Abstract
Background
Non‐steroidal anti‐inflammatory drugs (NSAIDs) have been widely used for the treatment of pain and fever associated with the common cold.
Objectives
To determine the effects of NSAIDs versus placebo (and other treatments) on signs and symptoms of the common cold, and to determine any adverse effects of NSAIDs in people with the common cold.
Search methods
We searched CENTRAL (2015, Issue 4, April), (January 1966 to April week 3, 2015), EMBASE (January 1980 to April 2015), CINAHL (January 1982 to April 2015) and ProQuest Digital Dissertations (January 1938 to April 2015).
Selection criteria
Randomised controlled trials (RCTs) of NSAIDS in adults or children with the common cold.
Data collection and analysis
Four review authors extracted data. We subdivided trials into placebo‐controlled RCTs and head‐to‐head comparisons of NSAIDs. We extracted and summarised data on global analgesic effects (such as reduction of headache and myalgia), non‐analgesic effects (such as reduction of nasal symptoms, cough, sputum and sneezing) and side effects. We expressed dichotomous outcomes as risk ratios (RR) with 95% confidence intervals (CI) and continuous data as mean differences (MD) or standardised mean differences (SMD). We pooled data using the fixed‐effect and random‐effects models.
Main results
We included nine RCTs with 1069 participants, describing 37 comparisons: six were NSAIDs versus placebo and three were NSAIDs versus NSAIDs. The overall risk of bias in the included studies was mixed. In a pooled analysis, NSAIDs did not significantly reduce the total symptom score (SMD ‐0.40, 95% CI ‐1.03 to 0.24, three studies, random‐effects model), or duration of colds (MD ‐0.23, 95% CI ‐1.75 to 1.29, two studies, random‐effects model). For respiratory symptoms, cough did not improve (SMD ‐0.05, 95% CI ‐0.66 to 0.56, two studies, random‐effects model) but the sneezing score significantly improved (SMD ‐0.44, 95% CI ‐0.75 to ‐0.12, two studies, random‐effects model). For outcomes related to the analgesic effects of NSAIDs (headache, ear pain, and muscle and joint pain) the treatment produced significant benefits. The risk of adverse effects was not high with NSAIDs (RR 2.94, 95% CI 0.51 to 17.03, two studies, random‐effects model) but it is difficult to conclude that such drugs are no different from placebo. The quality of the evidence may be estimated as 'moderate' because of imprecision. The major limitations of this review are that the results of the studies are quite diverse and the number of studies for one result is quite small.
Authors' conclusions
NSAIDs are somewhat effective in relieving the discomfort caused by a cold but there is no clear evidence of their effect in easing respiratory symptoms. The balance of benefit and harms needs to be considered when using NSAIDs for colds.
Plain language summary
Non‐steroidal anti‐inflammatory drugs for the common cold
Review question We carried out a review on the effects of non‐steroidal anti‐inflammatory drugs (NSAIDs) for treating pain or respiratory symptoms such as cough associated with the common cold.
Background The common cold is the most common and widespread illness known to humans. NSAIDs, for example, aspirin, ibuprofen and naproxen, have analgesic (pain‐reducing) and antipyretic (fever‐reducing) effects. NSAIDs have been widely used for over a century for the treatment of pain and fever associated with the common cold.
Study characteristics The evidence is current to April 2015. This review found nine studies (1069 participants of both genders, including children, adults and older people from the USA, Japan, Belgium and Denmark) that compared various NSAIDs either with each other or with an inactive substance that has no treatment value (placebo).
Key results Our findings suggest that NSAIDs may improve most analgesia‐related symptoms caused by the common cold (headache, ear pain, and muscle and joint pain), but there is no clear evidence that NSAIDs are effective in improving coughs and runny noses caused by the common cold. Some of the included trials reported gastrointestinal complaints, rash and oedema (fluid retention) in the NSAIDs group.
Quality of the evidence The quality of the evidence may be estimated as 'moderate' because of imprecision. The major limitations of this review are that the results of the studies are quite diverse and the number of studies for each outcome is quite small.
Conclusion NSAIDs are somewhat effective in relieving the discomfort caused by a cold but there is no clear evidence of their effect in easing respiratory symptoms. The balance of benefit and harms needs to be considered when using NSAIDs for colds.
Summary of findings
Summary of findings for the main comparison. Non‐steroidal anti‐inflammatory drugs for the common cold.
| Non‐steroidal anti‐inflammatory drugs for the common cold | ||||||
| Patient or population: patients with common cold Settings: community or care facilities or hospital Intervention: non‐steroidal anti‐inflammatory drugs | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Non‐steroidal anti‐inflammatory drugs | |||||
| Sum of overall symptom score | — | The mean sum of overall symptom score in the intervention groups was 0.4 standard deviations lower (1.03 lower to 0.24 higher) | — | 293 (3 studies) | ⊕⊕⊕⊝ moderate1 | — |
| Duration of colds | — | The mean duration of colds in the intervention groups was 0.23 lower (0 to 0 higher) | — | 214 (2 studies) | ⊕⊕⊕⊝ moderate2 | — |
| Throat irritation score | — | The mean throat irritation score in the intervention groups was 0.01 standard deviations lower (0.33 lower to 0.3 higher) | — | 159 (2 studies) | ⊕⊕⊕⊝ moderate2 | — |
| Headache score | — | The mean headache score in the intervention groups was 0.65 standard deviations lower (1.11 to 0.19 lower) | — | 159 (2 studies) | ⊕⊕⊕⊝ moderate2 | — |
| Score of pain in muscles/joints score | — | The mean pain in muscles/joints score in the intervention groups was 0.40 standard deviations lower (0.77 to 0.03 lower) | — | 0 (2 studies) | See comment | — |
| Cough score | — | The mean cough score in the intervention groups was 0.05 standard deviations lower (0.66 lower to 0.56 higher) | — | 159 (2 studies) | ⊕⊕⊕⊝ moderate2 | — |
| Rhinorrhoea score | — | The mean rhinorrhoea score in the intervention groups was 0.03 standard deviations higher (0.25 lower to 0.3 higher) | — | 199 (3 studies) | ⊕⊕⊕⊝ moderate2 | — |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1# NSAIDs group 141, placebo group 152. 2Too small sample size.
Background
Description of the condition
The common cold is an acute respiratory tract infection (ARTI) and is the most common and widespread illness known to humans, affecting all age groups. Young children suffer an average of six to eight colds a year, while adults experience approximately two to four colds a year. Although the common cold is usually mild, with symptoms lasting one to two weeks, it is a leading cause of medical visits and days missed from school and work (Heikkinen 2003). Nasal congestion, rhinorrhoea, sneezing and coughing accompanied by general malaise are typical symptoms of the common cold. Over 200 serologically different viral types are responsible for common colds, with the rhinovirus being the most common cause (Eccles 2005).
Description of the intervention
Despite ongoing research into antiviral drugs, there are no effective therapies for the prevention or treatment of the common cold. Therefore, treatment of colds is normally aimed at relieving the symptoms of the illness. Several classes of drugs are currently available, including decongestants, anticholinergics, antihistamines and antitussives. These are effective, to a greater or lesser extent, in treating symptoms of the common cold (AlBalawi 2013; De Sutter 2012; Li 2013; Ostberg 1997; Saraswat 2011; Smith 2014).
How the intervention might work
NSAIDs have been widely used for over a century for the treatment of pain and fever associated with the common cold. Despite their widespread present day use and the long medical history of the use of NSAIDs in relieving pain associated with the common cold, there is a lack of clinical data to support the efficacy of NSAIDs treating this condition. There is some evidence that cold symptoms might be the result of inflammatory mediators such as kinins and prostaglandins, which can be blocked by NSAIDs, rather than the result of the direct cytopathic effects of viruses (Eccles 2005; Gwaltney 2002).
Why it is important to do this review
Several studies have proposed that NSAIDs could be effective in alleviating common cold symptoms, including sneezing and coughing (Sperber 1989; Sperber 1992; Winther 2001). However, no consensus has been reached on this issue. This systematic review is an update of a Cochrane review first published in 2009 (Kim 2009).
Objectives
To determine the effects of NSAIDs versus placebo (and other treatments) on signs and symptoms of the common cold, and to determine any adverse effects of NSAIDs in people with the common cold.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) comparing NSAIDs used either alone or in combination with other medications versus placebo and other therapies for the treatment of signs and symptoms of the common cold in adults and children.
Types of participants
We included adults and children with the common cold, who had no other acute illness or severe, chronic conditions. The case definition of the common cold used was: recent onset of symptoms of runny or stuffy nose (or both), and sneezing, with or without symptoms of headache and cough. We excluded participants if they suffered from allergic rhinitis, had a concurrent lower or chronic respiratory infection or another chronic disease, atopic eczema, asthma, fever (> 38 °C), sinusitis or exudative pharyngitis.
Source populations were volunteers from the community, hospital or community outpatient departments, and primary care settings. We accessed additional evidence from studies of healthy volunteers exposed to rhinovirus in experimental conditions.
Types of interventions
NSAIDs versus placebo as a treatment for symptoms of the common cold. We considered variable doses and routes of administration of the NSAID treatments. We included trials that allowed concurrent use of other medications if they permitted equal access for patients in both the NSAIDs and placebo groups (Ta'i 2012).
Types of outcome measures
We did not consider objective assessments such as rhinometry and rhinoscopy.
Primary outcomes
Global evaluation of efficacy in the treatment of common cold symptoms.
Decrease in the number or duration of individual common cold symptoms. These symptoms were assessed by severity scale.
Secondary outcomes
Any reported side effects.
Search methods for identification of studies
Electronic searches
In the previous review we searched the Cochrane Central Register of Controlled Trials (CENTRAL) (2011, Issue 1), which contains the Cochrane Acute Respiratory Infections Group's Specialised Register, MEDLINE (January 1966 to March week 4, 2011), EMBASE (January 1980 to April 2011), CINAHL (January 1982 to April 2011) and ProQuest Digital Dissertations (January 1938 to April 2011).
For 2013 update we searched CENTRAL (2013, Issue 1), which contains the Cochrane Acute Respiratory Infections Group's Specialised Register, MEDLINE (January 2011 to March week 4, 2013), EMBASE (January 2011 to April 2013), CINAHL (January 2011 to April 2013) and ProQuest Digital Dissertations (January 2011 to April 2013).
For this 2015 update, we searched CENTRAL (2015, Issue 4, April), which contains the Cochrane Acute Respiratory Infections Group's Specialised Register, MEDLINE (January 2013 to April week 3, 2015), EMBASE (January 2013 to April 2015), CINAHL (January 2013 to April 2015) and ProQuest Digital Dissertations (January 2013 to April 2015).
See Appendix 1 and Appendix 2 for the EMBASE and CINAHL search strategies and Appendix 3 for the search strategy used for MEDLINE and CENTRAL. We combined the MEDLINE search terms with the highly sensitive search strategy designed by The Cochrane Collaboration for identifying RCTs (Lefebvre 2011). We adapted these search terms to search EMBASE.
We imposed no language or publication restrictions.
Searching other resources
We assessed non‐English language papers and, if necessary and possible, translated them, with the assistance of native language speakers. We searched reference lists of review articles and of all included studies to find other potentially eligible studies. We contacted authors of the included trials to request unpublished studies.
Data collection and analysis
Selection of studies
We used the search strategy detailed above to obtain titles and abstracts of studies that might be relevant to the review. Three review authors (YSM, YJC, YWH) independently screened titles and abstracts and one review author (SYK) collated the results. All review authors participated in resolving discrepancies until a consensus was reached.
Data extraction and management
The same review authors (YSM, YJC, YWH) independently carried out data extraction using standard data extraction forms. We translated studies reported in non‐English language journals before assessment. Where more than one publication of one trial existed, we included only the publication with the most complete data. We resolved disagreements by discussion.
Assessment of risk of bias in included studies
Three review authors (YSM, YJC, YWH) independently assessed the methodological quality of included studies using The Cochrane Collaboration's 'Risk of bias' tool (Higgins 2011). One review author (SYK) collated the results. All review authors participated in resolving discrepancies until a consensus was reached.
Measures of treatment effect
The effect of NSAIDs on common cold signs and symptoms was our primary measure of interest. We expressed results as risk ratios (RRs) with 95% confidence intervals (CIs) for dichotomous outcomes.
We used the standardised mean difference (SMD) where continuous scales of measurement were used to assess the effects of treatment (for example, mean severity scores and time to symptom relief), because different scales were used in most of the trials.
We summarised adverse effects when reported. We calculated the RR with 95% CI for each adverse effect, where possible, either compared to no treatment or to another treatment.
Unit of analysis issues
We split trials including more than two comparisons and analysed them as individual pair‐wise comparisons. By dividing the placebo cases, we ensured that we did not count cases in the placebo group more than once when conducting a meta‐analysis. We had no special issues in the analysis of studies with non‐standard designs.
Dealing with missing data
We attempted to contact the trial authors for additional information if data from the trial reports were unclear or missing. We have excluded data from the meta‐analysis and clearly stated the reason if we judged missing data to render the result uninterpretable.
Assessment of heterogeneity
We assessed heterogeneity amongst trials by using the Chi2 test for heterogeneity (with a 10% level of statistical significance) and the I2 statistic.
We considered other sources of heterogeneity, apart from differences in interventions, namely clinical diversity (children/adults, different classes of NSAIDs and different dosages) and study quality. Heterogeneity in treatments could be related to prior agent(s) used, and the agent, dose and duration of the therapy.
Assessment of reporting biases
There were insufficient trials for us to assess the likelihood of publication bias by examining a funnel plot for asymmetry.
Data synthesis
We pooled data using a fixed‐effect model if there was no significant heterogeneity (I2 statistic < 50%). If there was significant heterogeneity (I2 statistic ≥ 50%), we used the random‐effects model.
Subgroup analysis and investigation of heterogeneity
We intended to conduct subgroup analyses where data were available, for example, by age (adult, child), NSAID class and whether the common cold was artificial or natural.
Sensitivity analysis
We pooled data using the fixed‐effect model but we also analysed the random‐effects model to ensure robustness of the model chosen and susceptibility to outliers.
Results
Description of studies
In the vast majority of studies, the clinical symptoms of the common cold, requirements for inclusion, type and dose of NSAIDs, outcomes of trials and duration of therapy were quite diverse, which caused difficulties in quantitative analysis.
Results of the search
In the previous searches, we identified 60 trials; of these, nine met the inclusion criteria. In this 2015 updated search, we did not identify any potential new trials. All included studies were double‐blinded RCTs. Four of the six trials of community‐acquired colds were multicentre trials.
