Abstract
Background:
The role of donor-derived cell-free DNA (dd-cfDNA) in screening for cardiac allograft vasculopathy (CAV) is unknown. We hypothesized that dd-cfDNA correlates with CAV, markers of inflammation, and angiogenesis in stable heart transplant (HT) recipients.
Methods:
Sixty-five HT recipients ≥2 years post-transplant, without recent rejection, were stratified by high (≥0.12%) versus low levels (<0.12%) of dd-cfDNA. A targeted amplification, next-generation sequencing assay (AlloSure®; CareDx, Inc.) was used to detect dd-cfDNA. Peripheral blood inflammatory and angiogenesis markers were assessed using a multiplex immunoassay system (Bioplex®).
Results:
Of 65 patients, 58 patients had a known CAV status and were included. Thirty had high levels of dd-cfDNA (≥0.12%), and 28 had low levels (<0.12%). CAV was present in 63% of patients with high dd-cfDNA vs. 35% with low dd-cfDNA (p = .047).Donor-specific antibodies were present in 25% of patients with high dd-cfDNA vs. 3.8% in those with low dd-cfDNA (p = .03). There were no differences in rejection episodes, inflammatory, or angiogenesis markers. Importantly, dd-cfDNA levels were not different when stratified by time post-transplant.
Conclusions:
Higher dd-cfDNA levels were associated with CAV in stable chronic HT recipients. Further studies are warranted to investigate a possible association between dd-cfDNA levels and CAV severity and whether dd-cfDNA can predict CAV progression.
Keywords: cardiac allograft vasculopathy, dd-cfDNA, donor-derived cell-free DNA
1 |. INTRODUCTION
Donor-derived cell-free DNA (dd-cfDNA) has recently been introduced as a novel marker of graft injury manifesting as acute cellular (ACR) and antibody-mediated rejection (AMR) in solid organ transplants. 1–5 Notably, among heart transplant recipients, increased levels of dd-cfDNA levels detect acute rejection before it can be detected on endomyocardial biopsy (EMB).4–6 In the pivotal multicenter, 740 patient D-OAR study, dd-cfDNA levels <0.2% had a 97% negative predictive value for AMR, and the mean dd-cfDNA level was only 0.12% in patients without pAMR1 (pathologic AMR 1).1
The AlloMap assay (CareDx, Inc., Brisbane, CA) utilizes gene expression of peripheral blood T-cell activation and is widely accepted as a noninvasive monitoring tool for ACR with a >99% negative predictive value.7 The addition of dd-cfDNA (AlloSure®, CareDx, Inc.) as a marker of allograft injury may be a valuable clinical asset.8
Cardiac allograft vasculopathy (CAV) is a leading cause of post-transplant graft failure and mortality, making routine monitoring and adjustment of immunosuppressive regimen essential. The importance of this is highlighted by the fact that early development of CAV is a marker of aggressive disease with poor clinical outcome.9 Despite advancements in imaging strategies, the standard for CAV assessment remains coronary angiography with intravascular imaging. However, the safety of invasive and noninvasive imaging is frequently limited by concomitant chronic kidney disease in the patient population at risk. 10–13 While peripheral blood biomarkers of inflammation and angiogenesis have previously been associated with CAV, a reliable biomarker to noninvasively detect CAV remains lacking.14
Inflammation and the immune system are important contributors to the development of CAV. One pathway is mediated by vascular inflammation, as evidenced by the association between increased C-reactive protein (CRP) and the development of CAV development.15 Another trigger for coronary endothelial injury is through allorecognition, whereby the recipient’s immune system recognizes non-self-human leukocyte antigens (HLA) of the transplanted heart. Further, CAV has been linked with T-cell activation, formation of donor-specific antibodies, endothelial cell activation, and altered cytokine expression. Likewise, HLA mismatching, rejection episodes, and anti-endothelial antibody formation have all also been associated with CAV development. The consideration that CAV is deeply intertwined with antibody-mediated rejection is an area of active interest.16–22
This raises the possibility of dd-cfDNA serving as marker for CAV and a potential means to noninvasively diagnose this pathology.23,24
With knowledge that dd-cfDNA (AlloSure®, CareDx, Inc.) has demonstrated the ability to identify of de-novo DSA and allograft rejection,25 we hypothesized that dd-cfDNA levels would correlate with markers of inflammation, angiogenesis, and CAV in a stable post-transplant population in the absence of acute graft rejection.
2 |. METHODS
2.1 |. Study design
Clinically stable heart transplant recipients ≥2 years post-transplant were enrolled between August 2017 and February 2019 in a cross-sectional study design. Exclusion criteria were ACR or AMR in the preceding 6 months, infection requiring treatment in the preceding 2 months, active malignancy receiving treatment, and multi-organ transplantation. The study was a pilot proof of concept study and was not powered for clinical outcomes.
On the day of enrollment, a one-time blood sample was obtained. A targeted amplification, next-generation sequencing assay (AlloSure®; CareDx, Inc.) was used to detect dd-cfDNA. Peripheral blood protein expression of interluekin-6 (IL-6), interluekin-18 (IL-18), tumor necrosis factor-alpha (TNF-α), soluble Fas-ligand (sFASL), angiopoetin-2, vascular endothelial growth factor (VEGF) A, C and D and transforming growth factor-alpha (TGF-α) was assessed using a multiplex immunoassay system (Bioplex®).
CAV status as well as post-transplant data including AMR (Defined using 2013 ISHLT pAMR criteria)26 and ACR episodes (defined ≥ Grade 1R/1B using a combination of the 1990 and 2004 ISHLT Criteria),27,28 graft function, presence of de-novo DSA (new DSA after transplant), and graft function were obtained from the electronic medical record.
Significant CAV was defined as ≥ISHLT CAV 1 on angiography or Stanford class III-IV on intravascular ultrasound (IVUS). The Stanford class is assigned by grading the most significant coronary lesion based on degree of intimal thickening and circumferential involvement.29 The University of Chicago Institutional Review Board approved this cross-sectional study. All patients provided informed consent prior to enrollment.
