Abstract
In rheumatoid arthritis (RA), synovial tissue abundantly expresses CCL21, a chemokine strongly associated with RA susceptibility. In this study, we aimed to characterize the functional significance of CCL21/CCR7 signaling in different phases of RA pathogenesis. We determined that CCR7 is a hallmark of RA M1 synovial fluid (SF) macrophages, and its expression in RA monocytes and in vitro differentiated macrophages is closely associated with disease activity score (DAS28). In early stages of RA, monocytes infiltrate the synovial tissue. However, blockade of SF CCL21 or CCR7 prevents RA SF-mediated monocyte migration. CCR7 expression in the newly migrated macrophages can be accentuated by LPS and IFNγ and suppressed by IL-4 treatment. We also uncovered that CCL21 stimulation increases the number of M1-polarized macrophages (CD14+CD86+), resulting in elevated transcription of IL-6 and IL-23. These CCL21-induced M1 cytokines differentiate naïve T cells to Th17 cells, without affecting Th1 cell polarization. In the erosive stages of disease, CCL21 potentiates RA osteoclastogenesis through M1-driven Th17 polarization. Disruption of this intricate crosstalk, by blocking IL-6, IL-23, or IL-17 function, impairs the osteoclastogenic capacity of CCL21. Consistent with our in vitro findings, we establish that arthritis mediated by CCL21 expands the joint inflammation to bone erosion by connecting the differentiation of M1 macrophages with Th17 cells. Disease progression is further exacerbated by CCL21-induced neovascularization. We conclude that CCL21 is an attractive novel target for RA therapy, as blockade of its function may abrogate erosive arthritis modulated by M1 macrophages and Th17 cell crosstalk.
Keywords: Rheumatoid arthritis, M1 macrophages, Th17 cells, Osteoclastogenesis, CCL21, CCR7
Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune disease in which inflammatory cytokines mediate joint inflammation leading to erosive bone disease. The synovial tissue (ST) of RA patients produces excessive amounts of CCL21, primarily from fibroblasts and macrophages [1]. As shown in genome-wide association studies (GWAS), CCL21 is a susceptibility gene for RA with various gene polymorphisms having been associated with RA and psoriatic arthritis [2, 3]. Its receptor, CCR7, is also highly expressed in the RA ST, on lining and sublining macrophages and endothelial cells [4].
CCL21 recruits CCR7+ dendritic cells and T cells to lymphoid and non-lymphoid tissues [5–8]. Besides modulating immune cell infiltration, CCL21 expressed in the synovial fluid (SF) promotes angiogenesis by activating CCR7+ endothelial cell migration [4]. CCL21 also triggers angiogenesis indirectly, by activating RA fibroblasts and macrophages to secrete proangiogenic factors, such as vascular endothelial growth factor (VEGF), angiopoietin 1, and interleukin (IL)-8 [1]. These findings indicate that CCL21-stimulated immune cell migration and neovascularization collaboratively accelerate RA disease progression.
It was reported that CCL21 amplifies proliferation and antigen presentation of bone marrow (BM)-derived dendritic cells [9]. Moreover, CCL21 and CCR7 deficiencies have both been shown to prevent Th1/Th17-driven autoimmune disease, as evidenced in experimental autoimmune encephalomyelitis [10]. Immuno-neutralization or knockout of CCR7 protects mice from collagen-induced arthritis (CIA) by reducing CD3+ naïve T cell numbers and elevating the frequency of Tregs [11]. In agreement with these studies, CCL19/CCL21-deficient mice infected with Leishmania donovani produce lower IL-12 and higher IL-10 levels compared to wild-type (WT) mice [12]. Earlier studies have focused on understanding the effect of CCL21/CCR7 on T cells, but little is known about the implications of this pathway on myeloid cell function.
We and others have demonstrated that in early disease, surface expression of CCR7 is highly upregulated on monocytes [13], and as disease becomes more established, levels of this receptor are accentuated in RA ST macrophages [4]. We also show that transcriptional CCR7 expression in RA monocytes and macrophages closely correlates with patient disease activity score (DAS28). We, therefore, took a comprehensive approach to investigate the effects of CCL21/CCR7 activation on monocyte and macrophage functions in RA. Our findings indicate that local expression of CCL21 promotes joint swelling and osteoclast formation mainly through myeloid CCR7 function. In this paper, we uncover a novel mechanism by which CCL21 activation of myeloid CCR7 promotes RA monocyte migration, exacerbates joint inflammation, and causes bone destruction.
Materials and methods
Patient samples
In accordance with the protocol approved by the UIC Institutional Ethics Review Board, RA patients and healthy subjects (NL) were consented to donate peripheral blood (PB) and SF samples. RA patients were diagnosed according to the 1987 revised criteria of ACR [14]. All donors gave informed written consent. The collected samples were de-identified. An additional protocol was approved to gather information that allows for evaluation of disease activity and impact of treatment, after informed written consent by patients, as previously described [15].
To study the effect of anti-TNF treatment on CCR7 expression (Fig. 1d), PB was obtained from 68 patients, 64 women and 4 men (mean age 48.6 ± 14.6 years). At the time of evaluation, patients were either on non-biological disease-modifying anti-rheumatic drugs (DMARDs; n = 34, 3 male and 31 female, mean age 51.6 ± 16.2 years), or treatment with anti-TNF therapy (n = 34, 1 male, 33 female, mean age 45.7 ± 12.4). Treatment with DMARDs (n = 34) consisted of DMARDs alone (methotrexate, leflunomide, sulfasalazine, azathioprine, hydroxychloroquine, or minocycline) (n = 27 of which 2 were on hydroxychloroquine only), or treatment with DMARD plus prednisone (n = 7). Patients treated with anti-TNF therapy (n = 34) were either on anti-TNF therapy alone (n = 7), anti-TNF plus prednisone (n = 1), anti-TNF therapy plus DMARD (n = 21), or anti-TNF with both DMARD and prednisone (n = 5).
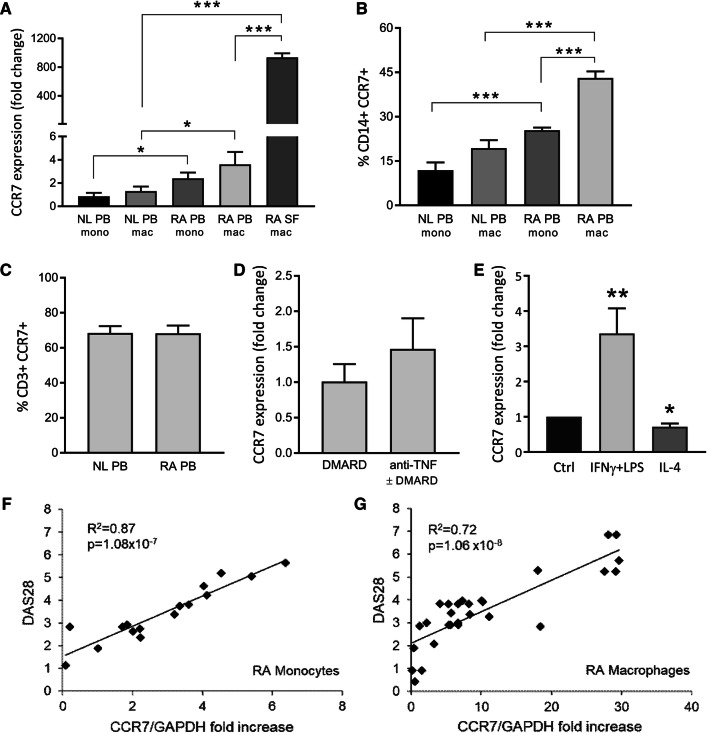
Fig. 1.
