INTRODUCTION
Functional neuroimaging provides a means to understand the relationship between brain structure and function.1 Functional MR (fMR) imaging can offer unique insight into preoperative planning for central nervous system (CNS) neoplasms by identifying areas of the brain affected or spared by the neoplasm.1 The development of fMR imaging presented a breakthrough in imaging acquisition and analysis1–3 as well as patient management. Since its discovery in 1992 by Ogawa and colleagues, the blood-oxygen-level-dependent (BOLD) fMR imaging technique has become the dominant in vivo imaging technique for functional brain imaging. BOLD fMR imaging provides functional information without requiring invasive electrodes, radiation, or intravenous contrast agent.1,3–5 The BOLD sequence uses differences in tissue magnetic susceptibility properties (T2* effect) between oxyhemoglobin (diamagnetic) and deoxyhemoglobin (paramagnetic).1,3,4,6 The BOLD fMR imaging signal depends on cerebral blood flow, cerebral blood volume, and cerebral metabolic rate. The net difference between tissue oxyhemoglobin and deoxyhemoglobin during the hemodynamic response (known as the hemodynamic response function [HRF]) is what generates the MR imaging signal. The “BOLD effect” assesses coupling of oxygenated blood flow and neuronal metabolism during functional tasks, resulting in a net difference in oxyhemoglobin and deoxyhemoglobin, which generates the BOLD signal.3,4,6 Hemoglobin’s magnetic properties depend on the reduction-oxidation of iron between Fe2+ and Fe3+ states (ie, oxygenated and deoxygenated states). These changes result in an increase in local tissue-derived signal intensity on T2*-weighted MR images.3–7 BOLD fMR imaging provides good spatial resolution for effective mapping of CNS function in patients whose tumor and/or peritumoral edema is adjacent to eloquent cortex.
This article discusses the applications, significance, and interpretation of BOLD fMR imaging and its relevance to presurgical planning in patients with CNS neoplasms.
BLOOD-OXYGEN-LEVEL–DEPENDENT FUNCTIONAL MR IMAGING AND THE ELOQUENT CORTEX
Sensorimotor
At many medical centers in the United States, BOLD fMR imaging is used to evaluate the sensorimotor system by providing an effective, low-risk, noninvasive means of evaluating the eloquent cortex, comparable with intraoperative mapping techniques.8 BOLD fMR imaging can be used to identify critical areas of interest to the neurosurgeon by discerning key functional areas of gray matter on structural MR imaging. This is particularly important in settings where tumor and/or peritumoral edema is in close proximity to eloquent cortex. The primary motor cortex, located in the precentral gyrus, is responsible for generating neural impulses that control motor movement. Any significant injury to this region can result in irreversible paresis.1,8,9 The primary sensory cortex is located in the postcentral gyrus.8 Separated by the central sulcus, the motor and sensory gyri are somatotopically organized.10