Included studies
The nine included studies involved 1069 participants suffering from colds. In six studies, 891 participants had community‐acquired colds, and in three studies, 178 participants were experimentally infected with cold viruses. For experimentally infected colds, inoculated populations were analysed. Only 72.5% of experimentally infected participants had cold symptoms. Therefore, we included non‐symptomatic infected participants in this analysis.
Three studies were performed in the USA, four in Japan, and one each in Belgium and Denmark. Trials took place in a total of 154 settings. Most were participants from hospitals, clinics and outpatient departments. One trial involved medical students and university staff. Three trials of experimentally infected colds did not report the trial setting. One trial involved mainly students and two trials reported participants only as volunteers.
Five trials compared NSAIDs with a placebo, three trials compared one NSAID with another, and one trial compared two NSAIDs with a placebo.
Five studies used ibuprofen, two used aspirin and two studies used loxoprofen. Ketoprofen, fenoprofen, fentiazac and naproxen were used in one study. Seven trials used visually identical capsules, one trial used a double‐dummy method and one trial used coded vials. The duration of treatment varied from a single dose to two daily doses for seven days.
Three studies used a general symptom score and five studies used a symptom severity score.
The Characteristics of included studies table includes a summary of the randomisation process, cold acquisition route, inclusion criteria, population, interventions and comparisons, outcome measures, adverse events and methodological quality.
Excluded studies
We excluded 51 trials: four studies were not randomised or the randomisation allocation was unclear; one study included febrile participants; 46 studies included participants with diagnoses other than common colds (see Characteristics of excluded studies table).
Risk of bias in included studies
The overall risk of bias in the included studies was mixed, largely due to missing information regarding randomisation procedures. We assessed two studies as being of high quality (Goto 2007; Ryan 1987).
Allocation
Out of the nine included studies (Goto 2007; Graham 1990; Itoh 1980; Katsu 1993; Nagaoka 1980; Ryan 1987; Sperber 1989; Sperber 1992; Winther 2001), two studies used a computer‐generated random numbers table to generate the allocation sequence (Goto 2007; Ryan 1987). The remaining studies contained insufficient information about the sequence generation process.
In four studies the allocation method was adequately concealed (Goto 2007; Itoh 1980; Nagaoka 1980; Ryan 1987). In two Japanese studies the randomisation process was carried out by two controllers who retained the key codes (Itoh 1980; Nagaoka 1980). In the remaining two studies, treatment was allocated by a third party (Goto 2007), or considered adequately concealed because the single oral dose was administered using a double‐blind method (Ryan 1987).
Blinding
All studies were described as 'double‐blind' and considered 'adequate'; either the active drug and placebo were identical, or an 'identical capsule double‐dummy' method was used.
Incomplete outcome data
Among the included studies, eight adequately addressed incomplete outcome data (Goto 2007; Graham 1990; Itoh 1980; Nagaoka 1980; Ryan 1987; Sperber 1989; Sperber 1992; Winther 2001). Three experimental rhinovirus cold trials excluded participants who were not infected, in which case the reason for exclusion may be justifiable (Graham 1990; Sperber 1989; Sperber 1992). In six studies the number of withdrawals was zero or very small (Itoh 1980; Nagaoka 1980; Ryan 1987; Sperber 1989; Sperber 1992; Winther 2001). One study had insufficient information to permit judgement of 'low risk' or 'high risk' of bias (Katsu 1993).
Selective reporting
We considered all studies as having 'unclear' risk of bias as all trials failed to include the study protocol. They had insufficient information to permit a judgement of either 'low risk' or 'high risk' of bias.
Other potential sources of bias
Amongst the included studies, none were stopped early or had reported claims of fraudulence against them. One study did not contain data to assess the baseline balance (Winther 2001). The overall quality of studies was mixed, largely due to missing information regarding randomisation procedures (Figure 1; Figure 2).
1.
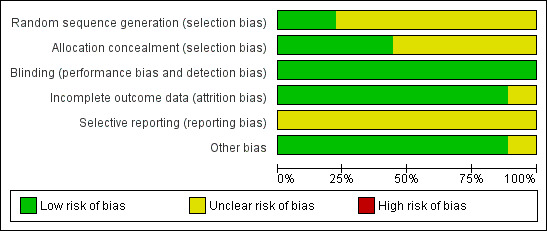
'Risk of bias' graph: review authors' judgements about each methodological quality item presented as percentages across all included studies
2.
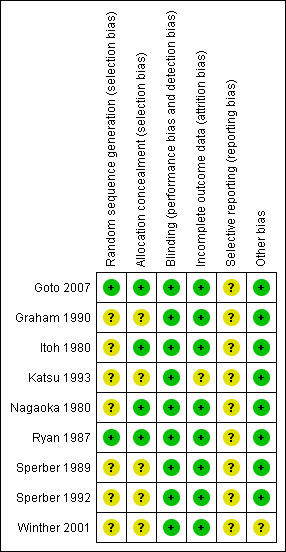
'Risk of bias' summary: review authors' judgements about each methodological quality item for each included study
Effects of interventions
See: Table 1
In total, we identified 37 outcomes. Eight studies assessed effectiveness and five studies assessed adverse effects. Twenty‐one (56.7%) of the 37 outcomes were assessed only by a single trial. Among the 16 outcomes assessed by two trials or more, six outcomes had an I2 statistic of ≥ 50% (overall symptom score, duration of colds, cough score, headache score, chills score and overall side effects).
Outcomes included in the meta‐analyses
One trial reported the daily symptom scores during six post‐challenge days and a six‐day cumulative symptom score (Sperber 1992). Other trials reported cumulative symptom scores, therefore we included the cumulative symptom score in the meta‐analysis for comparison.
One trial reported cumulative symptom scores for individual symptoms, such as rhinorrhoea and nasal obstruction, as well as cumulative symptom scores for individual areas (that is, nasal symptom score) (Sperber 1989). To prevent double counting and to compare data, we included only cumulative symptom scores of individual symptoms in the meta‐analysis.
Graham 1990 used aspirin (4 g/day) and ibuprofen (1.2 g/day). The dose of ibuprofen was the usual prescribed dose for the common cold and that of aspirin was not, therefore we chose to use the ibuprofen group in the meta‐analysis.
Primary outcomes
1. Global evaluation of efficacy in the treatment of common cold symptoms
i. Non‐steroidal anti‐inflammatory drugs (NSAIDs) versus placebo
Three trials assessed the total symptom score improvement of NSAIDs on the course of the common cold (Goto 2007; Sperber 1989; Sperber 1992). The first trial included 40 young adults and compared the effect of ibuprofen at a dose of 200 mg/four times a day for five days with that of a placebo (Sperber 1989). During six post‐challenge days, the daily total symptom score was not significantly different between the two groups. The second trial included 79 young adults and compared naproxen at a dose ranging from 3.0 g to 5.0 g for five days with placebo (Sperber 1992). The total five‐day symptom score judged by the modified Jackson criteria was reduced by 29% (95% confidence interval (CI) 16% to 42%) in the naproxen group compared with the placebo group. The third trial included 174 adults and compared the effects of loxoprofen at a dose of 60 mg/twice a day for seven days with placebo (Goto 2007). Duration of illness, number of days with limited daily activities and total symptom score were not significantly different between the two groups. We conducted a meta‐analysis of data from the three trials. The results of the pooled analysis were not significant (standardised mean difference (SMD) ‐0.40, 95% CI ‐1.03 to 0.24, random‐effects model) (Analysis 1.1) and there was heterogeneity (I2 statistic = 83%).
1.1. Analysis.
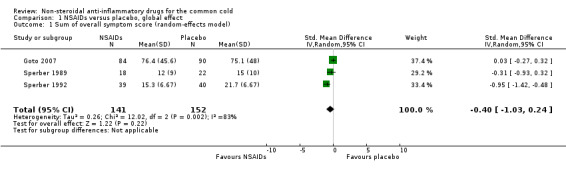
Comparison 1 NSAIDs versus placebo, global effect, Outcome 1 Sum of overall symptom score (random‐effects model).
Two trials assessed the duration of colds (Goto 2007; Sperber 1989). The results of the pooled analysis were not significant (mean difference (MD) ‐0.23, 95% CI ‐1.75 to 1.29, random‐effects model) (Analysis 1.3) and there was heterogeneity (I2 statistic = 80%).
1.3. Analysis.
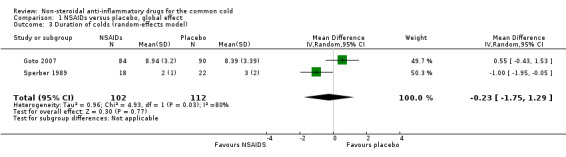
Comparison 1 NSAIDs versus placebo, global effect, Outcome 3 Duration of colds (random‐effects model).
One trial assessed the proportion of patients with symptoms of moderate to marked severity; no significant effect was detected (Sperber 1989).
ii. Head‐to‐head comparisons
Three trials involving participants with natural colds assessed the effect of one NSAID compared to other NSAID and ranked the severity of global symptoms on a five‐ to seven‐point scale; all three trials were performed in Japan (Itoh 1980; Katsu 1993; Nagaoka 1980).
Nagaoka 1980, which involved 222 participants, compared fentiazac (300 mg/day) with ibuprofen (600 mg/day). Katsu 1993 involved 167 participants and compared loxoprofen (80 mg/day) with ibuprofen (600 mg/day). Itoh 1980 enrolled 184 participants with upper respiratory tract infections and compared aspirin with ketoprofen. Itoh 1980 reported that there was no statistically significant difference between the groups in a subgroup analysis for the population with common colds, but the estimates and the number of participants included in the study population were not reported. Therefore, we could not use this result in a pooled analysis of efficacy.
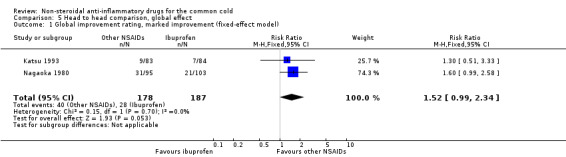
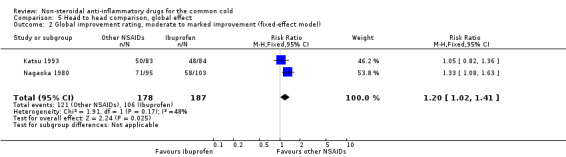
Marked improvement and moderate to marked improvement (on a global improvement rating) were significant in only one study (Nagaoka 1980).
2. Decrease in the number or duration of individual common cold symptoms
i. NSAIDs versus placebo: analgesic effects
Two trials measured nine outcomes evaluating the analgesic effects of NSAIDs (Sperber 1992; Winther 2001). The types of NSAIDs and the scale of outcomes differed between these studies.
As mentioned above, Sperber 1992 assessed the effect of naproxen in participants with an experimental cold and reported daily symptom scores and total (five‐day) symptom scores. Winther 2001 enrolled 80 participants with natural colds. The effect of ibuprofen at a dose of 400 mg/three times a day for three days was studied and the severity of symptoms was then ranked on a four‐point scale (not present, mild, moderate, severe) and a three‐day cumulative symptom score was reported.
Firstly, the cumulative throat irritation score was used in two trials (Sperber 1992; Winther 2001). In Sperber 1992, total (five‐day) and daily throat scores were not significantly different between the treatment groups. In Winther 2001, the total throat irritation/pain score was not significantly different between the treatment groups. As expected, the results of the pooled analysis were not significant (SMD ‐0.01, 95% CI ‐0.33 to 0.30, fixed‐effect model) (Analysis 2.1) and there was no heterogeneity.
2.1. Analysis.
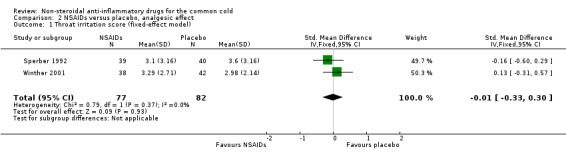
Comparison 2 NSAIDs versus placebo, analgesic effect, Outcome 1 Throat irritation score (fixed‐effect model).
Secondly, cumulative headache scores were reported in the same two trials (Sperber 1992; Winther 2001). All trials reported that headache scores were significantly lower in the NSAIDs groups than in the placebo groups. In a pooled analysis, NSAIDs significantly reduced headache scores (SMD ‐0.65, 95% CI ‐1.11 to ‐0.19, random‐effects model) (Analysis 2.2); there was marginal heterogeneity (I2 statistic = 51%).
2.2. Analysis.
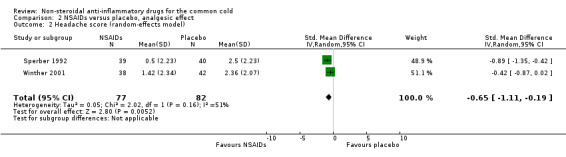
Comparison 2 NSAIDs versus placebo, analgesic effect, Outcome 2 Headache score (random‐effects model).
Thirdly, cumulative pain scores in the muscles and joints were also reported in these two trials (Sperber 1992; Winther 2001). In Winther 2001, the pain score in muscles and joints did not differ significantly between the treatment groups. In Sperber 1992, the myalgia score was significantly reduced in the naproxen group. In a pooled analysis, NSAIDs significantly reduced the score for pain in muscles and joints (SMD ‐0.40, 95% CI ‐0.77 to ‐0.03, fixed‐effect model) (Analysis 2.3); there was no heterogeneity.
2.3. Analysis.

Comparison 2 NSAIDs versus placebo, analgesic effect, Outcome 3 Score of pain in muscles/joints score (fixed‐effect model).
Fourthly, the two studies assessed a cumulative malaise score (Sperber 1992; Winther 2001). All trials reported that the malaise score was not significantly different between the two treatment groups. However, in a pooled analysis there was a trend towards reduction of malaise (SMD ‐0.29, 95% CI ‐0.6 to 0.03, fixed‐effect model) (Analysis 2.4).
2.4. Analysis.
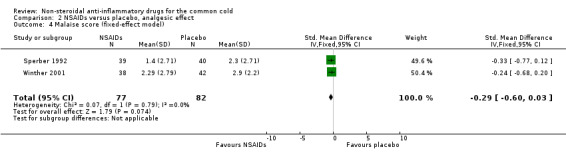
Comparison 2 NSAIDs versus placebo, analgesic effect, Outcome 4 Malaise score (fixed‐effect model).
