222–226. 10.2500/ajra.2012.26.3769 [DOI] [PubMed] [Google Scholar]
- Chen JX, Lindeborg M, Herman SD, Ishai R, Knoll RM, Remenschneider A, … Kozin ED (2018). Systematic review of hearing loss after traumatic brain injury without associated temporal bone fracture. American Journal of Otolaryngology, 39(3), 338–344. 10.1016/j.amjoto.2018.01.018 [DOI] [PubMed] [Google Scholar]
- Choi JS, Jang SS, Kim J, Hur K, Ference E, & Wrobel B (2021). Association between olfactory dysfunction and mortality in US adults. JAMA Otolaryngol Head Neck Surg, 147(1), 49–55. 10.1001/jamaoto.2020.3502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kruijk JR, Leffers P, Menheere PP, Meerhoff S, Rutten J, & Twijnstra A (2003). Olfactory function after mild traumatic brain injury. Brain Injury, 17(1), 73–78. 10.1080/0269905021000010221 [DOI] [PubMed] [Google Scholar]
- Deems DA, & Doty RL (1987). Age-related changes in the phenyl ethyl alcohol odor detection threshold. Transactions - Pennsylvania Academy of Ophthalmology and Otolaryngology, 39(1), 646–650. [PubMed] [Google Scholar]
- Doty RL (2006). Olfactory dysfunction and its measurement in the clinic and workplace. Int Arch Occup Environ Health, 79(4), 268–282. 10.1007/s00420-005-0055-6 [DOI] [PubMed] [Google Scholar]
- Doty RL (2015). Olfactory dysfunction and its measurement in the clinic. World J Otorhinolaryngol Head Neck Surg, 1(1), 28–33. 10.1016/j.wjorl.2015.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doty RL, Deems DA, & Stellar S (1988). Olfactory dysfunction in parkinsonism: A general deficit unrelated to neurologic signs, disease stage, or disease duration. Neurology, 38(8), 1237–1244. 10.1212/wnl.38.8.1237 [DOI] [PubMed] [Google Scholar]
- Doty RL, Marcus A, & Lee WW (1996). Development of the 12-item cross-cultural smell identification test (CC-SIT). Laryngoscope, 106(3 Pt 1), 353–356. 10.1097/00005537-199603000-00021 [DOI] [PubMed] [Google Scholar]
- Doty RL, Shaman P, Kimmelman CP, & Dann MS (1984). University of Pennsylvania Smell Identification Test: A rapid quantitative olfactory function test for the clinic. Laryngoscope, 94(2 Pt 1), 176–178. 10.1288/00005537-198402000-00004 [DOI] [PubMed] [Google Scholar]
- Doty RL, Yousem DM, Pham LT, Kreshak AA, Geckle R, & Lee WW (1997). Olfactory dysfunction in patients with head trauma. Archives of Neurology, 54(9), 1131–1140. 10.1001/archneur.1997.00550210061014 [DOI] [PubMed] [Google Scholar]
- Egger M, Davey Smith G, Schneider M, & Minder C (1997). Bias in meta-analysis detected by a simple, graphical test. BMJ, 315(7109), 629–634. 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrom I, Sjolund S, Nordin S, Nordin Adolfsson A, Adolfsson R, Nilsson LG, … Olofsson JK (2017). Smell loss predicts mortality risk regardless of dementia conversion. Journal of the American Geriatrics Society, 65(6), 1238–1243. 10.1111/jgs.14770 [DOI] [PubMed] [Google Scholar]
- Fortin A, Lefebvre MB, & Ptito M (2010). Traumatic brain injury and olfactory deficits: The tale of two smell tests! Brain Injury, 24(1), 27–33. 10.3109/02699050903446815 [DOI] [PubMed] [Google Scholar]
- Foster E, Bayley M, Langer L, Saverino C, Chandra T, Barnard C, & Comper P (2022). The Toronto Concussion Study: Sense of smell is not associated with concussion severity or recovery. Brain Injury, 1–9. 10.1080/02699052.2022.2037713 [DOI] [PubMed] [Google Scholar]