In the pre-BOLD fMR imaging era and in instances where BOLD fMR imaging is not available, traditional anatomic landmark approaches are used to identify the precentral gyrus and intraoperative electrodes are used to map out the hand-foot motor regions. Traditionally, the “reverse omega sign” is used to identify the hand motor region; however, this is not always reliable to because of anatomic variation and/or distortion of the homunculus by neoplasm or edema. Motor functions activated by the primary somatosensory cortex, such as the planning, execution, and control of specific behavior, is a complex neural process, and delineating neuronal function solely according to anatomic landmarks can be unreliable.11 In addition, the lack of reliable anatomic landmarks make it difficult to precisely localize the facial motor region on the precentral gyrus.12 Supported by clinical and neural data, 3 main motor areas (hand, face/lips/tongue, and foot) can be reliably identified on fMR imaging with good agreement between BOLD fMR imaging maps and intraoperative functional mapping.12,13 The foot motor region is usually located medially along the parasagittal aspect of the precentral gyrus at the level of the interhemispheric fissure (Figs. 1 and 2). The direct intraoperative cortical stimulation of this region is complicated by the presence of the adjacent superior sagittal sinus and can be further complicated by the presence of nearby edema, tumor, and/or aberrant vasculature such as a developmental venous anomaly. The 3 main functional areas (face, hand, foot) span the precentral gyrus (lateral to medial) and can be reliably assessed on task-based fMR imaging.1 It is important that patients with paresis can elicit motor and sensory activation with sensory stimulation of the hand, face, or foot through induced motor signals.14,15 During an fMR imaging examination, studies have shown paretic patients to induce more head motion as they experience difficulty moving the affected limb, leading to motion artifact and misregistration of BOLD signal.1
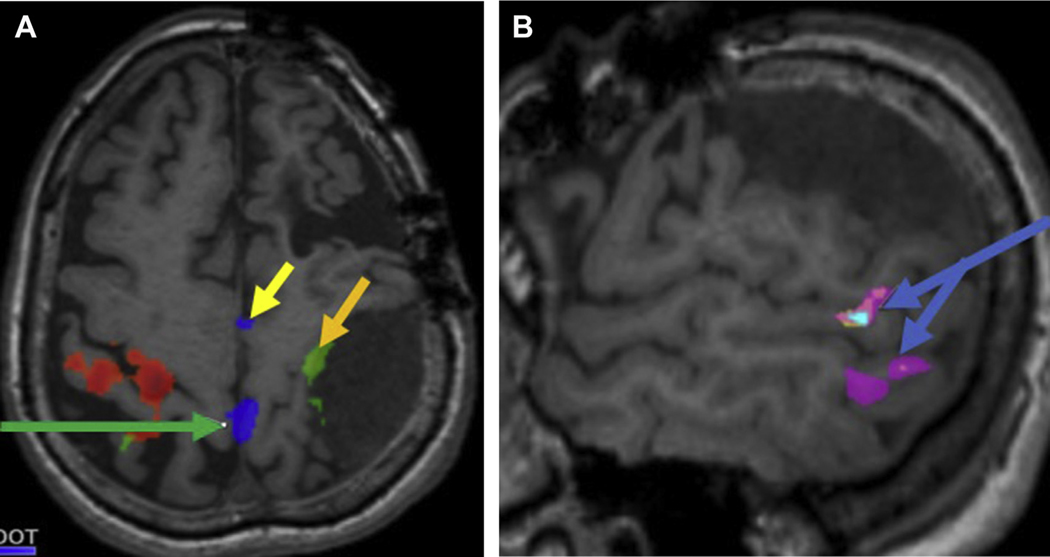
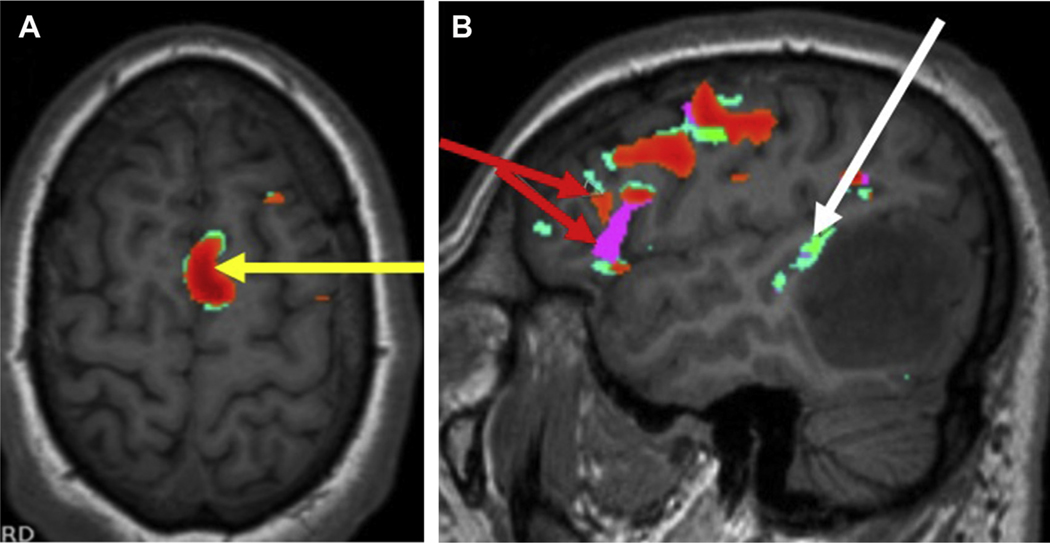
Fig. 1.
A 61-year-old woman with recurrent meningioma presented for presurgical evaluation of primary motor cortex. (A) Axial T1-precontrast series depicts recurrent extra-axial dural-based mass along the left posterior cerebral convexity with mass effect on the perirolandic structures. Task-fMR imaging BOLD signal depicts motor activation along the precentral gyrus (left foot, green arrow; left hand, orange arrow; SMA, yellow arrow). (B) Sagittal T1-precontrast images in the same subject depicts Wernicke area (blue arrows) along the posterolateral left temporal lobe with variant anatomy.
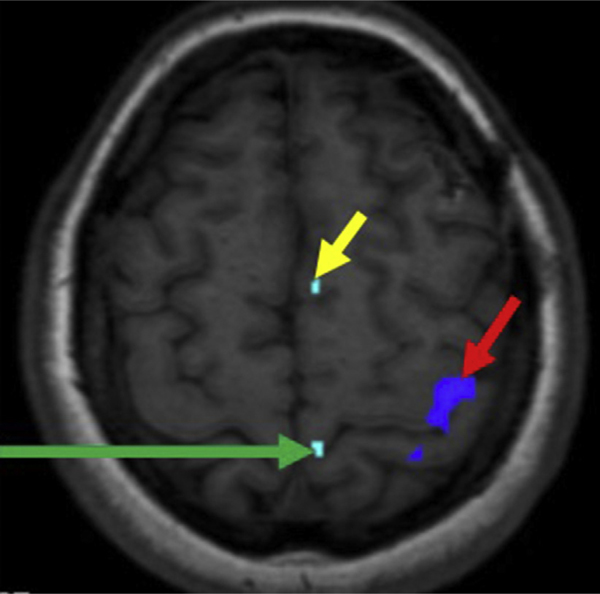
Fig. 2.
A 36-year-old woman with left insular glioma presented for preoperative eloquent cortex mapping. Left toe movement elicits activation in the left parasagittal precentral gyrus (green arrow), and left finger tap elicits activation along the lateral aspect of the left precentral gyrus (red arrow). The SMA is depicted by the yellow arrow.