Fifthly, the two studies assessed a cumulative chills score; the results were mixed. One trial reported a significant reduction (Sperber 1992) and the other reported a significant increase (Winther 2001). In a pooled analysis, the statistical significance of the difference disappeared and heterogeneity was detected (SMD ‐0.03, 95% CI ‐1.12 to 1.06, I2 statistic = 91.5%, random‐effects model) (Analysis 2.5).
2.5. Analysis.
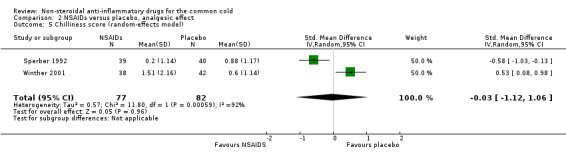
Comparison 2 NSAIDs versus placebo, analgesic effect, Outcome 5 Chilliness score (random‐effects model).
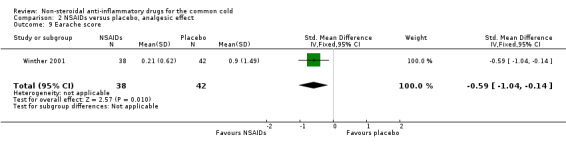
The cumulative earache score was significantly reduced in the ibuprofen group compared to the placebo group (Winther 2001).
ii. NSAIDs versus placebo: non‐analgesic effects
Four trials measured 15 outcomes irrelevant to the analgesic effect (Graham 1990; Sperber 1989; Sperber 1992; Winther 2001). The scales of outcomes were quite diverse. Three trials tested ibuprofen (Graham 1990; Sperber 1989; Winther 2001) and one trial tested naproxen (Sperber 1992).
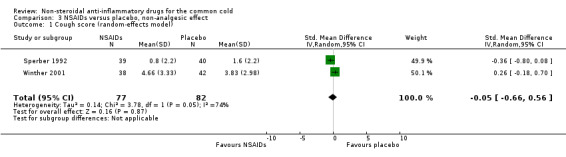
Firstly, two trials reported a cumulative cough score (Sperber 1992; Winther 2001). In Sperber 1992, the cumulative cough score was not significant (0.8 and 1.6, naproxen and placebo, respectively), but the daily score was significantly reduced at four days (P value < 0.01). Winther 2001 evaluated the cumulative cough score, but there was no difference between the groups. The results of a pooled analysis for cumulative cough score were not significant.
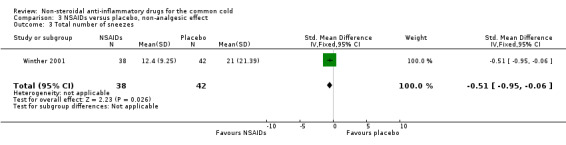
Secondly, two trials evaluated a cumulative sneezing score (Sperber 1992; Winther 2001). In Sperber 1992, the cumulative sneezing score was not significant (1.5 and 2.2, naproxen and placebo, respectively) but daily scores were reduced in the naproxen group at one and four days. The statistically insignificant differences between scores were at two and three days. In Winther 2001, the cumulative sneezing score was significantly reduced in the ibuprofen group, and the result of a pooled analysis supported this effect (SMD ‐0.44, 95% CI ‐0.75 to ‐0.12, the P value of the heterogeneity test was 0.44; fixed‐effect model) (Analysis 3.2). Winther also examined the total number of sneezes and the result was significant.
3.2. Analysis.
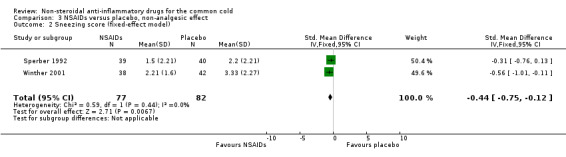
Comparison 3 NSAIDs versus placebo, non‐analgesic effect, Outcome 2 Sneezing score (fixed‐effect model).
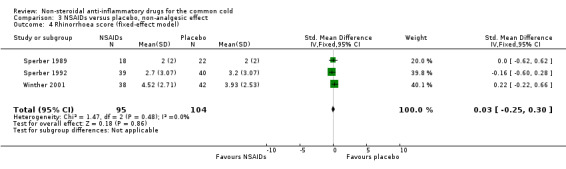
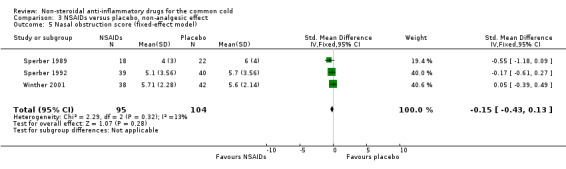
Three trials studied a cumulative rhinorrhoea score and a cumulative nasal obstruction score, and found no differences between the groups (Sperber 1989; Sperber 1992; Winther 2001).
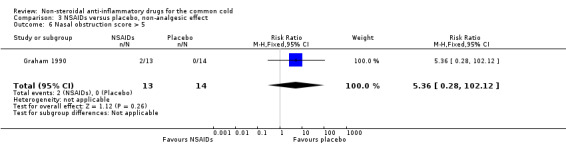
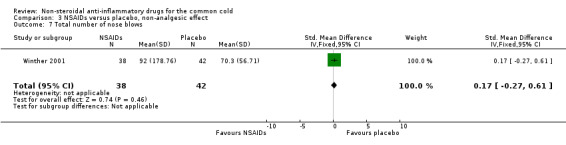
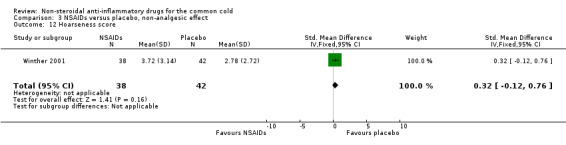
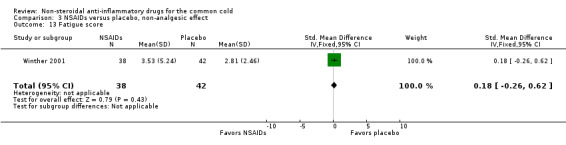
The proportion of nasal obstruction scores greater than five points (Graham 1990), total mucus weight, total tissue count (Sperber 1989), total number of nose blows, cumulative nasal dryness score, cumulative score for reduced sense of smell, cumulative hoarseness score, cumulative fatigue score and cumulative malaise score were quantified in a single study (Winther 2001) and the results were not significantly different between the treatment groups.
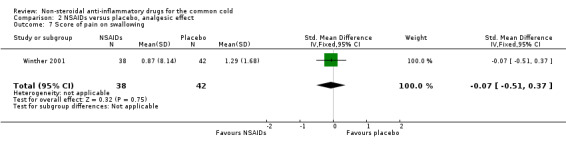
The cumulative nose irritation score, cumulative pain on swallowing score and cumulative eye itching score were also not significantly different between the treatment groups (Winther 2001).
Secondary outcomes
1. Any reported side effects
i. NSAIDs versus placebo: adverse effects
Five trials reported adverse effects. One study reported that adverse effects were more frequent in the loxoprofen group (9.5% versus 1.1%, P value < 0.05) (Goto 2007). Otherwise we could not find any evidence of an increased frequency of adverse effects in the active treatment groups. These outcomes included overall side effects, gastrointestinal complaints and other problems such as rash and oedema.
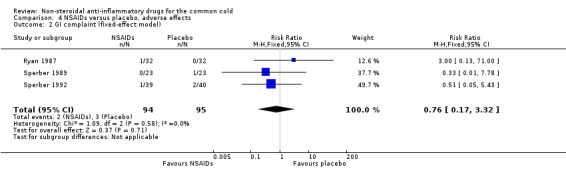
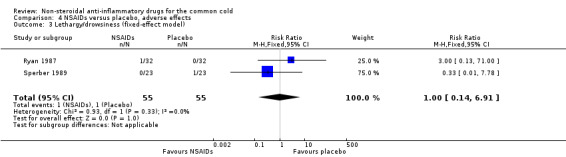
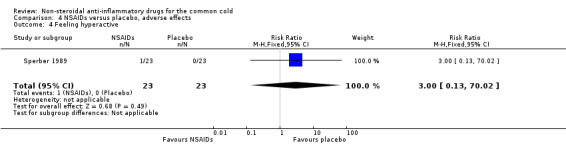
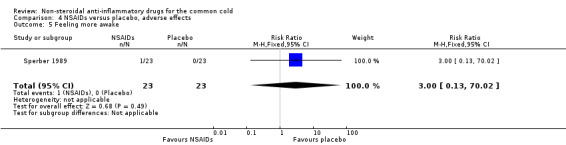
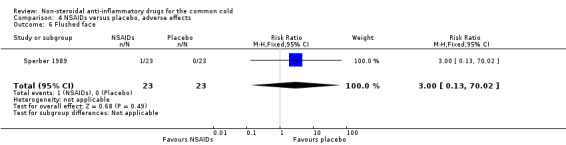
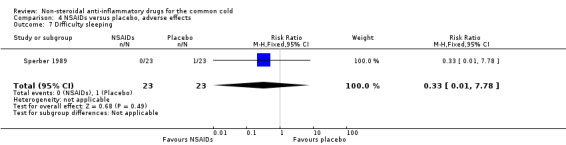
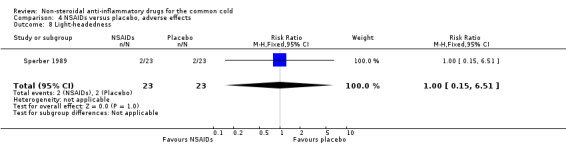
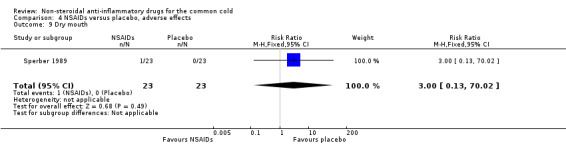
Two trials assessed the overall side effects of NSAIDs and there was moderate heterogeneity (Goto 2007; Sperber 1989). The results of a pooled analysis for overall side effects was not significant (risk ratio (RR) 2.94, 95% CI 0.51 to 17.03, random‐effects model) (Analysis 4.1). Three trials reported gastrointestinal adverse effects and found no differences between the groups (Ryan 1987; Sperber 1989; Sperber 1992). Lethargy/drowsiness, feeling hyperactive, feeling more awake, flushed face, difficulty sleeping, light‐headedness and dry mouth were reported in one or two trials and the results were not significantly different between the treatment groups.
4.1. Analysis.

Comparison 4 NSAIDs versus placebo, adverse effects, Outcome 1 Overall side effects (random‐effects model).
Discussion
In summary, if non‐steroidal anti‐inflammatory drugs (NSAIDs) are administered to community‐infected or experimentally infected cold patients, their analgesic effect against pain and irritation induced by the cold is relatively effective, but reports on whether they are helpful in relieving respiratory symptoms, such as coughing and sneezing, are not consistent and the evidence is insufficient.
Despite a comprehensive search, only nine studies met the inclusion criteria, six of which were placebo‐controlled randomised controlled trials (RCTs) and three of which were head‐to‐head RCTs. When we evaluated the methodological quality of the included studies using The Cochrane Collaboration's tool for assessing risk of bias (Higgins 2011), the overall quality of studies was mixed, largely due to missing information regarding randomisation procedures. We assessed two studies as being of high quality (Goto 2007; Ryan 1987). Our outcomes were mainly subjective and blinding of participants may be critical. All nine studies were described as 'double‐blind' and considered 'adequate'.
Among the results used to examine the effect of NSAIDs on the common cold, the ones looking at the analgesic effect evaluated headache, throat irritation, muscle and joint pain, ear pain, malaise and chills. Among them, headache, ear pain and muscle and joint pain showed significant results and malaise showed borderline significance. However, throat irritation was not improved, and chills showed mixed results. For some cases where symptoms did not improve, the reasons were uncertain. Whether the cold was community‐acquired or experimentally infected, the trial quality and dose of NSAIDs could not explain the differences. In the case of throat irritation, if the cold was an infection with a rhinovirus, there was the possibility that the treatment was not effective because throat pain disappeared naturally over a short period of time (Heikkinen 2003). There is also the possibility that the mechanism of throat pain may be different from that of headache and muscle pain. In the case of chills, NSAIDs were obviously effective in one trial, but worsened the symptoms in the other trial. Chills are known to happen mainly when the fever has lowered, therefore the measure of improvement may be different from the other symptoms and depend on whether there was a fever before the administration of treatment or not. However, because there was no information on the body temperature before starting the treatment in the two trials, we cannot draw a conclusion on this matter. Apart from these two symptoms, NSAIDs improved most of the analgesia‐related symptoms caused by a cold. Therefore, we recommend the use of NSAIDs for these symptoms.
Three trials studied whether NSAIDs had a comprehensive effect on various symptoms caused by the common cold (Goto 2007; Sperber 1989; Sperber 1992). Two of them were conducted with participants whose cold was experimentally infected by a rhinovirus (Sperber 1989; Sperber 1992). One of those showed a statistically significant difference in the effect of NSAIDs (Sperber 1992), and when we merged the results of the two studies the results were significant. However, one recently published trial reported that the total symptom score showed no significant difference between the two groups (Goto 2007). The results of the pooled analysis were not significant and there was heterogeneity, but the reason for this was unclear.
Among the studies two trials examined whether NSAIDs reduced the duration of a cold (Goto 2007; Sperber 1989). The results of the pooled analysis were not significant and there was heterogeneity. NSAIDs did not have any effect on the severity or duration of a cold. There were only two trials and the number of participants in the studies was small, therefore it is hard to draw a definite conclusion about the effects of NSAIDs on the duration of a cold.
One of the current issues related to the administration of NSAIDs for the common cold is whether NSAIDs are helpful in relieving respiratory symptoms such as cough. Many of the studies on the common cold recommend the administration of NSAIDs to ease coughing caused by a cold (Heikkinen 2003; Irwin 2000). The recently published American College of Chest Physicians (ACCP) guidelines recommend the combined administration of first‐generation antihistamine and nasal decongestant or the administration of naproxen for cough caused by a cold (Pratter 2006). Respiratory symptoms examined in this review were cough, nasal discharge and sneezing. The medication was not effective for cough in two trials and pooled results did not show a significant improvement (Sperber 1992; Winther 2001). None of the three trials showed a significant result for nasal discharge, and pooled results were not significant (Sperber 1989; Sperber 1992; Winther 2001). However, in the case of sneezing, one trial showed a significant improvement and pooled results showed a moderate effect (Winther 2001). Considering these results, which differ from existing guidelines, there is no clear evidence that NSAIDs are effective for coughs caused by a cold, or should be recommended in order to ease cough caused by a cold.
