Abstract
The fetal fraction (FF) is a function of both biological factors and bioinformatics algorithms used to interpret DNA sequencing results. It is an essential quality control component of noninvasive prenatal testing (NIPT) results. Clinicians need to understand the biological influences on FF to be able to provide optimal post-test counseling and clinical management. There are many different technologies available for the measurement of FF. Clinicians do not need to know the details behind the bioinformatics algorithms of FF measurements, but they do need to appreciate the significant variations between the different sequencing technologies used by different laboratories. There is no universal FF threshold that is applicable across all platforms and there have not been any differences demonstrated in NIPT performance by sequencing platform or method of FF calculation. Importantly, while FF should be routinely measured, there is not yet a consensus as to whether it should be routinely reported to the clinician. The clinician should know what to expect from a standard test report and whether reasons for failed NIPT results are revealed. Emerging solutions to the challenges of samples with low FF should reduce rates of failed NIPT in the future. In the meantime, having a “plan B” prepared for those patients for whom NIPT is unsuccessful is essential in today’s clinical practice.
1 |. INTRODUCTION
Over the past decade the advent of noninvasive prenatal testing (NIPT) has necessitated the rapid education of all clinicians involved in prenatal care. Not only did NIPT abruptly displace longstanding practices in aneuploidy screening—it was also the first clinical test based on the fundamentally novel principle of analyzing circulating cell free (cf) DNA. Clinicians have therefore had to promptly implement a new screening test, as well as grasping the new biology and technology that underlies its performance.
cfDNA refers to the DNA that exists as short fragments in plasma or other body fluids, which is distinct from the DNA contained within the nucleus of an intact cell. These cfDNA fragments are released from all organs during a range of cellular processes, including apoptosis, necrosis and microparticle secretion.1 Circulating plasma cfDNA has been intensively studied for a wide range of noninvasive “liquid biopsy” applications in oncology and general medicine but has had the most impactful translational success in prenatal screening for fetal aneuploidy.2
Maternal plasma cfDNA contains both maternal and fetal sources of cfDNA (Figure 1). The source of fetal DNA is the trophoblast,3–6 while the predominant source of maternal DNA is the hematopoietic system.7,8 All maternal organs contribute some cfDNA into maternal plasma, including solid tumors.9
FIGURE 1.
Multiple organ sources of cell-free DNA in maternal plasma
NIPT for fetal aneuploidy employs high throughput sequencing methods (“next generation sequencing”) to count the proportional representation of each chromosome in the plasma cfDNA.10 In the nonpregnant state, the proportional representation of each chromosome in the plasma cfDNA reflects the size of the chromosome and the karyotype of the individual. In a euploid pregnant woman, a deviation from the expected chromosome profile in plasma cfDNA due to excess or deficient cfDNA fragments from a particular chromosome suggests the presence of fetal trisomy or monosomy, respectively. A statistically significant variation in cfDNA fragment counts for a particular chromosome—commonly defined as a z-score > 3—constitutes a high-risk result.
Whole chromosome imbalances, as well as sub-chromosomal copy number variants (CNVs), can also be detected using cfDNA. These include genome-wide CNVs as small as 3 Mb in size11–13 and targeted microdeletion syndromes such as 5p− (cri du chat), 22q11.2 − (Di George syndrome), 15q− (Prader-Willi/Angelman syndrome), 4p − (Wolf-Hirschhorn syndrome), 11q− (Jacobsen syndrome), 8q−(Langer-Giedion syndrome), and 1p36−.14–16
1.1 |. What is fetal fraction?
Fetal fraction (FF) is the percentage of total maternal plasma cfDNA that is of fetoplacental origin [FF = fetal cfDNA/(fetal cfDNA + maternal cfDNA). It is therefore a function of both maternal and fetal cfDNA levels in the maternal plasma. The “fetal” DNA is actually placental.17 Between 10 and 20 weeks of gestation, the average FF is 10% to 15%.18,19
1.2 |. Why is FF important with regard to NIPT performance?
FF measurement is a crucial component of sample quality control and statistical confidence.20 Measuring the FF ensures that placental cfDNA is detectable in the maternal plasma in sufficient quantity to generate a meaningful result.21 The minimum FF threshold for adequate NIPT performance varies by assay (discussed below), but is typically between 2% and 4%. With a higher FF, there is greater statistical separation of aneuploid and euploid pregnancies, providing greater confidence in the final result (Figure 2). Detection rates decline with low FFs, such that a “no call” may be issued if the FF is below the lower limit of detection (LOD) for that laboratory. Higher sequencing depths can be used to compensate for very low FFs, but this has significant cost implications.