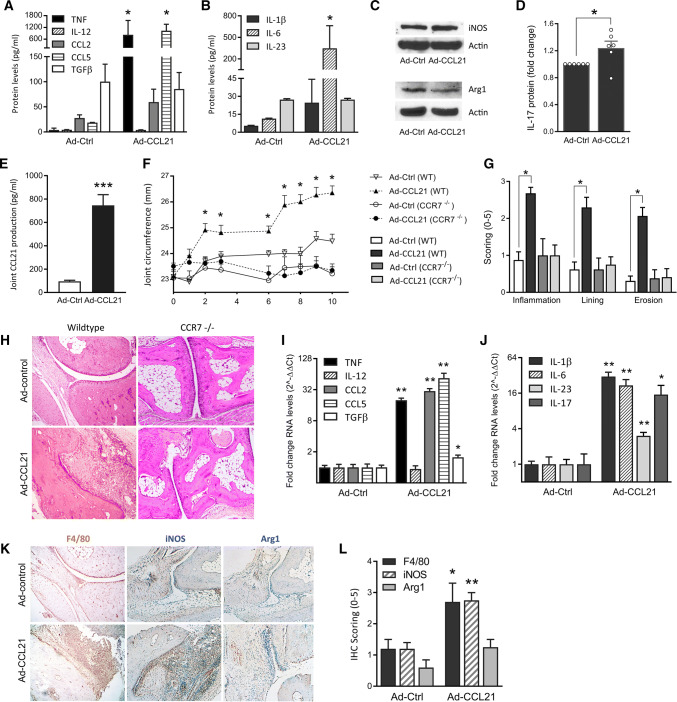
CCR7 is a marker for M1 macrophages in the synovial fluid of RA patients. PBMCs were isolated from blood (PB) and synovial fluid (SF) samples of RA patients and healthy subjects (NL). Through qRT-PCR (a) and FACS (b) analysis, expression of CCR7 was evaluated in PB monocytes (mono), in vitro differentiated macrophages (PB mac) and SF macrophages (SF mac) (a: n = 10–18; b: n = 6). c FACS analysis was also used to evaluate CCR7 expression in CD3+ T cells (n = 6). d Patient data were collected to distinguish CCR7 between patients who are and are not receiving anti-TNFα therapy (n = 34). e In vitro differentiated PB macrophages were polarized to M1 (100 ng/mL IFNγ + LPS) or M2 (100 ng/mL IL-4) and qRT-PCR was used to detect CCR7 RNA levels after polarization (n = 7). Data are presented as mean ± sem. f, g Disease activity (DAS28) was correlated to CCR7 RNA levels in RA monocytes (n = 16) and in vitro differentiated macrophages (n = 29). *p < 0.05, **p < 0.01, ***p < 0.005
To study the correlation between RA monocytes CCR7 expression and disease activity (Fig. 1f), PB was obtained from 14 women and 2 men (mean age, 55.7 ± 2.3 years). At the time of blood donation, patients were receiving no treatment (n = 1), taking DMARDs (methotrexate, leflunomide, and sulfasalazine azathioprine) alone (n = 1), taking DMARDs plus hydroxychloroquine (n = 1), taking DMARDs plus prednisone (n = 1), taking DMARDs plus rituximab (n = 3), taking DMARDs plus hydroxychloroquine and minocycline (n = 1), or taking a TNF inhibitor alone (n = 2), with a DMARD (n = 2), a DMARD plus prednisone (n = 2), or with a DMARD plus hydroxychloroquine and prednisone (n = 2).
PB mononuclear cells (PBMC) from 29 RA patients (25 women and 4 men; mean age, 51.7 ± 3.7 years) were differentiated into macrophages for 7 days to evaluate the correlation between macrophage CCR7 expression and disease activity (Fig. 1g). At the time of blood donation, patients were receiving no treatment (n = 4), taking DMARDs (methotrexate, leflunomide, and sulfasalazine azathioprine) alone (n = 2), taking DMARDs plus hydroxychloroquine (n = 3), DMARDs plus prednisone (n = 5), taking DMARDs plus rituximab (n = 3), taking DMARDs plus hydroxychloroquine plus minocycline (n = 2), or taking a TNF inhibitor alone (n = 3), with a DMARD (n = 2), a DMARD plus prednisone (n = 1), or with a DMARD plus hydroxychloroquine and prednisone (n = 4).
Cell isolation and culture
PBMCs and SF mononuclear cells were isolated by density gradient centrifugation (400g, 30 min), having been layered on Ficoll-Paque Premium (GE Healthcare). Negative selection EasySep kits (StemCell Technologies) were used as per manufacturer’s instructions to isolate monocytes or T cells. To differentiate monocytes to macrophages, adherent cells were cultured in 20% FBS/RPMI for 7 days. After overnight starvation (0% FBS/RPMI), cells were stimulated as indicated. In contrast, PBMCs or enriched T cells used for T cell polarization assays were stimulated immediately upon isolation.
Murine BM macrophages were isolated and cultured as previously described [16]. BM cells were pre-treated with 40 ng/ml mM-CSF in 10% FBS/RPMI for 4 days before use in cell induction experiments.
Flow cytometry
PB monocytes, in vitro differentiated macrophages, and T cells were stained with isotype control antibodies (Abs) (BD Pharmingen), CCR7 Ab (R&D Systems), CD14 Ab (BD Pharmingen), or CD3 Ab (Biolegend). The percentage of double-positive CD14+CCR7+ or CD3+CCR7+ was determined. When evaluating M1 polarization, macrophages were stained with CD86 Ab and CD14 Ab (Biolegend). Shown data represent the percentage of CD14+CD86+ among CD14+ gated cells.
Chemotaxis
Boyden chamber (Neuroprobe) chemotaxis was performed using NL monocytes. We evaluated the effects of stimulation with various concentrations of recombinant human CCL21 or 5% RA SF for 2 h. PBS and FMLP (10 nM) served as a − and + control, respectively. CCR7 and CCL21 were neutralized with CCR7 Ab and CCL21 Ab (10 µg/ml; R&D Systems), respectively, for 1 h prior to chemotactic stimulation. To determine relevant signaling pathways, monocytes were treated for 1 h with 5 µM inhibitors of NF-κB (MG132), ERK (PD98059), p38 (SB203580), or PI3K (LY294002) (Millipore). Subsequently, monocyte chemotaxis in response to 10 ng/ml of CCL21 was evaluated for 2 h.
Western blot (WB)
Activated signaling pathways in NL monocytes, treated with CCL21 (100 ng/mL; R&D Systems) for 5–65 min, were examined by WB analysis. Blots were probed for degradation of IκB (Santa Cruz; 1:3000), compared to β-Actin (Sigma; 1:3000) confirming equal loading. Blots were also incubated with Abs against ERK, MAPK p38 and Akt, detecting either total or phosphorylated (p) forms of the kinases (Cell Signaling; 1:1000). Cell lysates of murine bone BM macrophages, transfected ex vivo with either adenovirus (Ad)-Ctrl or Ad-CCL21 (109 vp/ml; Welgen Inc), were incubated with Abs to examine iNOS (1:1000; Santa Cruz) and Arginase 1 (Arg1) (1:500; Santa Cruz) content, compared to β-Actin to confirm equal loading.
qRT-PCR
RNA was isolated using TRIzol (Life Technologies). Conversion to cDNA and subsequent qRT-PCR analysis were performed using the High-Capacity cDNA Reverse Transcription Kit and Taqman Gene expression Master Mix (Applied Biosystems). Predesigned Taqman primer/probe mixes were purchased from Integrated DNA Technologies. Data are presented as fold changes in RNA levels compared to control treatment, calculated following the 2−ΔΔCt method, with GAPDH and Actin Beta serving as two internal control genes [17, 18].