NSAIDs draw attention due to their adverse effects. For some NSAIDs, their long‐term use increases the risk of cardiovascular disease (Matchaba 2004) and may cause gastrointestinal side effects (Ofman 2002). The frequency of gastrointestinal side effects increases in proportion to the dose and period of NSAID medication but the risk of gastrointestinal side effects cannot be excluded with short‐term use (Hernández‐Díaz 2000). In trials included in this review, the risk of side effects was not high but it is difficult to conclude that they are no different from placebo in terms of side effects.
In this review, three trials studied which specific NSAIDs were more effective in treating a cold (Itoh 1980; Katsu 1993; Nagaoka 1980). One study found that fentiazac was more effective than ibuprofen (Nagaoka 1980). However, this is probably because the dose of ibuprofen used in the trial was 600 mg/day, lower than that used in other trials.
The absence of epithelial destruction during rhinovirus infections has led to the idea that the clinical symptoms of the common cold may not be caused by a direct cytopathic effect of the viruses but instead are primarily caused by the inflammatory response of the host by media such as kinins, leukotrienes and histamines (Heikkinen 2003). Accordingly, NSAIDs are believed to ease not only fever and irritation but also respiratory symptoms such as coughing. However, this was not proven in the review. Further research is needed to examine their effects.
For analgesic effects on a cold, acetaminophen was also frequently used along with NSAIDs. However, in this review we did not examine which of the medications was superior in terms of effect and safety. Further research is needed to evaluate this.
Major limitations of this review are that the results of the research are quite diverse and the number of studies for each outcome is quite small. For this reason, it is somewhat difficult to draw clear conclusions.
In conclusion, NSAIDs are recommended for relieving irritation or pain caused by a cold but the notion that NSAIDs are effective in relieving respiratory symptoms such as cough and nasal discharge needs more solid evidence.
Summary of main results
If NSAIDs are administered to community‐infected or experimentally infected cold patients, their analgesic effect against pain and irritation induced by the cold is somewhat effective but reports on whether they are helpful in relieving respiratory symptoms such as coughing and sneezing are not consistent and the evidence is insufficient.
Overall completeness and applicability of evidence
The trials included in the analyses mainly involved young adults of both sexes. Therefore the results of these trials may not be applicable to children and older people.
Quality of the evidence
The quality of evidence was estimated as moderate because of imprecision of the evidence.
Potential biases in the review process
Among the analgesic effect outcomes of NSAIDs, headache, pain in muscles and joints, and earache were statistically significant. However, these findings were mainly based on only two trials.
Agreements and disagreements with other studies or reviews
Two studies (Heikkinen 2003; Irwin 2000) and the ACCP guidelines (Pratter 2006) recommend the administration of NSAIDs for coughs caused by a cold. However, this review concluded that there is no clear evidence that NSAIDs are effective for coughs caused by a cold.
Authors' conclusions
Implications for practice.
Non‐steroidal anti‐inflammatory drugs (NSAIDs) are somewhat effective in relieving the discomfort caused by a cold but there is no clear evidence of their effect in easing respiratory symptoms. The balance of benefit and harms needs to be considered when using NSAIDs for colds.
Implications for research.
We are unable to support the theory that NSAIDs are effective in reducing cough, based upon the data included in this review. A large trial to study the effect of NSAIDs on colds may make this relationship clearer. For analgesic effects on the common cold, acetaminophen is also frequently used along with NSAIDs. However, in this review we did not examine which of these treatments was superior in terms of effect or safety. For this evaluation, we consider another review necessary.
Feedback
Non‐steroidal anti‐inflammatory drugs for the common cold, 8 December 2009
Summary
In their Cochrane Review on non‐steroidal anti‐inflammatory drugs for the common cold, Kim et al. (1) "recommend NSAIDs for relieving discomfort or pain caused by the common cold” without any reservations. However, the common cold is a rather harmless condition, whereas NSAIDs can have serious and even lethal adverse effects (2‐4). The review also has methodological shortcomings.
One problem is the excessive number of outcomes; the review authors report on no less than 26 primary outcomes. Four of these, sneezing, headache, pain in muscles/joints and earache, were statistically significant, but the first 3 outcomes were based on only 2 trials, including 159 participants, and the last outcome on only 1 trial.
One of these 2 trials was an experimental study (5) of 87 healthy volunteers that were inoculated with rhinovirus. The trial had unclear sequence generation, unclear concealment of allocation and was not analysed using intention to treat, as 8 people were excluded from the analysis. The volunteers were treated with very high doses of naproxen, up to 1500 mg daily, which is higher than what has been approved for treatment of acute pain conditions (6), and as the risk of harms increases linearly with the dose (7), this is particularly problematic. This trial is also included in the analysis for global effects where it had the largest effect of the 3 included trials and contributed to substantial heterogeneity, which suggests bias or problems with generalisability. Further, as it can be problematic to generalise findings from experimental settings to patients (8), it is questionable to pool this trial with trials from clinical settings.
The second trial (9) included 80 patients with natural colds that received 1200 mg ibuprofen daily. This trial also had unclear sequence generation and unclear allocation concealment. Additionally, for analysis 2.9 and 2.10 of Chilliness score, the authors have erroneously extracted the results from the placebo arm of this trial as though they belonged to another trial (5) and vice versa. This raises the question whether there were other data extraction errors. Data extraction errors are frequent in meta‐analyses using SMD (10).
Adverse effects are not mentioned in the Discussion and only briefly in Results. According to the authors, 5 trials assessed adverse effects but they only reported data from 4 trials. The omitted trial (9) reported adverse effects (e.g. pain in abdomen, ear buzzing) as continuous outcomes, and not as binary (5). While it is reasonable not to pool trials with binary and continuous outcomes, we are puzzled as to why the authors omitted reporting any adverse effects data from this trial in their Cochrane Review. We wonder whether adverse effects from other trials were similarly ignored.
The 4 trials where the review authors reported adverse effects assessed 9 outcomes and for all outcomes, the confidence intervals were wide (e.g. for overall adverse effects, RR 2.94 [0.51, 17.03]). Based on this uncertainty, adverse effects of NSAIDs cannot be dismissed and it is therefore surprising that the authors did not refer to additional evidence, as recommended in The Cochrane Handbook (11). NSAIDs are known to cause serious harms (2‐4).
Additionally, in Methods the authors state “We assessed heterogeneity amongst trials by using the Chi2 test for heterogeneity with a 10% level of statistical significance and I2 test.” In their protocol the I2 is not mentioned at all. While there was substantial heterogeneity for overall side effects (I2 = 58%) the Chi2 test for heterogeneity was not statistically significant (P = 0.12). So, based on their own criteria the authors should have analysed the data using a fixed‐effect model, which would have shown a significant increase in overall side effects, relative risk 2.88 [1.11, 7.45] (P = 0.03).
Additionally, there are some discrepancies between what was reported in the protocol and what was done in the review. Kim et al. originally stated in their protocol (1) that they would search databases for unpublished trials, contact authors for missing data and examine publication bias, but apparently did not do any of this. The identified trials were all very small. It is therefore likely that the identified sample of published trials is biased (12), as small trials with non‐statistical findings are often not published.
In their abstract, the authors recommend NSAIDs for “reliving discomfort or pain”. This statement is highly misleading, as it indicates that NSAIDs have other clinical effects than their analgesic effect. The authors do not use the word “discomfort” anywhere else in the review, but we assume it refers to either global outcomes or non‐analgesic outcomes. However, the authors found no effect on global outcomes and the effect on “sneezing” is likely spurious, as it occurred for only one out of 13 non‐analgesic outcomes, and was based on the 2 problematic trials already described.
Based on these methodological problems, and the serious adverse effects of the drugs, we believe there is no sound basis for recommending NSAIDs for the common cold and urge the authors to present a more balanced view.
References
1) Kim SY, Chang YJ, Cho HM, Hwang YW, Moon YS. Non‐steroidal anti‐inflammatory drugs for the common cold. Cochrane Database of Systematic Reviews 2009, Issue 3. Art. No.: CD006362. DOI: 10.1002/14651858.CD006362.pub2. 2) Hernández‐Díaz S, Rodríguez LA. Association between nonsteroidal anti‐inflammatory drugs and upper gastrointestinal tract bleeding/perforation: an overview of epidemiologic studies published in the 1990s. Arch Intern Med. 2000;160:2093‐9. 3) Fosbøl EL, Gislason GH, Jacobsen S, Folke F, Hansen ML, Schramm TK, Sørensen R, Rasmussen JN, Andersen SS, Abildstrom SZ, Traerup J, Poulsen HE, Rasmussen S, Køber L, Torp‐Pedersen C. Risk of myocardial infarction and death associated with the use of nonsteroidal anti‐inflammatory drugs (NSAIDs) among healthy individuals: a nationwide cohort study. Clin Pharmacol Ther. 2009;85:190‐7. 4) Gislason GH, Rasmussen JN, Abildstrom SZ, Schramm TK, Hansen ML, Fosbøl EL, Sørensen R, Folke F, Buch P, Gadsbøll N, Rasmussen S, Poulsen HE, Køber L, Madsen M, Torp‐Pedersen C. Increased mortality and cardiovascular morbidity associated with use of nonsteroidal anti‐inflammatory drugs in chronic heart failure. Arch Intern Med. 2009;169:141‐9. 5) Sperber SJ, Hendley JO, Hayden FG, Riker DK, Sorrentino JV, Gwaltney JM Jr. Effects of naproxen on experimental rhinovirus colds. A randomised, double‐blind, controlled trial Annals of Internal Medicine. 1992;117:3741. 6) Naproxen. DrugDex ® Evaluations.Thomson Micromedex. Modified: 12 July 2009. [http://www.micromedex.dk/hcs/librarian/ND_T/HCS/ND_PR/Main/CS/726463/DUPLICATIONSHIELDSYNC/2A5F1A/ND_PG/PRIH/ND_B/HCS/SBK/4/ND_P/Main/PFActionId/hcs.common.RetrieveDocumentCommon/DocId/0004/ContentSetId/31#TopOfPage] (Accessed 19 November 2009) 7) Gøtzsche PC. NSAIDs. In: Young C, ed. Clinical Evidence Handbook. London: BMJ Publishing Group Limited, June 2009:384‐5. 8) Rothwell PM. External validity of randomized controlled trials: "to whom do the results of this trial apply?". Lancet. 2005;365:82‐93. 9) Winther B, Mygind N. The therapeutic effectiveness of ibuprofen on the symptoms of naturally acquired common colds. American Journal of Rhinology. 2001;15:23942. 10) Gøtzsche PC, Hróbjartsson A, Maric K, Tendal B. Data extraction errors in meta‐analyses that use standardized mean differences. JAMA. 2007;298:430‐7. Erratum in: JAMA. 2007;298:2264. 11) Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 [updated September 2009]. The Cochrane Collaboration, 2008. [www.cochrane‐handbook.org] (Accessed 19 November 2009) 12) Hopewell S, Loudon K, Clarke MJ, Oxman AD, Dickersin K. Publication bias in clinical trials due to statistical significance or direction of trial results. Cochrane Database of Systematic Reviews 2009, Issue 1. Art. No.: MR000006. DOI: 10.1002/14651858.MR000006.pub3.
Reply
Thank you for your feedback. I think that the comments fall into four main areas.
1. Adverse effects. 2. Discrepancy between the protocol and review. 3. The methodological issues of weak studies, and multiple outcomes. 4. The heterogeneity tests used, and the choice of random‐effects or fixed‐effect model.
We will discuss the feedback according to the four main areas.
1. Adverse effect issues Safety‐related issues of NSAIDs, in particular, the issues of cardiovascular disease and gastrointestinal disease have been reviewed in many studies but no clear conclusion has been drawn on what problems there can be in short‐term uses such as the use for a common cold. Of course, the risk of gastrointestinal side effects may increase even in short‐term usage. We agree with the commentators that there have not been many safety‐related discussions in the review and the power is not high enough to conclude on the safety of NSAIDs based on trials in this review. We also agree that review of other systematic reviews related to safety issues is necessary. As for the trial on which the commentators stated that it omitted safety‐related results, the trial author mentioned that there was no abnormal adverse events in the trial and the outcomes mentioned by the commentators classified it as effectiveness outcomes.
2. Discrepancy between the protocol and review ‐ search of unpublished trials and publication bias In the methods, we did make some efforts to search for unpublished trials. “We searched reference lists of review articles and of all included studies to find other potentially eligible studies. We contacted authors of the included trials to request unpublished studies”. However, we did not find any additional trials.
We did examine publication bias by funnel plot analysis. We omitted them because there were too many funnel plots in our review.
3. The methodological issues of weak studies, and multiple (26) outcomes As mentioned by the commentators, the number of results may be too large. This problem is mainly because outcomes of trials and duration or dose of therapy were quite diverse, so it was inevitable (in this sense). The effect of NSAIDs may not be different according to whether a cold is induced experimentally or happens naturally. A calculation error that the commentators pointed out was corrected. We added the following to the Discussion: “Major limitations of this review is that the results of the research are quite diverse and the number of studies for one result is quite small. For this reason, it is somewhat difficult to draw clear conclusions."
4. The heterogeneity tests used, and the choice of random‐effects or fixed‐effect model The reason for changing the protocol and review methodology in connection to heterogeneity is because The Cochrane Handbook was upgraded from 4.2 to 5.0 during the review and the 5.0 version recommends the use of I2 statistic and so we added it. In the Chi2 test, some heterogeneity was observed as I2 statistic = 58%, although not statistically significant, so in the actual analysis we presented both the fixed‐effect model and the random‐effects model. For the above reason, we are going to add new text to the Results, Discussion and Conclusions sections.
Results Two trials assessed the overall side effects of NSAIDs, and there was moderate heterogeneity. The results of a pooled analysis for overall side effects was significant in the fixed‐effect model (risk ratio (RR) 2.88 (95% CI 1.11 to 7.45), (P = 0.03), but not in random‐effects model (RR 2.94, 95% CI 0.51 to 17.03). Three trials reported gastrointestinal adverse effects and found no differences between the groups. Lethargy/drowsiness, feeling hyperactive, feeling more awake, flushed face, difficulty sleeping, light‐headedness and dry mouth were reported in one to two trials and the results were not significantly different between the treatment groups.
Discussion NSAIDs are drawing attention for their side effects. For some NSAIDs, their long‐term use increases the risk of cardiovascular disease and may cause gastrointestinal side effects. The frequency of gastrointestinal side effects increases in proportion to the dose and period of medication with NSAIDs but the risk of gastrointestinal side effects cannot be excluded in short‐term use. In trials included in this review, the risk of side effects was not evidently high; it is hard to conclude that they are not different from placebo in terms of side effects.