2.2 |. Study groups
Transplant recipients were stratified and divided into groups by high and low levels of dd-cfDNA. With the baseline of a stable patient reported as 0.07% 1, and previously reported median for low-grade AMR at 0.12%, we considered the comparison of patients with dd-cfDNA < and ≥0.12%.1
High levels of dd-cfDNA are defined as equal or above 0.12% (≥0.12%)
Low levels of dd-cfDNA are defined as below 0.12% (<0.12%)
2.3 |. Outcomes
The primary outcome was presence of cardiac allograft vasculopathy.
Secondary outcomes were peripheral blood levels of angiogenesis and inflammatory markers.
To analyze longitudinal fluctuations in dd-cfDNA levels, we stratified patients by time post-transplant (2–5 years, 5–10 years, ≥ 10 years).
2.4 |. Statistical analysis
Continuous variables were expressed as a median with interquartile range and compared using the Mann-Whitney U test. Categorical variables were compared using the chi-square test or Fisher’s exact test as appropriate. We stratified patients into two groups based on a dd-cfDNA level of 0.12% (≥0.12% vs. <0.12%) based on the previously described cutoff to detect AMR.1 To evaluate differences in outcome measures between groups, we used the Kruskal-Wallis test. All statistical analyses were performed using SPSS Statistics 22 (SPSS Inc, Chicago, IL, USA). A two-tailed p-value <.05 was considered significant.
3 |. RESULTS
3.1 |. Patient characteristics
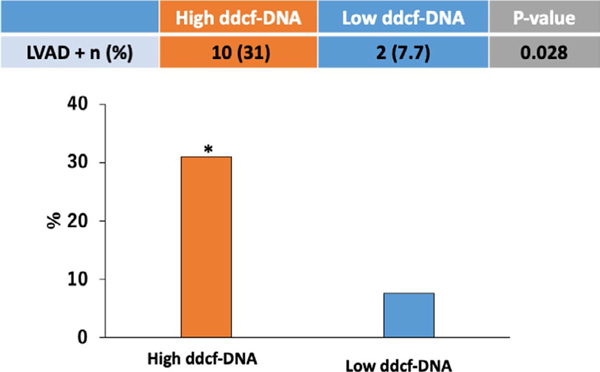
Sixty-five clinically eligible, stable heart transplant recipients were screened for enrollment. In 7 patients, the CAV status was not known, and these patients were not included in the analysis. Fifty-eight patients formed the study cohort with a median age of 61 (Interquartile Range [IQR] 52–69) years, a median time post-transplant of 90 (IQR 37–158) months, and 76 % were male (Table 1). The median level of dd-cfDNA was 0.14% with an IQR 0.06 to 0.26%. When patients were stratified based on dd-cfDNA level, 32 had high levels of dd-cfDNA (≥0.12%) and 26 had low levels (<0.12%).1 The baseline characteristics of the two groups are illustrated in Table 1. There were no differences in age, left ventricular ejection fraction, or time post-transplant between the two groups. In the high dd-cfDNA group, there was a higher proportion of patients who had an LVAD prior to transplant (31% vs. 7.7 %, p = .03) (Figure 1). The low dd-cfDNA group had a higher percentage of prior CMV viremia, 50 vs 25% (p = .049). There were no differences between the groups in the frequency of ACR (≥ ISHLT Grade 1R/1B)27,28 and AMR between the groups (p = .90 and .49 respectively).
TABLE 1.
Baseline Characteristics and group comparison of high vs low dd-cfDNA
| n=58 | High ddc-fDNA (≥0.12%, n=32) | Low dd-cfDNA (<0.12%, n=26) | p-value | |
|---|---|---|---|---|
| Age, years | 61 (52, 69) | 61 (48, 69) | 64 (59, 69) | .28 |
| LVEF, % | 63 (58, 66) | 63 (60, 66) | 60 (55, 66) | .43 |
| Time post-HT, months | 90 (37, 158) | 92 (53, 158) | 83 (32, 158) | .44 |
| Male, n (%) | 44 (76) | 23 (72) | 21 (81) | .43 |
| AA, n (%) | 27 (47) | 14 (44) | 13 (50) | .32 |
| ICM, n (%) | 19 (33) | 13 (41) | 6 (23) | .16 |
| Prior LVAD, n (%) | 12 (20) | 10 (31) | 2 (7.7) | .028* |
| History of CMV viremia, n (%) | 21 (36) | 8 (25) | 13 (50) | .049* |
| DSA+, n (%) | 9 (16) | 8 (25) | 1 (3.8) | .033* |
| ACR >1A, n (%) | 31 (53) | 17 (53) | 14 (54) | .94 |
| AMR, n (%) | 2 (3.4) | 2 (6.3) | 0 (0) | .5 |
| CAV+, n (%) | 29 (50) | 20 (63) | 9 (35) | .047* |
Abbreviations: AA, African American; ACR, Acute Cellular Rejection (ISHLT 1990); AMR, Antibody-mediated rejection; CAV, Cardiac allograft vasculopathy; CMV, Cytomegaly virus; dd-cfDNA, Donor-derived Cell-free DNA; DSA, Donor-specific antibodies; ICM, Ischemic cardiomyopathy; LVAD, Left ventricular assist device; LVEF, left ventricular ejection fraction.
p < .05.
FIGURE 1.