Also important are secondary motor areas, which when damaged can lead to significant morbidity, thus increasing the importance of precise fMR imaging localization.1 The secondary areas of the brain of interest for neurosurgical planning include the pre–supplementary motor area (pre-SMA) and the supplementary motor area (SMA).16 The SMA consists of a posterior SMA, which is most commonly identified adjacent to the precentral sulcus and is normally active during motor tasks (see Figs. 1 and 2).16 The anterior portion of the SMA (Fig. 3) is more active during language activation and its borders are less well defined. Recent studies have suggested that the motor region of the SMA is somatotopically arranged.16 Studies have shown that direct activation of the SMA influences speech, which is evident during different language tasks, such as silent verbal fluency and repetition.17 Motor planning is largely associated with the SMA. Within the SMA is a centralized region that remains active during both language and motor tasks. It is also acknowledged that the SMA plays an important role in the planning and execution of movements, and that both passive and active tasks can be reliably detected through BOLD fMR imaging.17
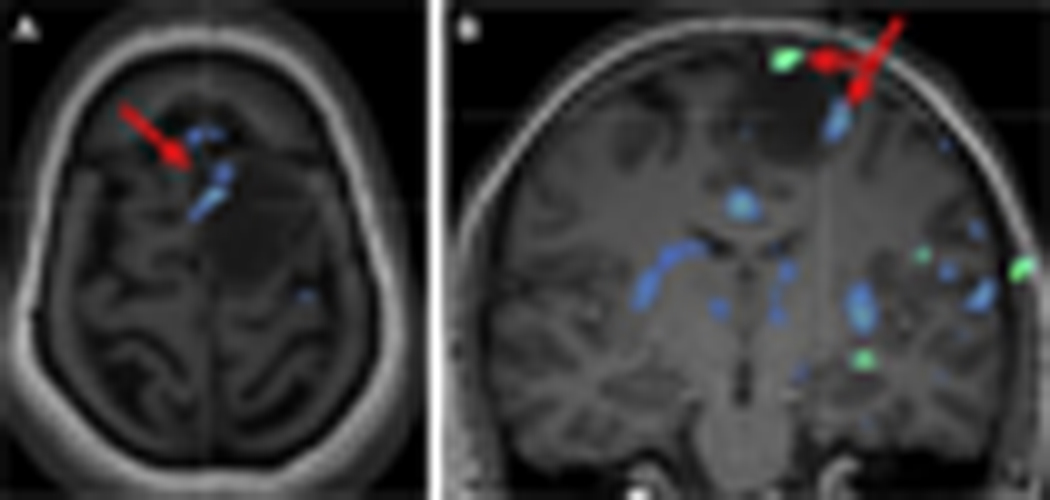
Fig. 3.
Axial (A) and coronal (B) presurgical task-fMR imaging in a 39-year-old woman with history of left frontal oligodendroglioma depicts SMA (red arrow) along the anteromedial and lateral margins of the neoplasm, which was confirmed on intraoperative functional mapping.
Because surgical resection of a brain neoplasm poses a risk to the eloquent cortex with potential for permanent neurologic damage, preoperative BOLD fMR imaging is particularly useful in cases where tumor and/or peritumoral edema is in close proximity to eloquent cortex. Using fMR imaging techniques, surgical teams can plan tumor resection through noninvasive visualization of the brain and analysis of lesion localization in relation to anatomic landmarks.18 Furthermore, fMR imaging derived information can also be used in counseling patients about the risks involved with surgical resection and in prospectively developing an appropriate surgical approach.19
Language (Broca and Wernicke Areas) and Memory
The Intracarotid Amobarbital Test, otherwise known as “Wada” testing, has been considered the gold standard for determining the language-dominant hemisphere.20 Although it is well known that the left cerebral hemisphere is usually the dominant center for language in most of the population, adequate preoperative testing is required to properly establish cerebral language dominance (Fig. 4). Owing to its high level of accuracy in predicting hemispheric language dominance and its noninvasive nature, task-fMR imaging is the standard of care in medical centers where it is available. Silent word-generation paradigms are most commonly used to elicit activation in the Broca area, most commonly located in the inferior frontal gyrus (Fig. 5). Silent sentence completion is the most frequently used paradigm to elicit activation in the Wernicke area (see Fig. 5). Of importance is that language regions (Wernicke more so than Broca) tend to be somewhat more broadly distributed and require careful image acquisition and postprocessing to ensure accurate depiction of the language network and limit overestimation or underestimation of these regions. To improve precision of localizing Broca and Wernicke regional activity, additional language paradigms are delivered including rhyming, antonym generation, object naming, and/or passive story listening.