- Fujiwara E, Schwartz ML, Gao F, Black SE, & Levine B (2008). Ventral frontal cortex functions and quantified MRI in traumatic brain injury. Neuropsychologia, 46(2), 461–474. 10.1016/j.neuropsychologia.2007.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghanizadeh A (2009). Loss of taste and smell during treatment with topiramate. Eat Weight Disord, 14(2–3), e137–138. 10.1007/BF03327811 [DOI] [PubMed] [Google Scholar]
- Giguere FL, Frasnelli A, De Guise E, & Frasnelli J (2019). Olfactory, cognitive and affective dysfunction assessed 24 hours and one year after a mild traumatic brain injury (mtbi). Brain Injury, 33(9), 1184–1193. 10.1080/02699052.2019.1631486 [DOI] [PubMed] [Google Scholar]
- Green P, & Iverson GL (2001). Effects of injury severity and cognitive exaggeration on olfactory deficits in head injury compensation claims. NeuroRehabilitation, 16(4), 237–243. [PubMed] [Google Scholar]
- Green P, Rohling ML, Iverson GL, & Gervais RO (2003). Relationships between olfactory discrimination and head injury severity. Brain Injury, 17(6), 479–496. 10.1080/0269905031000070242 [DOI] [PubMed] [Google Scholar]
- Gupta PK, Sayed N, Ding K, Agostini MA, Van Ness PC, Yablon S, … Diaz-Arrastia R (2014). Subtypes of post-traumatic epilepsy: Clinical, electrophysiological, and imaging features. Journal of Neurotrauma, 31(16), 1439–1443. 10.1089/neu.2013.3221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupte R, Brooks W, Vukas R, Pierce J, & Harris J (2019). Sex differences in traumatic brain injury: What we know and what we should know. Journal of Neurotrauma, 36(22), 3063–3091. 10.1089/neu.2018.6171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han P, Su T, Qin M, Chen H, & Hummel T (2021). A systematic review of olfactory related questionnaires and scales. Rhinology, 59(2), 133–143. 10.4193/Rhin20.291 [DOI] [PubMed] [Google Scholar]
- Han P, Winkler N, Hummel C, Hahner A, Gerber J, & Hummel T (2018a). Alterations of brain gray matter density and olfactory bulb volume in patients with olfactory loss after traumatic brain injury. Journal of Neurotrauma, 35(22), 2632–2640. 10.1089/neu.2017.5393 [DOI] [PubMed] [Google Scholar]
- Han P, Winkler N, Hummel C, Hahner A, Gerber J, & Hummel T (2018b). Impaired brain response to odors in patients with varied severity of olfactory loss after traumatic brain injury. Journal of Neurology, 265(10), 2322–2332. 10.1007/s00415-018-9003-8 [DOI] [PubMed] [Google Scholar]
- Haxel BR, Grant L, & Mackay-Sim A (2008). Olfactory dysfunction after head injury. Journal of Head Trauma Rehabilitation, 23(6), 407–413. 10.1097/01.HTR.0000341437.59627.ec [DOI] [PubMed] [Google Scholar]
- Howell J, Costanzo RM, & Reiter ER (2018). Head trauma and olfactory function. World J Otorhinolaryngol Head Neck Surg, 4(1), 39–45. 10.1016/j.wjorl.2018.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang T, Wei Y, & Wu D (2021). Effects of olfactory training on posttraumatic olfactory dysfunction: A systematic review and meta-analysis. Int Forum Allergy Rhinol, 11(7), 1102–1112. 10.1002/alr.22758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hura N, Xie DX, Choby GW, Schlosser RJ, Orlov CP, Seal SM, & Rowan NR (2020). Treatment of post-viral olfactory dysfunction: An evidence-based review with recommendations. Int Forum Allergy Rhinol, 10(9), 1065–1086. 10.1002/alr.22624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DH, Kim SW, Hwang SH, Kim BG, Kang JM, Cho JH, … Kim SW (2017). Prognosis of olfactory dysfunction according to etiology and timing of treatment. Otolaryngology and Head and Neck Surgery, 156(2), 371–377. 10.1177/0194599816679952 [DOI] [PubMed] [Google Scholar]