T cell polarization
Donor cells, either PBMCs or selected T cells, were seeded at 0% FBS/RPMI for 1 h. Instead of discarding the suspension cells when replacing the medium with 10% FBS/RPMI, they were centrifuged, resuspended in the new medium, and put back on the cell cultures. To allow T cell differentiation, the medium was supplemented with 1 µg/mL CD3 Ab and 1 µg/mL CD28 Ab (Biolegend). Simultaneously, various stimuli were added. Cells were control-treated (medium) or stimulated with CCL21, LPS or a combination of IL-6 and IL-1β (all at 100 ng/mL). After 7 days, cell media were collected. DuoSet ELISAs (R&D Systems) were used to quantify protein levels of IL-17 and IFNγ in previously collected cell media.
To confirm Th17 polarization in murine cells, we first isolated mouse BM cells and cultured them in 10% FBS/RPMI for 4 days in the presence of 40 ng/mL mM-CSF. On day 4, we homogenized fresh spleens to isolate live murine splenocytes. We removed the M-CSF-containing culture medium and seeded the splenocytes on top of the adherent BM cells (1:1; 4 × 106 cells each). These co-cultures were incubated for 7 days on 10% FBS/RPMI, supplemented with 1 µg/mL CD3 Ab and 1 µg/mL CD28 Ab, and stimulated with either recombinant murine proteins or recombinant adenoviruses. For reference, cultures were control-treated or stimulated with a combination of mIL-1β, mIL-6, and mTGFβ (all at 100 ng/mL). Alternatively, cells were incubated with an empty adenoviral vector (Ad-Ctrl) or with Ad-CCL21 (Welgen Inc) at 109 vp/mL. As a measure for Th17 polarization, a DuoSet ELISA (R&D Systems) was used to quantify mIL-17 in the media collected after 7 days.
Osteoclastogenesis
Osteoclastogenesis was evaluated following the previously described protocol [16]. We used Acid Phosphatase Leukocyte Kit (Sigma-Aldrich) to perform tartrate-resistant acid phosphatase (TRAP) staining. Microscopic images were taken at 10 × magnification and the number of TRAP+ osteoclasts per high-power field (HPF) was counted.
Animals
All animal studies were approved by UIC Animal Care and Use Committee. WT or CCR7-/- C57BL/6 mice (Jackson lab) (≥ 8 weeks) were injected intra-articularly with Ad-Ctrl or Ad-CCL21 (3 × 1010 vp/ankle), on days 0 and 7. Joint circumference was monitored as previously described [19], until mice were sacrificed on day 10. Both the anterior–posterior and the lateral diameter (a and b) were measured using a caliper and the circumference (c) was calculated using the formula c = 2π × √[(a2 + b2)/2]. Ankles were homogenized in 1 mL of TRIzol for RNA isolation and qRT-PCR, or fixed in 10% formalin, decalcified, and paraffin-imbedded for immunohistochemistry.
Immunohistochemistry
Slides were deparaffinized in xylene and antigens were unmasked by either incubating slides in Proteinase K digestion buffer (Roche, F4/80), 10 mM citrate (pH 6.0) + 0.05% Tween-20 (iNOS Arg1, CD3 and Vimentin), or 10 mM Tris (pH 9.0) + 1 mM EDTA (VWF), as previously described [15]. H&E-stained ankles were scored by two independent, blinded observers for inflammation, synovial lining thickness and bone erosion on a 0–5 scale. Alternatively, ankles were stained with F4/80 Ab (1:100; GeneTex), iNOS Ab (1:200; Santa Cruz), Arg1 Ab (1:200; Santa Cruz), CD3 Ab (1:100; Genetex), Vimentin (1:2000; Thermo Scientific), or VWF Ab (1:1000; Dako). TRAP staining was performed as previously described [16]. Osteoclastogenesis in the mouse ankles was quantified by counting the number of TRAP+ osteoclasts in each section.
Statistical analysis
GraphPad Prism 7 software was used both to create included figures and to perform statistical analysis. When comparing multiple groups, statistical significance was first confirmed by ANOVA. Differences between two groups were evaluated by unpaired t test, unless otherwise specified. p values below 0.05 were considered significant.
Results
Expression of myeloid CCR7 is highly upregulated in RA blood and synovial fluid
First, we characterized the expression and functional significance of CCR7 in RA patients. CCR7 mRNA levels were threefold higher in RA compared to NL PB monocytes and in vitro differentiated macrophages (Fig. 1a). Notably, RA SF macrophages express the highest CCR7 RNA levels, both compared to NL and RA PB differentiated macrophages (720- and 260-fold, respectively; p < 0.0001). Accordingly, the percentage of double-positive CD14+CCR7+cells was twofold higher in RA monocytes and macrophages compared to those in NL counterpart cells (Fig. 1b). In contrast, while T cells also express CCR7, no difference was observed in the CD3+CCR7+ cell population between NL and RA patients (Fig. 1c). Furthermore, patients treated with TNF Ab ± DMARD had similar CCR7 mRNA levels in RA monocytes as those receiving DMARD therapy (Fig. 1d). Since CCR7 expression was highly accentuated in RA SF macrophages, known to have a strong M1 profile, we next explored the connection between CCR7 and macrophage polarization (Fig. 1e). Differentiation of naïve macrophages into M1 by IFNγ + LPS treatment enhances CCR7 expression by 3.5-fold (p = 0.0061). In contrast, IL-4-induced M2 polarization reduces CCR7 levels (p = 0.0114). Moreover, regression analysis demonstrates that patients with greater DAS28 exhibit increased CCR7 expression in RA monocytes (R2 = 0.87) and macrophages (R2 = 0.72) (Fig. 1f, g). Our data suggest that CCR7 is highly upregulated in RA blood and SF myeloid cells and its levels are linked to M1 differentiation and RA disease activity.
CCL21 promotes CCR7+ monocyte accumulation in RA
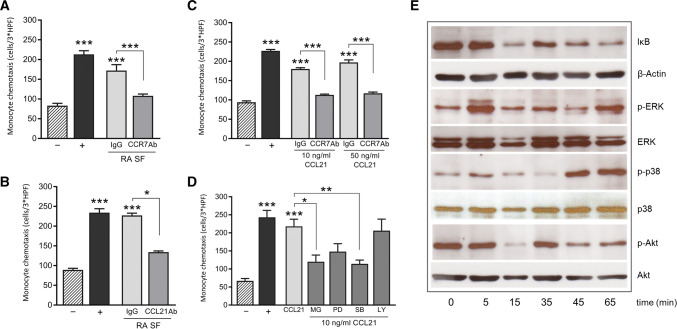
Because elevated myeloid CCR7 expression correlates with DAS28, we asked whether high levels of CCL21 in the RA SF are implicated in attracting circulating monocytes into the joint. Immuno-neutralization of CCR7 on monocytes or CCL21 in RA SF significantly reduces the chemotactic activity of RA SF (Fig. 2a, b; p = 0.0024). In parallel, recombinant CCL21 similarly induces monocyte chemotaxis, in a CCR7-dependent manner (Fig. 2c). Signaling studies reveal that CCL21 induces p38 and ERK phosphorylation and IκB degradation (Fig. 2e). Inhibition of NF-κB and p38 signaling confirms their contribution to CCL21-induced monocyte chemotaxis (Fig. 2e; p = 0.0226 and p = 0.0099, respectively). However, while we observed a trend, the ERK inhibitor did not significantly reduce monocyte migration (p = 0.0777). In contrast, Akt phosphorylation was not affected by CCL21, nor did PI3K inhibition impact monocyte chemotaxis (Figs. 2d, e). In short, joint CCL21 attracts CCR7+ monocytes by activating NF-κB and p38 pathways.