FIGURE 2.
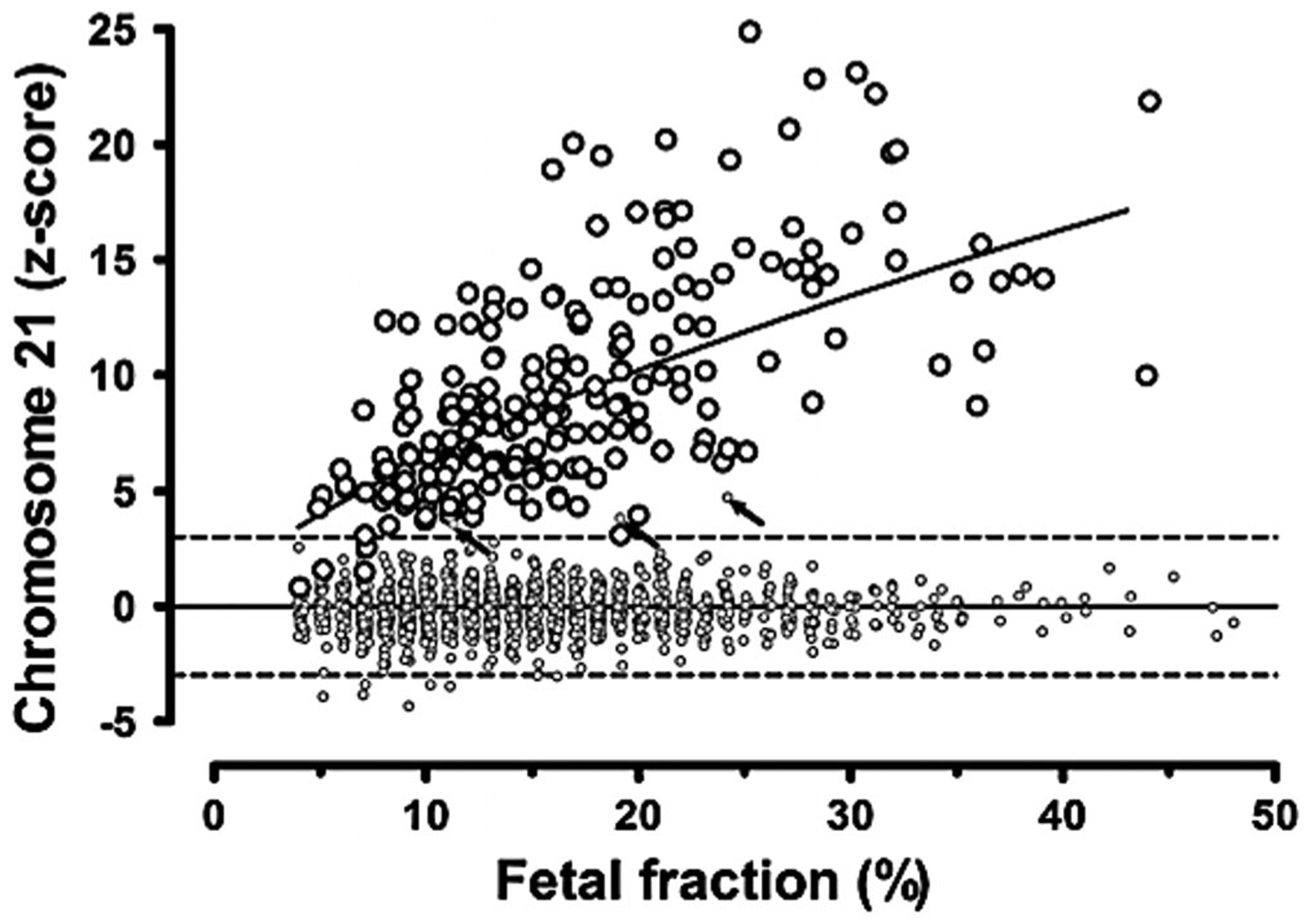
Relationship between fetal fraction and chromosome 21 z-score in euploid and pregnancies with Down syndrome. The large open circles indicate chromosome z-scores in first and second trimester pregnancies with Down syndrome. As fetal fraction increases, the average z-score also increases. The line indicates the change in the average z-score by fetal fraction. All but 4 of the 212 cases are above a z-score of 3, and these 4 occur at fetal fractions of 7% or less. Small open circles indicate chromosome 21 z-scores in 1484 euploid pregnancies. The z-scores are centered on zero and generally fall between −3 and +3. Only three euploid pregnancies fall above a z-score of 3 (arrows), and these are not associated with lower fetal fractions. Reproduced from Canick et al (2013) with permissions from Wiley-Blackwell Publishing20
1.3 |. What is the significance of high FF?
There has been considerable interest in determining whether high levels of total fetal cfDNA may be an early predictor for placental pathologies such as spontaneous preterm birth22 and preeclampsia.23 Results, however, have been inconsistent, with other studies showing no relationship between elevated placental cfDNA levels and pregnancy complications.24,25 There is currently no validated clinical test using absolute placental cfDNA levels or FF to predict pregnancy outcomes.
1.4 |. What is the significance of low FF?
Low FF can result in a test failure or a “no call” result. The rate of “no call” results range from 1% to 8% depending on the assay technology,26 with low FF being the most common cause. Approximately 50% to 60% of women with a failed NIPT result will have success with a second blood draw.27,28 However, the major concern with a failed result due to low FF is that this has been associated with a higher risk of aneuploidy across several sequencing platforms, ranging from 2.7% to 23.3%.29–31
The association between low FF and an increased risk of aneuploidy generates clinical concern after a failed NIPT result. For clinicians to understand the significance of low FF, they need to understand the biological factors that can affect FF and the bioinformatics behind the interpretation algorithms.
2 |. THE BIOLOGY OF FF
There is large interindividual variation in FF, with many biological factors influencing the maternal and placental cfDNA contributions. Any conditions that increase the maternal contribution and/or reduce the placental contribution may lower the FF. Table 1 summarizes some of the established feto-placental and maternal influences on FF.
TABLE 1.
Biological influences on fetal fraction
| Effect on fetal fraction | References | |
|---|---|---|
| Feto-placental factors | ||
| Gestational age (GA) | Increases with GA | 27 |
| Crown rump length (CRL) | Increases with CRL | 18 |
| Mosaicism | Decreases | 44 |
| Fetal aneuploidy | Variable | 18, 27, 31, 41 |
| Triploidy | Decreases | 43 |
| Multiple pregnancy | Total FF increases, but FF decreases per fetus | 47, 48 |
| Maternal factors | ||
| Maternal weight | Decreases with increasing maternal weight | 18, 19, 27 |
| Maternal autoimmune disease | Decreases with active maternal disease | 33, 35, 36 |
| Low molecular weight heparin | Possible decrease | 37–40 |
| Serum PaPP-A | ncreases | 18, 73 |
| Serum free beta-HCG | ncreases | 18, 74 |
| Ethnicity | Variable | 18, 48, 75 |
| Assisted reproductive technology conception | Decreases | 74 |
| Parity | Decreases | 48, 75 |
| Maternal age | Decreases | 48, 75 |
2.1 |. Maternal component
The most well-recognized and clinically significant influence on FF is maternal weight. The proportion of women with FF < 4% increases with maternal weight, estimated at 7%, 11% and 50% of women weighing 100, 110 and 160 kg respectively.18 This FF decline with maternal weight is thought to be due to an increase in maternal cfDNA contribution, possibly due to adipocyte inflammation and necrosis,32 with or without a simultaneous reduction in placental contribution.20
Maternal cfDNA levels are also increased by inflammatory conditions such as systemic lupus erythematosus33 and by B12 deficiency.34 Women with active autoimmune disease such as autoimmune neutropenia have been reported to experience repeated NIPT failures that appear to be resolved by suppression of disease activity.35,36 There are also emerging reports linking low molecular weight heparin (LMWH) with NIPT failures due to low FF.37,38 Failures due to low FF may occur in up to 18% of women on therapy, with a significant difference from untreated women even after controlling for maternal weight and hyper-tension.37 Adjusting the timing of blood sample collection to immediately prior to the next dose may improve the success of a redraw in these women.39 However, a conflicting report claims that the relationship between low FF and LMWH is due to the underlying disease rather than the treatment itself.40 The exact mechanism of the interaction between LWMH and NIPT failures remains to be elucidated.