- Kobal G, & Hummel T (1998). Olfactory and intranasal trigeminal event-related potentials in anosmic patients. Laryngoscope, 108(7), 1033–1035. 10.1097/00005537-199807000-00015 [DOI] [PubMed] [Google Scholar]
- Kobal G, Hummel T, Sekinger B, Barz S, Roscher S, & Wolf S (1996). “Sniffin’ Sticks:” Screening of olfactory performance. Rhinology, 34(4), 222–226. [PubMed] [Google Scholar]
- Kondo H, Matsuda T, Hashiba M, & Baba S (1998). A study of the relationship between the T&T olfactometer and the University of Pennsylvania Smell Identification Test in a Japanese population. Am J Rhinol, 12(5), 353–358. 10.2500/105065898780182390 [DOI] [PubMed] [Google Scholar]
- Konstantinidis I, Tsakiropoulou E, Bekiaridou P, Kazantzidou C, & Constantinidis J (2013). Use of olfactory training in post-traumatic and postinfectious olfactory dysfunction. Laryngoscope, 123(12), E85–90. 10.1002/lary.24390 [DOI] [PubMed] [Google Scholar]
- Kraus MF, Little DM, Donnell AJ, Reilly JL, Simonian N, & Sweeney JA (2007). Oculomotor function in chronic traumatic brain injury. Cognitive and Behavioral Neurology, 20(3), 170–178. 10.1097/WNN.0b013e318142badb [DOI] [PubMed] [Google Scholar]
- Levin HS, High WM, & Eisenberg HM (1985). Impairment of olfactory recognition after closed head injury. Brain, 108 (Pt 3), 579–591. 10.1093/brain/108.3.579 [DOI] [PubMed] [Google Scholar]
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, … Moher D (2009). The prisma statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ, 339, b2700. 10.1136/bmj.b2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Zong G, Doty RL, & Sun Q (2016). Prevalence and risk factors of taste and smell impairment in a nationwide representative sample of the us population: A cross-sectional study. BMJ Open, 6(11), e013246. 10.1136/bmjopen-2016-013246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas AI, Hukkelhoven CW, Marshall LF, & Steyerberg EW (2005). Prediction of outcome in traumatic brain injury with computed tomographic characteristics: A comparison between the computed tomographic classification and combinations of computed tomographic predictors. Neurosurgery, 57(6), 1173–1182. 10.1227/01.neu.0000186013.63046.6b [DOI] [PubMed] [Google Scholar]
- McCrea M (2008). Mild traumatic brain injury and postconcussion syndrome: The new evidence base for diagnosis and treatment. Oxford University Press. [Google Scholar]
- Merezhinskaya N, Mallia RK, Park D, Bryden DW, Mathur K, & Barker FM 2nd., (2019). Visual deficits and dysfunctions associated with traumatic brain injury: A systematic review and meta-analysis. Optometry and Vision Science, 96(8), 542–555. 10.1097/OPX.0000000000001407 [DOI] [PubMed] [Google Scholar]
- Merkonidis C, Grosse F, Ninh T, Hummel C, Haehner A, & Hummel T (2015). Characteristics of chemosensory disorders--results from a survey. European Archives of Oto-Rhino-Laryngology, 272(6), 1403–1416. 10.1007/s00405-014-3210-4 [DOI] [PubMed] [Google Scholar]
- Miao X, Yang L, Gu H, Ren Y, Chen G, Liu J, & Wei Y (2015). Evaluation of post-traumatic anosmia with MRI and chemosensory ERPs. European Archives of Oto-Rhino-Laryngology, 272(8), 1945–1953. 10.1007/s00405-014-3278-x [DOI] [PubMed] [Google Scholar]
- Murphy C, Schubert CR, Cruickshanks KJ, Klein BE, Klein R, & Nondahl DM (2002). Prevalence of olfactory impairment in older adults. JAMA, 288(18), 2307–2312. 10.1001/jama.288.18.2307 [DOI] [PubMed] [Google Scholar]