Patients with high levels of dd-cfDNA ≥ 0.12 had higher percentage of bridge to transplant with LVAD (31 vs. 7.7%, p 0.028). dd-cfDNA—Donor-derived Cell-free DNA; LVAD—left ventricular assist device
3.2 |. Cardiac allograft vasculopathy
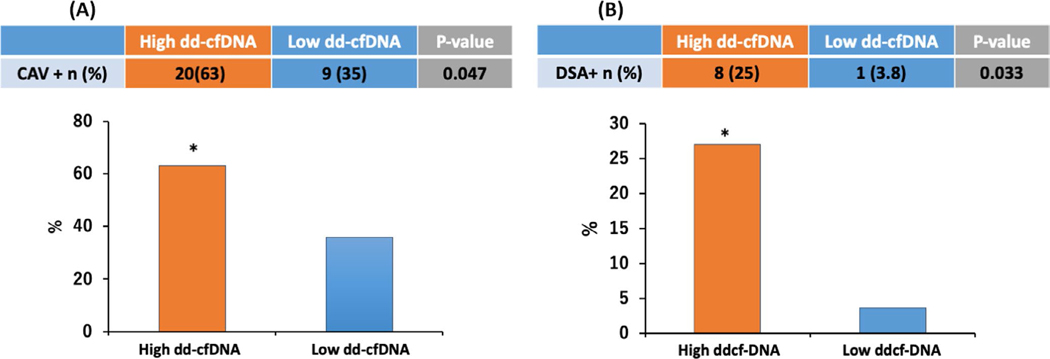
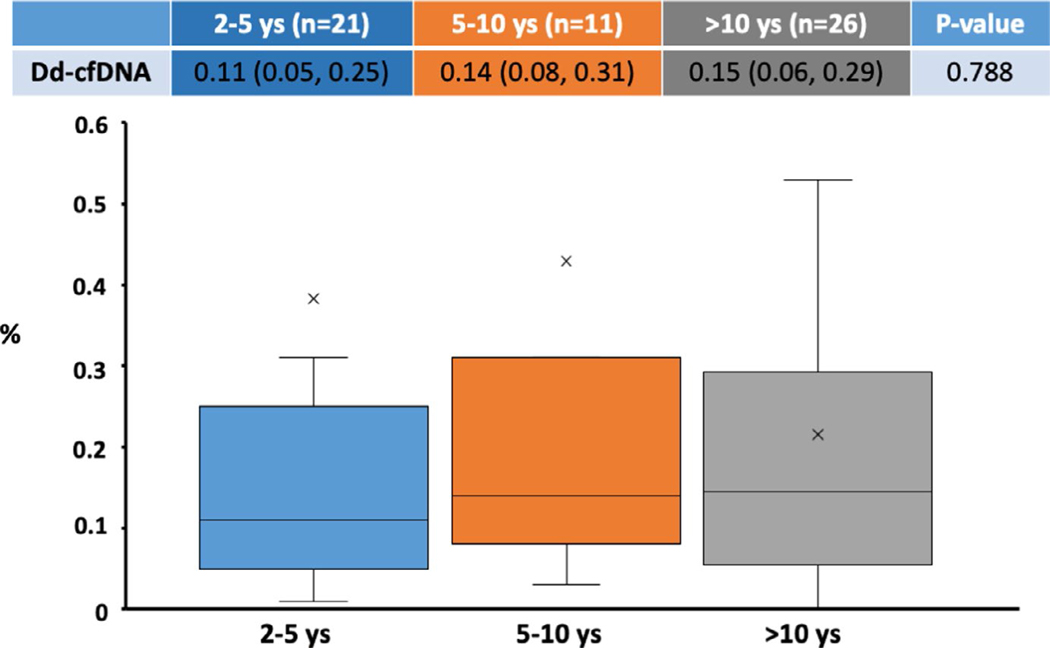
Significant CAV was present in 63% of patients with high levels of dd-cfDNA compared to 35% in the low dd-cfDNA group (p = .047, Table 1, Figure 2a). Graft function was preserved in both groups. Additionally, the proportion of patients with the presence of de-novo DSA formation was 25% in the high dd-cfDNA group as compared to 3.8% in the low dd-cfDNA group (p = .033, Table 1, Figure 2b). Notably, when stratified by time post-transplant (grouped by 2–5 years, 5–10 years, ≥ 10 years) dd-cfDNA fractions were similar across the groups (0.11% [IQR 0.05–0.25] vs. 0.14% [IQR 0.08–0.31] vs. 0.15% [IQR 0.06–0.29], p = .79) (Figure 3).
FIGURE 2.
A, When stratified for levels of dd-cfDNA below or above the median patients with high levels of dd-cfDNA ≥ 0.12 were significantly more likely to have CAV 63% vs. 35% when compared to those with low levels of dd-cfDNA (p 0.047). CAV—Cardiac allograft vasculopathy; dd-cfDNA—Donor-derived Cell-free DNA. B, Patients with high levels of dd-cfDNA ≥ 0.12 had higher percentage of de-novo DSA formation (25 vs. 3.8%, p 0.033). DSA—Donor-specific antibodies; dd-cfDNA—Donor-derived Cell-free DNA.
FIGURE 3.
When analyzed for time post-transplant, there were no significant differences in dd-cfDNA levels for times 2–5 years, 5–10, and >10 years post-transplant 0.11 (0.05, 0.25), 0.14 (0.08, 0.31), and 0.15 (0.06, 0.29) p 0.788
3.3 |. Inflammatory and angiogenesis markers
Interleukin-18 levels were statistically lower in the high dd-cfDNA group (p = .049). There were no significant differences in any of the other inflammatory and angiogenesis factors between the high and low dd-cfDNA groups (Table 2).
TABLE 2.
