Abstract
Identification of synaptic partners is a fundamental task for systems neuroscience. To date few reliable techniques exists for whole brain labelling of downstream synaptic partners in a cell-type dependent and monosynaptic manner. Herein we describe a novel monosynaptic anterograde tracing system based on the deletion of the gene UL6 from the genome of a cre-dependent version of the anterograde Herpes Simplex Virus 1 strain H1291. Given that this knockout blocks viral genome packaging and thus viral spread, we reasoned that co-infection of a HSV H129 ΔUL6 virus with a recombinant adeno-associated virus expressing UL6 in a cre-dependent manner would result in monosynaptic spread from target cre-expressing neuronal populations. Application of this system to five non-reciprocal neural circuits resulted in labeling of neurons in expected projection areas. While some caveats may preclude certain applications, this system provides a reliable method to label postsynaptic partners in a brain-wide fashion.
Introduction:
Herpes Simplex Virus 1 (HSV), as a member of the alphaherpesvirus family, is one of the few mammalian neurotropic viruses that enters and exits the axon terminal in the course of its natural life cycle2. The retrograde and anterograde trafficking of viral particles and components necessary to achieve this feat plays a significant role in the pathological aspects of infection and have been well investigated1,3–7. These characteristics have also made HSV1 and other alphaherpesviridae attractive candidates for neuronal circuit tracing 2,8–10.
The HSV1 strain H129 has attracted particular interest due to its propensity to spread predominantly in the anterograde direction11 – that is, from a neuron to its post-synaptic neuronal partners. Given this directional bias, H129 has found use in anatomical studies labeling downstream populations from specific brain regions 9,12–24. In 2011, such techniques were advanced with the modification of H129 to be cre-dependent 25. In this H129 LSL-TK-FP virus, as it will be denoted herein, the viral thymidine kinase - required for replication in non-dividing cells – has been replaced by a codon-modified human thymidine kinase (TK) and fluorescent protein (FP) expression cassette controlled by a lox-stop-lox site (LSL). As a result, this virus can only replicate in and spread from cre-positive neurons, allowing for cell type specificity when targeting starter cells. While this virus has found useful application14,26,27, the polysynaptic nature of the H129 LSL-TK-FP virus and its rapid rate of spread makes results from these experiments difficult to analyze.
To limit spread monosynaptically, we employed a trans-complementation strategy28, in which a viral gene necessary for spread is deleted from the virus and selectively rescued in a targeted neuron population from which the virus can then spread. To avoid the pitfalls and complexities found in previous attempts to apply this strategy to HSV1 H12929,30, we targeted the viral gene UL6, which forms a dodecamer pore on the surface of the viral capsid and is required for packaging of the viral genome (Fig. 1a).31–34. Besides its necessity for the spread of infectious particles, its relatively small size, no known role in viral life cycle, low per-viral-particle copy number, and lack of any known toxicity when expressed exogenously in mammalian cells make it an attractive target for deletion and transcomplementation.
Figure 1: Functional deletion of HSV UL6 in H129 LSL-TK-TT prohibits viral spread in vitro and in vivo.

(a) Illustration of HSV packaging of viral DNA into the capsid via UL6 portal. (b) UL6 deletion scheme. The UL6 locus (top) was targeted with a GFP expression cassette (middle) flanked by regions homologous to the UL6 gene, resulting in the virus HSV H129 LSL-TK-TT ΔUL6-GFP. Following purification, targeted removal of the GFP expression cassette by CRISPR/Cas9 resulted in a frameshift after 377 amino acids of the UL6 coding sequence and premature UL6 termination (bottom). (c) Insertion of the GFP expression cassette prohibits spread of the virus. Application of H129 LSL-TK-TT ΔUL6-GFP to monolayers of vero cells, vero cells constitutively expressing UL6 (31-UL6), and vero cells transfected prior to inoculation with the plasmid pNef-coUL6. (d) Brain tissue collected 5-days after injection of HSV H129 LSL-TK-TT (left column) and HSV H129 LSL-TK-TT ΔUL6 (right column) in primary visual cortex of Sim1-cre mice. Anti-HSV antibody staining in magenta, DAPI in blue.
Our strategy for monosynaptic restriction of anterograde tracing from a specific cell type involved the following approach. Following generation a new variant of H129-LSL-TK-FP with UL6 deleted (e.g. a TdTomato expressing version - H129 LSL-TK-TT ΔUL6) and a cre-dependent UL6-expressing helper virus (e.g. AAV-nef-2N-DIO-GFP-p2a-coUL6) the reagents can be used to identify the postsynaptic partners of a cre-expressing cell type. First, AAV-nef-2N-DIO-GFP-p2a-coUL6 will be injected into a region of interest in which a “starter” cell type of interest expresses cre. This will result in expression of GFP and UL6 in future starter cells. Later, H129 LSL-TK-TT ΔUL6 is injected into the same region. The presence of cre in starter cells removes the LSL sequence allowing TK and TT expression from the HSV genome and replication of the virus only in starter cells. Starter cells are marked by the expression of both TT and GFP. Transcomplementation with UL6 further allows for the production of infectious H129 TK-TT ΔUL6 particles that spread to and infect postsynaptic neurons. The postsynaptic neurons are labeled by expression of TK and TT from the HSV genome, but the UL6 deletion prevents further spread.
Herein we describe the monosynaptic restriction of H129-LSL-TK-FP via functional UL6 deletion. Transcomplementation with AAV expressing a codon-optimized UL6 in well described non-reciprocal circuits resulted in rescue of viral spread from cre-expressing populations, as evidenced by viral labeling of neurons in expected output regions. Further, we characterize the important caveats experimenters should consider in its successful application.
Materials & Methods:
Cells and Media
Vero cells (ATCC CCL-81) were used for propagation of UL6-intact viruses. The UL6-complementing Vero cell line 31-UL6 was kindly provided by Dr. Sandra Weller. Vero cell lines were grown in 10% Fetal Bovine Serum (FBS; HyClone SH 30070.03) DMEM media (Gibco 11995–040) and 31-UL6 cells were grown in a 5% FBS Sodium Pyruvate negative DMEM media (Gibco 11956–084) with 0.25 mg/ml Geneticin (Gibco 10131–027). For plaque picking, a 1% Methylcellulose (MC; MP Biomedicals 155496) media was made with the appropriate aforementioned medias. All cells were grown at 37C and 5% C02.
Viral Production and Titering
HSV H129 LSL TK-TT and TK-GFP were kindly provided by Dr. David Anderson 25. For viral production, a single plaque was collected from a 6-well plate, freeze-thawed thrice, and applied to a 10cm plate of confluent Vero or 31-UL6 cells. Once the plate had reached 100% cytopathic effect (cpe) or cells became noticeably loose, the media was collected and clarified by centrifugation. This media was then split and applied to ten 15cm plates of the appropriate cell line. Having reached 100% cpe (typically ~3 days), the media from these plates was clarified, filtered through a 0.45um filter (Millipore SCHVU02RE) and stored at 4C for a few days or −80C if longer than a week. To concentrate, the viral supernatant was spun at 19.4K rpm for two hours in a Beckman-coulter SW-28 rotor. Viral pellets were resuspended in 100ul of HBSS (Gibco 14175–095) and aggregated atop a 2.5ml 20% w/v sucrose in HBSS solution. This was then spun for two hours at 21K rpm in a SW55 rotor. The supernatant was removed and the pellet allowed to dry by placing the centrifuge tube upside down on a paper towel before resuspension in 50–100ul of HBSS. Virus was stored at 4C overnight before final pellet dissolution and aliquoting. Viral aliquots were stored at −80C.
To titer virus, 2ul of viral aliquot as added to 1998ul of warmed 31-UL6 cell media and serially diluted 10-fold 6 times to produce a 10−3 to 10−9 serial dilution. 250ul of each dilution was added to 3 wells of confluent 31-UL6 cells on a 24 well plate. Wells were monitored for 3 days to track the growth of plaques. Once easily discernable, plaques were counted for the dilution condition containing less than 100 plaques and counts were averaged across the three wells. This average was used to calculate the plaque forming units (pfu) per ml. The titers of the HSV preparations used here were as follows: H129 LSL-TK-TT, 1.86 × 109 pfu/ml; H129 LSL-TK-TT ΔU6, 1.066 × 107 pfu/ml; H129 LSL-TK-GFP-ΔUL6-mCherry, 1.2 × 107 pfu/ml.
All AAVs were prepared by the Salk Vector Core and use a UL6 coding sequence codon optimized for mouse by Genscript. Titer for the AAV8-nef-2N-DIO-GFP-p2a-coUL6 virus used for GPi, and dSub injections was 3.22 × 1012 GC/mL. Titer for the AAV2-nef-2N-DIO-GFP-p2a-coUL6 used for retina injections was 3.48 × 1011 GC/mL.
UL6-knockout production
To extract HSV DNA, media was first aspirated from a H129 LSL-TK-TT inoculated 15cm plate of Vero cells showing 100% cpe. 5mls of lysis buffer (1ml of 10% SDS in 49ml TE buffer, 10ug/ml DNAse-free RNAse A) was applied to the cells, swirled, and collected in a 15ml tube. 1ul of proteinase K (NEB #P8107S) was added and the solution was incubated at 37C for 90 minutes. Phenol:chloropform DNA extraction was then performed and DNA resuspended in TE buffer following purification.
