Abstract
Rationale:
At least six different types of anti-depressant treatments have been shown to either increase the neuroprotective kynurenine pathway (KP) metabolite, kynurenic acid (KynA), or decrease the neurotoxic KP metabolite, quinolinic acid (QA). Non-steroidal anti-inflammatory drugs (NSAIDs) including ibuprofen have shown some efficacy in the treatment of depression but their effects on the KP have not been studied in humans.
Objectives:
To evaluate the effect of ibuprofen on circulating KP metabolites.
Methods:
In a randomized, placebo-controlled, cross-over study, 20 healthy adults (10 women) received a single oral dose of 200mg ibuprofen, 600mg ibuprofen or placebo in a counterbalanced order (NCT02507219). Serum samples were drawn in the mid-afternoon, 5 hours after ibuprofen/placebo administration. KP metabolites were measured blind to visit by tandem mass spectrometry. Data were analyzed with linear mixed effect models. The primary outcome was KynA/QA and the secondary outcome was KynA.
Results:
After Bonferroni correction, there was a significant effect of treatment on KynA/QA. The effect was driven by an increase in KynA concentration after the 600mg dose but not the 200mg dose relative to placebo (Cohen’s d=1.71). In contrast, both the 200mg (d=1.03) and 600mg (d=2.05) doses of ibuprofen decreased tryptophan concentrations relative to placebo.
Conclusions:
Given its KynA-elevating effects, ibuprofen could have neuroprotective effects in the context of depression as well as other neuroinflammatory disorders that are characterized by a reduction in KynA.
Keywords: Ibuprofen, Non-steroidal anti-inflammatory drugs, Kynurenine Pathway, Kynurenic Acid, Depression, Tryptophan
INTRODUCTION
The putative role of inflammation in the etiology of mood disorders has led to interest in treating depression with anti-inflammatory medications, including widely-used non-steroidal anti-inflammatory drugs (NSAIDs) which reduce inflammation by inhibiting the cyclooxygenase (COX) enzymes, COX-1 and/or COX-2 (Yagami et al. 2016). A recent meta-analysis reported a therapeutic signal for the COX-2 inhibitor, celecoxib, when used as an add-on for the treatment of depression (Kohler-Forsberg et al. 2019). However, the largest study to date found no efficacy for celecoxib versus placebo in patients with bipolar depression (Husain et al. 2020). In fact, pharmacoepidemiological studies generally suggest that inhibition of COX-2 may be counterproductive but that preferential COX-1 inhibition such as in the form of low-dose aspirin may be protective (Hu et al. 2020; Kessing et al. 2019; Kohler et al. 2015; Stolk et al. 2010; Warner-Schmidt et al. 2011; Zhang et al. 2022). Consistent with these data, we found beneficial effects of adjunctive treatment with low-dose aspirin in the context of bipolar depression (Savitz et al. 2018) although a recent clinical trial of adjunctive low-dose aspirin in youth with major depressive disorder (MDD) was negative (Berk et al. 2020a) and another recent study reported that low-dose aspirin failed to protect against the development of depression in older adults (Berk et al. 2020b).
The anti-depressant effects of ibuprofen a non-selective NSAID that blocks both COX-1 and COX-2 isoforms, have been less well studied. Preclinical work has shown that ibuprofen alleviates depression-like behavior after Bacillus Calmette-Guerin inoculation (Saleh et al. 2014), the forced swim test (Norden et al. 2015), and chronic restraint stress (Seo et al. 2019). An epidemiological study of selective serotonin reuptake inhibitor (SSRI) users showed that concurrent use of ibuprofen reduced the risk of psychiatric contacts over the follow-up period (Kohler et al. 2015) while a pooled analysis of NSAID trials for osteoarthritis showed that 800mg of ibuprofen T.I.D for six weeks was associated with a small but significant reduction in depressive symptoms versus placebo (Iyengar et al. 2013). Data from the FDA’s safety and adverse event reporting program, “MedWatch”, suggested that ibuprofen might have anti-depressant effects in women but not men (Lehrer and Rheinstein 2019). Partially consistent with these data, an observational trial of women at high-risk for depression in the post-partum period reported that utilization of ibuprofen was protective although the effect was marginal (Kapulsky et al. 2021).
Like other NSAIDs, ibuprofen inhibits the COX enzymes that promote inflammation by catalyzing the formation of prostaglandins and thromboxanes from arachidonic acid (although it likely has many other “off-target effects”). One consequence of the inflammatory process is activation of the kynurenine pathway (KP) leading to the production of several neuroactive metabolites that modulate a range of physiological processes, including glutamatergic and dopaminergic neurotransmission (Dantzer et al. 2011; Schwarcz et al. 2012) (Figure 1). We and others have shown that depression is associated with a decrease in the circulating concentration of the NMDA receptor antagonist, kynurenic acid (KynA) relative to the NMDA receptor agonist, quinolinic acid (QA) (Bartoli et al. 2021; Bay-Richter et al. 2015; Myint et al. 2007; Paul et al. 2022; Savitz 2020; Wurfel et al. 2017). Some groups have also reported an increase in QA in post-mortem brain samples from patients with severe depression (Steiner et al. 2011) and in the cerebrospinal fluid (CSF) of suicide attempters (Erhardt et al. 2013). Further, several forms of anti-depressant therapy including escitalopram (Halaris et al. 2015), ketamine (Kadriu et al. 2019; Verdonk et al. 2019; Zhou et al. 2018), electroconvulsive therapy (Guloksuz et al. 2015; Schwieler et al. 2016), cognitive behavior therapy (Savitz et al. 2020), aerobic exercise (Agudelo et al. 2014; Javelle et al. 2021) and real-time fMRI neurofeedback (Tsuchiyagaito et al. 2021) have been shown to either increase KynA or decrease QA, raising the possibility of a common underlying mechanism of action. Given these data and the anti-depressant potential of NSAIDs (albeit equivocal) we were interested in the effect of acute ibuprofen administration on the KP. Here, we tested the effect of a single dose of ibuprofen on serum KP metabolites in healthy adults using a cross-over design with two different doses of ibuprofen (600 mg and 200 mg) and one dose of placebo. Consistent with our recent work (Savitz et al. 2020; Tsuchiyagaito et al. 2021; Zheng et al. 2022) we selected KynA/QA as the primary outcome in order to evaluate the balance between the putative neuroprotective and neurotoxic arms of the kynurenine pathway. We hypothesized that ibuprofen, especially at the 600mg dose, would increase serum concentrations of KynA and/or decrease concentrations of QA relative to placebo.