Inflammatory and angiogenesis markers
| pg/ml | High dd-cfDNA (≥0.12%, n=32) | Low dd-cfDNA (<0.12%, n=26) | p-value |
|---|---|---|---|
| IL-6 | 1.22 (0.29, 1.70) | 0.85 (0, 1.89) | .74 |
| IL-18 | 24.98 (19.15, 40.53) | 34.47 (27.17, 48.10) | .049* |
| TNF-a | 1.91 (1.44, 2.87) | 2.26 (1.22, 2.68) | .84 |
| sFASL | 24.01 (12.08, 76.49) | 69.76 (18.87, 91.34) | .066 |
| Ang-2 | 350.31 (123.10, 659.18) | 480.61 (274.96, 869.39) | .075 |
| VEGF-A | 58.05 (41.16, 76.66) | 75.61 (49.83, 98.65) | .13 |
| VEGF-C | 154.97 (109.74, 376.44) | 283.85 (151.58, 462.97) | .123 |
| VEGF-D | 79.02 (53.42, 178.47) | 155.71 (84.27, 176.70) | .12 |
| TGFa | 3.11 (2.43, 8.83) | 5.09 (3.42, 9.92) | .29 |
Abbreviations: Ang-2, Angiopoetin-2; dd-cfDNA, Donor-derived Cell-free DNA; IL-18,
Interluekin-18; IL-6, Interluekin-6; sFASL, Soluble Fas-ligand; TGF-α, Transforming growth factor-alpha; TNF-α, Tumor necrosis factor-alpha; VEGF, Vascular endothelial growth factor A, C and D.
p < .05.
4 |. DISCUSSION
In the current study, we reported presence of CAV and levels of inflammatory and angiogenic biomarkers in patients with high vs. low levels of dd-cfDNA. The main results are (1) In a stable transplant population, CAV was more prevalent in patients with high levels of dd-cfDNA; (2) High levels of dd-cfDNA were associated with an increased prevalence of de-novo DSA; (3) dd-cfDNA levels do not vary with time post-transplant; and (4) Biomarkers of inflammation and angiogenesis were not associated with increased fractions of dd-cfDNA in stable heart transplant recipients.
4.1 |. dd-cfDNA for CAV monitoring
Cell-free DNA is the most specific marker of graft injury since the donor genome is unique from the recipient.4,30 Several studies of heart transplant recipients showed that a significant increase in dd-cfDNA was correlated with acute rejection episodes, and importantly prior to clinical or biochemical manifestation of rejection.4–6,8 Recently, Khush et al. reported results of a multicenter study validating dd-cfDNA for both detection of AMR and ACR with an AUC of 0.64 and estimated NPV of 97.1% and PPV of 8.9%. The majority of the tests were done during months 3–12 post-transplant (81%). Notably, the median fraction of dd-cfDNA in the setting of mild AMR was 0.12%.1 Both ACR and AMR are associated with the development of CAV, which is present in 50% of patients at 10 years post-transplant and remains a major limiting factor to long-term survival post-transplantation.31,32 Currently, the standard of care for CAV screening is coronary angiography with intravascular imaging, although there has been significant development of noninvasive screening modalities, including positron emission tomography, perfusion magnetic resonance imaging, and coronary computed tomography angiography (CCTA).10–13 In a meta-analysis of 615 patients, CCTA was shown to have excellent sensitivity, specificity, and NPV for the detection of angiographic CAV.33 However its widespread use is limited by the need for ionizing contrast, which is problematic in the chronic heart transplant population due to prevalent chronic kidney disease.34,35 High sensitivity troponin has been shown to have diagnostic value for severe CAV and to predict adverse outcomes, but its value in the diagnosis of earlier forms of CAV is unknown. 36,37 Furthermore, troponin assays may be difficult to interpret in long-term transplant patients with CKD, whereas dd-cfDNA can assess myocardial injury and is not impacted by kidney function.38,39
This pilot study found an association between elevated dd-cfDNA ≥0.12% and the presence of CAV, indicating that CAV should be considered as a potential etiology of elevated dd-cfDNA in the absence of rejection. Further work to validate this pattern and assess dd-cfDNA as a biomarker to complement noninvasive imaging in the screening for subclinical CAV is encouraged, as well the potential to quantify and guide immunosuppression using dd-cfDNA when considering potential treatments as another area of future work. 40,41 Neither drift nor an increase in the baseline dd-cfDNA levels have been demonstrated, differentiating it from AlloMap. Importantly, we found no increase in dd-cfDNA as a function of time post-transplant when stratified by years post-transplant (grouped by 2–5 years, 5–10 years, ≥ 10 years, Figure 3). However, individual long-term trends of dd-cfDNA are not known.1 Our data support this stability, considering dd-cfDNA observation in a cross-sectional analysis across a wider span of post-transplant follow-up.
A potential use of dd-cfDNA could be to inform about the future risk of CAV development. Immune activation events in the early post-transplant course are known risk factors for CAV development. Recently, ACR ≥2R and class II DSA during the first post-transplant year have been identified as independent predictors of CAV trajectories.42 Consequently, serial early assessments of ddcf-DNA possibly reflecting these immune activation events could be useful to further inform about an individual’s risk of CAV later in the post-transplant course pending future longitudinal studies.
4.2 |. CAV and DSA
Development of de-novo class II DSA post-transplant is associated with CAV and adverse outcomes.43 And it has recently been hypothesized that early elevations of dd-cfDNA could be a risk factor for de-novo DSA. 44 In our study, we found that patients with high dd-cfDNA levels were more likely to have de-novo DSA, although the small numbers in this study precluded further stratification by human leukocyte antigen class or mean fluorescence intensity (MFI). Furthermore, we did not find a difference in the prevalence of prior AMR among patients with high or low levels of DSA. However, there were only two cases of AMR recorded in the entire cohort. It is possible that episodes of subclinical AMR have been missed in this cohort, which underlines the clinical benefit of serial dd-cfDNA assessments. Interestingly, we also found that patients with dd-cfDNA ≥0.12% had a higher percentage of prior LVAD use. This is in line with the well-established sensitizing effect of LVAD both as a consequence of blood transfusions and device-specific sensitizing effect. However, the clinical significance of allo-sensitization in LVAD patients is debated and might not affect mortality.45,46 The subgroup of patients bridged to transplant with LVAD may benefit from serial dd-cfDNA monitoring.