The UL6 knockout was generated using a modified two-step fluorescent protein insertion and deletion selection process, as outlined previously 35. For initial interruption of the UL6 gene, a linearized GFP expression cassette flanked upstream and downstream, respectively, by 1500bp and 1000bp regions homologous to the target site of integration in the HSV genome was transfected with PEI into a 6 well plate of 31-UL6 cells at a 1:3 ratio with H129 LSL-TK-TT DNA. This expression cassette was designed to split the UL6 coding sequence, as we found that full replacement the UL6 gene resulted in inhibition of viral growth (data not shown), likely due to disruption of cis-acting regions governing the expression of the neighboring UL5 and UL7 genes. Six days later the cells were collected, resuspended in 0.4ml of cold dPBS, and aliquoted to 100ul. The volume of each aliquot was brought up to 1ml with cold dPBS and stored at −80. A single aliquot was freeze-thawed three times in liquid nitrogen and a 37C water bath before being diluted 10−2, 10−3, and 10−4 in cold dPBS. 400ul of each dilution was added to two wells of a 6 well plate of confluent 31-UL6 cells and the media was replaced with 1% MC media one hour after inoculation. Within 3 days fluorescent plaques were visible.
To pick plaques, MC media was aspirated and the cells carefully washed with warm dPBS, followed by the addition of 1 – 2 mls of warm dPBS to the well. As many as six plaques were picked and transferred to PCR tubes, where the volume was brought up to 10ul with dPBS. Three rounds of freeze/thaw were performed using liquid nitrogen and a 37C water bath, and the viral plaque solution was diluted to 10−1, 10−2, and 10-3. Between 2 and 4ul of each dilution was applied to a single well of a 24 well plate of 31-UL6 cells, and the media was switched with 1% MC media one hour after inoculation. This plaque picking protocol was repeated every 2 to 4 days for at least 3 rounds past the point at which all plaques were fluorescent.
Once functional deletion of UL6 was confirmed, the fluorescent protein used for selection was removed similarly. In an effort to remove as much of the UL6 coding region as possible without interfering with cis-acting regions important for UL5 and UL7 regulation, six Cas9 guide RNA were designed targeting regions within the UL6 coding region, three upstream and three downstream of the inserted fluorescent protein expression cassette (Supp. Table 1). Guide sequences were cloned into CRISPR/Cas9 expression plasmid pX458 and 1 ug of each upstream and downstream plasmid combination was transfected with PEI in each well of a 12 well plate of 31-UL6 cells. Twenty-four hours after transfection, 0.1 MOI of H129 LSL-TK-TT ΔUL6-GFP or HSV H129 LSL-TK-GFP ΔUL6-mCherry was applied to each well. Once all cells displayed cytopathic effect, the cells were collected and the plaque picking of fluorescence negative plaques began as previously described.
Mice and Viral Injections
All procedures involving live animals were approved by the Institutional Animal Care and Use Committees at the Salk Institute for Biological Studies, Massachusetts Institute of Technology, and Cold Spring Harbor Laboratory.
The following mouse lines were used: Som-ires-Cre (Jackson Labs 013044), Slc17a6:Cre (Jackson Labs 016963), Sim1:Cre KJ18 (MGI:4367070), Ntsr1:Cre (MMRRC # 030648-UCD), and PV:Cre (Jackson Labs 017320). Male and female mice were used and injected between the ages of 45 and 101 days.
Tissue was collected 5 days after HSV injection in experiments using Slc17a6:Cre and Sim1:Cre mice to compare H129 LSL-TK-TT and H129 LSL-TK-TT ΔUL6 viruses. For transcomplementation experiments, all HSV injections occurred two weeks following AAV injection, and tissue was collected seven days after HSV injection. All HSV and AAV injections in the brain were performed using pressure and delivered a volume of 200nl for each virus. Som-ires-Cre mice were used to target globus pallidus internal segment using the stereotactic coordinates AP −1.1, ML 2.1, DV 4.2; Slc17a6:Cre mice were used to target dorsal subiculum using the stereotactic coordinates AP −4.04, ML 2, DV 1.5; primary visual cortex injections of Ntsr1:Cre and Sim1:Cre mice used the stereotactic coordinates AP −3.28, ML 2.5, DV 0.5; Slc17a6:Cre mice were used to target the subthalamic nucleus using the stereotactic coordinates AP −1.8, ML 1.4, DV −4.7; and PV:Cre mice were used to target the posterior substantia innominata with stereotactic coordinates AP −0.58, ML 1.75, DV −4.5. Intravitreal retina injections in Slc17a6:Cre mice were performed by creating a small incision with the tip of a 27G needle posterior to the scleral line and delivering 300nl of AAV via pressure. Retinal injections of HSV were 200nl. All injections were conducted using pulled glass pipettes (VWR 53432–706, approximate inner diameter of 0.357mm microns).
Tissue Processing and IHC
Mice were anesthetized with euthasol and intracardially perfused using 50mls of PBS and 100mls of 4% paraformaldahyde (PFA). Following perfusion, brains were dissected and placed in 5mls of 2% PFA and 15% sucrose in PBS at 4C until having sunk in this solution. Brains were then transferred to 5mls of 30% sucrose in PBS at 4C. Following sinking, brains were cut on a microtome at 50um and slices were stored in solution of 1% NaN3 in PBS.
Eyes were dissected along with the brain following perfusion and put in PBS. Retinas were immediately dissected and placed in 4% PFA for one hour at 4C. After being fixed, retinas were stored in 1% NaN3 in PBS at 4C until staining.
Tissue staining started with a 5 minutes wash of 0.1% Triton X-100 in PBS followed by a 2 to 3 hour block comprised of 5% normal donkey serum in 0.5% Triton X-100 in PBS at room temperature. Primary staining occurred overnight at 4C. Primary antibodies including rabbit anti-dsRed (Takara 632496), rabbit anti-HSV (Novus NB 120–9533), and goat anti-GFP (Rockland 600–101-215) were applied at 1:1000 in block. Following primary staining, tissue was washed for 10 minutes in PBS three times. Secondary antibodies were diluted at 1:500 in PBS and applied to the tissue for 2 to 3 hours at room temperature. Secondary antibodies included Alexaflour 568 donkey anti-rabbit (Invitrogen A10042), Alexaflour 647 donkey anti-rabbit (Invitrogen A31573), and Alexaflour 488 donkey anti-goat (Invitrogen A11055). Following secondary staining, tissue was washed thrice for 10 minutes in PBS. Ten micromolar DAPI was applied for 10 minutes following by three five-minute PBS washes. Tissue was stored in 1% NaN3 in PBS at 4C prior to mounting.
AAV/HSV recombination
Early transcomplementation experiments using an AAV-nef-DIO-GFP-p2a-coUL6 construct led to frequent GFP expression in off-target neuron populations when compared with injections of the AAV helper virus alone. As this fluorescence appeared to be mediated by the HSV, we hypothesized that cre-dependent recombination across the loxp sites present in both the HSV loxp-stop-loxp cassette and AAV DIO construct were resulting in integration of the GFP, and potentially coUL6, coding sequences that are present between the loxp sites in the AAV. We found this to be the case (Supp. Fig. 2c & d) and redesigned our DIO construct to use the orthogonal Cre recognition sites lox2722 and loxn in a construct deemed AAV2-nef-2N-DIO-GFP-p2a-coUL6. Users should take special care to avoid using constructs containing the loxp motif to avoid similar issues.
AAV DIO plasmids utilizing different orthogonal lox pairs were transfected into Vero cells 48 hours prior to H129 LSL-TK-TT ΔUL6 application at an MOI of 1. Forty-eight hours after viral application media from these wells was collected and clarified by centrifugation at 300rpm. 1ml of this media was applied to a confluent well of a 6-well plate of Vero cells. Images were taken 48 hours later using a dSLR camera mounted on a fluorescent microscope.
Flow cytometry
The shuttle plasmid used in the production of the HSV H129 LSL-TK-TT virus was kindly provided by Dr. David Anderson. 1ug of both the pHSV-CAG-LSL-TK-2a-TT plasmid and pAAV-EF1α-iCre were transfected by PEI into a single well of a 6-well plate of HEK 293 cells. As a control, 1 ug of both pAAV-nef-DIO-tdTomato and pAAV-Ef1α-iCre were transfected in a separate well in an identical fashion. Forty-eight hours after transfection cells were trypsinated, collected in 2ml Eppendorf tubes, and spun at 500 rcf for 3 minutes. The media was aspirated and the cellular pellet resuspended in 1ml of cold 1% FBS in dPBS. The cells were spun down again, the media aspirated, and cells resuspended in 1ml of cold 10um DAPI in 1% FBS in dPBS. Cells were spun down, the media aspirated, and cells resuspended in 500ul of cold 1% FBS in dPBS. This solution was moved to filter-topped tubes (Falcon 352235) on ice and run on a LSRII in the Salk Flow Cytometry Core Facility.