2.2 |. Fetoplacental component
FF is affected by different fetal aneuploidies.18,27,30,41 The higher FF associated with trisomy 21 is advantageous for the performance of NIPT for this condition. In contrast, trisomies 13 and 18 are associated with lower median FFs (approximately 0.7 MoM on SNP-based assay30) and lower overall detection rates compared with trisomy 21.42 Digynic triploidy is associated with extremely low FF (measurements < 0.5th percentile after correction for maternal weight and gestation).43 Aneuploidies reported after a failed NIPT result include: triploidy, trisomy 13, trisomy 18, trisomy 21, trisomy 16 mosaic, and monosomy X.29,30
It is now possible to measure the relative contribution of a trisomic chromosome to cfDNA and compare it to the overall FF. This is called the “trisomic fraction.”44 In a fetus with a high risk NIPT result for trisomy, the specific cfDNA fraction contributed by the trisomic chromosome may guide interpretation of the abnormal result. When the trisomic fraction is much less than the FF, it suggests the presence of placental mosaicism (ie, the number of trisomy 21 cells is less than the total placental cells contributing to FF). Conversely, if the trisomic fraction is much higher than the FF, then maternal mosaicism could be suspected as it suggests a maternal source of cfDNA (Figure 3).44,45 When the trisomic fraction approximates the FF, it suggests true aneuploidy. Furthermore, the risk of adverse pregnancy outcomes, including miscarriage, fetal death, fetal growth restriction, true fetal mosaicism, and uniparental disomy appears to be increased for this group.45
FIGURE 3.
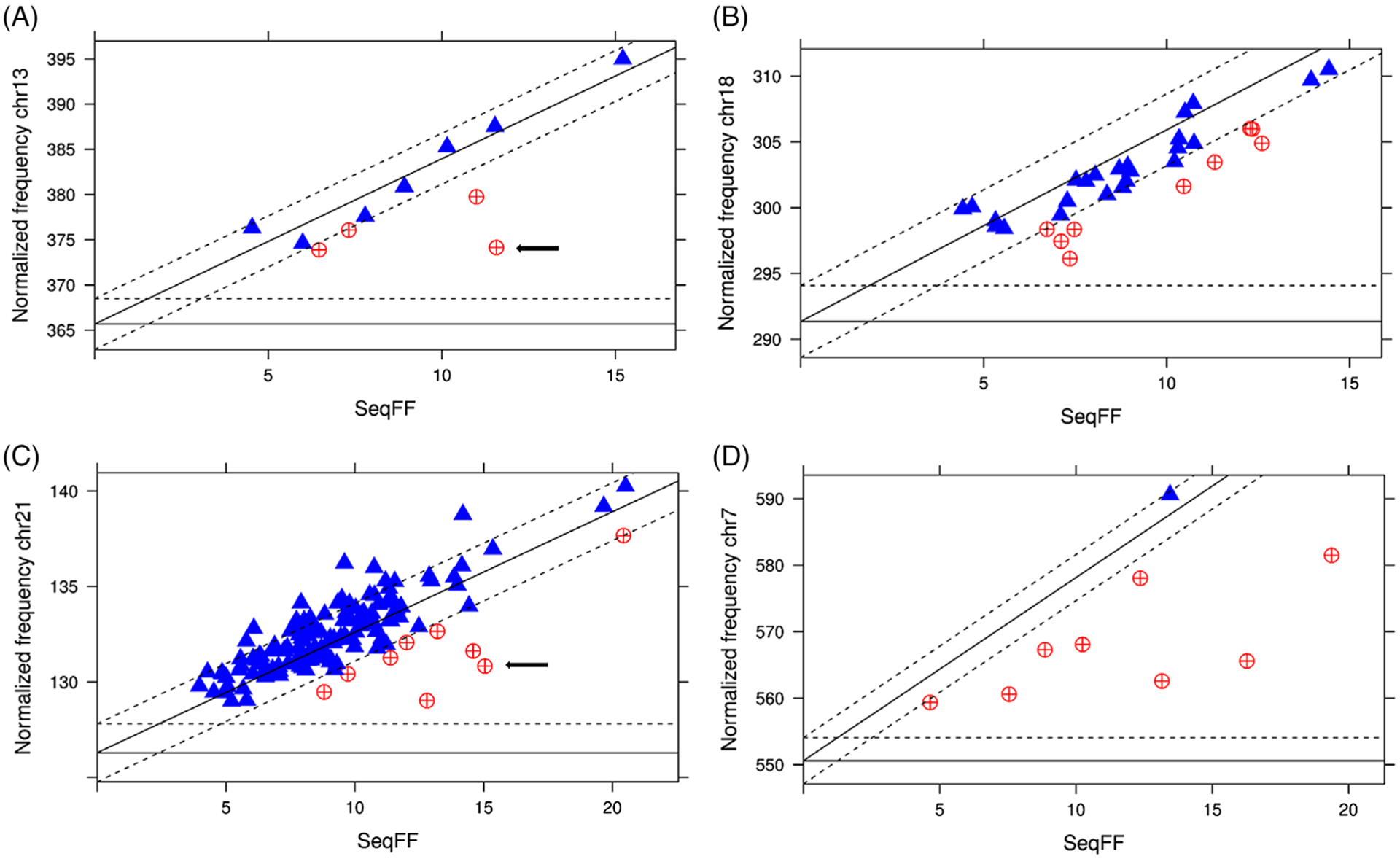
Normalized chromosomal read count in relation to the fetal fraction. Normalized chromosomal read count in relation to the fetal fraction (SeqFF) for chromosomes 13 (A), 18 (B), 21 (C), and 7 (D). The X-axis depicts the fetal fraction and the Y-axis the normalized chromosomal read count. Horizontal and diagonal lines, respectively, mark the predicted normal and trisomic normalized read counts (full lines) and ±3 SD as measured in the normal cases (X-axis and dashed lines). Values within 3 SD from expected trisomy counts are plotted as blue triangles. Values outside the area delineated by the X-axis and dashed lines represent cases with |Z| > 3 and |TriZ| > 3. These are plotted as red circles and indicate pregnancies at risk for fetoplacental mosaicism. The arrows indicate a set of discordant twins. Reproduced from Brison et al (2019) with permission from Wiley-Blackwell Publishing44
2.3 |. Multiple gestation
The available data from multiple gestations suggest that NIPT performs as well in twins as in singletons, though the point estimates are less precise due to smaller numbers.42 For comparable accuracy, the “per fetus” FF should be the same as that required for singletons. One might expect that women carrying twins would have twice as much circulating placental DNA, but that is not the case. Both dichorionic and monochorionic pregnancies, have lower “per fetus” FFs than singletons46 though arguably, only total fetal cfDNA is relevant in monochorionic twins because they are almost always monozygotic with concordant karyotypes. SNP-based cfDNA analysis has shown that the combined FFs for dizygous and monozygous fetuses were 35% and 26% greater than singletons, respectively. However, the “per fetus” FF in dizygous twins is 32% lower than singletons.47
Test failures due to low FF are accordingly generally higher in twins, and vary by chorionicity and cfDNA platform. Reported test failure rates due to low FF in targeted cfDNA assays are: 1.8% (433/23495) for singletons, 4.1% (5/122) for monochorionic twins, and 8.7% (70/806) for dichorionic diamniotic twins.48 For a SNP-based cfDNA assay with a 2.8% FF LOD, test failure rates are 1.7% (2102/121 446) for singletons, 0.8% (11/1454) for monozygous twins, and 5.6% (178/3161) for dizygous twins.