- Nelson LD, Temkin NR, Dikmen S, Barber J, Giacino JT, Yuh E, … Zafonte R (2019). Recovery after mild traumatic brain injury in patients presenting to US Level I Trauma Centers: A transforming research and clinical knowledge in traumatic brain injury (track-tbi) study. JAMA Neurol, 76(9), 1049–1059. 10.1001/jamaneurol.2019.1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann D, Zupan B, Babbage DR, Radnovich AJ, Tomita M, Hammond F, & Willer B (2012). Affect recognition, empathy, and dysosmia after traumatic brain injury. Archives of Physical Medicine and Rehabilitation, 93(8), 1414–1420. 10.1016/j.apmr.2012.03.009 [DOI] [PubMed] [Google Scholar]
- Nordin S, Monsch AU, & Murphy C (1995). Unawareness of smell loss in normal aging and Alzheimer’s disease: Discrepancy between self-reported and diagnosed smell sensitivity. Journals of Gerontology. Series B: Psychological Sciences and Social Sciences, 50(4), P187–192. 10.1093/geronb/50b.4.p187 [DOI] [PubMed] [Google Scholar]
- Osborne-Crowley K, & McDonald S (2016). Hyposmia, not emotion perception, is associated with psychosocial outcome after severe traumatic brain injury. Neuropsychology, 30(7), 820–829. 10.1037/neu0000293 [DOI] [PubMed] [Google Scholar]
- Parma V, Straulino E, Zanatto D, Cantagallo A, Tirindelli R, & Castiello U (2012). Implicit olfactory abilities in traumatic brain injured patients. Journal of Clinical and Experimental Neuropsychology, 34(9), 977–988. 10.1080/13803395.2012.711811 [DOI] [PubMed] [Google Scholar]
- Peterson AB, Zhou H, Thomas KE, & Daughertty J (2021). Surveillance report of traumatic brain injury-related hospitalizations and deaths by age group, sex, and mechanism of injury—United States, 2016 and 2017.
- Proskynitopoulos PJ, Stippler M, & Kasper EM (2016). Post-traumatic anosmia in patients with mild traumatic brain injury (mTBI): A systematic and illustrated review. Surgical Neurology International, 7(Suppl 10), S263–275. 10.4103/2152-7806.181981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawal S, Hoffman HJ, Bainbridge KE, Huedo-Medina TB, & Duffy VB (2016). Prevalence and risk factors of self-reported smell and taste alterations: Results from the 2011–2012 US National Health and Nutrition Examination Survey (NHANES). Chemical Senses, 41(1), 69–76. 10.1093/chemse/bjv057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandford AA, Davidson TM, Herrera N, Gilbert P, Magit AE, Haug K, … Murphy C (2006). Olfactory dysfunction: A sequela of pediatric blunt head trauma. International Journal of Pediatric Otorhinolaryngology, 70(6), 1015–1025. 10.1016/j.ijporl.2005.10.013 [DOI] [PubMed] [Google Scholar]
- Savage R, Combs DR, Pinkston JB, Advokat C, & Gouvier WD (2002). The role of temporal lobe and orbitofrontal cortices in olfactory memory function. Archives of Clinical Neuropsychology, 17(4), 305–318. [PubMed] [Google Scholar]
- Schafer L, Schriever VA, & Croy I (2021). Human olfactory dysfunction: Causes and consequences. Cell and Tissue Research, 383(1), 569–579. 10.1007/s00441-020-03381-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield PW, & Doty RL (2019). The influence of head injury on olfactory and gustatory function. Handbook of Clinical Neurology, 164, 409–429. 10.1016/B978-0-444-63855-7.00023-X [DOI] [PubMed] [Google Scholar]