Results:
Functional deletion of the UL6 gene in the H129 LSL-TK-TT virus leads to restriction of viral spread
The HSV1 protein UL6 is required for formation of the viral capsid pore, necessary for viral genome packaging and, therefore, production and spread of infectious particles31. Starting with the tdTomato version of the cre-dependent H129 virus (H129 LSL-TK-TT), we performed a knockout of the UL6 gene using a two-step selection process (Fig. 1b) which began with the insertion of a GFP expression cassette into the middle of the UL6 locus. Following several rounds of purification, the virus was applied to monolayers of Vero cells, Vero cells constitutively expressing UL6 (31-UL6 cells), and Vero cells transfected with a plasmid expressing a codon-optimized UL6 (Vero + pNef-coUL6; Fig 1c). The absence of plaques in Vero cells indicates that the GFP expression cassette insertion impairs viral spread (Fig 1c, left). Further, viral spread was rescued by UL6 transcomplementation, as GFP+ plaques were found in conditions in which UL6 expression was present either by constitutive expression or plasmid transfection (Fig 1c, center & right), indicating that restriction of viral spread is a function of UL6 disruption.
To test if spread of the ΔUL6 virus is also restricted in vivo, both the original H129 LSL-TK-TT and H129 LSL-TK-TT ΔUL6 virus were injected into the visual cortex of Sim1:Cre mice, which selectively express cre in sparse layer 5 cortical cells sending projections to superior colliculus. Having found tdTomato expression to be weak in the H129 LSL-TK-TT virus, likely due in part to the repetitive nature of the tdTomato gene and the high recombination rate of HSV (Supp. Fig. 1), we used an HSV antibody to label infected cells when using this virus. Five days following injection of H129 LSL-TK-TT into primary visual cortex diffuse, spread was observed throughout the brain, most notably in cortex, thalamus, hippocampus, and other subcortical areas (Fig 1d, left column). H129 LSL-TK-TT ΔUL6 injection resulting primarily in HSV antibody labeling near the injection site as well as some labelling in known regions containing cre-positive cells and providing input to V1, such as contralateral cortex (Fig 1d, right column), likely due to expected axonal uptake and retrograde spread 25. These results indicate that the ΔUL6 virus is unable to spread from the original site of infection in vivo.
Transcomplementation of the H129 LSL-TK-TT ΔUL6 virus with an AAV expressing UL6 leads to anterograde monosynaptic spread in well described circuits
To test if transcomplementation of the ΔUL6 virus in vivo leads to monosynaptic spread of the virus, while ensuring that any identified spread was not due to direct or transneuronal retrograde spread, we targeted three well described circuits containing long distance projections with no known direct reciprocity: retinal ganglion cells (RGCs; cell type VGLUT2; mouse line Slc17a6:Cre) to lateral geniculate nucleus (LGN) and superior colliculus (SC) 36; globus pallidus internal (GPi; cell type somatostatin; mouse line Som:Cre) to lateral habenula (LH) 37,38; and dorsal subiculum (dSub; cell line VGLUT2; mouse line Slc17a6:Cre) to mammillary body 39–41.
To better segregate starter cells from output cells to which the HSV has spread, we utilized a cre-dependent AAV system and an orthogonal fluorescent protein, in this case GFP, to differentiate AAV and HSV infected cells (Fig. 2a). Due to the large portion of the original UL6 coding region still present in the H129 LSL-TK-TT ΔUL6 virus and the propensity of the HSV genome to recombine with DNA containing homologous regions, we codon optimized the UL6 coding region for the mouse, termed here coUL6, to decrease the sequence homology of the UL6 gene present in the complementing AAV from that in the HSV genome. This modification would likely impart the further benefit of improved transgene expression. Finally, orthogonal lox sites were used in the AAV virus to avoid cre-dependent recombination between AAV and HSV genome, which we found resulted in reincorporation of UL6 into the HSV genome and polysynaptic spread (Supp. Fig. 2).
Figure 2: Transcomplementation of H129 LSL-TK-TT ΔUL6 with AAV in nonreciprocal circuits results in HSV labeled neurons in anterograde nuclei.
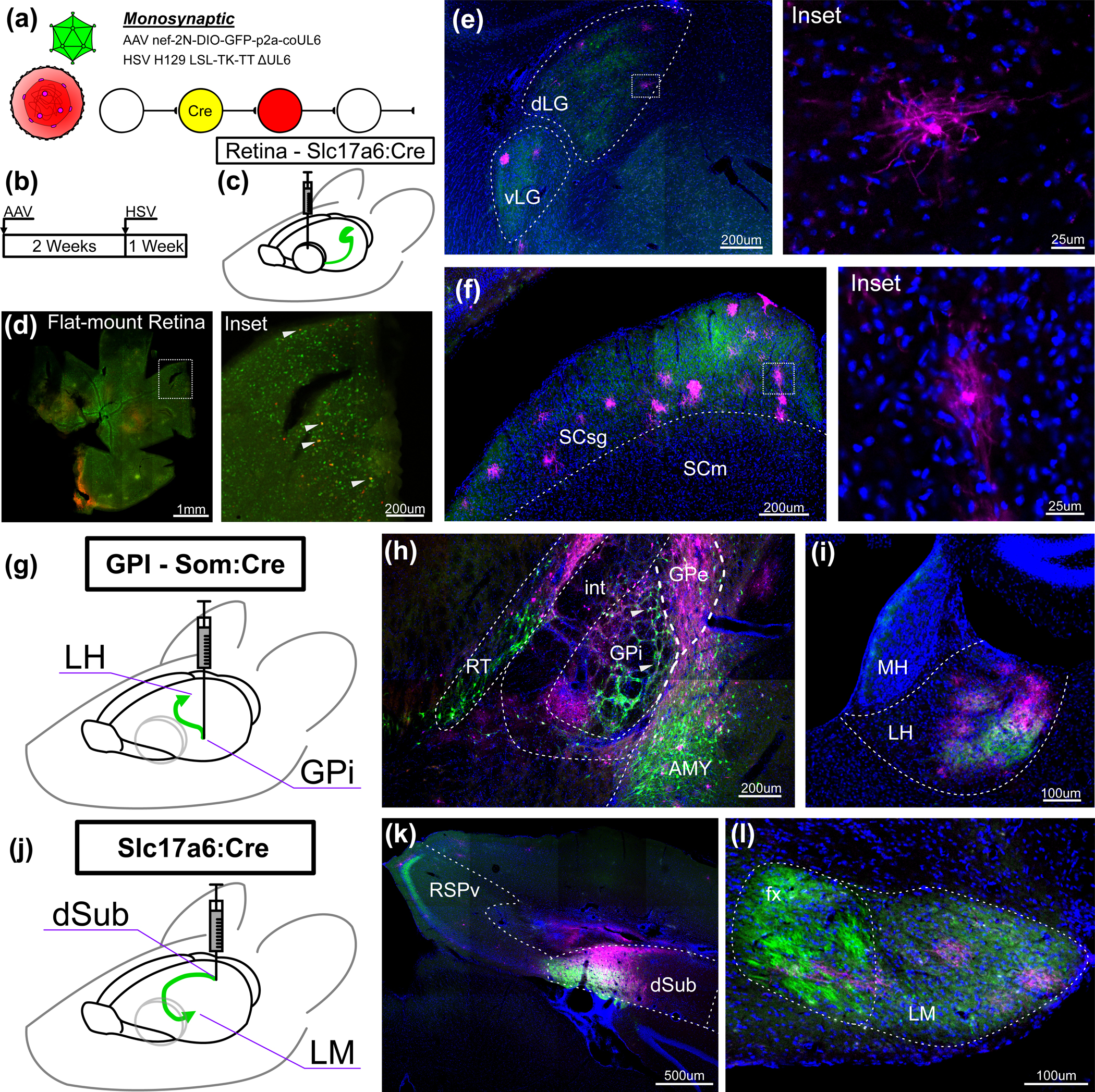
(a) Illustration of transcomplementation of H129 LSL-TK-TT ΔUL6 with AAV expression of codon-optimized UL6. (b) Experimental timeline of transcomplementation experiment. (c) Schematic of retina injection into Slc17a6:Cre mice. (d) Flat-mount retina in the right eye of a Slc17a6-cre mouse after intravitreal injection of H129 LSL-TK-TT ΔUL6. Inset shows dotted-box region of injection site; white arrows denote putative starter cells. AAV-expressed GFP in green, HSV-expressed tdTomato in red. (e) Brain slices from the same mouse showing HSV-positive neurons in visual thalamic nuclei. Example neuron shown in the inset at right. (f) Brain slices from the same mouse showing HSV-positive neurons in superior colliculus. Example neuron shown in the inset at right. (g) Schematic of experiment design targeting somatostatin positive neurons of the GPi and their postsynaptic targets in LH. (h) Injection site of AAV8-nef-2N-DIO-GFP-p2a-coUL6 and H129 LSL-TK-TT ΔUL6 in the GPi of a Som:cre mouse. White arrows denote putative starter cells. (i) Ipsilateral LH from the same mouse. (j) Schematic of experiment design targeting VGLUT2 positive neurons of the dSub and their postsynaptic targets in LM. (k) Injection site of AAV8-nef-2N-DIO-GFP-p2a-coUL6 and H129 LSL-TK-TT ΔUL6 in the dSub of a Slc17a6:Cre mouse. (l) Ispilateral LM from the same mouse. DAPI in blue, anti-HSV antibody in magenta. Tissue collected 7 days after HSV injections. dLG, dorsal lateral geniculate nucleus; vLG, ventral geniculate nucleus; LP, lateral posterior nucleus of the thalamus; SCsg, superior colliculus superficial gray layer; SCm, superior colliculus, motor related; GPe, globus pallidus external segment; GPi, globus pallidus internal segment; int, internal capsule; RT, reticular thalamus; AMY, amygdalar nuclei; MH, medial habenula; LH, lateral habenula; RSPv, ventral retrosplenial cortex; POST, postsubiculum; dSub, dorsal subiculum; fx, columns of the fornix; LM, lateral mammillary body.