Fig. 2.
CCL21/CCR7 signaling promotes monocyte chemotaxis. The chemotactic activity of monocytes, exposed to various stimuli for 2 h, was tested in a Boyden chamber. Data represent the number of monocytes counted over 3 HPF and are presented as mean ± sem. PBS was used as a negative control (−) and FMLP (10 nM) as a positive control (+). a, b Monocytes were stimulated with 5% RA SF in the presence of either a control Ab (IgG) or neutralizing Abs against CCR7 or CCL21 (n = 6). c CCL21-induced chemotaxis was also neutralized with CCR7 Ab (n = 3). d Monocytes treated with signaling inhibitors (5 μM), MG132 (MG), PD98059 (PD), SB203580 (SB) or LY294002 (LY), were stimulated with CCL21 (10 ng/mL) (n = 3). e Cell lysates of monocytes stimulated for 5–65 min with CCL21 (10 ng/mL) were processed for WB, to detect the phosphorylation status of signaling molecules ERK, p38 MAPK and Akt. IκB degradation was quantified as a measure for NFκB activity. The blot shown is one representative out of three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.005
CCL21 connects M1 macrophages to Th17 cell differentiation
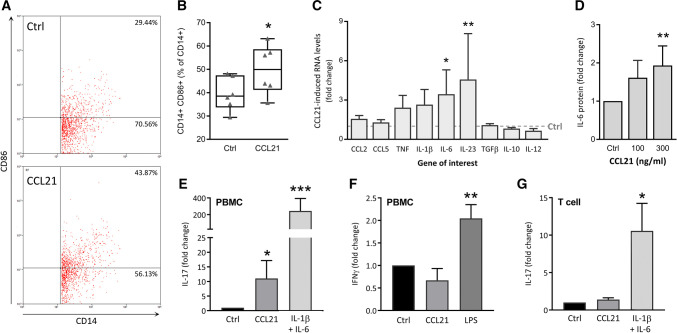
Given that the expression of CCR7 increases as monocytes differentiate into macrophages and following M1 polarization, we examined whether CCL21 can exacerbate expression of inflammatory M1 markers. Although the frequency of double-positive CD14+CD86+ cells is elevated by CCL21 stimulation of naïve macrophages (Fig. 3a, b), CCL21-polarized macrophages exhibit an atypical M1 chemokine profile. Expression of traditional M1 cytokines including CCL2, CCL5, and TNF, does not change upon CCL21 stimulation. However, we do note significantly elevated transcription of IL-6 and IL-23 in CCL21-polarized macrophages (Fig. 3c). In line with these findings, CCL21 stimulation dose-dependently upregulates IL-6 protein production (Fig. 3d).
Fig. 3.
CCL21 stimulates M1-driven Th17 polarization. a, b Differentiated PBMC were stimulated with CCL21 (100 ng/mL) for 24 h and then stained with Ab against CD14 (pan-macrophage marker) and CD86 (M1) for FACS analysis. a Flow chart of one representative experiment out of 6 is shown. b Percentage of double-positive CD14+CD86+ cells are represented as a box and whiskers plot, including individual percentages (n = 6). A paired t test was used to assess statistical significance of the difference between untreated (Ctrl) and CCL21-treated cells. c PBMCs untreated (Ctrl) or stimulated with CCL21 (300 ng/mL) for 24 h were processed for qRT-PCR analysis (n = 7). CCL21-induced gene expression is expressed as a fold change (2−ΔΔCt) of baseline gene expression (Ctrl = 1), analyzed by multiple t tests. d IL-6 protein levels were measured by ELISA in the cell media of NL PBMCs stimulated with different concentrations of CCL21 for 48 h (n = 5). e, f IL-17 (Th17) and IFNγ (Th1) protein levels were measured by ELISA in the cell media of PBMCs differentiated over 7 days in the presence of CD3 Ab and CD28 Ab, stimulated with CCL21, IL-1β and IL-6, LPS (all 100 ng/mL) or left untreated (Ctrl) (e: n = 7; f: n = 5). g Similarly, cell media of negatively selected T cells cultured under the same conditions were also collected for IL-17 ELISA (n = 4). d–g Protein levels were compared using a non-parametric Mann–Whitney test. Data are presented as mean ± sem. *p < 0.05, **p < 0.01, ***p < 0.005
As IL-6 and IL-23 are involved in Th17 differentiation and maintenance [20, 21], we evaluated the effect of CCL21 on T cell polarization. For this purpose, RA PBMCs were control-treated or stimulated with IL-1β + IL-6 (Th17 + Ctrl), LPS (Th1 + Ctrl), or CCL21 and conditioned media were screened for production of Th17 and Th1 markers (Fig. 3e, f). We found that PBMCs stimulated with CCL21 secrete higher protein levels of IL-17, indicative of Th17 polarization. In contrast to the positive control, Th1 differentiation or IFNγ production was not impacted by CCL21. Notably, when T cells are cultured in the absence of myeloid cells, CCL21 loses its ability to induce Th17 cell polarization (Fig. 3g; p = 0.3143). The data suggest that CCL21 does not induce Th17 differentiation directly. Nevertheless, it operates indirectly by affecting myeloid cell production of Th17-promoting cytokines. CCL21 stimulation of RA CCR7+ macrophages cultivates a unique subset of M1 macrophages that amplifies Th17 cell differentiation.