Whether the relationship of dd-cfDNA and CAV is confounded by DSA is difficult to interpret due to the sample size, but importantly the data support the hypothesis that the overall immunological composite of molecular inflammation, allograft injury, and de-novo DSA support the immunological etiology of CAV, showing it is very much intertwined and related. The directionality regarding these associations needs further mechanistic studies. 47
4.3 |. Angiogenesis/Inflammation and CAV
Cardiac allograft vasculopathy results from complex interaction between chronic immune activation via alloimmune and non-immune mechanisms leading to concentric and longitudinal intimal hyperplasia in the coronary arteries.36
In line with pathologies already outlined, complement activation with C3d deposition also predicts onset of CAV, interestingly C3d deposition in peritubular capillaries indicates renal allograft rejection, in addition to C4d, and so whether the same is true in heart transplant adds to the idea the CAV may be part of the allograft rejection spectrum.48,49 Graft endothelial cells express HLA Class I molecules, and host dendritic cells constantly present donor alloantigen leading to T-cell activation. Activated T cells invade the graft and contribute to the ongoing smoldering subendothelial immune activation and endothelial dysfunction via pro-inflammatory cytokine release.41,48,50 Infiltrating macrophages produce inflammatory cytokines, growth factors, and lead to matrix deposition as well as smooth muscle proliferation and neo-intima fibrosis.51,52
Endothelial injury and repair have previously been implicated in the pathogenesis of CAV. In a mouse model, vascular endothelial growth factor (VEGF) inhibition was shown to reduce severity and incidence of CAV, while attenuating myocardial edema and neo-angiogenesis.53 Daly et al. found vascular endothelial growth factor (VEGF)-C, VEGF-A, and platelet factor-4 (PF-4) as significant independent biomarkers of angiographically documented CAV (area under the curve [AUC] = 0.98; p < .001).14 However in our cohort, we did not find a difference in angiogenesis factors including VEGF-A, C, and D when stratified for high and low levels of dd-cfDNA. Our cohort was followed for a shorter time than the previous study (7.5 years vs. 12 years), potentially accounting for this difference. 14
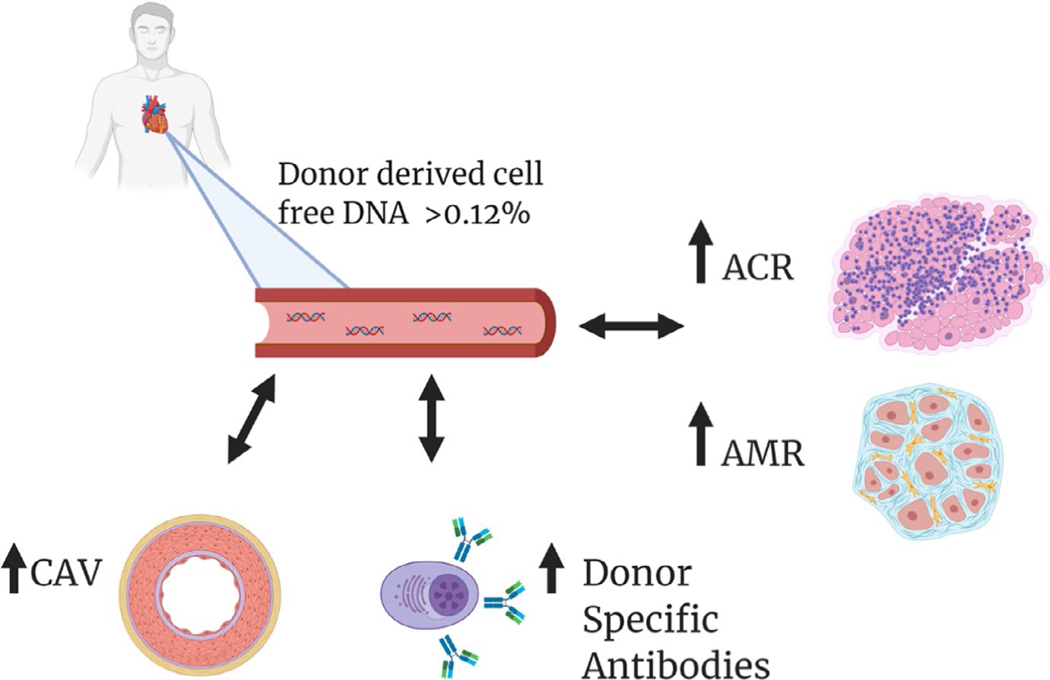
Our results are hypothesis generating and do not allow for a mechanistic link to CAV in the absence of an acute coronary event. We speculate that the higher dd-cfDNA levels in patients with CAV as a sign of chronic endothelial injury even in the clinical stable transplant recipient without graft dysfunction or active myocardial ischemia. Figure 4 provides an overview of our novel findings and previously reported implications of dd-cfDNA in heart transplant recipients.
FIGURE 4.
Central Figure, Elevated dd-cfDNA>0.12% is a marker of graft injury and can detect both ACR and AMR early post-transplant. These early elevations in dd-cfDNA have been implied as a potential risk factor for de-novo DSA, which are associated with development of CAV and adverse outcomes. We now show that de-novo DSA formation is indeed more common in long-term transplant recipients with elevated dd-cfDNA> 0.12% and importantly that these high levels of dd-cfDNA are associated with CAV. In these stable, long-term transplant recipients, dd-cfDNA might be elevated as a consequence of persistent endothelial injury even in the absence of graft dysfunction or active myocardial ischemia. Thus, dd-cfDNA possibly along with de-novo DSA formation could add to noninvasive screening and risk evaluation for CAV. DSA—Donor-specific Antibodies, ACR—Acute Cellular Rejection (ISHLT 1990), AMR—Antibody-mediated Rejection, CAV—Cardiac allograft vasculopathy, dd-cfDNA—Donor-derived Cell-free DNA
4.4 |. Inflammatory factors
Rosen et al. recently reported an association between serum inflammatory markers and CAV severity.54 Since our cohort included only stable heart transplant patients without recent rejection, it is not surprising that we did not find an association between inflammatory markers and dd-cfDNA levels other than the lower level of IL-18 in patients with dd-cfDNA>0.12%, which is of unclear significance. It is conceivable, yet speculative, that stable transplant recipients have lower baseline inflammatory markers than healthy controls given immunosuppressive therapy. Along this thought it is notable that our stable transplant patients irrespective of dd-cfDNA level had lower levels of TNF-a and IL-6 than healthy adults as previously reported.55 However, it has previously been shown that elevated serum C-reactive protein levels were associated with CAV and were responsive to statin therapy. 15,56,57 Thus in the future when investigating dd-cfDNA as a serial CAV screening test, it would be reasonable to further investigate a correlation with inflammatory markers.