Conclusion NSAIDs are somewhat effective in relieving discomfort caused by a cold, but there is no clear evidence of their effect in easing respiratory symptoms. The use of NSAIDs for a cold should be decided in consideration of side effects.
Contributors
Andreas Lundh, Britta Tendal. The Nordic Cochrane Centre, Rigshospitalet, Dept. 3343, Blegdamsvej 9, 2100 Copenhagen Ø, Denmark
What's new
| Date | Event | Description |
|---|---|---|
| 17 April 2015 | New citation required but conclusions have not changed | Our conclusions remain unchanged. |
| 17 April 2015 | New search has been performed | Searches updated. We did not identify any new trials for inclusion. |
History
Protocol first published: Issue 1, 2007 Review first published: Issue 3, 2009
| Date | Event | Description |
|---|---|---|
| 17 April 2013 | New search has been performed | Searches updated. Two new trials were identified and excluded (Azuma 2010; Azuma 2011). Our conclusions remain unchanged. |
Acknowledgements
We would like to acknowledge the helpful comments of the panel of experts who refereed our review. We are grateful to Liz Dooley and Hayley Edmonds, Cochrane ARI Group Managing Editor and former Assistant Managing Editor. We also wish to thank the following people for commenting on the draft review: Tracey Lloyd, Owen Hendley, Rick Shoemaker and Bruce Arroll.
Appendices
Appendix 1. EMBASE search strategy
/* COMMON COLD */
#1 'common cold'/exp OR (common cold*):ti,ab
#2 coryza:ti,ab
#3 ('upper respiratory infection'):ti,ab OR ('upper respiratory infections'):ti,ab
#4 ('upper respiratory tract infection'):ti,ab OR ('upper respiratory tract infections'):ti,ab
#5 urti:ti,ab
#6 ((respiratory tract infection:ti,ab) OR (respiratory tract infections:ti,ab)) AND upper:ti,ab
#7 'rhinitis'/exp OR rhinit*:ti,ab
#8 'pharyngitis'/exp OR pharyngit*:ti,ab
#9 'sore throat':ti,ab OR 'sore throats':ti,ab
#10 'rhinopharyngitis'/exp OR nasopharyngit*:ti,ab
#11 'laryngitis'/exp OR laryngit*:ti,ab
#12 'coughing'/exp OR cough*:ti,ab
#13 'nose obstruction'/exp OR 'nasal obstruction':ti,ab
#14 'sneezing'/exp OR sneez*:ti,ab
#15 'rhinovirus'/exp OR rhinovirus:ti,ab
#16 OR/#1‐#15
/* NSAIDS */
#17 'nonsteroid antiinflammatory agent'/exp OR nsaid*:ti,ab OR (((non‐steroid OR nonsteroid OR 'non steroid' OR 'non steroids') AND (anti‐inflammatory OR antiinflammatory OR 'anti inflammatory')):ti,ab)
#18 'azapropazone'/exp OR apazone:ti,ab
#19 'acetylsalicylic acid'/exp OR aspirin:ti,ab
#20 'celecoxib'/exp OR celecoxib:ti,ab
#21 'diclofenac'/exp OR diclofenac:ti,ab
#22 'diflunisal'/exp OR diflunisal:ti,ab
#23 'etodolac'/exp OR etodolac:ti,ab
#24 'fenoprofen'/exp OR fenoprofen:ti,ab
#25 'flurbiprofen'/exp OR flurbiprofen:ti,ab
#26 'ibuprofen'/exp OR ibuprofen:ti,ab
#27 'indometacin'/exp OR indomethacin:ti,ab
#28 'ketoprofen'/exp OR ketoprofen:ti,ab
#29 'ketorolac'/exp OR ketorolac:ti,ab
#30 'meclofenamic acid'/exp OR meclofenamate:ti,ab
#31 'meloxicam'/exp OR meloxicam:ti,ab
#32 'salicylic acid methyl ester'/exp OR methylsalicylate:ti,ab OR 'methyl salicylate':ti,ab
#33 'nabumetone'/exp OR nabumetone:ti,ab
#34 'naproxen'/exp OR naproxen:ti,ab
#35 'nimesulide'/exp OR nimesulide:ti,ab
#36 'oxaprozin'/exp OR oxaprozin:ti,ab
#37 'phenylbutazone'/exp OR phenylbutazone:ti,ab
#38 'piroxicam'/exp OR piroxicam:ti,ab
#39 'salicylic acid'/exp OR salicylate:ti,ab
#40 'sulindac'/exp OR sulindac:ti,ab
#41 'tenoxicam'/exp OR tenoxicam:ti,ab
#42 'tolmetin'/exp OR tolmetin:ti,ab
#43 OR/#17‐#42
/* RCT */
#44 'clinical trial'/exp OR 'clinical trial':ti,ab
#45 'randomized controlled trial'/exp OR 'randomized controlled trial':ti,ab
#46 'randomisation'/exp OR random*:ti,ab
#47 'single blind procedure'/exp OR (singl*:ti,ab AND (mask*:ti,ab OR blind*:ti,ab))
#48 'double blind procedure'/exp OR (doubl*:ti,ab AND (mask*:ti,ab OR blind*:ti,ab))
#49 'triple blind procedure'/exp OR (trip*:ti,ab AND (mask*:ti,ab OR blind*:ti,ab))
#50 'placebo'/exp OR placebo:ti,ab
#51 OR #44‐#50
/* Combine & Limit */
#52 #16 AND #43 AND #51
#53 #16 AND #43 AND [randomized controlled trial]/lim
#54 (#52 OR #53) AND [human]/lim
#55 #54 AND [2009‐2011]/py
Appendix 2. CINAHL search strategy
/* COMMON COLD */
S1 (MH "Common Cold") OR (TX "common cold*")
S2 TX coryza
S3 (MH "Respiratory Tract Infections") or TX "upper respiratory infection*"
S4 TX "upper respiratory tract infection*"
S5 TX URTI
S6 (TX "respiratory tract infection*") AND (TX upper)
S7 (MH "Rhinitis") OR (TX rhinit*)
S8 (MH "Pharyngitis") OR (TX pharyngit*)
S9 TX "sore throat*"
S10 (MH "Nasopharynx") OR (TX nasopharyngit*)
S11 (MH "Laryngitis") OR (TX laryngit*)
S12 (MH "Cough") OR (TX cough*)
S13 (MH "Nasal Obstruction") OR (TX nasal obstruction*)
S14 (MH "Sneezing") OR (TX sneez*)
S15 TX rhinovirus
S16 OR/S1‐S15
/* NSAIDS */
S17 (MH "Antiinflammatory Agents, Non‐Steroidal") OR (TX nsaid*) OR (TX (non‐steroid* OR nonsteroid* OR "non steroid*") AND TX (anti‐inflammator* OR antiinflammator* OR "anti inflammator*"))
S18 TX azapropazone
S19 MH "Aspirin" OR aspirin
S20 MH "Cox‐2 Inhibitors" OR TX celecoxib
S21 MH "Diclofenac" OR TX diclofenac
S22 TX diflunisal
S23 MH "Etodolac" OR TX etodolac
S24 TX fenoprofen
S25 MH "Flurbiprofen" OR TX flurbiprofen
S26 MH "Ibuprofen" OR TX ibuprofen
S27 MH "Indomethacin" OR TX indomethacin
S28 TX ketoprofen
S29 MH "Ketorolac" OR TX ketorolac
S30 TX meclofenamate
S31 TX meloxicam
S32 TX (methylsalicylate OR "methyl salicylate")
S33 TX nabumetone
S34 MH "Naproxen" OR TX naproxen
S35 TX nimesulide
S36 TX oxaprozin
S37 MH "Phenylbutazone" OR TX phenylbutazone
S38 MH "Piroxicam" OR TX piroxicam
S39 MH "Salicylic Acids" OR TX salicylate
S40 MH "Sulindac" OR TX sulindac
S41 TX tenoxicam
S42 MH "Tolmetin" OR TX tolmetin
S43 OR/S11‐S42
/* RCT */
S44 MH "Clinical trial" OR TX "clinical trial"
S45 MH "Randomized Controlled Trials" OR TX "randomized controlled trial"
S46 MH "Random Sample" OR TX random*
S47 MH "Single‐Blind Studies" OR TX (singl* AND (mask* OR blind*))
S48 MH "Double‐Blind Studies" OR TX (doubl* AND (mask* OR blind*))
S49 MH "Triple‐Blind Studies" OR TX (trilp AND (mask* OR blind*))
S50 MH "Placebos" OR TX placebo
S51 OR S44‐S50
/* Combine & Limit */
S52 S16 AND S43 AND S51
S53 S16 AND S43 AND [crinical trial]/lim
S54 S52 OR S53
S55 S54 AND [2009‐2011]/py
Appendix 3. MEDLINE and CENTRAL search strategy
MEDLINE (Ovid)
1 Common Cold/ 2 common cold*.tw. 3 coryza.tw. 4 upper respiratory infection*.tw. 5 upper respiratory tract infections*.tw. 6 urti.tw. 7 respiratory tract infections.sh. and upper.tw. 8 Rhinitis/ 9 rhinit*.tw. 10 exp Pharyngitis/ 11 pharyngit*.tw. 12 sore throat*.tw. 13 exp Nasopharyngitis/ 14 nasopharyngit*.tw. 15 exp Laryngitis/ 16 laryngit*.tw. 17 Cough/ 18 cough*.tw. 19 Nasal Obstruction/ 20 nasal obstruction*.tw. 21 Sneezing/ 22 sneez*.tw. 23 Rhinovirus/ 24 rhinovirus*.tw. 25 or/1‐24 26 exp Anti‐Inflammatory Agents, Non‐Steroidal/ 27 nsaid*.tw. 28 ((non‐steroid* or nonsteroid* or non steroid*) and (anti‐inflammator* or antiinflammator* or anti inflammator*)).tw. 29 Apazone.sh. or apazone.tw. 30 Aspirin.sh. or aspirin.tw. 31 celecoxib.nm. or celecoxib.tw. 32 diclofenac.sh. or diclofenac.tw. 33 diflunisal.sh. or diflunisal.tw. 34 etodolac.sh. or etodolac.tw. 35 fenoprofen.sh. or fenoprofen.tw. 36 flurbiprofen.sh. or flurbiprofen.tw. 37 ibuprofen.sh. or ibuprofen.tw. 38 indomethacin.sh. or indomethacin.tw. 39 ketoprofen.sh. or ketoprofen.tw. 40 ketorolac.sh. or ketorolac.tw. 41 Meclofenamic Acid/ 42 meclofenamate.tw. or meloxicam.nm. or meloxicam.tw. 43 methyl salicylate.nm. or methylsalicylate.tw. or methyl salicylate.tw. 44 nabumetone.nm. or nabumetone.tw. 45 naproxen.sh. or naproxen.tw. 46 nimesulide.nm. or nimesulide.tw. 47 oxaprozin.nm. or oxaprozin.tw. 48 phenylbutazone.sh. or phenylbutazone.tw. 49 piroxicam.sh. or piroxicam.tw. 50 salicylate.mp. 51 sulindac.sh. or sulindac.tw. 52 tenoxicam.nm. or tenoxicam.tw. 53 tolmetin.sh. or tolmetin.tw. 54 or/26‐53 55 25 and 54
Data and analyses
Comparison 1. NSAIDs versus placebo, global effect.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Sum of overall symptom score (random‐effects model) | 3 | 293 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐1.03, 0.24] |
| 2 Moderate to marked severity | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.18, 2.11] |
| 3 Duration of colds (random‐effects model) | 2 | 214 | Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐1.75, 1.29] |
| 4 Duration of restriction of daily activities | 1 | 174 | Mean Difference (IV, Fixed, 95% CI) | ‐0.56 [‐1.24, 0.12] |
1.2. Analysis.
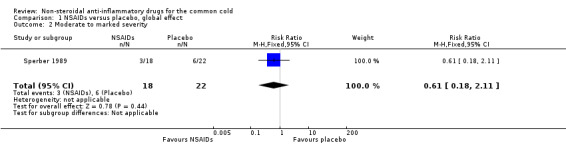
Comparison 1 NSAIDs versus placebo, global effect, Outcome 2 Moderate to marked severity.
1.4. Analysis.
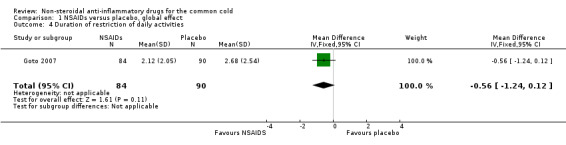
Comparison 1 NSAIDs versus placebo, global effect, Outcome 4 Duration of restriction of daily activities.
Comparison 2. NSAIDs versus placebo, analgesic effect.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Throat irritation score (fixed‐effect model) | 2 | 159 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.33, 0.30] |
| 2 Headache score (random‐effects model) | 2 | 159 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.65 [‐1.11, ‐0.19] |
| 3 Score of pain in muscles/joints score (fixed‐effect model) | 2 | 114 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐0.77, ‐0.03] |
| 4 Malaise score (fixed‐effect model) | 2 | 159 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.29 [‐0.60, 0.03] |
| 5 Chilliness score (random‐effects model) | 2 | 159 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐1.12, 1.06] |
| 6 Nose irritation score | 1 | 80 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.48, 0.40] |
| 7 Score of pain on swallowing | 1 | 80 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.07 [‐0.51, 0.37] |
| 8 Eye itching score | 1 | 80 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.14 [‐0.58, 0.30] |
| 9 Earache score | 1 | 80 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.59 [‐1.04, ‐0.14] |
2.6. Analysis.
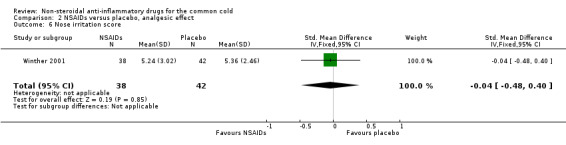
Comparison 2 NSAIDs versus placebo, analgesic effect, Outcome 6 Nose irritation score.
2.7. Analysis.
Comparison 2 NSAIDs versus placebo, analgesic effect, Outcome 7 Score of pain on swallowing.
2.8. Analysis.

Comparison 2 NSAIDs versus placebo, analgesic effect, Outcome 8 Eye itching score.