All transcomplementation experiments were conducted by injecting AAV-nef-2N-DIO-GFP-p2a-coUL6 two weeks prior to injection of H129 LSL-TK-TT ΔUL6. Tissue was collected seven days later. Both viruses were injected in the vitreous of the right eye of Slc17a6:Cre mice (Fig. 2b & c). Flat mount retina (Fig. 2d) revealed widespread GFP and tdTomato positive RGCs, indicative of AAV and HSV infection, respectively, and successful recombination leading to transgene expression. RGCs that are both red and green indicate putative starter cells (Fig 2d, inset). In the brain, HSV antibody staining in visual thalamus (Fig 2e) and superior colliculus (Fig 2f) revealed HSV positive neurons amongst GFP positive axons. While cell death was evident in some HSV infected neurons by membrane blebbing or lack of a cell body, non-neuronal HSV labelling was rare and appeared to be associated with glial response to dying neurons. Healthy neurons with discernable morphology were abundant amongst HSV labeled cells (Fig 2e & f, inset). Control injections, in which no AAV virus was injected, were conducted in two animals and found no evidence of spread (Supp. Fig. 3).
The same injection schedule was performed in Som:Cre mice targeting the GPi, which contains Som+ neurons sending projections to the LH (Fig 2g) 37,38. Labeling at the injection site showed both GFP and HSV positive neurons in the target region (Fig. 2h). GFP positive axons were found in LH amongst which many HSV positive neurons were also found (Fig. 2i). While some AAV and HSV infection was found in regions surrounding the injection site, particularly by AAV in the central amygdala nucleus, we have no reason believe this expression would lead to the noted HSV labeling in LH. No evidence of anterograde HSV spread was found in controls lacking the AAV helper virus (Supp. Fig. 4).
Injections were performed targeting VGLUT2 cells of the dSub using a Slc17a6:Cre mouse line. This population sends projections to the LM from which they receive no known direct reciprocal connection (Fig. 2j) 39–41. Transcomplementation experiments yielded GFP and HSV positive cells in dSub (Fig. 2k). Some HSV positive cells were also found amongst GFP positive axons projecting from this population to ventral retrosplenial cortex (RSPv), but due to the possibility of locally reciprocal connectivity between RSPv and dSub these cells may be the result of direct retrograde spread from the injection site and are not submitted here as evidence of monosynaptic anterograde spread. At the terminus of the columns of the fornix, which the projections from dSub to the LM comprise, HSV+ neurons are found amongst GFP+ axons in the LH (Fig. 2l), indicating the expected anterograde transynaptic spread from dSub neurons. As with the two previously tested circuits, no evidence of transsynaptic spread were found in the control injections of H129 LSL-TK TT ΔUL6 in this circuit.
These data collectively indicate monosynaptic restriction of the H129 LSL-TK-TT ΔUL6 virus from the targeted cre-expressing populations when complemented by an AAV helper virus expressing UL6.
Transcomplementation of H129 LSL-TK-TT ΔUL6 does not result in retrograde monosynaptic spread.
Previous studies have indicated that HSV H129 can travels in a transneuronal retrograde manner, however in a less efficient and delayed manner10,42–44. While it is apparent that there is direct axonal uptake of HSV1 viral particles from the injection site (direct retrograde spread), we endeavored to measure the extent to which transsynaptic retrograde spread was occurring in our system. To test this, we assessed whether H129 LSL-TK-TT ΔUL6 could spread retrogradely from trans-complemented starter neurons in the LGN to RGCs. The AAV helper virus was injected in the LGN of CRH:Cre mice, which selectively express cre in the visual thalamus. Two weeks later, H129 LSL-TK-TT was injected into primary visual cortex (Fig. 3a) so that the virus could retrogradely infect cre positive neurons in LGN via their axon terminals. This approach removed the possibility of any HSV labelling in RGCs as the result of direct retrograde spread from an LGN injection, while allowing for the possibility of transsynaptic retrograde spread following UL6 transcomplentation (Fig. 3b). Tissue was collected seven days after HSV injection. HSV antibody labelling at the injection site (Fig. 3c) and in LGN (Fig. 3d) are indicative of successful retrograde spread and overlap with the AAV injection, including HSV and GFP-expressing starter cells. Flat mounted retinas stained with the HSV antibody were unable to detect any labelled cells (Fig. 3e & f). While absence is by no means proof, we were unable to identify any instance of retrograde transsynaptic spread in this experiment, nor in any of the other circuits tested.
Figure 3: HSV H129 can travel by direct, but not transsynaptic, retrograde spread.

(a) Viral surgery overview. Injection of AAV8 nef-2N-DIO-GFP-p2a-coUL6 into the LGN of a CRH:Cre mouse, with restricted expression of Cre in the thalamus, was followed two weeks later by injection of HSV H129 LSL-TK-TT ΔUL6 into visual cortex. (b) Experimental overview. Direct axonal uptake of HSV infects LGN neurons that were infected directly by the AAV helper virus. HSV is injected in V1, and not LGN, to avoid direct retrograde infection of retinal ganglion cells. As such, viral spread found in the retina must come from transsynaptic retrograde spread. (c) HSV injection site in V1. (d) AAV injection site in LGN. Magenta labelling is the result of direct retrograde HSV spread. (e) The left and (f) right retinas, flat mounted. Tissue collected 7 days post-injection. HSV antibody staining in magenta, GFP in green, and DAPI in blue. V1, primary visual cortex; LGv, lateral geniculate nucleus, ventral; LGd, lateral geniculate nucleus, dorsal; LP, lateral posterior nucleus.
Cre-dependent activation of TK leads to rapid cell death and UL6 knock-out manipulates viral transgene expression
Cell death is an obvious hallmark of replication competent neurotropic viruses, HSV being no exception. While loose tissue and abscesses are common in experiments using the polysynaptic virus H129 LSL-TK-TT (Fig. 1d), dense DAPI staining indicative of gliosis was commonly seen at the injection sites of the ΔUL6 version of the virus (Fig. 4a). Cell death was evident in postsynaptic neurons labeled by HSV as well: LGN labeled neurons resulting from transcomplementation of H129 LSL-TK-TT ΔUL6 in the retina of Slc17a6 displayed both healthy morphologies and cytopathic effect (Fig. 4b) evident both in gross morphology and increased local DAPI density.
Figure 4: Transcomplementation leads to rapid cell death while UL6 knockout leads to latent-like state.