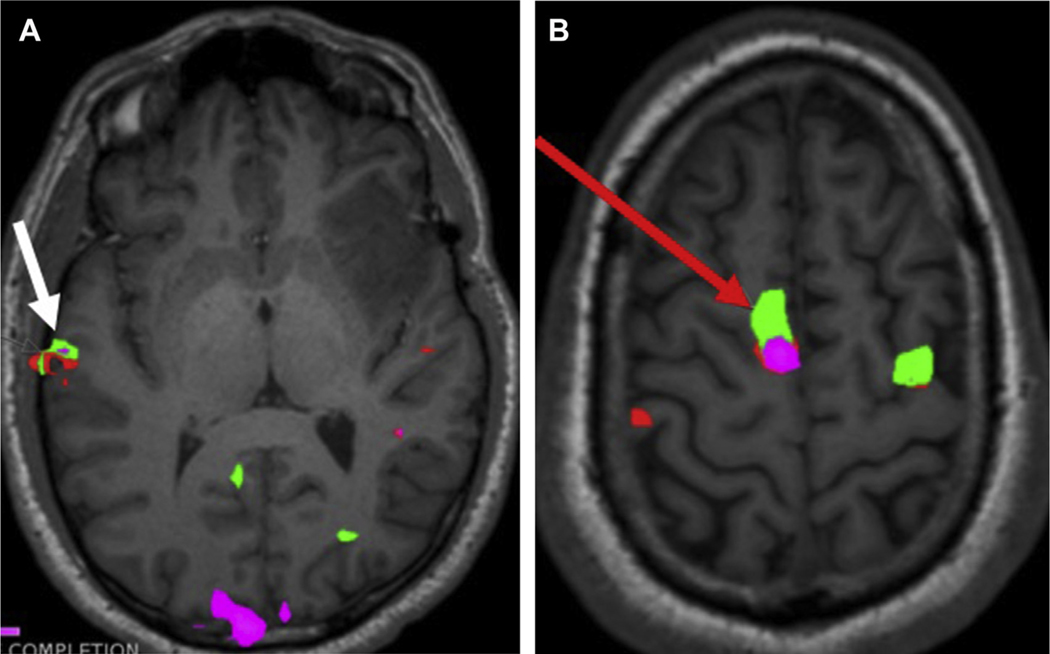
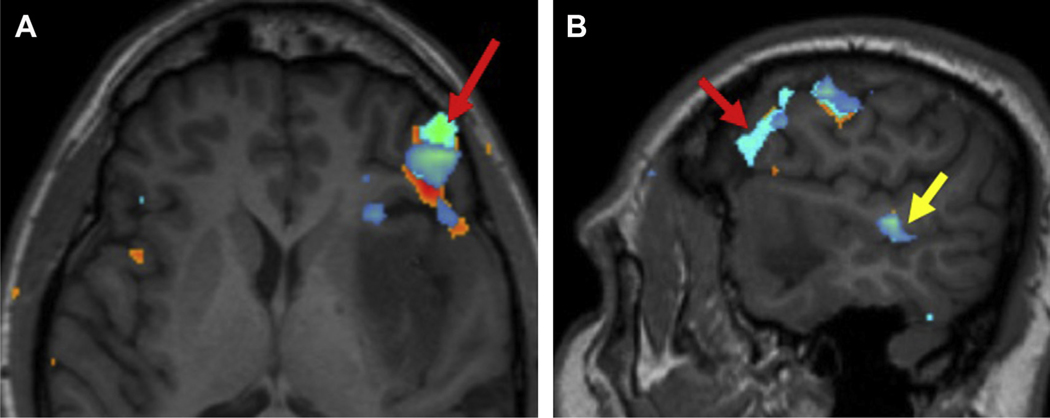
Fig. 4.
Preoperative axial task-fMR imaging (A) in a 29-year-old man with left frontoinsular glioma depicts right temporal dominance of Wernicke area (white arrow). More superiorly, (B) there is robust activation of the SMA in the right frontal lobe (red arrow).
Fig. 5.
Preoperative axial task-fMR imaging (A) in a 31-year-old man with left temporo-occipital glioma depicts left cerebral language dominance with robust activation of the SMA in the left frontal lobe (yellow arrow). Sagittal series (B) depicts activation in Broca (red arrow) and Wernicke (white arrow) regions.
Evaluation of memory lateralization is not well established on task-fMR imaging. A case study by Szaflarski and colleagues21 showed mixed results in the diagnostic accuracy of fMR imaging in predicting the memory outcomes of patients with epilepsy through presurgical evaluation. At present, additional research is required to develop a robust task-fMR or resting-state fMR (rs-fMR) imaging application for memory assessment. Such a development can be applied in the preoperative setting for patients with tumor or epilepsy, but can also be extended to evaluation of neurodegenerative diseases.10
PITFALLS OF BLOOD-OXYGEN-LEVEL–DEPENDENT FUNCTIONAL MR IMAGING
During an fMR imaging scan, it is common to find artifacts in patients with brain tumors. Artifacts in MR imaging can result from anything that disrupts the T2* imaging signal, and may be caused by the MR scanner hardware or patient interaction.22
Motion-related artifacts can be random or episodic (essential tremor, breathing, cardiac related) and are usually minor in amplitude. As such, most fMR imaging postprocessing software packages are robust enough to remove noise and adequately coregister BOLD and structural MR imaging series. Stimulus-related motion (eg, facial movement during word generation or sentence rhyming) can be difficult to remove. With modern statistical analysis, these motion-related signal artifacts may be removed as long as they vary enough from signal generated by the stimulus presentation. It is noteworthy that significant motion artifact can limit the BOLD series’ diagnostic value and can be difficult to remove with standard motion correction. This artifact can affect stimulus-related BOLD signal and structural correlation, and be mistaken for the stimulus-generated signal, adversely affecting the study.1 A strategy to overcome this limitation is to use the contralateral hemisphere as a reference control to simplify suppositions about displaced anatomy and affected BOLD signal magnitudes.23 In such cases, repeat acquisition is recommended.