Determining whether a twin pregnancy with a high risk NIPT result is discordant or concordant for aneuploidy can be estimated using the “trisomic fraction” approach described above. Where the trisomic fraction is approximately half of the total FF, this would suggest one euploid and one trisomic fetus, whereas twins with a trisomic fraction equal to the total FF would be suspected to be concordant for trisomy.44
The interpretation of NIPT results is complicated in the event of a single fetal demise in a twin gestation. The placental territory of a demised fetus may continue to release cfDNA into maternal blood for more than three months, which may cause a false positive result if the demised twin was aneuploid.46,49 It has also been reported that the changes in FF following single fetal demise are unpredictable, and not amenable to assigning a “safe” waiting period before performing NIPT.48 Therefore, cfDNA is not recommended for aneuploidy screening at any interval after single twin demise.
3 |. CLINICAL LABORATORY ASPECTS OF FF
Differences in single nucleotide polymorphisms (SNP) between the mother and the fetus can be used to distinguish fetal cfDNA from maternal cfDNA, thereby facilitating measurement of FF.50,51 However, accumulating knowledge about the unique biological characteristics of fetal cfDNA enable it to be distinguished from maternal DNA without knowledge of specific genotype or SNP differences. Placental cfDNA fragments are now known to be shorter, differentially methylated and have a different nucleosome “footprint” compared with cfNA of maternal origin.52,53
3.1 |. What methods are used to measure FF?
With the maturing knowledge base in the field and the recognition of the importance of FF for quality control, many NIPT providers routinely measure FF. However, the methods used to measure FF vary considerably and are not directly comparable, leading to calls for industry standardization in methods and reporting.54,55 While Y chromosome-based methods appear to be the closest to an accepted “gold standard” in this field,56–58 these are obviously limited by their applicability to only those pregnancies with a male fetus. Some advocate using a combination of methods to check for the presence of fetal cfDNA and FF calculation for female and male bearing pregnancies.57 Examples of current FF measurement approaches are provided in Table 2.
TABLE 2.
Technical approaches used to measure FF
| Method | Algorithm name | Selected references |
|---|---|---|
| Sex chromosome based | ||
| Y-chromosome based | 52, 76–78 | |
| DFRAG | 56, 57 79 | |
| cSMART | 78 | |
| X-chromosome based | PREFACE | 80 |
| Genotype-independent methods | ||
| Sequence read count | SeqFF | 56, 81 |
| Nucleosome profile | SANEFALCON | 82 |
| Fragment sized-based | 83, 84 | |
| MPS deduction: sequence counts | Fetal Quant | 85 |
| Methylation differences | 53, 86 | |
| SNP-based | ||
| Polymorphic loci quantified with microarray or sequencing | DANSR | 48, 51, 58 |
| SNP loci | 47, 50 | |
| Insertion/deletion polymorphisms | 87 | |
Bioinformatics tools profoundly influence FF measurement. They have been previously thoroughly reviewed by Peng and colleagues.59 Several attempts to compare different FF algorithms have been published that highlight the significant variation between methods.56,60 It is now apparent that individual clinical laboratories should optimize their platform-specific FF methods according to their lower LOD, and not rely on arbitrary FF cutoff values for quality control.
3.2 |. How does setting a cut-off value affect interpretation of NIPT results?
Optimizing the LOD involves a trade-off between higher statistical confidence and minimizing “no call” results. If an overly stringent FF cutoff is used, this could lead to more frequent “no call” results, with the associated health care costs of medical consultations, additional risk evaluation and possibly, invasive testing. In large population-based observational study involving three different NIPT platforms, when test failures were included with high risk calls as “screen positives,” the screen positive rate of NIPT was only 0.5% lower than combined first trimester screening (2.4% vs 2.9%).61
3.3 |. What can be done to reduce “no call” results on samples with low FF?
Addressing the problem of low FF is a high priority. Many approaches have been proposed to enrich FF by optimizing sequencing conditions, capitalizing on the biological differences between fetal and maternal DNA, and/or developing new statistical algorithms, including:
Deeper sequencing62 or improving sequencing efficiency of existing methods.50
Enrichment of fetal cfDNA based on their smaller fragment size using magnetic beads63 or e-gel electrophoresis.64
cfDNA recovery and repair with a commercial DNA kit used in forensics and archeology.65
Statistical algorithms to identify pregnancies at increased risk of trisomy 18, 13 or triploidy after a failed result due to low FF.66
3.4 |. Should clinical laboratories routinely report the FF to clinicians?
Laboratory reporting practices vary substantially with regard to FF. A workshop hosted by the Laboratory Methods Special Interest Group at the 2016 meeting of the International Society of Prenatal Diagnosis could not reach agreement on whether FF should be routinely reported. Only 11 of the 43 participating laboratories included FF on their submitted examples of NIPT reports; a further laboratory reported only that the FF was “good.” As no consensus was reached, no clear guidance on FF reporting arose from the workshop.55
A subsequent international pilot study of external quality assessment for NIPT also found a large variation in the content of reports, with key information frequently omitted or difficult to identify. Of 40 participants in the first pilot group, FF was reported by 51%, with 40% failing to report the fraction or whether it was measured at all. The remaining 9% of laboratories only reported FF for the “no abnormality detected” cases, or if it was insufficient to meet internal quality control standards.67
The American College of Medical Genetics and Genomics (ACMGG) recommends that all laboratories establish and monitor analytical and clinical validity for FF, and include a clearly visible FF on NIPT reports.68 The ACMGG also recommends that all laboratories should specify the reason for a no-call when reporting noninvasive prenatal screening results. In a recent evaluation of commercial laboratory practices in the United States, 9 of 10 participating companies provided FF in their reports but only five specified a reason for a no-call result.69
It remains debatable whether it is useful to provide a FF percentage to clinicians, given the significant variation in measurement between technologies, lack of universal FF cutoff, and laboratory-specific limits of detection. Some methodologies may be reliable with FFs as low as 2% while others use a fixed cutoff of 4%.70 Furthermore, some laboratories use multiple methods to determine FF, depending on fetal sex, so a single number may not be meaningful to a clinician. Ultimately, the intent of FF reporting is to provide assurance that fetal DNA has been detected and that the level was sufficient to pass laboratory internal quality assurance standards. If the FF is reported, its significance in relation to the test result should be made clear.55
4 |. “PLAN B”: CLINICAL MANAGEMENT AFTER A FAILED RESULT
It is recommended that women whose cfDNA screening results are not reported, indeterminate, or uninterpretable, receive further genetic counseling and be offered comprehensive ultrasound evaluation and diagnostic testing because of an increased risk of aneuploidy.71
4.1 |. Should women have a redraw, another aneuploidy screening method, or a diagnostic testing after a failed result due to low FF?