CCL21-induced Th17 polarization contributes to osteoclastogenesis
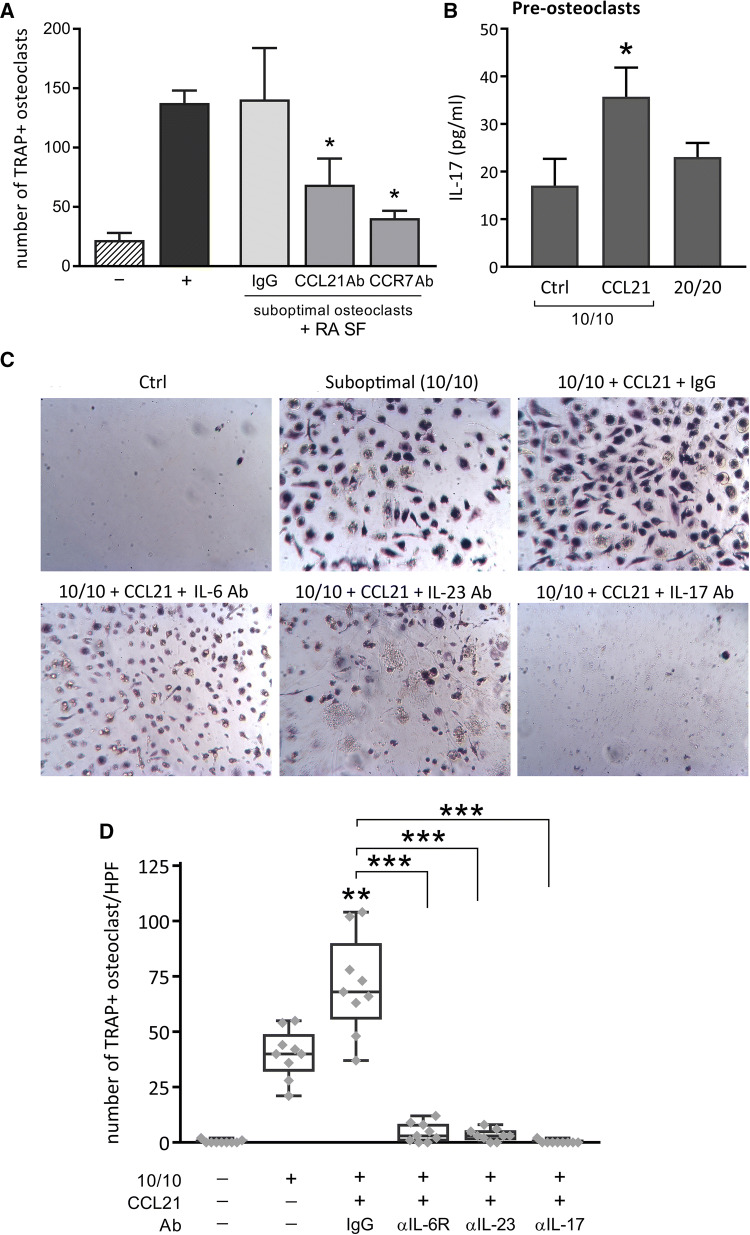
A defining step in the progression of RA is the formation of osteoclasts, which can actively destroy the bone and cartilage. Under suboptimal osteoclastogenic condition (10 ng/ml M-CSF/RANKL), RA SF can transform RA PBMCs into mature osteoclasts. Interestingly, neutralizing Abs to CCL21 or CCR7 protect against RA SF-induced osteoclastogenesis (Fig. 4a; p = 0.0455 and p = 0.0472, respectively). RA osteoclast progenitor cell cultures activated by CCL21 express IL-17 (Fig. 4b). As previously reported, Th17 cells/IL-17 and its inducers, including IL-6 and IL-23, play a central role in RA osteoclastogenesis [22]. We demonstrate that CCL21 can remodel RA PBMCs into TRAP+ osteoclasts. This function is impaired by blocking Abs against IL-6R, IL-23 and IL-17 (Fig. 4c, d) (p = 0.0016, p < 0.0001, and p < 0.0001, respectively). Collectively, our findings indicate that, by promoting Th17 polarization, M1 cytokines induced by CCL21 stimulate osteoclastogenesis.
Fig. 4.
CCL21/CCR7 promotes osteoclastogenesis, mediated by IL-6/IL-23-induced Th17 cells. a RA PBMCs cultured in suboptimal osteoclastogenic conditions (10 ng/mL M-CSF and RANKL; 10/10) were control-treated with only cell media (−) or stimulated with RA SF (2%), combined with IgG, CCL21 Ab or CCR7 Ab (10 µg/mL). As a positive control (+) cells were stimulated with optimal concentrations of M-CSF and RANKL (20 ng/mL). After 14–21 days of differentiation (fresh stimuli × 2/week), a TRAP staining was performed and the stained, multinuclear osteoclasts were counted (n = 6). b PBMCs conditioned in suboptimal osteoclastogenic conditions (10/10) were untreated or stimulated with either CCL21 (100 ng/mL) or 20 ng/mL M-CSF and RANKL (20/20) for 48 h. IL-17 protein levels were measured in the collected cell media through ELISA. Differences in IL-17 protein levels were assessed using a one-tailed unpaired t test (n = 3). Data are presented as mean ± sem. c, d Finally, NL PBMCs cultured in suboptimal osteoclastogenic conditions (10/10) were untreated or stimulated with CCL21 (100 ng/mL), in the presence of a control IgG or neutralizing Abs, IL-6R Ab, IL-23 Ab or IL-17 Ab (10 µg/mL). After 21 days of differentiation (fresh stimuli × 2/week) and following TRAP staining, osteoclasts were counted (n = 9; 3 donors tested in triplicate). c Images of TRAP+ cells (original magnification × 10) from one representative donor out of three are shown. d Counts are represented as a box and whiskers plot, including individual datapoints. *p < 0.05, **p < 0.01, ***p < 0.005
CCL21 induces erosive arthritis characterized by monocyte infiltration, M1/Th17 polarization, osteoclastogenesis, and vascularization
We next evaluated the effect of CCL21 on murine cells, by transfecting BM macrophages with an empty adenoviral vector (Ad-Ctrl) or Ad-CCL21 (Fig. 5a, d). Ad-CCL21 markedly increased the production of M1 cytokines TNF and CCL5 (Fig. 5a). Similar to our previous data, protein levels of IL-6 were highly upregulated in the presence of CCL21 (Fig. 5b). These findings were substantiated by WB analysis, showing higher levels of M1-associated iNOS and reduced M2-associated Arg1 following CCL21 stimulation (Fig. 5c). Accordingly, in CCL21-activated BM macrophage and splenocyte co-cultures, higher levels of IL-17 were detected (Fig. 5d). This suggests that similar to human studies, CCL21-induced IL-6 promotes murine Th17 polarization.
Fig. 5.
Ad-CCL21 induces M1-driven joint inflammation and Th17 polarization in mice. a–d Murine BM macrophages cultured ex vivo were transfected with recombinant adenoviruses Ad-Ctrl (empty vector) or Ad-CCL21. After 3 days, cell media was collected for ELISA (a, b; n = 3–4) and the adherent cells were lysed for western blot (c; 1 representative experiment out 3 shown). d Co-cultures of murine BM macrophages and splenocytes were incubated for 7 days to allow Th cell differentiation, after infection with Ad-Ctrl or Ad-CCL21. Mouse IL-17 protein levels were determined by ELISA and presented as a fold change in protein levels versus Ad-Ctrl-treated cultures (n = 6). e WT mouse joints, injected with Ad-Ctrl and Ad-CCL21, were homogenized on day 5 to validate local CCL21 production by ELISA (n = 8). f–l WT and CCR7-deficient (CCR7 −/−) mice were injected intra-articularly with Ad-Ctrl or Ad-CCL21, on day 0 and day 7. f Changes in joint circumference were monitored over the course of 10 days (n = 14 ankles/group). On day 10, joints were collected for immunohistochemistry and qRT-PCR. g, h Tissue sections were stained with H&E and scored for inflammation, synovial lining thickness and bone erosion on a 0–5 scale (n = 7). i, j WT adenovirus-treated joints were processed for qRT-PCR analysis. RNA levels of various cytokines related to macrophage (i) and Th17 (j) polarization were evaluated (n = 14). Gene expression is expressed as a fold change (2-ΔΔCt) of baseline gene expression (Ad-Ctrl). k, l Tissue sections were also stained for macrophage markers F4/80 (n = 7), iNOS and Arg1 (n = 4–5) and staining was scored on a 0–5 scale. Data are presented as mean ± sem. *p < 0.05, **p < 0.01, ***p < 0.005
Through intra-articular injection of Ad-CCL21, we induced local overexpression, resulting in sevenfold higher levels of CCL21 on day 5 postinjection relative to control (Fig. 5e). Local expression of CCL21 in WT mice increases ankle circumference as early as day 2 (Fig. 5f). Joint swelling plateaus thereafter, but inflammation is potentiated following a second injection on day 7. Conversely, both clinical and histological analyses demonstrate that local expression of CCL21 does not impact joint inflammation, synovial lining thickness, or osteoclastic bone erosion in CCR7−/− compared to WT mice (Fig. 5f, g).