4.5 |. Limitations
This is a single-center analysis with a limited sample size of 58 patients. However, these patients form a very well-characterized, stable cohort of long-term transplant survivors. Due to a small number of patients with CAV, we cannot analyze correlation of dd-cfDNA with CAV severity. This is also applicable to de-novo DSA formation.
In this study, we have obtained one-time samples of dd-cfDNA. It is important to note that despite inclusion of only clinically stable HT recipients there are inherent limitations to a single measurement given variabilities in immunological stability. Thus, future serial analysis of ddcf-DNA levels would provide additional information.
Importantly, the level of 0.12% to discriminate between high and low levels of dd-cfDNA chosen in this study does not immediately imply clinical practicability. More so, we describe an association between a higher percentage of CAV with higher dd-cfDNA levels in long-term transplant survivors. Interestingly, the median dd-cfDNA level in our 58 patients’ cohort was 0.14% with an IQR 0.06 to 0.26% without significant fluctuations amid increasing time post-transplant. Larger studies are needed to inform about trends of dd-cfDNA in long-term transplant survivors prior to establishing specific values.
5 |. CONCLUSION
In a cohort of stable transplant recipients >2 years post-HT, dd-cfDNA levels were associated with CAV and may identify those who will benefit from invasive assessment. Furthermore, CAV should be considered in the differential diagnosis of elevated dd-cfDNA. Inflammatory and angiogenesis cytokines were not associated with levels of dd-cfDNA in a stable post-transplant population. These results call for longitudinal studies to investigate the prognostic value of serial dd-cfDNA levels for the development and progression of CAV, and to assess whether dd-cfDNA levels are associated with CAV severity.
ACKNOWLEDGEMENTS
The authors would like to thank CareDx, Inc and the heart transplant recipients who generously participated in this study. The authors would also like to acknowledge BioRender for providing templates and the platform that were used for creating Figure 4.
Funding information
This study was conducted with the support of CareDx.
Footnotes
CONFLICT OF INTEREST
KJC is supported by the National Heart, Lung, and Blood Institute (Grant K23HL148528).
REFERENCES
- 1.Khush KK, Patel J, Pinney S, et al. Noninvasive detection of graft injury after heart transplant using donor-derived cell-free DNA: A prospective multicenter study. Am J Transplant. 2019;19(10):2889–2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agbor-Enoh S, Wang Y, Tunc I, et al. Donor-derived cell-free DNA predicts allograft failure and mortality after lung transplantation. EBioMedicine. 2019;40:541–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agbor-Enoh S, Tunc I, De Vlaminck I, et al. Applying rigor and reproducibility standards to assay donor-derived cell-free DNA as a non-invasive method for detection of acute rejection and graft injury after heart transplantation. J Heart Lung Transplant. 2017;36(9):1004–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beck J, Oellerich M, Schulz U, et al. Donor-Derived Cell-Free DNA Is a Novel Universal Biomarker for Allograft Rejection in Solid Organ Transplantation. Transplant Proc. 2015;47(8):2400–2403. [DOI] [PubMed] [Google Scholar]
- 5.Hidestrand M, Tomita-Mitchell A, Hidestrand PM, et al. Highly sensitive noninvasive cardiac transplant rejection monitoring using targeted quantification of donor-specific cell-free deoxyribonucleic acid. J Am Coll Cardiol. 2014;63(12):1224–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Vlaminck I, Valantine HA, Snyder TM, et al. Circulating cell-free DNA enables noninvasive diagnosis of heart transplant rejection. Sci Transl Med. 2014;6(241):241ra77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pham MX, Teuteberg JJ, Kfoury AG, et al. Gene-expression profiling for rejection surveillance after cardiac transplantation. N Engl J Med. 2010;362(20):1890–1900. [DOI] [PubMed] [Google Scholar]
- 8.Grskovic M, Hiller DJ, Eubank LA, et al. Validation of a Clinical-Grade Assay to Measure Donor-Derived Cell-Free DNA in Solid Organ Transplant Recipients. J Mol Diagn. 2016;18(6):890–902. [DOI] [PubMed] [Google Scholar]
- 9.Broichhausen C, Riquelme P, Geissler EK, Hutchinson JA. Regulatory macrophages as therapeutic targets and therapeutic agents in solid organ transplantation. Curr Opin Organ Transplant. 2012;17(4):332–342. [DOI] [PubMed] [Google Scholar]
- 10.Daly KP, Dearling JL, Seto T, et al. Use of [18F]FDG Positron Emission Tomography to Monitor the Development of Cardiac Allograft Rejection. Transplantation. 2015;99(9):e132–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bravo PE, Bergmark BA, Vita T, et al. Diagnostic and prognostic value of myocardial blood flow quantification as non-invasive indicator of cardiac allograft vasculopathy. Eur Heart J. 2018;39(4):316–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barthelemy O, Toledano D, Varnous S, et al. Multislice computed tomography to rule out coronary allograft vasculopathy in heart transplant patients. J Heart Lung Transplant. 2012;31(12):1262–1268. [DOI] [PubMed] [Google Scholar]