All of these options should be discussed with the woman, incorporating consideration of factors such as gestational age, background risk of aneuploidy, results of detailed fetal ultrasound examination, and patient preferences. Women should be made aware of the increased risk of aneuploidy after a failed result (in the absence of other obvious causes, such as incorrect dates), and the 40% to 50% failure rate of a repeat blood sample. A detailed ultrasound examination should be performed, and diagnostic testing recommended if a fetal abnormality is present. If the ultrasound is normal and the woman wishes to avoid diagnostic testing, she could be offered an alternative screening test (ideally the first trimester combined test), or a repeat blood draw for NIPT. If the woman elects to have repeat NIPT between 10 and 13 weeks, storing a serum sample at the time of the second NIPT blood draw will ensure that an opportunity for first trimester combined screening will not be missed should the second NIPT attempt fail.
For women with an increased risk of test failure (eg, maternal weight ≥ 100 kg) delaying NIPT collection until the time of a 12 week scan would be prudent to reduce NIPT failures due to borderline FF at 10 to 11 weeks. This would also provide an opportunity for a good early fetal morphological assessment with transvaginal ultrasound examination. If there are no fetal abnormalities seen at 12 weeks that would prompt an offer of prenatal diagnostic testing, then NIPT can be performed, with a back-up serum sample for the first trimester combined test collected in case of NIPT failure. While PaPP-A and free bHCG are positively correlated with FF (Table 1), there are no data on whether prior failed NIPT due to low FF affects the performance of the first trimester combined test or second trimester serum screening.
4.2 |. Summary: The 3 “B”s—biology, bioinformatics, and a plan “B”
FF is an essential quality control component of NIPT that is intimately related to both biology and technology. Clinicians need to understand the biological influences on FF to avoid test failures wherever possible, and to manage test failures due to low FF rationally (Box 1). FF can now also provide useful clinical information in the case of a high-risk result, with regard to the likelihood of placental mosaicism, maternal CNV or discordant aneuploidy in twins.
BOX 1. A fetal fraction toolbox for clinicians: Questions to ask yourself.
What is my patient’s weight?
Are there any possible relevant maternal medical conditions?
Is my patient on low molecular weight heparin?
Was this pregnancy conceived by assisted reproductive technology?
Have gestation and viability been determined with ultrasound examination?
Is this a multiple pregnancy? Is it dichorionic or monochorionic?
Are there sonographic features to suggest triploidy or aneuploidy?
There are many different technologies available for NIPT and FF measurement. Clinicians do not need to know the details behind bioinformatics algorithms used to measure FF, but they do need to appreciate the significant variation between sequencing laboratories and that methods used are not directly comparable. There is no universal fixed FF threshold that is applicable across all platforms and there have not been any demonstrated differences in NIPT performance by platform or FF method.72
Importantly, routine measurement of FF should be a factor in choosing an NIPT provider, although routine reporting of FF is still under debate. The clinician should know what to expect from a standard test report and whether reasons for failed NIPT are revealed (Box 2). Emerging solutions to the challenges of samples with low FF should reduce rates of failed NIPT results in the future. In the meantime, having a “plan B” prepared for those patients for whom NIPT is unsuccessful is essential in today’s clinical practice.
BOX 2. A fetal fraction toolbox for clinicians: Questions to ask the clinical laboratory providing your NIPT results.
Do you routinely measure FF as part of quality control procedures?
Has your laboratory established its own limit of detection?
Do you report FF on the clinical reports? If so, do you explain the significance of the FF to the clinician?
What is the rate of NIPT failures based on low FF? For singletons and twins?
Do you calculate per fetus FF for twins? Do you use chorionicity information?
Do you provide clinicians with the reason for a failed NIPT result?
What is the rate of successful repeat blood draws for women with a failed result?
What other support to you provide to clinicians and patients after a failed result?
What’s already known about this topic?
Fetal fraction (FF) is a crucial quality control parameter for interpretation of noninvasive prenatal testing (NIPT) results;
There are highly variable laboratory practices for FF measurement and reporting;
In some studies, failed NIPT results due to low FF are associated with an increased risk of aneuploidy.
What does this study add?
This article summarizes the relevant biological and technical aspects of FF measurement for the clinician;
The clinical management of women with failed NIPT results due to low FF should include detailed fetal ultra-sound examination and an individualized discussion of the available options including diagnostic testing, NIPT redraw, and alternative screening tests.
ACKNOWLEDGMENTS
L.H. is funded by a National Health and Medical Research Council Early Career Fellowship (1105603). D.B.’s work was supported in part by the National Human Genome Research Institute’s Intramural Research Program, National Institutes of Health, Bethesda, Maryland through grant ZIA HG200400-03.
Funding information
National Health and Medical Research Council, Grant/Award Number: 1105603; National Human Genome Research Institute, Grant/Award Number: Z1A HG200400-02
Footnotes
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
REFERENCES
- 1.Gahan PB, ed. Circulating nucleic acids in early diagnosis, prognosis and treatment monitoring: an introduction. Advances in predictive, preventive and personalised medicine. Vol 5. Netherlands: Springer Publishing; 2015. 10.1007/978-94-017-9168-7_11. [DOI] [Google Scholar]
- 2.Hui L, Bianchi DW. Noninvasive prenatal testing for aneuploidy – the vanguard of genomic medicine. Annu Rev Med. 2017;68:459–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Faas BH, de Ligt J, Janssen I, et al. Non-invasive prenatal diagnosis of fetal aneuploidies using massively parallel sequencing-by ligation and evidence that cell-free fetal DNA in the maternal plasma originates from cytotrophoblastic cells. Expert Opin Biol Ther. 2012;12: S19–S26. [DOI] [PubMed] [Google Scholar]