The BOLD fMR imaging signal can be attenuated by noise. Multi-echo echo-planar (EP) imaging, whereby the fMR imaging signal is collected at multiple echo times, is a method to improve sensitivity to BOLD responses.24 This allows for better noise reduction capability and offers advantages in imaging brain regions susceptible to distortion and signal dropout.24 It is important to routinely inspect T2*-weighted images in an attempt to correct any and all artifacts found during the fMR imaging scans. As BOLD fMR imaging is an EP imaging–based technique, susceptibility artifact can result in BOLD signal loss, for example in blood products (hemosiderin), air-tissue interface, and metal (eg, dental hardware, surgical hardware near cortical regions).1
Neurovascular uncoupling (NVU), which refers to disruption of coupling between neuronal activity and neurovascular response in areas within or surrounding the tumor, is a major pitfall to consider, as it can lead to false-negative neuronal activity. The BOLD effect is proportional to the net change in volume from oxygenated to deoxygenated blood in the tissue; this is true as long as the natural HRF between the neurovascular system and the brain is preserved. In certain conditions—for example, hypervascular neoplasms—the normal HRF is disrupted, predisposing to NVU. As such, lesions in the eloquent cortex with marked increased vascularity should be scrutinized closely in cases demonstrating limited to no significant neuronal activation.1
BLOOD-OXYGEN-LEVEL–DEPENDENT FUNCTIONAL MR IMAGING APPLICATIONS
BOLD fMR imaging can reliably map eloquent cortex presurgically and is sufficiently accurate for neurosurgical planning. HRF captured on BOLD fMR imaging allows for noninvasive in vivo assessment of eloquent cortical activation.25 BOLD fMR imaging is useful for identification of the primary sensorimotor cortex, especially in cases where gyral anatomy is distorted by neoplasm or other CNS disorder (see Fig. 1). In patients with brain tumors undergoing neurosurgical intervention, fMR imaging can decrease postoperative morbidity by identifying eloquent cortex prospectively to guide surgical intervention. BOLD fMR imaging can be performed to evaluate the sensorimotor regions with paradigms that are both volitional and passive.26 Normal brain anatomic variance (see Fig. 1) is another reason patients undergo fMR imaging, because the conventional anatomic landmarks associated with functional brain regions cannot be identified on structural imaging.26,27
Neuroplasticity refers to the reorganization of neural networks that can be seen in the setting of slow-growing neoplasms and is another key factor to be considered in functional recovery of patients with brain tumors. fMR imaging can help identify reorganization in cerebral networks prospectively, guiding appropriate surgical intervention and minimizing postoperative morbidity.28 In patients with CNS neoplasms and history of stroke or comorbidities (eg, hypertension, coronary artery disease, diabetes) placing them at risk for stroke, fMR imaging can help depict reorganization of functional networks. Studies have shown that performing simple motor tasks with the affected limb following a stroke is associated with higher brain activation in many cortical areas when compared with healthy volunteers, including regions of the dorsal motor cortex, the ventral motor cortex, and the SMA.28 Longitudinal fMR imaging studies have revealed that neural activity is often enhanced in motion-related areas in both hemispheres before returning to normal levels similar to those in healthy controls during the first 12 months after stroke.3
Numerous other considerations are important when performing BOLD fMR imaging in clinical practice. For example, patients with brain tumors benefit from shorter-length tasks because they find greater difficulty keeping their head still in comparison with healthy subjects. Numerous studies have shown that patients benefit from signal averaging via block design, meaning that brain regions associated with a specific task can be activated even if it is not essential to the task being performed.29 For example, since the primary visual cortex shows robust activation on language fMR imaging paradigms, the authors reduce the overall scan time in patients in whom the visual cortex is uninvolved by acquiring information of the primary visual cortex on language paradigms and excluding visual paradigms from the fMR imaging examinations. The authors perform fMR imaging examinations with dedicated visual paradigms in cases where the patient is symptomatic (visual deficits), or when the primary visual cortical region is infiltrated by neoplasm or distorted by mass effect. Similarly, in instances when eloquent cortex is distant from pathology and functional deficits are not clinically detected, fMR imaging examinations are curtailed to reduce scan time for patient comfort. This strategy is also helpful in instances where the patient is projected to undergo a longer than usual MR imaging evaluation (eg, MR imaging spectroscopy).
BOLD fMR imaging in the postoperative setting is especially helpful if a patient is expected to undergo staged and/or multistep surgical resection. For example, after a tumor is removed, regions of brain compressed by mass effect may demonstrate regained functionality because this tissue can become active, showing the BOLD effect on postoperative fMR imaging.
TASK-BASED VERSUS RESTING-STATE BLOOD-OXYGEN-LEVEL–DEPENDENT FUNCTIONAL MR IMAGING
To elicit neuronal activation for generation of BOLD signal, patients are trained before entering the MR imaging scanner and instructed to follow tasks delivered in functional paradigms given as visual cues. For motor cortex activation (see Fig. 2), foot movement, finger tapping, and lip pucker (or tongue movement) are most commonly used. For language network assessment (Fig. 6), silent word generation, sentence completion, and rhyming are the 3 most commonly used paradigms. To limit scan variability, the American Society of Functional Neuroradiology has provided a list of paradigms with recommended scanner parameters (https://www.asfnr.org/paradigms/).
Fig. 6.
A 42-year-old man with left insular glioma presented for presurgical language network mapping. fMR imaging depicts left cerebral dominance of the language network with robust activation in (A) Broca region (red arrow) and (B) Wernicke region (yellow arrow).
Task-fMR imaging depends on the patient’s ability to perform specific tasks that elicit BOLD signal alternation on MR imaging. The BOLD series is overlaid on the structural imaging (usually high-resolution T1 and/or T2-weighted sequence) to locate eloquent regions such as the primary language and motor and visual cortices.1–3 There are several Food and Drug Administration–approved commercial software packages available in clinical practice that have made evaluation of task-based fMR imaging less time consuming by automating most of the postprocessing (eg, motion correction, image registration).