In the ankles of CCL21 arthritic mice, we detected highly elevated expression of TNF, CCL2, and CCL5 (Fig. 5i). When the macrophage profile was analyzed, we accordingly noted that local CCL21 expression was responsible for remodeling F4/80 macrophages to a predominately iNOS + phenotype (Fig. 5k, l). Consistently, Arg1 staining and joint TGFβ expression levels were inferior to the levels of M1-associated markers expressed in the CCL21 arthritic mice. We also found that the transcription of Th17-promoting cytokines IL-6, IL-1β, and IL-23, as well as IL-17 was upregulated by CCL21 (Fig. 5j). In contrast, CCL21 expression did not impact IL-12, a Th1 inducer (Fig. 5i).
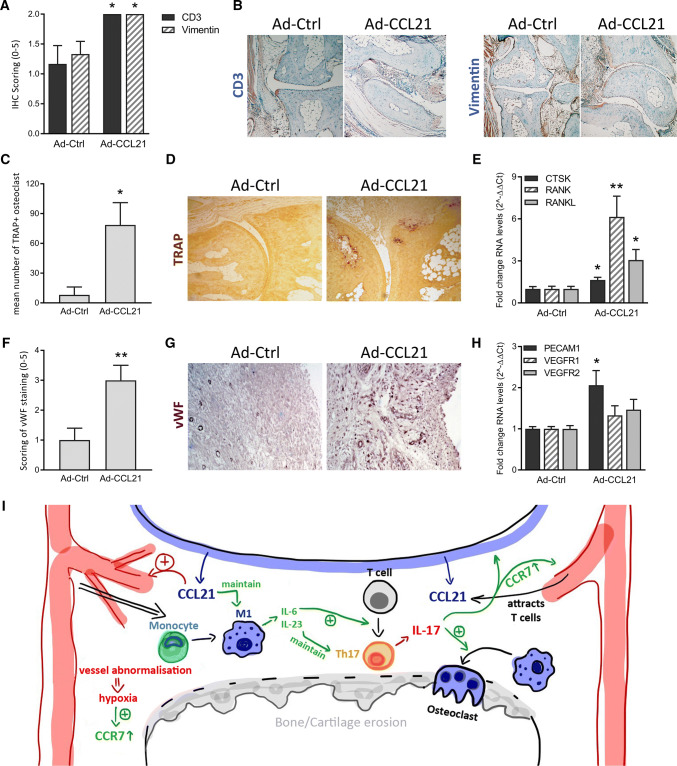
Interestingly, histological studies revealed a modest, yet significant upregulation of CD3+ T cells and Vimentin+ fibroblasts in the CCL21-induced arthritic joints (Fig. 6a, b). Hence, the presence of joint T cells and fibroblasts and their synergistic cell-to-cell interaction with myeloid cells may contribute to the broader panel of M1 cytokines observed in vivo relative to the more limited range produced by in vitro differentiated macrophages.
Fig. 6.
Macrophage-mediated CCL21/CCR7 pathway in RA pathogenesis. WT mice were injected intra-articularly with Ad-Ctrl or Ad-CCL21, on day 0 and day 7, before ankles were collected on day 10 for immunohistochemistry and qRT-PCR analysis. a, b Tissue sections were stained for CD3 and Vimentin (n = 6) and staining was scored on a 0-5 scale. c, d Tissue sections were TRAP stained to allow quantification of the number of TRAP+ osteoclasts in the treated joints (n = 7). e Gene expression of proteins related to osteoclastogenesis was evaluated, expressed as a fold change (2-ΔΔCt) of baseline gene expression (Ad-Ctrl) (n = 6). f, g Sections were also stained for endothelial cell marker VWF and staining was scored on a 0–5 scale (n = 7). h RNA levels related to the vasculature were evaluated (n = 6). All data are presented as mean ± sem. *p < 0.05 **p < 0.01 (i) CCL21-triggered immune cell accumulation and M1-Th17 crosstalk promote RA pathogenesis, leading to intra-articular angiogenesis, osteoclastogenesis and progressive joint destruction, as schematically summarized
Osteoclastogenesis was exacerbated by CCL21 as evidenced by the upregulation of RANKL, RANK, and CTSK transcription and numbers of TRAP+ cells in the arthritic joints compared to their control-treated counterparts (Fig. 6c–e). In addition, elevated expression levels of PECAM1, an endothelial marker, and upregulated VWF staining support the role of CCL21 in propagating erosive arthritis in part through joint vascularization (Fig. 6f–h). These findings validate the previously reported direct and indirect proangiogenic effects of CCL21 in RA [1, 4].
Discussion
The current study reveals the mechanism by which the CCL21/CCR7 pathway impacts different stages of RA pathogenesis. In the early stages of disease, CCL21 expressed in the RA SF attracts circulating monocytes. During RA progression, infiltrated macrophages are remodeled to M1 cells that produce IL-6 and IL-23 and polarize naïve T cells into pathogenic Th17 cells. M1-driven Th17 cell differentiation, facilitated by CCL21, in turn fosters osteoclastogenesis, thereby initiating the destructive phase of the disease. Taken together, local expression of CCL21 provokes erosive arthritis by connecting M1 and Th17 cells, while joint neovascularization and progressive immune cell influx further exacerbate disease (Fig. 6i).
Supporting the close association of DAS28 with myeloid CCR7 expression, others have observed that in the 1st year of RA diagnosis, CCR7 levels on monocytes correlate with C reactive protein levels [13]. Although CCL21 and CCR7 modulate physiological T cell homing, their effect on monocyte recruitment had not been explored. We now show that SF CCL21 plays an important role in recruiting CCR7+ RA monocytes. Interestingly, others have shown that CCL21 Ab therapy alleviates experimental myocardial infarction by indirectly downregulating neutrophil and monocyte extravasation [23]. They attributed this diminished immune cell recruitment to a reduction of secondary respective neutrophil and monocyte chemoattractant levels, observed after CCL21 neutralization. However, said study did not consider the potential for CCL21 to mediate immune cell influx directly. Some studies did evaluate the direct chemotactic effects of CCL21 on other inflammatory cells, focusing on dendritic cells. In atherosclerotic plaques, for example, oxidized LDL has been reported to reduce levels of CCR7 and CCL21, thereby suppressing dendritic cell infiltration [24]. Our findings do not only reveal that CCL21 actively attracts monocytes to the joints, but also that synovial macrophages continue to be crucial effector cells in CCL21-induced arthritic mice.