- 4.Alberry M, Maddocks D, Jones M, et al. Free fetal DNA in maternal plasma in anembryonic pregnancies: confirmation that the origin is the trophoblast. Prenat Diagn. 2007;27:415–418. [DOI] [PubMed] [Google Scholar]
- 5.Flori E, Doray B, Gautie E, et al. Circulating cell-free fetal DNA in maternal serum appears to originate from cyto- and syncytiotrophoblastic cells. Case report. Hum Reprod. 2004;19:723–724. [DOI] [PubMed] [Google Scholar]
- 6.Masuzaki H, Miura K, Oshiura KI, Yoshimura S, Niikawa N, Ishimaru T. Detection of cell free placental DNA in maternal plasma: direct evidence from three cases of confined placental mosaicism. J Med Genet. 2004;41:289–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lui YY, Chik KW, Chiu RW, Ho CY, Lam CW, Lo YM. Predominant hematopoietic origin of cell-free DNA in plasma and serum after sex-mismatched bone marrow transplantation. Clin Chem. 2002;48: 421–427. [PubMed] [Google Scholar]
- 8.Snyder MW, Kircher M, Hill AJ, Daza RM, Shendure J. Cell-free DNA comprises an in vivo nucleosome footprint that informs its tissues-of-origin. Cell. 2016;164(1–2):57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diaz LA Jr, Bardelli A. Liquid biopsies: genotyping circulating tumor DNA. J Clin Oncol. 2014;32(6):579–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chitty LS, Lo YM. Noninvasive prenatal screening for genetic diseases using massively parallel sequencing of maternal plasma DNA. Cold Spring Harb Perspect Med. 2015;5(9):a023085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Srinivasan A, Bianchi DW, Huang H, et al. Noninvasive detection of fetal subchromosome abnormalities via deep sequencing of maternal plasma. Am J Hum Genet. 2013;167–76(34):92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu SC, Jiang P, Choy KW, et al. Noninvasive prenatal molecular karyotyping from maternal plasma. PLoS One. 2013;8:e60968. 10.1371/journal.pone.0060968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lefkowitz RB, Tynan JA, Liu T, et al. Clinical validation of a noninvasive prenatal test for genome-wide detection of fetal copy number variants. Am J Obstet Gynecol. 2015;227:e1–e16. [DOI] [PubMed] [Google Scholar]
- 14.Wapner RJ, Babiarz JE, Levy B, et al. Expanding the scope of noninvasive prenatal testing: detection of fetal microdeletion syndromes. Am J Obstet Gynecol. 2015;212:332.e1–e9. [DOI] [PubMed] [Google Scholar]
- 15.Helgeson J, Wardrop J, Boomer T, et al. Clinical outcome of sub-chromosomal events detected by whole-genome noninvasive prenatal testing. Prenat Diagn. 2015;35:999–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gross SJ, Stosic M, McDonald-McGinn DM, et al. Clinical experience with single-nucleotide polymorphism-based non-invasive prenatal screening for 22q11.2 deletion syndrome. Ultrasound Obstet Gynecol. 2016;47:177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taglauer ES, Wilkins-Haug L, Bianchi DW. Cell-free fetal DNA in the maternal circulation as an indication of placental health and disease. Placenta. 2014;35 suppl:S64–S68. 10.1016/j.placenta.2013.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ashoor G, Syngelaki A, Poon LC, Rezende JC, Nicolaides KH. Fetal fraction in maternal plasma cell-free DNA at 11–13 weeks’ gestation: relation to maternal and fetal characteristics. Ultrasound Obstet Gynecol. 2013;41:26–32. [DOI] [PubMed] [Google Scholar]
- 19.Palomaki GE, Kloza EM, Lambert-Messerlian GM, et al. DNA sequencing of maternal plasma to detect Down syndrome: An international clinical validation study. Genet Med. 2011;13:913–920. [DOI] [PubMed] [Google Scholar]
- 20.Canick JA, Palomaki GE, Kloza EM, Lambert-Messerlian GM, Haddow JE. The impact of maternal plasma DNA fetal fraction on next generation sequencing tests for common fetal aneuploidies. Prenat Diagn. 2013;33:667–674. [DOI] [PubMed] [Google Scholar]
- 21.Takoudes T, Hamar B. Performance of non-invasive prenatal testing when fetal cell-free DNA is absent. Ultrasound Obstet Gynecol. 2015; 45(1):112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dugoff L, Barberio A, Whittaker PG, Schwartz N, Sehdev H, Bastek JA. Cell-free DNA fetal fraction and preterm birth. Am J Obstet Gynecol. 2016;215(2):231.e1–231.e7. [DOI] [PubMed] [Google Scholar]
- 23.Rafaeli-Yehudai T, Imterat M, Douvdevani A, et al. Maternal total cell-free DNA in preeclampsia and fetal growth restriction: evidence of differences in maternal response to abnormal implantation. PLoS One. 2018;13(7):e0200360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poon LC, Musci T, Song K, Syngelaki A, Nicolaides KH. Maternal plasma cell-free fetal and maternal DNA at 11–13 weeks’ gestation: relation to fetal and maternal characteristics and pregnancy outcomes. Fetal Diagn Ther. 2013;33(4):215–223. [DOI] [PubMed] [Google Scholar]
- 25.Rolnik DL, O’Gorman N, Fiolna M, van den Boom D, Nicolaides KH, Poon LC. Maternal plasma cell-free DNA in the prediction of preeclampsia. Ultrasound Obstet Gynecol. 2015;45(1):106–111. [DOI] [PubMed] [Google Scholar]
- 26.Yaron Y The implications of non-invasive prenatal testing failures: a review of an under-discussed phenomenon. Prenat Diagn. 2016;36: 391–396. [DOI] [PubMed] [Google Scholar]
- 27.Kinnings SL, Geis JA, Almasri E, et al. Factors affecting levels of circulating cell-free fetal DNA in maternal plasma and their implications for noninvasive prenatal testing. Prenat Diagn. 2015;35:816–822. [DOI] [PubMed] [Google Scholar]
- 28.Hui L, Teoh M, da Silva Costa F, et al. Clinical implementation of cell-free DNA based aneuploidy screening: perspectives from a national audit. Ultrasound Obstet Gynecol. 2015;45:10–15. [DOI] [PubMed] [Google Scholar]
- 29.Norton ME, Jacobsson B, Swamy GK, et al. Cell-free DNA analysis for noninvasive examination of trisomy. N Engl J Med. 2015;372(17): 1589–1597. [DOI] [PubMed] [Google Scholar]
- 30.Pergament E, Cuckle H, Zimmermann B, et al. Single-nucleotide polymorphism-based noninvasive prenatal screening in a high-risk and low-risk cohort. Obstet Gynecol. 2014;124:210–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palomaki GE, Kloza EM, Lambert-Messerlian GM, et al. Circulating cell free DNA testing: are some test failures informative? Prenat Diagn. 2015;35:289–293. [DOI] [PubMed] [Google Scholar]