Though reliable in most clinical settings, task-based fMR imaging presents certain limitations and disadvantages. For example, task-based fMR imaging cannot be performed in patients suffering from severe neurologic deficits (eg, dementia, complete paresis).30 Depending on language limitations and/or educational barriers, several different tasks may be required to assess different motor and language functions. Many trials may be required to achieve the desired activation maps, resulting in lengthy scanning sessions.30 Task-fMR imaging also has limited utility in the pediatric preadolescent setting (especially infants and toddlers) because of the required cooperation by patients, as these subjects cannot follow commands on task-paradigms that are usually delivered through visual cues.
rs-fMR imaging is another BOLD fMR imaging technique with potential for overcoming task-based limitations. rs-fMR imaging measures the BOLD effect over a period of time while the patient is in the MR scanner. Agarwal and colleagues31 had originally measured the resting state of the human brain motor cortex with fMR imaging and discovered that even during rest, different brain regions are able to communicate without an actively performed task. Because there are no prescan training requirements of the subject for rs-fMR imaging scanning, patients can be instructed to lie quietly with their eyes closed during the scanning process.31 rs-fMR imaging is emerging as a useful alternative to task-fMR imaging, especially in cases where task-fMR imaging cannot be performed. In the absence of task performance, rs-fMR imaging maps acquire BOLD signal corresponding to low-frequency neuronal signal fluctuations during rest, thus allowing for detection of multiple functional networks.31 By using the changes in the BOLD signal, a 4-dimensional time series of the brain can be constructed to reflect changing neuronal activity.6 As the data are processed through a series of steps including head motion correction, spatial smoothing, and noise and bandpass filtering, the data can be evaluated.6 Multiple subject comparison requires normalization of the data across all subjects followed by a method of targeting the connection between the regions.
The most popular analysis methods for targeting connections include seed-based analysis (SBA) and independent component analysis (ICA). SBA is a hypothesis-driven method that uses an extracted BOLD time course from the region of interest (ROI) to determine the connectivity of a seed relative to the rest of the brain.32 ICA is a mathematical technique used to define a region of functional connectivity. ICA is a data-driven technique used to decompose the data set into independent components with strong temporal coherence (intraconnected maps), each of which associates with the time course of the overall signal.4,32 Although the application of fMR imaging in neuro-oncology has become standard in localizing brain regions before surgery, there has been difficulty in transitioning from task-based localization to the resting state.
A key limitation in rs-fMR imaging has been the lack of standardization across many recent studies. Although new fMR imaging technologies are being developed, there is a lack of standardization of physiologic parameters, pharmacologic interventions, and characterization of disease-related vascular changes, with limited concrete data on how these changes affect the BOLD signal.33 Current methods to analyze data also have limitations. SBA is based on predetermined ROIs, which can be arbitrary or task-fMR imaging derived.24 Because some patients may have brain-distorting mass lesions, the collective database of ROIs may become difficult to use.6,24 Although the uses of task versus rs-fMR imaging techniques differ in reliability to functional mapping, certain approaches see similar reliability with respect to mapping the sensorimotor network of healthy subjects. rs-fMR imaging has shown great promise as a diagnostic tool, and with enough comparative studies showing lack of discrepancy between both task and rs-fMR imaging, it has potential to become the noninvasive standard of care for surgical planning and prognosis.30–33
COMBINATION FUNCTIONAL BRAIN IMAGING
For presurgical planning, many techniques can be implemented complementarily to fMR imaging. Techniques used in place of or in addition to fMR imaging are magnetoencephalography (MEG), electroencephalography (EEG), PET, and diffusion tractography (DTI).1 Different techniques can vary in spatial or temporal resolution, invasiveness, and how they localize or lateralize function.
MEG has been shown to predict the location of the gyrus of interest more accurately and specifically in comparison with fMR imaging.34 Because fMR imaging activates the entire network (both primary and secondary areas) for motor tasks, there is difficulty interpreting where the primary, secondary, and sensory gyri are localized because they are acting simultaneously. This is not an issue with MEG. Spatial resolution between the 2 methods vary such that fMR imaging can be as low as 1 mm, whereas MEG achieves 5 mm.34–36 Both techniques assist in more precise preoperative surgical planning compared with traditional MR markers, however, because MEG scanners are not as readily available as conventional MR imaging scanners, the latter being more commonly used.1
EEG is a method similar to MEG in that it directly measures electrical activity of the brain through small metal electrodes.34 Even though intraoperative EEG is traditionally more invasive than fMR imaging, integrating the 2 techniques into the neurosurgical navigation system is beneficial in certain cases.
DTI is an MR technique that measures water diffusivity along white matter tracts using eigenvectors.2,3 In particular, DTI is able to map and characterize diffusion as a function of spatial localization.2,3 The combination of DTI with other imaging methods can improve its specificity for complex diseases. Combining DTI with gray matter fMR imaging localization paints a more complete picture of the functional anatomy in the area surrounding a tumor, with feedback such as tissue infiltration, distortion, and/or destruction.1–3
PET imaging can provide functional information by using a radioactive imaging agent conjugated with another compound (eg, glucose, antibodies, small molecules) depending on the disease of interest.20,37 Fluorodeoxyglucose (FDG), a glucose analog, is the most commonly used PET radiotracer that provides metabolic information associated with tumor. FDG PET’s role is somewhat limited in the setting of CNS neoplasms because there is increased background brain parenchymal FDG uptake resulting from physiologic neuronal activity, which limits spatial discrimination between tumor and adjacent normal brain tissue.38 PET-MR imaging is a hybrid technology allowing for simultaneous acquisition of both PET and MR.39 The benefit of the PET-MR imaging is that it can provide improved spatial resolution of detected CNS abnormalities with less (about 50%) PET radiotracer-associated radiation exposure.39 In CNS disorders, PET-MR imaging scans are most commonly used for evaluation of neoplasms, epilepsy, and neurodegenerative disorders.