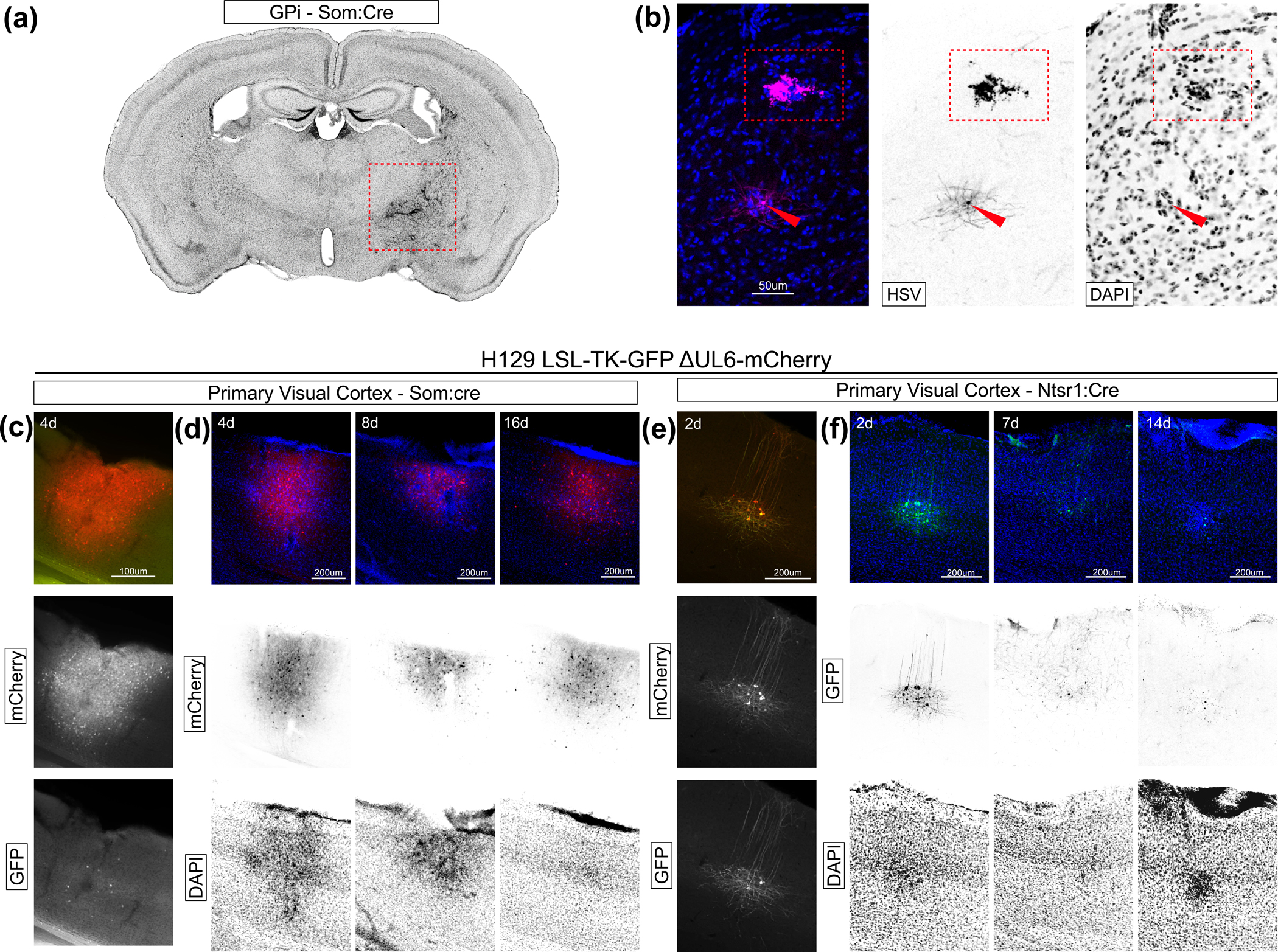
(a) Whole brain slice showing condensed DAPI staining at the injection site (dotted box). 7 days post injection of H129 LSL-TK-TT ΔUL6, 21 days post injection of AAV8 nef-2N-DIO-GFP-2A-coUL6. Injection targeting GPi. (b) Example HSV labeled neurons present in LGN after transcomplementation in retina of Slc17a6-cre mouse showing a live cell (red arrow) and a dead cell (red box). Anti-HSV antibody in magenta, DAPI in blue. Tissue collected 7 days after injection. Channels split at right. (c-f) Primary visual cortex tissue following injection of H129 LSL-TK-GFP ΔUL6-mCherry. (c) Fluorescent protein expression in tissue from a Som:Cre mouse four days after injection. GFP in green, mCherry in red. Channels split below. (d) Tissue collected at 4, 8, and 16 days post injection. GFP channel not shown. mCherry in red, DAPI in blue. Channels split below. (e) Fluorescent protein expression in tissue from a Ntsr1:Cre mouse two days after injection. GFP in green, mCherry in red. Channels split below. (f) Primary visual cortex tissue following injection of H129 LSL-TK-GFP ΔUL6-mCherry in Ntsr1-cre mice. Tissue collected 2, 7, and 14 days post injection. mCherry channel not shown. DAPI in blue, GFP in green. Split channels below.
We reasoned that cre-dependent TK expression, which was necessary for viral gene expression and replication, was responsible for cell death. To test this, we used H129 LSL-TK-GFP ΔUL6-mCherry, a GFP version of the cre-dependent H129 constitutively expressing mCherry in the UL6 locus. This virus should express TK and GFP only in cre positive cells, and mCherry in all infected cells, allowing us to probe the rate of cell death in a TK-dependent manner.
H129 LSL-TK-GFP ΔUL6-mCherry virus was injected into the primary visual cortex of Som:Cre (Som+ inhibitory cortical neurons) and Ntsr1:Cre (layer 6 corticothalamic neurons) mice and tissue was collected at varying time points. These two cell lines were selected due to their distinct morphologies and distributed sparse and compact distributions, respectively. Surprisingly, mCherry expression appeared to be restricted only to cells matching the expected morphology and distribution of the cre-positive population (Fig. 4c & e), despite the expression cassette being unrestricted and driven by a strong minimal Ef1a promoter. Further, GFP expression was initially strong but decreased across time points (Fig. 4f), suggesting rapid death or viral inactivation in these cells. On the other hand, mCherry expression remained robust (Fig. 4d), suggesting a continual latent-like infection in these cells.
These data show that cre-dependent TK expression rapidly activates the virus, likely contributing to cell death. These results further indicate two surprising outcomes: First, many cells infected with ΔUL6 virus enter a latent-like state, as the mCherry, but not GFP, is expressed in most cells after the first time point. Second, cre-activation of the TK gene is required to express from the UL6 locus, as mCherry expression, despite being driven from a strong pan-cellular promoter, is restricted to cells anatomically and morphologically consistent with the cre-expressing population. (This observation has possible implications for mechanisms of HSV suppression by TK-inhibiting drugs.) Along with the observation that trans-complementation leads to greater cell death than injection of the HSV virus alone (Supp. Fig. 3–5), these results suggest that the UL6 knockout affects the viral life cycle and transgene expression, perhaps due to buildup of unpackaged HSV genomes.
A GFP version of the cre-dependent HSV H129 yields improved post-synaptic labeling
Our data suggested that the weak expression found in the tdTomato version of the cre-dependent H129 virus could be due to either entry of the virus into a latent state in the absence of UL6 or due to an increased rate of recombination and thus mutation at the tdTomato locus, due to its repetitive nature and the propensity of HSV to recombine across homologous regions. To improve the labeling of post-synaptic cells without the use of an HSV antibody, we repeated the UL6 knockout in a GFP version of the cre-dependent HSV H129 virus, hypothesizing that a monomeric fluorophore would yield better expression.
We tested this virus, HSV H129 LSL-TK-GFP ΔUL6, in two nonreciprocal circuits. First, the helper virus AAV8-nef-2N-DIO-mCherry-p2a-coUL6 was injected into the subthalamic nucleus (STN) of a Slc17a6:Cre mouse followed by H129 LSL-TK-GFP ΔUL6 (Fig. 5a). As with previous transcomplementation experiments, HSV was injected two weeks after the AAV helper, and tissue was collected seven days post-HSV injection. Putative starter cells were found by GFP and mCherry colabelling in the STN (Fig. 5b & c). GFP positive cells were also found in the substantia nigra pars reticulata, a known postsynaptic partner to VGLUT2 cells of the STN 45, indicating successful anterograde monosynaptic spread.
Figure 5: Transcomplementation of H129 LSL-TK-GFP ΔUL6 with AAV in nonreciprocal circuits results in HSV labeled neurons in anterograde nuclei.

(a) Schematic of injection into the STN of Slc17a6:Cre mice. (b) Brain slice from tissue collected 8 days post-injection showing mCherry expression from the AAV8-nef-2N-DIO-mCherry-p2a-coUL6 helper virus. (c) Close up of putative starter cells expressing mCherry and GFP from the AAV and HSV viruses, respectively. (d) Brain slices from the same mouse showing GFP expression from the HSV virus in neurons of the SNr. A close-up shows example neuron in the inset at right. (e) Schematic of experiment design targeting Parvalbumin-positive neurons of the posterior Substantia Innominata (pSI) and their postsynaptic targets in S1. In this mouse, AAV helper virus and H129 LSL-TK-GFP ΔUL6 were injected in the left hemisphere, which H129 LSL-TK-GFP ΔUL6 alone was injected in the right. Tissue was collected (f) Brain slice showing the injection site of AAV8-nef-2N-DIO-mCherry-p2a-coUL6 and H129 LSL-TK-GFP ΔUL6 in the left pSI. (g) Brain slice showing GFP positive cells in left S1. (h) Brain slice showing the injection site of H129 LSL-TK-GFP ΔUL6 in the right pSI. (i) Brain slice showing no GFP positive cells in the right S1. PV, parvalbumin; STN, subthalamic nuclei; cp, cerebral peduncle; SNr, Substantia Nigra pars reticulata; pSI, posterior substantia innominata; GPi, globus pallidus internal segment; S1, somatosensory cortex.
The system was then tested in PV cells of the posterior substantia innominata (pSI), which sends projections to primary somatosentory cortex (S1). In this experiment, we injected the AAV helper and H129 LSL-TK-GFP ΔUL6 virus in the left hemisphere and the H129 LSL-TK-GFP ΔUL6 virus alone in the right hemisphere. Relative to HSV injection alone, co-injection led to extensive cell death at the injection site (Fig. 5f & h) and a few putative starter cells, displaying similar necrotic outcomes as we had seen with the tdTomato version of the virus. GFP positive cells were found in S1 of the same hemisphere as the co-injection, while no GFP positive cells were found ipsilateral to the H129 LSL-TK-GFP ΔUL6 injection alone (Fig. 5g & i).
These data confirm that the H129 LSL-TK-GFP ΔUL6 version of the virus can similarly be used for monosynaptic anterograde tracing without the need of HSV counterstaining.
Conclusions:
Identification of synaptic partners is a fundamental task of neural circuit analysis. Herein we show that the H129 LSL-TK-TT ΔUL6 and H129 LSL-TK-GFP ΔUL6 viruses can be used for cre-dependent monosynaptic anterograde spread when complemented with an AAV expressing UL6 (Fig 3 – 5). While this tool adds to the ever-growing viral toolkit available to the modern systems neuroscientist, potential users of this system should be aware of its limitations and caveats.