- 32.Vora NL, Johnson KL, Basu S, Catalano PM, Hauguel-de Mouzon S, Bianchi DW. A multifactorial relationship exists between total circulating cell-free DNA levels and maternal BMI. Prenat Diagn. 2012;32: 912–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chan RWY, Jiang P, Peng Z, et al. Plasma DNA aberrations in systemic lupus erythematosus revealed by genomic and methylomic sequencing. Proc Natl Acad Sci U S A. 2015;111:E5302–E5311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schuring-Blom H, Lichtenbelt K, van Galen K, et al. Maternal vitamin B12 deficiency and abnormal cell-free DNA results in pregnancy. Prenat Diagn. 2016;36(8):790–793. [DOI] [PubMed] [Google Scholar]
- 35.Hui L, Bethune M, Weeks A, Kelley J, Hayes L. Repeated failed noninvasive prenatal testing owing to low cell-free fetal DNA fraction and increased variance in a woman with severe autoimmune disease. Ultrasound Obstet Gynecol. 2014;44:242–243. [DOI] [PubMed] [Google Scholar]
- 36.Hui CY, Tan WC, Tan EL, Tan LK. Repeated failed non-invasive prenatal testing in a woman with immune thrombocytopenia and antiphospholipid syndrome: lessons learnt. BMJ Case Rep. 2016;2016. 10.1136/bcr-2016-216593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burns W, Koelper N, Barberio A, et al. The association between anti-coagulation therapy, maternal characteristics, and a failed cfDNA test due to a low fetal fraction. Prenat Diagn. 2017;37:1125–1129. [DOI] [PubMed] [Google Scholar]
- 38.Ma G, Wu W, Lee M, Lin Y, Chen M. Low-molecular-weight heparin associated with reduced fetal fraction and subsequent false-negative cell-free DNA test result for trisomy 21. Ultrasound Obstet Gynecol. 2018;51:276–277. [DOI] [PubMed] [Google Scholar]
- 39.Gromminger S, Erkan S, Schock U, et al. The influence of low molecular weight heparin medication on plasma DNA in pregnant women. Prenat Diagn. 2015;35:1155–1157. [DOI] [PubMed] [Google Scholar]
- 40.Dabi Y, Guterman S, Jani JC, et al. Autoimmune disorders but not heparin are associated with cell-free fetal DNA test failure. J Transl Med. 2018;16(1):335. 10.1186/s12967-018-1705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rava RP, Srinivasan A, Sehnert AJ, Bianchi DW. Circulating fetal cell-free DNA fractions differ in autosomal aneuploidies and monosomy X. Clin Chem. 2014;60:243–250. [DOI] [PubMed] [Google Scholar]
- 42.Gil MM, Accurti V, Santacruz B, Plana MN, Nicolaides KH. Analysis of cell-free DNA in maternal blood in screening for aneuploidies: updated meta-analysis. Ultrasound Obstet Gynecol. 2017;50:302–314. [DOI] [PubMed] [Google Scholar]
- 43.Nicolaides KH, Syngelaki A, del Mar Gil M, Quezada MS, Zinevich Y. Prenatal detection of fetal triploidy from cell-free DNA testing in maternal blood. Fetal Diagn Ther. 2014;35(3):212–217. [DOI] [PubMed] [Google Scholar]
- 44.Brison N, Neofytou M, Dehaspe L, et al. Predicting fetoplacental chromosomal mosaicism during noninvasive prenatal testing. Prenat Diagn. 2018;38(4):258–266. 10.1002/pd.5223. [DOI] [PubMed] [Google Scholar]
- 45.Pertile M, Halks-Miller M, Flowers N, et al. Rare autosomal trisomies, revealed by maternal plasma DNA sequencing, suggest increased risk of feto-placental disease. Sci Transl Med. 2017;9:eaan1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grömminger S, Yagmur E, Erkan S, et al. Fetal aneuploidy detection by cell-free DNA sequencing for multiple pregnancies and quality issues with vanishing twins. J Clin Med. 2014;3(3):679–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hedriana H, Martin K, Saltzman D, Billings P, Demko Z, Benn P. Cell Free DNA fetal fraction in twin gestations in single nucleotide polymorphism-based non-invasive prenatal screening. Prenat Diagn. 2019. 10.1002/pd.5609 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Galeva S, Gil MM, Konstantinidou L, Akolekar R, Nicolaides KH. First-trimester screening for trisomies by cfDNA testing of maternal blood in singleton and twin pregnancies: factors affecting test failure. Ultrasound Obstet Gynecol. 2019;53:804–809. [DOI] [PubMed] [Google Scholar]
- 49.Bevilacqua E, Chen K, Wang Y, et al. Cell-free DNA analysis after reduction in multifetal pregnancies. Ultrasound Obstet Gynecol. 2019. 10.1002/uog.20366. [DOI] [PubMed] [Google Scholar]
- 50.Ryan A, Hunkapiller N, Banjevic M, et al. Validation of an enhanced version of a single-nucleotide polymorphism-based noninvasive prenatal test for detection of fetal aneuploidies. Fetal Diagn Ther. 2016; 40(3):219–223. [DOI] [PubMed] [Google Scholar]
- 51.Sparks AB, Struble CA, Wang ET, Song K, Oliphant A. Noninvasive prenatal detection and selective analysis of cell-free DNA obtained from maternal blood: evaluation for trisomy 21 and trisomy 18. Am J Obstet Gynecol. 2012;206:319.e1–319.e9. [DOI] [PubMed] [Google Scholar]
- 52.Fan HC, Blumenfeld YJ, Chitkara U, Hudgins L, Quake SR. Noninvasive diagnosis of fetal aneuploidy by shotgun sequencing DNA from maternal blood. Proc Natl Acad Sci U S A. 2008;105(42):16266–16271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jensen TJ, Kim SK, Zhu Z, et al. Whole genome bisulfite sequencing of cell-free DNA and its cellular contributors uncovers placenta hypomethylated domains. Genome Biol. 2015;16:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wataganara T, Bui TH, Choy KW, Leung TY. Debates on fetal fraction measurement and DNA-based noninvasive prenatal screening: time for standardisation? BJOG. 2016;123(suppl 3):31–35. [DOI] [PubMed] [Google Scholar]
- 55.Deans ZC, Allen S, Jenkins L, et al. Recommended practice for laboratory reporting of non-invasive prenatal testing of trisomies 13, 18 and 21: a consensus opinion. Prenat Diagn. 2017;37(7): 699–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hestand MS, Bessem M, van Rijn P, et al. Fetal fraction evaluation in non-invasive prenatal screening. Eur J Hum Genet. 2019;27:198–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Van Beek DM, Straver R, Weiss MM, et al. Comparing methods for fetal fraction determination and quality control of NIPT samples. Prenat Diagn. 2017;37:769–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schmid M, White K, Stokowski R, et al. Accuracy and reproducibility of fetal-fraction measurement using relative quantitation at polymorphic loci with microarray. Ultrasound Obstet Gynecol. 2018;51: 813–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Peng XL, Jiang P. Bioinformatics approaches for fetal DNA fraction estimation in noninvasive prenatal testing. Int J Mol Sci. 2017;18: 453–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Arteri CG, Haverty C, Evans EA, et al. Noninvasive prenatal screening at low fetal fraction: comparing whole-genome sequencing and single-nucleotide polymorphism methods. Prenat Diagn. 2017;37: 482–490. [DOI] [PubMed] [Google Scholar]