- Schofield PW, Moore TM, & Gardner A (2014). Traumatic brain injury and olfaction: A systematic review. Frontiers in Neurology, 5, 5. 10.3389/fneur.2014.00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigurdardottir S, Andelic N, Skandsen T, Anke A, Roe C, Holthe OO, & Wehling E (2016). Olfactory identification and its relationship to executive functions, memory, and disability one year after severe traumatic brain injury. Neuropsychology, 30(1), 98–108. 10.1037/neu0000206 [DOI] [PubMed] [Google Scholar]
- Sorokowski P, Karwowski M, Misiak M, Marczak MK, Dziekan M, Hummel T, & Sorokowska A (2019). Sex differences in human olfaction: A meta-analysis. Front Psychol, 10, 242. 10.3389/fpsyg.2019.00242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Ivany A, & Schminkey D (2016). Intimate partner violence and traumatic brain injury: State of the science and next steps. Family and Community Health, 39(2), 129–137. 10.1097/FCH.0000000000000094 [DOI] [PubMed] [Google Scholar]
- Summers CR, Ivins B, & Schwab KA (2009). Traumatic brain injury in the United States: An epidemiologic overview. Mt Sinai J Med, 76(2), 105–110. 10.1002/msj.20100 [DOI] [PubMed] [Google Scholar]
- Sumner D (1964). Post-traumatic anosmia. Brain, 87, 107–120. 10.1093/brain/87.1.107 [DOI] [PubMed] [Google Scholar]
- Teasdale G, & Jennett B (1974). Assessment of coma and impaired consciousness. A practical scale. Lancet, 2(7872), 81–84. 10.1016/s0140-6736(74)91639-0 [DOI] [PubMed] [Google Scholar]
- Temmel AF, Quint C, Schickinger-Fischer B, Klimek L, Stoller E, & Hummel T (2002). Characteristics of olfactory disorders in relation to major causes of olfactory loss. Archives of Otolaryngology - Head and Neck Surgery, 128(6), 635–641. 10.1001/archotol.128.6.635 [DOI] [PubMed] [Google Scholar]
- Tobias A (1999). Assessing the influence of a single study in the meta-analysis estimate. Stata Technical Bulletin, 8(47). https://ideas.repec.org/a/tsj/stbull/y1999v8i47sbe26.html [Google Scholar]
- VA/DoD. (2021). VA/DOD clinical practice guideline for the management of concussion-mild trauamtic brain injury
- Wehling E, Nordin S, Espeseth T, Reinvang I, & Lundervold AJ (2011). Unawareness of olfactory dysfunction and its association with cognitive functioning in middle aged and old adults. Archives of Clinical Neuropsychology, 26(3), 260–269. 10.1093/arclin/acr019 [DOI] [PubMed] [Google Scholar]
- Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, & Tugwell P (2000). The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses.
- Xydakis MS, Mulligan LP, Smith AB, Olsen CH, Lyon DM, & Belluscio L (2015). Olfactory impairment and traumatic brain injury in blast-injured combat troops: A cohort study. Neurology, 84(15), 1559–1567. 10.1212/WNL.0000000000001475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaki T, Oka N, Odaki M, & Kobayashi S (2020). Usability of intravenous thiamine injection test compared with odor stick identification test for japanese patients with severe traumatic brain injury. Auris, Nasus, Larynx, 47(2), 233–237. 10.1016/j.anl.2019.07.004 [DOI] [PubMed] [Google Scholar]
- Yousem DM, Geckle RJ, Bilker WB, Kroger H, & Doty RL (1999). Posttraumatic smell loss: Relationship of psychophysical tests and volumes of the olfactory bulbs and tracts and the temporal lobes. Academic Radiology, 6(5), 264–272. 10.1016/s1076-6332(99)80449-8 [DOI] [PubMed] [Google Scholar]
- Yousem DM, Geckle RJ, Bilker WB, McKeown DA, & Doty RL (1996). Posttraumatic olfactory dysfunction: MR and clinical evaluation. AJNR: American Journal of Neuroradiology, 17(6), 1171–1179. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