Previous efforts towards development of a monosynaptic anterograde tracer using a variety of viruses29,46–48 have suffered from polysynaptic spread, low reproducibility, lack of cell-type targeting, or low expression levels. Most recently, the application of the yellow fever vaccine YFV-17D has shown promising results with regards to these shortcomings49. Similarly to previous reports using HSV H129, YFV-17D exhibits delayed retrograde spread, a shortcoming the authors circumvented through application of the Doxycycline-inducible system for short-lived temporal control of transgene expression. While we were unable to detect any retrograde transsynaptic spread in our system, perhaps due to starter cell death prior to timepoints relevant to retrograde transport, we expect application of a similar system to the helper AAV would similarly ameliorate unwanted retrograde spread.
Notably, HSV H129 differs from YFV-17D in being a DNA virus, allowing for direct cre-dependent control of expression and a simplified single-AAV helper virus system. While we demonstrated the application of this system using well defined neural circuits, this system has already been applied to elucidate novel downstream populations from specific cell types 50. As such, future iterations of this system could be made to carry other genetic cargo to functionally interrogate novel neural circuits, such FLP recombinase or channelrhodopsins.
Starter cell death is likely to make manipulations that require viable pre- and postsynaptic partners difficult if not impossible. Our experiments showed cell blebbing as soon as two days after injection. Our transcomplementation experiments indicate that starter cell populations are likely to die by the time transneuronal spread is evident, as evidenced by the scarcity of clearly overlapping HSV and AAV neurons at the injection site of most experiments in which anterograde spread was evident (Fig 2 & 3). Depending on the circuit, however, the incubation time may be shortened: while all data shown here came from experiments in which tissue was collected seven days following HSV injection, transneuronal spread was evident in as few as 5 days in experiments targeting RGCs and 3 days in those targeting the Ntsr1 cortical layer 5 cell population. Finally, care should be taken to ensure that both H129 LSL-TK-TT ΔUL6 and the AAV helper do not infect cre-positive cells present in neighboring areas to the targeted tissue, as rapid starter cell death may make this source of potential false positives difficult to detect. Control injections delivering only one of the constitutive parts of the tracing system will ensure the injection volume is appropriately restricted.
Assessing the efficiency of transsynaptic tracers is difficult, as the true number of connected neurons and synapses comprising those connections is unknown. Further, the rapid rate of cell death of starter neurons and our inability to reliably differentiate cre-activated starter cells from local cre-negative neurons infected with HSV made quantifying the number of labelled pre to post-synaptic neurons impossible. Investigators may need to dial in injection and tissue collection time points from those used here to improve the efficiency in their specific circuit of interest. Further, efficiencies may vary between cell types and synapse, as evidenced by the variety in the number of cells found in the output regions of the five circuits tested here.
HSV has a strong propensity for direct retrograde infection via axonal uptake from the site of injection. In systems in which cre-expressing populations exist presynaptic to the target region, HSV LSL-TK-FP ΔUL6 uptake at those axon terminals would lead to cre-mediated viral activation and potential falsely identified postsynaptic partners. Great care should be taken in avoiding such instances and results should always be compared to expression patterns seen in control injections of the H129 LSL-TK-FP ΔUL6 virus alone.
We found that HSV has a high propensity for recombination and cre-dependent incorporation. Experimenters should avoid introducing other genomes, such as those carried by AAV, that may have high homology to the HSV genome or carry loxp sites. If cre-dependency is needed in an additional AAV, use loxN or lox2722 as used in the AAV helpers described herein.
In our hands, tdTomato signal was weak from injections of the H129 LSL-TK-TT virus and its derivatives, warranting the use of an HSV antibody to identify infected cells. While there are several possible causes of this weak expression, it is our hypothesis that the repetitive nature of the tdTomato coding sequence makes it susceptible to recombination or mutation. Potential users should use the GFP version of the virus until a version containing a monomeric red fluorescent protein is developed. While we were able to label post-synaptic partners using the GFP version of the virus without counterstaining, we encourage experimenters to use the HSV antibody with this virus to protect against false positives due to possibility of HSV ΔUL6 entering a latency-like state.
Evidence for such a latency-like state was found in control injections of H129 LSL-TK-GFP ΔUL6-mCherry. In these injections, mCherry expression, but largely not GFP, was found in cre-positive neurons. This was surprising, given that GFP expression, and not mCherry, is directly cre-dependent. Indeed, GFP expression was only found at the earliest tissue collection time points. This suggests that cre-dependent activation of thymidine kinase, which is necessary for genome replication, is rapidly inhibited as the viral life cycle progresses in the ΔUL6 viruses, perhaps due to a buildup in unpackaged genomes. It is unclear what effect, if any, this deletion-dependent latency has on viral spread, but contributes additional evidence that the UL6 virus is largely nontoxic until transcomplementation with UL6. Further, it suggests that toxicity issues may be resolved in this or similar HSV H129-based systems as our understanding of the life cycle of the virus in the CNS improves.
Supplementary Material
Supp. Figure 1: Sporadic and weak expression of tdTomato from the H129 LSL-TK-TT ΔUL6 virus but not from expression cassette used to make the virus.
Examples of tdTomato expression from the cre-activated H129 LSL-TK-TT ΔUL6 virus show (a) weak expression in HSV antibody labeled neurons in lateral habenula (LH) from transcomplementation experiments targeting somatostatin neurons of the internal segment of glubus pallidus (GPi), and stronger expression of tdtomato (b) in HSV antibody labeled neurons of the retrosplenial cortex (RSP) from transcomplementation experiments targeting VGLUT2 neurons of the dorsal subiculum (dSub). All tissue collected 7 days after HSV injection. No antibody was used against tdTomato in these preparations. (c) Flow cytometry dot plots of HEK 293 cells transfected with the indicated plasmids.
Supp. Figure 2: Recombination across loxp sites present in in HSV and AAV leads to cre-dependent integration of the GFP-p2a-coUL6 expression cassette from AAV in the H129 LSL-TK-TT ΔUL6 virus.
(a) Injections of H129 LSL-TK-TT ΔUL6 following AAV8-nef-DIO-GFP-p2a-coUL6 in primary visual cortex of Ntsr1-cre mice leads to GFP+ cells outside of layer 5. Injection of AAV8-nef-DIO-GFP-p2a-coUL6 alone (left) results in restricted expression of GFP in the target layer 5 population. A similar injection followed two weeks later (right) shows tdTomato+ and GFP+ cells outside of layer 5. (b) pAAV-nef-DIO-GFP-p2a-coUL6 design (top) and table of tested orthogonal lox combinations. “2P” is the standard DIO configuration. (c) Vero cell monolayers 48hrs after application of H129 LSL-TK-TT ΔUL6 at a MOI 1. Transfection parameters are shown above each column. pAAV test conditions in the right 3 columns. (d) Vero cell monolayers 48hrs after application of 1 ml of filtered media from each condition in (c).
Supp. Figure 3: Transcomplementation of H129 LSL-TK-TT ΔUL6 with AAV in retinal ganglion cells results in HSV labeled neurons in lateral geniculate nucleus and superior colliculus.
(a) Flat-mount retina in the right eye of a Slc17a6-cre mouse after intravitreal injection of H129 LSL-TK-TT ΔUL6. Inset shows dotted-box region of injection site. Anti-HSV antibody in magenta. Brain slices from the same mouse showing visual thalamic nuclei (b) and superior colliculus (c). DAPI in blue, anti-HSV antibody in magenta. Each channel separated below. (d) Flat-mount retina following injection of AAV8-nef-2N-DIO-GFP-p2a-coUL6 and H129 LSL-TK-TT ΔUL6 in GPi of Som-ires-cre mouse. GFP in green, tdTomato in red. Inset show dotted-box region contain double labeled neurons. Brain slices from the same mouse show HSV positive neurons amongst GFP positive axons originating from retina in lateral geniculate nucleus (e) and superior colliculus (f). GFP in green, anti-HSV antibody in magenta, DAPI in blue. Center column shows split channels. Inset shows example HSV infected neuron within the dotted box. All tissue collected 7 days after HSV injection. dLG, dorsal lateral geniculate nucleus; vLG, ventral geniculate nucleus; LP, lateral posterior nucleus of the thalamus; SCsg, superior colliculus superficial gray layer; SCm, superior colliculus, motor related.
Supp. Figure 4: Transcomplementation of H129 LSL-TK-TT ΔUL6 with AAV in somatostatin neurons of the internal segment of the globus pallidus results in HSV labeled neurons in lateral habenula.
(a) Injection site of H129 LSL-TK-TT ΔUL6 in the internal segment of globus pallidus in an Som-ires-cre mouse. (b) Habenula from the same mouse. Channels split at right. (c) Injection site of AAV8-nef-2N-DIO-GFP-p2a-coUL6 and H129 LSL-TK-TT ΔUL6 in the internal segment of globus pallidus in a Som-ires-cre mouse. (d) HSV positive neurons amongst GFP positive axons originating from globus pallidus internal segment in lateral habenula. Channels split at right. GFP in green, anti-HSV antibody in magenta, DAPI in blue. All tissue collected 7 days following HSV injection. GPe, globus pallidus external segment; GPi, globus pallidus internal segment; int, internal capsule; RT, reticular thalamus; AMY, amygdalar nuclei; MH, medial habenula; LH, lateral habenula.