We document that CCL21 can transform naïve macrophages into a unique M1 phenotype that expresses IL-6 and IL-23, while production of typical M1 cytokines TNF, CCL2, and CCL5 remains unchanged. Perhaps, this indicates that CCL21 is less critical for the initial M1 polarization when CCR7 levels are modest. Although we show that monocyte-to-macrophage differentiation increases CCR7 cell surface expression, its presence is even more markedly enhanced by M1-promoting factors and is suppressed by M2 inducers. Hence, M1 macrophages with elevated levels of CCR7 could be more responsive to CCL21 activation than naïve macrophages. CCL21 could sustain the CCR7 + M1 phenotype and, furthermore, establish a distinct inflammatory profile, which differs from the typical M1 secretome. This is consistent with earlier findings suggesting that CCL21 activates signaling in M1, but not M2 macrophages [25]. Notably, local expression of CCL21 in mouse ankle joints induces a wider range of classical M1 factors. These differences may be due to the extended CCL21 exposure in the ankles, which can promote both direct and indirect effects, as well as the presence of multiple cell types that further amplify CCL21-induced inflammation. Authenticating the central role of CCL21 in M1 differentiation, we show the dominance of F4/80+iNOS+ over F4/80+Arg1+ cells in the ankles of arthritic mice and further document the dependency of CCL21-differentiated Th17 cells on M1 cytokines.
In contrast to earlier findings, we did not find any association between CCL21 and Th1 cell polarization, in part due to lack of IL-12 induction [26]. However, a strong link was identified between CCL21-stimulated M1 factors, IL-6 and IL-23, and arthritic Th17 cell differentiation. Interestingly, IL-17 modulates expression of CCR7 in RA fibroblasts and endothelial cells [4]. This may suggest that there is a feedback regulation between CCL21-induced Th17 polarization and responsiveness of RA fibroblast and endothelial to CCL21.
Current first-line therapy of RA targets either TNF or IL-6R. TNF Ab treatment does not impact the CCL21/CCR7 pathway. We also noted that the expression of CCR7 is not normalized in RA patients receiving TNF Ab therapy. Similarly, in humanized TNF transgenic mice, resolution of experimental arthritis mediated by TNF Ab treatment has no impact on CCR7 or CCL21 expression in joint macrophages [27]. We have demonstrated that IL-6, IL-23, and IL-17 are responsible for CCL21-potentiated osteoclast formation. Hence, IL-6R Ab therapy may partially suppress CCL21-induced Th17 polarization and osteoclastogenesis. However, IL-6R Ab treatment will not alleviate the proangiogenic effects of CCL21, or its direct chemotactic effect on various immune cells.
CCR7−/− mice are resistant to CIA, in part due to reduced CD3+ cells and increased Treg polarization [11]. However, severe defects in the thymic architecture of CCR7-/- mice have been observed [28], which indicates that long-term therapeutic inhibition of CCR7 may cause serious side effects. A previous study also demonstrated that response to infection is mitigated in CCL19/CCL21-deficient mice [12]. We postulate that targeted inhibition of CCL21 function, rather than suppressing both CCR7 ligands, may yield optimal results without the side effects.
GWAS studies have identified CCL21 as a risk factor for RA [2, 29], and while CCL19 and CCL21 can both increase T cell chemotaxis, only CCL21 promotes extravasation [7]. Moreover, CCL19 causes CCR7 internalization, resulting in receptor desensitization. In contrast, binding of CCL21 to CCR7 prevents efficient receptor internalization [30]. Taken together, these results suggest that CCL19 operates as a physiological CCR7 ligand, while CCL21 is mainly responsible for the pathological function of CCR7.
Our study is the first to characterize comprehensively the impact of CCL21/CCR7 in RA. CCL21-mediated T cell accumulation combined with its ability to recruit myeloid cells and activate IL-6 and IL-23 transcription enables a crosstalk between macrophages and T cells, necessary to drive Th17 polarization. Ultimately, this inflammatory milieu cultivated by CCL21 fosters osteoclastogenesis. In conclusion, blockade of CCL21 function could abrogate monocyte infiltration early in RA, prevent M1 and Th17 crosstalk and impair the progression of RA joint inflammation and bone destruction.
Acknowledgements
This work was supported in part by awards from the Department of Veteran’s Affairs MERIT Award 1I01BX002286, the National Institutes of Health AR056099 and AR065778 and the National Psoriasis Foundation (NPF). We also want to thank the clinical staff at the Division of Rheumatology at UIC, who have aided us to inform and involve patients in our studies and enable us to pursue clinically relevant, translational research.
Abbreviations
- Ab
Antibody
- Ad-CCL21
Recombinant adenovirus facilitating CCL21 expression
- Ad-Ctrl
Empty adenoviral vector
- Arg1
Arginase 1
- BM
Bone marrow
- CIA
Collagen-induced arthritis
- DAS28
Disease activity score (determined by 28 joint count)
- DMARD
Disease-modifying anti-rheumatic drugs
- GWAS
Genome-wide association studies
- HPF
High-power field
- IL
Interleukin
- NL
Healthy donor
- PB
Peripheral blood
- PBMC
Peripheral blood mononuclear cells
- RA
Rheumatoid arthritis
- SF
Synovial fluid
- ST
Synovial tissue
- TRAP
Tartrate-resistant acid phosphatase
- VEGF
Vascular endothelial growth factor
- WB
Western blot
- WT
Wild type
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pickens SR, Chamberlain ND, Volin MV, Pope RM, Mandelin AM, 2nd, Shahrara S. Characterization of CCL19 and CCL21 in rheumatoid arthritis. Arthritis Rheum. 2011;63:914–922. doi: 10.1002/art.30232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li G, Zhao J, Li B, Ma J, Zhao Q, Wang X, Lv Z, Li K, Du Z, Ma X, Liu J. Associations between CCL21 gene polymorphisms and susceptibility to rheumatoid arthritis: a meta-analysis. Rheumatol Int. 2017;37:1673–1681. doi: 10.1007/s00296-017-3784-4. [DOI] [PubMed] [Google Scholar]
- 3.Bowes J, Ho P, Flynn E, Ali F, Marzo-Ortega H, Coates LC, Warren RB, McManus R, Ryan AW, Kane D, Korendowych E, McHugh N, FitzGerald O, Packham J, Morgan AW, Bruce IN, Barton A. Comprehensive assessment of rheumatoid arthritis susceptibility loci in a large psoriatic arthritis cohort. Ann Rheum Dis. 2012;71:1350–1354. doi: 10.1136/annrheumdis-2011-200802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pickens SR, Chamberlain ND, Volin MV, Pope RM, Talarico NE, Mandelin AM, 2nd, Shahrara S. Role of the CCL21 and CCR4 pathways in rheumatoid arthritis angiogenesis. Arthritis Rheum. 2012;64:2471–2481. doi: 10.1002/art.34452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cosway EJ, Ohigashi I, Schauble K, Parnell SM, Jenkinson WE, Luther S, Takahama Y, Anderson G. Formation of the intrathymic dendritic cell pool requires CCL21-mediated recruitment of CCR5(+) progenitors to the thymus. J Immunol. 2018;201:516–523. doi: 10.4049/jimmunol.1800348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Forster R, Schubel A, Breitfeld D, Kremmer E, Renner-Muller I, Wolf E, Lipp M. CCR6 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 1999;99:23–33. doi: 10.1016/S0092-8674(00)80059-8. [DOI] [PubMed] [Google Scholar]