Figure 1,
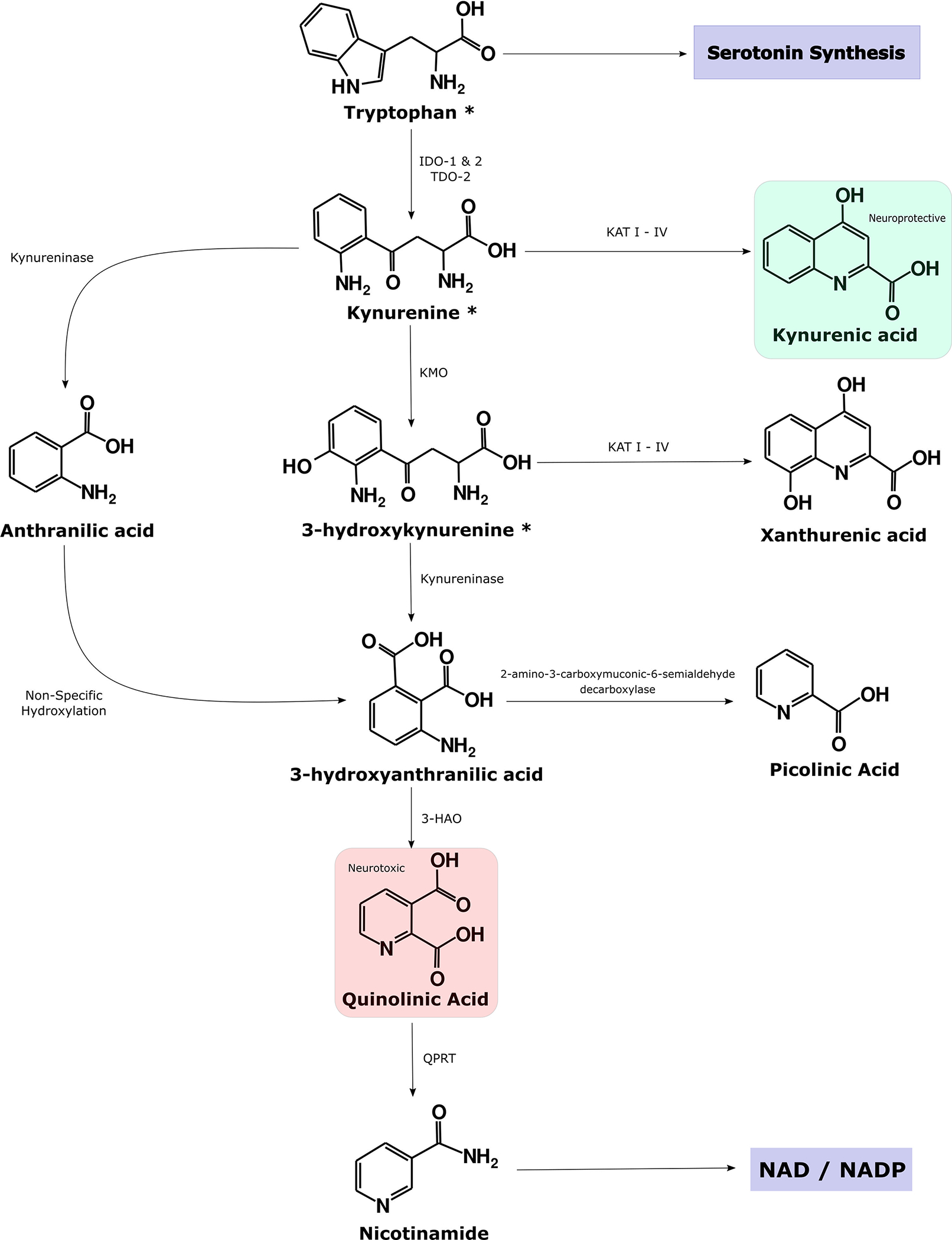
reproduced from (Savitz 2020), illustrates the main branches of the kynurenine pathway (KP). Approximately 95% of tryptophan is metabolized into kynurenine by the enzymes, indoleamine dioxygenase (IDO) and tryptophan dioxygenase (TDO). During inflammation IDO is upregulated and kynurenine is in turn preferentially converted down the quinolinic acid (QA) pathway at the expense of the neuroprotective metabolite, kynurenic acid (KynA). Theoretically, this rerouting of metabolism is in order to generate NAD for activated immune cells but may have adverse consequences in the form of the generation of neurotoxic metabolites, including the NMDA receptor agonist QA.
METHODS
Methodological details have been published elsewhere (Burrows et al. 2022; Cosgrove et al. 2021) and are summarized here. The research was conducted in accordance with the Helsinki Declaration of 1975. Participants provided written informed consent after receiving a full explanation of the study procedures and risks, as approved by the Western IRB. Participants were recruited between May and October of 2015. Twenty-two healthy participants (11 women) aged 18–50 years participated in a double-blind, randomized, crossover study (NCT02507219; Table 1). Two participants withdrew before completion, so that data from 20 participants were analyzed (n=10 women). The CONSORT diagram is shown in Figure 2. Exclusion criteria were as follows: (1) history of any psychiatric disorder (2) current or past 6-month alcohol or drug abuse; (3) regular use (> 15 days for past 30 days) of NSAIDS; (4) history of clinically significant hepatic, cardiac, renal, neurologic, cerebrovascular, metabolic, gastric, or pulmonary disease; (5) history of seizure disorders; (6) pregnancy or plan to become pregnant within the next 18 weeks; (7) women who are currently menstruating; (8) fMRI-related exclusion criteria (e.g. claustrophobia, metal implants).
Table 1.
Characteristics of the Study Participants
| Variable | |
|---|---|
| N | 20 |
| Age (years) | 32.4 ± 6.7 |
| Sex (% male) | 10 (50.0) |
| Body Mass Index (kg/m2) | 26.6 ± 5.8 |
| Ethnicity | |
| White | 12 (100%) |
| Hispanic or Latino | 0 (0%) |
| Educational status | |
| Some college, no degree | 3 |
| Associate degree | 11 |
| Bachelor’s degree | 2 |
| Master’s degree | 2 |
| Missing data | 2 |
| Concomitant medication | |
| Birth Control | 3 |
| OTC allergy medications | 3 |
| Phentermine | 1 |
| Clonazepam | 1 |
| Levothyroxine | 1 |
Figure 2. CONSORT Flow Diagram.