2.9. Analysis.
Comparison 2 NSAIDs versus placebo, analgesic effect, Outcome 9 Earache score.
Comparison 3. NSAIDs versus placebo, non‐analgesic effect.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cough score (random‐effects model) | 2 | 159 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.66, 0.56] |
| 2 Sneezing score (fixed‐effect model) | 2 | 159 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.44 [‐0.75, ‐0.12] |
| 3 Total number of sneezes | 1 | 80 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.51 [‐0.95, ‐0.06] |
| 4 Rhinorrhoea score (fixed‐effect model) | 3 | 199 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.25, 0.30] |
| 5 Nasal obstruction score (fixed‐effect model) | 3 | 199 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.15 [‐0.43, 0.13] |
| 6 Nasal obstruction score > 5 | 1 | 27 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.36 [0.28, 102.12] |
| 7 Total number of nose blows | 1 | 80 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.17 [‐0.27, 0.61] |
| 8 Total mucus weight | 1 | 40 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.13 [‐0.49, 0.76] |
| 9 Total tissue number count | 1 | 40 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.83, 0.42] |
| 10 Score of dryness in the nose | 1 | 80 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.04 [‐0.40, 0.48] |
| 11 Score of reduced sense of smell | 1 | 80 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.08 [‐0.36, 0.51] |
| 12 Hoarseness score | 1 | 80 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.32 [‐0.12, 0.76] |
| 13 Fatigue score | 1 | 80 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.18 [‐0.26, 0.62] |
3.1. Analysis.
Comparison 3 NSAIDs versus placebo, non‐analgesic effect, Outcome 1 Cough score (random‐effects model).
3.3. Analysis.
Comparison 3 NSAIDs versus placebo, non‐analgesic effect, Outcome 3 Total number of sneezes.
3.4. Analysis.
Comparison 3 NSAIDs versus placebo, non‐analgesic effect, Outcome 4 Rhinorrhoea score (fixed‐effect model).
3.5. Analysis.
Comparison 3 NSAIDs versus placebo, non‐analgesic effect, Outcome 5 Nasal obstruction score (fixed‐effect model).
3.6. Analysis.
Comparison 3 NSAIDs versus placebo, non‐analgesic effect, Outcome 6 Nasal obstruction score > 5.
3.7. Analysis.
Comparison 3 NSAIDs versus placebo, non‐analgesic effect, Outcome 7 Total number of nose blows.
3.8. Analysis.
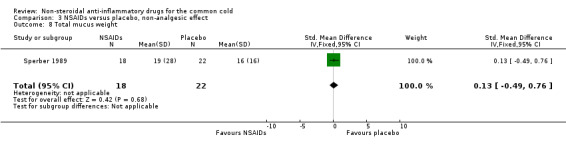
Comparison 3 NSAIDs versus placebo, non‐analgesic effect, Outcome 8 Total mucus weight.
3.9. Analysis.
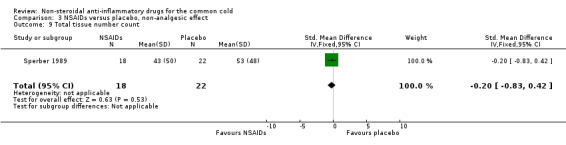
Comparison 3 NSAIDs versus placebo, non‐analgesic effect, Outcome 9 Total tissue number count.
3.10. Analysis.
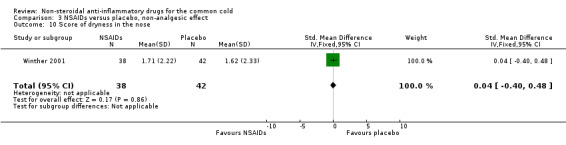
Comparison 3 NSAIDs versus placebo, non‐analgesic effect, Outcome 10 Score of dryness in the nose.
3.11. Analysis.
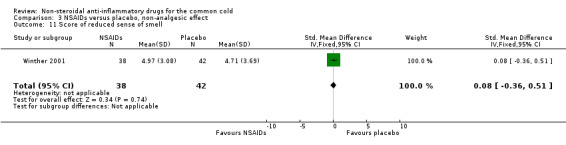
Comparison 3 NSAIDs versus placebo, non‐analgesic effect, Outcome 11 Score of reduced sense of smell.
3.12. Analysis.
Comparison 3 NSAIDs versus placebo, non‐analgesic effect, Outcome 12 Hoarseness score.
3.13. Analysis.
Comparison 3 NSAIDs versus placebo, non‐analgesic effect, Outcome 13 Fatigue score.
Comparison 4. NSAIDs versus placebo, adverse effects.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall side effects (random‐effects model) | 2 | 220 | Risk Ratio (M‐H, Random, 95% CI) | 2.94 [0.51, 17.03] |
| 2 GI complaint (fixed‐effect model) | 3 | 189 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.17, 3.32] |
| 3 Lethargy/drowsiness (fixed‐effect model) | 2 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.14, 6.91] |
| 4 Feeling hyperactive | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 70.02] |
| 5 Feeling more awake | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 70.02] |
| 6 Flushed face | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 70.02] |
| 7 Difficulty sleeping | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.78] |
| 8 Light‐headedness | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.15, 6.51] |
| 9 Dry mouth | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 70.02] |
4.2. Analysis.
Comparison 4 NSAIDs versus placebo, adverse effects, Outcome 2 GI complaint (fixed‐effect model).
4.3. Analysis.
Comparison 4 NSAIDs versus placebo, adverse effects, Outcome 3 Lethargy/drowsiness (fixed‐effect model).
4.4. Analysis.
Comparison 4 NSAIDs versus placebo, adverse effects, Outcome 4 Feeling hyperactive.
4.5. Analysis.
Comparison 4 NSAIDs versus placebo, adverse effects, Outcome 5 Feeling more awake.
4.6. Analysis.
Comparison 4 NSAIDs versus placebo, adverse effects, Outcome 6 Flushed face.
4.7. Analysis.
Comparison 4 NSAIDs versus placebo, adverse effects, Outcome 7 Difficulty sleeping.
4.8. Analysis.
Comparison 4 NSAIDs versus placebo, adverse effects, Outcome 8 Light‐headedness.
4.9. Analysis.
Comparison 4 NSAIDs versus placebo, adverse effects, Outcome 9 Dry mouth.
Comparison 5. Head to head comparison, global effect.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Global improvement rating, marked improvement (fixed‐effect model) | 2 | 365 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.52 [0.99, 2.34] |
| 2 Global improvement rating, moderate to marked improvement (fixed‐effect model) | 2 | 365 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [1.02, 1.41] |
5.1. Analysis.
Comparison 5 Head to head comparison, global effect, Outcome 1 Global improvement rating, marked improvement (fixed‐effect model).
5.2. Analysis.
Comparison 5 Head to head comparison, global effect, Outcome 2 Global improvement rating, moderate to marked improvement (fixed‐effect model).
Characteristics of studies
Characteristics of included studies [author‐defined order]
Graham 1990.
| Methods | Double‐blind, placebo‐controlled, experimental colds | |
| Participants | 59 inoculated; 42 colds. Mean age 20.1 years, 43.3% women, university students | |
| Interventions | 2 groups: aspirin 4 g/day and ibuprofen 1.2 g/day for 7 days | |
| Outcomes | The proportion of nasal obstruction score > 5 in the aspirin group (6/15) significantly differed from that in the placebo group (0/14, P value < 0.05) Mean mucus weight, mean tissue count, mean overall symptom score and mean overall side effect score were reported but any other statistical parameters such as SD, SE, 95% CI and P value for each group or the difference between these groups were not reported | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "... a randomised double‐blind, placebo‐controlled clinical trial" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "... identical capsules containing aspirin (500 mg), ibuprofen (200 mg) or placebo" Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "... 4 volunteers who were considered uninfected and were excluded from further analyses" Comment: probably done |
| Selective reporting (reporting bias) | Unclear risk | No protocol, no convincing text |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Goto 2007.
| Methods | Double‐blind, placebo‐controlled, natural colds | |
| Participants | 174 adults, age 18 to 65 years, 35% women, 23 outpatients facilities, URTI onset 2 days or less | |
| Interventions | Loxoprofen 60 mg 2 times for 7 days | |
| Outcomes | Duration of illness; the number of days with limited daily activities was not significantly different between groups | |
| Notes | The primary outcome was duration of illness in days | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation was based on simple computer‐generated random digits" Comment: probably done |
| Allocation concealment (selection bias) | Low risk | "... self‐drawing a sealed opaque envelope in the physician's sight....the correspondence between the digits and the group assignment was held in the central, secured location by a third party independent of the investigators until data collection was completed. Thus, allocation was concealed and masked from both patients and physicians" Comment: probably done |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "A double‐blind, randomised, placebo‐controlled trial"; "those in the control group were to take a placebo which was quite similar to active loxoprofen in shape and taste" Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "... six (two in loxoprofen group and four in placebo group) withdrew from the study, because two patients (one in loxoprofen and another in placebo) did not complete the diary; three patients (one in loxoprofen and the others in placebo) did not return the diary; and one patient (placebo) decided not to continue the study after the allocation. We excluded nine more participants (two in loxoprofen and seven in placebo) from analyses" Comment: probably done (missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups) |
| Selective reporting (reporting bias) | Unclear risk | No protocol, no convincing text |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Itoh 1980.
| Methods | Double‐blind, head‐to‐head comparison, natural colds | |
| Participants | 184 adults, mean age, sex not reported for the subgroup of colds, 29 centres, outpatient departments of hospitals and clinics, URTI onset ≤ 3 days | |
| Interventions | 2 groups: ketoprofen 50 mg 3 times and aspirin 500 mg 3 times for 3 days | |
| Outcomes | No significant difference in FGIR between 2 groups | |
| Notes | No available data on adverse effects for the subgroup of common colds | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Low risk | "... randomisation process was done by two controllers and key codes were kept by controllers (in Japanese)" Comment: probably done |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "... double‐blind method...active drug capsule and aspirin capsule were quite similar in shape (in Japanese)" Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "91/93 cases in ketoprofen group and 89/91 cases in aspirin group were finally analyzed" Number of withdrawals was too small to make any important difference to the estimated intervention effect Comment: probably done |
| Selective reporting (reporting bias) | Unclear risk | No protocol, no convincing text |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Katsu 1993.
| Methods | Double‐blind, double‐dummy, head‐to‐head comparison, natural colds | |
| Participants | 167 adults, mean age, sex not reported for the subgroup of colds, 32 centres, outpatient departments of hospitals and clinics, moderate to severe URTI, not requiring antibiotics | |
| Interventions | 2 groups: loxoprofen 180 mg/day and ibuprofen 600 mg for 3 days | |
| Outcomes | No significant difference in FGIR between 2 groups | |
| Notes | No available data on adverse effects for the subgroup of common colds | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "... were randomly assigned to receive" Comment: insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information about the allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "... double‐blind, double‐dummy method...active drug and placebo were quite similar in shape (in Japanese)" Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "112/130 of the CS‐600 group and 113/132 group were evaluated in the assessment improvement ratings" Comment: there are no reasons for missing participants. Insufficient reporting of attrition/exclusions to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | No protocol, no convincing text |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Nagaoka 1980.
| Methods | Double‐blind, head‐to‐head comparison, natural colds | |
| Participants | 222 adults, sex not reported for the subgroup of colds, 51 centres, outpatient departments of hospitals and clinics, URTI onset ≤ 2 days and fever ≤ 39 °C | |
| Interventions | 2 groups: fentiazac 300 mg/day and ibuprofen 600 mg/day for 3 days | |
| Outcomes | Moderate to marked improvement of FGIR was more frequent in the fenoprofen group than the placebo (P value < 0.05) | |
| Notes | No available data on adverse effects for the subgroup of common colds | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Low risk | "... randomisation process was done by two controllers and key codes were kept by controllers (in Japanese)" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "... double‐blind, double‐dummy method...active drug and placebo were quite similar in shape (in Japanese)" Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "243 out of 244 patients were analyzed after the elimination of 1 drop‐out case" Comment: number of withdrawals was too small to make any important difference to the estimated intervention effect Comment: probably done |
| Selective reporting (reporting bias) | Unclear risk | No protocol, no convincing text |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Ryan 1987.
| Methods | Double‐blind, placebo‐controlled, natural colds | |
| Participants | 64 adults, age range 18 to 60 years, 75% women, single family centre, fever ≤ 37.8 °C with moderate pain due to malaise/aches | |
| Interventions | Fenoprofen 200 mg single dose | |
| Outcomes | No available data on efficacy | |
| Notes | Only 2 adverse effects (1 stomach discomfort and 1 drowsiness), both in the fenoprofen group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "... assigned to one of three treatment groups via a computer‐generated random table" |
| Allocation concealment (selection bias) | Low risk | "Each dose of medication was dispensed in identically appearing capsules" Single oral dose was given Comment: probably done |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Each dose of medication was dispensed in identically appearing capsules in double‐blind method" Single oral dose was given Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants who entered the study completed treatment and were included in the assessment of effectiveness and side effects |
| Selective reporting (reporting bias) | Unclear risk | No protocol, no convincing text |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Sperber 1989.
| Methods | Double‐blind, placebo‐controlled, experimental colds | |
| Participants | 40 inoculated, 31 colds, mean age 21 years, 39.1% women, setting not reported, fever ≤ 37.7 °C | |
| Interventions | Ibuprofen 200 mg, 2 doses for the first day and 4 doses for the subsequent 4 days | |
| Outcomes | 4‐point scale. Moderate to marked severity (2‐ to 3‐point) was reduced in the ibuprofen group (18% versus 29%) but statistical significance was not reported | |
| Notes | Adverse effects were slightly more frequent in the ibuprofen group (6/23) than in the control group (4/23) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "... were randomly assigned to receive" Comment: insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information about the allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "... two identically appearing capsules" Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Among 58 inoculated participants, 8 were excluded (7 not infected, 1 infected with wild type virus), 1 was withdrawn |
| Selective reporting (reporting bias) | Unclear risk | No protocol, no convincing text |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Sperber 1992.