- 13.Torres HJ, Merello L, Ramos SA, et al. Prevalence of cardiac allograft vasculopathy assessed with coronary angiography versus coronary vascular ultrasound and virtual histology. Transplant Proc. 2011;43(6):2318–2321. [DOI] [PubMed] [Google Scholar]
- 14.Daly KP, Seifert ME, Chandraker A, et al. VEGF-C, VEGF-A and related angiogenesis factors as biomarkers of allograft vasculopathy in cardiac transplant recipients. J Heart Lung Transplant. 2013;32(1):120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hognestad A, Endresen K, Wergeland R, et al. Plasma C-reactive protein as a marker of cardiac allograft vasculopathy in heart transplant recipients. J Am Coll Cardiol. 2003;42(3):477–482. [DOI] [PubMed] [Google Scholar]
- 16.Taylor DO, Edwards LB, Boucek MM, et al. Registry of the International Society for Heart and Lung Transplantation: twenty-fourth official adult heart transplant report–2007. J Heart Lung Transplant. 2007;26(8):769–781. [DOI] [PubMed] [Google Scholar]
- 17.Lindelow B, Bergh C, Lamm C, Andersson B, Waagstein F. Graft coronary artery disease is strongly related to the aetiology of heart failure and cellular rejections. Eur Heart J. 1999;20(18):1326–1334. [DOI] [PubMed] [Google Scholar]
- 18.Stoica SC, Cafferty F, Pauriah M, et al. The cumulative effect of acute rejection on development of cardiac allograft vasculopathy. J Heart Lung Transplant. 2006;25(4):420–425. [DOI] [PubMed] [Google Scholar]
- 19.Raichlin E, Edwards BS, Kremers WK, et al. Acute cellular rejection and the subsequent development of allograft vasculopathy after cardiac transplantation. J Heart Lung Transplant. 2009;28(4):320–327. [DOI] [PubMed] [Google Scholar]
- 20.Reed EF, Demetris AJ, Hammond E, et al. Acute antibody-mediated rejection of cardiac transplants. J Heart Lung Transplant. 2006;25(2):153–159. [DOI] [PubMed] [Google Scholar]
- 21.Fredrich R, Toyoda M, Czer LS, et al. The clinical significance of antibodies to human vascular endothelial cells after cardiac transplantation. Transplantation. 1999;67(3):385–391. [DOI] [PubMed] [Google Scholar]
- 22.Dunn MJ, Crisp SJ, Rose ML, Taylor PM, Yacoub MH. Antiendothelial antibodies and coronary artery disease after cardiac transplantation. Lancet. 1992;339(8809):1566–1570. [DOI] [PubMed] [Google Scholar]
- 23.Kaczmarek I, Deutsch MA, Kauke T, et al. Donor-specific HLA alloantibodies: long-term impact on cardiac allograft vasculopathy and mortality after heart transplant. Exp Clin Transplant. 2008;6(3):229–235. [PubMed] [Google Scholar]
- 24.Cocanougher B, Ballantyne CM, Pollack MS, et al. Degree of HLA mismatch as a predictor of death from allograft arteriopathy after heart transplant. Transplant Proc. 1993;25(1 Pt 1):233–236. [PubMed] [Google Scholar]
- 25.Kobashigawa JA, Patel J, Kransdorf E, et al. Does Cell-Free DNA Detect the Development of De Novo Donor Specific Antibodies. J Heart Lung Transplant. 2019;38(4):S288. [Google Scholar]
- 26.Berry GJ, Burke MM, Andersen C, et al. The 2013 International Society for Heart and Lung Transplantation Working Formulation for the standardization of nomenclature in the pathologic diagnosis of antibody-mediated rejection in heart transplantation. J Heart Lung Transplant. 2013;32(12):1147–1162. [DOI] [PubMed] [Google Scholar]
- 27.Stewart S, Winters GL, Fishbein MC, et al. Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. J Heart Lung Transplant. 2005;24(11):1710–1720. [DOI] [PubMed] [Google Scholar]
- 28.Billingham ME, Cary NR, Hammond ME, et al. A working formulation for the standardization of nomenclature in the diagnosis of heart and lung rejection: Heart Rejection Study Group. The International Society for Heart Transplantation. J Heart Transplant. 1990;9(6):587–593. [PubMed] [Google Scholar]
- 29.St Goar FG, Pinto FJ, Alderman EL, et al. Intracoronary ultrasound in cardiac transplant recipients. In vivo evidence of “angiographically silent” intimal thickening. Circulation. 1992;85(3):979–987. [DOI] [PubMed] [Google Scholar]
- 30.Tong YK, Lo YM. Diagnostic developments involving cell-free (circulating) nucleic acids. Clin Chim Acta. 2006;363(1–2):187–196. [DOI] [PubMed] [Google Scholar]
- 31.Davis MK, Hunt SA. State of the art: Cardiac transplantation. Trends Cardiovasc Med. 2014;24(8):341–349. [DOI] [PubMed] [Google Scholar]
- 32.Prada-Delgado O, Estevez-Loureiro R, Paniagua-Martin MJ, Lopez-Sainz A, Crespo-Leiro MG. Prevalence and prognostic value of cardiac allograft vasculopathy 1 year after heart transplantation according to the ISHLT recommended nomenclature. J Heart Lung Transplant. 2012;31(3):332–333. [DOI] [PubMed] [Google Scholar]
- 33.Wever-Pinzon O, Romero J, Kelesidis I, et al. Coronary computed tomography angiography for the detection of cardiac allograft vasculopathy: a meta-analysis of prospective trials. J Am Coll Cardiol. 2014;63(19):1992–2004. [DOI] [PubMed] [Google Scholar]
- 34.Soderlund C, Lofdahl E, Nilsson J, Reitan O, Higgins T, Radegran G Chronic kidney disease after heart transplantation: a single-centre retrospective study at Skane University Hospital in Lund 1988–2010. Transpl Int. 2016;29(5):529–539. [DOI] [PubMed] [Google Scholar]