- 61.Lindquist A, Poulton A, Kluckow E, et al. The Victorian Perinatal Record Linkage study. Paper presented at: Abstract accepted for oral plenary at the World Congress of the International Society for Ultra-sound in Obstetrics and Gynecology; October, 2019; Berlin, Germany. [Google Scholar]
- 62.Benn P, Cuckle H. Theoretical performance of non-invasive prenatal testing for chromosome imbalances using counting of cell-free DNA fragments in maternal plasma. Prenat Diagn. 2014;34(8):778–783. [DOI] [PubMed] [Google Scholar]
- 63.Hu P, Liang D, Chen Y, et al. An enrichment method to increase cell-free fetal DNA fraction and significantly reduce false negatives and test failures for non-invasive prenatal screening: a feasibility study. J Transl Med. 2019;17(1):124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liang B, Li H, He Q, et al. Enrichment of the fetal fraction in noninvasive prenatal screening reduces background maternal interference. Sci Rep. 2018;8:17675. 10.1038/s41598-018-35738-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vong JSL, Jiang P, Cheng S-H, et al. Enrichment of fetal and maternal long cell-free DNA fragments from maternal plasma following DNA repair. Prenat Diagn. 2019;39:88–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McKanna T, Ryan A, Krinshpun S, et al. Fetal fraction-based risk algorithm for non-invasive prenatal testing: screening for trisomies 13 and 18 and triploidy in women with low cell-free fetal DNA. Ultra-sound Obstet Gynecol. 2019;53(1):73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Deans ZC, Allen S, Jenkins L, et al. Ensuring high standards for the delivery of NIPT world-wide: Development of an international external quality assessment scheme. Prenat Diagn. 2019;39(5):379–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gregg AR, Skotko BG, Benkendorf JL, et al. Noninvasive prenatal screening for fetal aneuploidy, 2016 update: a position statement of the American College of Medical Genetics and Genomics. Genet Med. 2016;18(10):1056–1065. [DOI] [PubMed] [Google Scholar]
- 69.Skotko BG, Allyse MA, Bajaj K, et al. Adherence of cell-free DNA noninvasive prenatal screens to ACMG recommendations. Genet Med. 2019;21(10):2285–2292. 10.1038/s41436-019-0485-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fiorentino F, Bobo S, Pizzuti F, et al. The importance of determining the limit of detection of non-invasive prenatal testing methods. Prenat. Prenat Diagn. 2016;36:304–311. [DOI] [PubMed] [Google Scholar]
- 71.American College of Obstetricians and Gynecologists. Practice bulletin No. 163 summary: screening for fetal aneuploidy. Obstet Gynecol. 2016;127(5):979–981. [DOI] [PubMed] [Google Scholar]
- 72.Rousseau F, Langlois S, Johnson J, et al. Prospective head to head comparison of accuracy of two sequencing platforms for screening for fetal aneuploidy by cell-free DNA: the PEGASUS study. Eur J Hum Genet. 2019;27(11):1701–1715. 10.1038/s41431-019-0443-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Scott FP, Menezes M, Palma-Dias R, et al. Factors affecting cell-free DNA fetal fraction and the consequences for test accuracy. J Matern Fetal Neonatal Med. 2018;31(14):1865–1872. [DOI] [PubMed] [Google Scholar]
- 74.Lee TJ, Rolnik DL, Menezes MA, McLennan AC, da Silva Costa F. Cell-free fetal DNA testing in singleton IVF conceptions. Hum Reprod. 2018;33(4):572–578. [DOI] [PubMed] [Google Scholar]
- 75.Rolnik DL, Da Silva Costa F, Lee TJ, Schmid M, McLennan AC. Association between fetal fraction on cell-free DNA testing and first trimester markers for pre-eclampsia. Ultrasound Obstet Gynecol. 2019. 10.1002/uog.18993. [DOI] [PubMed] [Google Scholar]
- 76.Chiu RW, Chan KC, Gao Y, et al. Noninvasive prenatal diagnosis of fetal chromosomal aneuploidy by massively parallel genomic sequencing of DNA in maternal plasma. Proc Natl Acad Sci U S A. 2008;105 (51):20458–20463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xu XP, Gan HY, Li FX, et al. A method to quantify cell-free fetal DNA fraction in maternal plasma using next generation sequencing: its application in non-invasive prenatal chromosomal aneuploidy detection. PLoS One. 2016;11(1):e0146997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Song Y, Zhou X, Huang S, et al. Quantitation of fetal DNA fraction in maternal plasma using circulating single molecule amplification and re-sequencing technology (cSMART). Clin Chim Acta. 2016;456: 151–156. [DOI] [PubMed] [Google Scholar]
- 79.Bayindir B, Dehaspe L, Brison N, et al. Noninvasive prenatal testing using a novel analysis pipeline to screen for all autosomal fetal aneuploidies improves pregnancy management. Eur J Hum Genet. 2015;23 (10):1286–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Raman L, Baetens M, De Smet M, Dheedene A, Van Dorpe J, Menten B. PREFACE: in silico pipeline for accurate cell-free fetal DNA fraction prediction. Prenat Diagn. 2019;39:925–933. 10.1002/pd.5508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kim SK, Hannum G, Geis J, et al. Determination of fetal DNA fraction from the plasma of pregnant women using sequence read counts. Prenat Diagn. 2015;35:810–815. [DOI] [PubMed] [Google Scholar]
- 82.Straver R, Oudejans CB, Sistermans EA, Reinders MJ. Calculating the fetal fraction for noninvasive prenatal testing based on genome-wide nucleosome profiles. Prenat Diagn. 2016;36(7):614–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yu SC, Chan KC, Zheng YW, et al. Size-based molecular diagnostics using plasma DNA for noninvasive prenatal testing. Proc Natl Acad Sci U S A. 2014;111(23):8583–8588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fan HC, Blumenfeld YJ, Chitkara U, Hudgins L, Quake SR. Analysis of the size distributions of fetal and maternal cell-free DNA by paired-end sequencing. Clin Chem. 2010;56(8):1279–1286. [DOI] [PubMed] [Google Scholar]
- 85.Jiang P, Chan KC, Liao GJ, et al. FetalQuant: deducing fractional fetal DNA concentration from massively parallel sequencing of DNA in maternal plasma. Bioinformatics. 2012;28(22):2883–2890. [DOI] [PubMed] [Google Scholar]
- 86.Nygren AOH, Dean J, Jensen TJ, et al. Quantification of fetal DNA by use of methylation-based DNA discrimination. Clin Chem. 2010;56: 1627–1635. [DOI] [PubMed] [Google Scholar]
- 87.Barrett AN, Xiong L, Tan TZ, et al. Measurement of fetal fraction in cell-free DNA from maternal plasma using a panel of insertion/deletion polymorphisms. PLoS One. 2017;12(10):e0186771. 10.1371/journal.pone.0186771. [DOI] [PMC free article] [PubMed] [Google Scholar]