Supp Figure 5: Transcomplementation of H129 LSL-TK-TT ΔUL6 with AAV in dorsal Subiculum VGLUT2 neurons results in HSV labeled neurons in mammillary body.
(a) Injection site of an H129 LSL-TK-TT injection targeting dorsal subiculum in a Slc17a6-cre mouse. (b) Columns of the fornix and mammilary body from the same mouse. Split channels at right. (c) Injection site of AAV8-nef-2N-DIO-GFP-p2a-coUL6 and H129 LSL-TK-TT ΔUL6 injections targeting dorsal subiculum in a Slc17a6-cre mouse. (d) HSV positive neurons amongst GFP positive axons originating from dSub in the mammilary body. GFP in green, anti-HSV antibody in magenta, DAPI in blue. Right column shows split channels. RSPv, ventral retrosplenial cortex; POST, postsubiculum; dSub, dorsal subiculum; fx, columns of the fornix; LM, lateral mammillary body.
Supp. Table 1: Guide RNA sequences used for removal of the GFP expression cassette from the UL6 locus for functional deletion of UL6
Acknowledgements
This work was supported by grants from the National Science Foundation (IOS-1707261) and the National Institutes of Health (EY022577, MH063912) to E.C; Stanley Center at the Broad Institute, Hock E. Tan and K. Lisa Yang Center for Autism Research at MIT, James and Patricia Poitras Center for Psychiatric Disorders Research at MIT, and NIH BRAIN Initiative (U01MH114819) to G.F.; and NINDS R01NS075531 to A.K.
Footnotes
Conflicts of Interest
All authors declare they have no conflicts of interest.
Data Availability
Data will be made available upon reasonable request from the corresponding author.
References:
- 1.Kratchmarov R, Taylor MP & Enquist LW Role of Us9 phosphorylation in axonal sorting and anterograde transport of pseudorabies virus. PLoS One 8, e58776 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Norgren RB et al. Anterograde transport of HSV-1 and HSV-2 in the visual system. Brain Res. Bull. 28, 393–399 (1992). [DOI] [PubMed] [Google Scholar]
- 3.Enquist LW, Tomishima MJ, Gross S & Smith G. a. Directional spread of an alphaherpesvirus in the nervous system. Vet. Microbiol. 86, 5–16 (2002). [DOI] [PubMed] [Google Scholar]
- 4.Diefenbach RJ, Miranda-Saksena M, Douglas MW & Cunningham AL Transport and egress of herpes simplex virus in neurons. Rev. Med. Virol. 18, 35–51 (2008). [DOI] [PubMed] [Google Scholar]
- 5.Kratchmarov R et al. Glycoproteins gE and gI are required for efficient KIF1A-dependent anterograde axonal transport of alphaherpesvirus particles in neurons. J. Virol. 87, 9431–40 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kratchmarov R, Taylor MP & Enquist LW Making the case : Married versus Separate models of alphaherpes virus anterograde transport in axons. Rev. Med. Virol 378–391 (2012) doi: 10.1002/rmv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith G. a, Gross SP & Enquist LW Herpesviruses use bidirectional fast-axonal transport to spread in sensory neurons. Proc. Natl. Acad. Sci. U. S. A. 98, 3466–70 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strack AM, Sawyer WB, Hughes JH, Platt KB & Loewy AD A general pattern of CNS innervation of the sympathetic outflow demonstrated by transneuronal pseudorabies viral infections. Brain Res. 491, 156–162 (1989). [DOI] [PubMed] [Google Scholar]
- 9.Rinaman L & Schwartz G Anterograde transneuronal viral tracing of central viscerosensory pathways in rats. J. Neurosci. 24, 2782–6 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Card JP & Enquist LW Transneuronal circuit analysis with pseudorabies viruses. Curr. Protoc. Neurosci 1–39 (2014) doi: 10.1002/0471142301.ns0105s68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dix RD, McKendall RR & Baringer JR Comparative neurovirulence of herpes simplex virus type 1 strains after peripheral or intracerebral inoculation of BALB/c mice. Infect. Immun. 40, 103–112 (1983). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vaughan CH & Bartness TJ Anterograde transneuronal viral tract tracing reveals central sensory circuits from brown fat and sensory denervation alters its thermogenic responses. Am. J. Physiol. Integr. Comp. Physiol. 302, R1049–R1058 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ryu V, Watts AG, Xue B & Bartness TJ Bidirectional crosstalk between the sensory and sympathetic motor systems innervating brown and white adipose tissue in male Siberian hamsters. Am. J. Physiol. - Regul. Integr. Comp. Physiol. 312, R324–R337 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGovern AE et al. Distinct Brainstem and Forebrain Circuits Receiving Tracheal Sensory Neuron Inputs Revealed Using a Novel Conditional Anterograde Transsynaptic Viral Tracing System. J. Neurosci. 35, 7041–7055 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Futai K, Doty CD, Baek B, Ryu J & Sheng M Specific trans-synaptic interaction with inhibitory interneuronal neurexin underlies differential ability of neuroligins to induce functional inhibitory synapses. J. Neurosci. 33, 3612–23 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ryu V, Garretson JT, Liu Y, Vaughan CH & Bartness TJ Brown Adipose Tissue Has Sympathetic-Sensory Feedback Circuits. J. Neurosci. 35, 2181–2190 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun N, Cassell MD & Perlman S Anterograde, transneuronal transport of herpes simplex virus type 1 strain H129 in the murine visual system. Anterograde, Transneuronal Transport of Herpes Simplex Virus Type 1 Strain H129 in the Murine Visual System. 70, (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dum RP, Levinthal DJ & Strick PL The Spinothalamic System Targets Motor and Sensory Areas in the Cerebral Cortex of Monkeys. J. Neurosci. 29, 14223–14235 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kelly RM & Strick PL Cerebellar Loops with Motor Cortex and Prefrontal Cortex of a Nonhuman Primate. J. Neurosci. 23, 8432–8444 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McGovern AE et al. Anterograde neuronal circuit tracing using a genetically modified herpes simplex virus expressing EGFP. J. Neurosci. Methods 209, 158–167 (2012). [DOI] [PubMed] [Google Scholar]
- 21.McGovern AE, Davis-Poynter N, Farrell MJ & Mazzone SB Transneuronal tracing of airways-related sensory circuitry using herpes simplex virus 1, strain H129. Neuroscience 207, 148–166 (2012). [DOI] [PubMed] [Google Scholar]
- 22.Song CK, Schwartz GJ & Bartness TJ Anterograde transneuronal viral tract tracing reveals central sensory circuits from white adipose tissue. Am J Physiol Regul Integr Comp Physiol 296, 501–511 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barnett EM, Evans GD, Sun N, Perlman S & Cassell MD Anterograde tracing of trigeminal afferent pathways from the murine tooth pulp to cortex using herpes simplex virus type 1. J. Neurosci. 15, 2972–2984 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang H et al. Brain-wide map of projections from mice ventral subiculum. Neurosci. Lett. 629, 171–179 (2016). [DOI] [PubMed] [Google Scholar]
- 25.Lo L & Anderson DJ A Cre-dependent, anterograde transsynaptic viral tracer for mapping output pathways of genetically marked neurons. Neuron 72, 938–50 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scott N, Prigge M, Yizhar O & Kimchi T A sexually dimorphic hypothalamic circuit controls maternal care and oxytocin secretion. Nature 525, 519–522 (2015). [DOI] [PubMed] [Google Scholar]
- 27.Padilla SL et al. Agouti-related peptide neural circuits mediate adaptive behaviors in the starved state. Nat. Neurosci. 19, 734–741 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wickersham IR et al. Monosynaptic Restriction of Transsynaptic Tracing from Single, Genetically Targeted Neurons. Neuron (2007) doi: 10.1016/j.neuron.2007.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zeng WB et al. Anterograde monosynaptic transneuronal tracers derived from herpes simplex virus 1 strain H129. Mol. Neurodegener. 12, 1–17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Su P et al. Rigorous anterograde trans-monosynaptic tracing of genetic defined neurons with retargeted HSV1 H129. bioRxiv (2020) doi: 10.1101/2020.12.01.407312. [DOI] [Google Scholar]
- 31.Lamberti C & Weller SK The Herpes simplex virus type 1 UL6 protein is essential for cleavage and packaging but not for genomic inversion. Virology 226, 403–407 (1996). [DOI] [PubMed] [Google Scholar]
- 32.Albright BS, Nellissery J, Szczepaniak R & Weller SK Disulfide Bond Formation in the Herpes Simplex Virus 1 UL6 Protein Is Required for Portal Ring Formation and Genome Encapsidation. J. Virol. 85, 8616–8624 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Newcomb WW et al. Assembly of the herpes simplex virus capsid: characterization of intermediates observed during cell-free capsid formation. J. Mol. Biol. 263, 432–46 (1996). [DOI] [PubMed] [Google Scholar]