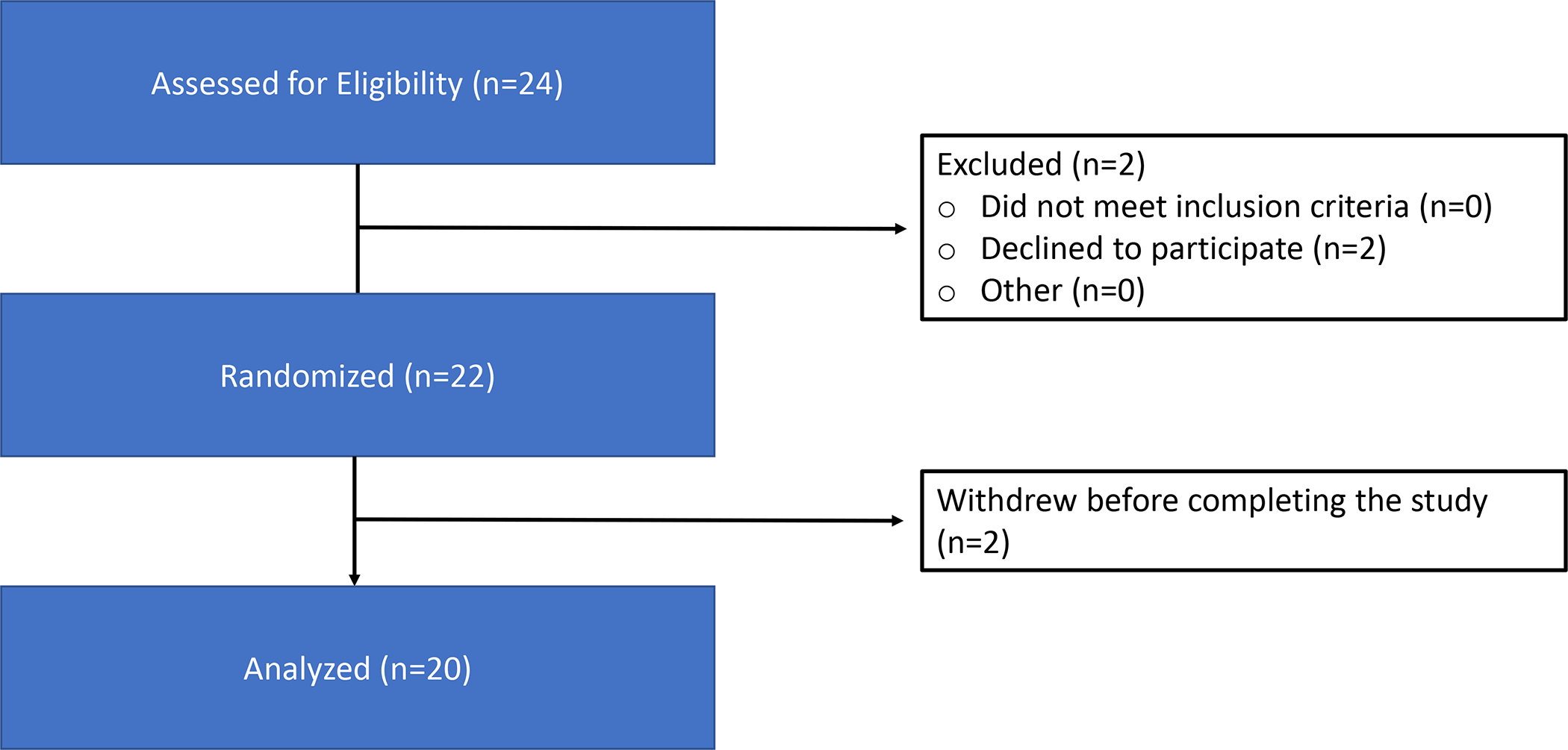
illustrates the number of participants recruited, randomized, and analyzed in the study.
Each participant completed a baseline screening assessment and was scheduled for three subsequent visits. During each visit, scheduled 2 weeks apart to preclude any potential carryover effects, participants received one oral pill containing placebo, 200 mg of ibuprofen, or 600 mg of ibuprofen, so that all participants received a total of 3 different doses. Ibuprofen was capsuled, and identical placebo capsules were produced by a local compounding pharmacy. The medication was administered together with a snack after an overnight fast at either 8am or 10am. Subsequently, participants completed an MRI scan, rating scales, and behavioral tasks. Blood was drawn approximately 5 hours post administration of ibuprofen/placebo in the mid-afternoon. Administration order was counterbalanced across participants, and experimenters were blinded to the medication codes. Although participants reported occasional over-the-counter NSAID use during the study period, they never took an NSAID within 48 hours of a study visit.
Serum samples were collected with BD Vacutainer serum tubes, processed according to the standard BD Vacutainer protocol, and stored at −80 C. Serum concentrations of tryptophan (TRP), kynurenine, kynurenic acid (KynA), 3-hydroxykynurenine (3HK), and quinolinic acid (QA) were measured blind to visit by Charles River Laboratories in January 2018. The KP metabolite concentrations were determined by high performance liquid chromatography (HPLC) with tandem mass spectrometry (MS/MS) detection using their standard protocols. The lowest level of quantification and intra-assay percentage of coefficient of variation for each of the KP metabolites were as follows: TRP: 3 μM, 1.4%; kynurenine: 0.225 μM, 1.6%; KynA: 7.5 nM, 6.6%; 3HK: 5 nM, 3.9%, and QA: 50 nM and 8.6%.
Data were analyzed with linear mixed effect models with treatment condition (i.e. 600mg, 200 mg, or placebo) as the independent variable and participant as a random factor. Covariate inclusion was determined by Bayesian Information Criterion (BIC) model comparison for all combinations of age, sex, and body mass index (BMI). Carry over effects were ruled out by testing the interaction between visit number and treatment condition for each outcome variable. Given the above-mentioned effect of antidepressant treatments on the KP, we selected the ratio of KynA to QA as the primary outcome. The secondary outcome was the change in KynA and the other KP metabolites were included as exploratory outcomes. We performed a Bonferroni correction to control for multiple comparisons (P<0.006, 2-tailed test).
RESULTS
There was a significant effect of treatment on the primary outcome, KynA/QA (F2,38 =5.79, p=0.006) such that KynA/QA concentration was increased after the 600mg dose of ibuprofen (Cohen’s d=0.99) relative to placebo (Table 2; Figure 3). The effect was driven by a significant increase in the secondary outcome variable, KynA (F2,38 =8.03, p=0.001) after the 600mg dose of ibuprofen (Cohen’s d=1.71) relative to the 200mg dose and placebo (Figure 3). Consistent with these results, there was a significant increase in KynA/Kyn (F2,38 =9.87, p<0.001) after the 600mg dose of ibuprofen (Cohen’s d=1.42) relative to the 200mg dose and placebo. There was no significant effect of treatment condition on QA, 3-HK, kynurenine, and Kyn/TRP concentrations (all p’s >0.01). There was, however, a significant effect of treatment on TRP concentrations (F2,38 = 10.1, p<0.001) such that TRP concentrations were decreased after both the 200mg dose (Cohen’s d=1.03) and 600mg dose (Cohen’s d=2.05) of ibuprofen relative to placebo (Figure 3).