SUMMARY
Functional neuroimaging is integral to current clinical practice in various disciplines including neurooncology, neurodegenerative disorders, and epilepsy. BOLD fMR imaging has been shown to help in presurgical planning, minimizing the risk of postsurgical morbidity while reducing operative time and decreasing craniotomy size.3,6 Task-based BOLD fMR imaging is the most commonly used technique for noninvasive assessment of eloquent cortex2,3,6 and can reliably elucidate cortical regions involved in sensorimotor, language, and visual functions. Though reliable, it is important to be aware of pitfalls of this technique, such as neurovascular uncoupling and susceptibility artifact, so as to provide the most accurate functional assessment.
KEY POINTS.
Functional MR imaging provides reliable in vivo assessment of the eloquent cortex and can be used to identify sensorimotor, language, and visual regions.
- Key limitations of BOLD task-fMR imaging include:
- Necessity of patient cooperation and ability of patients to perform the required task, thus limiting its application in young and elderly patients as well as those with neurocognitive limitations.
- BOLD fMR imaging is motion sensitive.
- In instances where tumor involves the eloquent cortex, postoperative changes limit BOLD fMR imaging assessment of perisurgical sites.
- Tumor and tumor microenvironment can affect normal hemodynamic response, resulting in neurovascular uncoupling and leading to false-negative BOLD fMR imaging signal changes.
ACKNOWLEDGMENTS
Funding support: Dr A.A. Chaudhry received funding support from NIH 5K12CA001727–23 and City of Hope Young Investigator Award. The authors would like to acknowledge Seth Hilliard for his assistance with the literature search on this article.
Footnotes
Disclosure Statement: The authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Gabriel M, Brennan NP, Peck KK, et al. Blood oxygen level dependent functional magnetic resonance imaging for presurgical planning. Neuroimaging Clin N Am 2014;24(4):557–71. [DOI] [PubMed] [Google Scholar]
- 2.Ulmer JL, Klein AP, Mueller WM, et al. Preoperative diffusion tensor imaging: improving neurosurgical outcomes in brain tumor patients. Neuroimaging Clin N Am 2014;24(4):599–617. [DOI] [PubMed] [Google Scholar]
- 3.Filippi M, Agosta F. Diffusion tensor imaging and functional MRI. Handb Clin Neurol 2016;136: 1065–87. [DOI] [PubMed] [Google Scholar]
- 4.Yeh CJ, Tseng YS, Lin YR, et al. Resting-state functional magnetic resonance imaging: the impact of regression analysis. J Neuroimaging 2015;25(1): 117–23. [DOI] [PubMed] [Google Scholar]
- 5.Buchbinder BR. Functional magnetic resonance imaging. Handb Clin Neurol 2016;135:61–92. [DOI] [PubMed] [Google Scholar]
- 6.Lang S, Duncan N, Northoff G. Resting-state functional magnetic resonance imaging: review of neurosurgical applications. Neurosurgery 2014;74(5): 453–64 [discussion: 464–5]. [DOI] [PubMed] [Google Scholar]
- 7.Dimou S, Battisti RA, Hermens DF, et al. A systematic review of functional magnetic resonance imaging anddiffusiontensorimagingmodalitiesusedinpresurgical planning of brain tumour resection. Neurosurg Rev 2013;36(2):205–14 [discussion: 214]. [DOI] [PubMed] [Google Scholar]
- 8.Buxton RB. The physics of functional magnetic resonance imaging (fMRI). Rep Prog Phys 2013;76(9): 096601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mangraviti A, Casali C, Cordella R, et al. Practical assessment of preoperative functional mapping techniques: navigated transcranial magnetic stimulation and functional magnetic resonance imaging. Neurol Sci 2013;34(9):1551–7. [DOI] [PubMed] [Google Scholar]
- 10.Barras CD, Asadi H, Baldeweg T, et al. Functional magnetic resonance imaging in clinical practice: state of the art and science. Aust Fam Physician 2016;45(11):798–803. [PubMed] [Google Scholar]
- 11.Borich MR, Brodie SM, Gray WA, et al. Understanding the role of the primary somatosensory cortex: opportunities for rehabilitation. Neuropsychologia 2015;79(Pt B):246–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silva MA, See AP, Essayed WI, et al. Challenges and techniques for presurgical brain mapping with functional MRI. Neuroimage Clin 2018;17:794–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leisman G, Moustafa AA, Shafir T. Thinking, walking, talking: integratory motor and cognitive brain function. Front Public Health 2016;4:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reid LB, Boyd RN, Cunnington R, et al. Interpreting intervention induced neuroplasticity with fMRI: the case for multimodal imaging strategies. Neural Plast 2016;2016:2643491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hassa T, de Jel E, Tuescher O, et al. Functional networks of motor inhibition in conversion disorder patients and feigning subjects. Neuroimage Clin 2016;11:719–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Potgieser AR, de Jong BM, Wagemakers M, et al. Insights from the supplementary motor area syndrome in balancing movement initiation and inhibition. Front Hum Neurosci 2014;8:960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vergani F, Lacerda L, Martino J, et al. White matter connections of the supplementary motor area in humans. J Neurol Neurosurg Psychiatry 2014;85(12): 1377–85. [DOI] [PubMed] [Google Scholar]