| Methods | Double‐blind, placebo‐controlled, experimental colds | |
| Participants | 79 inoculated (first cohort 34, second cohort 24 and third cohort 21); 56 colds. Mean age 21.4 years, 52% women. Setting not reported | |
| Interventions | For first cohort, naproxen loading dose of 400 mg followed by 200 mg 3 times daily, and for second and third cohort, naproxen loading dose of 500 mg followed by 500 mg 3 times daily for 5 days | |
| Outcomes | 5‐point symptom score. Total cumulative 5‐day score for headache was lower in the naproxen group (0.5 versus 2.5, P value < 0.001) | |
| Notes | 1 in the naproxen group and 2 in the placebo group experienced gastrointestinal complaints | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Participants were randomly assigned to receive..." Comment: insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information about the allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "The study drug and placebo were supplied in identically appearing capsules" Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Among 87 volunteers completed, 79 were considered evaluable" The reason for exclusion (infected with wild type rhinovirus, not infected, missed dose of study drug) is unlikely to be related to the outcome of the trial (symptomatic improvement of common cold symptoms) Comment: probably done |
| Selective reporting (reporting bias) | Unclear risk | No protocol, no convincing text |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Winther 2001.
| Methods | Double‐blind, placebo‐controlled, natural colds | |
| Participants | 80 adults, mean age 30.1 years, 60% women, single centre, medical students and members of the staff at the university | |
| Interventions | Ibuprofen 400 mg 3 times for 3 days | |
| Outcomes | 4‐point symptom score by patients. Sneezing, earache, headache, and pain in muscles and joints were significantly reduced in the ibuprofen group compared with the placebo group. Number of sneezing episodes was also reduced (21.33 ± 3.3 and 12.44 ± 1.5, P value = 0.02) | |
| Notes | No adverse effects in either group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "... randomised study of two parallel groups" Comment: insufficient information about the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information about the allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Coded vials with ibuprofen and placebo tablets were provided by Benzon Pharma" Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "All patients who entered the study completed treatment and were included in the assessment of effectiveness and side effects" Comment: probably done |
| Selective reporting (reporting bias) | Unclear risk | No protocol, no convincing text |
| Other bias | Unclear risk | No data on baseline imbalance |
CI: confidence interval FGIR: final global improvement rating SD: standard deviation SE: standard error URTI: upper respiratory tract infection
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aggarwal 1997 | Not common cold |
| Azuma 2010 | Not common cold |
| Azuma 2011 | Not common cold |
| Bachert 2005 | Febrile URTI |
| Banchini 1993 | Not common cold |
| Batista 1985 | Not common cold |
| Bellussi 1993 | Not common cold |
| Bellussi 1996 | Not common cold |
| Benrimoj 2001 | Not common cold |
| Bernstein 1974 | Not common cold |
| Blagden 2002 | Not common cold |
| Bonifaci 1977 | Not common cold |
| Cappella 1993 | Not common cold |
| Chachtel 2011 | Not common cold |
| Ebel 1985 | Not common cold |
| Eccles 2003 | Not common cold |
| Fujimori 1982 | Not randomised |
| Fujimori 1983 | Not common cold |
| Gehanno 2003 | Not common cold |
| Gruber 1977 | Not common cold |
| Kandoth 1984 | Not common cold |
| Katsu 1977 | Not common cold |
| Katsu 1978 | Not common cold |
| Katsu 1982 | Randomisation is not clear |
| Katsu 1983 | Not common cold |
| Kierszenbaum 1991 | Not common cold |
| Lopes 1991 | Not common cold |
| Martinez Gallardo 1994 | Randomisation is not clear |
| Matsumoto 1984 | Not common cold |
| Moore 2002 | Not common cold |
| Nagaoka 1985 | Not common cold |
| Nagaoka 1986a | Not common cold |
| Nagaoka 1986b | Not common cold |
| Nouri 1984 | Not common cold |
| Nouri 1993 | Not common cold |
| Pagella 2001 | Not common cold |
| Passali 1989 | Not common cold |
| Passali 1997 | Not common cold |
| Reiner 1983 | Not common cold |
| Ruperto 2011 | Not common cold |
| Russo 2013 | Not common cold |
| Salmon 1993 | Not common cold |
| Salzberg 1993 | Not common cold |
| Sanchez 1999 | Not common cold |
| Schachtel 1993 | Not common cold |
| Schachtel 2002 | Not common cold |
| Stanley 1975 | Randomisation is not clear |
| Tamura 1984 | Not common cold |
| Ulukol 1999 | Not common cold |
| Vauzelle 1996 | Not common cold |
| Watson 2000 | Not common cold |
URTI: upper respiratory tract infection
Contributions of authors
Soo young Kim (SYK), Yoon‐Jung Chang (YJC), Ye‐won Hwang (YWH) and Yoo Sun Moon (YSM) were responsible for study selection, methodological quality assessment, data extraction and analyses, and writing the review. Hye Min Cho (HMC) was responsible for the literature search and writing the review.
Declarations of interest
Soo Young Kim: none known. Yoon‐Jung Chang: none known. Hye Min Cho: none known. Ye‐Won Hwang: none known. Yoo Sun Moon: none known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Goto 2007 {published data only}
- Goto M, Kawamura T, Shimbo T, Takahashi O, Ando M, Miyaki K, et al. Influence of loxoprofen use on recovery from naturally acquired upper respiratory tract infections: a randomized controlled trial. Internal Medicine 2006;46(15):1179‐86. [DOI] [PubMed] [Google Scholar]
Graham 1990 {published data only}
- Graham NM, Burrell CJ, Douglas RM, Debelle P, Davies L. Adverse effects of aspirin, acetaminophen, and ibuprofen on immune function, viral shedding, and clinical status in rhinovirus‐infected volunteers. Journal of Infectious Diseases 1990;162(6):1277‐82. [DOI] [PubMed] [Google Scholar]
Itoh 1980 {published data only}
- Itoh K, Nagaoka S, Hamada A, Noguchi E, Suzuki H, Taniai T, et al. A double‐blind clinical study of ketoprofen on acute upper respiratory infection ‐ comparison with aspirin. Clinical Evaluation 1980;8(2):457‐79. [Google Scholar]
Katsu 1993 {published data only}
- Katsu M, Oishi A, Nakamura H, Matsuoka Y, Irimajiri S, Kobayashi H, et al. Clinical evaluation of loxoprofen sodium (CS‐600E) on upper respiratory tract inflammation: a double blind controlled study in comparison with ibuprofen. Journal of Clinical Therapeutics and Medicines 1993;9(10):2299‐320. [Google Scholar]
Nagaoka 1980 {published data only}
- Nagaoka S, Nagahama F, Kunno K, Haga T, Ito K, Ito F, et al. Therapeutic effects of fentiazac on common cold ‐ a double‐blind clinical study. Clinical Evaluation 1980;8(3):757‐88. [Google Scholar]
Ryan 1987 {published data only}
- Ryan PB, Rush DR, Nicholas TA, Graham DG. A double‐blind comparison of fenoprofen calcium, acetaminophen, and placebo in the palliative treatment of common nonbacterial upper respiratory infections. Current Therapeutic Research, Clinical and Experimental 1987;41(1):17‐23. [Google Scholar]
Sperber 1989 {published data only}
- Sperber SJ, Sorrentino JV, Riker DK, Hayden FG. Evaluation of an alpha agonist alone and in combination with a nonsteroidal antiinflammatory agent in the treatment of experimental rhinovirus colds. Bulletin of the New York Academy of Medicine 1989;65(1):145‐60. [PMC free article] [PubMed] [Google Scholar]
Sperber 1992 {published data only}
- Sperber SJ, Hendley JO, Hayden FG, Riker DK, Sorrentino JV, Gwaltney JM Jr. Effects of naproxen on experimental rhinovirus colds. A randomized, double‐blind, controlled trial. Annals of Internal Medicine 1992;117(1):37‐41. [DOI] [PubMed] [Google Scholar]
Winther 2001 {published data only}
- Winther B, Mygind N. The therapeutic effectiveness of ibuprofen on the symptoms of naturally acquired common colds. American Journal of Rhinology 2001;15(4):239‐42. [PubMed] [Google Scholar]
References to studies excluded from this review
Aggarwal 1997 {published data only}
- Aggarwal P, Ambrose C, Kumar M, Bhavani V, Bose E, Bose S, et al. Piroxicam versus ibuprofen as adjuncts to antibiotic therapy for symptomatic relief in upper respiratory infections: a perspective, randomized, comparative, multicentre study. Indian Practitioner 1997;50(11):939‐47. [Google Scholar]
Azuma 2010 {published data only}
- Azuma A, Kudoh S, Nakashima M, Nagatake T. A double‐blind study of zaltoprofen for the treatment of upper respiratory tract infection. Pharmacology 2010;85(1):41‐7. [DOI] [PubMed] [Google Scholar]
Azuma 2011 {published data only}
- Azuma A, Kudoh S, Nakashima M, Nagatake T. Antipyretic and analgesic effects of zaltoprofen for the treatment of acute upper respiratory tract infection: verification of a non‐inferiority hypothesis using loxoprofen sodium. Pharmacology 2011;87(3‐4):204‐13. [DOI] [PubMed] [Google Scholar]
Bachert 2005 {published data only}
- Bachert C, Chuchalin AG, Eisebitt R, Netayzhenko VZ, Voelker M. Aspirin compared with acetaminophen in the treatment of fever and other symptoms of upper respiratory tract infection in adults; a multicenter, randomized, double‐blind, double‐dummy, placebo‐controlled, parallel‐group, single‐dose, 6‐hour dose‐ranging study. Clinical Therapeutics 2005;27(7):993‐1003. [DOI] [PubMed] [Google Scholar]
Banchini 1993 {published data only}
- Banchini G, Scaricabarozzi I, Montecorboli U, Ceccarelli A, Chiesa F, Ditri L, et al. Double‐blind study of nimesulide in divers with inflammatory disorders of the ear, nose and throat. Drugs 1993;46(Suppl 1):100‐2. [DOI] [PubMed] [Google Scholar]
Batista 1985 {published data only}
- Batista NA, Zago MC, Schincarioli SM, Nader MC, Almeida J. Clinical evaluation of diclofenac resinate drops, associated to antibiotics, in the treatment of upper respiratory tract infections. Comparative study in pediatrics. Arquivos Brasileiros de Medicina 1985;59(6):479‐84. [Google Scholar]
Bellussi 1993 {published data only}
- Bellussi L, Passali D. Treatment of upper airways inflammation with nimesulide. Drugs 1993;46(Suppl 1):107‐10. [DOI] [PubMed] [Google Scholar]
Bellussi 1996 {published data only}
- Bellussi L, Biagini C, Calearo C, Cenacchi V, Campora E, Girolamo A, et al. Antiphlogistic therapy with ketoprofen lysine salt vs nimesulide in secretive otitis media, rhinitis/rhinosinusitis, pharyngitis/tonsillitis/tracheitis. Otorinolaringologia 1996;46(1):49‐57. [Google Scholar]
Benrimoj 2001 {published data only}
- Benrimoj SI, Langford JH, Christian J, Charlesworth A, Steans A. Efficacy and tolerability of the anti‐inflammatory throat lozenge flurbiprofen 8.75mg in the treatment of sore throat: a randomised, double‐blind, placebo‐controlled study. Clinical Drug Investigation 2001;21(3):183‐93. [DOI] [PubMed] [Google Scholar]
Bernstein 1974 {published data only}
- Bernstein JE, Nelson FK. A double blind evaluation of an aspirin containing gum tablet for relief of the pain of common sore throat. Journal of International Medical Research 1974;2(1):76‐80. [Google Scholar]
Blagden 2002 {published data only}
- Blagden M, Christian J, Miller K, Charlesworth A. Multidose flurbiprofen 8.75 mg lozenges in the treatment of sore throat: a randomised, double‐blind, placebo‐controlled study in UK general practice centres. International Journal of Clinical Practice 2002;56(2):95‐100. [PubMed] [Google Scholar]
Bonifaci 1977 {published data only}
- Bonifaci E, Giorgi CM, Vibelli C. Symptomatic management of acute inflammation of the upper airways in paediatric patients. Minerva Pediatrica 1977;29(5):285‐96. [PubMed] [Google Scholar]
Cappella 1993 {published data only}
- Cappella L, Guerra A, Laudizi L, Cavazzuti GB. Efficacy and tolerability of nimesulide and lysine‐acetylsalicylate in the treatment of paediatric acute upper respiratory tract inflammation. Drugs 1993;46(Suppl 1):222‐5. [DOI] [PubMed] [Google Scholar]
Chachtel 2011 {published data only}
- Chachtel BP, McCabe D, Berger M, Zhang R, Sanner KM, Savino L, et al. Efficacy of low‐dose celecoxib in patients with acute pain. Journal of Pain 2011;12(7):756. [DOI] [PubMed] [Google Scholar]
Ebel 1985 {published data only}
- Ebel DL, Shih WJ, Rhymer AR. A multi‐centre, double‐blind randomized study to assess the efficacy and tolerance of sulindac versus placebo in the symptomatic treatment of patients with upper respiratory tract infection. Current Medical Research and Opinion 1985;9(10):666‐75. [DOI] [PubMed] [Google Scholar]
Eccles 2003 {published data only}
- Eccles R, Loose I, Jawad M, Nyman L. Effects of acetylsalicylic acid on sore throat pain and other pain symptoms associated with acute upper respiratory tract infection. Pain Medicine 2003;4(2):118‐24. [DOI] [PubMed] [Google Scholar]
Fujimori 1982 {published data only}
- Fujimori I, Kono M, Takeda Y, Sekita K, Katsu M, Adachi M, et al. Clinical evaluation of Clinoril tablets in acute upper respiratory tract infections. Kansenshogaku Zasshi 1982;56(12):1186‐95. [DOI] [PubMed] [Google Scholar]
Fujimori 1983 {published data only}
- Fujimori I, Kono M, Sekita T, Takeda Y, Ogihara K, Nakagawa H, et al. A double‐blind clinical evaluation of suprofen on acute upper respiratory infection. Comparison with aspirin. Kansenshogaku Zasshi 1983;57(1):62‐81. [DOI] [PubMed] [Google Scholar]
Gehanno 2003 {published data only}