Table 2:
Kynurenine Metabolites by IBU Dosage. Mean (SEM) and Cohen’s D of each metabolite and ratio grouped by IBU dose condition. Unadjusted Cohen’s D statistics are given for the difference between the IBU dose condition and placebo (reference). Units in ratios converted to nM where necessary.
| IBU Dose | Placebo | 200mg | 600mg | ||
|---|---|---|---|---|---|
| Mean(SEM) | Mean(SEM) | Cohen’s D | Mean(SEM) | Cohen’s D | |
| TRP (μM) | 51.5 (2.1) | 46.0 (1.8) | 0.64 | 44.0 (1.9) | 0.86 |
| KYN (μM) | 1.64 (0.07) | 1.58 (0.06) | 0.22 | 1.60 (0.07) | 0.13 |
| KynA (nM) | 30.4 (2.5) | 31.3 (2.0) | 0.09 | 37.0 (2.7) | 0.57 |
| 3HK (nM) | 22.8 (1.9) | 22.3 (0.9) | 0.06 | 24.2 (1.3) | 0.19 |
| QA (nM) | 262 (18) | 261 (14) | <0.01 | 264 (15) | 0.03 |
| KYN/TRP | 0.032 (0.001) | 0.035 (0.02) | 0.39 | 0.07 (0.002) | 0.63 |
| KynA/KYN | 0.019 (0.001) | 0.020 (0.002) | 0.28 | 0.024 (0.002) | 0.71 |
| KynA/3HK | 1.43 (0.11) | 1.45 (0.12) | 0.04 | 1.57 (0.12) | 0.29 |
| KynA/QA | 0.12 (0.01) | 0.13 (0.01) | 0.04 | 0.15 (0.02) | 0.45 |
IBU = ibuprofen, TRP = tryptophan, KYN = kynurenine, KynA = kynurenic acid, 3HK = 3-hydroxykynurenine, QA = quinolinic acid, SEM = standard error of the mean
Figure 3.
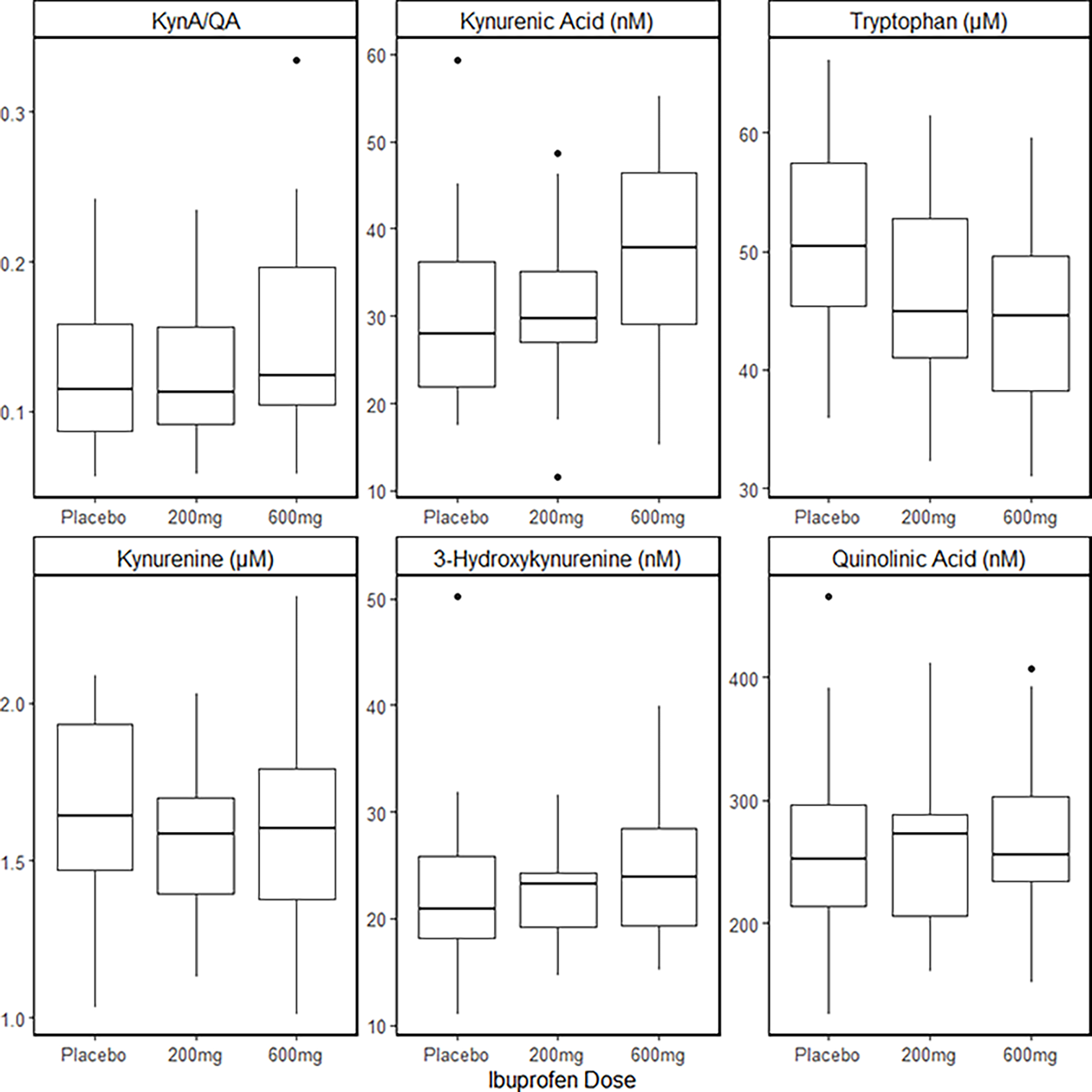
Boxplots illustrating the mean concentrations of KynA/QA, KynA, TRP, Kyn, 3-HK, and QA in serum approximately five hours after a single oral dose of placebo, 200mg of ibuprofen, or 600mg of ibuprofen. The solid line in BOLD represents the median, the boxes represent the 25th-75th percentiles, and the tails, the 95% confidence interval.