- 18.Yu ZB, Lv YB, Song LH, et al. Functional connectivity differences in the insular sub-regions in migraine without aura: a resting-state functional magnetic resonance imaging study. Front Behav Neurosci 2017;11:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abalkhail TM, MacDonald DB, Al Thubaiti I, et al. Intraoperative direct cortical stimulation motor evoked potentials: stimulus parameter recommendations based on rheobase and chronaxie. Clin Neurophysiol 2017;128(11):2300–8. [DOI] [PubMed] [Google Scholar]
- 20.Benjamin CFA, Dhingra I, Li AX, et al. Presurgical language fMRI: technical practices in epilepsy surgical planning. Hum Brain Mapp 2018;39(10): 4032–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Szaflarski JP, Gloss D, Binder JR, et al. Practice guideline summary: use of fMRI in the presurgical evaluation of patients with epilepsy: report of the guideline development, dissemination, and implementation Subcommittee of the American Academy of Neurology. Neurology 2017;88(4):395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krupa K, Bekiesinska-Figatowska M. Artifacts in magnetic resonance imaging. Pol J Radiol 2015; 80:93–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Middlebrooks EH, Frost CJ, Tuna IS, et al. Reduction of motion artifacts and noise using independent component analysis in task-based functional MRI for preoperative planning in patients with brain tumor. AJNR Am J Neuroradiol 2017;38(2):336–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caballero-Gaudes C, Reynolds RC. Methods for cleaning the BOLD fMRI signal. Neuroimage 2017; 154:128–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dewiputri WI, Auer T. Functional magnetic resonance imaging (FMRI) neurofeedback: implementations and applications. Malays J Med Sci 2013; 20(5):5–15. [PMC free article] [PubMed] [Google Scholar]
- 26.Choudhri AF, Patel RM, Siddiqui A, et al. Cortical activation through passive-motion functional MRI. AJNR Am J Neuroradiol 2015;36(9):1675–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Durning SJ, Costanzo M, Artino AR Jr, et al. Using functional magnetic resonance imaging to improve how we understand, teach, and assess clinical reasoning. J Contin Educ Health Prof 2014;34(1): 76–82. [DOI] [PubMed] [Google Scholar]
- 28.Li W, Wang M, Li Y, et al. A novel brain network construction method for exploring age-related functional reorganization. Comput Intell Neurosci 2016;2016: 2429691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abd-El-Barr MM, Saleh E, Huang RY, et al. Effect of disease and recovery on functional anatomy in brain tumor patients: insights from functional MRI and diffusion tensor imaging. Imaging Med 2013;5(4): 333–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palacios EM, Sala-Llonch R, Junque C, et al. Resting-state functional magnetic resonance imaging activity and connectivity and cognitive outcome in traumatic brain injury. JAMA Neurol 2013;70(7): 845–51. [DOI] [PubMed] [Google Scholar]
- 31.Agarwal S, Lu H, Pillai JJ. Value of frequency domain resting-state functional magnetic resonance imaging metrics amplitude of low-frequency fluctuation and fractional amplitude of low-frequency fluctuation in the assessment of brain tumor-induced neurovascular uncoupling. Brain Connect 2017;7(6):382–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu L, Caprihan A, Bustillo J, et al. An approach to directly link ICA and seed-based functional connectivity: application to schizophrenia. Neuroimage 2018;179:448–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen JE, Glover GH. Functional magnetic resonance imaging methods. Neuropsychol Rev 2015; 25(3):289–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Proudfoot M, Woolrich MW, Nobre AC, et al. Magnetoencephalography. Pract Neurol 2014;14(5): 336–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schmid E, Thomschewski A, Taylor A, et al. , E-PILEPSY consortium. Diagnostic accuracy of functional magnetic resonance imaging, Wada test, magnetoencephalography, and functional transcranial Doppler sonography for memory and language outcome after epilepsy surgery: a systematic review. Epilepsia 2018. 10.1111/epi.14588. [DOI] [PubMed] [Google Scholar]
- 36.Sollmann N, Ille S, Boeckh-Behrens T, et al. Mapping of cortical language function by functional magnetic resonance imaging and repetitive navigated transcranial magnetic stimulation in 40 healthy subjects. Acta Neurochir (Wien) 2016;158(7):1303–16. [DOI] [PubMed] [Google Scholar]
- 37.Bruinsma TJ, Sarma VV, Oh Y, et al. The relationship between dopamine neurotransmitter dynamics and the blood-oxygen-level-dependent (BOLD) Signal: a review of pharmacological functional magnetic resonance imaging. Front Neurosci 2018;12:238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smucny J, Wylie KP, Tregellas JR. Functional magnetic resonance imaging of intrinsic brain networks for translational drug discovery. Trends Pharmacol Sci 2014;35(8):397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matthews R, Choi M. Clinical utility of positron emission tomography magnetic resonance imaging (PET-MRI) in gastrointestinal cancers. Diagnostics (Basel) 2016; 6(3). 10.3390/diagnostics6030035. [DOI] [PMC free article] [PubMed] [Google Scholar]