- Gehanno P, Dreiser RL, Ionescu E, Gold M, Liu JM. Lowest effective single dose of diclofenac for antipyretic and analgesic effects in acute febrile sore throat. Clinical Drug Investigation 2003;23(4):263‐71. [DOI] [PubMed] [Google Scholar]
Gruber 1977 {published data only}
- Gruber CM Jr, Collins T. Dose response to fenoprofen in an antipyretic study of fenoprofen and propoxyphene. Journal of Medicine 1977;8(1):27‐34. [PubMed] [Google Scholar]
Kandoth 1984 {published data only}
- Kandoth PW, Joshi MK, Joshi VR, Satoskar RS. Comparative evaluation of antipyretic activity of ibuprofen and aspirin in children with pyrexia of varied aetiology. Journal of International Medical Research 1984;12(5):292‐7. [DOI] [PubMed] [Google Scholar]
Katsu 1977 {published data only}
- Katsu M, Mashita H, Fujimori I, Shimada S, Fujii T. Therapeutic effects of fenbufen in common cold: multi‐clinic double‐blind study. Kansenshogaku Zasshi 1977;51(4):184‐96. [DOI] [PubMed] [Google Scholar]
Katsu 1978 {published data only}
- Katsu M, Fujii T, Fujimori I, Katayama T, Harada K, Koizumi H, et al. Therapeutic utility of naproxen on acute upper respiratory infection: multi‐clinical double‐blind study. Kansenshogaku Zasshi 1978;52(5):148‐63. [DOI] [PubMed] [Google Scholar]
Katsu 1982 {published data only}
- Katsu M, Hayakawa M, Kawai M, Fujimori I, Kohno M, Takeda Y, et al. Double blind controlled study of miroprofen in acute upper respiratory tract infections ‐ comparison with ibuprofen. Kansenshogaku Zasshi 1982;56(5):434‐53. [DOI] [PubMed] [Google Scholar]
Katsu 1983 {published data only}
- Katsu M, Adachi M, Hayakawa M, Fujimori I, Kohno M, Takeda Y, et al. Clinical evaluation of sulindac (CLINORIL) in the treatment of acute upper respiratory tract infection: double‐blind comparison with ibuprofen. Kansenshogaku Zasshi 1983;57(3):260‐72. [DOI] [PubMed] [Google Scholar]
Kierszenbaum 1991 {published data only}
- Kierszenbaum J, Vitral BG, Kierszenbaum AML, Sousa PR. Evaluation of nimesulid oral suspension versus diclofenac resinate in upper respiratory tract infections in the pediatrics. Pediatria Moderna 1991;27(7):560‐2. [Google Scholar]
Lopes 1991 {published data only}
- Lopes FO. Amoxicillin versus amoxicillin + nimesulide in otolaryngologic infections ‐ a randomized study. Folha Medica 1991;102(3):81‐5. [Google Scholar]
Martinez Gallardo 1994 {published data only}
- Martinez Gallardo F, Lopez Fiesco A, Zamora G. Symptomatic treatment of common cold in children with a new combination of naproxen sodium plus pseudoephedrine hydrochloride: a comparative trial against pseudoephedrine syrup. Proceedings of the Western Pharmacology Society 1994;37:157‐8. [PubMed] [Google Scholar]
Matsumoto 1984 {published data only}
- Matsumoto K, Fujimori I, Tsubura E, Sakuma A, Hayashi M, Kudo K, et al. Clinical evaluation of oxaprozin in the treatment of acute upper respiratory tract inflammations: a double‐blind comparative study using ibuprofen as the control drug. Kansenshogaku Zasshi 1984;58(7):628‐46. [DOI] [PubMed] [Google Scholar]
Moore 2002 {published data only}
- Moore N, Parc JM, Ganse E, Wall R, Schneid H, Cairns R. Tolerability of ibuprofen, aspirin and paracetamol for the treatment of cold and flu symptoms and sore throat pain. International Journal of Clinical Practice 2002;56(10):732‐4. [PubMed] [Google Scholar]
Nagaoka 1985 {published data only}
- Nagaoka S, Fumio N, Ohito O, Hideyo N, Morise M, Osamu T, et al. Clinical effect of floctafenine in acute upper respiratory infection: a double blind test in comparison with aspirin. Japanese Pharmacology and Therapeutics 1985;13(2):981‐1010. [Google Scholar]
Nagaoka 1986a {published data only}
- Nagaoka S, Hiraga H, Ohito O, Hideyo N, Suetsugu S, Osamu T, et al. Clinical effect of emorfazone in acute upper respiratory infection: a double‐blind test in comparison with aspirin. Japanese Pharmacology and Therapeutics 1986;14(9):5883‐900. [Google Scholar]
Nagaoka 1986b {published data only}
- Nagaoka S, Nakamura S, Umehara Y, Nagahama F, Okayasu M, Noguchi E, et al. Clinical evaluation of tolfenamic acid on acute upper respiratory tract inflammation: multi‐center double‐blind study with ibuprofen. Journal of Clinical Therapeutics and Medicines 1986;2(2):221‐50. [Google Scholar]
Nouri 1984 {published data only}
- Nouri ME. Nimesulide for treatment of acute inflammation of the upper respiratory tract. Clinical Therapeutics 1984;6(2):142‐50. [PubMed] [Google Scholar]
Nouri 1993 {published data only}
- Nouri E, Monti T. Nimesulide granules for the treatment of acute inflammation of the ear, nose or throat. Drugs 1993;46(Suppl 1):103‐6. [DOI] [PubMed] [Google Scholar]
Pagella 2001 {published data only}
- Pagella F, Rossi V, Zanoletti E, Benazzo M, Mira E. Efficacy and tolerability of a mouthwash based on diclofenac in the treatment of disorders of the upper respiratory tract. Double blind study versus flurbiprofen mouthwash. Otorinolaringologia 2001;51(2):77‐81. [Google Scholar]
Passali 1989 {published data only}
- Passali D, Bellussi L, Ciferri G, Scaricabarozzi I. Use of nimesulide in inflammations of the upper respiratory tract. Clinica Terapeutica 1989;128(2):105‐11. [PubMed] [Google Scholar]
Passali 1997 {published data only}
- Passali D, Gorga A, Ferri R, Bellussi L. Controlled clinical study on the efficacy and tolerability of methoxibutropate versus nimesulide in otorhinolaryngology. Otorinolaringologia 1997;47(3):145‐50. [Google Scholar]
Reiner 1983 {published data only}
- Reiner M. Nimesulide and antibiotics in the treatment of acute infections of the respiratory tract. Current Medical Research and Opinion 1983;8(7):487‐92. [DOI] [PubMed] [Google Scholar]
Ruperto 2011 {published data only}
- Ruperto N, Carozzino L, Jamone R, Freschi F, Picollo G, Zera M, et al. A randomized, double‐blind, placebo‐controlled trial of paracetamol and ketoprofren lysine salt for pain control in children with pharyngotonsillitis cared by family pediatricians. Italian Journal of Pediatrics 2011;37:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Russo 2013 {published data only}
- Russo M, Bloch M, Looze FD, Morris C, Shephard A. Flurbiprofen microgranules for relief of sore throat: a randomised, double‐blind trial. British Journal of General Practice 2013;63:607. [DOI] [PMC free article] [PubMed] [Google Scholar]
Salmon 1993 {published data only}
- Salmon Rodriguez LE, Arista Viveros HA, Lopez E, Trujillo C, Maciel R, Lujan M. Evaluation of the efficacy and safety of nimesulide and naproxen in the symptomatic treatment of upper respiratory tract infections in children. A comparative blind study. Investigacion Medica Internacional 1993;20(2):43‐54. [Google Scholar]
Salzberg 1993 {published data only}
- Salzberg R, Giambonini S, Maurizio M, Roulet D, Zahn J, Monti T. A double‐blind comparison of nimesulide and mefenamic acid in the treatment of acute upper respiratory tract infections in children. Drugs 1993;46(Suppl 1):208‐11. [DOI] [PubMed] [Google Scholar]
Sanchez 1999 {published data only}
- Sanchez Gonzalez A, Gonzalez Galindo T, Santana Hurtado O. Efficacy and safety assessment of tolmetin sodium (400 mg tid) vs naproxen sodium (275 mg tid) for the treatment of acute upper respiratory tract infection symptoms. Investigacion Medica Internacional 1999;26(1):3‐8. [Google Scholar]
Schachtel 1993 {published data only}
- Schachtel BP, Thoden WR. A placebo‐controlled model for assaying systemic analgesics in children. Clinical Pharmacology and Therapeutics 1993;53(5):593‐601. [DOI] [PubMed] [Google Scholar]
Schachtel 2002 {published data only}
- Schachtel BP, Homan HD, Gibb IA, Christian J. Demonstration of dose response of flurbiprofen lozenges with the sore throat pain model. Clinical Pharmacology and Therapeutics 2002;71(5):375‐80. [DOI] [PubMed] [Google Scholar]
Stanley 1975 {published data only}
- Stanley ED, Jackson GG, Panusarn C, Rubenis M, Dirda V. Increased virus shedding with aspirin treatment of rhinovirus infection. JAMA 1975;231(12):1248‐51. [PubMed] [Google Scholar]
Tamura 1984 {published data only}
- Tamura M, Ito T, Sudo M, Tazawa M, Sayama T, Yoshida M, et al. A double‐blind clinical evaluation of flurbiprofen on acute upper respiratory tract inflammation‐comparative study with ibuprofen. Kansenshogaku Zasshi 1984;58(12):1289‐303. [DOI] [PubMed] [Google Scholar]
Ulukol 1999 {published data only}
- Ulukol B, Koksal Y, Cin S. Assessment of the efficacy and safety of paracetamol, ibuprofen and nimesulide in children with upper respiratory tract infections. European Journal of Clinical Pharmacology 1999;55(9):615‐8. [DOI] [PubMed] [Google Scholar]
Vauzelle 1996 {published data only}
- Vauzelle‐Kervroedan F, Revzani Y, Pons G, Consten L, Pariente‐Khayat A, D'Athis P, et al. Antipyretic efficacy of tiaprofenic acid in febrile children. Fundamental Clinical Pharmacology 1996;10(1):56‐9. [DOI] [PubMed] [Google Scholar]
Watson 2000 {published data only}
- Watson N, Nimmo WS, Christian J, Charlesworth A, Speight J, Miller K. Relief of sore throat with the anti‐inflammatory throat lozenge flurbiprofen 8.75 mg: a randomised, double‐blind, placebo‐controlled study of efficacy and safety. International Journal of Clinical Practice 2000;54(8):490‐6. [PubMed] [Google Scholar]
Additional references
AlBalawi 2013
- AlBalawi ZH, Othman SS, AlFaleh K. Intranasal ipratropium bromide for the common cold. Cochrane Database of Systematic Reviews 2013, Issue 6. [DOI: 10.1002/14651858.CD008231.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
De Sutter 2012
- Sutter A, Driel M, Kenyon L. Oral antihistamine‐decongestant‐analgesic combinations for the common cold. Cochrane Database of Systematic Reviews 2012, Issue 2. [DOI: 10.1002/14651858.CD004976.pub2] [DOI] [PubMed] [Google Scholar]
Eccles 2005
- Eccles R. Understanding the symptoms of the common cold and influenza. Lancet Infectious Diseases 2005;5(11):718‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gwaltney 2002
- Gwaltney JM. Viral respiratory infection therapy: historical perspectives and current trials. American Journal of Medicine 2002;112(Suppl 6A):33‐41. [DOI] [PubMed] [Google Scholar]
Heikkinen 2003
- Heikkinen T, Jarvinen A. The common cold. Lancet 2003;361(9351):51‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hernández‐Díaz 2000
- Hernández‐Díaz S, Rodriguez LA. Association between nonsteroidal anti‐inflammatory drugs and upper gastrointestinal tract bleeding/perforation: an overview of epidemiologic studies published in the 1990s. Archives of Internal Medicine 2000;160(14):2093‐9. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Irwin 2000
- Irwin RS, Madison JM. The diagnosis and treatment of cough. New England Journal of Medicine 2000;343(23):1715‐21. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Li 2013
- Li S, Yue J, Dong BR, Yang M, Lin X, Wu T. Acetaminophen (paracetamol) for the common cold in adults. Cochrane Database of Systematic Reviews 2013, Issue 7. [DOI: 10.1002/14651858.CD008800.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Matchaba 2004
- Matchaba P, Gitton X, Krammer G, Ehrsam E, Sloan VS, Olson M, et al. Risk of cardiovascular events and rofecoxib: cumulative meta‐analysis. Lancet 2004;364(9450):2021‐9. [DOI] [PubMed] [Google Scholar]
Ofman 2002
- Ofman JJ, MacLean CH, Straus WL, Morton SC, Berger ML, Roth EA, et al. A meta‐analysis of severe upper gastrointestinal complications of nonsteroidal antiinflammatory drugs. Journal of Rheumatology 2002;29(4):804‐12. [PubMed] [Google Scholar]
Ostberg 1997
- Ostberg B, Winther B, Borum P, Mygind N. Common cold and high‐dose ipratropium bromide: use of anticholinergic medication as an indicator of reflex‐mediated hypersecretion. Rhinology 1997;35(2):58‐62. [PubMed] [Google Scholar]
Pratter 2006
- Pratter MR. Cough and the common cold: ACCP evidence‐based clinical practice guidelines. Chest 2006;129(Suppl 1):72‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Saraswat 2011
- Saraswat A, Driel ML, Sutter AIM. Antihistamines for the common cold. Cochrane Database of Systematic Reviews 2011, Issue 10. [DOI: 10.1002/14651858.CD009345] [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 2014
- Smith SM, Schroeder K, Fahey T. Over‐the‐counter (OTC) medications for acute cough in children and adults in community settings. Cochrane Database of Systematic Reviews 2014, Issue 11. [DOI: 10.1002/14651858.CD001831.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ta'i 2012
- Ta'i SH, Ferguson KAM, Singh HK, Sharma AN, Kumar S, Driel ML, et al. Nasal decongestants for the common cold. Cochrane Database of Systematic Reviews 2012, Issue 2. [DOI: 10.1002/14651858.CD009612] [DOI] [Google Scholar]
References to other published versions of this review
Kim 2007
- Kim SY, Chang YJ, Cho HM, Hwang YW, Moon YS. Non‐steroidal anti‐inflammatory drugs for the common cold. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD006362] [DOI] [Google Scholar]
Kim 2009
- Kim Sy, Chang Y‐J, Cho HM, Hwang Y‐w, Moon YS. Non‐steroidal anti‐inflammatory drugs for the common cold. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD006362.pub2] [DOI] [PubMed] [Google Scholar]
Kim 2011
- Kim Sy, Chang Y‐J, Cho HM, Hwang Y‐w, Moon YS. Non‐steroidal anti‐inflammatory drugs for the common cold. Cochrane Database of Systematic Reviews 2011, Issue 10. [DOI: 10.1002/14651858.CD006362.pub2] [DOI] [Google Scholar]
Kim 2013
- Kim SY, Chang YJ, Cho HM, Hwang YW, Moon YS. Non‐steroidal anti‐inflammatory drugs for the common cold. Cochrane Database of Systematic Reviews 2013, Issue 6. [DOI: 10.1002/14651858.CD006362.pub3] [DOI] [PubMed] [Google Scholar]