DISCUSSION
This study revealed that oral ingestion of a 600mg dose of ibuprofen induced a significant (~25%) increase in serum KynA concentrations relative to placebo. This ibuprofen-associated increase in circulating KynA is consistent with several preclinical studies. Edwards et al. initially showed that a single dose of the non-selective COX inhibitor, diclofenac (40mg/kg, s.c.), increased KynA levels in the rat brain one hour after administration (Edwards et al. 2000). Subsequent work confirmed this effect at 50mg/kg, i.p. 3.5 hours after diclofenac administration and extended this finding to indomethacin (50 mg/kg, i.p.), a preferential COX-1 inhibitor (Schwieler et al. 2005). A follow-up study showed that the same dose of indomethacin increased KynA by 150–300% and increased the tonic firing of dopamine neurons in the ventral tegmental area (VTA) (Schwieler et al. 2006). More recently, KynA concentrations in both plasma and hippocampus were shown to be significantly increased after a single ibuprofen dose in rats (Maciejak et al. 2013). The increase in KynA was significant for both 75 and 150mg/kg i.p. doses but the effect was strongest at the higher dose. In contrast, the selective COX-2 inhibitors, parecoxib and meloxicam decreased brain concentrations of KynA (Schwieler et al. 2005) and decreased firing rate and burst firing activity in the VTA (Schwieler et al. 2006). The decrease in KynA observed in the case of selective COX-2 inhibitors versus the increase in KynA associated with non-selective NSAIDs could conceivably explain why selective COX-2 inhibitors have been reported to worsen depression while COX-1 inhibitors or non-selective NSAIDs may have anti-depressant properties (Hu et al. 2020; Kessing et al. 2019; Kohler et al. 2015; Savitz et al. 2018; Stolk et al. 2010; Warner-Schmidt et al. 2011; Zhang et al. 2022). Further research is needed to test this hypothesis.
The mechanism through which ibuprofen increases KynA is unclear. Schwieler and colleagues found that they could block the indomethacin-induced increase in brain KynA with the prostaglandin E1/E2 agonist, misoprostol, suggesting that the effect may be mediated by the inhibitory action of prostaglandins on the synthesis of KynA or its precursor, kynurenine (Schwieler et al. 2005). Thus, it is possible that KynA concentrations are altered because of upstream changes in the KP. However, we did not observe a significant effect of treatment on kynurenine concentration in this study. Third, there is some evidence that the non-selective NSAID, diclofenac inhibits kynurenine monooxygenase (KMO), the enzyme that converts kynurenine to 3-HK (Modoux et al. 2021). Auer and colleagues compared the molecular structure of known KMO inhibitors to a database of existing medications and found a match with diclofenac. The in silico data were then confirmed with an in vitro KMO cell lysate enzymatic inhibition assay which showed that diclofenac had inhibitory effects on KMO (Shave et al. 2018). It is conceivable that ibuprofen and perhaps other non-selective NSAIDs also possess inhibitory activity against KMO which would then bias metabolism of kynurenine towards KynA. However, in a post-hoc analysis, we observed that ibuprofen was associated with a non-significant increase in 3HK/Kyn, which we would not expect if ibuprofen was inhibiting KMO.
The other result of interest was the decrease in serum TRP observed with both the 200mg and 600mg doses of ibuprofen relative to placebo. The decrease in TRP concentration is consistent with a preclinical study that showed that single doses of both 75mg/kg and 150mg/kg i.p. of ibuprofen resulted in significant decreases in plasma TRP in rats (Maciejak et al. 2013). The decrease in serum TRP concentration is likely a consequence of TRP being displaced from its binding site on albumin by ibuprofen, resulting in greater renal clearance. Albumin is a serum protein that serves as a transport vehicle for many ligands and drugs, including TRP and ibuprofen, respectively. A recent study showed that infusion of 800mg of ibuprofen during hemodialysis treatment could displace several toxins as well as TRP from albumin augmenting their removal and decreasing their concentrations in serum (Madero et al. 2019).
According to the monoamine theory of depression, a decrease in TRP, the precursor of serotonin, would likely worsen depressive symptoms (Schildkraut and Kety 1967). However, this hypothesis has fallen into disfavor in recent years, in part because no convincing mechanism of monoamine loss has been discovered. Interest in the KP was originally viewed through the lens of the monoamine theory of depression. That is, a key pathophysiological mechanism was thought to be the preferential breakdown of TRP into kynurenine at the expense of serotonin (Capuron et al. 2002; Lapin and Oxenkrug 1969; Widner et al. 2002). However, several preclinical studies have demonstrated that inflammatory stimuli actually increase rather than decrease brain TRP and serotonin (Dunn and Welch 1991; O’Connor et al. 2009). Moreover, Dantzer and colleagues demonstrated that the depressive effects of lipopolysaccharide (LPS) could be blocked with an inhibitor of indoleamine 2’3 dioxygenase (IDO) without affecting brain TRP and serotonin turnover (O’Connor et al. 2009). Thus, the current model postulates that an imbalance between downstream neuroprotective and neurotoxic KP metabolites such as KynA and QA is the critical pathophysiological mechanism underlying depression (Myint and Kim 2003; Savitz 2020; Savitz et al. 2015; Wichers et al. 2005). If this hypothesis is correct and ibuprofen does increase KynA concentrations in the brain, then this raises the possibility that ibuprofen may have neuroprotective effects not just in the case of depression, but in several other neuroinflammatory disorders characterized by a decrease in KynA. For instance, epidemiological data suggest that ibuprofen reduces the risk for Parkinson’s Disease (PD) (Ascherio and Schwarzschild 2016; Gao et al. 2011) and decreased concentrations of KynA have been reported in the cerebrospinal fluid and plasma of patients with PD (Heilman et al. 2020; Lim et al. 2017; Sorgdrager et al. 2019). Conceivably, ibuprofen could be protecting against PD by increasing KynA levels and thereby limiting the excitotoxic effects of QA on the NMDA receptor.
The main limitation of this work is that the data were collected from medically healthy individuals with no history of psychiatric disorders. Thus, we were unable to test whether ibuprofen had any anti-depressant effects and whether these effects were mediated by changes in inflammatory mediators or KP metabolites. Similarly, we were unable to evaluate the effect of ibuprofen on pain. For instance, Schwieler and colleagues have previously proposed that increased concentrations of brain KynA may contribute to the analgesic effects of non-selective NSAIDs (Schwieler et al. 2005). Second, we did not obtain measurements of COX activity or prostaglandin and thromboxane metabolites although given the healthy nature of the sample we may have had limited sensitivity. Third, since this was a pilot study, the sample size was small, and we did not perform an a priori power analysis. Fourth, KP concentrations were measured in the serum and do not necessarily extrapolate to the brain parenchyma - for a recent discussion of this issue see (Savitz 2022). Nevertheless, it is worth noting that ibuprofen was reported to increase KynA concentrations in both the hippocampus and plasma of rats (Maciejak et al. 2013). Finally, we used a single-dose design which eliminated the problem of compliance but raises the issue of whether similar effects on the KP would also occur after chronic treatment with ibuprofen.