- 34.Newcomb WW, Homa FL & Brown JC Involvement of the Portal at an Early Step in Herpes Simplex Virus Capsid Assembly Involvement of the Portal at an Early Step in Herpes Simplex Virus Capsid Assembly. 79, 10540–10546 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sawtell NM & Thompson RL Herpes Simplex Virus Mutant Generation and Dual-Detection Methods for Gaining Insight into Latent/Lytic Cycles in Vivo. 1144, 129–147 (2014). [DOI] [PubMed] [Google Scholar]
- 36.Martersteck EM et al. Diverse Central Projection Patterns of Retinal Ganglion Cells. Cell Rep. 18, 2058–2072 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wallace ML et al. Genetically Distinct Parallel Pathways in the Entopeduncular Nucleus for Limbic and Sensorimotor Output of the Basal Ganglia. Neuron 94, 138–152.e5 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shabel SJ, Proulx CD, Trias A, Murphy RT & Malinow R Input to the Lateral Habenula from the Basal Ganglia Is Excitatory, Aversive, and Suppressed by Serotonin. Neuron 74, 475–481 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roy DS et al. Distinct Neural Circuits for the Formation and Retrieval of Episodic Memories. Cell 170, 1000–1012.e19 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kinnavane L, Vann SD, Nelson AJD, O’Mara SM & Aggleton JP Collateral Projections Innervate the Mammillary Bodies and Retrosplenial Cortex: A New Category of Hippocampal Cells. Eneuro 5, ENEURO.0383–17.2018 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Witter MP Connections of the subiculum of the rat: Topography in relation to columnar and laminar organization. Behav. Brain Res. 174, 251–264 (2006). [DOI] [PubMed] [Google Scholar]
- 42.Wojaczynski GJ, Engel EA, Steren KE, Enquist LW & Patrick Card J The neuroinvasive profiles of H129 (herpes simplex virus type 1) recombinants with putative anterograde-only transneuronal spread properties. Brain Struct. Funct. 220, 1395–1420 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Archin NM, Van den Boom L, Perelygina L, Hilliard JM & Atherton SS Delayed spread and reduction in virus titer after anterior chamber inoculation of a recombinant of HSV-1 expressing IL-16. Investig. Ophthalmol. Vis. Sci. 44, 3066–3076 (2003). [DOI] [PubMed] [Google Scholar]
- 44.Archin NM & Atherton SS Infiltration of T-lymphocytes in the brain after anterior chamber inoculation of a neurovirulent and neuroinvasive strain of HSV-1. J. Neuroimmunol. 130, 117–127 (2002). [DOI] [PubMed] [Google Scholar]
- 45.Bosch C, Degos B, Deniau JM & Venance L Subthalamic nucleus high-frequency stimulation generates a concomitant synaptic excitation-inhibition in substantia nigra pars reticulata. J. Physiol. 589, 4189–4207 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Beier KT et al. Anterograde or retrograde transsynaptic labeling of CNS neurons with vesicular stomatitis virus vectors. Proc. Natl. Acad. Sci. U. S. A. 108, 15414–9 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zingg B et al. AAV-Mediated Anterograde Transsynaptic Tagging: Mapping Corticocollicular Input-Defined Neural Pathways for Defense Behaviors. Neuron 93, 33–47 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zingg B, Peng B, Huang JJ, Tao HW & Zhang LI Anterograde Trans-Synaptic AAV Strategies for Probing Neural Circuitry. bioRxiv 2019.12.24.888172 (2019) doi: 10.1101/2019.12.24.888172. [DOI] [Google Scholar]
- 49.Li E et al. Anterograde transneuronal tracing and genetic control with engineered yellow fever vaccine YFV-17D. Nat. Methods 18, 1542–1551 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang Y et al. Targeting thalamic circuits rescues motor and mood deficits in PD mice. Nature 607, 321–329 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supp. Figure 1: Sporadic and weak expression of tdTomato from the H129 LSL-TK-TT ΔUL6 virus but not from expression cassette used to make the virus.
Examples of tdTomato expression from the cre-activated H129 LSL-TK-TT ΔUL6 virus show (a) weak expression in HSV antibody labeled neurons in lateral habenula (LH) from transcomplementation experiments targeting somatostatin neurons of the internal segment of glubus pallidus (GPi), and stronger expression of tdtomato (b) in HSV antibody labeled neurons of the retrosplenial cortex (RSP) from transcomplementation experiments targeting VGLUT2 neurons of the dorsal subiculum (dSub). All tissue collected 7 days after HSV injection. No antibody was used against tdTomato in these preparations. (c) Flow cytometry dot plots of HEK 293 cells transfected with the indicated plasmids.
Supp. Figure 2: Recombination across loxp sites present in in HSV and AAV leads to cre-dependent integration of the GFP-p2a-coUL6 expression cassette from AAV in the H129 LSL-TK-TT ΔUL6 virus.
(a) Injections of H129 LSL-TK-TT ΔUL6 following AAV8-nef-DIO-GFP-p2a-coUL6 in primary visual cortex of Ntsr1-cre mice leads to GFP+ cells outside of layer 5. Injection of AAV8-nef-DIO-GFP-p2a-coUL6 alone (left) results in restricted expression of GFP in the target layer 5 population. A similar injection followed two weeks later (right) shows tdTomato+ and GFP+ cells outside of layer 5. (b) pAAV-nef-DIO-GFP-p2a-coUL6 design (top) and table of tested orthogonal lox combinations. “2P” is the standard DIO configuration. (c) Vero cell monolayers 48hrs after application of H129 LSL-TK-TT ΔUL6 at a MOI 1. Transfection parameters are shown above each column. pAAV test conditions in the right 3 columns. (d) Vero cell monolayers 48hrs after application of 1 ml of filtered media from each condition in (c).
Supp. Figure 3: Transcomplementation of H129 LSL-TK-TT ΔUL6 with AAV in retinal ganglion cells results in HSV labeled neurons in lateral geniculate nucleus and superior colliculus.
(a) Flat-mount retina in the right eye of a Slc17a6-cre mouse after intravitreal injection of H129 LSL-TK-TT ΔUL6. Inset shows dotted-box region of injection site. Anti-HSV antibody in magenta. Brain slices from the same mouse showing visual thalamic nuclei (b) and superior colliculus (c). DAPI in blue, anti-HSV antibody in magenta. Each channel separated below. (d) Flat-mount retina following injection of AAV8-nef-2N-DIO-GFP-p2a-coUL6 and H129 LSL-TK-TT ΔUL6 in GPi of Som-ires-cre mouse. GFP in green, tdTomato in red. Inset show dotted-box region contain double labeled neurons. Brain slices from the same mouse show HSV positive neurons amongst GFP positive axons originating from retina in lateral geniculate nucleus (e) and superior colliculus (f). GFP in green, anti-HSV antibody in magenta, DAPI in blue. Center column shows split channels. Inset shows example HSV infected neuron within the dotted box. All tissue collected 7 days after HSV injection. dLG, dorsal lateral geniculate nucleus; vLG, ventral geniculate nucleus; LP, lateral posterior nucleus of the thalamus; SCsg, superior colliculus superficial gray layer; SCm, superior colliculus, motor related.
Supp. Figure 4: Transcomplementation of H129 LSL-TK-TT ΔUL6 with AAV in somatostatin neurons of the internal segment of the globus pallidus results in HSV labeled neurons in lateral habenula.
(a) Injection site of H129 LSL-TK-TT ΔUL6 in the internal segment of globus pallidus in an Som-ires-cre mouse. (b) Habenula from the same mouse. Channels split at right. (c) Injection site of AAV8-nef-2N-DIO-GFP-p2a-coUL6 and H129 LSL-TK-TT ΔUL6 in the internal segment of globus pallidus in a Som-ires-cre mouse. (d) HSV positive neurons amongst GFP positive axons originating from globus pallidus internal segment in lateral habenula. Channels split at right. GFP in green, anti-HSV antibody in magenta, DAPI in blue. All tissue collected 7 days following HSV injection. GPe, globus pallidus external segment; GPi, globus pallidus internal segment; int, internal capsule; RT, reticular thalamus; AMY, amygdalar nuclei; MH, medial habenula; LH, lateral habenula.
Supp Figure 5: Transcomplementation of H129 LSL-TK-TT ΔUL6 with AAV in dorsal Subiculum VGLUT2 neurons results in HSV labeled neurons in mammillary body.
(a) Injection site of an H129 LSL-TK-TT injection targeting dorsal subiculum in a Slc17a6-cre mouse. (b) Columns of the fornix and mammilary body from the same mouse. Split channels at right. (c) Injection site of AAV8-nef-2N-DIO-GFP-p2a-coUL6 and H129 LSL-TK-TT ΔUL6 injections targeting dorsal subiculum in a Slc17a6-cre mouse. (d) HSV positive neurons amongst GFP positive axons originating from dSub in the mammilary body. GFP in green, anti-HSV antibody in magenta, DAPI in blue. Right column shows split channels. RSPv, ventral retrosplenial cortex; POST, postsubiculum; dSub, dorsal subiculum; fx, columns of the fornix; LM, lateral mammillary body.
Supp. Table 1: Guide RNA sequences used for removal of the GFP expression cassette from the UL6 locus for functional deletion of UL6
Data Availability Statement
Data will be made available upon reasonable request from the corresponding author.