- 7.Weninger W, Carlsen HS, Goodarzi M, Moazed F, Crowley MA, Baekkevold ES, Cavanagh LL, von Andrian UH. Naive T cell recruitment to nonlymphoid tissues: a role for endothelium-expressed CC chemokine ligand 21 in autoimmune disease and lymphoid neogenesis. J Immunol. 2003;170:4638–4648. doi: 10.4049/jimmunol.170.9.4638. [DOI] [PubMed] [Google Scholar]
- 8.Gunn MD, Kyuwa S, Tam C, Kakiuchi T, Matsuzawa A, Williams LT, Nakano H. Mice lacking expression of secondary lymphoid organ chemokine have defects in lymphocyte homing and dendritic cell localization. J Exp Med. 1999;189:451–460. doi: 10.1084/jem.189.3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou S, Li R, Qin J, Zhong C, Liang C. SLC/CCR9 stimulates the proliferation of BMDCs by the pNF-kappaB p65 pathway. Anat Rec (Hoboken) 2010;293:48–54. doi: 10.1002/ar.21015. [DOI] [PubMed] [Google Scholar]
- 10.Kuwabara T, Ishikawa F, Yasuda T, Aritomi K, Nakano H, Tanaka Y, Okada Y, Lipp M, Kakiuchi T. CCR10 ligands are required for development of experimental autoimmune encephalomyelitis through generating IL-23-dependent Th17 cells. J Immunol. 2009;183:2513–2521. doi: 10.4049/jimmunol.0800729. [DOI] [PubMed] [Google Scholar]
- 11.Moschovakis GL, Bubke A, Friedrichsen M, Ristenpart J, Back JW, Falk CS, Kremmer E, Forster R. The chemokine receptor CCR1 is a promising target for rheumatoid arthritis therapy. Cell Mol Immunol. 2018 doi: 10.1038/s41423-018-0056-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ato M, Maroof A, Zubairi S, Nakano H, Kakiuchi T, Kaye PM. Loss of dendritic cell migration and impaired resistance to Leishmania donovani infection in mice deficient in CCL19 and CCL21. J Immunol. 2006;176:5486–5493. doi: 10.4049/jimmunol.176.9.5486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ellingsen T, Hansen I, Thorsen J, Moller BK, Tarp U, Lottenburger T, Andersen LS, Skjodt H, Pedersen JK, Lauridsen UB, Svendsen A, Lindegaard H, Jacobsen S, Ostergaard M, Vestergaard A, Jurik AG, Junker P, Christensen AF, Hetland ML, Horslev-Petersen K, Stengaard-Pedersen K. Upregulated baseline plasma CCL19 and CCR13 cell-surface expression on monocytes in early rheumatoid arthritis normalized during treatment and CCL19 correlated with radiographic progression. Scand J Rheumatol. 2014;43:91–100. doi: 10.3109/03009742.2013.803149. [DOI] [PubMed] [Google Scholar]
- 14.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 15.Chamberlain ND, Vila OM, Volin MV, Volkov S, Pope RM, Swedler W, Mandelin AM, 2nd, Shahrara S. TLR5, a novel and unidentified inflammatory mediator in rheumatoid arthritis that correlates with disease activity score and joint TNF-alpha levels. J Immunol. 2012;189:475–483. doi: 10.4049/jimmunol.1102977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim SJ, Chen Z, Chamberlain ND, Essani AB, Volin MV, Amin MA, Volkov S, Gravallese EM, Arami S, Swedler W, Lane NE, Mehta A, Sweiss N, Shahrara S. Ligation of TLR5 promotes myeloid cell infiltration and differentiation into mature osteoclasts in rheumatoid arthritis and experimental arthritis. J Immunol. 2014;193:3902–3913. doi: 10.4049/jimmunol.1302998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 18.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:RESEARCH0034. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pickens SR, Chamberlain ND, Volin MV, Mandelin AM, 2nd, Agrawal H, Matsui M, Yoshimoto T, Shahrara S. Local expression of interleukin-27 ameliorates collagen-induced arthritis. Arthritis Rheum. 2011;63:2289–2298. doi: 10.1002/art.30324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McGeachy MJ, Bak-Jensen KS, Chen Y, Tato CM, Blumenschein W, McClanahan T, Cua DJ. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat Immunol. 2007;8:1390–1397. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- 21.Revu S, Wu J, Henkel M, Rittenhouse N, Menk A, Delgoffe GM, Poholek AC, McGeachy MJ. IL-23 and IL-1beta drive human Th17 cell differentiation and metabolic reprogramming in absence of CD28 costimulation. Cell Rep. 2018;22:2642–2653. doi: 10.1016/j.celrep.2018.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim KW, Kim HR, Kim BM, Cho ML, Lee SH. Th17 cytokines regulate osteoclastogenesis in rheumatoid arthritis. Am J Pathol. 2015;185:3011–3024. doi: 10.1016/j.ajpath.2015.07.017. [DOI] [PubMed] [Google Scholar]
- 23.Jiang Y, Bai J, Tang L, Zhang P, Pu J. Anti-CCL21 antibody attenuates infarct size and improves cardiac remodeling after myocardial infarction. Cell Physiol Biochem. 2015;37:979–990. doi: 10.1159/000430224. [DOI] [PubMed] [Google Scholar]
- 24.Nickel T, Pfeiler S, Summo C, Kopp R, Meimarakis G, Sicic Z, Lambert M, Lackermair K, David R, Beiras-Fernandez A, Kaczmarek I, Weis M. oxLDL downregulates the dendritic cell homing factors CCR24 and CCL21. Mediators Inflamm. 2012;2012:320953. doi: 10.1155/2012/320953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xuan W, Qu Q, Zheng B, Xiong S, Fan GH. The chemotaxis of M1 and M2 macrophages is regulated by different chemokines. J Leukoc Biol. 2015;97:61–69. doi: 10.1189/jlb.1A0314-170R. [DOI] [PubMed] [Google Scholar]
- 26.Flanagan K, Moroziewicz D, Kwak H, Horig H, Kaufman HL. The lymphoid chemokine CCL21 costimulates naive T cell expansion and Th1 polarization of non-regulatory CD4+ T cells. Cell Immunol. 2004;231:75–84. doi: 10.1016/j.cellimm.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 27.Huang QQ, Birkett R, Doyle R, Shi B, Roberts EL, Mao Q, Pope RM. The role of macrophages in the response to TNF inhibition in experimental arthritis. J Immunol. 2018;200:130–138. doi: 10.4049/jimmunol.1700229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Misslitz A, Pabst O, Hintzen G, Ohl L, Kremmer E, Petrie HT, Forster R. Thymic T cell development and progenitor localization depend on CCR28. J Exp Med. 2004;200:481–491. doi: 10.1084/jem.20040383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raychaudhuri S, Remmers EF, Lee AT, Hackett R, Guiducci C, Burtt NP, Gianniny L, Korman BD, Padyukov L, Kurreeman FA, Chang M, Catanese JJ, Ding B, Wong S, van der Helm-van Mil AH, Neale BM, Coblyn J, Cui J, Tak PP, Wolbink GJ, Crusius JB, van der Horst-Bruinsma IE, Criswell LA, Amos CI, Seldin MF, Kastner DL, Ardlie KG, Alfredsson L, Costenbader KH, Altshuler D, Huizinga TW, Shadick NA, Weinblatt ME, de Vries N, Worthington J, Seielstad M, Toes RE, Karlson EW, Begovich AB, Klareskog L, Gregersen PK, Daly MJ, Plenge RM. Common variants at CD40 and other loci confer risk of rheumatoid arthritis. Nat Genet. 2008;40:1216–1223. doi: 10.1038/ng.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Otero C, Groettrup M, Legler DF. Opposite fate of endocytosed CCR30 and its ligands: recycling versus degradation. J Immunol. 2006;177:2314–2323. doi: 10.4049/jimmunol.177.4.2314. [DOI] [PubMed] [Google Scholar]