In sum, this randomized, placebo controlled, cross-over design provides preliminary evidence that ibuprofen acutely increases the serum concentration of the neuroprotective KP metabolite, KynA. This result raises the possibility that ibuprofen could have therapeutic benefits in the context of diseases that are characterized by reductions in KynA such as mood disorders. Further, the data raise the possibility that ibuprofen could be used as an experimental tool to manipulate the KP in order to better understand its role in the pathophysiology of neurological and psychiatric disorders.
FUNDING
This work was supported by the William K. Warren Foundation, the National Institute of General Medical Sciences (grant number: P20GM121312 to MPP), and the National Institute of Mental Health (grant number: R01MH123652 to JS).
Footnotes
STATEMENT OF INTEREST
None
REFERENCES
- Agudelo LZ, Femenia T, Orhan F, Porsmyr-Palmertz M, Goiny M, Martinez-Redondo V, Correia JC, Izadi M, Bhat M, Schuppe-Koistinen I, Pettersson AT, Ferreira DM, Krook A, Barres R, Zierath JR, Erhardt S, Lindskog M, Ruas JL (2014) Skeletal muscle PGC-1alpha1 modulates kynurenine metabolism and mediates resilience to stress-induced depression. Cell 159: 33–45. [DOI] [PubMed] [Google Scholar]
- Ascherio A, Schwarzschild MA (2016) The epidemiology of Parkinson’s disease: risk factors and prevention. Lancet Neurol 15: 1257–1272. [DOI] [PubMed] [Google Scholar]
- Bartoli F, Misiak B, Callovini T, Cavaleri D, Cioni RM, Crocamo C, Savitz JB, Carra G (2021) The kynurenine pathway in bipolar disorder: a meta-analysis on the peripheral blood levels of tryptophan and related metabolites. Mol Psychiatry 26: 3419–3429. [DOI] [PubMed] [Google Scholar]
- Bay-Richter C, Linderholm KR, Lim CK, Samuelsson M, Traskman-Bendz L, Guillemin GJ, Erhardt S, Brundin L (2015) A role for inflammatory metabolites as modulators of the glutamate N-methyl-d-aspartate receptor in depression and suicidality. Brain Behav Immun 43: 110–7. [DOI] [PubMed] [Google Scholar]
- Berk M, Mohebbi M, Dean OM, Cotton SM, Chanen AM, Dodd S, Ratheesh A, Amminger GP, Phelan M, Weller A, Mackinnon A, Giorlando F, Baird S, Incerti L, Brodie RE, Ferguson NO, Rice S, Schafer MR, Mullen E, Hetrick S, Kerr M, Harrigan SM, Quinn AL, Mazza C, McGorry P, Davey CG (2020a) Youth Depression Alleviation with Anti-inflammatory Agents (YoDA-A): a randomised clinical trial of rosuvastatin and aspirin. BMC Med 18: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk M, Woods RL, Nelson MR, Shah RC, Reid CM, Storey E, Fitzgerald S, Lockery JE, Wolfe R, Mohebbi M, Dodd S, Murray AM, Stocks N, Fitzgerald PB, Mazza C, Agustini B, McNeil JJ (2020b) Effect of Aspirin vs Placebo on the Prevention of Depression in Older People: A Randomized Clinical Trial. JAMA Psychiatry 77: 1012–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrows K, Figueroa-Hall LK, Kuplicki R, Stewart JL, Alarbi AM, Ramesh R, Savitz JB, Teague TK, Risbrough VB, Paulus MP (2022) Neuronally-enriched exosomal microRNA-27b mediates acute effects of ibuprofen on reward-related brain activity in healthy adults: a randomized, placebo-controlled, double-blind trial. Sci Rep 12: 861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuron L, Ravaud A, Neveu PJ, Miller AH, Maes M, Dantzer R (2002) Association between decreased serum tryptophan concentrations and depressive symptoms in cancer patients undergoing cytokine therapy. Molecular psychiatry 7: 468–73. [DOI] [PubMed] [Google Scholar]
- Cosgrove KT, Kuplicki R, Savitz J, Burrows K, Simmons WK, Khalsa SS, Teague TK, Aupperle RL, Paulus MP (2021) Impact of ibuprofen and peroxisome proliferator-activated receptor gamma on emotion-related neural activation: A randomized, placebo-controlled trial. Brain Behav Immun 96: 135–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Lawson MA, Kelley KW (2011) Inflammation-associated depression: from serotonin to kynurenine. Psychoneuroendocrinology 36: 426–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn AJ, Welch J (1991) Stress- and endotoxin-induced increases in brain tryptophan and serotonin metabolism depend on sympathetic nervous system activity. J Neurochem 57: 1615–22. [DOI] [PubMed] [Google Scholar]
- Edwards SR, Mather LE, Lin Y, Power I, Cousins MJ (2000) Glutamate and kynurenate in the rat central nervous system following treatments with tail ischaemia or diclofenac. J Pharm Pharmacol 52: 59–66. [DOI] [PubMed] [Google Scholar]
- Erhardt S, Lim CK, Linderholm KR, Janelidze S, Lindqvist D, Samuelsson M, Lundberg K, Postolache TT, Traskman-Bendz L, Guillemin GJ, Brundin L (2013) Connecting inflammation with glutamate agonism in suicidality. Neuropsychopharmacology 38: 743–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Chen H, Schwarzschild MA, Ascherio A (2011) Use of ibuprofen and risk of Parkinson disease. Neurology 76: 863–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guloksuz S, Arts B, Walter S, Drukker M, Rodriguez L, Myint AM, Schwarz MJ, Ponds R, van Os J, Kenis G, Rutten BP (2015) The impact of electroconvulsive therapy on the tryptophan-kynurenine metabolic pathway. Brain Behav Immun 48: 48–52. [DOI] [PubMed] [Google Scholar]
- Halaris A, Myint AM, Savant V, Meresh E, Lim E, Guillemin G, Hoppensteadt D, Fareed J, Sinacore J (2015) Does escitalopram reduce neurotoxicity in major depression? J Psychiatr Res 66–67: 118–26. [DOI] [PubMed] [Google Scholar]