- 35.Thomas HL, Banner NR, Murphy CL, et al. Incidence, determinants, and outcome of chronic kidney disease after adult heart transplantation in the United Kingdom. Transplantation. 2012;93(11):1151–1157. [DOI] [PubMed] [Google Scholar]
- 36.Garrido IP, Garcia-Lara J, Pinar E, et al. Optical coherence tomography and highly sensitivity troponin T for evaluating cardiac allograft vasculopathy. Am J Cardiol. 2012;110(5):655–661. [DOI] [PubMed] [Google Scholar]
- 37.Hofmann NP, Steuer C, Voss A, et al. Comprehensive bio-imaging using myocardial perfusion reserve index during cardiac magnetic resonance imaging and high-sensitive troponin T for the prediction of outcomes in heart transplant recipients. Am J Transplant. 2014;14(11):2607–2616. [DOI] [PubMed] [Google Scholar]
- 38.Stacy SR, Suarez-Cuervo C, Berger Z, et al. Role of troponin in patients with chronic kidney disease and suspected acute coronary syndrome: a systematic review. Ann Intern Med. 2014;161(7):502–512. [DOI] [PubMed] [Google Scholar]
- 39.McGuire AL, Urosevic N, Chan DT, Dogra G, Inglis TJ, Chakera A. The impact of chronic kidney disease and short-term treatment with rosiglitazone on plasma cell-free DNA levels. PPAR Res. 2014;2014:643189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jennings DL, Lange N, Shullo M, et al. Outcomes associated with mammalian target of rapamycin (mTOR) inhibitors in heart transplant recipients: A meta-analysis. Int J Cardiol. 2018;265:71–76. [DOI] [PubMed] [Google Scholar]
- 41.Nikolova AP, Kobashigawa JA. Cardiac Allograft Vasculopathy: The Enduring Enemy of Cardiac Transplantation. Transplantation. 2019;103(7):1338–1348. [DOI] [PubMed] [Google Scholar]
- 42.Loupy A, Coutance G, Bonnet G, et al. Identification and Characterization of Trajectories of Cardiac Allograft Vasculopathy After Heart Transplantation: A Population-Based Study. Circulation. 2020;141(24):1954–1967. [DOI] [PubMed] [Google Scholar]
- 43.Smith JD, Banner NR, Hamour IM, et al. De novo donor HLA-specific antibodies after heart transplantation are an independent predictor of poor patient survival. Am J Transplant. 2011;11(2):312–319. [DOI] [PubMed] [Google Scholar]
- 44.Jackson AM, Cochrane AB, Nathan S, et al. Elevated Donor-Derived Cell-Free DNA (ddcfDNA) as an Early Risk Factor for the Development and Persistence of De Novo Donor Specific HLA Antibody. J Heart Lung Transplant. 2018;37(4):S16–S17. [Google Scholar]
- 45.Chiu P, Schaffer JM, Oyer PE, et al. Influence of durable mechanical circulatory support and allosensitization on mortality after heart transplantation. J Heart Lung Transplant. 2016;35(6):731–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arnaoutakis GJ, George TJ, Kilic A, et al. Effect of sensitization in US heart transplant recipients bridged with a ventricular assist device: update in a modern cohort. J Thorac Cardiovasc Surg. 2011;142(5):1236–1245, 1245 e1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dholakia S, De Vlaminck I, Khush KK. Adding Insult on Injury: Immunogenic Role for Donor-derived Cell-free DNA? Transplantation. 2020;104(11):2266–2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moseley EL, Atkinson C, Sharples LD, Wallwork J, Goddard MJ. Deposition of C4d and C3d in cardiac transplants: a factor in the development of coronary artery vasculopathy. J Heart Lung Transplant. 2010;29(4):417–423. [DOI] [PubMed] [Google Scholar]
- 49.Kuypers DR, Lerut E, Evenepoel P, Maes B, Vanrenterghem Y, Van Damme B. C3D deposition in peritubular capillaries indicates a variant of acute renal allograft rejection characterized by a worse clinical outcome. Transplantation. 2003;76(1):102–108. [DOI] [PubMed] [Google Scholar]
- 50.Vasilescu ER, Ho EK, de la Torre L, et al. Anti-HLA antibodies in heart transplantation. Transpl Immunol. 2004;12(2):177–183. [DOI] [PubMed] [Google Scholar]
- 51.Billingham ME. Histopathology of graft coronary disease. J Heart Lung Transplant. 1992;11(3 Pt 2):S38–44. [PubMed] [Google Scholar]
- 52.Jansen MA, Otten HG, de Weger RA, Huibers MM. Immunological and Fibrotic Mechanisms in Cardiac Allograft Vasculopathy. Transplantation. 2015;99(12):2467–2475. [DOI] [PubMed] [Google Scholar]
- 53.Chatur S, Wong BW, Carthy JM, McManus BM. Inhibition of vascular endothelial growth factor reduces cardiac allograft vasculopathy. J Heart Lung Transplant. 2016;35(9):1124–1130. [DOI] [PubMed] [Google Scholar]
- 54.Rosen SA, Burch M, Fenton M. The Effect of Inflammation and Severity of Cardiac Allograft Vasculopathy on Coronary Artery Distensibility After Paediatric Heart Transplantation. J Heart Lung Transplant. 2018;37(4):S398–S399. [Google Scholar]
- 55.Kleiner G, Marcuzzi A, Zani V, Monasta L, Zauli G Cytokine levels in the serum of healthy subjects. Mediators Inflamm. 2013;2013:434010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pahl E, Naftel DC, Kuhn MA, et al. The impact and outcome of transplant coronary artery disease in a pediatric population: a 9-year multi-institutional study. J Heart Lung Transplant. 2005;24(6):645–651. [DOI] [PubMed] [Google Scholar]
- 57.Pethig K, Heublein B, Kutschka I, Haverich A. Systemic inflammatory response in cardiac allograft vasculopathy: high-sensitive C-reactive protein is associated with progressive luminal obstruction. Circulation. 2000;102(suppl 3):III-233–III-236. [DOI] [PubMed] [Google Scholar]