- Heilman PL, Wang EW, Lewis MM, Krzyzanowski S, Capan CD, Burmeister AR, Du G, Escobar Galvis ML, Brundin P, Huang X, Brundin L (2020) Tryptophan Metabolites Are Associated With Symptoms and Nigral Pathology in Parkinson’s Disease. Mov Disord 35: 2028–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu K, Sjolander A, Lu D, Walker AK, Sloan EK, Fall K, Valdimarsdottir U, Hall P, Smedby KE, Fang F (2020) Aspirin and other non-steroidal anti-inflammatory drugs and depression, anxiety, and stress-related disorders following a cancer diagnosis: a nationwide register-based cohort study. BMC Med 18: 238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain MI, Chaudhry IB, Khoso AB, Husain MO, Hodsoll J, Ansari MA, Naqvi HA, Minhas FA, Carvalho AF, Meyer JH, Deakin B, Mulsant BH, Husain N, Young AH (2020) Minocycline and celecoxib as adjunctive treatments for bipolar depression: a multicentre, factorial design randomised controlled trial. Lancet Psychiatry 7: 515–527. [DOI] [PubMed] [Google Scholar]
- Iyengar RL, Gandhi S, Aneja A, Thorpe K, Razzouk L, Greenberg J, Mosovich S, Farkouh ME (2013) NSAIDs are associated with lower depression scores in patients with osteoarthritis. Am J Med 126: 1017 e11–8. [DOI] [PubMed] [Google Scholar]
- Javelle F, Bloch W, Knoop A, Guillemin GJ, Zimmer P (2021) Toward a neuroprotective shift: Eight weeks of high intensity interval training reduces the neurotoxic kynurenine activity concurrently to impulsivity in emotionally impulsive humans - A randomized controlled trial. Brain Behav Immun 96: 7–17. [DOI] [PubMed] [Google Scholar]
- Kadriu B, Farmer CA, Yuan P, Park LT, Deng ZD, Moaddel R, Henter ID, Shovestul B, Ballard ED, Kraus C, Gold PW, Machado-Vieira R, Zarate CA Jr. (2019) The kynurenine pathway and bipolar disorder: intersection of the monoaminergic and glutamatergic systems and immune response. Mol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapulsky L, Christos P, Ilagan J, Kocsis J (2021) The Effects of Ibuprofen Consumption on the Incidence of Postpartum Depression. Clin Neuropharmacol 44: 117–122. [DOI] [PubMed] [Google Scholar]
- Kessing LV, Rytgaard HC, Gerds TA, Berk M, Ekstrom CT, Andersen PK (2019) New drug candidates for depression - a nationwide population-based study. Acta Psychiatr Scand 139: 68–77. [DOI] [PubMed] [Google Scholar]
- Kohler O, Petersen L, Mors O, Gasse C (2015) Inflammation and depression: combined use of selective serotonin reuptake inhibitors and NSAIDs or paracetamol and psychiatric outcomes. Brain Behav 5: e00338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler-Forsberg O, C NL, Hjorthoj C, Nordentoft M, Mors O, Benros ME (2019) Efficacy of anti-inflammatory treatment on major depressive disorder or depressive symptoms: meta-analysis of clinical trials. Acta Psychiatr Scand 139: 404–419. [DOI] [PubMed] [Google Scholar]
- Lapin IP, Oxenkrug GF (1969) Intensification of the central serotoninergic processes as a possible determinant of the thymoleptic effect. Lancet 1: 132–6. [DOI] [PubMed] [Google Scholar]
- Lehrer S, Rheinstein PH (2019) Nonsteroidal anti-inflammatory drugs (NSAIDs) reduce suicidal ideation and depression. Discov Med 28: 205–212. [PubMed] [Google Scholar]
- Lim CK, Fernandez-Gomez FJ, Braidy N, Estrada C, Costa C, Costa S, Bessede A, Fernandez-Villalba E, Zinger A, Herrero MT, Guillemin GJ (2017) Involvement of the kynurenine pathway in the pathogenesis of Parkinson’s disease. Prog Neurobiol 155: 76–95. [DOI] [PubMed] [Google Scholar]
- Maciejak P, Szyndler J, Turzynska D, Sobolewska A, Kolosowska K, Lehner M, Plaznik A (2013) The kynurenine pathway: a missing piece in the puzzle of valproate action? Neuroscience 234: 135–45. [DOI] [PubMed] [Google Scholar]
- Madero M, Cano KB, Campos I, Tao X, Maheshwari V, Brown J, Cornejo B, Handelman G, Thijssen S, Kotanko P (2019) Removal of Protein-Bound Uremic Toxins during Hemodialysis Using a Binding Competitor. Clin J Am Soc Nephrol 14: 394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modoux M, Rolhion N, Mani S, Sokol H (2021) Tryptophan Metabolism as a Pharmacological Target. Trends Pharmacol Sci 42: 60–73. [DOI] [PubMed] [Google Scholar]
- Myint AM, Kim YK (2003) Cytokine-serotonin interaction through IDO: a neurodegeneration hypothesis of depression. Med Hypotheses 61: 519–25. [DOI] [PubMed] [Google Scholar]
- Myint AM, Kim YK, Verkerk R, Scharpe S, Steinbusch H, Leonard B (2007) Kynurenine pathway in major depression: evidence of impaired neuroprotection. J Affect Disord 98: 143–51. [DOI] [PubMed] [Google Scholar]
- Norden DM, McCarthy DO, Bicer S, Devine RD, Reiser PJ, Godbout JP, Wold LE (2015) Ibuprofen ameliorates fatigue- and depressive-like behavior in tumor-bearing mice. Life Sci 143: 65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor JC, Lawson MA, Andre C, Moreau M, Lestage J, Castanon N, Kelley KW, Dantzer R (2009) Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol Psychiatry 14: 511–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul ER, Schwieler L, Erhardt S, Boda S, Trepci A, Kampe R, Asratian A, Holm L, Yngve A, Dantzer R, Heilig M, Hamilton JP, Samuelsson M (2022) Peripheral and central kynurenine pathway abnormalities in major depression. Brain Behav Immun 101: 136–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh LA, Hamza M, El Gayar NH, Abd El-Samad AA, Nasr EA, Masoud SI (2014) Ibuprofen suppresses depressive like behavior induced by BCG inoculation in mice: role of nitric oxide and prostaglandin. Pharmacol Biochem Behav 125: 29–39. [DOI] [PubMed] [Google Scholar]
- Savitz J (2020) The kynurenine pathway: a finger in every pie. Mol Psychiatry 25: 131–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz J (2022) Blood versus cerebrospinal fluid: Kynurenine pathway metabolites in depression. Brain Behav Immun 101: 333–334. [DOI] [PubMed] [Google Scholar]
- Savitz J, Drevets WC, Smith CM, Victor TA, Wurfel BE, Bellgowan PS, Bodurka J, Teague TK, Dantzer R (2015) Putative neuroprotective and neurotoxic kynurenine pathway metabolites are associated with hippocampal and amygdalar volumes in subjects with major depressive disorder. Neuropsychopharmacology 40: 463–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz J, Ford BN, Yeh HW, Akeman E, Cosgrove K, Clausen AN, Martell C, Kirlic N, Santiago J, Teague TK, Irwin MR, Paulus MP, Aupperle RL (2020) Behavioral activation therapy for depression is associated with a reduction in the concentration of circulating quinolinic acid. Psychol Med: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz JB, Teague TK, Misaki M, Macaluso M, Wurfel BE, Meyer M, Drevets D, Yates W, Gleason O, Drevets WC, Preskorn SH (2018) Treatment of bipolar depression with minocycline and/or aspirin: an adaptive, 2×2 double-blind, randomized, placebo-controlled, phase IIA clinical trial. Transl Psychiatry 8: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schildkraut JJ, Kety SS (1967) Biogenic amines and emotion. Science 156: 21–37. [DOI] [PubMed] [Google Scholar]
- Schwarcz R, Bruno JP, Muchowski PJ, Wu HQ (2012) Kynurenines in the mammalian brain: when physiology meets pathology. Nature reviews Neuroscience 13: 465–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwieler L, Erhardt S, Erhardt C, Engberg G (2005) Prostaglandin-mediated control of rat brain kynurenic acid synthesis--opposite actions by COX-1 and COX-2 isoforms. J Neural Transm (Vienna) 112: 863–72. [DOI] [PubMed] [Google Scholar]
- Schwieler L, Erhardt S, Nilsson L, Linderholm K, Engberg G (2006) Effects of COX-1 and COX-2 inhibitors on the firing of rat midbrain dopaminergic neurons--possible involvement of endogenous kynurenic acid. Synapse 59: 290–8. [DOI] [PubMed] [Google Scholar]
- Schwieler L, Samuelsson M, Frye MA, Bhat M, Schuppe-Koistinen I, Jungholm O, Johansson AG, Landen M, Sellgren CM, Erhardt S (2016) Electroconvulsive therapy suppresses the neurotoxic branch of the kynurenine pathway in treatment-resistant depressed patients. J Neuroinflammation 13: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo MK, Lee JG, Park SW (2019) Effects of escitalopram and ibuprofen on a depression-like phenotype induced by chronic stress in rats. Neurosci Lett 696: 168–173. [DOI] [PubMed] [Google Scholar]
- Shave S, McGuire K, Pham NT, Mole DJ, Webster SP, Auer M (2018) Diclofenac Identified as a Kynurenine 3-Monooxygenase Binder and Inhibitor by Molecular Similarity Techniques. ACS Omega 3: 2564–2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorgdrager FJH, Vermeiren Y, Van Faassen M, van der Ley C, Nollen EAA, Kema IP, De Deyn PP (2019) Age- and disease-specific changes of the kynurenine pathway in Parkinson’s and Alzheimer’s disease. J Neurochem 151: 656–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner J, Walter M, Gos T, Guillemin GJ, Bernstein HG, Sarnyai Z, Mawrin C, Brisch R, Bielau H, Meyer zu Schwabedissen L, Bogerts B, Myint AM (2011) Severe depression is associated with increased microglial quinolinic acid in subregions of the anterior cingulate gyrus: evidence for an immune-modulated glutamatergic neurotransmission? Journal of neuroinflammation 8: 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolk P, Souverein PC, Wilting I, Leufkens HG, Klein DF, Rapoport SI, Heerdink ER (2010) Is aspirin useful in patients on lithium? A pharmacoepidemiological study related to bipolar disorder. Prostaglandins Leukot Essent Fatty Acids 82: 9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiyagaito A, Smith JL, El-Sabbagh N, Zotev V, Misaki M, Al Zoubi O, Kent Teague T, Paulus MP, Bodurka J, Savitz J (2021) Real-time fMRI neurofeedback amygdala training may influence kynurenine pathway metabolism in major depressive disorder. Neuroimage Clin 29: 102559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdonk F, Petit AC, Abdel-Ahad P, Vinckier F, Jouvion G, de Maricourt P, De Medeiros GF, Danckaert A, Van Steenwinckel J, Blatzer M, Maignan A, Langeron O, Sharshar T, Callebert J, Launay JM, Chretien F, Gaillard R (2019) Microglial production of quinolinic acid as a target and a biomarker of the antidepressant effect of ketamine. Brain Behav Immun 81: 361–373. [DOI] [PubMed] [Google Scholar]
- Warner-Schmidt JL, Vanover KE, Chen EY, Marshall JJ, Greengard P (2011) Antidepressant effects of selective serotonin reuptake inhibitors (SSRIs) are attenuated by antiinflammatory drugs in mice and humans. Proc Natl Acad Sci U S A 108: 9262–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichers MC, Koek GH, Robaeys G, Verkerk R, Scharpe S, Maes M (2005) IDO and interferon-alpha-induced depressive symptoms: a shift in hypothesis from tryptophan depletion to neurotoxicity. Mol Psychiatry 10: 538–44. [DOI] [PubMed] [Google Scholar]
- Widner B, Laich A, Sperner-Unterweger B, Ledochowski M, Fuchs D (2002) Neopterin production, tryptophan degradation, and mental depression--what is the link? Brain Behav Immun 16: 590–5. [DOI] [PubMed] [Google Scholar]
- Wurfel BE, Drevets WC, Bliss SA, McMillin JR, Suzuki H, Ford BN, Morris HM, Teague TK, Dantzer R, Savitz JB (2017) Serum kynurenic acid is reduced in affective psychosis. Transl Psychiatry 7: e1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagami T, Koma H, Yamamoto Y (2016) Pathophysiological Roles of Cyclooxygenases and Prostaglandins in the Central Nervous System. Mol Neurobiol 53: 4754–71. [DOI] [PubMed] [Google Scholar]
- Zhang L, Bao Y, Tao S, Zhao Y, Liu M (2022) The association between cardiovascular drugs and depression/anxiety in patients with cardiovascular disease: A meta-analysis. Pharmacol Res 175: 106024. [DOI] [PubMed] [Google Scholar]
- Zheng H, Teague TK, Yeh FC, Burrows K, Figueroa-Hall LK, Aupperle RL, Khalsa SS, Paulus MP, Savitz J (2022) C-Reactive protein and the kynurenic acid to quinolinic acid ratio are independently associated with white matter integrity in major depressive disorder. Brain Behav Immun 105: 180–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Zheng W, Liu W, Wang C, Zhan Y, Li H, Chen L, Li M, Ning Y (2018) Antidepressant effect of repeated ketamine administration on kynurenine pathway metabolites in patients with unipolar and bipolar depression. Brain Behav Immun 74: 205–212. [DOI] [PubMed] [Google Scholar]


